Abstract
Ovarian cancer (OC) is the 7th most common cancer in women world-wide and the 3rd most common female cancer. For the treatment of OC, there is no successful therapeutic. The medications that are currently available have significant side effects and a low therapeutic index. This work aimed to evaluate the anticancer activity of organoselenium pseudopeptide compound against OC cell lines. After treatment with 50 μM of compound 4 (CPD 4), the viability was determined. The anticancer activity was further investigated by different methods including cell cycle and apoptosis analysis, colony formation assay, zymography, comet assay and Western blot. In comparison to a positive control, compound 4 showed cytotoxicity toward A2780CP cells rather than A2780 and SKOV-3 cells. Compound 4 was more selective to OC cells rather than HSF cells. Moreover, Compound 4 was able to inhibit cell migration and proliferation. The anticancer effect of compound 4 was found to be partially via cell cycle arrest, overexpression of p27 cell cycle inhibitor and induction of apoptosis through DNA fragmentation and activated production of ROS. Compound 4 had a differential effect on the modulation of PI3K/AKT/mTOR signaling pathway in the OC treated cell lines, also inhibited lipogenesis process via downregulation of FASN expression. Conclusion: This work highlights the unique role of Compound 4 against OC via modulation of oxidative stress, inhibition of survival PI3K/AKT/mTOR pathway. Compound 4 was found to be a promising alternative therapy for the treatment of OC in this investigation.
Keywords: Ovarian cancer, Reactive oxygen species, DNA fragmentation Metalloproteniases, PI3K/AKT/mTOR, Lipogenesis
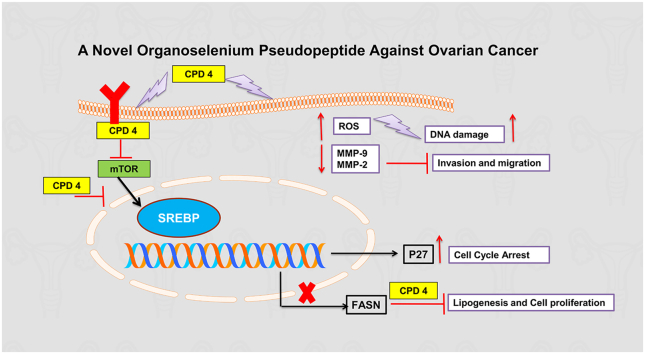
Graphical abstract
Highlights
-
•
Ovarian cancer is the 7th most common cancer in women world-wide.
-
•
Organoselenium compounds have anti-cancer activity.
-
•
In this study a novel organoselenium pseudopeptide has been studied for its anticancer activity against ovarian cancer.
-
•
Organoselenium pseudopeptide modulated ROS, cell cycle, induced apoptosis, DNA damage and attenuated the PI3K/Akt pathway.
1. Introduction
Ovarian cancer (OC) is a type of gynaecological cancer that is extremely deadly (Koshiyama et al., 2017). Ovarian cancer is the world's 7th most prevalent cancer in women world-wide, and the 3rd most common female cancer after breast and cervix cancer (Palomba et al., 2020). As there are no symptoms and sign, 70% of patients are diagnosed late, with an extremely low overall survival rate. Therefore, ovarian cancer is called silent killer (Subhi and Hassan, 2019; Mansha et al., 2019). Older women (>65 years old) are more susceptible to ovarian cancer than younger one (Jayasree and Madhavi, 2019). In 2020, Among all cancer cases there were 313,959 ovarian cancer estimated cases and 207,252 death cases (Sung et al., 2021). In Egypt, ovarian cancer represents 4.1% of all new cases according to International Agency of Research on Cancer (Bray et al., 2018).
Studies of molecular genetics and epidemiologic showed a vast assortment of risk and protective factors. The family history is the most significant risk factor a where about 90% of hereditary ovarian cancer cases are due to mutation in the BRCA1 and BRCA2 tumor suppressor genes (Holschneider and Berek, 2000; Jones et al., 2017). About 16.4% of women with BRCA1 and 7% of women with BRCA2 are suffering from ovarian cancer (Jones et al., 2017). Other risk factors include hormone replacement therapy (HRT) and age (Reid et al., 2017). In addition to genetic factors, there are environmental factors such as ionizing radiation, talc powder, asbestos, pesticides and herbicides exposures. Lifestyle is considered as another possible risk factor such as cigarette smoking, obesity and diet (Rasool et al., 2016; Salehi et al., 2008). Oral contraceptive, tubal ligation and pregnancy are considered protective-factors against ovarian cancer (Doherty et al., 2017).
The first line in ovarian cancer therapy is surgical cytoreduction followed by platinum/taxane combination therapy (El-Senduny et al., 2016; Coward et al., 2015). Alternative ovarian cancer treatments include molecular targeted therapy (such as PARP inhibitors) (Walsh, 2018), hyperthermic intraperitoneal chemotherapy (HIPEC) (Cortez et al., 2018), immunotherapy (Pujade-Lauraine, 2017), stereotactic body radiation therapy (SBRT) (Iftode et al., 2018) and hormonal therapy (Gockley and Wright, 2018). However, most of the patients acquire platinum resistance and suffer from side effects such as vomiting, nausea, fatigue, premature menopause, anxiety, depression and hair loss (Ataseven et al., 2020; Guan and Lu, 2018; Kayl and Meyers, 2006). Therefore, there is continuous need for discovery of new chemotherapeutic drug.
In the beginning of 19th century, selenium was isolated from chalcopyrite by Swedish chemist Berzelius. Selenium is an essential micronutrient and obtained from diet or nutritional supplement (Chen et al., 2020). Selenium is incorporated in organic and inorganic compounds. The bioavailability of organoselenium compounds is higher than inorganoselenium compounds (Rusetskaya et al., 2019). Recent reports showed that Selenium (Se)-containing compounds have anti-cancer activity via their selectivity and high efficacy toward cancer cells exerting their cytotoxic effects by working as a pro-oxidants that disrupt cellular redox homeostasis (Gandin et al., 2018) and induction of apoptosis via different pathways such as death receptors, P53, AMP-activated protein kinase (AMPK) and mitochondria (Chen et al., 2019). Additionally, selenium(Se)-containing compounds have antibacterial and antifungal activity (Narajji et al., 2007).
The goal of this study is to investigate the anticancer activity of new organoselenium compound against ovarian cancer cell lines and study the mechanism of activity by evaluating the change in apoptosis, cell cycle, wound healing, colony formation, and activity of metalloproteinase.
2. Material and method
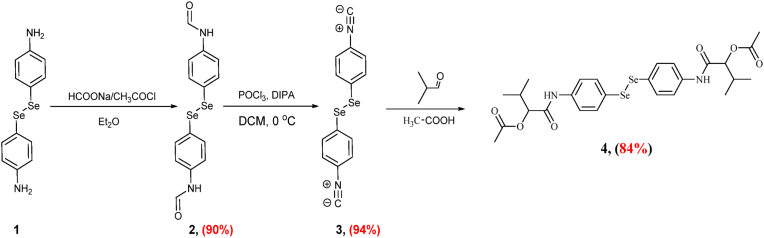
Synthesis of ((diselanediylbis (4,1-phenylene)) bis (azanediyl)) bis(3-methyl-1-oxobutane-1,2-diyl) diacetate (compound 4) by the Passerini reaction of diselenide diisonitrile 6, with acetic acid, and isobutyraldehyde in dichloromethane at room temperature (Scheme 1, Supplementary File) (Shaaban et al., 2021a).
Scheme 1.
Synthesis of ((diselanediylbis (4,1-phenylene)) bis (azanediyl)) bis(3-methyl-1-oxobutane-1,2-diyl) diacetate (compound 4).
