Abstract
Background:
Ischemic ECG changes are subtle and transient in patients with suspected non-ST-segment elevation acute coronary syndrome (NSTE-ACS), yet the prehospital (PH)-ECG is not routinely used during subsequent evaluation at the emergency department (ED). We sought to compare the diagnostic performance of PH- and ED-ECG and evaluate the incremental gain of artificial intelligence (AI)-augmented ECG analysis.
Methods:
This prospective observational cohort study recruited patients with prehospital chest pain. We retrieved PH-ECG obtained by paramedics in the field and first ED-ECG obtained by nurses during in-hospital evaluation. Two independent and blinded reviewers interpreted ECG dyads in mixed order as per practice recommendations. Using 179 morphological ECG features, we trained, cross-validated, and tested a random forest classifier to augment NSTE-ACS diagnosis.
Results:
Our sample included 2,122 patients (age 59 (16); 53% females; 44% Black, 13.5% confirmed ACS). The rate of diagnostic ST elevation and ST depression were 5.9% and 16.2% on PH-ECG and 6.1% and 12.4% on ED-ECG, with ~40% of changes seen on PH-ECG persisting and ~60% resolving. Using PH-ECG alone gave poor baseline performance with AUROC, sensitivity, and negative predictive value of 0.69, 0.50, and 0.92. Using serial ECG changes enhanced this performance (0.80, 0.61, and 0.93). Interestingly, augmenting the PH-ECG alone with AI algorithms boosted its performance (0.83, 0.75, 0.95), yielding NRI of 29.5% against expert ECG interpretation.
Conclusion:
In this study, sixty percent of diagnostic ST changes resolved prior to hospital arrival, making the ED-ECG suboptimal for in-hospital evaluation of NSTE-ACS. Using serial ECG changes or incorporating AI-augmented analyses would allow correctly reclassifying one in four patients with suspected NSTE-ACS.
Keywords: prehospital ECG, acute coronary syndrome, dynamic ECG changes, machine learning, artificial intelligence
INTRODUCTION
The 12-lead electrocardiogram (ECG) remains the initial diagnostic test for evaluating the 7 million Americans presenting annually to an emergency department (ED) for a chief complain of non-traumatic chest pain.1 With the goal of expeditiously identifying acute coronary syndrome (ACS), guidelines now recommend the acquisition of a 12-lead ECG in the prehospital (PH) setting (i.e., during transport by emergency medical services) and transmitting it to the receiving hospital.2 The practice of acquiring and transmitting a PH-ECG in patients with the high pretest probability of disease has been shown to dramatically improve outcomes in patients with ST-elevation ACS (STE-ACS).3–5 However, the clinical impact of this practice is mostly confined to reducing first medical contact-to-intervention time via early catheterization laboratory (CATH) activation for those with STE-ACS.6,7 In the absence of ST-segment elevation, prehospital personnel frequently do not transmit the PH-ECG, and ED clinicians primarily rely on initial findings seen on ED-ECG in conjunction with guideline-recommended biomarker-driven evaluations. Thus, the PH-ECG is not routinely used as an informative data point in the comprehensive in-hospital evaluation of all patients with suspected ACS. While the practice of PH-ECG implementation and integration into systems originated over a decade ago, a lack of systematic inclusion of PH-ECG into the diagnostic workup beyond STE-ACS still remains8. This lack of inclusion is further aggravated by the variability of PH ECG acquisition practices and the poor integration of prehospital and in-hospital electronic health records, which often leaves the PH-ECG unavailable to ED clinicians during the initial patient evaluation.9,10
Nearly two thirds of ACS cases are considered non-ST elevation ACS (NSTE-ACS).11 Due to the heterogeneity of findings when compared to STE-ACS,12 the diagnostic workup of NSTE-ACS often involves a lengthy monitoring and assessment process, including frequent examinations, serial cardiac biomarker assays, and repeated ECG evaluation during their ED and hospital stay.2 This is further complicated by the fact that the STEMI vs not-a-STEMI diagnostic paradigm has its own limitations when deciding the optimal treatment strategy.13 Nearly 40% of STEMI-ECGs having no total coronary occlusions and 25% of those with not-a-STEMI-ECG having a total coronary occlusions requiring intervention.14 Integrating the PH-ECG into this paradigm of in-hospital evaluation of NSTE-ACS is not yet established due to the dearth of data regarding its potential incremental value in identifying NSTE-ACS. However, it is known that around 20% of diagnostic ST-segment elevations seen on PH-ECG resolve by the time the first ED-ECG is acquired, which has important implications in STE-ACS detection.15–17 The pathogenesis of NSTE-ACS suggests that coronary occlusions are more likely to be transient and/or unstable, especially when first-line anti-ischemic therapies (e.g., aspirin, nitroglycerin) are administered by PH personnel, hence it is plausible that the PH-ECG might play an even bigger role in NSTE-ACS detection18. Unfortunately, data on such a diagnostic potential are scarce.
Another challenge posed by ECG detection of NSTE-ACS is that 12-lead ECG changes are subtle and are highly multi-dimensional, hence requiring advanced algorithms to identify changes that cannot be detected otherwise.19 Subtle ECG changes are also dynamic over time and their evolution prior to hospital arrival might provide further diagnostic value for detecting NSTE-ACS. Harvesting subtle and significant ischemia ECG patterns other than ST amplitude has been shown to significantly improve the diagnosis of occlusion MI (OMI), especially when initial ECG findings do not meet STEMI criteria.20 Thus, the recent incorporation of explainable artificial intelligence (AI) algorithms for cardiac ischemia detection from 12-lead ECG data can provide a powerful tool to help identify cases of NSTE-ACS that can otherwise be missed by clinicians.21 The role of AI-augmented ECG diagnosis of NSTE-ACS is yet to be explored.
Herein, we report findings from a large PH-ECG database of patients calling 9-1-1 for chest pain in the United States. The specific aims of this analysis were to (1) examine whether incorporating the PH-ECG in serial ECG analysis (i.e., classical interpretation of ST amplitude) results in any increase in diagnostic gain of NSTE-ACS; and (2) given the PH-ECG is more likely to capture transient subtle ischemic patterns, does the use of AI-ECG (i.e., mining for important ischemic patterns other than ST amplitude) improve the diagnostic gain of NSTE-ACS.
METHODS
Design, Setting and Sample
Subjects for this sub-analysis were obtained from the EMPIRE study (ECG Methods for the Prompt Identification of Coronary Events)19. Study methods are described in detail elsewhere and are published on ClinicalTrials.gov (NCT04237688). Briefly, the study was a prospective, observational study of non-traumatic chest pain patients that called 9-1-1 for a chief complaint of chest pain or other atypical, suspicious symptoms (e.g., shortness of breath, epigastric pain, and syncope) requiring ECG evaluation. Between 2013 to 2018, we prospectively enrolled consecutive patients who called 9-1-1 in the City of Pittsburgh and were transported by Pittsburgh Emergency Medical Services to three separate University of Pittsburgh Medical Center (UPMC) hospitals: UPMC Shadyside, UPMC Presbyterian, and UPMC Mercy. As part of routine care for patients with symptoms suspicious for ACS, all enrolled patients had their 12-lead ECG transmitted to the UPMC Medical Command Center for further evaluation by a physician. For this sub analysis, we included patients who had both a PH ECG and an ED ECG. We excluded patients with PH CATH laboratory activation for suspected STE-ACS identified in the field by paramedics, ventricular fibrillation/tachycardia, or with secondary repolarization changes confounding ischemia evaluation (e.g., ventricular pacing, bundle branch block, or left ventricular hypertrophy with strain pattern). The patients were recruited for the study under a waiver of informed consent, and the University of Pittsburgh Institutional Review Board approved this study.
