Abstract
Poly 2-vinyl-4,4-dimethylazlactone (PVDMA) has received much attention as a “reactive platform” to prepare charge-shifting polycations via post-polymerization modification with tertiary amines that possess primary amine or hydroxyl reactive handles. Upon hydrolysis of the resulting amide or ester linkages, the polymers can undergo a gradual transition in net charge from cationic to anionic. Herein, a systematic investigation of the hydrolysis rate of PVDMA-derived charge-shifting polymers is described. PVDMA was modified with tertiary amines bearing either primary amine, hydroxyl, or thiol reactive handles. The resulting polymers possessed tertiary amine side chains connected to the backbone via amide, ester, or thioester linkages. The hydrolysis rates of each PVDMA derivative were monitored at 25°C and 50°C at pH values of 5.5, 7.5, and 8.5. While the hydrolysis rate of the amide-functionalized PVDMA was negligible over the period investigated, the hydrolysis rates of the ester- and thioester-functionalized PVDMA increased with increasing temperature and pH. Interestingly, the hydrolysis rate of the thioester-functionalized PVDMA appears to be more rapid than the ester-functionalized PVDMA at all pH values and temperatures investigated. We believe these results can be utilized to inform the future preparation of PVDMA-based charge-shifting polymers for biomedical applications.
Keywords: azlactone, charge-shifting polymers, hydrolysis, pH-responsive
Graphical Abstract
The hydrolysis rates of PVDMA-derived charge-shifting polymers with tertiary amine side chains containing amide, ester, and thioester linkages were investigated over a range of pH values and temperatures. While the amide-modified polymers did not hydrolyze, the hydrolysis of the ester- and thioester-functionalized PVDMA increased with both increases in pH and temperature, with the thioesters degrading more quickly.

1. Introduction
Charge-shifting polymers are a class of polyelectrolytes that can gradually change net charge in response to stimuli such as changes in pH or chemical stimuli.[1–4] While examples include anionic-to-cationic[5] and cationic-to-zwitterionic shifts,[4,6] polymers exhibiting cationic-to-anionic shifts are the most widely employed.[1] Charge-shifting polycations can form a strong inter-polyelectrolyte complex with polyanions which will dissociate upon a reduction in the net cationic charge of the polymer. This property makes charge-shifting polycations intriguing for several biomedical applications, including DNA,[6–12] RNA,[13],[14],[15] drug,[16] and protein delivery.[17],[18] A common example of a charge-shifting polycation is poly(dimethylamino)ethyl acrylate (PDMAEA), which has side chains that possess tertiary amine moieties connected to the polymer via an ester bond.[19],[20] Since esters can undergo base-catalyzed hydrolytic degradation to form negatively charged carboxylic acids, PDMAEA can undergo a cationic-to-anionic net charge transition after cleavage of the tertiary amine via hydrolysis of the ester bond.[19]
2-Vinyl-4,4-dimethylazlactone (VDMA) has garnered attention for the synthesis of charge-shifting polymers as the azlactone moiety can undergo ring-opening reactions when exposed to a variety of nucleophilic species, including primary amines, hydroxyls, and thiols.[21],[22] VDMA can be polymerized utilizing controlled polymerization techniques like reversible addition-fragmentation chain transfer (RAFT) polymerization allowing the facile synthesis of a “reactive platform” for post-polymerization modifications to afford a broad range of polymers derived from the same parent polymer, thus having the same degree of polymerization while retaining the azlactone functionality.[23–26] For example, PVDMA can be reacted with primary amines to prepare: thin films with superhydrophobic properties;[27],[28] protein-polymer conjugates;[29],[30],[31] and thermo-responsive materials.