2.1. Ovarian cancer cell lines and the drugs
SKOV-3 (p53 and glutathione S-transferase mutant) cells are resistant to cisplatin, paclitaxel and Adriamycin (Choi et al., 2016; Gonera et al., 2014). A2780 (wild type p53) is cisplatin-sensitive and A2780CP is the resistant one. SKOV-3 cell line was obtained from (Nawah Scientific, Egypt). The cisplatin-sensitive (A2780) and cisplatin-resistant cells (A2780CP) was generously provided by Jan Brábek, BIOCEV, Czech Republic. The cells were maintained in 37 °C and 5% CO2 in RPMI-1640 medium (BioWhittaker® Lonza, Cat.No.12–702F). Fetal bovine serum (10%) supplement was added to the medium (FBS) (Cat No: S–001B-BR, Life Science Group L UK) and (100 IU/mL) penicillin/streptomycin (Lonza, 17-602E) (100 μg/mL). To sustain the resistance one μM of cisplatin was added to the medium, which obtained from Sigma-Aldrich and dissolved in 0.9% saline then stored as 1666 μM stock solution at −20 °C. Compound 4 was dissolved in DMSO (Dimethyl sulfoxide) (Serva, Heidelberg, Germany, Cat. No. 20385.02) and stored at −20 °C (Shaaban et al., 2021b).
2.2. Cell viability assay
In a 96-well plate, cells were seeded (100 μl/well). At 37 °C and 5% CO2 condition, cells were incubated with various concentrations of compound 4 (50, 25, 12.5, 6.25, 3.125, and 1.56 μM). A positive control was Cisplatin, while a negative control was DMSO (0.5% V/V). MTT (3-(4,5-dimethylthiazoyl)-2,5-diphenyl-tetrazolium bromide (MTT) (5 mg/mL Phosphate Buffered Saline (PBS) was added after 48 h of incubation and the plate was incubated for another 4 h. The formazan crystals were then solubilized with an acidified sodium dodecyl sulphate (SDS) solution (10% SDS containing 0.01N HCl in 1x PBS). The absorbance was measured at λ570- 630 nm by Biotek plate reader (Gen5™) after 14 h of incubation. GraphPad Prism 8 was used to calculate the IC50 value of compounds.
2.3. Proliferation assay
In a 96-well plate, cells were seeded (100 μl/well). The cells were treated with compound 4 after 2 h of incubation at 37 °C and 5% CO2. After 4 days of incubation, the plate was incubated with MTT for 4 h. After that, the crystals were dissolved and absorbance was measured as mentioned above.
2.4. Selectivity index (SI) calculations
Human skin fibroblast cells (HSF) were seeded at 1 × 05 (cells/mL) in a 96-well plate and incubated overnight at 37 °C and 5% CO2 to examine the selectivity of the most active compound against ovarian cancer. The most active compound was serially diluted the next day. The viability of HSF cells was measured using the MTT assay as described above, after 48 h of incubation, and (SI) was calculated using Equation 1.
Equation (1): calculation of selectivity index where IC50 normal = the concentration of the tested compound that kills 50% of normal cells, IC50 cancer = the concentration of the tested compound that kills 50% of cancer cells
| Equation 1 |
2.5. Cell cycle analysis
According to Gray et al., (Gray and Coffino, 1979; Pozarowski et al., 2004). In a six-well plate Ovarian cancer cells seeded (2 mL/well) and permitted to attach overnight at 37 °C and 5% CO2. The IC50 value of each cell line was used to treat the cells. [Table 1]. Cells were washed twice with ice-cold 1x PBS after 48 h of culture and collected after trypsinization. The detached cells were centrifuged for 5 min at 4 °C at 500×g. The collected cells were resuspended and were fixed by using 70% ethanol. After 2 h of incubation at −20 °C, the fixed cells were washed and stained with propidium (PI)/RNase (USA, NJ, BD Biosciences, BDB550825) and incubated for 30 min at room temperature (RT) in dark. Within 1 h, DNA content in each phase was measured by flow cytometry (BD Accuri C6 Plus flow cytometer, NJ, USA) (El-Senduny et al., 2019, 2021).
Table 1.
The IC50 (μM) of the most cytotoxic compounds against ovarian cancer cell lines and selectivity index (SI), ± indicates the standard deviation from three separated trials.
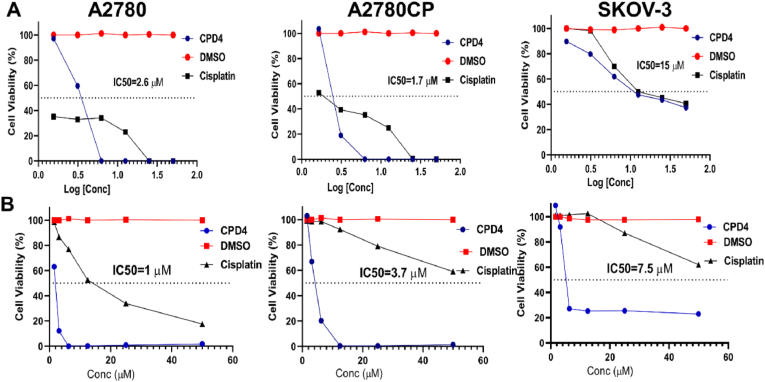
| Cell line | IC50 (μM) | SI |
|---|---|---|
| A2780 | 2.6 ± 0.4 | 43.3 |
| A2780CP | 1.7 ± 0.1 | 66.3 |
| SKOV-3 | 15 ± 0.8 | 7.5 |
| HSF | 50 |
2.6. Apoptosis assay
The apoptotic cells were measured by using PI and Anexin-V-FITC DXN kit (Thermo Fisher Scientific™, USA, eBioscience, 51-66121E). In a six-well plate, the cells were seeded (2 mL/well) and permitted to attach overnight at 5% CO2 and 37 °C and. Cells were treated with IC50 value of each cell line showed in [Table 1] for 48 h. After trypsinization, the cells were harvested and washed two times. After that, cells were incubated for 15 min with PI and annexin-V-FITC in the binding buffer. The cell count in each phase was detecting by BD Accuri C6 Plus flow cytometer, NJ, USA (El-Senduny et al., 2021; Barakat et al., 2018).
2.7. Comet assay
Comet assay is a bio monitoring tool to examine the effect of compound 4 on levels of DNA damage. According to Dhawan et al. procedures (Dhawan et al., 2003), In a six-well plate, ovarian cancer cells were seeded and incubated for 24 h. The cells were treated with IC50 value of compound 4 showed in [Table 1]. After 48 h of incubation at 37 °C and 5% CO2, cells were washed twice with 1x PBS. Live cells were counted after trypsinization with trypan blue (0.4% w/w). Cells were suspended in low melting agarose (0.5% LMPA/PBS). The cells were spread on frosted microscope slides (coated with a layer of normal melting agarose (1% NMA/H2O)) (Thermo Scientific™ R0491). After solidification, the cells were lysed in lysis buffer for 2 h at 4 °C. Slides were then placed in the electrophoresis solution for 20 min to allow DNA unwinding. Electrophoresis was carried out (24 V and 300 mA for 30 min). The neutralization step was carried out for 5 min and repeated three times (0.4 M Tris-HCl, pH 7.5). The DNA was visualized by staining with ethidium bromide (20 μg/mL). To avoid further DNA damage, all of these procedures were carried out in the dark. The pictures were captured with an Olympus BX43 camera attached to an Olympus PEN Lite E-PL3 camera.
2.8. Colony formation assay
In a six-well plate, cells were seeded at 500 (cells/mL) and incubated overnight at 37 °C and 5% CO2. Drug was added with the concentrations mentioned in the [Table 1]. In the untreated control, cells were left without changing media until colonies were observed, A2780 (10 days), A2780CP (13 days) and SKOV-3 (14 days). Colonies were washed and fixed with ice-cold methanol for 20 min and stained with 0.5% crystal violet (El-Senduny et al., 2019).
