Abstract
Rationale and Objective
The safety and efficacy of long-term exercise training in reducing physical functional loss in older adults with advanced CKD and co-morbidity is uncertain.
Study Design
Multi-center, parallel group, randomized controlled trial.
Settings and Participants
Adults 55 years and older with CKD stage G3b-4 enrolled from centers in Baltimore and Boston.
Intervention
Twelve months of in-center supervised exercise training incorporating majority aerobic but also muscle strengthening activities or a group health education control intervention, randomly assigned in 1:1 ratio.
Outcomes
Primary outcomes were cardiorespiratory fitness and submaximal gait at 6 and 12 months quantified by peak oxygen consumption (VO2peak) on graded exercise treadmill test and distance walked on the six-minute walk test, respectively. Secondary outcomes were changes in lower extremity function, renal function, glycemia, blood pressure, and body mass index.
Results
Among 99 participants, mean age was 68 years, 62% were African American, and mean eGFR 33 mL/min/1.73m2; 59% had diabetes and 29% had coronary artery disease. Among those randomized to exercise, 59% of exercise sessions were attended in the initial 6 months. Exercise was well tolerated without excess occurrence of adverse events. At 6 months, aerobic capacity was higher among exercise participants (17.9±5.5 vs. 15.9±7.0 ml/kg/min, p=0.03), but differences were not sustained at 12-months. The 6-minute walk distance improved more in the exercise (adjusted differences: 98 feet, p=0.02; p=0.03 for treatment-by-time interaction). The exercise group had greater improvements on the get up and go test (p=0.04) but not the short physical performance battery (p=0.8).
Limitations
Planned sample size was not reached. Loss to follow-up and dropout were greater than anticipated.
Conclusions.
Among adults ≥55 years with CKD stages G3b-4 and a high level of medical comorbidity, a 12-month program of in-center aerobic and resistance exercise training was safe and associated with improvements in physical functioning.
Funding:
NIH.
Trial Registration:
Keywords: Chronic Kidney Disease, Clinical Trial, Exercise, Physical Function, Aging
Introduction
Chronic kidney disease (CKD) is common among older adults. With advancing age, sarcopenia, the age-associated decline in muscle mass and strength also becomes increasingly common.1 Sarcopenia risk may be enhanced by concurrent CKD, given described catabolic losses in muscle among people with CKD.2 Accordingly, older adults with CKD often have worse physical function than those without CKD along with a higher prevalence of frailty
Exercise may reduce physical function losses or even improve physical function in older adults with CKD;3 however most studies that have evaluated exercise in older adults with CKD have been modest in sample size, of relatively short duration, and either focused on individuals receiving dialysis or individuals with earlier stages of CKD.4-13 The longer-term impacts of sustained exercise training in older adults with non-dialysis advanced CKD and the feasibility of such an intervention in this population with extensive comorbidity remain uncertain. The best data to date derive from a study of older adults in Australia, demonstrating that, in a population with mean age of 61 years and mean estimated glomerular filtration rate (eGFR) of 39 mL/min/1.73m2, an 8 week exercise program followed by a 10 month home maintenance training program was safe and associated with early improvements in physical function that waned during the maintenance phase.7 Our objective in this randomized trial was to determine the effect of 12 months of exercise training on cardiorespiratory fitness and physical performance in a diverse sample of adults ≥55 years with advanced (stage G3b-4) CKD.
Methods
Study Design and Oversight
The Aerobics, Weights, and Renal Disease (AWARD) study was a multicenter, parallel-group randomized controlled trial designed to test the effect of 12 months of center-based supervised exercise training compared to a health education control intervention on changes in physical performance and cardiorespiratory fitness among adults ≥55 years with CKD stages G3b and G4. Full details of the protocol are available in the supplemental material (Supplementary File 1). Participants were enrolled from the University of Maryland Medical Center, VA Maryland Healthcare System, Tufts Medical Center, and Partners Healthcare. All participants provided written informed consent. The study protocol was approved by the University of Maryland, Baltimore and Tufts University Health Sciences Institutional Review Boards and by the Human Subjects Subcommittee of the VAMHCS. The study protocol was registered on clinicatrials.gov (NCT01462097). Data and Safety Monitoring was performed by an independent board.