[32–34] Interestingly, when PVDMA is reacted with hydroxyl groups, it forms an ester bond that is susceptible to hydrolytic degradation.[1],[2],[34] If the hydroxyl moiety contains a tertiary amine, then upon hydrolysis, the tertiary amine-functionalized PVDMA changes net charge (owing to the resulting carboxylic acid groups on the hydrolyzed PVDMA). Depending on the pH in which this charge transition occurs, the polymer could transition from cationic-to-anionic, neutral-to-anionic, or cationic-to-neutral. This opens the door to applications such as the delivery of biomolecules where anionic species can be released from positively-charged PVDMA-based polymers upon hydrolysis and subsequent cationic-to-anionic transition. For example, Lynn et al. have used PVDMA functionalized with tertiary amine side chains via ester linkages to prepare multilayered films that can release plasmid DNA (pDNA) upon hydrolysis of the side chains.[2] In a subsequent study, they prepared pDNA-containing ultrathin films based on PVDMA functionalized with tertiary amine side chains connected to the backbone via amide and ester linkages.[1] The rate of hydrolysis (and release of pDNA) could be controlled for up to three months under physiological conditions. In another notable study, Lynn et al. investigated the rate of hydrolytic degradation of PVDMA functionalized with tertiary amine-functionalized alcohols where the length of the alkyl spacer between the amine and resulting ester bond is either two or three carbons.[2] Under physiological conditions (pH 7.4 and 37 °C), the authors reported that the half-life was six days for polymers with a two-carbon spacer. In contrast, the half-life was 200 days for a three-carbon spacer, allowing the hydrolytic degradation to be tuned by manipulating the ratio of these moieties in PVDMA. Lynn et al. hypothesized that the reasons for differing degradation rates arise from a combination of two influences: differences in the hydrophobicity of the side chains and the mechanisms for the ester hydrolysis with the potential of intramolecularly-assisted hydrolysis more likely for the polymer with the shorter alkyl spacer. In 2020, Ros et al. investigated the hydrolytic degradation of a structurally-similar charge shifting polymer, PDMAEA, with an alkyl spacer of two carbons between the tertiary amine and ester bond at various pHs and temperatures.[19] They proposed that the amine group can assist hydrolysis of the ester group via a 5-membered or 7-membered intramolecular mechanism under acidic and basic conditions, respectively.
While the hydrolysis of esters can be slow depending on pH and temperature, the rate of hydrolysis of thioesters can be orders of magnitude higher depending on pH, temperature, and sterics around the thioester bond.[35] Additionally, thioesters are susceptible to degradation via exposure to glutathione (GSH), which can accelerate the degradation under physiologically-relevant conditions and can broaden the “kinetic window,” making thioesters particularly intriguing for biological applications such as intracellular delivery. Therefore, the functionalization of PVDMA with thiols could result in materials that undergo hydrolytic degradation significantly faster than the corresponding esters, which could open the door to applications requiring a faster rate of polymer hydrolysis and charge-shifting. Although there are a few reports of thiol-functionalized PVDMA,[36,37] the hydrolytic degradation rates of such thioesters have not been reported to our knowledge.
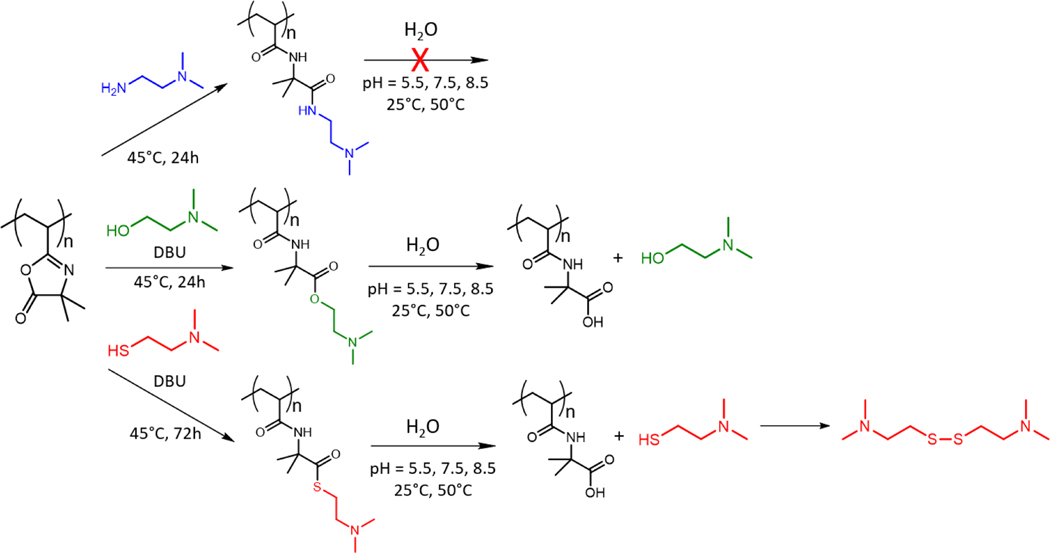
Herein, we investigate the hydrolytic degradation of PVDMA functionalized with a library of tertiary amine-containing side chains. Specifically, we investigate side chains connected to the polymer backbone via amide, ester, or thioester linkages to examine the effect of temperature and pH on the rate of degradation of these groups to provide a greater understanding of the kinetics of hydrolysis of the PVDMA-based charge shifting polycations.
