Abstract
MicroRNAs (miRNAs) are discovered in science about 23 years ago. These are short, a series of non-coding, single-stranded and evolutionary conserved RNA molecules found in eukaryotic cells. It involved post-transcriptional fine-tune protein expression and repressing the target of mRNA in different biological processes. These miRNAs binds with the 3′-UTR region of specific mRNAs to phosphorylate the mRNA degradation and inhibit the translation process in various tissues. Therefore, aberrant expression in miRNAs induces numerous cardiovascular diseases and developmental defects. Subsequently, the miRNAs and Wnt singling pathway are regulating a cellular process in cardiac development and regeneration, maintain the homeostasis and associated heart diseases. In Wnt signaling pathway majority of the signaling components are expressed and regulated by miRNAs, whereas the inhibition or dysfunction of the Wnt signaling pathway induces cardiovascular diseases. Moreover, inadequate studies about the important role of miRNAs in heart development and diseases through Wnt signaling pathway has been exist still now. For this reason in present review we summarize and update the involvement of miRNAs and the role of Wnt signaling in cardiovascular diseases. We have discussed the mechanism of miRNA functions which regulates the Wnt components in cellular signaling pathway. The fundamental understanding of Wnt signaling regulation and mechanisms of miRNAs is quite essential for study of heart development and related diseases. This approach definitely enlighten the future research to provide a new strategy for formulation of novel therapeutic approaches against cardiovascular diseases.
Keywords: MicroRNAs, Wnt, Cardiovascular diseases, Heart development, Homeostasis
Graphical abstract
Highlights
-
•
Specific involvement of microRNAs and its role of Wnt signaling in cardiovascular diseases.
-
•
Cell activation and microRNAs in cardiovascular diseases.
-
•
MicroRNAs act as biomarkers and potential therapeutic targets for cardiovascular diseases.
1. Introduction
Heart disease is a leading cause of human morbidity, mortality, and physical disability worldwide. Present day the heart diseases are rising, and it has been found that about 10% of people globally will be suffering from heart failure of the total population in the year 2030 (Salinas and Lin, 2019). Major organ and multiple gene encoded protein signaling also affected by biological, genetic and vascular risk factors that influence several type heart diseases. Heart failure is caused by numbers of related heart diseases like myocardial ischemia, valve diseases, heart rhythm disorders, pericardial abnormalities and cardiac dysfunction (Ingall, 2004).
Wnt and Fzd (Frizzled) is significant protein molecules of signal transduction pathway where Wnt act as ligand for the Fzd proteins family. This signal transduction pathway mainly control tissue development, cells migrate and polarity processes (Qiang and Walsh, 2005). It also noted that a total 19 families of Wnt genes are identified in mammalian genomes, which have glycosylated protein containing 350–400 amino acids and consist with 22–24 cysteine residues (Janda and Garcia, 2015; Clevers, 2006). In molecular signaling pathway Wnt has two binding sites and consist of low-density receptor-related protein (LPR5/LPR6) (Bhanot and Brink, 1996; Joiner and Ke, 2013). The complex receptor of Wnt activates the β-catenin protein signaling, considering as a secondary messenger of canonical signaling pathway. It is phosphorylated by the ubiquitin pathway, consist with the destruction protein complex of axin, adenomatous polyposis coli (APC), casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK-3β) to showing its imminent activity (Yost and Torres, 1996). Therefore, when the lipoprotein receptor-like protein is activated, it formed intracellular adaptor disheveled protein (Dv1) to the plasma membrane. That dissociates and accumulates the β-catenin protein complex. The β-catenin protein migrates to the nucleus as secondary messenger and interacts with the transcription factors of TCF and LEF, and become activated the gene expression (Fagotto and Glück, 1998; MacDonald and Tamai, 2009). Several secondary signaling pathways are activated by Wnt protein, such as planar cell polarity (PCP) and Ca2+ pathway, to activate the protein kinase C (PKC) protein by β-catenin mediated molecular signaling (Veeman and Axelrod, 2003). Planer cell polarity pathway activates the Rock and Jnk-kinase pathway by G-protein Rho and Rac receptor and controls the cell orientation to adjacent cells (Carvajal-Gonzalez and Roman, 2016). But in Wnt/Ca2+ pathway activated phospholipase C (PLC) via heterotrimeric G proteins. That results in Ca2+ dependent enzyme rising in the cell that leads to activation of heart physiology like protein kinase C (PKC), calcium dependent kinase-II and calcineurin proteins (Kühl and Sheldahl, 2000).
The Wnt signaling pathway performed a major role in the gene expression pattern of cardiovascular diseases. A new point of intervention of the Wnt pathway may lead to low molecular compounds targeted by novel drugs. The pathway inhibitors outlined the cancer treatment and the effects of cardiac diseases of human being. We are providing an overview of the Wnt signaling pathway regulating mechanism with the role of β-catenin in gene expression and the effect of miRNAs against the cardiovascular diseases. We also summarize the key roles and figure out the involvement of miRNAs in Wnt signaling in the cardiovascular diseases. Thus, the signaling modulates an active condition in heart diseases by pharmacological events. Probably this is the first report may provide a novel direction for miRNAs mediated molecular signaling pathway for the treatment of heart diseases.
Recently, modern medical biotechnology demonstrated that the miRNAs have essential regulatory function and emerged as promising diagnostic and therapeutic tools against the high risk cardiac diseases (Ouyang and Wei, 2021). MicroRNAs are single-stranded, small size (19–25 nucleotides), evolutionary conserved RNA molecules with non-coding sequences (Mencía Castaño and Raftery, 2020). Generally, these are the epigenetic regulators that modulate the gene expression and primarily associate with posttranslational modifications (Ardekani and Naeini, 2010). In post transcriptional modification event's the miRNAs target messenger RNAs (mRNAs) to inhibit the translation and loaded into the RNA-induced silencing complex (RISC) (Vaghf and Khansarinejad, 2021). The RISC has endonuclease slicer that required for shaping of mRNAs and other structural core protein, e.g Argonaut protein (Ago2) (Hammond and Boettcher, 2001). The Ago2 protein associate with miRNAs complex that able to bind with ssRNAs and dsRNAs (Martinez and Patkaniowska, 2002).
