Abstract
Objectives
Infection-related childhood hearing loss is one of the few preventable chronic health conditions that can affect a child’s lifelong trajectory. This study sought to quantify relationships between infection-mediated hearing loss and middle ear disease and environmental factors, such as exposure to wood smoke, cigarette smoke, household crowding, and lack of access to plumbed (running) water, in a northwest region of rural Alaska.
Design
This study is a cross-sectional analysis to estimate environmental factors of infection-related hearing loss in children aged 3 to 21 years. School hearing screenings were performed as part of two cluster randomized trials in rural Alaska over two academic years (2017–2018 and 2018–2019). The first available screening for each child was used for this analysis. Sociodemographic questionnaires were completed by parent/guardians upon entry into the study. Multivariable regression was performed to estimate prevalence differences and prevalence ratios. A priori knowledge about the prevalence of middle ear disease and the difficulty inherent in obtaining objective hearing loss data in younger children led to analysis of children by age (3–6 years versus 7 years and older) and a separate multiple imputation sensitivity analysis for pure-tone average-based infection-related hearing loss measures.
Results
A total of 1634 children participated. Hearing loss was present in 11.1% of children sampled based on otoacoustic emission as the primary indicator of hearing loss and was not associated with exposure to cigarette smoke (PR=1.07, 95% CI, 0.48–2.38), use of a wood-burning stove (PR=0.85 95% CI, 0.55–1.32), number of persons living in the household (PR=1.06 95% CI, 0.97–1.16), or lack of access to running water (PR=1.38 95% CI, 0.80–2.39). Using pure-tone average as a secondary indicator of hearing loss also showed no association with environmental factors. Middle ear disease was present in 17.4% of children. There was a higher prevalence of middle ear disease in homes without running water versus those with access to running water (PR=1.53, 95% CI, 1.03–2.27). There was little evidence to support any cumulative effects of environmental factors. Heterogeneity of effect models by age found sample prevalence of hearing loss higher for children aged 3–6 years (12.2%, 95% CI, 9.3–15.7) compared to children 7 years and older (10.6%, 95% CI, 8.9–2.6), as well as for sample prevalence of middle ear disease (22.7%, 95% CI, 18.9–26.9 and 15.3%, 95% CI, 13.3–17.5, respectively).
Conclusions
Lack of access to running water in the home was associated with increased prevalence of middle ear disease in this rural, Alaska Native population, particularly among younger children (aged 3–6 years). There was little evidence in this study that cigarette smoke, wood-burning stoves, and greater numbers of persons in the household were associated with infection-mediated hearing loss or middle ear disease. Future research with larger sample sizes and more sensitive measures of environmental exposure is necessary to further evaluate these relationships. Children who live in homes without access to running water may benefit from earlier and more frequent hearing health visits.
INTRODUCTION
Childhood hearing loss has a well-known, negative impact on early language development, academic performance, relationships with others, and future vocational opportunities (Bess et al., 1998; Kennedy et al., 2006; Lieu, 2004). The World Health Organization estimates that 60% of hearing loss in children is preventable (Krug et al., 2016). The majority of preventable childhood hearing loss is infection-related. For example, acute otitis media and other infectious illnesses lead to chronic middle ear disease and ultimately hearing loss (Krug et al., 2016). Ear infections are particularly common in rural areas, including rural Alaska, where the population is primarily Alaska Native. Alaska Native infants and children develop otitis media at 4–5 times the national average (Reed et al., 1967; Singleton et al., 2018).
In rural northwest Alaska, hearing is integral to the Yup’ik, Iñupiaq, and Siberian Yup’ik peoples. All three groups have a unique culture and speak diverse languages/dialects. In the Norton Sound region, subsistence, drumming and dancing, and the role of elders in the community (such as the passing down of oral traditional knowledge and history through storytelling) are important, and hearing plays an essential role. To improve the identification of childhood hearing loss in the region, two mixed-methods cluster randomized trials entitled Hearing Norton Sound evaluated an enhanced mHealth hearing screening and specialty telemedicine referral pathway in 15 communities in the region (Emmett et al., In Press; Emmett, Robler, Gallo, et al., 2019; Emmett, Robler, Wang, et al., 2019). These trials were conducted to determine the best methods for identifying infection-related hearing loss and improving follow-up from school hearing screening for school-aged children (3–21 years of age).
Elevated hearing loss prevalence in rural Alaska and in rural communities across the world is multifactorial, with nutritional, environmental, and genetic risk factors contributing (Amusa et al., 2005; Chonmaitree et al., 2016; Emmett et al., 2018; Korvel-Hanquist et al., 2017). Risk factors for ear infections in Alaska Native children have not yet been evaluated.
Environmental exposures, such as smoke from a wood-burning stove used to cook or heat the home, have been shown to increase the risk of ear infections in other rural regions, such as in West Africa (Amusa et al., 2005). Additionally, exposure to cigarette smoke is a well-established risk factor for acute otitis media in young children, regardless of urban or rural status (American Academy of Family Physicians et al., 2004). Household crowding has been shown to be a risk factor in the development of acute otitis media (Bowie et al., 2014), as well as other infectious diseases, such as meningococcal meningitis (Baker et al., 2000) and acute respiratory infections (Murray et al., 2012). Finally, infants and children who live in households without access to plumbed (running) water are more susceptible to upper respiratory infections and other bacterial infections (Hennessy & Bressler, 2016; Hennessy et al., 2008), including ear infections.
Addressing hearing health disparities in rural communities is important (Tsimpida et al., 2021). To better understand the health disparities in infection-related childhood hearing loss, we used data from the Hearing Norton Sound trials to quantify relationships between several environmental factors and infection-related hearing loss and middle ear disease in a cohort of rural Alaskan children.