Clinical Data and Outcome Adjudication
Independent reviewers manually abstracted the key in-hospital data elements from the electronic health records as recommended by the American College of Cardiology for measuring the management and outcomes of patients with ACS, including22: demographics, past medical history, home medications, clinical presentation and course of hospitalization, laboratory tests, imaging studies, cardiac catheterization, treatments, and in-hospital complications.
The primary outcome of the study was the diagnosis of ACS any time during the indexed admission, which included unstable angina, NSTE-ACS, and STE-ACS. Two independent physician reviewers adjudicated the primary outcome of ACS as per the following Universal Definition of Myocardial Infarction criteria:12 (1) rise and fall in cardiac troponin I (≥99th percentile according to location criteria); (2) diagnostic ST elevation or ST depression in two contiguous ECG leads;12 (3) echocardiographic evidence of new loss of viable myocardium or new regional wall motion abnormalities; or (4) coronary angiographic or nuclear imaging demonstrating greater than 70% stenosis of a major coronary artery with or without treatment.23 Patients were considered to have a confirmed ACS diagnosis if they displayed any or all of these criteria.
ECG Signal Processing
All ECGs were obtained as part of routine medical care. The PH ECG were obtained by paramedics in the field using HeartStart MRX monitors (Philips Healthcare, Cambridge, MA). We obtained the digital raw XML files transmitted to our medical command center and stored them for offline analysis. The ED ECG was obtained by ED staff using MAC VUE360 Resting ECG devices (GE Healthcare, Milwaukee, WI). We obtained the digital vectorized PDF files stored in the in-hospital electronic health record system and stored them for offline analysis. For the purpose of serial ECG analyses, we selected the patients’ first PH ECG and first ED ECG as the corresponding study ECGs.
PH ECGs were processed by manufacturer-specific software (Advanced Algorithm Research Center, Philips Healthcare, Andover, MA), whereas ED ECGs were processed by CALECG software (AMPS LLC, New York, NY). Noise, artifact, and ectopic beats were removed, and time-synchronized median beats were calculated per ECG lead. Next, a total of 179 ECG features were calculated from each ECG, including: (1) multi-lead global ECG intervals (k=8); (2) frontal-plane axes (k=3); (3) lead-specific amplitude, duration, and/or area of P wave, Q wave, R / R` wave, S / S` wave, QRS complex, ST80 segment, and T wave (k=144); and (4) lead-specific PR interval and QT interval (k=24).
Expert ECG Interpretation
Each ECG was reviewed by two independent physicians who were blinded to the study outcome. The aim of expert interpretation was to capture the performance of physicians in reviewing ECG and adjudicating for cardiac ischemia when patients are presenting with symptoms suggestive of ACS. The performance of these independent reviewers was given the title “reference standard”. The independent physicians adjudicated the presence of diagnostic territorial ST-segment elevation (STE) or depression (STD) as per the Universal Definition of Myocardial Infarction recommendation as two contiguous leads with:12 (1) STE ≥ 2 mm in V2–V3 in men ≥ 40 years, ≥ 2.5 mm in men < 40 years, or ≥ 1.5 mm in women; or STE ≥ 1 mm in other leads; or (2) new horizontal or downsloping STD ≥ 0.5 mm in any lead with or without T-wave inversion > 1 mm in leads with prominent R wave or R/S ratio > 1. Any disagreements between the reviewers were resolved by review by a board-certified cardiologist. ST changes on the PH-ECG or ED-ECG were documented per patient in the anterior, lateral, or inferior myocardial walls as either no changes (0), ST depression (1), or ST-elevation (2). This coding scheme yielded an ordinal scale variable with range 0 to 6, which was used in a logistic regression model to generate predicted probability of ACS and for AUC analysis. Next, temporal changes between PH-ECG and ED-ECG were also documented in the anterior, lateral, and inferior myocardial walls as either no changes (0); resolution of changes seen on PH-ECG (1); evolution of new changes not seen on PH-ECG (2); and persistence of changes at the ED as seen on the PH-ECG (3). This coding scheme yielded an ordinal scale variable with range 0 to 9, which was also used in a logistic regression model to generate predicted probability of ACS and for AUC analysis.
AI-Augmented ECG Analysis
We divided our dataset of PH-ECG and ED-ECG dyads into 80% training and 20% testing subsets. The training and testing subsets were each preprocessed using imputation of missing values with the mean or mode of the corresponding feature for continuous or categorical variables, respectively, and normalization with the L2 norm. We ran a 10-fold cross-validation to obtain results for the training subset, then used the remaining unseen data set for testing.
Next, we used a Random Forest classifier to build our AI models for predicting confirmed ACS cases. Beside its robustness to outliers, data skewness, missingness, and unbalanced outcome distribution,24 we have previously shown that the random forest classifier is well suited to handle the multidimensionality observed in 12-lead ECG data. The Random Forest (RF) classifier was implemented with 1000 trees with fixed criterion to measure the quality of a split using ‘entropy’ (for information gain). The ‘balanced subsample’ mode was selected where weights were computed for the output values automatically and inversely proportional to class frequencies in the bootstrap sample for every tree. These parameters were tuned during the 10-fold cross-validation training stage. An unseen hold-out set of patients was then used to assess the generalizability of the model during the testing stage. For model explainability, we used algorithm agonist approach based on feature importance. The traditional feature importance based on mean decrease in impurity shows bias towards high cardinality features, even if they are random and unrelated to the outcome, so it tends to overfit using these features. Therefore, we used the permutation importance method and plotted the importance ranking using the test set, which would reflect the usefulness of the features in making generalizable predictions instead of reflecting an overfitting model.
Using the modeling approach described above, we built four random forest classifiers: (1) AI-PH-ECG, (2) AI-ED-ECG, (3) AI-Serial-ECG, and (4) AI-ECG-Clinical. We used the 179 features from the PH ECG to build the first classifier and used the 179 features from the ED ECG to build the second classifier. For the third classifier, we used the 179 features from PH ECG (baseline) and the delta change in each value between the PH ECG and the ED ECG dyads. The final classifier included the 179 features from PH ECG, the 179 corresponding delta changes in features between the two ECG dyad, and the clinical data available at triage. The clinical data elements from the latter included age, sex, race, comorbidities (hypertension, diabetes, smoking, dyslipidemia, heart failure, known CAD, old MI, COPD, prior catheterization), and prehospital interventions (morphine, oxygen, nitroglycerin, aspirin).