Recent study revealed that the miRNAs regulates Wnt signaling pathways, and both are interlinking each other to implicated different biological processes in animal body. MicroRNAs are participating at every step of Wnt signaling pathway to performed a key role in Wnt protein regulation and expression (Ueno and Hirata, 2013). During cardiac vascular diseases miRNAs execute the positive or negative regulation of Wnt protein in Wnt signaling pathway (Su, Zhang et al., 2012; Inui, Martello et al., 2010). MicroRNAs are might be potential vital element in cardiac diseases as these are play an important role for signal transduction and cellular development of an organism (Bartels and Tsongalis, 2009). Therefore, the basic knowledge about the miRNAs mediated role of cellular mechanisms are used in therapeutic tools and diagnostic approaches for biological activities, and diseases at cellular level (Wang and Kwong, 2013; Espinoza-Lewis and Wang, 2012; CDC, 2001). Recently, scientific communities are still investigating about the therapeutic tools of miRNAs against heart diseases (Balatti and Acunzo, 2016). Moreover, in this review we briefly reviewed the biogenesis and function of miRNAs, mechanism of action in Wnt signaling pathway, and finally highlighted the functional aspects of miRNAs that are regulates to targeting the Wnt signaling pathway linked with the cardiovascular diseases.
2. Biosynthesis pathway of miRNAs
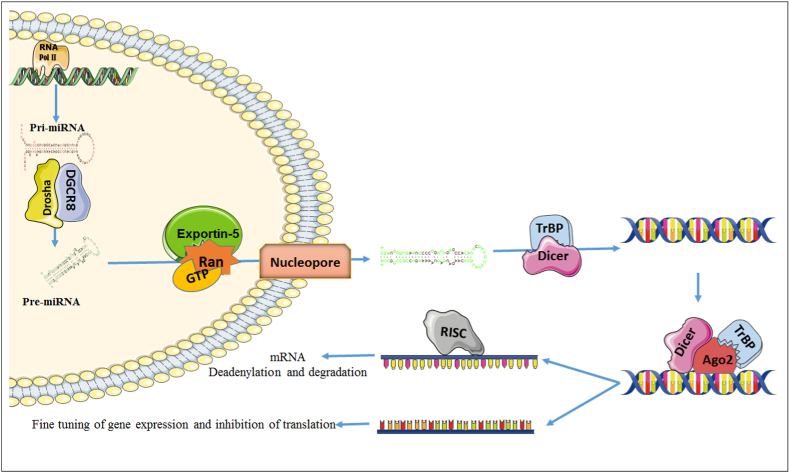
In miRNA biogenesis pathway multiple protein components regulates the mature miRNA structure and its functions (Cheloufi and Dos Santos, 2010). Similar to proteins, the genes coding for miRNAs are exist in DNA of the nucleus. Each gene is transcribed by RNA polymerase II and produces either a regulatory or messenger RNA. The miRNA profiling, gene regulation, and understanding their biogenesis mechanism as well as the expression patterns is very much significant in defining miRNAs biological roles (Yang and Lai, 2011; Ledda and Ottaggio, 2020). Molecular pathway of this whole process is accurate, organized, and stepwise composite procedure which initiated from the inter-nuclear region and persistent to the cytoplasmic part of the cell until its final production completed.
Firstly, the long primary miRNA (pri-miRNA) was sliced by drosha protein along with its co-factor the Di George syndrome chromosomal region 8 (DGCR8). These protein remove the tail portion and developed a hairpin or stem-loop likes precursor miRNA structure (Bhattacharya and Sharma, 2020). Afterwards one of the nucleocytoplasmic transporter proteins, exportin-5 of karyopherin family and Ran-GTP cofactor recognized the pre-microRNA and brings it to the cytoplasm region of the cell (Melo and Melo, 2014). Here, the RNase-III endonuclease together with Dicer complex and its cofactor element, trans-activator RNA binding protein (TRBP)/PKR-activating protein (PACT) remove the loop, resulting as formation of an asymmetrical double stranded mature microRNA (Maurin and Cazalla, 2012). This synthesized the miRNA is of 20–25 nucleotides long. Subsequently, the matured miRNA loaded on Ago2 protein and interacts with dicer molecule to bind the short chain of miRNA. Now the miRNA is unwound, and one strand is completely released. The remaining strand, called as guide strand link with Ago2 and some additional proteins to form the RISC (RNA induced silencing complex). The RISC can now leading to its target and inactivates one or multiple genes of mRNA sequence. The 3′UTR of mRNA (messenger RNA) of a targeted gene is complementary to the sequence of the miRNA, which enables the base pairing of match nucleotides pair (Fig. 1). Once it bound, there are three ways of RISC to inactivate the function of messenger RNA. Within the complex proteins can cut the mRNA chain and further degraded by deadenylation in the cell. Considering the inhibition of translation mechanism; the RISC complex inhibits the ribosomal subunits from binding to the specific mRNA strand. Therefore, in both cases mRNA will unable to translate any protein, and the gene will be silenced (Krol and Loedige, 2010). Besides that, miRNAs also participate in various biological functions and detected all types of human biological fluids. Therefore, it confirmed that miRNAs play an important role as biomarker in human diseases and developmental process (Bhattacharya and Sharma, 2020).
Fig. 1.
Graphical model of miRNA biogenesis, expression and their biological function in cell.
