Background:
Negative-pressure wound therapy (NPWT) has improved split-thickness skin graft (STSG) survival rates, but prolonged application increases bacterial bioburden. Antimicrobial NPWT adjuncts have demonstrated efficacy, but strong evidence is lacking. We hypothesized that simultaneously replacing NPWT dressings within 48–72 hours and cleansing with Dakin's solution—a well-known antimicrobial agent—would increase STSG take.
Methods:
We performed a controlled retrospective case series on three groups of STSG patients treated between January 2014 and December 2020: bolster dressings, continuous NPWT (C-NPWT), and Dakin's NPWT (D-NPWT). Patients with documented measurements of STSG survival were included. The primary outcome was the percentage of STSG take calculated by survival area using surgical tape measures 2 weeks after surgery.
Results:
Fifty-nine patients were followed up for greater than or equal to 3 months. Average wound size for bolsters was smaller than that for D-NPWT (83 cm2 versus 204 cm2; P < 0.05). Average treatment time was 6.4 ± 2.4 days (bolsters), 6.5 ± 0.9 days (C-NPWT), and 2.8 ± 0.9 days (D-NPWT; P < 0.01). Average percentage of STSG take was 92% ± 0% (bolsters), 82% ± 0% (C-NPWT), and 99% ± 0% (D-NPWT; P < 0.01); there were significant differences between bolsters versus C-NPWT (P < 0.05) and C-NPWT versus D-NPWT (P < 0.05), but not between bolsters and D-NPWT.
Conclusions:
Interrupting NPWT with 0.125% Dakin’s solution cleansing is associated with increased STSG survival compared with standard NPWT protocols, but not bolster dressings. These findings warrant further investigation due to limitations of this retrospective case series.
Takeaways
Question: What methods can be utilized to optimize survival of split-thickness skin grafts?
Findings: The combination of NPWT and midtherapy cleansing with 0.125% Dakin’s solution is associated with increased STSG survival and shares comparable results with other antimicrobial NPWT modalities. This technique, therefore, warrants further investigation in larger and more diverse patient cohorts to arrive at more generalizable conclusions.
Meaning: Practitioners should consider utilizing Dakin’s solution rinses in between wound vacuum applications to optimize skin graft healing.
INTRODUCTION
Split-thickness skin grafts (STSGs) are commonly utilized for wound reconstruction.1 However, failure rates are variable and have been reported to range from 32%2,3 to as high as 66% in a prospective observational study of 73 lower extremity wounds published in 2015.3,4 Typical postoperative STSG protocols include conventional bolster dressings (ie, tie over techniques) and negative-pressure wound therapy (NPWT). NPWT has revolutionized the field by providing greater protection from shear forces and reducing complication rates compared with conventional methods.5–7 For example, in 2011, a prospective randomized controlled trial of 30 burn patients demonstrated a statistically significant improvement in STSG take using a negative-pressure dressing (97%) compared with the conventional dressing group consisting of Vaseline gauze and cotton pads (88%).6
Despite the manufacturer’s guidelines that NPWT dressings should be changed every 48 to 72 hours,8 there is published evidence that advocates for a timeline of up to 7 consecutive days for specific indications.9 For STSGs, it is common practice to maintain NPWT for 5–7 days to enable incorporation with the wound bed;10–12 however, in the senior author’s (G.D.X) experience, this length of time would sometimes result in the development of a malodorous bacterial biofilm over the graft site. This finding is reinforced by data from a retrospective review (n = 25) and prospective randomized controlled trial (n = 54), showing a statistically significant increase in bacterial bioburden after NPWT application over a variety of wounds.13,14 In the senior author’s experience, prolonged application of NPWT using the commonly accepted timeline of 7 days prohibited interval examination and potential treatment of the graft site.