2.9. Wound healing assay
In a six-well plate, cells were seeded. On the next day, afresh medium containing the concentration of drug as mentioned in [Table 1] was added after removing the old medium. Scratch was made with sterile P200 tip and pictures were taken at zero and healing was monitored. After the untreated control closed, the cells were washed, fixed and stained with crystal violet. Image J 1.51 software was used to determine the size of the wound (El-Senduny et al., 2019, 2021).
2.10. Zymography assay
In serum-starved ovarian cancer cells, gelatin zymography was employed to assess the levels of secreted matrix metalloproteinase (MMP-9 and MMP-2) gelatinolytic activity (Toth and Fridman, 2001; Toth et al., 2012). The medium was collected after 48 h of incubation with compound 4 and electrophoresed in a 10% polyacrylamide gel containing gelatin as a substrate. After electrophoresis, the gels were treated for 2 h in renaturation buffer and then incubated in developing buffer overnight at 37 °C. The gels were stained for 1 h with 0.5% Coomassie brilliant blue R-250 and then destained with (30% methanol-10% acetic acid). Against the background of Coomassie blue-stained gelatin, gelatinolytic activity was observed as an unstained band. Bands quantified by Image J v.1.50i software.
2.11. Detection of ROS
According to DCFDA-Cellular Reactive Oxygen Species Detection Assay Kit (ab 113851) instructions, cells were seeded and cultured overnight for adhesion in a 25 cm2 tissue culture flask. After trypsinization, the cells were harvested and washed. The cells were re-suspended in 20 μM DCFDA stain for 30 min. After that, cells were incubated with the concentration of the drug mentioned in [Table 1] using TBHP as a positive control for 4 h. The ROS level was determined using flow cytometry BD Accuri C6 Plus to measure the level of H2O2 (El-Senduny et al., 2021).
2.12. Western blot
The cells were seeded and treated as mentioned above. Cells were lysed by RIPA buffer containing 1x phosphatase and protease inhibitor after 48 h of incubation with compound 4. Supernatant was collected followed by detection of protein concentration by using Pierce™ BCA Protein Assay Kit (Cat.No.23225). SDS-PAGE (10%, 12% or 15%) was used for 45 min at 100 V. A nitrocellulose membrane 0.2 or 0.45 μM was used for protein transferring for 70 or 90 min at 100 or 90 V, respectively. Primary antibodies were added to the membrane after blocking then incubated overnight. As a loading control, GAPDH was used. Anti-mouse or anti rabbit HRP-linked antibody (Cat. No. 7076S and 7074S) were used. The signal was recorded by a ChemiDoc BioRad documentation system (El-Senduny et al., 2021; Ismail et al., 2022).
3. Results
3.1. Compound 4 was more cytotoxic and proliferative agent than other organoselenium compounds
In the current study, compound 4's anticancer activity was tested using the MTT assay on three ovarian cancer cell lines (A2780, A2780CP, and SKOV-3). The results reveled that compound 4 was more potent against resistant cells (A2780CP) rather than sensitive cells (A2780) and SKOV-3 [Table 1]. Additionally, compound 4 was tested against normal human skin fibroblast cells (HSF) to investigate the selectivity index towards ovarian cancer cells with the selectivity index computed using [Equation (1)]. Compound 4 proved to be selective to ovarian cancer cells (A2780CP, A2780 and SKOV-3) rather than normal cells HSF.
Compound 4's antiproliferative potential was tested using the MTT assay against ovarian cancer cell lines and it was efficient to act as an antiproliferative agent. The results reveled that compound 4 was more potent against A2780 (IC50 = 1 μM) rather than A2780CP (IC50 = 3.7 μM) and SKOV-3 (7.5 μM) as shown in (Fig. 1).
Fig. 1.
(A) Cytotoxicity of compound 4 on ovarian cancer cells, sensitive cells (A2780), resistant cells (A2780CP) and SKOV-3 cells were incubated with compound 4 for 48 h and as a negative control, DMSO was used. MTT assay was used to determine the cell viability. (B) Proliferation activity of compound 4 against ovarian cancer cells was determined by incubation of cells with compound 4 for 4 days. The cell viability was determined by MTT assay. Biotek plate reader (Gen5™) was used to measure the absorbance at λ570- 630.
3.2. Cell migration and proliferation was inhibited by compound 4
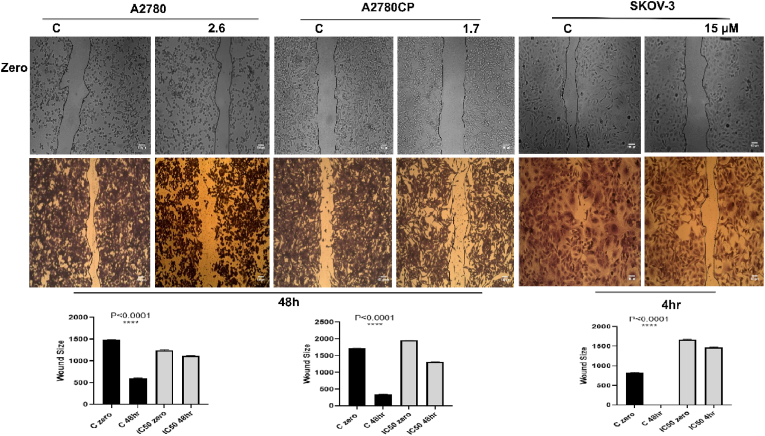
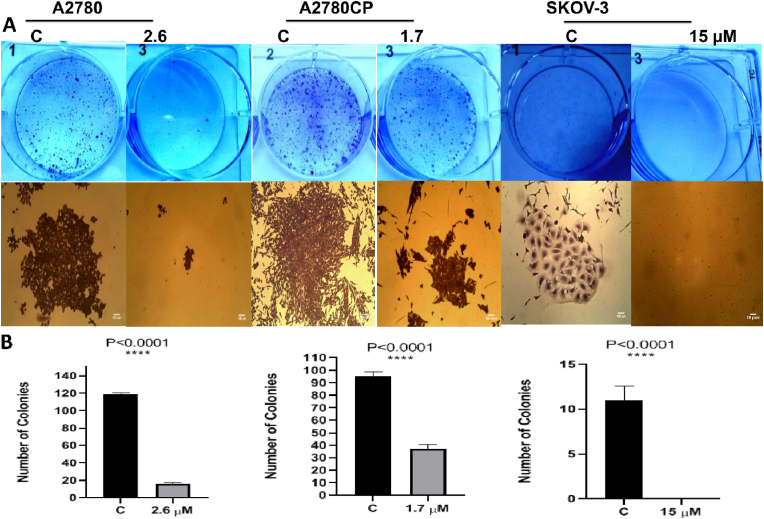
Wound healing assay was performed to investigate the role of compound 4 in inhibiting the migration in vitro (Rodriguez et al., 2005). Comparing to the negative control (DMSO), compound 4 revealed a significant inhibition, restricting cells from closing and healing the wound (Fig. 2). Compound 4 was used to investigate if it could stop single cells from growing and forming colonies. The results showed that compound 4 was able to completely inhibit the formation of the colonies in the three ovarian cancer cell lines comparing to the negative control (DMSO) (Fig. 3).
Fig. 2.
(A) Effect of compound 4 on wound healing of ovarian cancer cells. The wound healing rate was monitored at zero time and 48 h. Image J software was used to measure the size of the wound. Results reveled that compound 4 inhibited cell migration and prevented cells from metastasis. (B): One-Way ANOVA was used to analyze data and significance was ∗∗∗∗P < 0.0001.
Fig. 3.
(A): The images showed the inhibitory effect of compound 4 on ovarian cancer cells through decreasing the number of colonies after treatment in comparison to negative control (DMSO). (B): One-Way ANOVA was used to analyze data and significance ∗∗∗∗P < 0.0001.
