Abstract
Objectives:
The purpose of this study was to investigate the feasibility of Community Health Workers as patient-site facilitators in teleaudiology-facilitated hearing aid services to improve hearing aid rehabilitation outcomes for older Hispanic/Latino adults in a medically underserved, rural, US-Mexico border community.
Design:
A total of 28 adults (aged 55–89) with bilateral hearing loss participated in this study. Individuals were randomized to one of two teleaudiology intervention arms that differed at the level of the patient-site facilitator. Participants in the experimental group were assisted locally by trained Community Health Worker facilitators. Participants in the control group were assisted locally by trained university student facilitators. Synchronous (real-time) teleaudiology hearing aid services took place with participants located at a rural community health center and the clinician located a university 70 miles away. The results of this feasibility study are presented within the RE-AIM (reach, effectiveness, adoption, implementation fidelity, maintenance) implementation framework.
Results:
Regarding reach, the participants in this study population are historically under-represented in research (primarily low-income Hispanic/Latino older adults). A total of 57 individuals were recruited, 47 were consented and assessed for eligibility and 28 individuals met inclusion criteria and were randomized. The average age of participants was 73.9 years, (range: 55 to 89 years) and most individuals were female (75%). Most participants (86%) reported having incomes less than $20,000 annually. Effectiveness results (via the Self Efficacy for Situational Communication Management Questionnaire) showed that both groups (CHW and control) significantly improved listening self-efficacy from pre-fitting baseline and no difference between groups was observed. Regarding datalogging, at the short-term follow-up, participants in the CHW group wore their hearing aids for more hours/day on average compared to participants in the control group. Implementation fidelity was high for both groups. Long-term maintenance of CHW-supported teleaudiology appears feasible given that training and institutional support is in place.
Conclusions:
Teleaudiology-delivered hearing aid services were feasible when facilitated locally by trained CHWs. Future efficacy and effectiveness research is warranted with CHWs and teleaudiology, potentially leading to a significant reduction in barriers for rural and medically under-resourced communities.
Keywords: teleaudiology, Community Health Worker, service delivery, feasibility, social determinants of health, community based participatory research
I. INTRODUCTION
Unaddressed age-related hearing loss in older adults poses a significant public health problem. Hearing loss affects approximately two in three adults over age 70, and prevalence increases with age (Goman & Lin, 2016; Lin et al., 2011). Hearing loss is associated with psychosocial impacts, including lower health-related quality of life, depression, and social isolation (Cosh et al., 2019; Lin et al., 2011), poorer physical functioning (Dalton et al., 2003), and cognitive decline (Lin et al., 2013). The most common strategy for managing the impacts of hearing loss centers on the use of hearing aids (Valente et al., 2006). Research shows that hearing aids help improve health-related quality of life by reducing the psychological, social, and emotional effects of hearing loss (Chisolm et al., 2007). Hearing aids may also help slow the rate of cognitive decline (Amieva et al., 2015). However, hearing aid use is low; only 14–33% of older adults with hearing loss report that they use hearing aids (Bainbridge & Ramachandran, 2014; Chien & Lin, 2012).
While the national (US) prevalence of hearing aid use is low, there are significant disparities in the use of hearing aids across multiple dimensions. A health disparity is a systematic difference that exists among particular populations or groups in access to, utilization of, and quality of health care, or in the prevalence of an adverse health condition, contributing to health inequity (Baciu et al., 2017; Braveman et al., 2018). Like many US health disparities, hearing aid use is lower among racial/ethnic minorities, individuals with lower income (Bainbridge & Ramachandran 2014; Mamo, Nieman & Lin, 2016), and those without private health insurance (Nieman et al., 2016). Additionally, a patient’s geographic (rural) location has been shown to be associated with delays in access to care (Chan et al., 2017).