2. Results and Discussion
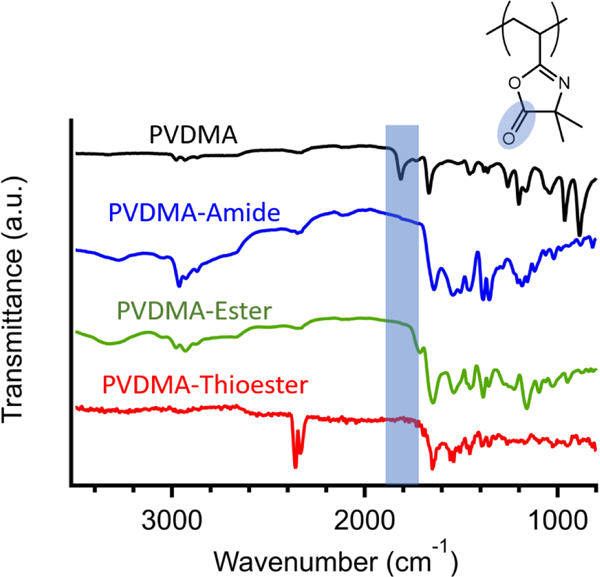
In order to study the hydrolytic degradation of PVDMA functionalized with side chains connected to the backbone via amide, ester, and thioester moieties, we first prepared PVDMA via RAFT polymerization using a similar procedure to previous literature reports.[33],[34] The number-average molecular weight (Mn) and dispersity (Ð) of the obtained PVDMA were analyzed via gel-permeation chromatography (GPC) and were found to be 22,600 g/mol and 1.03, respectively (Figure 1). 1H NMR spectroscopy was performed on this product, and the characteristic resonance peaks were analyzed. (See Supporting Information Figure S1). Since PVDMA can be utilized as a reactive platform, we functionalized this product with a small library of structurally-similar tertiary amine moieties bearing various nucleophilic reactive handles, including primary amine, hydroxyl, or thiol moieties (Scheme 1). The resulting modified polymers possessed side chains with tertiary amines connected to the backbone via amide bonds, ester bonds, and thioester bonds. The complete conversion of the azlactone was verified via Fourier transform infrared (FTIR) spectroscopy, and the spectra are shown in Figure 2. As can be seen, the PVDMA precursor exhibited a strong absorbance band at 1810 cm−1, which is characteristic of the azlactone carbonyl bond.[34] Upon post-polymerization modification with each of the small molecule amines, this absorbance band disappeared, indicating complete conversion of the PVDMA scaffold into the functionalized product. 1H NMR spectroscopy was used to identify the characteristic peaks associated with the modified PVDMA scaffolds, and details are provided in the Supporting Information. Additionally, GPC elution time shifts of ester- and amide-modified PVDMA are shown in the Figures S3 and S5 respectively.
Figure 1.
GPC elution time trace of the PVDMA scaffold. Mn = 22,600 g/mol, Ð = 1.03.
Scheme 1.