MATERIALS AND METHODS
Study Overview
In the Hearing Norton Sound trials, all children within the Bering Strait School District and early education programs (preschool through 12th grade) were eligible. The main trial ran for two school years (2017–2019) and enrolled children kindergarten through 12th grade. An ancillary trial was added in the second year (2018–2019) and expanded enrollment to preschool children. On screening day, enrolled children received the school hearing screening (distortion product otoacoustic emissions), an mHealth pure-tone plus tympanometry screening, and a gold standard audiometric evaluation. Full details of the trial are available elsewhere (Emmett, Robler, Gallo, et al., 2019; Emmett, Robler, Wang, et al., 2019). Parents/guardians of enrolled children were asked to complete a sociodemographic survey (see Supplemental Digital Content) at the time of enrollment. The survey was created based on community member input and stakeholder feedback. Children in the main trial were screened in one or both years, and in the ancillary trial, enrolled preschool children were screened once. Data from the initial hearing screening for each enrolled child were included in the analysis.
Definition of Infection-Related Hearing Loss and Middle Ear Disease
For this analysis, hearing loss was defined by the objective distortion product otoacoustic emissions (DPOAE; Natus/Bio-Logic AuDX) screening conducted as part of the school hearing screening. Although a full audiometric assessment was conducted, the necessity for a behavioral response for assessment of hearing loss using pure-tone audiometry led to significant missing data in the younger study participants, and thus a pure-tone average (PTA) from the gold standard assessment was not used as the primary indicator. The DPOAE screening was automated and involved school staff placing a soft tip in the ear and recording a pass or refer for each ear. Emissions were measured at 2 kHz, 3 kHz, 4 kHz, and 5 kHz in each ear (primary levels of 65/55 dB SPL for f1/f2) using an overall pass/refer criterion (6 dB SPL minimum signal-to-noise ratio) of three of four frequencies meeting predetermined response conditions (Gorga et al., 1997).
The DPOAE screen was chosen as the primary indicator of hearing loss due to its availability across the entire age spectrum. However, a second measure was calculated using air conduction audiometry at 0.5 kHz, 1 kHz, 2 kHz, and 4 kHz with a validated tablet-based audiometer and supra-aural earphones (Shoebox, Clearwater Clinical, Canada). Hearing loss was defined as a pure-tone average (PTA) greater than 25 dB on either ear (Krug et al., 2016). Air conduction audiometry was more difficult to obtain in younger children (aged 3–6 years) due to the requirement for conditioned response and thus was considered a secondary measurement.
Middle ear disease was defined by otoscopy and tympanometry findings. Digital otoscopy obtained using a USB digital otoscope (Otocam, Otometrics, Denmark) was used to determine presence of pathology based on review by the audiologist. Tympanometry was performed using a Bluetooth tympanometer (Otoflex 100, Otometrics, Denmark). Presence of middle ear disease was determined by Type B (flat) or Type C tympanogram (<−200 daPa) or positive findings on otoscopy (FitzZaland & Zink, 1984). Positive findings on otoscopy included one of the following categories requiring referral as determined by the audiologist: retraction, effusion, acute otitis media, otorrhea, perforation, patent tube, plugged tube, and external otitis. These categories were selected as clinical indicators for middle ear disease, consistent with a diagnosis of infection-related pathology needing management. Infection-related hearing loss was defined as having referral status for otoacoustic emission screening combined with tympanometry or otoscopic conditions consistent with middle ear disease.
Definition of Potential Factors of Hearing Loss and Middle Ear Disease
We used the following questions, completed by the primary caregivers of enrolled children, to define the potential factors: how many people were currently living in the house (including the child), the number of smokers in the house (none, 1–2, or 3+ smokers), whether a wood-burning stove or fireplace was used to cook or help heat the house, and whether there was running water in the house.
In addition to environmental characteristics of the home, basic sociodemographic characteristics were collected, including child’s age and grade, biological sex, and race and ethnicity.
Statistical Analysis
The analysis sample included the first available screening and survey completion record for each enrolled participant. Since newly eligible children could enter in year 2, the first screening may have occurred in the first or second year of the study.
Descriptive statistics were used to describe the environmental and sociodemographic characteristics of the analytic study sample. Counts and percentages were calculated for categorical variables and medians and interquartile ranges were calculated for continuous variables.
Bivariate relationships between household environmental factors and ear/hearing-related outcomes were estimated using modified Poisson regression (Zou & Donner, 2013). Separate models using a log link and identity link were fitted to data to estimate prevalence ratios and prevalence differences, respectively. The study team hypothesized that there may be community-level clustering in the prevalence of hearing loss and middle ear disease; thus, generalized estimating equations with an independence working correlation structure were specified. Due to the small number of communities (k=15), a Kauermann-Carroll correction (Gallis et al., 2020; Li & Redden, 2015) was also applied to the estimation of the standard errors to limit Type I error. To increase precision and reduce bias in estimation of the magnitude of the association between the environmental factors and study outcomes, each regression was also adjusted for age (in years), sex, and Alaska Native race. Quasilikelihood under the Independence model Criterion (QIC) (Pan, 2001) was used to select the best functional form for continuous covariates (number in household and age) from linear, quadratic, and cubic forms.
To ascertain whether there was any apparent “cumulative” effect of the factors (i.e., does prevalence of ear/hearing related issues increase with the number of risk factors that a child has), a variable containing the count of risk factors present was constructed as its own independent correlate in a risk factor regression. The count of risk factors was a sum total of binary indicators for having no running water, using a wood-burning stove or fireplace, and having 3 or more smokers in the house (range 0 to 3). Three or more smokers was chosen based on the expectation that as the highest value for the categorical number of smokers, it presented the most substantial risk.