3.3. Inhibition of wound healing via MMP-2 and MMP-9 inhibition
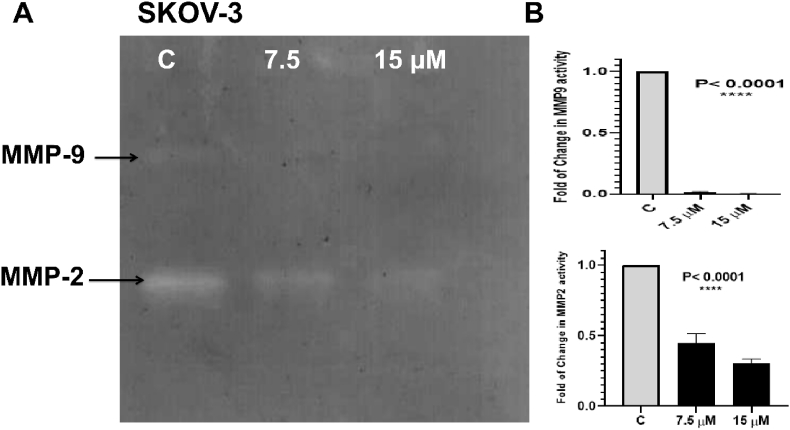
Invasion, migration, adhesion and proliferation depend on the level of metalloproteinases in the media where they degrade extracellular matrix (ECM) components and cell surface proteins. In this study the activity of MMP-2 and MMP-9 was evaluated by zymography. The results showed that compound 4 treatment significantly reduced the activity of MMP-9 and MMP-2 in the conditioned media of treated SKOV-3 (Fig. 4). Metalloproteinases are not detected in sensitive cells (A2780) and resistant cells (A2780CP).
Fig. 4.
(A): Inhibition of MMP-2 and MMP-9 after compound 4 treatments. Serum starved SKOV-3 cells were seeded as described in the methods section, and then treated for 48 h with (½ IC50 and IC50) of compound 4. Media were collected following treatment and total protein content was analyzed by BCA assay then gelatin zymography was performed. (B): Statistical significance was indicated: ∗∗∗∗p < 0.0001.
3.4. Compound 4 arrested ovarian cancer cells
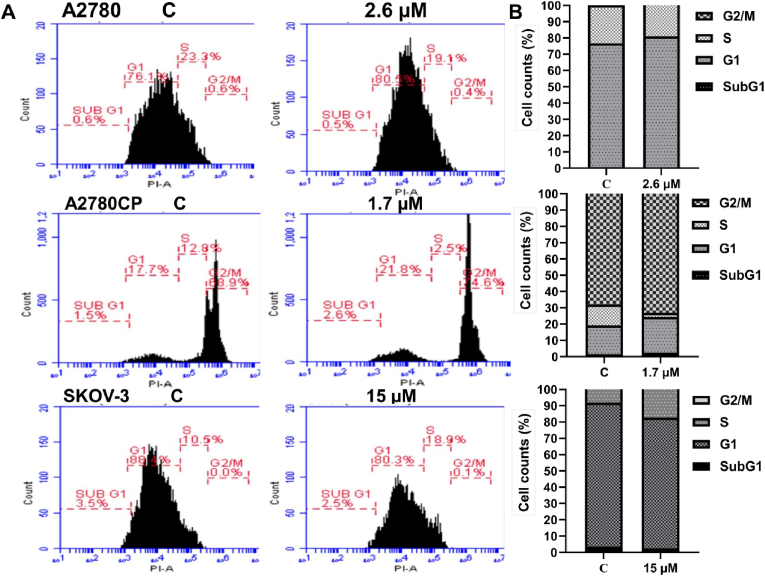
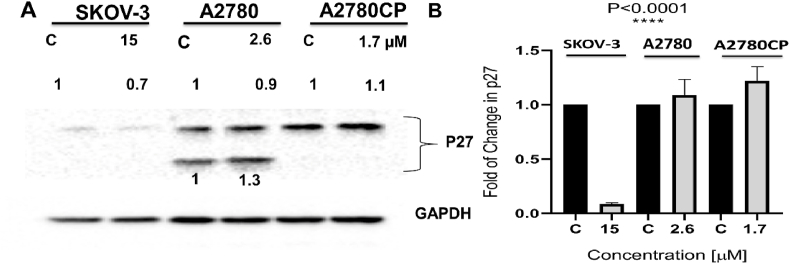
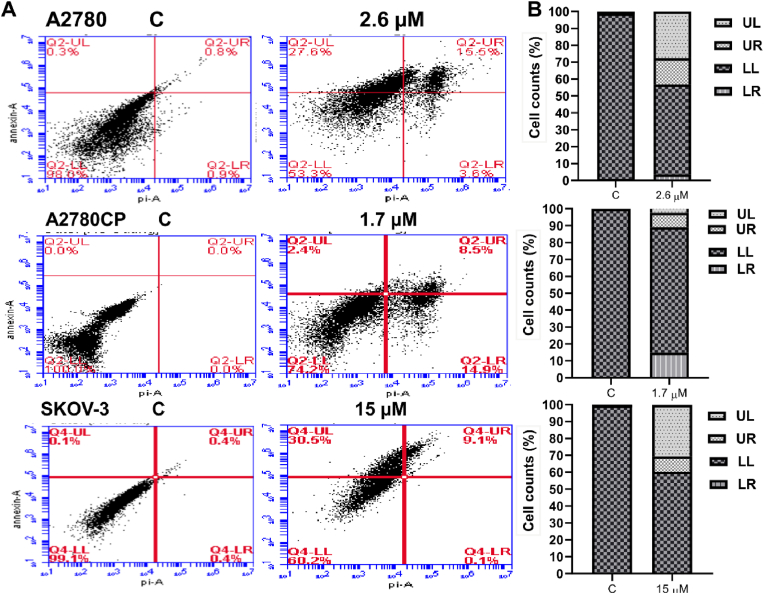
Compound 4's IC50 value [Table 1] was applied to ovarian cancer cells for 48 h. After that, propidium iodide was used to determine the amount of DNA content in each phase of the cell cycle. Results showed that compound 4 arrested sensitive cells (A2780) at G1 phase (80.5%) and resistant cells (A2780CP) (21.8%). On the other hand, compound 4 arrested SKOV-3 at S phase (18.9%). P27 is considered as a tumor suppressor which responsible for cell cycle regulation and is abundant in early G1 phase and quiescent (G0) (Abbastabar et al., 2018). There is a differential effect in the expression level of p27 in three tested cell lines. The resulted showed an increase in the expression of p27 in A2780CP but decreased in A2780 and SKOV-3 cell lines as shown in (Fig. 5, Fig. 6). The cell population undergoing either earlier events or late events of apoptosis was measured by flow cytometry after compound 4 treatment. The analysis revealed that compound 4 induced the apoptosis in the three ovarian cancer cell lines A2780 (15.5% vs. 0.8% for DMSO-treated cells), A2780CP (14.9% vs. 0% for DMSO-treated cells and around 30.5% for SKOV-3 cell line vs. 0% for control (Fig. 7).
Fig. 5.
(A): The effect of compound 4 treatment on cell cycle phases for cancerous cells. (B): Represent the number of cells in each phase of cell cycle.
Fig. 6.
(A) Effectiveness of compound 4 treatment on the expression level of p27, cells were treated with IC50 value of compound 4 as mentioned above for 48 h. As a loading control, glyceraldehyde3-phosphate dehydrogenase (GAPDH) was used. (B): One-Way ANOVA was used to analyze the data, and the significance was ∗∗∗∗P < 0.0001.
Fig. 7.