3. Wnt signaling pathway and miRNAs regulation
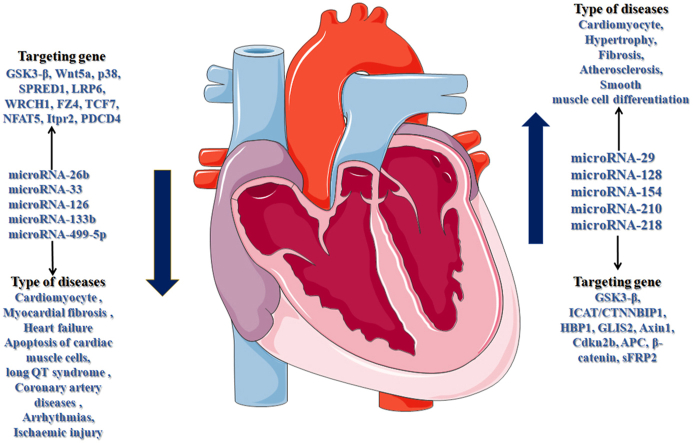
Wnt (Wingless and int-1) signaling pathways are evolutionarily conserved and are classified as canonical β-catenin-independent, non-canonical β-catenin-dependent, Wnt cell polarity and calcium pathways (Villarroel and del Valle-Pérez, 2020). It consists of 19 secreted glycoproteins, Wnt ligands, Fzd (Frizzled) receptors, associate co-receptor and scaffolding proteins (Astudillo, 2020). These proteins are employed in a diverse plethora of cellular activity. While these proteins are not functioning in cellular system, this signaling pathway is correlated with various diseases (Wang, de Marco et al., 2019; Cici, Corrado et al., 2019; Huang and Wei, 2018). From transcriptional regulation in the cytoplasm to post-translation modification of Wnt protein is tightly regulated at every level cellular expression (Ma and Hottiger, 2016). It also noted that the Wnt signaling mechanism is absent to result in phosphorylation of cytoplasmic β-catenin degraded by destruction complex with Axin, β-catenin, adenomatous polyposis coli (APC), Ser/Thr kinases GSK-3β and casein kinase I α (CKIα) protein subunit (MacDonald and Tamai, 2009; Tolwinski and Wieschaus, 2004). Simultaneously the destruction complex of GSK-3β and CKIα phosphorylate β-catenin also be degraded by ubiquitination (Clevers and Nusse, 2012). Whereas, the β-catenin play a vital role in Wnt signaling pathways since the lack of β-catenin in the nucleus results to inhibit the TCF-mediated activation of targeted signaling genes. When the TCF protein is present and accumulates the β-catenin in the cytoplasm, the complex has undergoes in nuclear translocation, activation and finally initiates the transcription of heart related genes (Rao and Kühl 2010). Subsequently, the inhibitory factors protein Dickkof (DKK) bind to the extracellular Wnt ligands, and secreted the Frizzled-related protein (sFRP) family, LRP6 or LRP5 and Wnt inhibitory factor 1 (WIF1) that inhibits the active status of β-catenin (Kawano and Kypta, 2003; He and Semenov, 2004; Clevers, 2006). Dickkof protein have consist four members of secreted glycoproteins, these are bind with low-density lipoprotein receptor related protein (LPR) and initiate the Wnt signal inhibition with high affinities (Bafico and Liu, 2001). Recent studies have seen the numbers of cardiovascular disease are performed a very important factor in sudden heart attacks. These diseases are cardiac hypertrophy, arrhythmias, fibrosis, coronary artery diseases, myocardial infarction and heart failure due to the lack of Wnt signaling protein activation (Calore and Lorenzon, 2019; Raso and Dirkx, 2019). The genetic mutation in Wnt protein families in Wnt signaling pathway causes cardiovascular diseases within the human body. Thus, the Wnt protein performed a crucial role in cardiovascular diseases at recent era of modern peoples (Fig. 2). The regulation mechanism of mRNA directly depends on miRNAs binding to the corresponding 3′-UTR sequence in the target mRNA gene at diverse levels, and controlling the Wnt signal transduction in cardiovascular diseases (Bhat and Jarmolowski, 2016). Several studies found that miRNAs act as a vital element in Wnt signaling pathway, these are miR-1, miR-16, miR-27b, miR-30d, miR-126, and miR-133 (Thum and Catalucci, 2008; Ardekani and Naeini, 2010). The human miRNAs are annotated by their gene ID of human genome organization and its mature sequences (Hayashi and Chuva de Sousa Lopes, 2008). These all miRNAs which regulate the Wnt signaling pathway associated components and have significant impacts on various cardiovascular diseases is listed in Table 1.
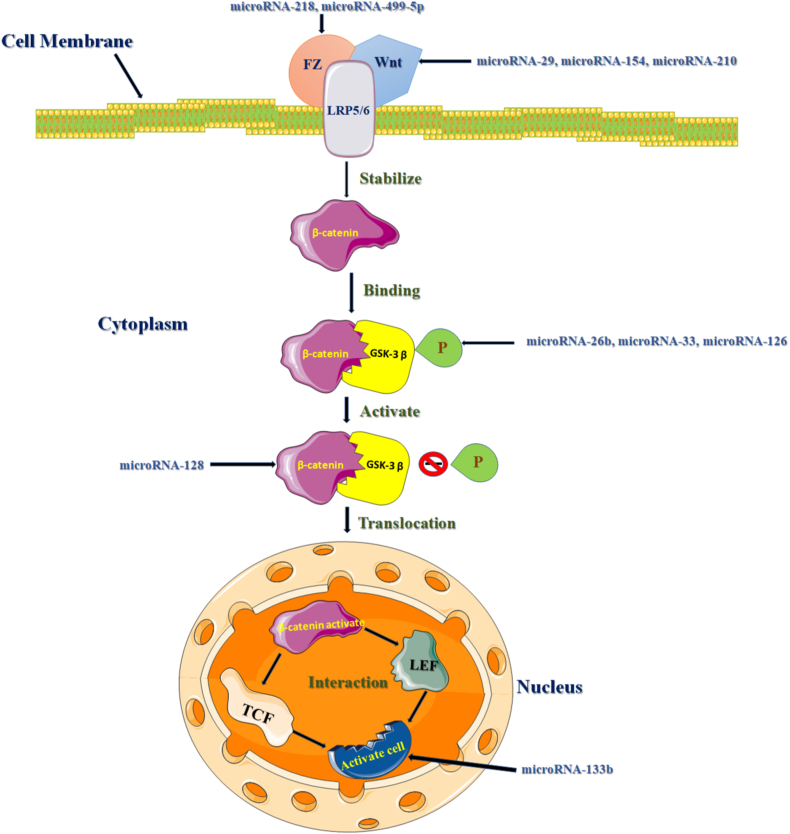
Fig. 2.
Concise model shows that intervention in Wnt signaling pathway regulated by miRNAs to activate the cardiac cells.
Table 1.
A Brief outline of miRNAs regulates the Wnt signaling pathway associated components and its impacts on various cardiovascular diseases.