Heterogeneity of relationships between environmental factors and ear/hearing-related outcomes by age of the child were ascertained by specifying an interaction term indicating that a child was age 3–6 years versus age 7 years and older. These age groups were selected because middle ear disease is more prevalent in children aged 3–6 years old. Linear combinations were computed to produce separate measures of effect by age group, and interaction terms with 95% confidence intervals (CI) were presented to quantify the magnitude of the difference and its precision.
Proportion of missing values were calculated for the ear/hearing-related outcomes, as well as for the covariates included in the models. Simple bivariate associations were also computed using Fisher’s exact tests to ascertain whether missingness was informed by any environmental variables. The proportion of missingness in outcomes and covariates was considered together with bivariate associations to determine whether a sensitivity analysis was necessary to correct for potential selection bias induced by informative missingness for DPOAE-based infection-related hearing loss and middle ear disease. A priori knowledge about the difficulty in obtaining pure-tone audiometry through conditioned response in younger children, coupled with a belief that missingness in pure-tone audiometry could be informative of hearing loss (e.g., children with hearing loss may be more difficult to condition for testing), led to a decision to use multiple imputation as a sensitivity analysis for the PTA-based infection-related hearing loss measure. Details about the multiple imputation process can be found in Supplemental Digital Content.
Results for all regression analyses, including those using imputed data, were also summarized visually using forest plots. For ease of interpretability, estimates and 95% CIs were graphed on a linearized log scale with axes labeled with exponentiated values of the estimates (interpreted as prevalence ratios).
This analysis was exploratory in nature, and thus no adjustments for multiplicity were made (Althouse, 2016). The focus of analysis was on magnitudes of estimated effect size and 95% CIs rather than confirmatory hypothesis testing. All quantitative analysis was conducted in Stata/MP 16.1 (College Station, TX). The study was reviewed and approved by the appropriate Institutional Review Boards. This study adhered to the STROBE guidelines (von Elm et al., 2007).
RESULTS
Descriptive Statistics
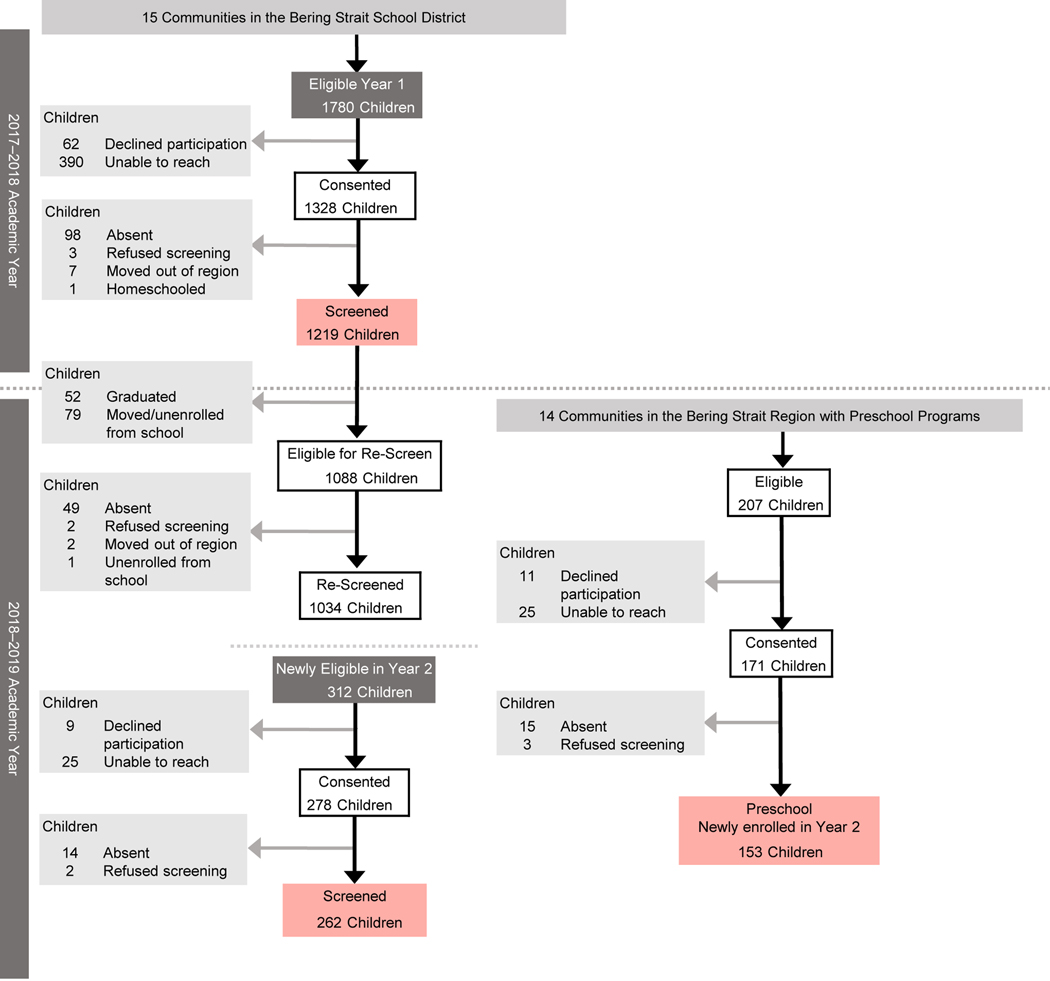
A total of 1634 children were enrolled in the study (n=1481 in the main trial, n=153 in the ancillary trial; Figure 1). The demographic characteristics of the enrolled sample are shown in Table 1. The median age was 9.0 years (Interquartile Range [IQR] 6.0–13.0); 52.5% were male, and 95.7% were Alaska Native or American Indian. Parents/guardians of 32.5% of study children reported lack of running water in the home, and 37.2% had a wood-burning stove for cooking or heating. The most common response regarding the number of smokers in the home was 1–2 smokers (52.8%). Two thirds of the sample had at least one risk factor (response of 3 or more smokers in the home, no access to running water in the home, and/or a wood-burning stove in the home). A little more than half (52.1%) the sample had a single risk factor. The sample prevalence of infection-related hearing loss was 11.1% (95% CI, 9.6–12.7) for DPOAE-based hearing loss (Table 2), and 6.2% (95% CI, 5.0–7.5) for PTA-based hearing loss (Supplemental Table 1). Sample prevalence of middle ear disease was 17.4% (95% CI, 15.5–19.3; Table 3).