The current prevailing model for hearing rehabilitation poses multiple obstacles for potentially millions of individuals (Mamo et al., 2016). Research is needed on interventions that target the barriers that limit access and contribute to hearing health care disparities. Given the negative impacts associated with unaddressed hearing loss, increasing the proportion of adults with hearing loss who obtain and use hearing aids has been recognized as a national priority (NASEM, 2016; NIDCD, 2017; PCAST, 2015), including by using different modes of service delivery to improve health equity. The current study tested the feasibility of an innovative approach to hearing aid service delivery, combining teleaudiology with the local support of Community Health Workers to minimize access barriers for a cohort of rural, low-income Hispanic/Latino older adults with hearing loss.
Teleaudiology
Teleaudiology, or the remote provision of hearing health care services, improves access by narrowing the geographic distance between patient and provider. Research has primarily focused on evaluating the feasibility and validity of teleaudiology service delivery, which has been demonstrated in video-otoscopy (Biagio et al., 2014; Kokesh et al., 2008; Lancaster et al., 2008), probe microphone hearing aid measurements (Ferrari & Bernardez-Braga, 2009), and hearing aid services (Campos & Ferrari, 2012; Muñoz et al., 2017; Pearce et al., 2009; Penteado et al., 2012; Pross et al., 2016). Despite such evidence, teleaudiology is not widespread. However, evidence suggests that adoption may be increasing due to restrictions on in-person care in the global COVID-19 pandemic (Eikelboom et al., 2022; Saunders & Roughley, 2020).
In teleaudiology, a facilitator is often needed at the patient site to carry out hands-on tasks. Patient-site facilitators can perform a number of duties, including technology-related tasks (e.g., establishing a local videoconferencing session), as well as tasks specific to the hearing health care service (e.g., preparing and placing electrodes on a client for Auditory Brainstem Response testing by a remote audiologist). A recent scoping review on patient-site facilitators in teleaudiology revealed that individuals from a variety of backgrounds in this role, including students, physicians, and technicians (Coco, Davidson, & Marrone, 2020). Interestingly, most reviewed studies placed an audiologist in the role of a facilitator. Given that one of the benefits of teleaudiology is the opportunity to expand capacity of the audiology workforce in rural and other underserved areas, real-world clinics using teleaudiology would not likely place an audiologist in the role of the facilitator. This finding helps underscore that there is a lack of implementation-focused teleaudiology research in real-world settings.
As teleaudiology research progresses from validation studies to evaluating feasibility and implementation, data is needed from teleaudiology studies in real-world practice settings, where audiologists would not necessarily be available to serve as facilitators, such as areas with limited local resources for providers. There is also a lack of research investigating facilitators’ potential impact on patients’ outcomes using pragmatic research designs. Such evidence will be crucial in order to translate research findings into sustainable, accessible, and equitable hearing health care services.
Community Health Workers.
Community Health Workers (CHWs) are non-medical frontline public health workers from the community who serve as a bridge between patients and the healthcare system by delivering culturally-relevant health education, connecting patients with services, and promoting preventative care measures such as vaccines (APHA, 2020; Balcazar et al., 2011; Lujan, Ostwald, & Ortiz, 2007; Rothschild et al., 2014). CHWs work in a variety of settings, and their specific roles are determined by community needs and organizational priorities, and therefore their day-to-day activities vary by region (Lhemann & Sanders, 2007). However, across locales, CHWs are defined by their expertise in understanding of their communities’ challenges and resources, aiding them in addressing social determinants of health (Lhemann & Sanders, 2007). Research demonstrates that the CHW workforce can improve patient-provider communication, enhance patient advocacy, improve access to care and improve patient health (review: Perry, Zulliger, & Rogers, 2014).
