Abstract
Innate resistance to Toxoplasma gondii is dependent on the ability of interleukin-12 (IL-12) to stimulate natural killer (NK) cell production of gamma interferon (IFN-γ). Since IL-18 is a potent enhancer of IL-12-induced production of IFN-γ by NK cells, SCID mice (which lack an adaptive immune response) were used to assess the role of IL-18 in innate resistance to T. gondii. Administration of anti-IL-18 to SCID mice infected with T. gondii resulted in an early reduction in serum levels of IFN-γ but did not significantly decrease resistance to this infection. In contrast, administration of exogenous IL-18 to infected SCID mice resulted in increased production of IFN-γ, reduced parasite burden, and a delay in time to death. The protective effects of IL-18 treatment correlated with increased NK cell numbers and cytotoxic activity at the local site of administration and with elevated levels of inducible nitrous oxide synthose in the spleens of treated mice. In addition, in vivo depletion studies demonstrated that the ability of exogenous IL-18 to enhance resistance to T. gondii was dependent on IL-12, IFN-γ, and NK cells. Together, these studies demonstrate that although endogenous IL-18 appears to have a limited role in innate resistance to T. gondii, treatment with IL-18 can augment NK cell-mediated immunity to this pathogen.
Interleukin-18 (IL-18) is a cytokine which was identified based on its ability to induce production of gamma interferon (IFN-γ) by T cells and enhance natural killer (NK) cell cytolytic activity (23, 38). IL-18 is structurally related to members of the IL-1 family (2) and is processed by IL-1 converting enzyme (ICE) (16, 17). In addition, IL-18 uses an IL-1-like signaling pathway that leads to the activation of NF-κB (31, 52). Although IL-18 is a member of the IL-1 family, it is functionally similar to IL-12. Thus, like IL-12, IL-18 increases production of IFN-γ by NK and T cells (23, 43, 56, 58, 59), augments cytotoxic activity of NK and CD8+ T cells (6, 18), and enhances immunity to tumors and infection (3, 5, 30, 37). Moreover, IL-18-deficient mice have impaired IFN-γ responses following infection with intracellular pathogens (26, 51, 55).
Innate resistance to toxoplasmosis is dependent on the ability of IL-12 to stimulate NK cell production of IFN-γ (15, 22). However, the development of optimal NK cell responses required for resistance to T. gondii is dependent on soluble and cell-bound ligands (CD28, IL-1, and TNF-α) which enhance the IL-12-induced NK cell production of IFN-γ (14, 20, 21, 25, 47). Since NK cells constitutively express the IL-18 receptor (24) and IL-18 is a potent enhancer of NK cell activity and synergizes with IL-12 to stimulate NK cell production of IFN-γ (23, 54, 59), it is a likely candidate to be involved in the regulation of innate resistance to T. gondii. The studies presented here suggest that endogenous IL-18 has a minor role in resistance to T. gondii but demonstrate that exogenous IL-18 can enhance NK cell-mediated resistance to this pathogen.
MATERIALS AND METHODS
Antibodies and cytokines.
A two-site enzyme-linked immunosorbent assay (ELISA) was employed to assay levels of IFN-γ as previously described (44). IL-18 levels were measured using a rat monoclonal antibody (MAb) specific for IL-18 as capture antibody and a polyclonal goat anti-IL-18 antibody (both antibodies were supplied by R&D Systems, Inc., Minneapolis, Minn.), in combination with a peroxidase-conjugated donkey anti-goat immunoglobulin G (IgG; Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) for detection. The sensitivity of this assay was routinely 39 pg of recombinant murine (rm) IL-18 per ml. The rabbit polyclonal anti-IL-18 used for in vivo neutralization studies was generated by multiple immunization of a rabbit with rmIL-18 provided by DNAX (5). In vitro assays showed that 20 μg of anti-IL-18 per ml could completely inhibit the production of IFN-γ induced by 10 ng of IL-18 per ml (data not shown). IL-12p40 levels were measured using MAb C17.8 and biotinylated MAb C15.6 prepared from hybridomas provided by G. Trinchieri (Wistar Institute). rmIFN-γ was purchased from Genzyme (Cambridge, Mass.). rmIL-18 was purchased from Pepro Tech, Inc. (Rocky Hill, N.J.). rmIL-12 was supplied by the Immunology Department of Genetics Institute (Cambridge, Mass.). Anti-asialoGM1 was purchased from Wako Chemicals, USA, Inc. (Richmond, Va.). Rabbit IgG and rat IgG were purchased from Sigma (St. Louis, Mo.).
Mice, infection, and cytokine treatment.
SCID B/6 mice were bred and maintained in Thoren caging units within the animal facility in the Gene Therapy Animal Facility of the University of Pennsylvania or purchased from Jackson Laboratory (Bar Harbor, Maine) and were 6 to 8 weeks of age when used in the experiments. Mice were routinely infected intraperitoneally (i.p.) with 20 cysts of the ME-49 strain of T. gondii. CBA/ca mice were purchased from Jackson Laboratory and were used to maintain the ME-49 strain of T. gondii and as a source of cysts for infection. To assess parasite burden at the local site of infection, 3 ml of phosphate-buffered saline (PBS) were injected into the peritoneal cavity of infected mice; cells were collected, and cytospins were prepared and stained with Diff-Quik (Dade Diagnostics of P.R. Inc., Aguada, Puerto Rico), and the percentage of peritoneal exudate cells (PECs) infected was estimated by microscopy. The percentage of cells infected was estimated by counting >500 cells/cytospin. To determine the effects of exogenous IL-18 on resistance to T. gondii, SCID mice were given 200 ng of IL-18 i.p. 1 day before infection and daily thereafter.