Figure 1.
STROBE Diagram
Final analytic study sample highlighted in red
Table 1.
Baseline characteristics of analytic sample
| Variable | Total (N = 1634) |
|---|---|
| Child’s age (years) | |
| Median (Q1, Q3) | 9.0 (6.0, 13.0) |
| N (% Missing) | 1618 (1.0%) |
| How many people currently live in the household? | |
| Median (Q1, Q3) | 6.0 (5.0, 7.0) |
| N (% Missing) | 1601 (2.0%) |
| Child’s Age Range (years) | |
| 3–6 | 449 (27.8%) |
| 7–9 | 404 (25.0%) |
| 10–12 | 331 (20.5%) |
| 13–15 | 241 (14.9%) |
| 16+ | 193 (11.9%) |
| Missing | 16 |
| Child’s sex | |
| Male | 858 (52.5%) |
| Female | 776 (47.5%) |
| Missing | 0 |
| Race of child | |
| Non-Native | 71 (4.3%) |
| Alaska Native/American Indian | 1563 (95.7%) |
| Missing | 0 |
| Grade Level | |
| ECE | 153 (9.4%) |
| K-5 | 865 (52.9%) |
| 6–8 | 318 (19.5%) |
| 9–12 | 298 (18.2%) |
| Missing | 0 |
| Highest education level of any caregiver | |
| <12 grade | 93 (5.8%) |
| HS Diploma or GED | 1027 (64.4%) |
| Some College | 320 (20.1%) |
| College Degree | 155 (9.7%) |
| Missing | 39 |
| Do you have running water in your house? | |
| Water in house | 1081 (67.5%) |
| No water in house | 520 (32.5%) |
| Missing | 33 |
| Do you use a wood-burning stove or fireplace? | |
| No | 1001 (62.8%) |
| Yes | 593 (37.2%) |
| Missing | 40 |
| Number of smokers in the house? | |
| None | 541 (33.8%) |
| 1–2 Smokers | 844 (52.8%) |
| 3 or more smokers | 215 (13.4%) |
| Missing | 34 |
| Count of risk factors | |
| 0 | 522 (32.8%) |
| 1 | 830 (52.1%) |
| 2 | 228 (14.3%) |
| 3 | 13 (0.8%) |
| Missing | 41 |
ECE: Early Childhood Education; GED: General Education Development
Table 2.
Regression estimated prevalence ratios and prevalence differences for environmental associations with hearing loss1
| Variable | Sample prevalence | Number of observations used2 | Prevalence ratio3 (95% CI) | Prevalence difference4 (95% CI) |
|---|---|---|---|---|
| How many people currently live in the household? | 1533 | 1.06 (0.97,1.16) | 0.7 (−0.5,1.9) | |
| Do you have running (plumbed) water in your house? | 1533 | |||
| Water in house | 9.7 (8.0,11.6) | (ref) | (ref) | |
| No water in house | 13.5 (10.6,16.9) | 1.38 (0.80,2.39) | 3.7 (−2.7,10.1) | |
| Do you use a wood-burning stove or fireplace to cook or help heat the house? | 1526 | |||
| No | 11.6 (9.6,13.8) | (ref) | (ref) | |
| Yes | 9.9 (7.6,12.7) | 0.85 (0.55,1.32) | −1.7 (−6.5,3.0) | |
| Number of smokers in the house? | 1532 | |||
| None | 11.3 (8.7,14.3) | (ref) | (ref) | |
| 1–2 Smokers | 10.4 (8.4,12.7) | 0.92 (0.53,1.58) | −0.9 (−7.0,5.2) | |
| 3 or more smokers | 12.1 (8.0,17.4) | 1.07 (0.48,2.38) | 0.8 (−8.5,10.1) | |
| Count of risk factors5 | 1525 | 1.11 (0.86,1.44) | 1.2 (−1.6,3.9) | |
| Overall prevalence of Hearing Loss | 11.1 (9.6,12.7) |
Hearing loss defined as having referral status for otoacoustic emission screening combined with referral for tympanometry or otoscopic conditions consistent with middle ear disease.
Number of observations used for regression analysis
Estimated using modified Poisson with generalized estimating equations with clustering at the community level (n=15) and small sample correction, adjusted for age, sex, and Alaska Native race
Estimated using generalized estimating equations with normal distribution and identity linking, accounting for clustering at the community level (n=15) and small sample correction, adjusted for age, sex, and Alaska Native race
Count of binary indicators for lack of water, wood burning stove/fireplace, and 3+smokers in house (range 0–3)
Table 3.