Given the data on NPWT and bacterial bioburden, we reasoned that interrupting the standard NPWT timeline of 7 days would mitigate biofilm formation and improve STSG take. Notably, previous microbiological studies showed that even despite routine foam changes, NPWT alone does not limit bacterial overgrowth compared with conventional therapy; in a prospective randomized trial from 2004, the bacterial loads were found to have no statistically significant difference.13–15 Because of this, studies to introduce antimicrobial agents such as 0.2% polyhexamethylene biguanide (PHMB)-impregnated dressings as an adjunct to NPWT have been performed, demonstrating STSG survival of up to 100% in a retrospective case-control study published in 2015.16
Considering the positive effect of adjunctive antimicrobial agents for NPWT, we hypothesized that two key elements would be essential to optimizing STSG survival: (1) replacing the NPWT sponge within 48–72 hours after initial application and (2) performing an antimicrobial cleanse at the time of sponge change. Because Dakin’s solution is a well-known, accessible, and efficacious antimicrobial agent with widespread use across various specialties,17 we hypothesized that midtherapy interruption of NPWT with a 0.125% Dakin’s solution rinse improves STSG survival and can be a comparable alternative to other antimicrobial NPWT modalities.
METHODS
A retrospective case series was performed. Patient information was extracted from the electronic medical records from the Long Island Plastic Surgical Group, PC (Garden City, N.Y.). All patients who underwent split-thickness skin grafting by a single surgeon (G.D.X.) from January 2014 to December 2020 were selected for data collection and analysis. All patients with documented measurements of STSG take were included.
The study involved comparing three patient groups: one group receiving bolster dressings, a second group undergoing the current NPWT protocol with a midtherapy Dakin’s solution rinse (D-NPWT), and a third group undergoing the previous, continuous NPWT (C-NPWT) protocol. The current protocol is as follows: application of NPWT at the time of the procedure, removal after 48–72 hours, cleaning the graft site with 0.125% Dakin’s solution, and reapplication of NPWT for an additional 72 hours. All NPWT dressings were removed by postoperative day 6, at which time the wound was once again cleansed with 0.125% Dakin’s solution followed by local wound care using either petrolatum gauze (Xeroform) or a silver-impregnated hydrocolloid dressing (Aquacel Ag) depending on the level of moisture of the graft site. The previous protocol involved continuous application of NPWT for 5–7 days, after which the site would be cleansed with 0.125% Dakin’s solution followed by local wound care as described above. The decision to utilize a bolster dressing or NPWT depended on anatomic location and wound size. Bolster dressings were applied to wounds on the head, face, and neck due to practicality and anticipated patient discomfort with NPWT; outside of those locations, any wounds larger than 150 cm2 were treated with NPWT.
Institutional review board exemption status for this study was obtained through BRANY institutional review board services. We collected the following information: age at the time of surgery, the method of skin graft dressing used, the length of time over which the dressings were applied, wound type, wound size, and percentage of STSG take. The primary outcome was STSG survival, and the primary objective was to demonstrate a statistically significant increase in STSG survival using the D-NPWT protocol compared with the previous C-NPWT protocol and the use of bolster dressings. The STSG wound site areas were estimated by multiplying the longest width by the longest length (in millimeters, measured by a surgical paper ruler). STSG survival was calculated at the end of each treatment period using the following formula:
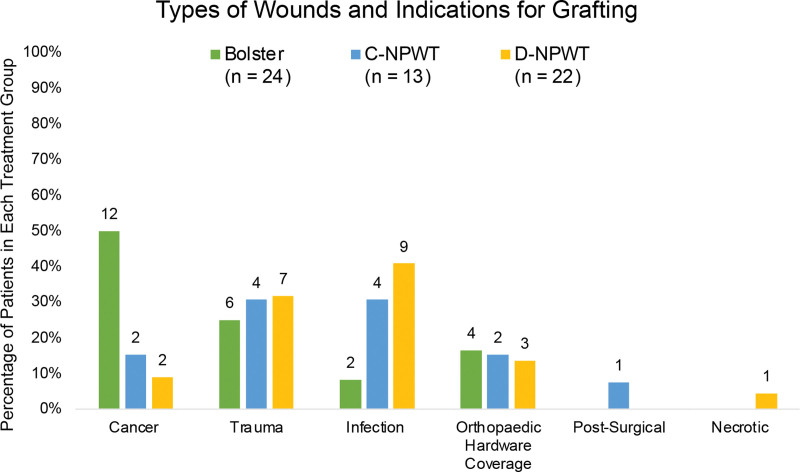
For patients with multiple wounds that were grafted on the same day, the total wound size was calculated by adding the multiple wound areas together. Every patient was evaluated at 1 week, 2 weeks, 1 month, and 3 months postoperatively. STSG survival was calculated at the 2-week interval for every patient. Wound types and indications for grafting were categorized into six different groups: cancer, trauma, infection, orthopedic hardware coverage, postsurgical, and necrotic wounds.