In vivo depletion.
To deplete NK cells, SCID mice were treated i.p. with 50 μg of anti-asialoGM1 3 days before infection and every 3 days thereafter. Fluorescence-activated cell sorting (FACS) analysis showed at least 98% depletion of NK1.1+ cells. Rabbit IgG was used to treat control mice. To assess the role of endogenous IL-18, SCID mice were treated with anti-IL-18 antibody or an isotype control at a dose of 2 mg/mouse on days −1, 1, and 3. For depletion of IL-12 or IFN-γ, SCID mice were given 0.75 mg of anti-IL-12 (C17.8) or 1 mg of anti-IFN-γ MAb (XMG6) or isotype control antibodies i.p. on days −1 and 3.
Analysis of IFN-γ production by NK cells.
Splenocytes from SCID mice were prepared as previously described (22). Briefly, spleens were dissociated in complete RPMI (10% heat-inactivated fetal calf serum, 2 mM glutamine, 1,000 U of penicillin per ml, 10 μg of streptomycin per ml, 0.25 mg of amphotericin B [Fungizone], 10 mM HEPES [Gibco, Grand Island, N.Y.], 1 mM sodium pyruvate, 1% [vol/vol] nonessential amino acids [Gibco], 5 × 10−5 M 2-mercaptoethanol) to give a single cell suspension. After lysis of erythrocytes, cells were washed twice and plated out at 105/well in a final volume of 200 μl. Cultures were stimulated with cytokines and/or RH strain tachyzoite lysate antigen (TLA) or cytokine(s) with or without antibody for 24 h. For the depletion of cytokines in the cultures, 30 μg of anti-IL-12 (C17.8), 20 μg of rat anti-IL-18 MAb (R&D Systems, Inc.), or 30 μg of rabbit anti-IL-18 polyclonal antibody per ml was added to the culture. All experiments were performed in triplicate.
Nitric oxide (NO) assay.
PECs were harvested and plated out at 105/well in a final volume of 200 ml per well. The cells were cultured in the medium alone for 24 h, and the levels of NO were measured using the Greiss assay. Briefly, 100 μl of the samples (supernatant) or the standard (NaNO2) was mixed with 100 μl of solution C, which was prepared by mixing an equal volume of solution A (sulfanilamide [1%] in 2.5% H3PO4 [phosphoric acid]) and solution B (napthylethylenediamine dihydrochloride [0.1%] in 2.5% H3PO4). The reaction was developed for 10 min at room temperature before reading it at 562 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader.
Cytotoxicity assay.
Cytotoxicity assays were performed as previously described (53). Briefly, YAC-1 cells (American Type Culture Collections, Rockville, Md.) were labeled with 100 μCi of 51Cr (Amersham, Arlington Heights, Ill.) for 1 h at 37°C, washed, and used as targets. PECs or splenocytes from mice were harvested and then washed twice, and the number of live cells was estimated based on trypan blue exclusion. These cells were plated at different effector target ratios and incubated at 37°C for 4 h. Supernatants were harvested with a Skatron cell press (Sterling, Va.), the amount of 51Cr released was estimated using a gamma counter (Packard, Meriden, Conn.), and the specific lysis was calculated as previously described.
Immunohistochemistry.
For histological analysis of tissues from infected mice, samples of livers, lungs, and spleens were removed from each mouse and fixed overnight in Accustain 10% Formalin neutral buffered solution (Sigma Diagnostics, St. Louis, Mo.) and then embedded in paraffin. Next, 5-μm paraffin sections were incubated for 1 h at 60°C and then rehydrated. Sections were incubated for 30 min with 0.3% H2O2–0.2 M NaN3 to quench endogenous peroxidase activity, followed by blocking with 10% goat serum (Vector Laboratories, Burlingame, Calif.) in Hanks balanced salt solution (HBSS). Sections were then incubated for 1 h at room temperature with primary rabbit antibody against iNOS (Transduction Laboratories, Lexington, Ky.) or T. gondii (from Fausto, G. Araujo, Palo Alto Medical Foundation) or an isotype control antibody (Sigma). After being washed in HBSS, sections were incubated with biotinylated anti-rabbit IgG antibody (Vector Laboratories). To visualize specific staining, sections were incubated with a peroxidase-conjugated avidin-biotin complex (Vectastain Elite ABC kit; Vector) according to the manufacturer's instructions, followed by incubation with 3,3′-diaminobenzidine (Vector), and counterstained with hematoxylin, dehydrated, and mounted in Permount (Fisher Scientific, Fair Lawn, N.J.).
FACS analysis.
The phycoerythrin (PE)-labeled anti-NK1.1 MAb was purchased from Pharmingen. For FACS analysis of NK cells, cells were incubated with purified anti-mouse CD32/CD16 to block nonspecific binding of MAbs to Fc receptors, followed by incubation with a PE-labeled anti-NK1.1 MAb for 30 min at 4°C. Background fluorescence was assessed using an irrelevant isotype control MAb (Pharmingen). Stained cells were analyzed with a FACScan cytoflurometer (Becton Dickinson Co., Mountain View, Calif.).
RNase protection assay (RPA).