Regression estimated prevalence ratios and prevalence differences for environmental associations with middle ear disease1
| Variable | Sample prevalence | Number of observations used2 | Prevalence ratio3 (95% CI) | Prevalence difference4 (95% CI) |
|---|---|---|---|---|
| How many people currently live in the household? | 1564 | 1.05 (0.99,1.10) | 0.8 (−0.1,1.8) | |
| Do you have running water in your house? | 1564 | |||
| Water in house | 14.5 (12.5,16.8) | (ref) | (ref) | |
| No water in house | 22.4 (18.8,26.2) | 1.53 (1.03,2.27) | 7.6 (−0.1,15.4) | |
| Do you use a wood-burning stove or fireplace? | 1557 | |||
| No | 18.0 (15.7,20.6) | (ref) | (ref) | |
| Yes | 15.5 (12.7,18.7) | 0.86 (0.64,1.17) | −2.4 (−7.3,2.5) | |
| Number of smokers in the house? | 1563 | |||
| None | 17.3 (14.2,20.8) | (ref) | (ref) | |
| 1–2 Smokers | 16.8 (14.4,19.6) | 0.96 (0.57,1.61) | −0.5 (−9.5,8.4) | |
| 3 or more smokers | 17.5 (12.7,23.4) | 1.02 (0.55,1.92) | 0.5 (−10.3,11.2) | |
| Count of risk factors5 | 1556 | 1.16 (0.90,1.49) | 2.5 (−2.0,7.0) | |
| Overall prevalence of middle ear disease | 17.4 (15.5,19.3) | 1599 |
Presence of middle ear disease was determined by Type B (flat) or Type C tympanogram (<−200 daPa) or positive findings on otoscopy.
Number of observations used for regression analysis
Estimated using modified Poisson with generalized estimating equations with clustering at the community level (n=15) and small sample correction, adjusted for age, sex, and Alaska Native race.
Estimated using generalized estimating equations with normal distribution and identity linking, accounting for clustering at the community level (n=15) and small sample correction, adjusted for age, sex, and Alaska Native race.
Count of binary indicators for lack of water, wood burning stove/fireplace, and 3+smokers in house (range 0–3)
Associations with Infection-Related Hearing Loss
The estimated associations between household environmental variables and prevalence of infection-related hearing loss, adjusting for age, sex, and Alaska Native race, are summarized in Table 2 (visualization in Supplemental Figure 1). Based on comparisons of QIC, a linear function was found to fit the data best for the two continuous independent variables (number in household and age), as well as the discrete count of risk factors. There was little evidence to suggest that infection-related hearing loss was associated with the number of people living in the household (PR=1.06 95% CI, 0.97–1.16), lack of indoor plumbing (PR=1.38 95% CI, 0.80–2.39), 3 or more smokers in the house (PR=1.07 95% CI, 0.48–2.38), or having a wood-burning stove (PR=0.85, 95% CI, 0.55–1.32). When considering a count of the number of risk factors, there was little evidence for any cumulative effect of environmental factors (PR=1.11, 95% CI, 0.86–1.44). The secondary indicator of PTA-based infection-related hearing loss largely agreed with DPOAE-based results for the total sample (Supplemental Tables 1 and 2). Prevalence difference estimates varied, however, given the lower overall sample prevalence for the PTA-based hearing loss compared with DPOAE-based.
Associations With Middle Ear Disease
Little association was found between middle ear disease and number of people living in the household, 3 or more smokers in the house, or presence of a wood-burning stove in the house (Table 3). However, there was evidence that a lack of running water in the house was associated with a 53% higher relative prevalence of middle ear disease (PR=1.53, 95% CI, 1.03–2.27) compared to children in homes with running water, an effect of higher magnitude and greater precision than that seen with infection-related hearing loss.
Heterogeneity
The results of the heterogeneity of effect models by age are presented in Supplemental Digital Content (Supplemental Tables 3 and 4 for infection-related hearing loss; Supplemental Tables 5 and 6 for middle ear disease). Visualizations can be found in Supplemental Figures 1 and 2, respectively. Overall, the sample prevalence of hearing loss and middle ear disease was higher for children aged 3–6 years (hearing loss=12.2%, 95% CI, 9.3–15.7; middle ear disease=22.7%, 95% CI, 18.9–26.9) than children aged 7 years and older (hearing loss=10.6%, 95% CI, 8.9–12.6; middle ear disease=15.3%, 95% CI, 13.3–17.5). An increase of one person in the household was associated with a 17% higher prevalence of hearing loss (PR=1.17, 95% CI, 1.05–1.31) and an 11% higher prevalence of middle ear disease (PR=1.11, 95% CI, 1.03–1.21) for children aged 3–6 years, with no association for older children. Use of a PTA- rather than a DPOAE-based measure changed the magnitude and direction of association for some risk factors (wood-burning stove, count of risk factors, and 3 or more smokers) for children aged 3–6 years but left associations largely similar between DPOAE- and PTA-based measures for children aged 7 years and older.
Lack of running water was also associated with higher prevalence of middle ear disease in children aged 3–6 years. Number of smokers in the house had estimated effects in opposing directions for children aged 3 to 6 years versus those aged 7 years and older, suggesting some evidence of heterogeneity by age.
Missing Data
A total of 65 (3.98%) children were missing data for the main hearing loss outcome (DPOAE-based), 96 (5.9%) for the PTA-based hearing loss outcome, and 34 (2.08%) were missing data for middle ear disease (otoscopy and tympanometry), with much of the missing data concentrated in children 3–6 years of age (Supplemental Table 7). Bivariate associations between the missingness of the outcome and the missingness of the covariates can be found in Supplemental Table 8. Covariate missingness (found in Supplemental Table 9) was non-differential by age group (age 3–6 years vs 7 years and older); therefore, outcome-only imputation was used to generate prevalence and regression estimated associations as a sensitivity analysis.
DISCUSSION
This is the first study examining environmental risk factors for hearing loss and middle ear disease among children in rural Alaskan environments. We examined the effect of subjective report on cigarette smoking, presence of a wood-burning stove, running water, and number of people living in the household on infection-mediated hearing loss and middle ear disease. Results from our study were mixed. We observed that lack of running water in the home was correlated with a higher prevalence of middle ear disease when compared to those who live in homes with running water, but we found little, if any, evidence of a correlation between hearing loss or middle ear disease and poor indoor air quality or number of people living in the home.