Statistical Analysis
Variables were reported as mean (standard deviation) or count (%). Groups were compared using chi-square for categorical variables or independent samples t-test for continuous variables. Trend evolution between PH- and ED-ECG were compared between groups using repeated-measures ANOVA. The diagnostic performance of STE and STD seen on PH-ECG, ED-ECG, or their dynamic changes between the two timepoints were evaluated for predicting confirmed ACS using multivariate logistic regression. Predicted probabilities were used to evaluated classification performance using the area under the receiver operating characteristic (AUROC) curve. The presence of at least one wall with diagnostic STE or STD were used to build the confusion matrix and calculate sensitivity, specificity, positive predictive value, and negative predictive value. We used McNemar’s test to compare the reclassification performance between different classifiers.
For AI algorithms, the training results of RF classifier on 10-fold cross validation were reported as mean (standard error). We generated binary predictions using Youden index on the ROC curves. The mean of thresholds resulting from training was used to produce the confusion matrices for the testing sets. We then computed the performance metrics described above along with F1 score and net reclassification improvement (NRI) index as compared to a reference standard. In addition, the comparison between the performance of the models was rigorously tested using the Wilcoxon signed-rank test on the two groups of AUROC values formed, each, by the results of the ten folds. Each group corresponds to the model having one of these sets of input variables: PH-ECG and ED-ECG variables. To compare the two paired groups of values, this method was chosen because it is the non-parametric alternative of the paired t-test since we are dealing with data that does not necessarily satisfy the assumptions of the t-test. For testing, we used bootstrapping on the test set to generate a group of ten AUROC values for each model and compared them using Wilcoxon signed-rank test as well. Statistical analyses were completed using SPSS v. 24.0 and AI models were implemented using Python v. 3.7. The level of significance was set at α = 0.05 for two-tailed hypothesis testing.
RESULTS
The study enrolled 2,400 patients in total. For the purposes of this study, we excluded 89 patients with PH CATH laboratory activation for STE-ACS identified in the field by paramedics, 22 patients with ventricular fibrillation/tachycardia, and 154 patients with secondary repolarization changes confounding ischemia evaluation, and 13 patients with either missing in-hospital or prehospital ECG. The final population for this study included 2,122 patients with suspected NSTE-ACS (age 59 (16); 53% females; 44% Black). Approximately 69% of our population had hypertension, 38% had a known history of coronary artery disease (CAD), and 28% had diabetes mellitus. Demographic and clinical characteristics of the population are summarized in Table 1. There were 288 (13.6%) cases of confirmed ACS during in-hospital evaluation. Among those with confirmed in-hospital ACS, 37% had subsequent evolution of STE-ACS that was not apparent during initial evaluation. The inter-rater agreement between the reviewers ranged from Kappa 0.86 to 0.91.
Table 1:
Demographics and Clinical Characteristics
| Clinical Characteristics | All Patients (n=2122) |
|---|---|
| Demographic | |
| Age (years) | 58 (16) |
| Female Sex | 1001 (47%) |
| Race | |
| White | 1216 (58%) |
| Black | 859 (40%) |
| Other | 47 (2%) |
| Ethnicity | |
| Hispanic or Latino | 15 (0.7%) |
| Not Hispanic or Latino | 1973 (93%) |
| Unspecified | 134 (6.3%) |
| Past Medical History | |
| Hypertension | 1469 (69%) |
| Ever Smoked | 1293 (68%) |
| Hyperlipidemia | 833 (39%) |
| Known CAD | 715 (38%) |
| Previous PCI or CABG | 668 (31%) |
| Diabetes Mellitus | 593 (28%) |
| Heart Failure | 337 (16%) |
| Diagnostics | |
| Positive initial troponin | 166 (8%) |
| Positive serial troponin | 253 (12%) |
| Stress test with SPECT scan | 278 (13.1%) |
| Focal evidence of ischemia | 29 (1.4%) |
| Outcomes & Course of Hospitalization | |
| Confirmed ACS | 288 (13.6%) |
| Final Discharge Diagnosis of NSTE-ACS | 179 (8%) |
| Subsequent In-Hospital Evolution of STE-ACS | 109 (5%) |
| Treatment with PCI or CABG | 197 (9%) |
| 30-Day Complication or Adverse Events | 256 (12%) |
Most patients (89.4%) were in normal sinus rhythm and 10.6% were in atrial fibrillation. Figure 1 shows the initial ischemic findings on PH- and ED-ECGs for the entire cohort (n = 2,122 patients). On PH-ECG, 125 patients (5.9%) had diagnostic STE and 343 (16.2%) had diagnostic STD, with rate of confirmed ACS in these subgroups of 62% and 32%, respectively (Figure 1A). Similarly, there were 129 (6.1%) and 263 (12.4%) diagnostic STE and STD on ED-ECG, with rate of confirmed ACS of 64% and 39% respectively (Figure 1B). These ischemic findings on the PH- and ED-ECG had poor classification performance of ACS events with AUROC of 0.692 (0.65–0.73) and 0.693 (0.66–0.73), sensitivity of 0.50 (0.44–0.56) and 0.479 (0.42–0.54), and specificity of 0.845 (0.83–0.86) and 0.864 (0.84–0.88), respectively. More interestingly, considering both ECGs together shows that only 49% and 37% of diagnostic STE and STD seen on PH-ECG persisted until ED-ECG, with 51% and 63% of PH diagnostic changes resolving prior to ED arrival. Figure 1C shows the rate of confirmed ACS in those who had resolving, new, or persistent diagnostic STE or STD. An approach based on the presence of dynamic ECG changes between PH and ED timepoints achieved a very good classification performance of confirmed ACS (AUROC 0.798 [0.77–0.83]).
Figure 1: The relationship between ischemic ECG findings and acute coronary syndrome.