Statistical analysis was performed using Microsoft Excel (Microsoft Corp., Redmond, Wash.). Comparison of means was executed using single-factor analysis of variance. A post-hoc Tukey-Kramer honest significance test for unequal sample sizes was performed to determine which means were significantly different from each other. The alpha-level for statistical significance was 0.05.
RESULTS
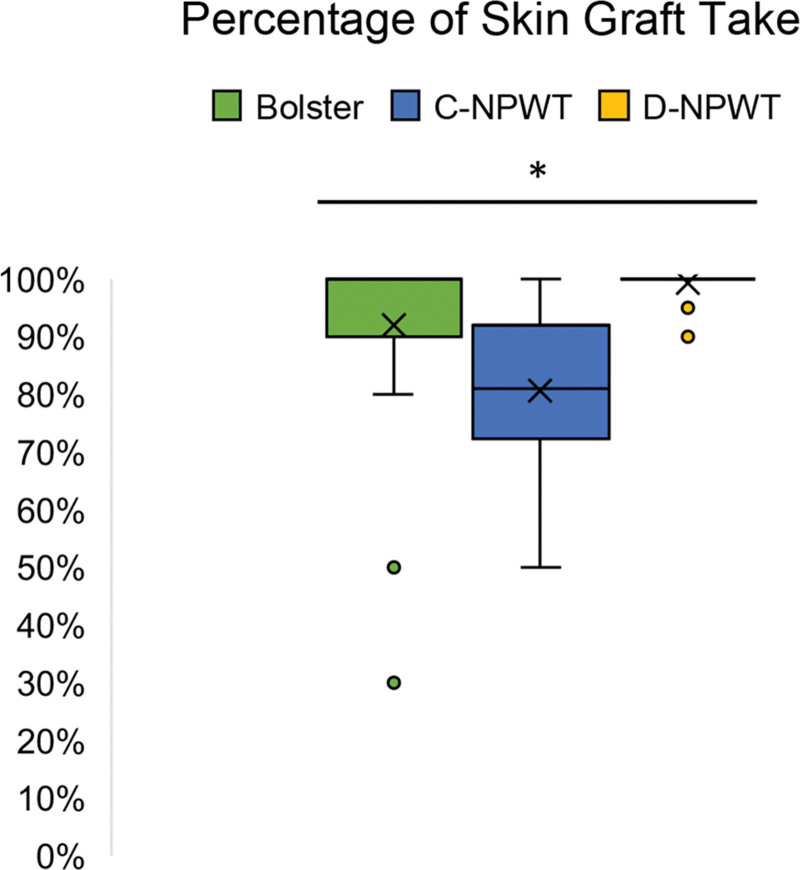
A summary of the results is illustrated in Table 1 and Figures 1 and 2. A total of 59 patients (40 men and 19 women) were followed up for a minimum of 6 months. All grafted wounds were on the lower extremity except for one upper extremity in the bolster group and one upper extremity in the D-NPWT group (97% of patients underwent lower extremity grafting). The patients were stratified into three groups: bolster dressing (n = 24), previous C-NPWT protocol (n = 13), and current D-NPWT (n = 22) protocol. The average age at the time of surgery was 66 ± 15 years for bolsters, 59 ± 16 years for C-NPWT, and 59 ± 17 years for D-NPWT. The average length of time over which the dressing was applied before initial removal was 6.4 ± 2.4 days for bolsters, 6.5 ± 0.9 days for C-NPWT, and 2.8 ± 0.9 days for D-NPWT. The average length of time over which the dressing was applied before removal was 6.4 ± 2.4 days for bolsters, 6.5 ± 0.9 days for C-NPWT, and 2.8 ± 0.9 days for D-NPWT. The average wound size was 83 ± 114 cm2 for bolsters, 153 ± 199 cm2 for C-NPWT, and 204 ± 189 cm2 for D-NPWT. The average percentage of STSG take was 92% ± 0% (range: 30%–100%) for bolsters, 82% ± 0% (range: 50%–100%) for C-NPWT, and 99% ± 0% (range: 90%–100%) for D-NPWT (Fig. 1). Documented traumatic etiologies included falls, burns, sharp injuries, animal bites, motor vehicle accidents, and fasciotomy wounds. Infectious etiologies included necrotizing fasciitis, infected orthopedic hardware, postsurgical infections, and unspecified infected wounds (Fig. 2).