(A): Induction of apoptosis in ovarian cancer cells as a result of compound 4 treatment. (B): Represent the number of the cells in each apoptotic phase.
3.5. Compound 4 induced apoptosis through DNA strand breaks in ovarian cancer single cell
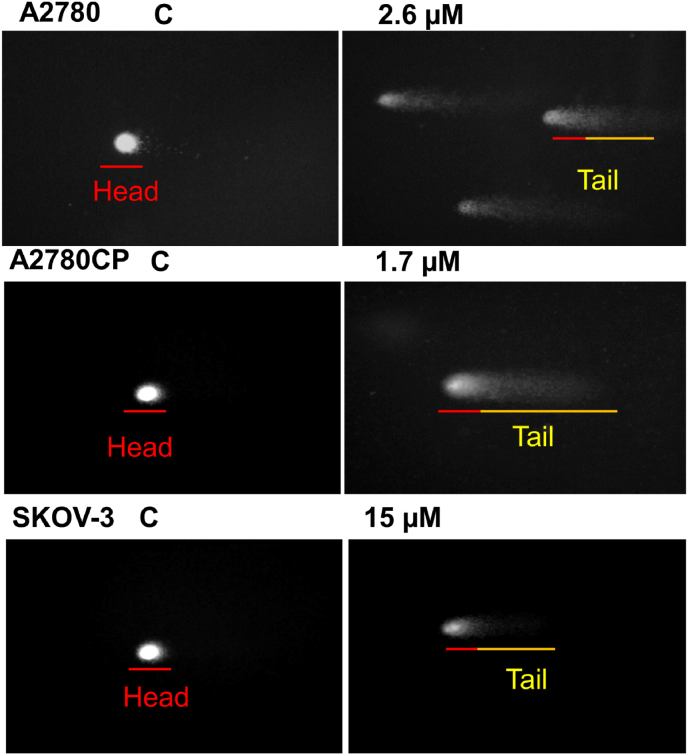
The comet assay is characterized by its simplicity, sensitivity, versatility, speed, and economy so it is an essential method for measuring DNA breaks (Collins, 2004). This technique was used to confirm the induction of apoptosis. Ovarian cancer cells were treated with IC50 value of compound 4 [Table 1]. The results showed the breakage of DNA strands and the length of the tail increase by increasing concentration of the drug (Fig. 8).
Fig. 8.
Effect of compound 4 treatment on DNA strand breakage. Ovarian cancer cells were treated with compound 4 for 48 h live cells were spread on a frosted microscope slide. After many steps mentioned in material and method section, Olympus PEN Lite E-PL3 camera connected with Olympus BX43 was used to take pictures.
3.6. Compound 4 triggers the production of ROS in ovarian cancer cell lines
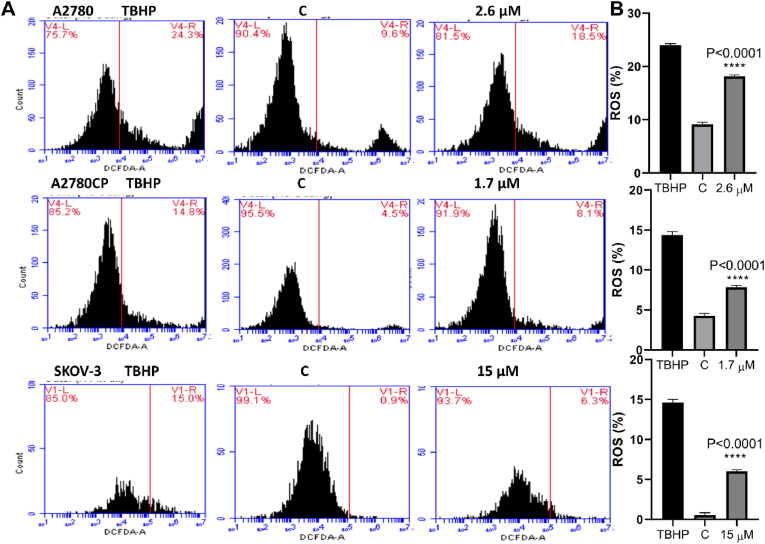
Superoxide (O2•–), hydroxyl radical (•OH), and hydrogen peroxide (H2O2) are all extremely reactive oxygen species that act as DNA damage modulators (Liou and Storz, 2010). Using the DCFDA – Cellular Reactive Oxygen Species Detection Assay kit, the level of ROS generation was determined. From the outcomes of this study the intracellular ROS level in ovarian cancer cells increased compared to untreated cells (Fig. 9). Our result suggested that compound 4 is a potent molecule for activating ROS in ovarian cancer cells.
Fig. 9.
(A): Compound 4 causes intracellular ROS production in ovarian cancer cells. For 30 min, cells were loaded with 20 μM DCFDA stain and then treated with IC50 value of compound 4 mentioned above and incubated for additional 4 h. ROS production was determined by gauging the level of H2O2 by flow cytometry BD Accuri C6 Plus DCF fluorescence intensity. (B): One-Way ANOVA was used to analyze the data and significance was ∗∗∗∗P < 0.0001.
3.7. Effect of compound 4 on PI3K/AKT/mTOR pathway and its correlation to lipogenesis and cell cycle
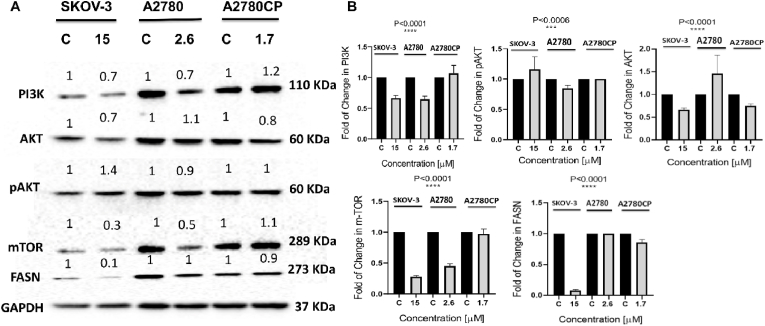
In comparison to other signaling pathways, the PI3K/AKT/mTOR pathway is critical in the regulation of a variety of biological processes including cell proliferation, metabolism, apoptosis, and angiogenesis (Xu et al., 2020; Chen et al., 2021). To investigate the effect of compound 4 treatment on PI3K/AKT/mTOR signaling pathway, western blotting was carried out. There is a differential effect in the three tested ovarian cancer cell lines. The results showed downregulation in the level of PI3k p110α, total AKT, pAKT and mTOR proteins in the treated A2780 cell line. Compound 4 treatment led to downregulation in the level of PI3k p110α, total AKT and mTOR proteins despite the increase in phosphorylated AKT. On the other hand, compound 4 treated A2780CP cells showed a decrease in the level of AKT and its phosphorylated form and mTOR while PI3K p110α was upregulated (Fig. 10). Thus, it may be concluded that compound 4 induces anticancer partly via PI3K/AKT/mTOR signaling pathway and involvement of other pathways in the cisplatin-resistant ovarian cancer cell line A2780CP. FASN is a major biosynthetic enzyme involved in de novo lipogenesis and the generation of long-chain fatty acids from malonyl-CoA and acetyl-CoA (Menendez and Lupu, 2017a, 2017b; Kuhajda et al., 2000; Kuhajda, 2000). In cancerous cells, fatty acid synthase is highly expressed for fatty acids synthesis which is required for energy production and membrane formation (Flavin et al., 2010). In our results, there is a different effect in three tested ovarian cancer cells. The results showed downregulation in the level of FASN in treated SKOV-3 and (A2780CP) resistant-ovarian cancer cells. On the other hand, compound 4 treated A2780 cells showed no effect on the level of FASN (Fig. 10).
Fig. 10.