Study Population
Community-dwelling adults 55 years and over with an estimated glomerular filtration rate (eGFR) using the CKD-Epi equation of 15 to <45 ml/min/1.73m2 were eligible. Exclusion criteria included: participation in more than 185 minutes weekly of self-reported moderate to vigorous physical activity, prior disabling stroke, severe anemia (hemoglobin <9g/dL), uncontrolled diabetes (glycated hemoglobin>10%), vascular claudication impairing physical functioning, dementia or severe cognitive impairment (Mini Mental State Exam score<20/30), NYHA class III/IV heart failure, and acute cardiovascular event (stroke, myocardial infarction, decompensated heart failure, or coronary revascularization) within the prior 6 months. Individuals with controlled diabetes and/or stable cardiovascular disease were not excluded. Potentially eligible patients who provided informed consent completed a screening procedure by a study physician including detailed medical history, physical exam, and laboratory testing to confirm eligibility. Participants who met all eligibility criteria next completed graded exercise treadmill testing (see below); those with evidence of inducible ischemia or clinically significant arrhythmia were excluded from subsequent participation.
Intervention
Eligible participants were randomized in a 1:1 ratio to the exercise intervention group or a health education control group, with stratification on site. A permuted block randomization protocol was utilized, with blocks varying in size and between the two sites. Given the nature of the study intervention, neither the participants nor investigators were blinded to group assignment. Participants in the Exercise Intervention Group were assigned to thrice weekly in-center exercise training for the first 6 months of the trial. Each session consisted of aerobic exercise, utilizing a treadmill (walking or light jogging) preferentially, with stationery cycle ergometry permitted in certain situations (e.g., for joint pain). Exercise was initiated during the first week for 20 minutes, with a target heart rate of 50%-60% of heart rate reserve (calculated according to Karvonen et al).14 Duration and intensity of exercise were progressively increased over 7 weeks for a goal of 40 minutes at 70%-80% of heart rate reserve. For two sessions each week following treadmill activities, participants were guided through a 10-minute routine of upper and lower extremity resistance training exercises, incorporating standing leg curls, knee extension and side hip raises with ankle weights, arm raises, biceps curls and overhead raises with hand weights, and leg squats and toe stands. Exercise intensity was targeted to a rating of 15 (“somewhat hard”) to 16 (“hard”) according to the scale of Borg (range, 6 to 20). Each session was preceded by a brief warm-up and followed by a brief cool-down period. Exercise was performed in research centers at the Baltimore VAMC or the Jean Mayer Human Nutrition Research Center on Aging at Tufts University under supervision by trained research staff. After a 6-month phase of thrice weekly in-center training, patients were transitioned to an additional 6-month maintenance phase, in which one of three weekly sessions could be completed at home, with identical duration and intensity as the in-center sessions. Adherence to exercise during home sessions was documented by participant log.
The Health Education group was assigned to group-based educational sessions, modeled after the successful aging intervention in the LIFE clinical trial in older adults15. Sessions were held weekly during the initial 6 month phase, and then monthly for the second (maintenance) 6 months, and were led variably by research staff, study investigators, nurses, and dieticians. Topics included healthy diet, chronic kidney disease, diabetes and blood pressure management, and other topics of relevance to older adults with chronic disease. Each session ended with light stretching.
Study Outcomes
All outcomes were measured at baseline and after approximately 6 and 12 months. The primary study outcomes were cardiorespiratory fitness (as quantified by peak oxygen consumption during a graded exercise treadmill test [GXT]) and performance on the six-minute walk test. Briefly, GXT was performed on a motorized treadmill using a modified Balke protocol.16 Participants walked on a treadmill at a pre-selected speed with progressive increases in grade (incline), until volitional fatigue. Oxygen consumption, CO2 production, and minute ventilation were measured continuously using a metabolic cart; values averaged for final 20 seconds prior to exhaustion were considered peak oxygen consumption (VO2peak). The six-minute walk test assesses submaximal exercise capacity, often approximating an exertional level representative of community-based tasks.17 Patients were instructed to “walk as far as possible” for six minutes, walking back and forth over a flat 100-foot surface. Total distance traveled over the six minutes was recorded.