This study supports the finding that ear infections should be considered a water-washed illness, and our evidence is consistent with the literature. It is well-established that presence of running water in the home leads to a decreased rate of water-washed infections (Gessner, 2008; Hennessy et al., 2008; Thomas et al., 2016; Wenger et al., 2010). Personal sanitation practices involving water disrupt transmission of pathogens implicated in water-washed infection, such as upper respiratory, gastrointestinal, and skin infections. A 2008 analysis of infection rates in western Alaska demonstrated that communities where <10% of homes had running water had significantly higher rates of hospitalization for infant pneumonia and skin infections when compared to communities where >80% of homes had running water (Hennessy et al., 2008; Thomas et al., 2016).
Both presence of a wood-burning stove and smoking in the home contribute to poor air quality (Noonan et al., 2011), which can lead to more lower and upper respiratory infections, as well as meningococcal meningitis in children (Amusa et al., 2005; Hodgson et al., 2001; Morris et al., 1990). Within otitis media pathophysiology, poor air quality can disrupt ciliary function and beat-frequency which can lead to upper respiratory tract infections (Heinrich & Raghuyamshi, 2004). Despite this, data from our study do not provide evidence to suggest that wood-burning stove exposure is correlated with increased prevalence of middle ear disease.
While there is strong evidence in the literature to suggest there is a correlation between cigarette smoke exposure and ear infections (Ey et al., 1995; Klein, 2000), we did not find convincing evidence in our study. However, estimation of this effect is complicated by the challenge of ascertaining smoking habits due to the social stigmatization of smoking in the 21st century. Socially undesirable behaviors are often underreported, and tobacco-reporting behaviors are no exception (Fendrich et al., 2005). Many individuals recognize the risks associated with smoking; therefore, fear of stigma associated with answering positively to how many smokers live in the home may have prevented the true level of exposure from being recorded. It is also possible that this recognition of the risk of indoor smoke exposure has resulted in less individuals smoking inside the home.
We did not find a strong association between number of individuals living in the home and hearing loss or middle ear disease. This is inconsistent with previous literature that found associations with poor health outcomes and increased spread of infectious diseases in children (Baker et al., 2000; Bowie et al., 2014). One possible reason for our null finding is that we limited the analysis to the number of people in the household versus the ratio of people to rooms, due to inconsistent responses related to number of rooms in the household.
Our analysis assessing the role of age as an effect modifier provides evidence that the effects of some environmental exposures on infection-related hearing loss likely differ by age. It may be important to account for age when measuring relationships between these variables. However, we cannot rule out the possibility that this heterogeneity was due to missing data or measurement error in these exposures and is not a true modifying effect of age. Additionally, we observed heterogeneity in the associations between risk factors and infection-related hearing loss by type of tool used to define hearing loss (DPOAE vs. PTA) in the youngest children. For example, the magnitude of associations was smaller with opposite direction of effect for some factors for children 3–6 years of age when PTA was used as the indicator of hearing loss. It is possible this is related to the need to use multiply imputed data to estimate the effect due to missing data or the imprecision of the pure-tone average in the detection of infection-related hearing loss. Nonetheless, prevalence of hearing loss and middle ear disease tend to be higher in younger children, and more research is needed to identify underlying mechanisms that drive the differential effects of risk factors and screening tools.
There are several limitations to this study. This secondary analysis was not specifically powered to detect the effects of environmental factors on ear/hearing outcomes, nor was it powered to examine differential effects by age. Data were also cross-sectional, therefore causal relationships cannot be determined due to temporal and incidence-prevalence biases inherent in the data. Duration of middle ear disease and hearing loss, might be differential by exposure status for some independent variables, such as indoor plumbing. If, for example, duration of middle ear disease is longer for those children without indoor plumbing compared to those with running water, then the prevalence ratio would be larger than the corresponding risk ratio and the relative risk of this exposure would be overestimated. Like many environmental studies, our data are prone to measurement error, and it can be difficult to examine exposures using a brief self-report tool that relies on subjective interpretation and response (Fendrich et al., 2005). To mitigate this limitation, the demographic questionnaire for the study was created with local stakeholder involvement and maintained a low readability score. Yet despite these efforts, questions were often answered inconsistently, such as number of rooms in the home. Inconsistencies were addressed by calling and clarifying with the parent/guardian when possible. Future studies on environmental risk factors with increased measurement accuracy and detail, such as type, duration, and extent of environmental exposure, are warranted.
It is worth mentioning that the World Health Organization (WHO) criteria for hearing loss changed from a PTA of >25 dB to PTA of >20 dB (World Health Organization, 2021), after study administration was complete. Changing the definition could impact the results of the gold standard audiometric assessment, with normal hearing from the former definition now defined as hearing loss with the new definition. PTA was used as a secondary indicator, however, with DPOAE used as the primary indicator for this analysis.
A strength of this study is the inclusion of a large sample population representative of rural northwest Alaska. This is also the first study to our knowledge that identified lack of access to running water as a risk factor for middle ear disease.
This study supports the current evidence that lack of access to running water in the household is associated with a higher prevalence of infectious disease, such as acute otitis media, in rural Alaska. This is an important finding for public health in Alaska. Middle ear disease and infection-related hearing loss are preventable illnesses, and children who live in homes without running water, particularly younger children (ages 3–6 years), may benefit from earlier and more frequent hearing health evaluations. Preventive hearing health services could include hearing screenings at well child visits, school screenings, and regular visits with hearing specialists, such as audiologists. Policies and funding that support access to clean, running water in all parts of Alaska are imperative. Furthermore, hearing health services, particularly for children younger than 6 years, are also crucial. These efforts will not only promote healthy development of children at a young age but also vocational and academic success later in life (Tsimpida et al., 2021).