Total RNA was extracted from spleens using Tri-Reagent (Sigma) and was assessed for cytokine mRNA content using the RiboQuant MultiProbe RNase Protection Assay System (Pharmingen). Briefly, 10 μg of RNA from each sample was hybridized in solution with the radiolabeled mCK-2b (containing IL-12p35, IL-12p40, IL-10, IL-1α, IL-1β, IL-1Ra, IL-18, IL-6, and IFN-γ) antisense RNA probe. Following hybridization, free probe and remaining single-stranded RNA were digested with RNase, and the protected probes were purified and resolved on 5% denaturing polyacrylamide gels using UltraPure Sequagel reagents (National Diagnostics, Atlanta, Ga.). Dried gels were then exposed to phosphorimaging screens, and protected fragments were visualized using a phosphorimager (GS-525; Bio-Rad, Culver City, Calif.). The quantity of protected RNA was determined using MultiAnalyst software (Bio-Rad) by measuring the density of each sample and subtracting the background levels. The relative expression of cytokine mRNA was determined by calculating the ratio of cytokine mRNA to housekeeping gene mRNA.
Statistics.
Statistical analysis (unpaired Student's t test, Mann-Whitney) was performed using INSTAT software (GraphPad Software, San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
Production of IL-18 during toxoplasmosis.
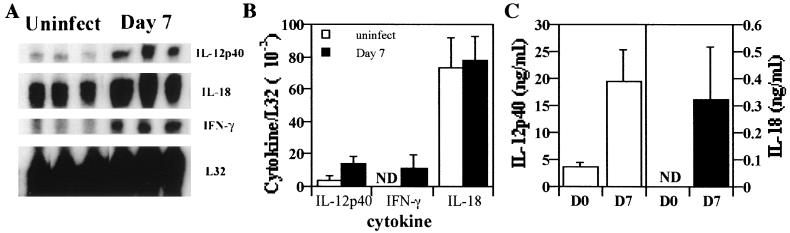
Previous studies have demonstrated that IL-18 is a proinflammatory cytokine which enhances production of IFN-γ by NK and T cells and is upregulated in response to infection (33, 41, 42). To determine if infection with T. gondii results in increased expression of IL-18, SCID mice were infected with T. gondii, and the levels of IL-18 mRNA in the spleen and IL-18 protein in the serum on day 7 postinfection were assessed. RPA analysis showed that, whereas levels of IL-12 and IFN-γ mRNA in the spleen were upregulated following infection, there were constitutive levels of mRNA for IL-18 in uninfected mice, and these were not significantly altered following infection (Fig. 1A and B). However, increased levels of IL-18 were detected in the serum of SCID mice following infection (Fig. 1C), and similar results were also observed by day 3 postinfection (data not shown). These data demonstrate that infection with T. gondii does result in increased serum levels of IL-18 mRNA but not of IL-18 mRNA. These data are consistent with a posttranslational mechanism to regulate secretion of IL-18 (16, 17).
FIG. 1.
Expression of IL-18 during toxoplasmosis. (A) Total RNA from the spleens of uninfected SCID mice or SCID mice infected for 7 days was extracted, and RPA analysis was performed as described in Materials and Methods. Similar results were observed in two experiments. (B) Densitometric analysis of RNA levels and expression relative to the housekeeping L32 gene. The results shown are the means ± the SD of the data presented. (C) Levels of IL-18 and IL-12 were measured by using ELISA in the serum from uninfected mice or mice infected for 7 days. The data shown are the means ± the SD from four independent experiments with three to six mice per group.
Role of endogenous IL-18 in resistance to T. gondii.
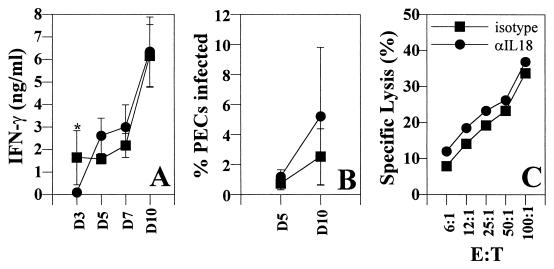
To explore the role of endogenous IL-18 in innate resistance to T. gondii, SCID mice were treated with a neutralizing rabbit polyclonal antibody specific for IL-18. Administration of anti-IL-18 to infected mice abrogated the infection-induced increase in serum levels of IL-18 on all days tested (days 3, 5, 7, and 10 [data not shown]) and resulted in a decrease in serum IFN-γ levels on day 3 postinfection (Fig. 2A). However, by days 7 and 10 postinfection, there was no significant difference in the serum levels of IFN-γ between experimental groups. Moreover, treatment with anti-IL-18 had no effect on parasite burden on either day 5 or day 10 postinfection (Fig. 2B) or on the infection induced increase in NK cell cytotoxicity on day 5 postinfection (Fig. 2C). In addition, treatment with anti-IL-18 did not alter the numbers of PECs recovered (isotype control = 4.3 × 106 ± 1.2 × 106; anti-IL-18 = 5.3 × 106 ± 2.1 × 106) or the total numbers of splenocytes (isotype control = 7.6 × 106 ± 1.3 × 106; anti-IL-18 = 8.2 × 106 ± 2.2 × 106) from mice infected for 5 days. The data presented are the means ± the standard deviations derived from an experiment representative of three experiments performed with three mice per group. Consistent with these results, administration of anti-IL-18 in a single experiment had no effect on the time to death of SCID mice infected with T. gondii (data not shown).
FIG. 2.