Secondary physical function study outcomes included the Timed Up and Go Test (TUG) and the Short Physical Performance Battery (SBBP).18 The TUG is a functional test of mobility, lower extremity strength, maximal gait speed, and balance. Participants begin seated in a straight-backed chair, and are instructed to stand up, wake 10 feet and turn around a cone, walk back to the chair and sit down, all as fast as possible. The fastest time to complete 2 trials was recorded. The SBBP is a widely used test of lower extremity in older adults, consisting of three components: 1) usual gait speed over a 4-meter course; 2) five repeated chair stands; 3) standing balance battery. Each component is scored 0-4, and the total scores of each component are summed (range, 0-12).19 Consistent with the LIFE clinical trial in older adults, we defined impaired performance as a total score of <10.20
Additional measurements collected including laboratory measurements, blood pressure, and anthropometrics. GFR was estimated from serum creatinine using the CKD-Epi estimating equation. Albuminuria was quantified on spot morning samples, and urine albumin standardized to urine creatinine concentration. Blood pressure was measured in the seated position after 5 minutes of rest 3 times at 1-minute intervals using automated sphygmomanometers; the average of these measurements was recorded. Anthropometric measurements included the body mass index and the ratio of waist to hip circumferences. Self-reported moderate-vigorous physical activity was estimated using the validated CHAMPS questionnaire.21
Safety and Adverse Events
Standardized safety procedures included querying about interim acute medical events or symptoms, vital sign measurements in all participants before and after each exercise session, and capillary glucose measurement before and after exercise in all diabetic participants on glucose-lowering medications. Individual exercise training sessions were held or delayed if hypo/hyperglycemia, severe hypertension, hypotension, or symptoms suggestive of new cardiovascular disease were present, or if acute illness otherwise precluded safe exercise. For both exercise and health education groups, adverse events (AE) were prospectively identified and were classified as expected or unexpected and as serious or non-serious according to standard definitions.
Statistical Methods
Sample size estimation was based on published and preliminary data on expected effect sizes on the change in the six minute walk test in older adults with CKD. We estimated that N=120 subjects would need to be randomized to have 80% power to detect a true difference in distance walked in 6 minutes of 75 feet, assuming a standard deviation of this difference of 180 feet and a 20% drop out rate. We estimated that a minimal required sample size of N=66 to provide 80% power to detect a difference in VO2peak of 2.5±4.5 ml/kg/min. A 5% type I error rate was assumed. We used linear mixed models with an unstructured covariance matrix to test the associations of treatment groups and their interaction with study visit (baseline, 6 months 12 months) with each individual primary and secondary physical function outcomes (dependent variables), accounting for changes in the outcome variable over time within study participant (see supplemental file S2 for analytic plan). The treatment-by-study visit interaction was the primary test of the difference in physical function between the randomized groups. For this analysis, all completed outcome measurements were used, including 6-month assessments on those participants who were unable to complete the 12-month assessments. We did not impute missing or unavailable outcome measurements, and did not adjust the type 1 error rate for multiple comparisons. We also conducted ANCOVA analysis to assess for significant differences in outcomes between treatment groups from at each follow-up visit separately, adjusting for baseline levels. In non-prespecified analysis, the rates of serious adverse events were compared formally between the two groups using Poisson regression.
Results:
Characteristics of Study Participants
From March 2012 through June 2016, 194 participants provided informed consent for a screening research visit. Of these, 99 were eligible, amenable to participating and randomized (Figure 1); N=49 to exercise, N=50 to health education). Accrual was terminated prior to achieving the prespecified goal due to funding and recruitment challenges. Characteristics were similar between participants in each randomized group (Table 1). Among all participants, mean age was 68.0 ± 8.2 years, mean eGFR was 33.3 ± 10.5 ml/kg/1.73m2, a majority were Black, obese, and had diabetes and hypertension, and 29% had stable coronary heart disease. On average, study participants were highly sedentary, with mean 78± 150 self-reported minutes weekly of moderate to vigorous physical activity.