Functionalization of PVDMA with primary-amine, hydroxyl, and thiol functional tertiary amines followed by the hydrolysis of the modified PVDMA.
Figure 2.
FTIR spectra of PVDMA (black), amide-modified PVDMA (blue), ester-modified PVDMA (green), and thioester-modified PVDMA (red). The characteristic carbonyl stretching band associated with lactone at 1810 cm1 is shaded in blue and is not present in the modified samples indicating complete conversion.
In order to investigate the rate of hydrolysis of the PVDMA-derived charge-shifting polycations, hydrolysis experiments were conducted with each of the modified PVDMA species in pH 5.5 (acetic acid/sodium acetate), 7.5 (potassium phosphate), and 8.5 (trizma) buffered solutions at 25°C and 50°C. The rates were monitored via 1H NMR spectroscopy by observing the changes in the peaks associated with the side chains at various time intervals (see Supporting Information for more details). If the functionalized polymer undergoes hydrolysis, the small-molecule tertiary amines located on the side chains are cleaved, resulting in carboxylic acids (Scheme 1) and, depending on the system’s pH, could result in a full or partial shift of the net polymer charge from cationic to anionic.
The kinetics of hydrolysis for the ester- and amide-modified PVDMA were obtained by 1H NMR spectroscopy and are shown in Figure 3. Exemplary 1H NMR data are shown in Figures S7 and S8. At all pH values and temperatures, the hydrolysis rate of the PVDMA functionalized via an amide linkage was negligible over 300 hours. This is unsurprising as amide bonds are relatively stable and hydrolyze slowly compared to more labile bonds such as esters.[5] The hydrolysis rates of the PVDMA functionalized via ester linkages exhibited a temperature and pH dependence, where the degradation rate increased with both increasing pH and temperature. This trend is in line with a previous report examining the hydrolysis rates of the structurally-similar polymer PDMAEA.[19] The half-lives of ester-modified PVDMA in various buffers were obtained by linear interpolation when possible and linear extrapolation when a discernable trend was observed and are shown in Table 1. The pH 5.5 ester-modified sample at 25°C did not exhibit an appreciable hydrolysis rate during the observation timeframe. However, the degradation half-life decreases to approximately 330 hours when the temperature is elevated to 50°C. The pH 7.5 samples showed degradation half-lives of approximately 1,800 hours and 40 hours at 25°C and 50°C, respectively, and the pH 8.5 samples showed degradation half-lives of 225 hours and 30 hours at 25°C and 50°C, respectively. These results demonstrate that the hydrolysis rates of ester-modified PVDMA can be tuned by varying both pH and temperature.
Figure 3.
The kinetics of the hydrolysis of ester-modified and amide-modified PVDMA at 25°C and 50°C and pH 5.5, pH 7.5, and pH 8.5. The rate of hydrolysis of the ester-modified PVDMA increased with both temperature and pH, while no noticeable degradation of the amide-modified PVDMA was observed during the periods of the experiments.
Table 1.
The temperature- and pH-dependent hydrolytic half-lives of tertiary amine-functionalized PVDMA connected via esters.