Table 1.
Summary of Skin Graft Survival Categorized by Protocol
| Bolsters, n = 24 | C-NPWT, n = 13 | D-NPWT, n = 22 | |
|---|---|---|---|
| Age (y) | 66 ± 15 | 59 ± 16 | 59 ± 17 |
| Length of time (d) | 6.4 ± 2.4 | 6.5 ± 0.9 | 2.8 ± 0.9 |
| Wound size (cm2) | 83 ± 114 | 153 ± 199* | 204 ± 189† |
| Percentage take (%) | 92 ± 0 | 82 ± 0 | 99 ± 0 |
The total number of wound measurements was 18.
The total number of wound measurements was 24.
Fig. 1.
Box-and-whisker plot illustrating percentage of split-thickness skin graft take categorized by dressing change protocol (*P < 0.01 from single-factor analysis of variance).
Fig. 2.
Percentage of wound types and indications for skin grafting in each treatment group. Each bar is labeled with the number of patients in that specific group.
The average age was not significantly different among the treatment groups (P = 0.2756). The average length of time to initial dressing removal was significantly different among the groups (P < 0.01), specifically between bolsters versus D-NPWT (P < 0.05) and C-NPWT versus D-NPWT (P < 0.05). There was also a significant difference in average wound size among the groups (P < 0.05), specifically between the bolster and D-NPWT groups (P < 0.05). Finally, the average percentage of STSG take was found to be statistically different among the three groups (P < 0.01; Fig. 1); specifically, there were significant differences between bolsters versus C-NPWT (P < 0.05) and C-NPWT versus D-NPWT (P < 0.05), but not between bolsters and D-NPWT.
DISCUSSION
Compared with standard NPWT protocols, our data show a statistically significant association with increased STSG success using the described D-NPWT protocol. Interestingly, STSG take using bolster dressings significantly exceeded that of C-NPWT, which could be attributed to the occlusive nature of NPWT providing a favorable environment for bacterial growth.18 Moreover, it should be noted that a bolster dressing is generally used more frequently with smaller skin graft sizes (as in the current study), which could bias these results and potentially explain the lack of statistical significance between D-NPWT and bolster dressings.
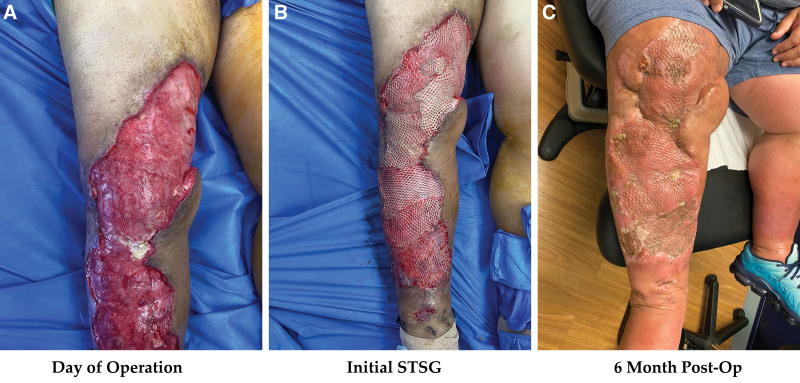
NPWT with instillation of dilute Dakin’s has demonstrated significant benefits in a retrospective case series of five patients with large venous stasis ulcers (>200 cm2) colonized with over 105 bacteria; all patients went on to heal completely with skin grafting.19 In wound therapy and skin graft studies, frequently isolated bacteria include Gram-negative rods (Pseudomonas aeruginosa), S. aureus, enterobacteriaceae, anaerobes, and Acinetobacter.14,20 Dakin’s solution has been shown to be effective against a broad spectrum of aerobic and anaerobic bacteria—including those previously mentioned—and can dissolve necrotic tissue debris.21,22 Taken together, these data support the combination of NPWT with antimicrobial agents (such as Dakin’s solution) to optimize skin graft take as with our own protocol (Table 1; Fig. 3). Nevertheless, a comparative study of Dakin’s and other cleansing solutions (eg, normal saline) as a control would provide useful insight into whether Dakin’s solution confers any unique advantage.