Effect of administration of anti-IL-18 during toxoplasmosis. SCID mice were infected i.p. with 20 ME-49 cysts and treated with a rabbit isotype control antibody or rabbit anti-IL-18 as described in Materials and Methods. The levels of IFN-γ in serum were measured by ELISA (A), and the percentage of PECs infected was estimated by using cytospins (B). The data shown are the means ± the SD of three pooled experiments with three to six mice per group (∗, P < 0.05). (C) NK cell (spleen) cytolytic activity against YAC-1 cells on day 7 postinfection were measured. The data shown are representative of three independent experiments.
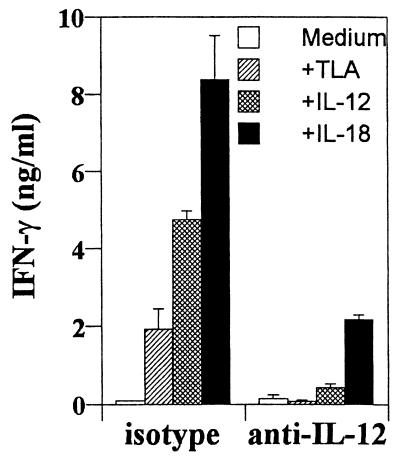
In vitro analysis revealed that splenocytes from infected SCID mice stimulated with TLA produced low levels of IFN-γ, and this was enhanced by the addition of IL-18 or IL-12 (Fig. 3). Moreover, the addition of IL-12 plus IL-18 in the presence of TLA resulted in at least two- to threefold-higher levels of IFN-γ compared to either cytokine alone (data not shown). The production of IFN-γ in these cultures was suppressed by the addition of anti-IL-12, and the ability of exogenous IL-18 to enhance production of IFN-γ was reduced by more than 70% when anti-IL-12 was added (Fig. 3). Furthermore, whereas IL-12p40 was readily detectable in cultures stimulated with TLA (ca. 10 ng/ml), IL-18 was not detected. Consistent with these latter results, the addition of anti-IL-18 did not significantly affect the ability of TLA alone or in combination with IL-12 to stimulate the production of IFN-γ (data not shown). These results indicate that the ability of IL-18 to enhance production of IFN-γ in this experimental system is largely dependent on endogenous IL-12. Together with the in vivo studies, these data suggest that endogenous IL-18 has a minor role in regulating the production of IFN-γ required for innate immunity to T. gondii.
FIG. 3.
IL-18 induced production of IFN-γ by splenocytes from infected SCID mice is dependent on IL-12. Splenocytes from SCID mice infected for 7 days were stimulated with 30 μg of TLA, 10 ng of IL-12, or 10 ng of IL-18 per ml in combination with 30 μg of rat IgG or anti-IL-12 (C17.8) per ml for 24 h, and the production of IFN-γ in the supernatants was measured by ELISA. The data shown are the means ± the SD from a single experiment done in triplicate. Similar results were observed in two additional experiments.
Administration of IL-18 enhances innate resistance to T. gondii.
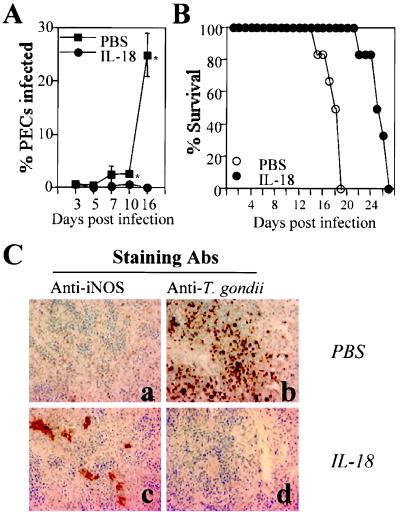
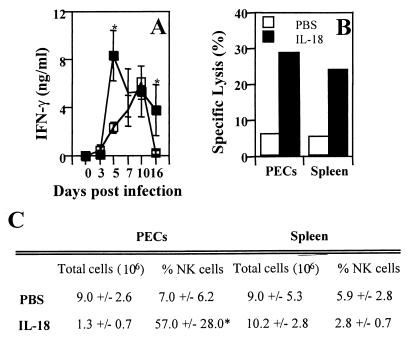
Since in vitro studies showed that IL-18 could enhance the parasite-induced production of IFN-γ by splenocytes, IL-18 was administered to SCID mice to determine if this treatment could enhance resistance to T. gondii. Administration of IL-18 to SCID mice 1 day before infection and daily thereafter resulted in a significant decrease in parasite burden on days 10 and 16 postinfection (Fig. 4A) and a 5- to 6-day delay in time to death (Fig. 4B). The total numbers of PECs recovered from mice infected for 16 days and treated with PBS or IL-18 were 15.0 × 106 ± 4.2 × 106 or 2.17 × 106 ± 1.15 × 106, respectively, and the total numbers of splenocytes recovered were 26.2 × 106 ± 2.5 (PBS) and 61.0 × 106 ± 4.2 × 106 (IL-18). The data presented are the means ± the SD of a representative experiment of three performed with three mice per group. Note that the numbers of PECs and splenocytes from uninfected SCID mice were typically 1 × 106 and 3 × 106 to 5 × 106, respectively. Immunohistochemical analysis of spleens from infected mice revealed that administration of IL-18 to SCID mice resulted in enhanced expression of iNOS associated with a decreased parasite burden (Fig. 4). Similar results were observed at the local site of infection. Thus, PECs isolated from infected mice treated with IL-18 for 7 days and incubated in medium alone for 24 h produced 38.3 ± 11.3 μM of nitrite, whereas PECs from control infected mice produced <0.1 μM (n = 3 mice per group). In addition, analysis of the number of cysts in the brains of mice on day 16 postinfection revealed that IL-18 treatment resulted in no detectable cysts (n = 3), while control mice treated with PBS had a mean of 4,166 ± 650 cysts (n = 3).