There is a small but growing body of evidence showing that CHWs have collaborated with audiology to help improve hearing health care access in vulnerable populations (review: O’Donovan, Verkerk, Winters, Chadha, & Bhutta, 2019). In examples reviewed by O’Donovan and colleagues, CHWs have been involved in screening for hearing loss (Akilan, Vidya, & Roopa, 2014; Olusanya & Akinyemi, 2009), motivating community members to attend screenings (Shrestha, Baral, & Weir, 2001), and delivering ear washing treatment for ear disease (Couzos, Lea, Mueller, Murray, & Culbong, 2003). One study illustrated the feasibility of trained CHWs in India to provide hearing aid services for adults in their community (Emerson et al., 2013). The majority of studies involving CHWs and audiology have occurred in Low and Middle Income Countries (O’Donovan et al., 2019). There are few examples of US-based interventions that involve a CHW model, either by engaging CHW staff within an existing health system (Ingram et al., 2016; N. Marrone et al., 2017; Robler et al., 2020; Sánchez et al., 2017), or by identifying and training individuals from within the community as peer-educators (Nieman et al., 2017; Suen et al., 2021).
A small cohort of studies shows that CHWs have also been engaged to use technology, including aspects of teleaudiology, to expand access to hearing health care in their communities (e.g., Gupta et al. 2017;protocols: Emmett, Robler, Gallo, et al., 2019; Emmett, Robler, Wang, et al., 2019). However, to date, research on CHWs and teleaudiology is limited, particularly in the United States, and has largely focused on the pediatric population.
Research gap
Despite the prevalence of unaddressed hearing loss in older adults, higher among disparity populations, there is limited research on strategies to address underlying barriers and increase hearing aid use. Separately, teleaudiology and CHWs each have decades of evidence showing their effectiveness as separate intervention strategies, yet few studies have integrated them. There are no known published investigations on the feasibility of trained US-based CHWs as patient-site facilitators in teleaudiology hearing aid services to improve hearing-related outcomes among older adults.
As indicated by past and ongoing research, combining aspects of teleaudiology with local support from CHWs can be a successful strategy for improving access to hearing health care for rural and other disparity populations. However, we lack information on CHWs assisting with adult hearing aid services delivered via synchronous teleaudiology. Such research could lead to evidence-based pathways to increase hearing aid access and use for older adults who experience barriers, helping to eliminate disparities and contribute to health and healthcare equity.
Theoretical framework and logic model
This research is framed by Andersen’s Healthcare Utilization Model, a widely used theoretical model for studying the predisposing, enabling, and need-related factors that contribute to health care use (Andersen, 1968; Andersen, Davidson, & Baumeister, 2007). Our logic model (Figure 1) is an adaptation of Andersen’s Model based on contributions from Bandura’s Social Cognitive Theory (Bandura, 1977, 1986). According to Bandura’s theory, learning occurs in a social context with reciprocal interactions of the person, behavior, and environment, including learning by modeling of others (Bandura, 1977). As seen in Figure 1, CHWs are conceptualized as an enabling resource in teleaudiology service delivery, and, as specified by Bandura’s Theory, their contribution is a mediator of improved hearing-related outcomes.
Figure 1.
Logic model showing the factors associated with hearing health outcomes in the Conexiones RCT, adapted from Andersen (1995).
Feasibility evaluation
The purpose of this study, Conexiones (Connections), was to investigate the feasibility of CHWs as patient-site facilitators in teleaudiology-facilitated hearing aid services to improve hearing aid rehabilitation outcomes for older Hispanic/Latino adults in a medically underserved, rural, US-Mexico border community. Feasibility outcomes were operationalized into the dimensions of the RE-AIM (Reach, Effectiveness, Adoption, Implementation fidelity, and Maintenance) evaluation framework (Glasgow et al., 1999). RE-AIM provides researchers with a specific and standard guide for reporting and evaluating the feasibility and impact of public health interventions.