| Sl. no | Names of the miRNA | Target gene | Possible role in Wnt signaling pathway | Type of cardiovascular diseases | References |
|---|---|---|---|---|---|
| 1. | hsa-miR-26b | GSK3-β and Wnt5a | Inhibition of β-catenin activity | Cardiomyocyte | (Wang and Liu, 2017) |
| 2. | hsa-miR-29a | GSK3-β, ICAT/CTNNBIP1, HBP1, and GLIS2 | Regulate the Wnt/β-catenin signaling activity | Hypertrophic cardiomyopathy, fibrosis | (Rubiś and Totoń-Żurańska, 2017) (Sassi and Avramopoulos, 2017) |
| 3. | hsa-mir-29c | GSK3-β, ICAT/CTNNBIP1, HBP1, and GLIS2 | Regulate the Wnt/β-catenin signaling activity | Cardiomyocyte hypertrophy, fibrosis | (Roncarati and Viviani Anselmi, 2014) (Sassi and Avramopoulos, 2017) |
| 4. | hsa-mir-33a | GSK3-β, p38 | Negatively Regulates Wnt/β-Catenin signaling pathway | Myocardial fibrosis | (Fang and Feng, 2013) (Chen and Ding, 2018) |
| 5. | hsa-miR-126 | GSK3-β, SPRED1, LRP6, WRCH1, FZ4 | Suppressing Wnt/β-catenin signaling | Heart failure | (Kura and Parikh, 2019) (Song and Nigam, 2015) |
| 6. | hsa-mir-128 | Axin1 | Promotes Wnt1/β-catenin activation | Cardiac hypertrophy | (Li and Li, 2021) |
| 7. | hsa-miR-133b | TCF7, NFAT5, Itpr2 | Wnt signalling mediates for the transcriptional control of Dnmt3b in cardiac cells | Apoptosis of cardiac muscle cells long QT syndrome and dilated cardiomyopathy, coronary artery diseases | (Wang and Rao, 2019) (Katoh and Katoh, 2006) (Rubiś and Totoń-Żurańska, 2017) (Di Mauro and Crasto, 2019) |
| 8. | hsa-mir-154 | Cdkn2b | – | Hypertrophy | (Bernardo and Nguyen, 2016) |
| 9. | hsa-miR-210 | APC | Regulating Wnt/β-catenin signaling activity | Atherosclerosis, smooth muscle cell differentiation | (Eken and Jin, 2017) |
| 10. | hsa-miR-218 | β-catenin, P-FZD, sFRP2 | enhanced Wnt/β-catenin signaling activity | Induced hypertrophy in cardiomyocytes | (Song and Nigam, 2015) (Liu and Zhao, 2016) (Hu and Sun, 2017) |
| 11. | hsa-miR-499-5p | PDCD4 | – | Arrhythmogenic cardiomyopathy/arrhythmias, ischaemic injury | (Calore and Lorenzon, 2019) (Li and Lu, 2016) |
3.1. GSK-3β protein
The GSK-3β (glycogen synthase kinase-3β) protein is associated with cardiovascular diseases and also involved in the Wnt signaling pathway. This protein based enzyme is a multifunctional proline rich serine-threonine kinase residue coded by the human GSK-3β gene and a key regulator of insulin-dependent glycogen synthesis (Stambolic and Woodgett, 1994; Doble and Woodgett, 2003; Luo, 2009). The GSK-3β protein is inactivating mediator and a phosphorylating agent of enzyme glycogen synthase within the eIF2B enzymes component. It also observed that GSK-3β protein is directly involved in heart growth, metabolism and developmental stages of embryonic conditions. Basically, the GSK-3β protein undergoes phosphorylation at position of Ser9/21 amino acid as an inhibitory domain that inactivates in the heart failure patients (Haq and Choukroun, 2001; Doble and Woodgett, 2003; Woodgett, 2001). When the GSK-3β protein is phosphorylated the overexpression of protein prevents heart development and cardiac diseases (Michael and Haq, 2004). But in active cases, the GSK-3β protein primarily induces heart failure. Accumulation of β-catenin in the nucleus built a protein complex with TCF/LEF during the inactivation of GSK-3β protein functions (Simon and Grandage, 2005). Besides that, the cellular regulations also carried out by different proteins such as β-catenin, c-myc, cyclin D1, cycline E, and p21CIP1 these are phosporylated by GSK-3β. Researcher also noted that the GSK-3β protein act as a vital role in the destruction of ubiquitin-dependent Wnt signaling pathway (Stakheev, 2020).
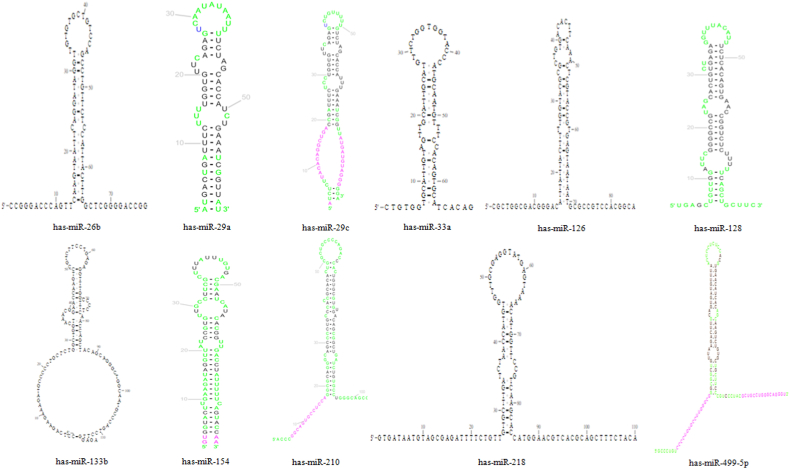
Earlier study aso found that miR-26b is bound with the GSK-3β encoded gene. This miRNA consist with 77 nts long nucleotides and encoded from chromosome Ch2q35 in human (Gao and Ye, 2021). It affects β-catenin activities and translocation to the nucleus with downregulated function subsequently the up regulation of the protein C-myc and cyclin D1 expression (El Sabeh and Saha, 2021; Xie and Li, 2021). Conversely, the miRNA-33a is retained on chromosome Ch22q13.2 position of MIR33A gene and exist as 69 nts sequence in human. It has a vital role in Wnt signaling pathway regulation in cardiac diseases. Several studies have found that the expression of miRNA-33a is mediated the down regulation of Pim-3 kinase enzyme caused the pancreatic diseases (Liang and Yu, 2015; Romaine and Tomaszewski, 2015). Both the miRNA-33a and miRNA-26b together inhibit the functions of β-catenin and GSK-3β protein which is lead the cardiovascular diseases. Recently, some findings demonstrated that the GSK-3β protein phosphorylates by the action of miRNA-126. This miRNA has situated at human chromosome ch9q34.3 and 85 nts long sequence of the miRNA-126 gene. The miRNA-126 is involved in reduced phosphorylation activity of GSK-3β which might be disturbed throughout the heart tissue (Guo and Ji, 2022). It also regulates the IGF-I, PI3K, insulin, and AKT signaling pathway in human body (Huang and Fang, 2013; Sonntag and Woo, 2012; Lechman and Gentner, 2012). Furthermore, the potential phosphorylation activity of miRNA-33a and miRNA-26b initiates cardiac diseases via targeting the GSK-3β protein within the Wnt signaling pathway (Zhang and Han, 2021). The significant human miRNAs stem-loop or secondary structure interlinking with the Wnt signaling pathway in cardiovascular diseases are shown in Fig. 3.
Fig. 3.
Collection of human miRNAs stem-loop or secondary structure interlinking with Wnt signaling pathway in cardiovascular diseases, (collected from the https://rnacentral.org/and https://asia.ensembl.org/index.html).
One abundant miRNA (miR-128) expressed high level in various types of cardiovascular diseases. It exists in the chromosomal position of Ch2q21.3 and consist 82 nts long chain of nucleotide bases. This miRNA regulates the upstream direction and inhibits the GSK-3β protein function, and also accumulates β-catenin for translocate to the nucleus. The β -catenin bind to the TCF/LEF protein that might leads to the gene activation of cardiac cells and promote associate cardiovascular diseases (Foulquier and Daskalopoulos, 2018).