(A): Western blot data showed decrease or increase in proteins SKOV-3, A2780 and A2780CP were treated with the IC50 value of compound 4 for 48 h. The cells were lysed in RIPA buffer containing phosphatase and protease inhibitor. BCA assay was used to determine protein concentration. 30 μg of protein were loaded in the gel. Proteins were transferred onto nitrocellulose membrane and subjected to the primary antibodies overnight at 4 °C then incubated with HRP-conjugated secondary antibody. As a loading control GAPDH was used. The level of change in proteins expression was normalized to the amount of GAPDH. (B): Statistical analysis of data was calculated by One-way ANOVA and significance was ∗∗∗∗P < 0.0001 and ∗∗∗P < 0.0006.
4. Discussion
Ovarian cancer (OC) is a type of gynecological cancer that is extremely deadly. the vast majority of patients present with advanced stage of ovarian cancer due to lack of early detection markers or symptoms (Koshiyama et al., 2017). As prooxidants and chemopreventive agents, organoselenium compounds have been a prominent focus of modern drug development (Narajji et al., 2007; Drake, 2006). The anticancer activity of oraganoselenium compound (compound 4) was studied in this work, and a part of its mechanism of action was described. Most of organoselenium compounds especially pseudopeptides revealed cytotoxicity against variety of cancer cells such as MCF-7 and HepG2 cells without showing any side effects on normal cells (Shaaban et al., 2019). Additionally, oraganoselenium pseudopeptides possess anticancer and chemosensitivity activity against liver cancer cell line (HepG2) (Shaaban et al., 2021b). In this work, compound 4 showed potent cytotoxicity against the cisplatin-resistant cell line (A2780CP), rather than its sensitive cell line (A2780) and the ovarian ascetic cell line (SKOV-3) without affecting the viability of normal human skin fibroblast cells (HSF).
One of the first methods for evaluating directional cell migration in vitro is the wound healing assay. It's easy, cost-effective, and simple to use. It's ideal for studying how cell-matrix and cell-cell interactions affect cell migration. (Rodriguez et al., 2005). Matrix metalloproteases (MMPs) are known to play an important role in cell migration, angiogenesis, metastasis differentiation, host defenses and apoptosis (Lou et al., 2013). In this study, compound 4 could effectively inhibit cell migration of the three tested cell lines which confirms the ability of compound 4 to stop migration.
The mechanism of inhibition of wound healing was evaluated by detecting the metalloproteinase 9 and 2 by zymography technique. We found that compound 4 decreased the activity of both MMP-9 and -2 in a dose-dependent manner. It has been reported that, the migration of SKOV-3 cells independent on the secretion of MMPs (Roomi et al., 2010). On the other hand, both A2780 and A2780CP did not secrete MMPs in the medium in our culture system. A2780 and A2780CP requires fibronectin in the culture plates (Tian et al., 2002). Our results are in accordance with the previously reported anti-metastatic activity of methylselenol which is a selenium metabolite against HT1080 fibrosarcoma cells via overproduction of tissue inhibitor of metalloproteinase-1 and -2 (Zeng et al., 2006).
The colony formation test is an in vitro cell survival experiment that measures the ability of a single cell to expand and form a colony. The colony is defined as the group of cells with at least 50 cells (Franken et al., 2006). The anti-tumor property of compound 4 was confirmed by performing colony formation assay. It was revealed that compound 4 reduced the size and the number of colonies in A2780 and A2780CP. Moreover, colony formation by compound 4-treated SKOV-3 was completely inhibited. Therefore, compound 4 might be a promising alternative to delay cancer cell proliferation.
Suzuki et al., revealed that the organoselenium compounds induce apoptosis via the intrinsic mitochondrial and endoplasmic reticulum apoptotic pathway (Suzuki et al., 2010). In this study, compound 4 induced cell death in the three tested cell lines. Under normal aerobic growth circumstances, intracellular reactive oxygen species like superoxide anion, hydrogen peroxide, and hydroxyl radical are formed, but they are enhanced when external stimuli are applied. Intracellular ROS causes oxidative damage by attacking cellular membrane, proteins, lipids, and DNA (Moloney and Cotter, 2018). It has been reported that selenocysteine caused elevation in the level of ROS and caused DNA strand breaks in cancer cells (Chen and Wong, 2009). Therefore, in our study, the level of ROS was evaluated by flowcytometry. The analysis showed that compound 4 led to an increase in the production of ROS and caused damage in DNA which was confirmed by comet assay. Therefore, compound 4 might be a promising prooxidant candidate for ovarian cancer.
Cell cycle is modulated by CDK inhibitors (CDKIs) and cyclin-dependent kinases (CDKs). The Cip/Kip family members (p57, p27, and p21) are mainly inhibitors for cyclin A, B, E, and D-dependent kinase complexes (Leal-Esteban and Fajas, 2020). P27 is a tumor suppressor and is prominent in early G1 phase and quiescent (G0) phase which interacts with Cdk2/Cyclin E to block entrance into the S phase, and its overexpression results in a G1 phase arrest (Abbastabar et al., 2018). According to Shi, C., et al. report, the organoselenium compound 1,2-[bis(1,2-Benzisoselenazolone-3(2H)-ketone)] ethane induced cell cycle arrest at S phase for both DU145 and PC-3 human prostate cancer cell lines via increasing protein levels of p21, cyclin E, and A while decreasing the levels of Cdk4, cyclin D1 and cyclin B1 (Shi et al., 2003). In this study, compound 4 showed the ability to arrest both sensitive (A2780) and resistant cells (A2780CP) at G1 phase while SKOV-3 cells were arrested at S phase. Western blot analysis revealed a decrease in the level of p27 in this investigation. after 48 h of treatment with compound 4 which could be explained by the degradation of p27 through ubiquitin-dependent or independent proteolytic pathway (Shirane et al., 1999).
The PI3K/AKT pathway is activated during the cell cycle's G1/S transition and regulates numerous critical cell cycle regulators, including the protein stability of p21Cip1, Cyclin D, and p27Kip1. Inhibition of the PI3K/AKT pathway by a modest pharmacological inhibitor or overexpression of the tumor suppressor PTEN (Phosphatase and Tensin Homolog) can result in nuclear accumulation of p27Kip1 and G1 cell cycle arrest in a variety of cell types (Prasad et al., 2015; Kim et al., 2009) and led to inhibition of metastasis via down regulation of MMP-9 and MMP-2 (Wang et al., 2015). In our study the inhibitory activity of compound 4 was evaluated by Western blot and showed a marked decrease in the level of downstream proteins such as mTOR which confirms the anti-cancer activity of compound 4.
Fatty Acid Synthase (FASN) is a major biosynthetic enzyme involved in de novo lipogenesis and the generation of long-chain fatty acids from malonyl-CoA and acetyl-CoA (Menendez and Lupu, 2017a; Kuhajda, 2000). FASN activity and expression are low in normal cells which is regulated via growth factors, hormones, and diet (Abdel-Magid, 2015). On the other hand, fatty acid synthase is widely expressed in many cancers such as ovarian, prostate, breast, colorectal, and hepatocellular for fatty acids synthesis which is required for energy production and membrane formation (Flavin et al., 2010). In cancer cells, regulation of fatty acid syntheses is complex which is regulated hormonally or through signaling pathways such as AKT and MAPK (mitogen-activated protein kinase) transduction pathways.