Fig. 3.
Clinical photographs and results of a patient who underwent split-thickness skin grafting of the right lower extremity and postoperative management using the D-NPWT protocol. A, day of operation. B, Initial STSG. C, Results at 6 months postoperative.
Other previously published antimicrobial NPWT adjuncts for improving STSG survival include povidone-iodine gauzes23 and the previously mentioned 0.2% PHMB-impregnated dressings.16 The povidone-iodine study was limited to a six-patient case series,23 but the PHMB study showed equally promising results in a retrospective case-control cohort of 40 patients.16 Our results are comparable to both studies with the common thread being the use of a strong antimicrobial adjunct to NPWT, and future comparative studies would be useful to determine the most cost-effective strategy. Additionally, a recent publication by Hahn et al24 on 66 lower extremity wounds demonstrated the efficacy of silver-impregnated NPWT sponges in reducing bacterial colonization rates compared with conventional NPWT, potentially offering another effective antimicrobial alternative.
The primary advantage of this study is that all patients were managed by a single surgeon, but it was limited by a small cohort size. Additionally, there are a variety of factors other than infection rate or bacterial load that can significantly influence skin graft survival, such as the nature of the wound bed (ie, clean versus traumatic versus contaminated) and patient comorbidities—notably, these factors were not specifically examined or stratified, thereby limiting our analysis. Conducting a matched-cohort study in the future could potentially account for these confounders. Furthermore, bacterial load alone is not always linearly correlated with tissue invasion and does not reliably predict graft loss.25,26 Although bacterial load was not directly measured in the current report, recording that data along with the rate of infection would be useful for future studies. There is no evidence to suggest that infection was the most influential variable on graft survival in this case series, but the impact of our D-NPWT protocol suggests that minimizing the likelihood of infection and bioburden can positively influence graft survival. Finally, this is the initial report of our D-NPWT protocol, and optimization of this protocol over time and economic cost-effectiveness evaluations will be the subject of future work. Overall, our data provide a useful analysis of the introduction of quarter-strength Dakin’s rinses into a skin graft protocol utilizing conventional NPWT.
CONCLUSIONS
Compared with NPWT alone, the combination of NPWT and midtherapy 0.125% Dakin’s solution is associated with increased STSG survival and shares comparable results with other antimicrobial NPWT modalities. Notably, there was no statistically significant difference in STSG take using this protocol compared with bolster dressings. This technique warrants further investigation in larger and more diverse patient cohorts to arrive at more generalizable conclusions.