3.2. Protein complex (FZD-WNT-LRP5/6)
In the activation state of the Wnt signaling pathway, the Wnt ligand protein bind with cellular membrane protein (frizzled receptor and LRP5/6 proteins). The frizzled protein is a G-protein coupled receptor that is more essential for Wnt pathway (Malbon, 2004). These trimeric protein complex phosphorylated by the protein kinase and inhibits the activity of β-catenin and block the phosphorylation of GSK-3β. Consequently the β-catenin is accumulates in the cytoplasm and followed by nuclear translocation in the nucleus. The GSK-3β protein dissociates with β-catenin and result into the binding with transcription factors TCF/LEF in the nucleus. Whereas, the transcription factors activated the Wnt responsive gene and expressed the linked disorders (Wang and Shu, 2005; Nusse and Clevers, 2017). This trimeric protein complex controls the numbers of biological activities like growth, metabolisms and development of cardiovascular diseases. These cardiovascular diseases are negatively regulated by miRNA-154, miRNA-499 and positively regulated by miRNA-210, miRNA-218 and miRNA-29 (Sun and Liu, 2021; Foulquier and Daskalopoulos, 2018). The miRNA-154 has encoded from the chromosomal position Ch14q32.31 and 84 nts long, whereas microRNA-499 has 122 nts base pair length and coded from the Ch20q11.22 chromosome position in human (Ibrahim and Fritz, 2021; Wang and Zhang, 2021). However, the miRNA-29 family, namely miRNA-29a/c found in chromosome position is Ch7q32.3 and Ch1q32.2, respectively. It also noted that the miRNA-154 and miRNA-499 suppress the Wnt signaling pathway. The miRNA-210 and miRNA-218 are equal 110 nts long and induce the Wnt signaling by blocking the APC, DKK and sFRPs receptor protein. This phenomena offered the Wnt protein plays a vital role in cardiovascular diseases.
3.3. Transcription factors family protein
The TCF/LEF protein family member plays vital role for heart development during the embryonic stage and different types of heart disease in adult condition. The transcription factors family protein interlinked with the cofactors that regulated by the Wnt protein subunit. The TCF7L1 protein is stage dependent and very enigmatic; it may be specially required for heart formation. Conversely deletion of TCF7L1 protein led to cardiomyocyte formation as the study was carried out on the mouse model (Athanasouli and Balli, 2022). TCF7L12 is the main TCF (T-cell factor) member of β-catenin protein expressed in CMs (cardiomyocytes), particularly during the late stage of cardiogenesis. During heart development and maturation stage the TCF7 and LEF1 protein decreased while TCF7L1 and TCF7L12 remain moderately constant level (Ye and Li, 2019). The TCF7L12 targets and restrict the cardiac function of heart in diseased condition (Iyer and Nagarajan, 2018). However, multiple factors promote the target gene of cardiovascular diseases because the activation of TCF/LEF protein stimulates the heart functions at the cardiovascular disease stage. The miRNA-133b/a functionally lowering the activities of β-catenin and TCF/LEF (Lin and Lin, 2021). The miRNA-133b/a consist with 119 nts and encoded from the Ch6p12.2 in humans. Previously, limited number of studies shown on the involvement of miRNAs in cardiovascular diseases through Wnt signalling pathway. Therefore, it is essential to do more scientific research on miRNAs as considering potential and functional aspects of molecular biology. Additional experimental and laboratory based studies are also required to identify how the Wnt signalling protein family regulates heart diseases coupled with the miRNAs.
4. miRNAs mediated cell activation of cardiovascular diseases
The miR-133b/a and miR-1 are dynamic muscle-specific miRNAs which helps in calcium signaling, cell growth and cellular development (Dong and Chen, 2010). In intracellular Ca2+ regulation both the miR-133b/a and miR-1 reduced cardiac hypertrophy with the down regulation of mRNA and calcineurin protein (Dong and Chen, 2010; Liu and Bezprozvannaya, 2008). These miRNAs also blocked cardiomyocyte hypertrophy by the effector's gene NFATC4. The GSK-3β inhibits by Wnt protein complex and activates the 3β-catenine protein unit. This 3β-catenine protein also phosphorylate and transfer from cytoplasm to the nucleus and attached with the transcription factors protein (TCF/LEF) to regulates the Wnt signaling pathway. The miRNA-1 also promotes cardiomyocyte hypertrophy in human embryonic stem cell, which is a multipotent progenitor that suppress Wnt signaling pathway (Lu and Lin, 2013). Another pro-hypertrophic miRNA-29 positively regulates the cardiac fibrosis. It prevents TAC (Transverse aortic constriction)-induced cardiomyocyte hypertrophy and inhibits the genetic deficiency. But during the overexpression of miR-29 it directly promotes phenylephrine-induced cardiomyocyte hypertrophy (Sassi and Avramopoulos, 2017; Roncarati and Viviani Anselmi, 2014). Such phenomenon was induced by the Wnt signaling inhibitory factors such as GSK3β, ICAT/CTNNBIP1, HBP1 and GLIS2 proteins (Wehbe and Nasser, 2019). Additionally, the human miRNAs and their HGNC ID, chromosome location, gene regulation, pre-miRNAs nucleotide length, the mature sequence and the map accession number interrelated with the Wnt signaling pathway in cardiovascular diseases are also listed in Table 2.
Table 2.
Various human miRNAs and their HGNC ID, chromosome location, gene regulation, pre-microRNAs nucleotide length, the mature sequence and the map accession number interrelated with Wnt signaling pathway in cardiovascular diseases.