FIG. 4.
Administration of IL-18 to SCID mice enhances resistance to T. gondii. SCID mice infected with T. gondii were treated with IL-18 (200 ng per mouse) or PBS beginning 1 day before infection and daily thereafter. (A) The percentages of PECs infected were calculated as described in Materials and Methods. Results shown are the means ± the SD of four pooled experiments with three to six mice per group (∗, P < 0.05). (B) SCID mice were infected with T. gondii and treated daily with PBS or IL-18, and the survival was monitored. The results presented are the pooled data from five independent experiments with a total of 15 mice per experimental group. (C) Immunohistochemical detection of T. gondii and iNOS in the spleens of SCID mice infected for 16 days and treated with PBS or IL-18. Similar results were observed in three other mice.
Administration of IL-18 to SCID mice resulted in a significant increase in serum levels of IFN-γ on days 5 and 16 postinfection (Fig. 5) but did not alter the infection-induced increase in serum levels of IL-12 (data not shown). FACS analysis revealed that administration of IL-18 to infected mice resulted in an increased percentage of NK cells at the local site of infection on day 16 postinfection, but there was no change in the total number of NK cells at this site compared to infected controls (Fig. 5C, P < 0.05). This increase in the percentage of NK cells was not seen in either the spleens of treated mice on day 16 postinfection or in the PECs and splenocytes from mice infected for 7 days which were treated with PBS or IL-18. However, both PECs (Fig. 5B) and splenocytes from IL-18-treated SCID mice had higher levels of NK cell cytolytic activity (using YAC-1 cells as target cells) compared to control SCID mice on day 16 postinfection.
FIG. 5.
Administration of IL-18 to SCID mice enhances production of IFN-γ and NK cell activity during toxoplasmosis. (A) Serum levels of IFN-γ from SCID mice treated with PBS or IL-18 were measured by ELISA. The results shown are the means ± the SD of four pooled experiments with three to six mice per group (∗, P < 0.05). (B) Administration of IL-18 to SCID mice enhances NK activity (effector/target ratio = 25) on day 16 postinfection of T. gondii. The data shown are representative of three independent experiments with three mice per group. (C) Effect of administration of IL-18 on NK cell numbers during toxoplasmosis. On day 16 postinfection, PECs and splenocytes from SCID mice treated with IL-18 or PBS were prepared and counted, and FACS analysis was performed to measure the percentage of NK cells. The data shown are the means ± the SD of three pooled experiments with three mice per group (∗, IL-18-treated mice had significantly more NK cells in the peritoneal cavity than PBS control mice; P < 0.05).
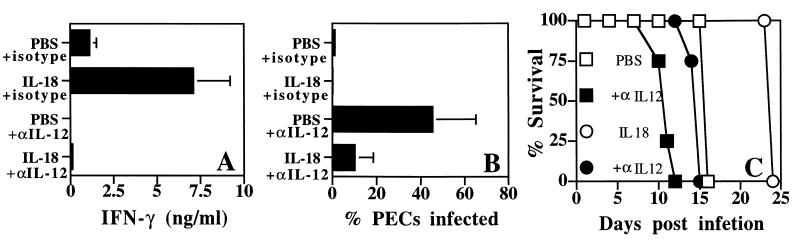
To assess the basis for the protective effects of exogenous IL-18, the roles of IL-12, IFN-γ, and NK cells in treated mice were analyzed. Depletion of IL-12 resulted in reduced serum levels of IFN-γ in both IL-18- and PBS-treated mice (Fig. 6A). In addition, our results showed that in the absence of IL-12, both IL-18- and PBS-treated SCID mice showed an increase in the percentage of infected PECs compared to isotype control mice on day 7 postinfection (Fig. 6B). The total numbers of PECs recovered from mice treated with PBS plus isotype, PBS plus anti-IL-12, IL-18 plus isotype, and IL-18 plus anti-IL-12 on day 7 postinfection were 2.7 × 106 ± 2.4 × 106, 9.8 × 106 ± 3.1 × 106, 0.9 × 106 ± 0.7 × 106, and 1.8 × 106 ± 0.4 × 106, respectively, whereas splenocytes from these four groups were 11.0 × 106 ± 1.8 × 106, 3.9 × 106 ± 1.8 × 106, 23.2 × 106 ± 4.5 × 106, and 4.5 × 106 ± 3.1 × 106, respectively. However, mice treated with IL-18 plus anti-IL-12 had fewer parasites than mice treated with anti-IL-12 alone (P < 0.05) (Fig. 6B). This reduction in parasite numbers correlated with a 2- to 3-day delay in time to death (P = 0.0025) (Fig. 6C). These results suggest that, although the protective effects of exogenous IL-18 are largely dependent on endogenous IL-12, there is an IL-18-dependent, IL-12-independent pathway that can enhance resistance to T. gondii.
FIG. 6.
IL-18-mediated resistance is dependent on endogenous IL-12. (A) SCID mice were infected with T. gondii and treated with IL-18 or PBS alone or in combination with anti-IL-12 or rat IgG. The treatment with anti-IL-12 resulted in complete abrogation of serum levels of IL-12p40 on days 3, 5, and 7 postinfection. The serum levels of IFN-γ on day 5 postinfection were measured by ELISA (A), and the percentage of infected PECs on day 7 postinfection calculated (B) as described in Materials and Methods. The data shown are the means ± the SD of the pooled data from three experiments with three to five mice per group. (C) Effect of depletion of endogenous IL-12 on the survival of SCID mice treated with IL-18. Similar results were observed in a repeat experiment with four mice per group.