Footnotes
Disclosures: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Braza ME, Fahrenkopf MP. Split-thickness skin grafts. StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing. [Updated 2020 Jul 31]. Available at https://www.ncbi.nlm.nih.gov/books/NBK551561/. Accessed March 6, 2021. [PubMed] [Google Scholar]
- 2.Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract. 2014;2014:582080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turissini JD, Elmarsafi T, Evans KK, et al. Major risk factors contributing to split thickness skin graft failure. Georg Med Rev. 2019;3:7755. [Google Scholar]
- 4.Stankiewicz M, Coyer F, Webster J, et al. Incidence and predictors of lower limb split-skin graft failure and primary closure dehiscence in day-case surgical patients. Dermatol Surg. 2015;41:775–783. [DOI] [PubMed] [Google Scholar]
- 5.Gkotsoulias E. Split thickness skin graft of the foot and ankle bolstered with negative pressure wound therapy in a diabetic population: the results of a retrospective review and review of the literature. Foot Ankle Spec. 2020;13:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petkar KS, Dhanraj P, Kingsly PM, et al. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns. 2011;37:925–929. [DOI] [PubMed] [Google Scholar]
- 7.Blume PA, Key JJ, Thakor P, et al. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C. therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinetic Concepts I (KCI). V.A.C therapy patient guide. 2009.. Available at https://www.mykci.com/-/media/Project/Acelity/Acelity-Base-Sites/USA-PDFs/New-Page-PDF/VACtherapypatientguide0.pdf. Accessed June 30, 2022
- 9.Kim YH, Hwang KT, Kim JT, et al. What is the ideal interval between dressing changes during negative pressure wound therapy for open traumatic fractures? J Wound Care. 2015;24:536,536,538–536,536,542. [DOI] [PubMed] [Google Scholar]
- 10.Senchenkov A, Petty PM, Knoetgen J, III, et al. Outcomes of skin graft reconstructions with the use of vacuum assisted closure (VAC(R)) dressing for irradiated extremity sarcoma defects. World J Surg Oncol. 2007;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TQ, Franczyk M, Lee JC, et al. Prospective randomized controlled trial comparing two methods of securing skin grafts using negative pressure wound therapy: vacuum-assisted closure and gauze suction. J Burn Care Res. 2015;36:324–328. [DOI] [PubMed] [Google Scholar]
- 12.Clark JM, Rychlik S, Harris J, et al. Donor site morbidity following radial forearm free flap reconstruction with split thickness skin grafts using negative pressure wound therapy. J Otolaryngol Head Neck Surg. 2019;48:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276–279. [DOI] [PubMed] [Google Scholar]
- 14.Mouës CM, Vos MC, van den Bemd G-JCM, et al. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf E, Jordan X, Clauss M, et al. High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound repair Regen. 2013;21:677–681. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, Chew KY, Chen CC, et al. Antimicrobial-impregnated dressing combined with negative-pressure wound therapy increases split-thickness skin graft engraftment: a simple effective technique. Adv Skin Wound Care. 2015;28:21–27. [DOI] [PubMed] [Google Scholar]
- 17.Ueno CM, Mullens CL, Luh JH, et al. Historical review of Dakin’s solution applications. J Plast Reconstr Aesthet Surg. 2018;71:e49–e55. [DOI] [PubMed] [Google Scholar]
- 18.Kwa KAA, Krijnen P, Bernards AT, et al. Bacterial species and load increase during negative pressure wound therapy: a prospective cohort study. Wounds a Compend Clin Res Pract. 2020;32:74–80. [PubMed] [Google Scholar]
- 19.Raad W, Lantis JC, II, Tyrie L, et al. Vacuum-assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unal S, Ersoz G, Demirkan F, et al. Analysis of skin-graft loss due to infection: infection-related graft loss. Ann Plast Surg. 2005;55:102–106. [DOI] [PubMed] [Google Scholar]
- 21.Georgiadis J, Nascimento VB, Donat C, et al. Dakin’s solution: “one of the most important and far-reaching contributions to the armamentarium of the surgeons.” Burns. 2019;45:1509–1517. [DOI] [PubMed] [Google Scholar]
- 22.Keyes M, Jamal Z, Thibodeau R. Dakin Solution. StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 23.Chang KP, Tsai CC, Lin TM, et al. An alternative dressing for skin graft immobilization: negative pressure dressing. Burns. 2001;27:839–842. [DOI] [PubMed] [Google Scholar]
- 24.Hahn HM, Lee IJ, Woo K-J, et al. Silver-impregnated negative-pressure wound therapy for the treatment of lower-extremity open wounds: a prospective randomized clinical study. Adv Skin Wound Care. 2019;32:370–377. [DOI] [PubMed] [Google Scholar]
- 25.McManus AT, Kim SH, McManus WF, et al. Comparison of quantitative microbiology and histopathology in divided burn-wound biopsy specimens. Arch Surg. 1987;122:74–76. [DOI] [PubMed] [Google Scholar]
- 26.Steer JA, Papini RP, Wilson AP, et al. Quantitative microbiology in the management of burn patients. II. Relationship between bacterial counts obtained by burn wound biopsy culture and surface alginate swab culture, with clinical outcome following burn surgery and change of dressings. Burns. 1996;22:177–181. [DOI] [PubMed] [Google Scholar]