Supplementary Material
Financial disclosures:
This study was funded by PCORI AD-1602-34571 and NIDCD R21DC018399.
Footnotes
conflicts of interest:
There are no conflicts of interest, financial, or otherwise.
Clinicaltrials.gov registration numbers: NCT03309553, NCT03662256
REFERENCES
- Althouse AD (2016, May). Adjust for Multiple Comparisons? It’s Not That Simple. Ann Thorac Surg, 101(5), 1644–1645. 10.1016/j.athoracsur.2015.11.024 [DOI] [PubMed] [Google Scholar]
- American Academy of Family Physicians, American Academy of Otolaryngology Head Neck Surgery, & American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. (2004). Otitis Media With Effusion. Pediatrics, 113(5), 1412–1429. 10.1542/peds.113.5.1412 [DOI] [PubMed] [Google Scholar]
- Amusa YB, Ijadunola IK, & Onayade OO (2005, Jul-Sep). Epidemiology of otitis media in a local tropical African population. West Afr J Med, 24(3), 227–230. 10.4314/wajm.v24i3.28202 [DOI] [PubMed] [Google Scholar]
- Baker M, McNicholas A, Garrett N, Jones N, Stewart J, Koberstein V, & Lennon D. (2000, Oct). Household crowding a major risk factor for epidemic meningococcal disease in Auckland children. Pediatr Infect Dis J, 19(10), 983–990. 10.1097/00006454-200010000-00009 [DOI] [PubMed] [Google Scholar]
- Bess FH, Dodd-Murphy J, & Parker RA (1998, Oct). Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear, 19(5), 339–354. https://www.ncbi.nlm.nih.gov/pubmed/9796643 [DOI] [PubMed] [Google Scholar]
- Bowie C, Pearson AL, Campbell M, & Barnett R. (2014, Jun). Household crowding associated with childhood otitis media hospitalisations in New Zealand. Aust N Z J Public Health, 38(3), 211–215. 10.1111/1753-6405.12162 [DOI] [PubMed] [Google Scholar]
- Chonmaitree T, Trujillo R, Jennings K, Alvarez-Fernandez P, Patel JA, Loeffelholz MJ, Nokso-Koivisto J, Matalon R, Pyles RB, Miller AL, & McCormick DP (2016, Apr). Acute Otitis Media and Other Complications of Viral Respiratory Infection. Pediatrics, 137(4). 10.1542/peds.2015-3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Platt A, Turner EL, Gallo JJ, Labrique A, Inglis SM, Jenson CD, Parnell HE, Wang N-Y, Hicks KL, Egger JR, Halpin PF, Yong M, Ballreich J, & Robler SK (In Press). mHealth School Screening and Telemedicine Referral to Improve Access to Specialty Care in Rural Alaska: A Cluster-Randomized Trial. Lancet Global Health. [DOI] [PMC free article] [PubMed]
- Emmett SD, Robler SK, Gallo JJ, Wang NY, Labrique A, & Hofstetter P. (2019, Jan 22). Hearing Norton Sound: mixed methods protocol of a community randomised trial to address childhood hearing loss in rural Alaska. BMJ Open, 9(1), e023081. 10.1136/bmjopen-2018-023081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Robler SK, Wang NY, Labrique A, Gallo JJ, & Hofstetter P. (2019, Jan 15). Hearing Norton Sound: a community randomised trial protocol to address childhood hearing loss in rural Alaska. BMJ Open, 9(1), e023078. 10.1136/bmjopen-2018-023078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Schmitz J, Karna SL, Khatry SK, Wu L, LeClerq SC, Pillion J, & West KP Jr. (2018, Feb 1). Early childhood undernutrition increases risk of hearing loss in young adulthood in rural Nepal. Am J Clin Nutr, 107(2), 268–277. 10.1093/ajcn/nqx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey JL, Holberg CJ, Aldous MB, Wright AL, Martinez FD, & Taussig LM (1995, May). Passive smoke exposure and otitis media in the first year of life. Group Health Medical Associates. Pediatrics, 95(5), 670–677. https://www.ncbi.nlm.nih.gov/pubmed/7724301 [PubMed] [Google Scholar]
- Fendrich M, Mackesy-Amiti ME, Johnson TP, Hubbell A, & Wislar JS (2005, Jan). Tobacco-reporting validity in an epidemiological drug-use survey. Addict Behav, 30(1), 175–181. 10.1016/j.addbeh.2004.04.009 [DOI] [PubMed] [Google Scholar]
- FitzZaland RE, & Zink GD (1984). A comparative study of hearing screening procedures. Ear and Hearing, 5(4), 205–210. [DOI] [PubMed] [Google Scholar]
- Gallis JA, Li F, & Turner EL (2020). xtgeebcv: A command for bias-corrected sandwich variance estimation for GEE analyses of cluster randomized trials. The Stata Journal, 20(2), 363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner BD (2008, May). Lack of piped water and sewage services is associated with pediatric lower respiratory tract infection in Alaska. J Pediatr, 152(5), 666–670. 10.1016/j.jpeds.2007.10.049 [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, & Peters J. (1997, Dec). From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear, 18(6), 440–455. 10.1097/00003446-199712000-00003 [DOI] [PubMed] [Google Scholar]
- Heinrich J, & Raghuyamshi VS (2004, Jul). Air pollution and otitis media: a review of evidence from epidemiologic studies. Curr Allergy Asthma Rep, 4(4), 302–309. 10.1007/s11882-004-0075-4 [DOI] [PubMed] [Google Scholar]