| Temperature (°C) | Half-Life (hrs) | ||
|---|---|---|---|
| pH 5.5 | pH 7.5 | pH 8.5 | |
|
| |||
| 25 | - | 1800 | 105 |
| 50 | 330 | 40 | 15 |
The thioester-functionalized PVDMA exhibited pH- and temperature-dependent hydrolysis similar to the ester-functionalized PVDMA. Interestingly, the hydrolysis rate of thioester-functionalized PVDMA appears to be more rapid than ester-modified PVDMA. Unfortunately, the hydrolysis rate of thioester-functionalized PVDMA could not be accurately quantified via 1H NMR spectroscopy owing to significant overlap between peaks associated with the functionalized polymer and small-molecule disulfide byproducts as shown in Figure 4 for the 50°C hydrolysis. However, thioester degradation can be monitored qualitatively over time by 1H NMR spectra, as shown in Figure 4b for the samples at 50°C. The resonance peak associated with the methylene protons adjacent to the thioester functionality on the polymer (red) decreases. In contrast, the peak corresponding to the same hydrogen on the small molecule (green) begins to appear in an overlapping region. By monitoring these peak changes, we again observed pH-dependent hydrolysis of the thioesters, where the thioester-functionalized PVDMA seems to be fully hydrolyzed by 15 hrs at 50°C for both pH 7.5 and 8.5. In contrast, the hydrolysis rate of the thioester at pH 5.5 appears to be much slower, and complete degradation seems to take at least 137 hrs at 50°C. Interestingly, a side reaction occurs between the small-molecule thiol byproducts that becomes more prominent in pH 7.5 and 8.5, as evidenced by the disappearance of the resonance peaks associated with the small-molecule thiol with the simultaneous appearance of resonance peaks associated with the byproduct (green and orange). These resonance peak shifts are consistent with the formation of disulfide bonds between the small-molecule thiols.[38] To confirm the identity of this byproduct, 1H NMR and ultraviolet-visible spectroscopy (UV-Vis) were performed on samples of the small-molecule thiol incubated in the various buffers at 50°C and the structure was confirmed as a disulfide that forms between the small-molecule thiols as shown in Figure 4a (See Supporting information and Figure S10 for experimental details). The 1H NMR spectra of the thioester-modified PVDMA samples incubated in pH 5.5, pH 7.5, and pH 8.5 buffer at 25°C are shown in the Supporting Information and exhibit similar pH and temperature-dependent trends in hydrolysis rates. Although the hydrolysis rate for the thioester-modified PVDMA could not be quantified via 1H NMR spectroscopy, a few interesting qualitative observations can be made. For example, the hydrolysis rate increases with increasing temperature and pH, the same trend as the ester-modified PVDMA. Additionally, the thioester moiety appears to be almost fully hydrolyzed at pH 7.5 and 8.5 after 15 hrs at 50°C. These degradation rates appear similar to those shown by the ester-modified PVDMA, if not significantly faster. Since thioesters are considered more susceptible to nucleophilic attack than esters and thiolate anions are more stable than oxalate anions, we hypothesize that the degradation of thioester-modified PVDMA will prove to be more rapid than esters which would extend the kinetic window for the charge transformation of PVDMA-based charge shifting polycations allowing for more rapid payload delivery in potential delivery applications.[39] Furthermore, the degradation of PVDMA modified with thioester linkages may proceed even more rapidly in a cellular environment that is high in concentrations of thiols like glutathione, which has been demonstrated to cleave thioester linkages more rapidly than hydrolysis alone.[35] This could be intriguing for applications requiring rapid payload delivery whereby amine-modified PVDMA with thioester linkages could deliver negatively-charged biomolecules to cellular environments in minutes to hours.
Figure 4.
(a) Hydrolysis of the thioester-modified PVDMA and subsequent thiol-thiol coupling of the small-molecule disulfide byproduct and (b) 1H NMR spectra monitoring the hydrolysis of the thioester-modified PVDMA at 50°C for different times and pH values (bottom). The shaded regions of the spectra correspond to the protons labeled with the same color in the top scheme. As hydrolysis proceeds, peaks associated with the small-molecule thiol product appear along with peaks associated with the disulfide byproduct formed from the small-molecule thiols.