Figure 1 –
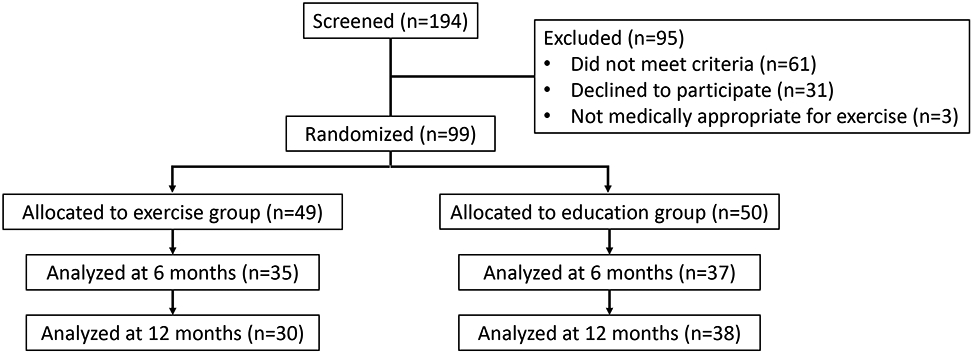
CONSORT diagram
Table 1:
Characteristics of participants at baseline:
| Study Sample (N=99) |
Exercise (N=49) | Health Education Control (N=50) |
p-value comparing randomized groups |
|
|---|---|---|---|---|
| Age (years) | 68.8 (8.2) | 67.9 (7.7) | 68.1 (8.8) | 0.9 |
| Female | 25 (25.3%) | 15 (30.6%) | 10 (20.0%) | 0.2 |
| Race | 0.4 | |||
| African American | 61 (61.6%) | 27 (55.1%) | 34 (68%) | |
| Caucasian | 31 (31.3%) | 18 (36.7%) | 13 (26.0%) | |
| Other | 7 (7.1%) | 4 (8.1%) | 3 (6%) | |
| eGFR (ml/min/1.73m2) | 33.3 (10.5) | 34.1 (9.5) | 32.6 (11.4) | 0.5 |
| Hb (g/dL) | 12.4 (1.6) | 12.5 (1.6) | 12.3(1.7) | 0.5 |
| BMI (kg/m2) | 31.1 (6.5) | 30.3 (7.2) | 31.8 (5.8) | 0.3 |
| Self-reported Moderate-vigorous physical activity (minutes/week) | 78 (150) | 72 (126) | 90 (174) | 0.6 |
| CAD | 29 (29.3%) | 16 (32.7%) | 13 (26.0%) | 0.5 |
| Diabetes | 58 (58.6%) | 30 (61.2%) | 28 (56.0%) | 0.6 |
| Hypertension | 92 (92.9%) | 44 (89.8%) | 48 (96.0%) | 0.3 |
| CHF 1 | 10 (10.1%) | 6 (12.2%) | 4(8.0%) | 0.5 |
| Peripheral vascular disease2 | 9 (9.1%) | 3 (6.1%) | 6 (12%) | |
| Smoking | 0.5 | |||
| Current | 16 (16.2%) | 10 (20.4%) | 6 (12.0%) | |
| Former | 58 (58.6%) | 28 (57.1%) | 30 (60.0%) | |
| Never | 25 (25.3%) | 11 (22.4%) | 14 (28.0%) | |
| Stroke or TIA | 16 (16.2%) | 9 (18.4%) | 7 (14.0%) | |
| COPD | 13 (13.1%) | 5 (10.2%) | 8 (16.0%) | 0.5 |
| Site | ||||
| Tufts | 43 (43.4%) | 21 (42.9%) | 22 (44.0%) | 0.9 |
| Univ of Maryland | 56.6%) | 28 (57.1%) | 28 (56.0%) |
CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; COPD: Chronic Obstructive Pulmonary Disease; TIA: Transient Ischemic Attack
Patient with NYHA Class III-IV symptoms were excluded.
Patients with symptomatic claudication were excluded.
Patients with disabling stroke were excluded.
Follow-up and Exercise Adherence
Nineteen (39%) of 49 participants randomized to exercise and N=12 (24%) of 50 health education participants dropped out, were lost to follow-up, or were otherwise unable to complete functional testing by the final 12 month follow-up (Figure 1). Participants who did not complete the 12-month outcome assessments tended to have lower eGFR and were more likely to have CAD, CHF, and peripheral vascular disease (Table S1). Among those in the exercise group, 1851 (59%) of 3111 prescribed exercise sessions were attended during the first 6 months (Table 2; excluding those sessions not prescribed due to illness, but considering patients lost to follow-up or dropped out for non-medical reasons as non-adherent). Adherence was somewhat lower (48.9%) during the maintenance 6-month phase of the study.