Growth factor receptors EGF (Epidermal Growth Factor) and ERBB-2 interact and stimulate downstream PI3K/AKT and MAPK, which express FASN via modification of sterol regulatory element-binding protein expression (SREBP)-1c which binds to FASN promoter hence activates FASN transcription (Kuhajda, 2006; Walz et al., 2018). FASN is thought to be a promising target for cancer treatment, there are many compounds to inhibit FASN expression such as C75, cerulenin, C93, orlistat and green tea polyphenol epigallocatechin-3-gallate (EGCG) (Lupu and Menendez, 2006). SKOV-3 ovarian cancer cells have constitutive active AKT which leads to overexpression of FASN (Wang et al., 2005). In this study, compound 4 down regulated the expression of AKT in both SKOV-3 and resistant cells (A2780CP) which in turn led to down regulation of FASN. In contrast, compound 4 increased expression level of AKT in sensitive cells (A2780) and did not affect on FASN expression level. These results revealed that compound 4 can inhibit lipogenesis via blocking PI3K/AKT/mTOR pathway leading to down regulation of FASN expression. Organoselenium compounds have been proven to act as multi-kinase inhibitors leading to apoptotic cancer cell death and autophagic through blocking of PI3K/AKT/mTOR pathway (Ibanez et al., 2012).
5. Conclusion
This work highlights the cytotoxicity of compound 4 (Pseudopeptides) against ovarian cancer cell lines (A2780, A2780CP and SKOV-3). The presented result showed that compound 4 inhibited wound healing and colony formation through decreasing the activity level of MMPs (MMP-2 and -9). Compound 4 induced apoptosis through increasing the production of ROS which leads to DNA strand breaks, shown by comet assay, also used as a cell cycle regulator through regulation of PI3K/AKT pathway which in turn regulates the expression level of p27. Compound 4 regulates FASN expression and so plays a significant role in lipogenesis. In summary, Compound 4 could be utilized as an alternative to chemotherapy in the treatment of ovarian cancer.
Credit author contribution
FFE and MMY conceived and designed the experiments. SSH synthesized the tested compounds. FAB designed the experiments, contributed reagents and materials. AME, MAA and FFE performed the experiments, analyzed and interpreted the data analysis. All authors have revised and approved the manuscript.
Fund
This study was partially funded by the Academy of Scientific Research and Technology ASRT, Egypt in cooperation with the Indian government (Project: Liposome Encapsulated Azadiradione for Triple Negative Breast Cancer Treatment).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank the Chemistry Department, Faculty of Science, Mansoura University, Egypt for providing some of the facilities required during this study. We would like to thank the Center of Excellence for Genome and Cancer Research, Urology and Nephrology Centre, Mansoura University, Mansoura, Egypt for providing Bio-Rad Imaging facility.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphar.2022.100134.
Contributor Information
Abeer M. El-Saudi, Email: abeermohamedelsaudi246@gmail.com.
Saad Shaaban, Email: dr.saad.chem@gmail.com.
Farid A. Badria, Email: badri002@mans.edu.eg.
Magdy M. Youssef, Email: mmm_youssef@mans.edu.eg.
Fardous F. El-Senduny, Email: fxe123@miami.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Abbastabar M., et al. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: a double-edged sword protein. DNA Repair. 2018;69:63–72. doi: 10.1016/j.dnarep.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Abdel-Magid A.F. Fatty acid synthase (FASN) inhibitors as potential treatment for cancer, obesity, and liver related disorders. ACS Med. Chem. Lett. 2015;6(8):838–839. doi: 10.1021/acsmedchemlett.5b00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataseven B., et al. Perception of side effects associated with anticancer treatment in women with breast or ovarian cancer (KEM-GO-1): a prospective trial. Support. Care Cancer. 2020;28(8):3605–3615. doi: 10.1007/s00520-019-05216-y. [DOI] [PubMed] [Google Scholar]
- Barakat A., et al. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018;8(26):14335–14346. doi: 10.1039/c8ra02358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chen T., Wong Y.S. Selenocystine induces reactive oxygen species-mediated apoptosis in human cancer cells. Biomed. Pharmacother. 2009;63(2):105–113. doi: 10.1016/j.biopha.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Chen Z., et al. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2019;56(2):179–196. doi: 10.1039/c9cc07683b. [DOI] [PubMed] [Google Scholar]
- Chen Z., et al. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020;56(2):179–196. doi: 10.1039/c9cc07683b. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., et al. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol. Med. Rep. 2021;23(1):88. doi: 10.3892/mmr.2020.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., et al. FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells. Oncotarget. 2016;7(3):3506–3519. doi: 10.18632/oncotarget.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Cortez A.J., et al. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018;81(1):17–38. doi: 10.1007/s00280-017-3501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward J.I., Middleton K., Murphy F. New perspectives on targeted therapy in ovarian cancer. Int. J. Womens Health. 2015;7:189–203. doi: 10.2147/IJWH.S52379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan A., et al. Protocol for the single cell gel electrophoresis/comet assay for rapid genotoxicity assessment. ITRC. 2003;1077(1) [Google Scholar]
- Doherty J.A., et al. Current gaps in ovarian cancer epidemiology: the need for new population-based research. J. Natl. Cancer Inst. 2017;109(10):1–7. doi: 10.1093/jnci/djx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake E.N. Cancer chemoprevention: selenium as a prooxidant, not an antioxidant. Med. Hypotheses. 2006;67(2):318–322. doi: 10.1016/j.mehy.2006.01.058. [DOI] [PubMed] [Google Scholar]
- El-Senduny F.F., et al. Approach for chemosensitization of cisplatin-resistant ovarian cancer by cucurbitacin B. Tumour Biol. 2016;37(1):685–698. doi: 10.1007/s13277-015-3773-8. [DOI] [PubMed] [Google Scholar]
- El-Senduny F.F., et al. An approach to treatment of liver cancer by novel glycyrrhizin derivative. Anti Cancer Agents Med. Chem. 2019;19(15):1863–1873. doi: 10.2174/1871520619666190411114718. [DOI] [PubMed] [Google Scholar]
- El-Senduny F.F., et al. Urea-functionalized organoselenium compounds as promising anti-HepG2 and apoptosis-inducing agents. Future Med. Chem. 2021;13(19):1655–1677. doi: 10.4155/fmc-2021-0114. [DOI] [PubMed] [Google Scholar]
- Flavin R., et al. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6(4):551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken N.A., et al. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Gandin V., et al. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018;127:80–97. doi: 10.1016/j.freeradbiomed.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Gockley A., Wright A. Living through ovarian cancer treatment: acute and long-term toxicities of chemotherapy for advanced-stage disease. Hematol. Oncol. Clin. N. Am. 2018;32(6):1073–1085. doi: 10.1016/j.hoc.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Gonera A., et al. SKOV-3 and Me45 cell response to cisplatin-based chemotherapy: an in vitro study. Folia Biol. 2014;60(5):213–219. doi: 10.14712/fb2014060050213. [DOI] [PubMed] [Google Scholar]
- Gray J., Coffino P. Methods in Enzymology. Elsevier; 1979. [19] Cell cycle analysis by flow cytometry; pp. 233–248. [DOI] [PubMed] [Google Scholar]
- Guan L.Y., Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov. Med. 2018;26(144):219–229. [PubMed] [Google Scholar]
- Holschneider C.H., Berek J.S. Seminars in Surgical Oncology. Wiley Online Library; 2000. Ovarian cancer: epidemiology, biology, and prognostic factors. [DOI] [PubMed] [Google Scholar]
- Ibanez E., et al. The quinoline imidoselenocarbamate EI201 blocks the AKT/mTOR pathway and targets cancer stem cells leading to a strong antitumor activity. Curr. Med. Chem. 2012;19(18):3031–3043. doi: 10.2174/092986712800672076. [DOI] [PubMed] [Google Scholar]