| Names of the miRNA | HGNC | Chromosome location | Gene regulation | Nucleotide length (nt) of pre-miRNA | Mature sequence of miRNA | miRNA map accession no. |
|---|---|---|---|---|---|---|
| hsa-miR-26b | 31612 | Ch2q35 | DOWN | 77 nt | 47 - CCUGUUCUCCAUUACUUGGCU - 67 | MI0000084 |
| hsa-miR-29a | 31616 | Ch7q32.3 | UP | 64 nt | 42 -UAGCACCAUCUGAAAUCGGUUA - 63 | MI0000087 |
| hsa-mir-29c | 31621 | Ch1q32.2 | UP | 88 nt | 54| UAGCACCAUUUGAAAUCGGUUA |75 | MI0000735 |
| hsa-mir-33a | 31634 | Ch22q13.2 | DOWN | 69 nt | 46| CAAUGUUUCCACAGUGCAUCAC |67 | MI0000091 |
| hsa-miR-126 | 31508 | Ch9q34.3 | DOWN | 85 nt | 52 - UCGUACCGUGAGUAAUAAUGCG - 73 | MI0000471 |
| hsa-mir-128 | 31510 | Ch2q21.3 | UP | 82 nt | 50-UCACAGUGAACCGGUCUCUUU-70 | MI0000447 |
| hsa-miR-133b | 31759 | Ch6p12.2 | DOWN | 119 nt | 66 - UUUGGUCCCCUUCAACCAGCUA - 87 | MI0000822 |
| hsa-mir-154 | 31541 | Ch14q32.31 | UP | 84 nt | 51-AAUCAUACACGGUUGACCUAUU-72 | MI0000480 |
| hsa-miR-210 | 31587 | Ch11p15.5 | UP | 110 nt | 66 - CUGUGCGUGUGACAGCGGCUGA - 87 | MI0000286 |
| hsa-miR-218 | 31595 | Ch4p15.31 | UP | 110 nt | 68-AUGGUUCCGUCAAGCACCAUGG-89 | MI0000294 |
| hsa-miR-499-5p | 32133 | Ch20q11.22 | DOWN | 122 nt | 33 - UUAAGACUUGCAGUGAUGUUU - 53 | MI0003183 |
5. Future perspectives
The miRNA-based research and its future applications for clinical purposes are increasing in faster ways. Within a short period (last decade) from the first reporting of miRNAs in human body has been considered an effective novel tool for diagnosis and therapeutics purposes linked with cardiovascular diseases. Nevertheless, there also still much more significant things that remain to understand. In the outlook of the current research viewpoint, more advanced studies are required to illuminate this precise approach. In fact the miRNAs are competent to suppress the protein translation and initiate the process of mRNA degradation pathways. Likewise, the particular sources, positions and roles of miRNA in light of the cell to cell molecular communication are need to be understood in a well-defined state.
Furthermore, the miRNAs also served as important prognostic and diagnostic biomarkers in the perception of diverse clinical approaches. The superior clinical trials or research analysis are prerequisites factor to establishing whether the current miRNAs candidates offered added advantages over and above those of other prevailing known biomarkers of cardiovascular diseases. Currently, more technological progress are essential to facilitated the quick, consistent and reproducible results targeted to the absolute quantification of cardiovascular diseases linked with miRNAs to facilitated the transition into an effective clinical practices.
Lastly, while there continue a number of vital challenges to overwhelmed, researchers should be aware about the impending arrival of miRNA-based therapeutics within the specified domain of clinical medicine for cardiovascular diseases.
6. Conclusions
Nowadays, human miRNAs can possibly be used as the new promising elements for novel personalized therapies. Numerous scientific reports previously highlighted about the usefulness of miRNA based treatments (in vivo) model. Even through, more significant studies are urgently required to translate these miRNA regulated gene interaction results into the bench to clinical applications. Additionally, the foremost effort also needs to address to test the multiple safety parameters of the miRNAs delivery systems, dosage, and mode of administration, time span of the treatments, the occurrence and also the prevention of side effects specific for the cardiovascular diseases.
Thus, it has been scientifically confirmed that the miRNAs performed a crucial role in numerous types of cardiovascular pathologies. The preliminary studies of Wnt signaling pathway and miRNAs already shown high promises for the scientific uses of miRNA as novel biomarkers and even therapeutic targets in the near future.
CRediT authorship contribution statement
Jiban Kumar Behera: Writing – original draft, Validation, Investigation. Manojit Bhattacharya: Conceptualization, Formal analysis, Writing – review & editing. Pabitra Mishra: Visualization, Data curation, Formal analysis. Akansha Mishra: Visualization, Validation. Adya Anindita Dash: Visualization, Validation. Niladri Bhusan Kar: Visualization, Validation. Bhaskar Behera: Validation, Formal analysis. Bidhan Chandra Patra: Supervision, Validation, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank to the authorities of Fakir Mohan University and Vidyasagar University.
Abbreviation
- miRNA
MicroRNA
- UTR
Untranslated region
- Fzd
Frizzled
- APC
Adenomatous polyposis coli
- CK1
Casein kinase 1
- GSK-3β
Glycogen synthase kinase 3β
- Dv1
Disheveled protein
- PKC
Protein kinase C
- TCF
T-cell factor
- LEF
Lymphoid enhancer factor
- PCP
Planar cell polarity
- PLC
Phospholipase C
- RISC
RNA-induced silencing complex
- Ago
Argonaut
- DGCR8
Di George syndrome chromosomal region 8
- TRBP
Trans-activator RNA binding protein
- PACt
PKR-activating protein
- sFRP
Frizzled-related protein
Data availability
No data was used for the research described in the article.
References
- Ardekani A.M., Naeini M.M. The role of microRNAs in human diseases. Avicenna J. Med. Biotechnol. (AJMB) 2010;2(4):161. [PMC free article] [PubMed] [Google Scholar]
- Astudillo P. Wnt5a signaling in gastric cancer. Front. Cell Dev. Biol. 2020;8:110. doi: 10.3389/fcell.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasouli P., Balli M., et al. The Wnt/TCF7L1 transcriptional repressor axis drives primitive endoderm formation by antagonizing naive and formative pluripotency. bioRxiv. 2022 doi: 10.1101/2022.05.18.492419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A., Liu G., et al. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001;3(7):683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Balatti V., Acunzo M., et al. Novel mechanisms of regulation of miRNAs in CLL. Trend. Cancer. 2016;2(3):134–143. doi: 10.1016/j.trecan.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C.L., Tsongalis G.J. MicroRNAs: novel biomarkers for human cancer. Clin. Chem. 2009;55(4):623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- Bernardo B.C., Nguyen S.S., et al. Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P., Brink M., et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382(6588):225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bhat S.S., Jarmolowski A., et al. MicroRNA biogenesis: epigenetic modifications as another layer of complexity to the microRNA expression regulation. Acta Biochim. Pol. 2016;63(4):717–723. doi: 10.18388/abp.2016_1370. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M., Sharma A.R., et al. Interaction between miRNAs and signaling cascades of Wnt pathway in chronic lymphocytic leukemia. J. Cell. Biochem. 2020;121(11):4654–4666. doi: 10.1002/jcb.29683. [DOI] [PubMed] [Google Scholar]
- Calore M., Lorenzon A., et al. A novel murine model for arrhythmogenic cardiomyopathy points to a pathogenic role of Wnt signalling and miRNA dysregulation. Cardiovasc. Res. 2019;115(4):739–751. doi: 10.1093/cvr/cvy253. [DOI] [PubMed] [Google Scholar]
- Carvajal-Gonzalez J.M., Roman A.-C., et al. Positioning of centrioles is a conserved readout of Frizzled planar cell polarity signalling. Nat. Commun. 2016;7(1):1–9. doi: 10.1038/ncomms11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloufi S., Dos Santos C.O., et al. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Ding H.-S., et al. MiR-33 promotes myocardial fibrosis by inhibiting MMP16 and stimulating p38 MAPK signaling. Oncotarget. 2018;9(31) doi: 10.18632/oncotarget.25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cici D., Corrado A., et al. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int. J. Mol. Sci. 2019;20(22):5552. doi: 10.3390/ijms20225552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- CDC Prevalence of disabilities and associated health conditions among adults--United States, 1999. Morbid. Mortal. Week. Rep. 2001;50(7):120–125. [PubMed] [Google Scholar]