3. Conclusion
In conclusion, we investigated the pH- and temperature-dependent hydrolysis rates of tertiary amine-modified PVDMA with amide, ester, and thioester linkages. While prior studies mainly focused on controlling the hydrolysis rate of PVDMA-derivatives by changing the nature of the linker moieties from either ester or amide, we extended this chemistry to include the thioester linker. Additionally, we performed a systematic study of the influence of pH and temperature on the hydrolytic degradation of these various linker moieties. While the amide linkages showed no detectable hydrolysis over 300 hours for any of the pH and temperatures investigated, the ester and thioester linkages exhibited increasing hydrolysis rates with both increasing temperature and pH. The ester-modified PVDMA exhibited hydrolytic half-lives that varied from over 1,800 hrs at pH 7.5 and 25°C to 30 hrs at pH 8.5 and 50°C, suggesting that the degradation (and hence the rate of charge shifting) of these materials can be tuned via temperature and pH. The thioester-modified PVDMA hydrolysis rates also increased with both temperature and pH and appeared to be faster than esters, although further quantification is needed to confirm this observation. We believe that these results can be utilized for the future design of PVDMA-based charge-shifting polymers by offering temperature- and pH-dependent hydrolysis rates and introducing a novel thioester-based charge-shifting polycation that appears to degrade more rapidly than its ester counterpart. These tunable hydrolysis rates could enable applications such as the delivery of bioactive agents that require precise control of the hydrolysis in timeframes varying from minutes to hours.
Supplementary Material
Acknowledgements
TAW would like to thank the National Institutes of Health (#P20 GM130460-01A1 7937) and the National Science Foundation (CAREER 2141666). AES would like to thank the National Science Foundation for support through CBET 1605894 and CBET 2031021.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- [1].Zhang J, Lynn DM, Adv. Mater 2007, 19, 4218–4223. [Google Scholar]
- [2].Sun B, Lynn DM, Control J. Release 2010, 148, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu X, Yang JW, Miller AD, Nack EA, Lynn DM, Macromolecules 2005, 38, 7907–7914. [Google Scholar]
- [4].Liu X, Yang JW, Lynn DM, Biomacromolecules 2008, 9, 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu X, Zhang J, Lynn DM, Soft Matter 2008, 4, 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sinclair A, Bai T, Carr LR, Ella-Menye JR, Zhang L, Jiang S, Biomacromolecules 2013, 14, 1587–1593. [DOI] [PubMed] [Google Scholar]
- [7].Luten J, van Nostrum CF, De Smedt SC, Hennink WE, Control J. Release 2008, 126, 97–110. [DOI] [PubMed] [Google Scholar]
- [8].Kumar R, Santa Chalarca CF, Bockman MR, Van Bruggen C, Grimme CJ, Dalal RJ, Hanson MG, Hexum JK, Reineke TM, Chem. Rev 2021, 121, 11527–11652. [DOI] [PubMed] [Google Scholar]
- [9].Luten J, Akeroyd N, Funhoff A, Lok MC, Talsma H, Hennink WE, Bioconjug. Chem 2006, 17, 1077–1084. [DOI] [PubMed] [Google Scholar]
- [10].Ros S, Freitag JS, Smith DM, Stöver HDH, ACS Omega 2020, 5, 9114–9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ooi YJ, Wen Y, Zhu J, Song X, Li J, Biomacromolecules 2020, 21, 1136–1148. [DOI] [PubMed] [Google Scholar]
- [12].Wang G, Zhu D, Zhou Z, Piao Y, Tang J, Shen Y, ACS Appl. Mater. Interfaces 2020, 12, 14825–14838. [DOI] [PubMed] [Google Scholar]