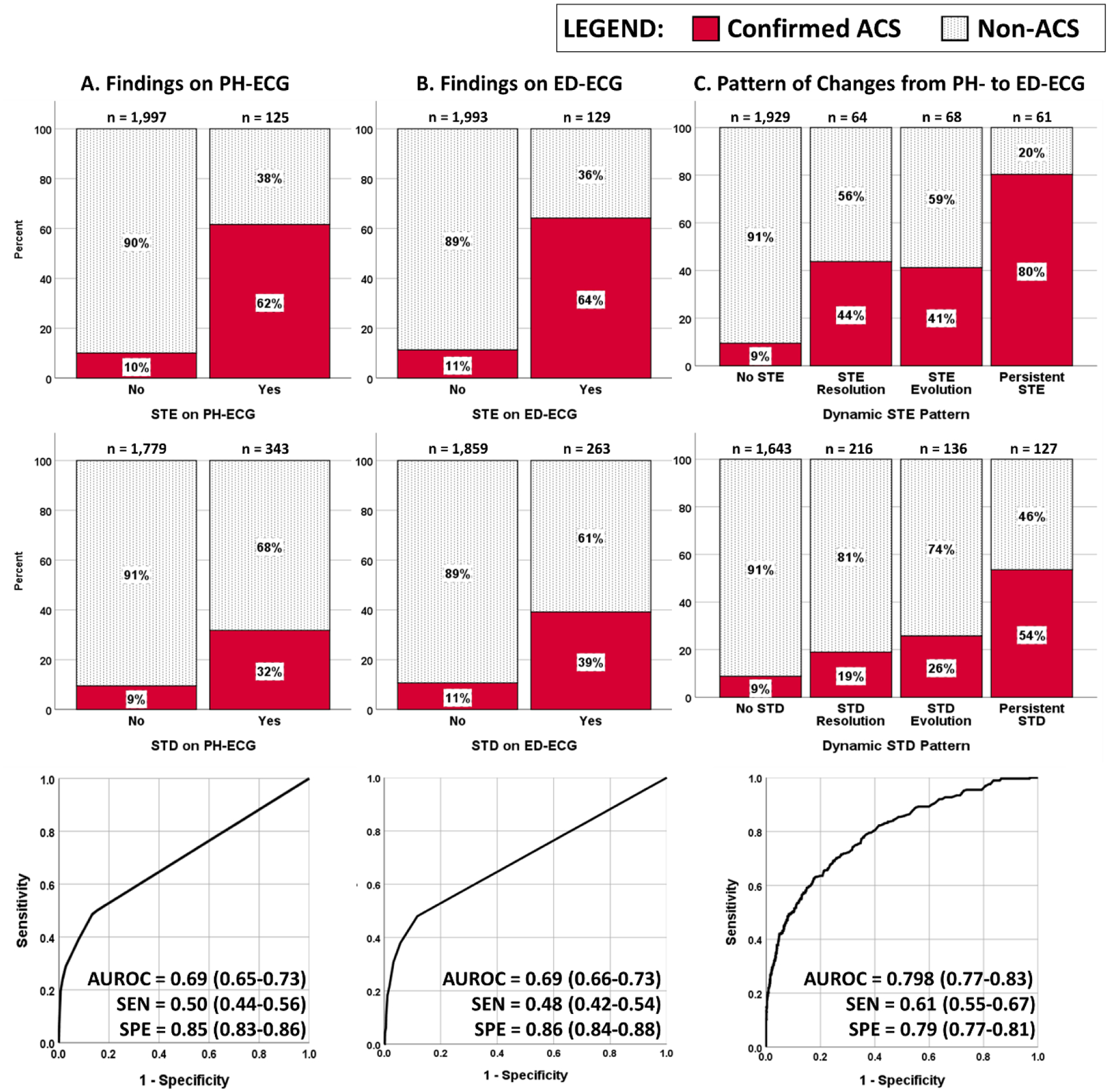
This figure shows how diagnostic ST changes correlated with acute coronary syndrome (ACS) on the prehospital (PH)-ECG (A), emergency department (ED)-ECG (B), and serial dynamic changes between both ECGs (C). ECG changes included diagnostic ST elevation or ST depression interpreted retrospectively by independent reviewers as per the 4th universal definition of MI guidelines.12 We excluded from this analysis patients with prehospital CATH laboratory activation for suspected STE-ACS identified in the field by paramedics. Area under ROC (AUROC) curves are based on a logistic regression classifier using the ST changes seen on each ECGs or their dynamic patterns. SEN: sensitivity, SPE: specificity. AUROC, SEN, and SPE are reported as value (95% CI).
Next, we explored the value of AI-augmenting analysis of PH- and ED-ECG. Figure 2 shows the results of algorithm performance on training subset (n=1699, 14% confirmed ACS) and testing subset (n=423, 13% confirmed ACS) as compared to the baseline classification performance of diagnostic findings on ED-ECG. During algorithm testing, both AI-PH-ECG and AI-ED-ECG algorithms had significantly higher performance compared to the reference standard (AUROC 0.83 [0.77–0.90] and 0.79 [0.73–0.86] vs. 0.62 [0.53–0.70], respectively). The AI-PH-ECG algorithm outperformed the reference standard with sensitivity, specificity, positive and negative predictive values of 0.75 (0.62–0.86), 0.76 (0.71–0.80), 0.32 (0.29–0.39), and 0.95 (0.92–0.97) versus 0.36 (0.23–0.50), 0.86 (0.82–0.89), 0.28 (0.21–0.39), and 0.90 (0.87–0.90) for expert ECG interpretation, respectively. This significant gain in performance translates into NRI index of 29.5% (p<0.001). We then investigated the incremental gain in classification performance of AI-PH-ECG when supplemented by serial temporal ECG changes (model 3: AI-serial-ECG) and the addition of clinical data elements available during triage (model 4: AI-ECG-clinical). Figure 3 shows there was no significant gain in AUROC for either of the two latter models during both training and testing, with peak classification performance achieved by PH-ECG alone, plateauing at AUROC of 0.82 (0.76–0.88).
Figure 2: Classification performance of NSTE-ACS using AI-augmented ECG analysis.
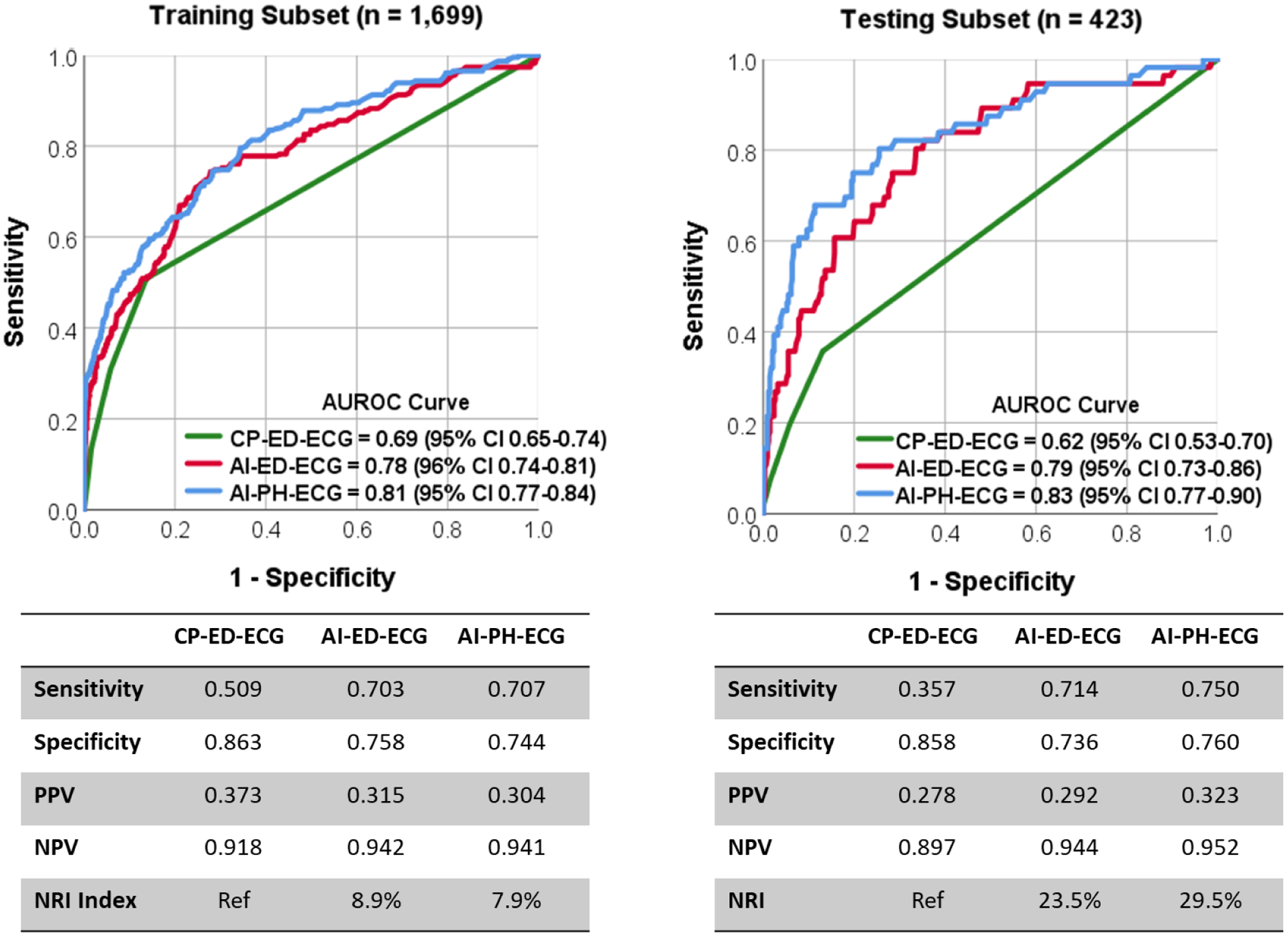
This figure shows random forest classification performance using features from prehospital ECG (AI-PH-ECG) or the emergency department (AI-ED-ECG) as compared to clinical practice based on ED evaluation (CP-ED-ECG) on both training subset (left) and testing subset (right). The tables show the diagnostic accuracy measures and the net reclassification performance (NRI) index as compared to CP-ED-ECG as a reference standard (Ref).
Figure 3: Classification performance of AI-augmented ECG analysis supplemented by serial ECG and clinical data.
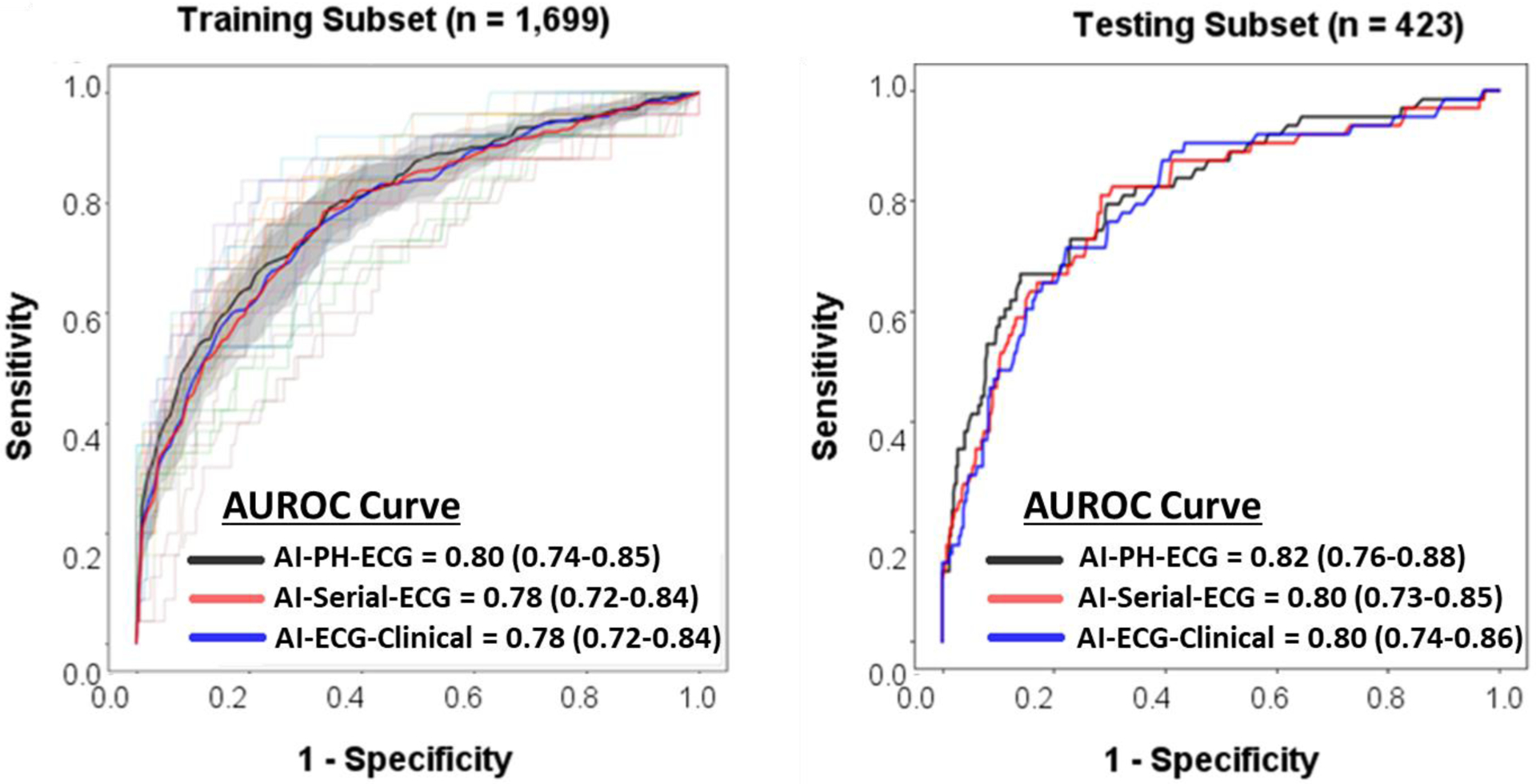
This figure shows the baseline classification performance of random forest model using features from prehospital ECG (AI-PH-ECG), both prehospital and ED ECGs (AI-serial-ECG), and serial ECG plus clinical data typically available during triage (AI-ECG-Clinical) on both training subset (left) and testing subset (right). This figure demonstrates that AI augmented ECG analysis reaches its classification performance plateau with PH-ECG alone, with no additional gain in performance when adding serial ECG or any other clinical data elements. In the training set, the lighter lines correspond to the results obtained for the individual folds during the 10-fold cross-validation, whereas the thicker lines correspond to the mean results for each model. The shaded areas highlight the space englobing all curves within 2 standard error around the mean curves.