- Di Mauro V., Crasto S., et al. Wnt signalling mediates miR-133a nuclear re-localization for the transcriptional control of Dnmt3b in cardiac cells. Sci. Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-45818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble B.W., Woodgett J.R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116(7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D.-L., Chen C., et al. Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy. Hypertension. 2010;55(4):946–952. doi: 10.1161/HYPERTENSIONAHA.109.139519. [DOI] [PubMed] [Google Scholar]
- Eken S.M., Jin H., et al. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ. Res. 2017;120(4):633–644. doi: 10.1161/CIRCRESAHA.116.309318. [DOI] [PubMed] [Google Scholar]
- El Sabeh M., Saha S.K., et al. Simvastatin inhibits Wnt/β-catenin pathway in uterine leiomyoma. Endocrinology. 2021;162(12):bqab211. doi: 10.1210/endocr/bqab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza-Lewis R.A., Wang D.-Z. MicroRNAs in heart development. Curr. Top. Dev. Biol. 2012;100:279–317. doi: 10.1016/B978-0-12-387786-4.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Glück U., et al. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 1998;8(4):181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Fang Y., Feng Y., et al. Aflatoxin B1 negatively regulates Wnt/β-catenin signaling pathway through activating miR-33a. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0073004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier S., Daskalopoulos E.P., et al. WNT signaling in cardiac and vascular disease. Pharmacol. Rev. 2018;70(1):68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Ye X., et al. miR-26b regulates cell proliferation and apoptosis of CD117+ CD44+ ovarian cancer stem cells by targeting PTEN. Eur. J. Histochem.: EJH. 2021;65(1) doi: 10.4081/ejh.2021.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Ji Q., et al. Naringin attenuates acute myocardial ischemia-reperfusion injury via miR-126/GSK-3β/β-catenin signaling pathway. Acta Cir. Bras. 2022;37 doi: 10.1590/acb370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S.M., Boettcher S., et al. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293(5532):1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Haq S., Choukroun G., et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103(5):670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Chuva de Sousa Lopes S.M., et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One. 2008;3(3) doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Semenov M., et al. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Hu F., Sun B., et al. MiR-218 induces neuronal differentiation of ASCs in a temporally sequential manner with fibroblast growth factor by regulation of the Wnt signaling pathway. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/srep39427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Fang Z.-f., et al. Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biol. Chem. 2013;394(9):1223–1233. doi: 10.1515/hsz-2013-0107. [DOI] [PubMed] [Google Scholar]
- Huang G.-R., Wei S.-J., et al. Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. World J. Gastroenterol. 2018;24(36):4178. doi: 10.3748/wjg.v24.i36.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim E., Fritz A., et al. A putative tumor suppressing role of hsa-miR-154 in breast cancer that acts by targeting CLOCK gene. Res. Square. 2021 doi: 10.21203/rs.3.rs-278396/v1. [DOI] [Google Scholar]
- Ingall T. Stroke-incidence, mortality, morbidity and risk. J. Insur. Med. 2004;36:143–152. [PubMed] [Google Scholar]
- Inui M., Martello G., et al. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010;11(4):252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- Iyer L.M., Nagarajan S., et al. A context-specific cardiac β-catenin and GATA4 interaction influences TCF7L2 occupancy and remodels chromatin driving disease progression in the adult heart. Nucleic Acids Res. 2018;46(6):2850–2867. doi: 10.1093/nar/gky049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C.Y., Garcia K.C. Wnt acylation and its functional implication in Wnt signalling regulation. Biochem. Soc. Trans. 2015;43(2):211–216. doi: 10.1042/BST20140249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner D.M., Ke J., et al. LRP5 and LRP6 in development and disease. Trends Endocrinol. Metabol. 2013;24(1):31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M., Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3ß to regulate ß-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 2006;5(9):1059–1064. doi: 10.4161/cbt.5.9.3151. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116(13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Krol J., Loedige I., et al. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Kühl M., Sheldahl L.C., et al. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16(7):279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kura B., Parikh M., et al. The influence of diet on microRNAs that impact cardiovascular disease. Molecules. 2019;24(8):1509. doi: 10.3390/molecules24081509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechman E.R., Gentner B., et al. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell. 2012;11(6):799–811. doi: 10.1016/j.stem.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda B., Ottaggio L., et al. Small RNAs in eucaryotes: new clues for amplifying microRNA benefits. Cell Biosci. 2020;10(1):1–13. doi: 10.1186/s13578-019-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Li X.-C., et al. Upregulation of miR-128 mediates heart injury by activating wnt/β-catenin signaling pathway in heart failure mice. Organogenesis. 2021;17(3–4):27–39. doi: 10.1080/15476278.2021.2020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Lu J., et al. MiR-499-5p protects cardiomyocytes against ischaemic injury via anti-apoptosis by targeting PDCD4. Oncotarget. 2016;7(24) doi: 10.18632/oncotarget.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Yu X.-J., et al. MicroRNA-33a-mediated downregulation of Pim-3 kinase expression renders human pancreatic cancer cells sensitivity to gemcitabine. Oncotarget. 2015;6(16) doi: 10.18632/oncotarget.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Lin F., et al. Effect of miR-133b on progression and cisplatin resistance of triple-negative breast cancer through FGFR1-wnt-β-catenin axis. Am. J. Tourism Res. 2021;13(6):5969. [PMC free article] [PubMed] [Google Scholar]
- Liu J.-J., Zhao C.-M., et al. miR-218 involvement in cardiomyocyte hypertrophy is likely through targeting REST. Int. J. Mol. Sci. 2016;17(6):848. doi: 10.3390/ijms17060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Bezprozvannaya S., et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22(23):3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T.-Y., Lin B., et al. Overexpression of microRNA-1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J. Mol. Cell. Cardiol. 2013;63:146–154. doi: 10.1016/j.yjmcc.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273(2):194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Hottiger M.O. Crosstalk between Wnt/β-catenin and NF-κB signaling pathway during inflammation. Front. Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B.T., Tamai K., et al. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C.C. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front. Biosci. Landmark. 2004;9(2):1048–1058. doi: 10.2741/1308. [DOI] [PubMed] [Google Scholar]