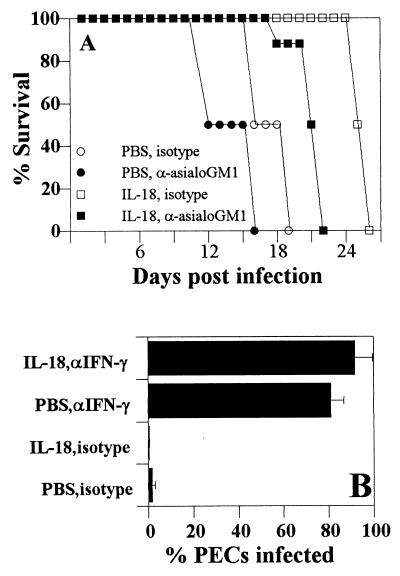
Depletion of NK cells in SCID mice treated with IL-18 antagonized the delay in time to death seen in these mice (Fig. 7A). NK cells are thought to be the major source of IFN-γ in SCID mice and, in our studies, depletion of NK cells led to >90% reduction in serum levels of IFN-γ during infection. However, low levels of IFN-γ were detected in NK-depleted, IL-18-treated SCID mice, suggesting either incomplete depletion of NK cells or that there are alternative sources of IFN-γ in SCID mice (12, 34, 57). Nevertheless, depletion of IFN-γ completely inhibited the protective effects of IL-18 (Fig. 7B), and mice died by day 9 postinfection (data not shown). On day 7 postinfection, the total numbers of PECs recovered from mice treated with PBS plus isotype, IL-18 plus isotype, PBS plus anti-IFN-γ and IL-18 plus anti-IFN-γ were 0.9 × 106, 1.3 × 106 ± 1.2 × 106, 11.6 × 106 ± 2.9 × 106, and 4.4 × 106 ± 2.5 × 106, respectively, and the total numbers of splenocytes of these four groups were 10 × 106 ± 0, 30 × 106 ± 10 × 106, 7.6 × 106 ± 4.3 × 106, and 5.0 × 106 ± 1.4 × 106, respectively. The data shown are the means ± the SD from a representative experiment with three mice per group. Together, these data indicate that IL-18 mediated resistance is dependent on the production of IFN-γ by NK cells.
FIG. 7.
IL-18-mediated resistance to T. gondii is dependent on NK cells and IFN-γ. (A) SCID mice (n = 8) were infected T. gondii and treated with IL-18 or PBS alone or in combination with rabbit IgG or rabbit anti-asialoGM1 as described in Materials and Methods, and survival was monitored. Similar results were observed in a repeat experiment. (B) SCID mice were infected with T. gondii and treated with IL-18 or PBS alone or in combination with anti-IFN-γ or rat IgG as described in Materials and Methods, and the percentage of infected PECs on day 7 postinfection was estimated. The data shown are the means ± the SD of three pooled experiments with three mice per group.
DISCUSSION
Previous studies have shown that infection with T. gondii results in increased production of IL-12 that is necessary for NK cell production of IFN-γ required for innate resistance to this pathogen (13, 49, 50). The data presented here demonstrate that, although infection resulted in an increase in serum levels of IL-18, these levels were low compared to other situations in which IL-18 has been shown to have a functional role (17). Accordingly, depletion of endogenous IL-18 during toxoplasmosis resulted in only a transient reduction in levels of IFN-γ and did not significantly affect parasite burden or survival. Thus, despite its functional similarity to IL-12, upregulation of IL-18 following infection appears to have a minor role in innate resistance to T. gondii. In support of this conclusion, mice deficient in the ICE (which is involved in the processing and secretion of IL-18) infected with T. gondii also have an early defect in their ability to produce IFN-γ but are resistant to toxoplasmosis (G. Cai and C. A. Hunter, manuscript in preparation). Together, these studies suggest a limited role for endogenous IL-18 in T-cell-independent resistance to T. gondii. Nevertheless, future studies using IL-18−/− mice will be necessary to confirm our findings on the role of endogenous IL-18 in resistance to toxoplasmosis. Consistent with our findings, other researchers have demonstrated that, during infection with Salmonella enterica serovar Typhimurium, endogenous IL-18 has a minor role in the production of IFN-γ required for clearance of this intracellular pathogen (8, 9). Interestingly, T. gondii and serovar Typhimurium induce high levels of nitric oxide during the acute stage of infection, and studies by Kim et al. demonstrated that nitric oxide can inhibit the production of IL-18 (28), which may explain the low levels of IL-18 detected following infection with these pathogens. This may be a common mechanism to limit potentially pathogenic immune responses or a strategy for intracellular pathogens to inhibit protective immune responses.