- Iftode C., et al. Stereotactic body radiation therapy in oligometastatic ovarian cancer: a promising therapeutic approach. Int. J. Gynecol. Cancer. 2018;28(8):1507–1513. doi: 10.1097/IGC.0000000000001324. [DOI] [PubMed] [Google Scholar]
- Ismail M.A., et al. Synthesis of new thienylnicotinamidines: proapoptotic profile and cell cycle arrest of HepG2 cells. Arch. Pharm. (Weinheim) 2022;355(9) doi: 10.1002/ardp.202100385. [DOI] [PubMed] [Google Scholar]
- Jayasree V., Madhavi N. Study of prevalence of ovarian tumours Among ovarian mass lesions in tertiary rural hospital. J. Basic Clin. Res. 2019;6(1):1–6. [Google Scholar]
- Jones M.R., et al. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecol. Oncol. 2017;147(3):705–713. doi: 10.1016/j.ygyno.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Kayl A.E., Meyers C.A. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr. Opin. Obstet. Gynecol. 2006;18(1):24–28. doi: 10.1097/01.gco.0000192996.20040.24. [DOI] [PubMed] [Google Scholar]
- Kim J., et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin. Cancer Res. 2009;15(1):81–90. doi: 10.1158/1078-0432.CCR-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama M., Matsumura N., Konishi I. Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics. 2017;7(1):12. doi: 10.3390/diagnostics7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F.P. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Kuhajda F.P. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66(12):5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- Kuhajda F.P., et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. U. S. A. 2000;97(7):3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Esteban L.C., Fajas L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866(5) doi: 10.1016/j.bbadis.2020.165715. [DOI] [PubMed] [Google Scholar]
- Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X., et al. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: new molecular leads for therapeutic intervention. OMICS. 2013;17(10):510–518. doi: 10.1089/omi.2013.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu R., Menendez J.A. Pharmacological inhibitors of Fatty Acid Synthase (FASN)--catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr. Pharmaceut. Biotechnol. 2006;7(6):483–493. doi: 10.2174/138920106779116928. [DOI] [PubMed] [Google Scholar]
- Mansha M., Gill A., Thomson P.C. Potential risk factors of ovarian cancer and analysis of CA125, a biomarker used for its monitoring and diagnosis. Mol. Biol. Rep. 2019;46(3):3325–3332. doi: 10.1007/s11033-019-04794-8. [DOI] [PubMed] [Google Scholar]
- Menendez J.A., Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin. Ther. Targets. 2017;21(11):1001–1016. doi: 10.1080/14728222.2017.1381087. [DOI] [PubMed] [Google Scholar]
- Menendez J.A., Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin. Ther. Targets. 2017;21(11):1001–1016. doi: 10.1080/14728222.2017.1381087. [DOI] [PubMed] [Google Scholar]
- Moloney J.N., Cotter T.G. Seminars in Cell & Developmental Biology. Elsevier; 2018. ROS signalling in the biology of cancer. [DOI] [PubMed] [Google Scholar]
- Narajji C., Karvekar M., Das A.J. Biological importance of organoselenium compounds. Indian J. Pharmaceut. Sci. 2007;69(3):344–351. [Google Scholar]
- Palomba G., et al. In: Breast Cancer and Breast Reconstruction. Tejedor L., editor. IntechOpen; London: 2020. Epidemiology and genetic susceptibility of breast and ovarian cancer in Sardinian population; pp. 48–63. [Google Scholar]
- Pozarowski P., Darzynkiewicz Z. In: Checkpoint Controls and Cancer: Volume 2: Activation and Regulation Protocols. Schönthal A.H., editor. Humana Press; Totowa, NJ: 2004. Analysis of cell cycle by flow cytometry; pp. 301–311. [Google Scholar]
- Prasad S.B., et al. PI3K/AKT pathway-mediated regulation of p27(Kip1) is associated with cell cycle arrest and apoptosis in cervical cancer. Cell. Oncol. 2015;38(3):215–225. doi: 10.1007/s13402-015-0224-x. [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E. New treatments in ovarian cancer. Ann. Oncol. 2017;28(Suppl. l_8):viii57–viii60. doi: 10.1093/annonc/mdx442. [DOI] [PubMed] [Google Scholar]
- Rasool M., et al. Evaluation of matrix metalloproteinases, cytokines and their potential role in the development of ovarian cancer. PLoS One. 2016;11(11):1–10. doi: 10.1371/journal.pone.0167149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B.M., Permuth J.B., Sellers T.A. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L.G., Wu X., Guan J.-L. Cell Migration. Springer; 2005. Wound-healing assay; pp. 23–29. [Google Scholar]
- Roomi M.W., et al. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol. Rep. 2010;23(3):605–614. doi: 10.3892/or_00000675. [DOI] [PubMed] [Google Scholar]
- Rusetskaya N.Y., et al. [Selenium compounds in redox regulation of inflammation and apoptosis] Biomed. Khim. 2019;65(3):165–179. doi: 10.18097/PBMC20196503165. [DOI] [PubMed] [Google Scholar]
- Salehi F., et al. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J. Toxicol. Environ. Health B Crit. Rev. 2008;11(3–4):301–321. doi: 10.1080/10937400701876095. [DOI] [PubMed] [Google Scholar]
- Shaaban S., et al. Synthesis and biochemical studies of novel organic selenides with increased selectivity for hepatocellular carcinoma and breast adenocarcinoma. Eur. J. Med. Chem. 2019;179:515–526. doi: 10.1016/j.ejmech.2019.06.075. [DOI] [PubMed] [Google Scholar]
- Shaaban S., et al. Enhancing the chemosensitivity of HepG2 cells towards cisplatin by organoselenium pseudopeptides. Bioorg. Chem. 2021;109 doi: 10.1016/j.bioorg.2021.104713. [DOI] [PubMed] [Google Scholar]
- Shaaban S., et al. Enhancing the chemosensitivity of HepG2 cells towards cisplatin by organoselenium pseudopeptides. Bioorg. Chem. 2021;109 doi: 10.1016/j.bioorg.2021.104713. [DOI] [PubMed] [Google Scholar]
- Shi C., et al. A novel organoselenium compound induces cell cycle arrest and apoptosis in prostate cancer cell lines. Biochem. Biophys. Res. Commun. 2003;309(3):578–583. doi: 10.1016/j.bbrc.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Shirane M., et al. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem. 1999;274(20):13886–13893. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- Subhi T.A., Hassan F.A. Role of some enzymes in early diagnosis of ovarian cancer. Indian Journal of Public Health Research Development. 2019;10(10):2206–2209. [Google Scholar]
- Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Suzuki M., et al. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother. Pharmacol. 2010;66(3):475–484. doi: 10.1007/s00280-009-1183-6. [DOI] [PubMed] [Google Scholar]
- Tian F., et al. Adhesion induces matrix metalloproteinase-9 gene expression in ovarian cancer cells. Chin. J. Cancer Res. 2002;14(4):251–253. [PubMed] [Google Scholar]
- Toth M., Fridman R. Metastasis Research Protocols. Springer; 2001. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography; pp. 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M., Sohail A., Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 2012;878:121–135. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- Walsh C. Targeted therapy for ovarian cancer: the rapidly evolving landscape of PARP inhibitor use. Minerva Ginecol. 2018;70(2):150–170. doi: 10.23736/S0026-4784.17.04152-1. [DOI] [PubMed] [Google Scholar]
- Walz J.Z., et al. Fatty acid synthase as a potential therapeutic target in feline oral squamous cell carcinoma. Vet. Comp. Oncol. 2018;16(1):E99–E108. doi: 10.1111/vco.12341. [DOI] [PubMed] [Google Scholar]
- Wang H.Q., et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24(22):3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- Wang X.J., et al. Inhibition of leucine aminopeptidase 3 suppresses invasion of ovarian cancer cells through down-regulation of fascin and MMP-2/9. Eur. J. Pharmacol. 2015;768:116–122. doi: 10.1016/j.ejphar.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Xu F., et al. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10(1):54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zeng H., et al. The selenium metabolite methylselenol inhibits the migration and invasion potential of HT1080 tumor cells. J. Nutr. 2006;136(6):1528–1532. doi: 10.1093/jn/136.6.1528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.