- Hennessy TW, & Bressler JM (2016). Improving health in the Arctic region through safe and affordable access to household running water and sewer services: an Arctic Council initiative. Int J Circumpolar Health, 75, 31149. 10.3402/ijch.v75.31149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy TW, Ritter T, Holman RC, Bruden DL, Yorita KL, Bulkow L, Cheek JE, Singleton RJ, & Smith J. (2008, Nov). The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health, 98(11), 2072–2078. 10.2105/AJPH.2007.115618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A, Smith T, Gagneux S, Adjuik M, Pluschke G, Mensah NK, Binka F, & Genton B. (2001, Sep-Oct). Risk factors for meningococcal meningitis in northern Ghana. Trans R Soc Trop Med Hyg, 95(5), 477–480. 10.1016/s0035-9203(01)90007-0 [DOI] [PubMed] [Google Scholar]
- Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, Petrou S, Watkin P, Worsfold S, Yuen HM, & Stevenson J. (2006, May 18). Language ability after early detection of permanent childhood hearing impairment. N Engl J Med, 354(20), 2131–2141. 10.1056/NEJMoa054915 [DOI] [PubMed] [Google Scholar]
- Klein JO (2000, Dec 8). The burden of otitis media. Vaccine, 19 Suppl 1, S2–8. 10.1016/s0264-410x(00)00271-1 [DOI] [PubMed] [Google Scholar]
- Korvel-Hanquist A, Djurhuus BD, & Homoe P. (2017, Jul). The Effect of Breastfeeding on Childhood Otitis Media. Curr Allergy Asthma Rep, 17(7), 45. 10.1007/s11882-017-0712-3 [DOI] [PubMed] [Google Scholar]
- Krug E, Cieza A, Chadha S, Sminkey L, Martinez R, Stevens GA, White KR, Neumann K, Olusanya B, Stringer P, Kameswaran M, Vaughan G, Warick R, Bohnert A, Henderson L, Basanez I, LeGeoff M, Fougner V, & Bright T. (2016). Childhood Hearing Loss − Act Now, Here’s How! World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/204507/WHO_NMH_NVI_16.1_eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- Li P, & Redden DT (2015, Jan 30). Small sample performance of bias-corrected sandwich estimators for cluster-randomized trials with binary outcomes. Stat Med, 34(2), 281–296. 10.1002/sim.6344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JE (2004, May). Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg, 130(5), 524–530. 10.1001/archotol.130.5.524 [DOI] [PubMed] [Google Scholar]
- Morris K, Morgenlander M, Coulehan JL, Gahagen S, & Arena VC (1990, Jan). Wood-burning stoves and lower respiratory tract infection in American Indian children. Am J Dis Child, 144(1), 105–108. 10.1001/archpedi.1990.02150250117047 [DOI] [PubMed] [Google Scholar]
- Murray EL, Klein M, Brondi L, McGowan JE Jr., van Mels C, Brooks WA, Kleinbaum D, Goswami D, Ryan PB, & Bridges CB (2012, Jan). Rainfall, household crowding, and acute respiratory infections in the tropics. Epidemiol Infect, 140(1), 78–86. 10.1017/S0950268811000252 [DOI] [PubMed] [Google Scholar]
- Noonan CW, Ward TJ, Navidi W, Sheppard L, Bergauff M, Palmer C, & Committee HEIHR (2011, Dec). Assessing the impact of a wood stove replacement program on air quality and children’s health. Res Rep Health Eff Inst(162), 3–37; discussion 39–47. https://www.ncbi.nlm.nih.gov/pubmed/22852484 [PubMed]
- Pan W. (2001, Mar). Akaike’s information criterion in generalized estimating equations. Biometrics, 57(1), 120–125. 10.1111/j.0006-341x.2001.00120.x [DOI] [PubMed] [Google Scholar]
- Reed D, Struve S, & Maynard JE (1967, Sep). Otitis media and hearing deficiency among Eskimo children: a cohort study. Am J Public Health Nations Health, 57(9), 1657–1662. 10.2105/ajph.57.9.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R, Seeman S, Grinnell M, Bulkow L, Kokesh J, Emmett S, Holve S, McCollum J, & Hennessy T. (2018, Jan). Trends in Otitis Media and Myringotomy With Tube Placement Among American Indian and Alaska Native Children and the US General Population of Children After Introduction of the 13-valent Pneumococcal Conjugate Vaccine. Pediatr Infect Dis J, 37(1), e6–e12. 10.1097/inf.0000000000001704 [DOI] [PubMed] [Google Scholar]
- Thomas TK, Ritter T, Bruden D, Bruce M, Byrd K, Goldberger R, Dobson J, Hickel K, Smith J, & Hennessy T. (2016, Feb). Impact of providing in-home water service on the rates of infectious diseases: results from four communities in Western Alaska. J Water Health, 14(1), 132–141. 10.2166/wh.2015.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimpida D, Kontopantelis E, Ashcroft DM, & Panagioti M. (2021, Jan-Dec). Conceptual Model of Hearing Health Inequalities (HHI Model): A Critical Interpretive Synthesis. Trends Hear, 25, 23312165211002963. 10.1177/23312165211002963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, & Initiative S. (2007, Oct 16). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med, 147(8), 573–577. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, Parks D, Rudolph K, Hurlburt D, Ritter T, Klejka J, & Hennessy T. (2010, Mar). Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J, 29(3), 251–256. 10.1097/INF.0b013e3181bdbed5 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021). World Report on Hearing. WHO Press. Retrieved 1/15/2022 from https://www.who.int/publications/i/item/world-report-on-hearing [Google Scholar]
- Zou GY, & Donner A. (2013, Dec). Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res, 22(6), 661–670. 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.