- [13].Gurnani P, Blakney AK, Terracciano R, Petch JE, Blok AJ, Bouton CR, McKay PF, Shattock RJ, Alexander C, Biomacromolecules 2020, 21, 3242–3253. [DOI] [PubMed] [Google Scholar]
- [14].Cook AB, Peltier R, Hartlieb M, Whitfield R, Moriceau G, Burns JA, Haddleton DM, Perrier S, Polym. Chem 2018, 9, 4025–4035. [Google Scholar]
- [15].Whitfield R, Anastasaki A, Truong NP, Cook AB, Omedes-Pujol M, Loczenski Rose V, Nguyen TAH, Burns JA, Perrier S, Davis TP, Haddleton DM, ACS Macro Lett. 2018, 7, 909–915. [DOI] [PubMed] [Google Scholar]
- [16].Zhang M, Chen X, Li C, Shen X, Control J. Release 2020, 319, 46–62. [DOI] [PubMed] [Google Scholar]
- [17].Hong J, Kim BS, Char K, Hammond PT, Biomacromolecules 2011, 12, 2975–2981. [DOI] [PubMed] [Google Scholar]
- [18].Lee Y, Ishii T, Cabral H, Kim HJ, Seo JH, Nishiyama N, Oshima H, Osada K, Kataoka K, Angew. Chemie - Int. Ed 2009, 48, 5309–5312. [DOI] [PubMed] [Google Scholar]
- [19].Ros S, Wang J, Burke NAD, Stöver HDH, Macromolecules 2020, 53, 3514–3523. [Google Scholar]
- [20].Ros S, Kleinberger RM, Burke NAD, Rossi NAA, Stöver HDH, Macromolecules 2018, 51, 5752–5761. [Google Scholar]
- [21].Buck ME, Lynn DM, Polym. Chem 2012, 3, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leiske MN, Mahmoud AM, Warne NM, Goos JACM, Pascual S, Montembault V, Fontaine L, Davis TP, Whittaker MR, Kempe K, Polym. Chem 2020, 11, 5681–5692. [Google Scholar]
- [23].Hansen RR, Hinestrosa JP, Shubert KR, Morrell-Falvey JL, Pelletier DA, Messman JM, Kilbey SM, Lokitz BS, Retterer ST, Biomacromolecules 2013, 14, 3742–3748. [DOI] [PubMed] [Google Scholar]
- [24].Lokitz BS, Messman JM, Hinestrosa JP, Alonzo J, Verduzco R, Brown RH, Osa M, Ankner JF, Kilbey SM, Macromolecules 2009, 42, 9018–9026. [Google Scholar]
- [25].Lokitz BS, Wei J, Hinestrosa JP, Ivanov I, Browning JF, Ankner JF, Kilbey SM, Messman JM, Macromolecules 2012, 45, 6438–6449. [Google Scholar]
- [26].Messman JM, Lokitz BS, Pickel JM, Kilbey SM, Macromolecules 2009, 42, 3933–3941. [Google Scholar]
- [27].Carter MCD, Wong MS, Wang F, Lynn DM, Chem. Mater 2020, 32, 6935–6946. [Google Scholar]
- [28].Buck ME, Schwartz SC, Lynn DM, Chem. Mater 2010, 22, 6319–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim JS, Sirois AR, Vazquez Cegla AJ, Jumai’An E, Murata N, Buck ME, Moore SJ, Bioconjug. Chem 2019, 30, 1220–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cullen SP, Mandel IC, Gopalan P, Langmuir 2008, 24, 13701–13709. [DOI] [PubMed] [Google Scholar]
- [31].Ho HT, Bénard A, Forcher G, Le Bohec M, Montembault V, Pascual S, Fontaine L, Org. Biomol. Chem 2018, 16, 7124–7128. [DOI] [PubMed] [Google Scholar]
- [32].Levere ME, Ho HT, Pascual S, Fontaine L, Polym. Chem 2011, 2, 2878–2887. [Google Scholar]
- [33].Quek JY, Zhu Y, Roth PJ, Davis TP, Lowe AB, Macromolecules 2013, 46, 7290–7302. [Google Scholar]
- [34].Zhu Y, Quek JY, Lowe AB, Roth PJ, Macromolecules 2013, 46, 6475–6484. [Google Scholar]
- [35].Chen J, Zhao M, Feng F, Sizovs A, Wang J, J. Am. Chem. Soc 2013, 135, 10938–10941. [DOI] [PubMed] [Google Scholar]
- [36].Carter MCD, Lynn DM, Chem. Mater 2016, 28, 5063–5072. [Google Scholar]
- [37].Liebscher J, Teßmar J, Groll J, Macromol. Chem. Phys 2020, 221, 1900500. [Google Scholar]
- [38].Mohanty AK, Ye J, Ahn J, Yun T, Lee T, Kim KS, Jeon HB, Chang T, Paik HJ, Macromolecules 2018, 51, 5313–5322. [Google Scholar]
- [39].Aksakal S, Aksakal R, Becer CR, Polym. Chem 2018, 9, 4507–4516. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.