Finally, we used RF permutation importance ranking to add explainability to the observed gain in NRI index using AI-PH-ECG model. Among the 179 features used in that model, the most important classical features were ST amplitude in leads aVL, I, III, V2, aVR, V4, V3, and V6; T amplitude in leads aVL, V2, III, V3 and I; and T area in leads aVL, III, V2, and I. The most important novel features were global Tpeak−Tend interval (rank #3), mean QRS−T angle (rank #8); spatial T axis (rank #15), and relative T to R amplitude ratio on RMS signal (Root Mean Square) (rank #12). Figure 4A shows the mean group differences in global Tpeak−Tend interval and QRS−T angle on PH- and ED-ECGs. Patients with confirmed ACS had significantly longer global Tpeak−Tend interval and wider QRS−T angle compared to their counterparts, with more pronounced dispersion on PH-ECG. To understand the multidimensional complexity of the 12 lead ECG, Figure 4B shows the 3-D scatterplot of the three most important features in RF classification delineating a non-linear hyperplane of ACS cases characterized by prolonged global Tpeak−Tend interval, STE in lead III, and distorted ST-segment in lead aVL (STE or STD).
Figure 4: Correlation between the most important ECG features in the diagnosis of acute coronary syndrome.
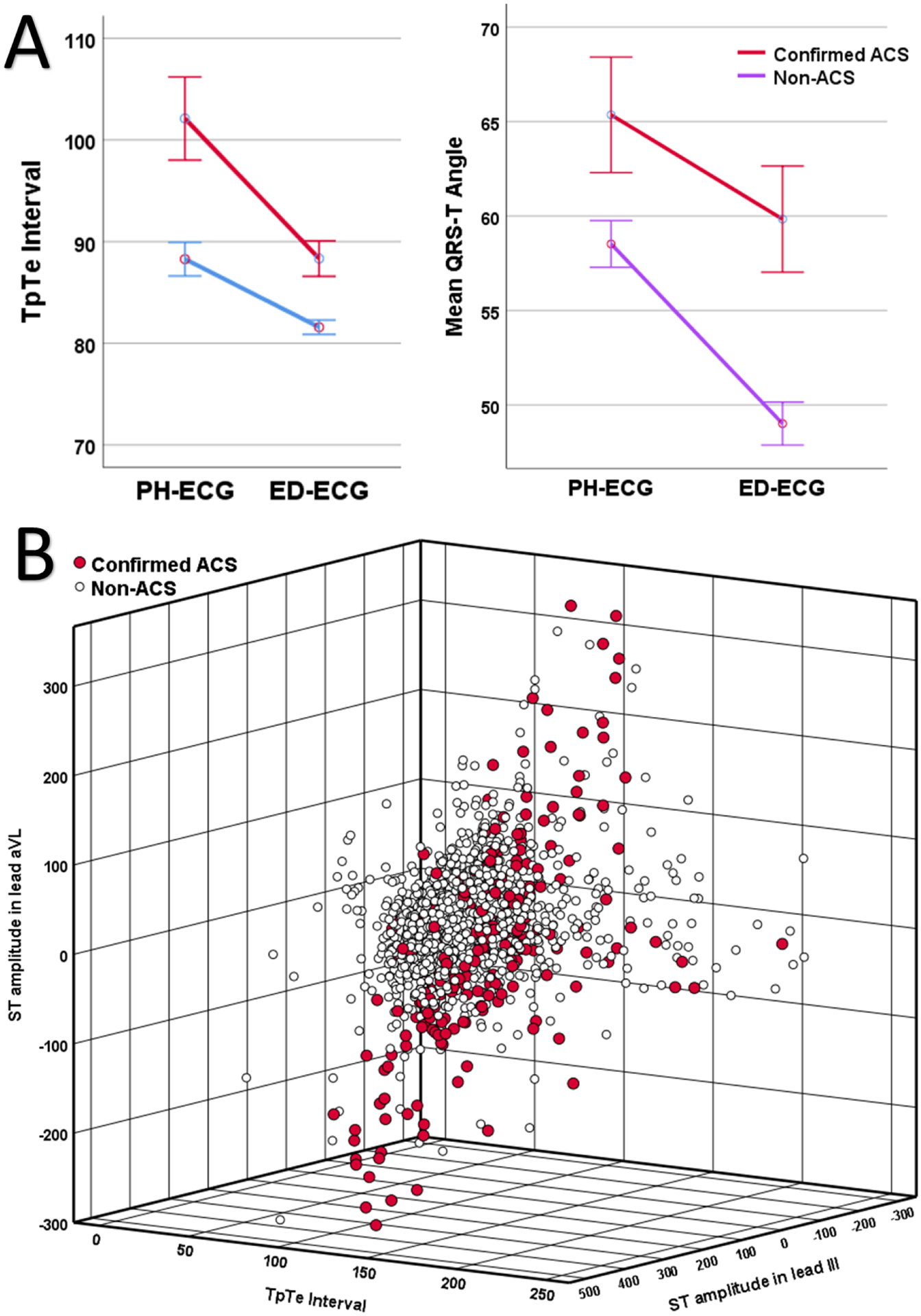
Plot A shows mean group differences in Tpeak−Tend interval (left) and QRS−T angle (right) on prehospital (PH)-ECG and emergency department (ED)-ECG in those with or without acute coronary syndrome (ACS). Plot B shows the 3-D scatterplot of the three most important features in the random forest delineating a non-linear hyperplane of ACS cases characterized by prolonged global Tpeak−Tend interval, ST elevation in lead III, and distorted ST-segment (elevation or depression) in lead aVL.
LIMITATIONS
Our study has a few limitations that should be considered when interpreting our findings. First, patients with secondary repolarization abnormalities (i.e., pacing, bundle branch block, left ventricular hypertrophy, or ventricular rhythm) were excluded from the study. These patients have a different course and are usually sicker, therefore our results are not generalizable to this population. Second, the findings of our study are based on a single healthcare system, therefore, it is difficult to generalize our results to different system. Testing our AI models on an independent system in necessary before establishing clinical utility. Finally, the PH-ECG and the ED-ECG were processed by different manufacturer-specific software. A classical review paper previously looked at the systematic differences among automated ECG interval measurements by seven widely used computer-based ECG interpretation algorithms, including AMPS and Philips (the two we used). The paper indicated the differences in measurement are clinically negligible (e.g., difference in QRS duration between AMPS and Philips is 4 milliseconds on average).25 Thus, the differences captured in the delta values are likely physiological rather than technical in nature.
DISCUSSION
In this study, we compared the diagnostic value of PH- and ED-ECG for classifying patients with suspected ACS and evaluated the diagnostic gain of using AI-augmented analysis of 12-lead ECG data. We found that more than one half of diagnostic STE and STD resolve prior to ED arrival. We demonstrated that using these temporal dynamic changes between PH-and ED-ECG yields very good classification performance (AUROC ~ 0.80), which far exceeds the diagnostic value of the ECG at each timepoint separately. However, using AI-augmented analysis of the 12-lead ECG yields a NRI index of ~24%−30% compared to current expert overread of ECG data during ED evaluation, a gain that can be achieved by using only the PH-ECG without the need for serial ECG changes or other clinical data elements. This gain in performance is based on subtle multi-dimensional changes in STT waveform and other novel markers of ventricular repolarization dispersion. These findings support the notion that the PH-ECG should be systematically considered as an important predictive data point in the diagnostic workup of suspected NSTE-ACS, especially when augmented by powerful AI tools.