Table 2:
Adherence to exercise intervention, by phase (Months 1-6 vs. 7-12)
| Study Period | Prescribed Sessions^ |
Attended Sessions |
Total Adherence |
Median Individual Adherence |
% with Adherence >66% |
|---|---|---|---|---|---|
| Months 1-6 | 3111 | 1851 | 59.5% | 60.7% | 45.8% |
| Months 7-12 | 2496 | 1221 | 48.9% | 38.2% | 30.2% |
Excluding those sessions for which exercise was prohibited due to acute medical reasons (e.g., acute infection, hospitalization). However, sessions in patients who had dropped out were considered “prescribed sessions” for calculating adherence.
Change in Physical Performance
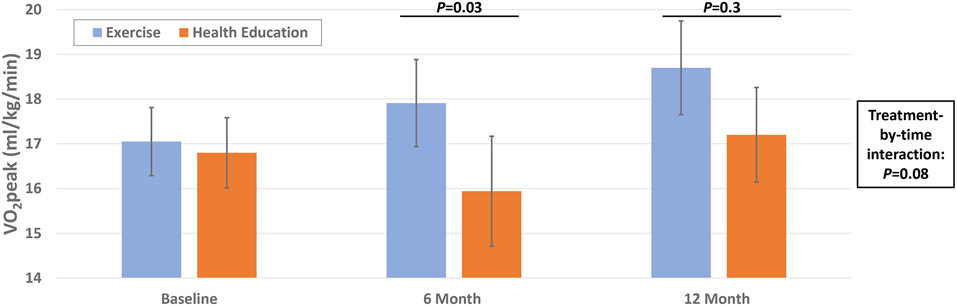
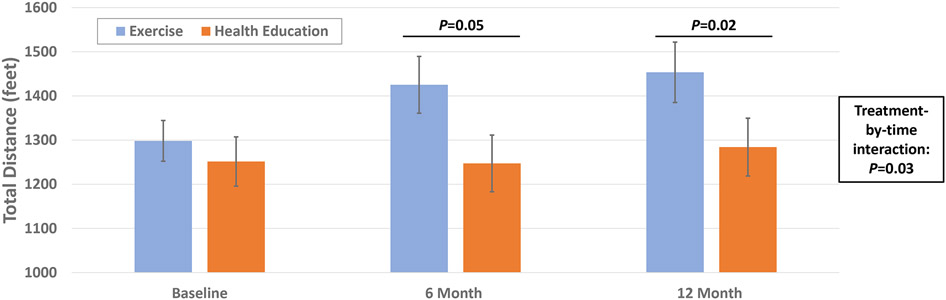
Aerobic capacity at baseline was similar between the two randomized groups (suppl table 2). At 6 months, aerobic capacity was higher in the exercise vs. patient education group (17.9±5.5 vs. 15.9±7.0 ml/kg/min, p=0.03), but these differences were not sustained at 12 months (Figure 2a, Table S2), and there was no significant difference in the change in aerobic capacity across the 12 months (treatment-by-visit interaction: p=0.08). Submaximal gait as quantified by the six-minute walk test was similar at baseline (Exercise group: 1298±323 feet vs. Health Education: 1251±394 feet). After 6 and 12 months, the distanced walked in six minutes was greater in the exercise versus health education groups (6-months: p=0.05, 12-months: p=0.03, Table S2). The rate of improvement in distance walked was significantly greater in the exercise group compared to the health education group (p=0.03 for treatment-by-time interaction; figure 2b).
Figure 2a and 2b.
Effect of Exercise training vs. Health education on V02peak (A) and 6 minute walk (B)
Performance on the TUG was similar between the two groups at baseline (suppl table 2). After 6 and 12 months, performance in the health education group was significantly slower (i.e., poorer) compared to the exercise group, with a significantly greater rate of worsening over time (Figure S1a, Table S2); treatment-by-time interaction p=0.04). Impaired lower extremity performance on the SPPB test (defined by total test score <10 out of 12 points) did not differ at baseline or over time between treatment groups (Figure S1b, Table S2).