Although endogenous IL-18 does not appear to be critical for innate resistance to T. gondii, administration of IL-18 to SCID mice did result in a significant reduction in the parasite burden associated with enhanced production of IFN-γ. In vivo depletion studies revealed that the protective effects of IL-18 were dependent on IL-12, IFN-γ, and NK cells, suggesting that the ability of IL-18 to synergize with IL-12 to stimulate NK cell production of IFN-γ is the basis for the protective effects of exogenous IL-18. In support of this, the administration of IL-18 to uninfected SCID mice, in which there are low levels of endogenous IL-12, did not increase the total number of splenocytes or the NK cell activity or serum IFN-γ levels compared to PBS-treated control mice. In contrast, the administration of IL-18 to infected SCID mice led to an increased percentage of NK cells in the peritoneal cavity, suggesting that IL-18 may enhance the recruitment of NK cells to the local sites. This may be due to the ability of IL-18 to stimulate chemokine production (11) or its ability to upregulate adhesion molecule expression (29). However, these data have to be interpreted with care since many of the treatments used in these studies resulted in large changes in numbers of cells in the peritoneum and spleen. For example, administration of IL-18 to infected mice resulted in a twofold increase in the numbers of spleen cells, but a similar change was not observed at the local site of infection; rather, infected mice treated with IL-18 had sevenfold fewer PECs compared to untreated mice. One interpretation of these data is that these changes may be a function of the immune regulatory effects of exogenous IL-18 and a function of parasite burden. Thus, the immune effects of IL-18 may account for the increase in the numbers of cells in the spleen. In contrast, the lower numbers of inflammatory cells in the peritoneum are likely a reflection of the reduced parasite burden in mice treated with IL-18. This balance between immune regulatory effects and parasite burden could also explain why treatment with anti-IL-12 inhibited expansion of immune populations in the spleen but the 5- to 10-fold increase in parasite numbers in the peritoneum led to a 3-fold increase in the numbers of inflammatory cells at this site. Nevertheless, SCID mice treated with IL-18 still succumbed to toxoplasmosis within 4 weeks, and immunohistochemical analysis revealed that these mice could not control parasite growth in peripheral tissues (lungs, hearts, and brains) but did control parasite replication at the local site of infection and treatment (peritoneal cavity). This contrasts with untreated SCID mice that had large numbers of parasites in the peritoneum at the time they succumb to the infection on day 20. Why IL-18 can protect at the local site of treatment but not in other organs may be explained by the ultimate requirement for T cells for long-term resistance to T. gondii or a restricted ability of IL-18 activated NK cells to traffic to, and mediate protection at, different sites.
There are several reports of an IL-12-independent pathway that allows the generation of IFN-γ-dependent resistance to T. gondii (10, 45), as well as to other intracellular pathogens (27, 40). Our studies suggest that IL-18 can decrease parasite burden and delay time to death of SCID mice treated with anti-IL-12. This IL-12-independent protective effect is likely to be due to the ability of IL-18 alone to stimulate NK cells to produce low levels of IFN-γ (4, 38). Thus, although serum levels of IFN-γ were greatly reduced in mice treated with anti-IL-12, administration of IL-18 to these mice did enhance levels of IFN-γ mRNA in the spleen (data not shown). Furthermore, mice deficient in the transcription factor STAT4 (required for IL-12-mediated signaling) are highly susceptible to toxoplasmosis; administration of IL-18 results in a reduced parasite burden but ultimately fails to protect these mice (G. Cai and C. A. Hunter, submitted for publication). Together, these studies suggest that exogenous IL-18 is a potent enhancer of IL-12-mediated resistance to T. gondii and, although it can enhance resistance to toxoplasmosis independently of IL-12, this is a relatively minor effect.
Recent studies have demonstrated the importance of endogenous IL-18 for enhancing the production of IFN-γ following infection with Leishmania major, Staphylococcus aureus (55), Mycobacterium tuberculosis (48), and murine cytomegalovirus infection (26). However, it appears that endogenous IL-18 is not required for innate resistance to T. gondii (this study) or serovar Typhimurium (9). Thus, although IL-12 and IFN-γ are central mediators of resistance to many of these pathogens (1, 7, 19, 46), the requirement for IL-18 varies between pathogens. In addition, our studies demonstrate that the ability of IL-18 to mediate resistance to T. gondii is largely dependent on endogenous IL-12. Since it has been proposed that IL-18 may be useful for the treatment of infectious diseases (36) and cancer (5, 32, 35, 39), the studies presented here add to our knowledge of the interactions between IL-12 and IL-18 necessary for optimal innate responses.
ACKNOWLEDGMENTS
This work was supported by a grant from the NIH (AI 42334-01) and center grant P30 DK50306. DNAX is supported by the Schering-Plough Corporation. C.A.H. is a Burroughs Wellcome New investigator in Molecular Parasitology.