This study demonstrates that exclusively relying on the ED-ECG during in-hospital evaluation comes with poor classification performance (AUROC < 0.70), significantly limiting providers’ ability to rule in or out ACS. It is known that ischemic ECG changes are often transient in nature. Acquiring an ECG during acute symptoms when patients are undergoing ischemic distress is more likely to elicit important prognostic information. We show that more than half the ischemic changes seen on PH-ECG resolve prior to ED arrival. The reason for this possibly reflects the timing the PH-ECG is acquired in the continuum of care, including the acquisition prior to initiation of any anti-ischemic therapies. Such interventions could transiently improve the underlying cardiac ischemia and blunt ECG findings by the time the ECG is acquired in the ED.26,27 It is also well established that ACS has an unstable course, meaning ischemic ECG findings could spontaneously resolve by the time patients are evaluated in the ED.16
Clinical practice guidelines emphasize the importance of PH-ECG use for clinical decision-making and advocates for its systematic incorporation in systems of care as a class I recommendation.4,28 Moreover, it is well established that detecting transient ischemic ECG changes in ACS, including those detected in the prehospital setting, can help identify patients with higher risk for adverse events.29,30 Yet, in clinical practice, the primary emphasis remains focused on identifying STE requiring catheterization lab activation, and few studies have previously analyzed the diagnostic value of PH-ECG in suspected NSTE-ACS. Some studies report that subtle changes on PH-ECG are associated with adverse outcomes in this population, demonstrating a positive impact on processes of care, including early disposition, timely interventions, and improved survival rate.31–33 Our study supports the notion that significant information gets lost by excluding the PH-ECG during in-hospital decision-making when evaluating NSTE-ACS. This has important clinical implications, as often no permanent record is kept of PH-ECGs in the in-hospital electronic health records, hence losing a valuable diagnostic data point in the lengthy process of patient evaluation. We demonstrate that using temporal dynamic patterns of STE and STD between PH- and ED-ECG yields very good classification performance compared to using either one separately, which aligns well with current literature.34–36
It is well established that ECG findings in NSTE-ACS are not always grossly evident and often require novel methods for identification.19 There are numerous reasons for these shortcomings; the infarct might be relatively small, the location of the infarct might be in a location only weakly sensed by the lead fields of the standard 12-lead ECG, or the infarct is slowly developing.37 Intriguingly, myocardial ischemia affects the configuration of both the QRS complex and ST-T waveform. Thus, an evolving infarct would translate into progressive regional changes in ST amplitude and slope, T wave amplitude and morphology, and QRS duration and configuration. These subtle and interrelated changes in ECG features as measured from the different 12 leads of the ECG open an important opportunity for AI-augmented analysis of ECG data to learn multi-dimensional patterns in these features that would otherwise be missed by humans. This explains the superior performance of AI-augmented analysis of ECG when compared to expert ECG interpretation; allowing clinicians to correctly reclassifying at least one in four patients with suspected ACS in our study. Interestingly, such AI-based pattern recognition of subtle ECG changes achieved the maximum gain in diagnostic performance using only the PH-ECG, without serial ECG changes or other clinical data elements. This again emphasizes the value of systematically incorporating the PH-ECG into systems of care while evaluating patients with suspected NSTE-ACS. This still does not undermine the value of serial ECG in NSTE-ACS given the complexity of temporality and the specific characteristics of these subtle changes.35,36
It is worth noting that many novel ECG features can globally quantify the subtle changes in QRS and ST-T waveform morphologies, greatly improving the sensitivity of the ECG for ischemia as well as drastically reducing the time required to diagnose NSTE-ACS.37 For instance, Tpeak−Tend interval is indicative of global repolarization dispersion and QRS-T angle is a general toolkit for identifying abnormalities in conduction and repolarization.38 Enriching our AI models with such features might have played a significant role in the observed diagnostic gain as compared to expert ECG interpretation based on practice recommendations. Nevertheless, elucidating novel ECG feature beyond STE vs. NSTE clinical practice paradigm can dramatically change care at the bedside.
This study has important clinical implications. First, in the absence of STE on presenting ECG, ED providers still need to consider abnormalities seen on the PH-ECG and their dynamic changes in the overall diagnostic workup of patients with suspected NSTE-ACS. This requires hospitals and systems of care to develop new tools or adopt existing ones to systematically incorporate the PH-ECG into the in-hospital electronic health record. Second, deploying AI-based automated ECG interpretation algorithms on PH-ECG can provide real-time decision support for PH and ED providers, which has important implications for improving patient safety (infarct size, adverse events), nursing surveillance and care (frequency of monitoring, caseload mixture, staff allocation), and care delivery systems (ED overcrowding, regionalization of care, resource utilization, admission unit availability, higher cost vs. lower cost bed allocation, catheterization lab activation). Moreover, the implementation of an AI-based automated ECG interpretation can offer a way to identify ACS cases that do not display criteria fulfilling STEMI criteria, such as occlusion MI (OMI).20 This can help identify OMI patients early, particularly since they often display inly subtle changes and consequently face treatment delays.
CONCLUSIONS
In this study, in three hospitals with coordinated EMS care, we found that more than one half of diagnostic STE and STD changes on a PH ECG resolve prior to ED arrival. Exclusively relying on ED-ECG during in-hospital evaluation of NSTE-ACS comes with poor classification performance, which can be overcome by evaluating the temporal dynamic changes between PH- and ED-ECG in response to prehospital interventions. These findings come with a number of clear takeaways: (1) serial negative ECGs do not significantly increase the negative predictive value of ECG, (2) serial positive ECGs do increase positive predictive value, and (3) a single positive ECG (i.e., positive PH-ECG and negative ED-ECG, or vice versa) has an intermediate predictive value. This pattern seems to hold true for both STE and STD on the 12-lead ECG. Moreover, this study demonstrates that AI-based analytics on a single ECG obtained during ongoing ischemia (i.e., PH-ECG) can capture subtle patterns indicative of NSTE-ACS without the need for serial ECG, which has important and immediate clinical implications for ED practice. This enhanced interpretability may lead to reclassification of one in four patients with suspected NSTE-ACS. This suggests a need for hospitals to develop tools to incorporate PH-ECG into systems of care as informative data points in the in-hospital evaluation of patients with suspected ACS. These findings require future validation in other EMS systems.