Change in risk factors and obesity
At baseline, the exercise and health education groups were well-balanced with regards to kidney function, albuminuria, blood pressure, HbA1C, and body mass index (Table S3). There were no significant differences in the overall change in any of these secondary outcomes between the two randomized groups, nor were there any significant differences in these outcomes at 6 or 12 months of follow-up.
Safety
A total of 182 adverse events occurred during the course of the study. Of these, N=72 (39.6%) were classified as serious adverse events, and 44% were considered expected based on the intervention or comorbid conditions. Three participants died (2 in health education group, 1 in exercise group) and 4 required chronic kidney replacement therapy (3 in health education group, 1 in exercise group). There were no serious adverse events judged related to exercise training (defined as occurring during or within 1 hour after an exercise session). The overall rate of adverse events in the exercise vs. health education groups (11.9 per 100 person-months vs. 12.6 per 100 person-months; incidence rate ratio [IRR]=0.92 [0.66-1.27], p=0.6) and serious adverse events (0.037 per 100 person-months vs. 0.054 per 100 person-months; IRR=0.70, [0.41, 1.20]; p=0.2) were not significantly different (table 3).
Table 3.
Frequency and rates of all Adverse Events and Serious Adverse events, by randomization group
| All Adverse Events | Serious Adverse Events | Death | Chronic Dialysis |
|||
|---|---|---|---|---|---|---|
| Total | Rate (per 100 p- months) |
Total | Rate (per 100 p- months) |
|||
| Exercise | 66 | 11.9 | 21 | 0.037 | 1 | 1 |
| Education | 79 | 12.6 | 34 | 0.054 | 2 | 3 |
Discussion
Among adults ≥55 years with CKD stages G3b-4 and with a high level of co-morbidity, a 12-month program of in-center aerobic and resistance exercise training was safe, feasible, and compared to health education control was associated with improvements in submaximal gait and mobility, as quantified by validated objective measure of physical performance. The exercise program also improved measured aerobic exercise capacity after 6 months compared to the health education group, although this difference was not sustained at 12 months. In contrast, there was no impact on secondary outcomes of cardiovascular risk, including blood pressure, lipids, kidney function, and body mass index when compared to health education control. The latter finding may be explained by insufficient statistical power, a true absence of effect of the exercise training program on these risk factors, or (for blood pressure and glycemia) post-randomization changes in medical therapy.
Compared to trials of similar interventions, adherence to the thrice weekly exercise regimen was moderately high, especially for the initial 6-month intervention period, though with a decline for the 2nd 6-month period despite the option for at-home exercise once weekly. Although there are no clear consensus criteria for categorizing exercise adherence in older adults,22 adherence in this study compares favorably with exercise adherence in the multi-center LIFE-P study, in which relatively healthy adults completed 65% of planned twice weekly exercise session over 6 months.23 Adherence to supervised exercise training in the CKD participants in the LANDMARK-3 trial was modestly higher at 70%, although this phase of the exercise intervention lasted only 8 weeks, with notably lower adherence after transition to home-based exercise. Of note, our estimates of adherence are conservative in that they assign long-term non-adherence status to participants who dropped out of the intervention during follow-up for non-medical reasons. However, more substantial changes in physical function may have been attained with greater adherence especially during the second 6-months period, and future studies should investigate novel methods to improve long-term exercise adherence in CKD patients. Overall safety of this intervention in older CKD patients was demonstrated by the absence of any exercise-related serious adverse events, and by similar overall serious adverse event rates to those subjects receiving health education only. Likewise, In the LANDMARK-3 trial, rates of hospitalizations (one subtype of serious adverse event) were equal in the exercise and health education groups, and no exercise-related SAEs were observed. The overall higher rates of SAEs in the present trial compared to the prior LANDMARK-3 trial are likely explained by the older population with a higher burden of comorbid conditions. These safety findings provide further support that older CKD patients with common CKD-related co-morbidities can safely engage in prolonged exercise training regimens, provided they are appropriately screened and selected.