REFERENCES
- 1.Altare F, Durandy A, Lammas D, Emile J-F, Lamhamedi S, Deist F L, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F, Jeppsson O, Gollob J A, Meinl E, Segal A W, Fischer A, Kumararatne D, Casanova J-L. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 2.Bazan J F, Timans J C, Kastelein R. A newly defined interleukin-1? Nature. 1996;379:591–591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 3.Bohn E, Sing A, Zumbihl R, Biefeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 4.Cai G, Kastelein R A, Hunter C A. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-γ when combined with IL-18. Eur J Immunol. 1999;29:2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin C M, Salhany K E, Wysocka M, Aruga E, Kurzawa H, Chang A E, Hunter C A, Fox J C, Trinchieri G, Lee W M F. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Investig. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dao T, Mehal W Z, Crispe I N. IL-18 augements perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–2222. [PubMed] [Google Scholar]
- 7.de Jong R, Altare F, Haagen I-A, Elferink D G, Boer T, van Breda Vriesman P J, Kabel P J, Draaisma J M T, van Dissel J T, Kroon F P, Casanova J-L, Ottenhoff T H M. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 8.Dybing J K, Walters N, Pascual D W. Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect Immun. 1999;67:6242–6248. doi: 10.1128/iai.67.12.6242-6248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elhofy A, Bost K L. Limited interleukin-18 response in Salmonella-infected murine macrophages and in Salmonella-infected mice. Infect Immun. 1999;67:5021–5026. doi: 10.1128/iai.67.10.5021-5026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely K H, Kasper L H, Khan I A. Augmentation of the CD8+ T cell response by IFN-γ in IL-12-deficient mice during Toxoplasma gondii infection. J Immunol. 1999;162:5449–5454. [PubMed] [Google Scholar]
- 11.Fehniger T A, Shah M H, Turner M J, VanDeusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 12.Fukao T, Matsuda S, Koyasu S. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-γ production by dendritic cells. J Immunol. 2000;164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Denkers E Y, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993;2:139–149. [PubMed] [Google Scholar]
- 14.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expressin of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin-12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livington D J, Su M S S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto W, Osaki T, Okamura H, Robbins P D, Kurimoto M, Nagata S, Lotze M T, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163:583–589. [PubMed] [Google Scholar]
- 19.Hunter C A, Candolfi E, Subauste C S, Cleave V V, Remington J S. Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology. 1995;84:16–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter C A, Chizzonite R, Remington J S. IL-1β is required for IL-12 to induce production of IFN-γ by NK cells, a role for IL-1β in the T-cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 21.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 22.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter C A, Timans J C, Pisacane P, Menon S, Cai G, Walker W, Aste-Amezaga M, Chizzonite R, Bazan J F, Kastelein R A. Comparison of the effects of interleukin-1α, interleukin-1β and interferon-γ-inducing factor on the production of interferon-γ by natural killer. Eur J Immunol. 1997;27:2787–2792. doi: 10.1002/eji.1830271107. [DOI] [PubMed] [Google Scholar]
- 24.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 25.Johnson L L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992;60:1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakaraj P, Ngo K, Wu Y, Angulo A, Ghazal P, Harris C A, Siekierka J J, Peterson P A, Fung-Leung W-P. Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J Exp Med. 1999;189:1129–1138. doi: 10.1084/jem.189.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan M H, Wurster A L, Grusby M J. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998;188:1191–1196. doi: 10.1084/jem.188.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y-M, Talanian R V, Li J, Billiar T R. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme) J Immunol. 1998;161:4122–4128. [PubMed] [Google Scholar]
- 29.Kohka H, Yoshino T, Iwagaki H, Sakuma I, Tanimoto T, Matsuo Y, Kurimoto M, Orita K, Akagi T, Tanaka N. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J Leukoc Biol. 1998;64:519–527. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- 30.Mastroeni P, Clare S, Khan S, Harrison J A, Hormaeche C E, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Interleukin-18 activates NF-κB in murine T helper type 1 cells. Biochem Biophys Res Commun. 1997;234:454–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 32.Micallef M J, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57:4557–4563. [PubMed] [Google Scholar]
- 33.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 34.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai H, Hara I, Horikawa T, Fujii M, Kurimoto M, Kamidono S, Ichihashi M. Antitumor effects on mouse melanoma elicited by local secretion of interleukin-12 and their enhancement by treatment with interleukin-18. Cancer Investig. 2000;18:206–213. doi: 10.3109/07357900009031825. [DOI] [PubMed] [Google Scholar]
- 36.Ohkusu K, Yoshimoto T, Takeda K, Ogura T, Kashiwamura S-I, Iwakura Y, Akira S, Okamura H, Nakanishi K. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect Immun. 2000;68:2449–2456. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 38.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukata Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 39.Osaki T, Okamura H, Cai Q, Robbins P D, Kurimoto M, Lotze M T, Tahara H. IFN-γ-inducing factor/IL-18 administration mediates IFN-γ- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]
- 40.Oxenius A, Karrer U, Zinkernagel R M, Hengartner H. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J Immunol. 1999;162:965–973. [PubMed] [Google Scholar]
- 41.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 42.Pizarro T T, Michie M H, Bentz M, Woraratanadharm J, Smith M F, Foley E, Moskaluk C A, Bickston S J, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 43.Qureshi M H, Zhang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto M, Kawakami K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–649. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Sander B, Höidén I, Andersson U, Möller E, Abrams J S. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. J Immunol Methods. 1993;166:201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- 45.Scharton-Kersten T, Caspar P, Sher A, Denkers E Y. Toxoplasma gondii: evidence for interleukin-12-dependent and -independent pathways of interferon-γ production induced by an attenuated parasite strain. Exp Parasitol. 1996;84:102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 46.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 47.Sharma S D, Hofflin J M, Remington J S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985;135:4160–4163. [PubMed] [Google Scholar]
- 48.Sugawara I, Yamada H, Kaneko H, Mizumo S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for the prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 50.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 52.Thomassen E, Bird T A, Renshaw B R, Kennedy M K, Sims J E. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interferon Cytokine Res. 1998;18:1077–1088. doi: 10.1089/jir.1998.18.1077. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker W, Aste-Amezaga M, Kastelein R A, Trinchieri G, Hunter C A. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFNγ. J Immunol. 1999;162:5894–5901. [PubMed] [Google Scholar]
- 55.Wei X, Leung B P, Niedbala W, Piedrafita D, Feng G, Sweet M, Dobbie L, Smith A J H, Liew F Y. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–2828. [PubMed] [Google Scholar]
- 56.Yang J, Murphy T L, Ouyang W, Murphy K M. Induction of interferon-γ production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999;29:548–555. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimoto T, Okamura H, Tagawa Y-I, Iwakura Y, Nakanishi K. Interleukin-18 together with interleukin-12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimoto T, Takeda K, Tanaka Y, Ohkusu K, Kashiwamura S-I, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 59.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]