Funding:
National Institute of Health grant # R01HL137761
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: ClinicalTrials.gov # NCT04237688
References
- 1.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010(26):1–31. [PubMed] [Google Scholar]
- 2.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. [DOI] [PubMed] [Google Scholar]
- 5.Bradley EH, Herrin J, Wang Y, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med. 2006;355(22):2308–2320. [DOI] [PubMed] [Google Scholar]
- 6.Adams GL, Campbell PT, Adams JM, et al. Effectiveness of prehospital wireless transmission of electrocardiograms to a cardiologist via hand-held device for patients with acute myocardial infarction (from the Timely Intervention in Myocardial Emergency, NorthEast Experience [TIME-NE]). Am J Cardiol. 2006;98(9):1160–1164. [DOI] [PubMed] [Google Scholar]
- 7.Diercks DB, Kontos MC, Chen AY, et al. Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. J Am Coll Cardiol. 2009;53(2):161–166. [DOI] [PubMed] [Google Scholar]
- 8.Ting HH, Krumholz HM, Bradley EH, et al. Implementation and integration of prehospital ECGs into systems of care for acute coronary syndrome: a scientific statement from the American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research, Emergency Cardiovascular Care Committee, Council on Cardiovascular Nursing, and Council on Clinical Cardiology. Circulation. 2008;118(10):1066–1079. [DOI] [PubMed] [Google Scholar]
- 9.Al-Zaiti S, Shusterman V, Carey MG. Novel Technical Solutions for Wireless ECG Transmission & Analysis in the Age of the Internet Cloud. Journal of Electrocardiology. 2013;46(6):540–545. [DOI] [PubMed] [Google Scholar]
- 10.Zègre-Hemsey JK, Asafu-Adjei J, Fernandez A, Brice J. Characteristics of Prehospital Electrocardiogram Use in North Carolina Using a Novel Linkage of Emergency Medical Services and Emergency Department Data. Prehospital Emergency Care. 2019;23(6):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers HP, Bracey A, Lee D, et al. Comparison of the ST-elevation myocardial infarction (STEMI) vs. NSTEMI and occlusion MI (OMI) vs. NOMI paradigms of acute MI. The Journal of emergency medicine. 2021;60(3):273–284. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum Y, de Luna AB, Fiol M, et al. Common pitfalls in the interpretation of electrocardiograms from patients with acute coronary syndromes with narrow QRS: a consensus report. Journal of Electrocardiology. 2012;45(5):463–475. [DOI] [PubMed] [Google Scholar]
- 14.Al-Zaiti S, Macleod MR, Van Dam PM, Smith SW, Birnbaum Y. Emerging ECG Methods for Acute Coronary Syndrome Detection: Recommendations & Future Opportunities. Journal of Electrocardiology. 2022;75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouwmeester S, van Hellemond IE, Maynard C, et al. The stability of the ST segment estimation of myocardial area at risk between the prehospital and hospital electrocardiograms in patients with ST elevation myocardial infarction. Journal of electrocardiology. 2011;44(3):363–369. [DOI] [PubMed] [Google Scholar]
- 16.Ownbey M, Suffoletto B, Frisch A, Guyette FX, Martin-Gill C. Prevalence and interventional outcomes of patients with resolution of ST-segment elevation between prehospital and in-hospital ECG. Prehosp Emerg Care. 2014;18(2):174–179. [DOI] [PubMed] [Google Scholar]
- 17.Badings EA, Remkes WS, The SH, et al. Early or late intervention in patients with transient ST-segment elevation acute coronary syndrome: Subgroup analysis of the ELISA-3 trial. Catheter Cardiovasc Interv. 2016;88(5):755–764. [DOI] [PubMed] [Google Scholar]
- 18.Al-Zaiti SS, Callaway CW, Kozik TM, Carey MG, Pelter MM. Clinical Utility of Ventricular Repolarization Dispersion for Real-Time Detection of Non-ST Elevation Myocardial Infarction in Emergency Departments. J Am Heart Assoc. 2015;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Zaiti SS, Martin-Gill C, Sejdic E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48(6):921–926. [DOI] [PubMed] [Google Scholar]
- 20.Meyers HP, Bracey A, Lee D, et al. Accuracy of OMI ECG findings versus STEMI criteria for diagnosis of acute coronary occlusion myocardial infarction. IJC Heart & Vasculature. 2021;33:100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Zaiti S, Besomi L, Bouzid Z, et al. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat Commun. 2020;11(1):3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon CP, Brindis RG, Chaitman BR, et al. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. J Am Coll Cardiol. 2013;61(9):992–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021:CIR0000000000001038. [DOI] [PubMed] [Google Scholar]
- 24.Hastie T, Tibshirani R, Friedman J. Random forests. In: The elements of statistical learning. Springer; 2009:587–604. [Google Scholar]
- 25.Kligfield P, Badilini F, Denjoy I, et al. Comparison of automated interval measurements by widely used algorithms in digital electrocardiographs. American heart journal. 2018;200:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Awan NA, Amsterdam EA, Vera Z, DeMaria AN, Miller RR, Mason DT. Reduction of ischemic injury by sublingual nitroglycerin in patients with acute myocardial infarction. Circulation. 1976;54(5):761–765. [DOI] [PubMed] [Google Scholar]
- 27.Beltrame JF, Stewart S, Leslie S, Poropat S, Horowitz JD. Resolution of ST-segment elevation following intravenous administration of nitroglycerin and verapamil. Am J Cardiol. 2002;89(4):452–455. [DOI] [PubMed] [Google Scholar]
- 28.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary. Journal of the American College of Cardiology. 2021;0(0). [Google Scholar]
- 29.Pelter MM, Adams MG, Drew BJ. Transient myocardial ischemia is an independent predictor of adverse in-hospital outcomes in patients with acute coronary syndromes treated in the telemetry unit. Heart Lung. 2003;32(2):71–78. [DOI] [PubMed] [Google Scholar]
- 30.Zègre Hemsey JK, Dracup K, Fleischmann KE, Sommargren CE, Paul SM, Drew BJ. Prehospital electrocardiographic manifestations of acute myocardial ischemia independently predict adverse hospital outcomes. J Emerg Med. 2013;44(5):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn T, Johnsen S, Gale CP, et al. Effects of prehospital 12-lead ECG on processes of care and mortality in acute coronary syndrome: a linked cohort study from the Myocardial Ischaemia National Audit Project. Heart. 2014;100(12):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravn-Fischer A, Karlsson T, Johanson P, Herlitz J. Prehospital ECG signs of acute coronary occlusion are associated with reduced one-year mortality. Int J Cardiol. 2013;168(4):3594–3598. [DOI] [PubMed] [Google Scholar]
- 33.Hemsey JKZ, Drew BJ. Prehospital electrocardiography: a review of the literature. Journal of emergency nursing. 2012;38(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. [DOI] [PubMed] [Google Scholar]
- 35.Lehmacher J, Neumann JT, Sorensen NA, et al. Predictive Value of Serial ECGs in Patients with Suspected Myocardial Infarction. J Clin Med. 2020;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarak B, Goodman SG, Yan RT, et al. Prognostic value of dynamic electrocardiographic T wave changes in non-ST elevation acute coronary syndrome. Heart. 2016;102(17):1396–1402. [DOI] [PubMed] [Google Scholar]
- 37.Lux RL. Non-ST-Segment Elevation Myocardial Infarction: A Novel and Robust Approach for Early Detection of Patients at Risk. Journal of the American Heart Association. 2015;4(7):e002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lux RL. Basis and ECG measurement of global ventricular repolarization. Journal of Electrocardiology. 2017;50(6):792–797. [DOI] [PubMed] [Google Scholar]