The long-term effects of exercise training on physical function in this trial were quantified using validated and reliable objective instruments. The six-minute walk test is a widely utilized measure of submaximal gait that quantifies levels of ambulatory tolerance reflective of real-world mobility in older adults. Performance on this test is on average more than 30% lower in individuals with CKD than among healthy adults, and performance is highly predictive of short-term and long-term mortality among patients with CKD24 as well as among adults with other serious chronic conditions. Peak aerobic capacity during GXT is an objective and reliable measure of cardiorespiratory fitness. VO2peak is strongly prognostic of mortality in patients receiving dialysis, and has been commonly used as an outcome measure in aerobic exercise interventional trials. The Timed Up and Go test in an integrated test of mobility, motor speed, balance, and lower extremity power.
There are notable strengths and unique features of the present study. In contrast to many prior exercise studies in CKD patients, this trial included an active comparator group, which provided similar (albeit less intensive) group social interaction to that experienced by participants in the supervised exercise training. Therefore, the observed differences in physical function are more likely to represent true physiologic effects of exercise training and are less likely to be explained by greater motivation among the exercise group from greater interactions with healthcare providers and other patients. Furthermore, the exercise intensity goal was individualized for each subject, as a fixed range of each participant’s heart rate reserve. This accounted for inter-individual differences in maximal heart rate due to factors such as chronotropic incompetence and pharmacological beta-blockade. Study participants received 12 months of exercise training, testing the durability of the benefits of such interventions, in contrast to the 2-6 months of training in the majority of prior studies in kidney disease patients 8,12,25. Finally, the study population included a racially diverse CKD population with a high burden of comorbidity including chronic cardiovascular diseases, reflective of the characteristics of many CKD patients in the US, thus enhancing the external validity of the study results.
There also important limitations of the study. Despite extension of the accrual period, the study did not meet its target, and drop-out and loss to follow-up were greater than expected, often due to intercurrent medical events that precluded safe or feasible continuation of exercise training. Therefore, the power to detect significant differences in physical performance may have been limited. A moderately greater loss to follow-up among the group randomized to exercise training, and systemic differences between those who completed vs. did not complete the 12 month study, may also have introduced bias into the comparison of between-group differences in the primary and secondary outcomes. The lack of blinding of outcome assessors may have also introduced bias. Of note, the study was not designed to detect differences in “hard” clinical outcomes such as hospitalization or death. However, the similar rates of serious adverse events between the two randomized intervention groups does support the overall safety of the long-term supervised exercise training program. Our study was not designed to have sufficient statistical power to detect effects of exercise on kidney function, and such studies would require longer duration of follow-up and a substantially larger number of participants. We did not adjust our analyses of the two primary endpoints for multiple comparisons, such that the risk of erroneously rejecting a true null hypothesis was increased.
In conclusion, a 12-month program of supervised aerobic and resistance exercise training in adults ≥55 years with stage G3b and G4 CKD is safe, feasible, and effective at improving physical performance, albeit with a higher than expected drop-out. Future studies should examine the long-term effects of exercise training on kidney outcomes and major cardiovascular risk factors in older CKD patients, whether similar safety and efficacy can be achieved with home-based exercise without in-person supervision, and novel methods to improve long-term adherence.
Data Sharing:
The authors will consider written requests for sharing of deidentified data from this clinical trial to qualified investigators. Due to information security restriction of the Veteran’s Health Administration, deidentified study data is unable to be shared on publicly-facing websites.
Supplementary Material
Figure S1: Effect of Exercise training vs. health education on performance on Timed Up and Go and Short Physical Performance Battery tests.
Table S1: characteristics of participants with and without 12-month functional outcome measures, by randomized group.
Table S2: Change in physical function, by randomization group
Table S3: Change in renal function and cardiovascular risk factors, by randomization group
Support:
The research was supported by funding from NIDDK (R01DK090401) and NIA (NIA K23AG057813, P30AG028747 and P30AG031679). The funders had no role in the analysis, reporting, or the decision to submit for publication.