- Martinez J., Patkaniowska A., et al. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110(5):563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Maurin T., Cazalla D., et al. RNase III-independent microRNA biogenesis in mammalian cells. RNA. 2012;18(12):2166–2173. doi: 10.1261/rna.036194.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C.A., Melo S.A. Springer; 2014. MicroRNA Biogenesis: Dicing Assay; pp. 219–226. RNA Mapping. [DOI] [PubMed] [Google Scholar]
- Mencía Castaño I., Raftery R.M., et al. Cell Engineering and Regeneration; 2020. microRNA Modulation; pp. 511–576. [Google Scholar]
- Michael A., Haq S., et al. Glycogen synthase kinase-3β regulates growth, calcium homeostasis, and diastolic function in the heart. J. Biol. Chem. 2004;279(20):21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Ouyang Z., Wei K. miRNA in cardiac development and regeneration. Cell Regen. 2021;10(1):1–21. doi: 10.1186/s13619-021-00077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Y.-W., Walsh K., et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106(5):1786–1793. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T.P., Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ. Res. 2010;106(12):1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Raso A., Dirkx E., et al. Therapeutic delivery of miR-148a suppresses ventricular dilation in heart failure. Mol. Ther. 2019;27(3):584–599. doi: 10.1016/j.ymthe.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaine S.P., Tomaszewski M., et al. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101(12):921–928. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarati R., Viviani Anselmi C., et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2014;63(9):920–927. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- Rubiś P., Totoń-Żurańska J., et al. Relations between circulating microRNAs (miR-21, miR-26, miR-29, miR-30 and miR-133a), extracellular matrix fibrosis and serum markers of fibrosis in dilated cardiomyopathy. Int. J. Cardiol. 2017;231:201–206. doi: 10.1016/j.ijcard.2016.11.279. [DOI] [PubMed] [Google Scholar]
- Salinas J., Lin H., et al. Whole blood microRNA expression associated with stroke: results from the Framingham Heart Study. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0219261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi Y., Avramopoulos P., et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017;8(1):1–11. doi: 10.1038/s41467-017-01737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Grandage V.L., et al. Constitutive activation of the Wnt/β-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24(14):2410–2420. doi: 10.1038/sj.onc.1208431. [DOI] [PubMed] [Google Scholar]
- Song J.L., Nigam P., et al. microRNA regulation of Wnt signaling pathways in development and disease. Cell. Signal. 2015;27(7):1380–1391. doi: 10.1016/j.cellsig.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag K.C., Woo T.-U.W., et al. Converging miRNA functions in diverse brain disorders: a case for miR-124 and miR-126. Exp. Neurol. 2012;235(2):427–435. doi: 10.1016/j.expneurol.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakheev D. Wnt/beta-catenin and mTOR signaling in regulation of T-cell phenotype and cytotoxic activity for adoptive cellular immunotherapy of cancer. 2020. http://hdl.handle.net/20.500.11956/121237
- Stambolic V., Woodgett J.R. Mitogen inactivation of glycogen synthase kinase-3 β in intact cells via serine 9 phosphorylation. Biochem. J. 1994;303(3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Zhang A., et al. MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int. J. Oncol. 2012;40(4):1162–1170. doi: 10.3892/ijo.2011.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Liu S., et al. Current status of microRNAs that target the wnt signaling pathway in regulation of osteogenesis and bone metabolism: a review. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res.: Int. Med. J. Exper. Clin. Res. 2021;27 doi: 10.12659/MSM.929510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T., Catalucci D., et al. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc. Res. 2008;79(4):562–570. doi: 10.1093/cvr/cvn137. [DOI] [PubMed] [Google Scholar]
- Tolwinski N.S., Wieschaus E. Rethinking WNT signaling. Trends Genet. 2004;20(4):177–181. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Ueno K., Hirata H., et al. Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int. J. Cancer. 2013;132(8):1731–1740. doi: 10.1002/ijc.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghf A., Khansarinejad B., et al. The role of microRNAs in diseases and related signaling pathways. Mol. Biol. Rep. 2021:1–13. doi: 10.1007/s11033-021-06725-y. [DOI] [PubMed] [Google Scholar]
- Veeman M.T., Axelrod J.D., et al. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Villarroel A., del Valle-Pérez B., et al. Src and Fyn define a new signaling cascade activated by canonical and non-canonical Wnt ligands and required for gene transcription and cell invasion. Cell. Mol. Life Sci. 2020;77(5):919–935. doi: 10.1007/s00018-019-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liu C., et al. Impact of miR-26b on cardiomyocyte differentiation in P19 cells through regulating canonical/non-canonical Wnt signalling. Cell Prolif. 2017;50(6) doi: 10.1111/cpr.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Rao N., et al. Analysis of potential roles of combinatorial microRNA regulation in occurrence of valvular heart disease with atrial fibrillation based on computational evidences. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.Q., Kwong Y.L., et al. Epigenetic inactivation of miR-9 family microRNAs in chronic lymphocytic leukemia-implications on constitutive activation of NFκB pathway. Mol. Cancer. 2013;12(1):1–9. doi: 10.1186/1476-4598-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., de Marco P., et al. Update on the role of the non-canonical Wnt/planar cell polarity pathway in neural tube defects. Cells. 2019;8(10):1198. doi: 10.3390/cells8101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang F., et al. Resolution factor 15-epi-Lipoxin A4 modulates miRNA-499 induced differentiation of cardiosphere-derived stem cells through dual inhibition of Wnt/β-catenin and TGFβ/SMAD signalling axes. J. King Saud Univ. Sci. 2021;33(7) [Google Scholar]
- Wang Z., Shu W., et al. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol. Cell Biol. 2005;25(12):5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbe N., Nasser S.A., et al. MicroRNAs in cardiac hypertrophy. Int. J. Mol. Sci. 2019;20(19):4714. doi: 10.3390/ijms20194714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J.R. Judging a protein by more than its name: GSK-3. Sci. STKE. 2001;(100):re12. doi: 10.1126/stke.2001.100.re12. 2001. [DOI] [PubMed] [Google Scholar]
- Xie B., Li L., et al. MicroRNA-1246 by targeting AXIN2 and GSK-3β overcomes drug resistance and induces apoptosis in chemo-resistant leukemia cells. J. Cancer. 2021;12(14):4196. doi: 10.7150/jca.58522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-S., Lai E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell. 2011;43(6):892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B., Li L., et al. Opposing roles of TCF7/LEF1 and TCF7L2 in cyclin D2 and Bmp4 expression and cardiomyocyte cell cycle control during late heart development. Lab. Invest. 2019;99(6):807–818. doi: 10.1038/s41374-019-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C., Torres M., et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10(12):1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han P., et al. Oncogenic landscape of somatic mutations perturbing pan-cancer lncRNA-ceRNA regulation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.658346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.