Footnotes
Financial Disclosure: There authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moreno-Gonzalez R, Corbella X, Mattace-Raso F, et al. Prevalence of sarcopenia in community-dwelling older adults using the updated EWGSOP2 definition according to kidney function and albuminuria : The Screening for CKD among Older People across Europe (SCOPE) study. BMC Gerlatr. Oct 2 2020;20(Suppl 1):327. doi: 10.1186/s12877-020-01700-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang XH, Mitch WE. Muscle wasting from kidney failure-a model for catabolic conditions. Int J Biochem Cell Biol. Oct 2013;45(10):2230–8. doi: 10.1016/j.biocel.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fielding RA, Guralnik JM, King AC, et al. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: Results from the LIFE study randomized trial. PLoS One. 2017;12(8):e0182155. doi: 10.1371/journal.pone.0182155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcellos FC, Del Vecchio FB, Reges A, et al. Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens. Jun 2018;32(6):397–407. doi: 10.1038/s41371-018-0055-0 [DOI] [PubMed] [Google Scholar]
- 5.Castaneda C, Gordon PL, Uhlin KL, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. Dec 4 2001;135(11):965–76. doi: 10.7326/0003-4819-135-11-200112040-00008 [DOI] [PubMed] [Google Scholar]
- 6.Hiraki K, Shibagaki Y, Izawa KP, et al. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: a randomized pilot and feasibility trial. BMC Nephrol. Jun 17 2017;18(1):198. doi: 10.1186/s12882-017-0613-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, Isbel NM. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. Apr 2015;65(4):583–91. doi: 10.1053/j.ajkd.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 8.Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J Am Soc Nephrol. Jan 2018;29(1):250–259. doi: 10.1681/ASN.2017010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leehey DJ, Collins E, Kramer HJ, et al. Structured Exercise in Obese Diabetic Patients with Chronic Kidney Disease: A Randomized Controlled Trial. Am J Nephrol. 2016;44(1):54–62. doi: 10.1159/000447703 [DOI] [PubMed] [Google Scholar]
- 10.Leehey DJ, Moinuddin I, Bast JP, et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. Dec 9 2009;8:62. doi: 10.1186/1475-2840-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. Dec 2011;43(4):1133–41. doi: 10.1007/s11255-010-9823-7 [DOI] [PubMed] [Google Scholar]
- 12.Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. Dec 5 2014;9(12):2052–8. doi: 10.2215/CJN.11791113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson EL, Greening NJ, Viana JL, et al. Progressive Resistance Exercise Training in CKD: A Feasibility Study. Am J Kidney Dis. Aug 2015;66(2):249–57. doi: 10.1053/j.ajkd.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 14.Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. May 1988;5(5):303–11. doi: 10.2165/00007256-198805050-00002 [DOI] [PubMed] [Google Scholar]
- 15.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. Nov 2011;66(11):1226–37. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. Jun 1959;10(6):675–88. [PubMed] [Google Scholar]
- 17.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. Feb 2003;123(2):387–98. doi: 10.1378/chest.123.2.387 [DOI] [PubMed] [Google Scholar]
- 18.Piva SR, Fitzgerald GK, Irrgang JJ, Bouzubar F, Starz TW. Get up and go test in patients with knee osteoarthritis. Arch Phys Med Rehabil. Feb 2004;85(2):284–9. doi: 10.1016/j.apmr.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. Mar 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 20.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. Jun 18 2014;311(23):2387–96. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. Jul 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 22.Hawley-Hague H, Horne M, Skelton DA, Todd C. Review of how we should define (and measure) adherence in studies examining older adults' participation in exercise classes. BMJ Open. Jun 23 2016;6(6):e011560. doi: 10.1136/bmjopen-2016-011560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fielding RA, Katula J, Miller ME, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. Nov 2007;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d [DOI] [PubMed] [Google Scholar]
- 24.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. Apr 2013;24(5):822–30. doi: 10.1681/ASN.2012070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, et al. Effect of Moderate Aerobic Exercise Training on Endothelial Function and Arterial Stiffness in CKD Stages 3–4: A Randomized Controlled Trial. Am J Kidney Dis. Aug 2015;66(2):285–96. doi: 10.1053/j.ajkd.2015.03.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Effect of Exercise training vs. health education on performance on Timed Up and Go and Short Physical Performance Battery tests.
Table S1: characteristics of participants with and without 12-month functional outcome measures, by randomized group.
Table S2: Change in physical function, by randomization group
Table S3: Change in renal function and cardiovascular risk factors, by randomization group