II. METHODS
Overview
In this Randomized Controlled Trial, we evaluated the feasibility of hearing aid services delivered via synchronous teleaudiology in a rural community of Hispanic/Latino adults assisted locally by CHW facilitators. Individuals were randomized to one of two groups. The experimental group received teleaudiology-delivered hearing aid services with patient-site hands-on support from trained CHWs, and the control group received teleaudiology-delivered hearing aid services with patient-site hands-on support from trained non-CHWs (university students). The study involved two intervention visits (hearing aid fitting and follow-up), and two longitudinal outcomes visits over the course of 17 weeks. This study had both quantitative and qualitative methods. The current paper focuses on quantitative results, and a subsequent publication will focus on qualitative outcomes. We expected all participants to improve from baseline to follow-up on the primary outcome, a survey of communication self-efficacy. We also expected to observe a difference between groups, with the CHW group improved relative to the control group on the primary outcome (communication self-efficacy), as well as a secondary outcome (datalogging). Ethical approval was obtained by the Institutional Review Board of the University of Arizona and all participants signed an informed consent. Clinical trial registration is at clinicaltrials.gov, NCT03864003.
Feasibility evaluation: RE-AIM
The reach component of RE-AIM refers to the absolute number and representativeness of the individuals who are willing to participate in the intervention. Effectiveness is the impact of an intervention on individual measured outcomes. Adoption refers to why individuals are willing to adopt the intervention. Implementation fidelity refers to the extent to which the protocol was followed. Maintenance is the resources needed to maintain the intervention or program over time. The dimensions of RE-AIM that are addressed in this study are further described in the following section. Data from post-intervention qualitative interviews were used as indicators of adoption and will be reported in a separate upcoming publication.
RE-AIM feasibility outcomes.
Reach.
The reach component of RE-AIM measures participation rate and characteristics of individuals who are willing to participate in the intervention (Harden et al., 2015). In the Conexiones trial, participants completed a study-generated demographic questionnaire at the eligibility screening, and hearing thresholds were collected as part of establishing study eligibility. We also measured participants’ health literacy, or the application of literacy skills to a health context, to help characterize the study population. Both subjective and objective health literacy measures were collected: a subjective single-item screening question, “How confident are you filling out medical forms by yourself?” (Chew et al., 2008) and an objective 18-item Short Assessment of Health Literacy-Spanish (Lee, Stucky, Lee, Rozier, & Bender, 2010).
Effectiveness.
The effectiveness component of RE-AIM measures the impacts of the intervention on outcomes of interest (Harden et al., 2015). Conexiones trial outcome measures were selected to align with Andersen’s Model, and were collected at baseline, and at three subsequent timepoints (3 weeks, 10 weeks, and 17 weeks post-baseline) to evaluate short and long-term effects of the intervention. The primary outcome was subjective communication self-efficacy using the Self-Efficacy for Situational Communication Management Questionnaire (SESMQ; Jennings, 2005; Jennings et al., 2014), collected at immediately before the hearing aid fitting (baseline), and at two subsequent timepoints (10 weeks and 17 weeks post-fitting) to evaluate short and long-term effects of the intervention. On the SESMQ, items are presented as 20 real-world listening and communication situations and are rated on two scales: hearing ability (SESMQ-H) and perceived listening self-efficacy (SESMQ-C). Individuals respond based on how well they can hear in each situation from 0 (not well at all) to 10 (very well), as well as their perceived confidence or self-efficacy, from 0 (not confident at all) to 10 (very confident). Total scores on each scale range from 0 to 200. with higher scores indicating better functioning.
Researchers also collected the average number of hours of hearing aid use per day from the datalogging feature of the hearing aid programming. Mean daily hearing aid usage is considered the total number of hours used divided by total calendar days (Humes et al., 1996, 2019). The study hearing aids manage datalogging by recording use (the amount of time that the battery door of the hearing aid is closed) and updating every 60 minutes in the hearing aid memory. The datalogging system of the hearing aid programming records information about usage since the last fitting or follow-up session.
Implementation fidelity.
The implementation fidelity component of RE-AIM addresses how consistently the intervention was delivered. Our approach to studying implementation fidelity follows relevant recommendations from the Workgroup of the National Institutes of Health Behavior Changes Consortium (BCC) for evidence-based guidance on measuring and reporting implementation fidelity across the areas of study design, training of interventionists, and delivery of the intervention (Bellg et al., 2004). Congruent with the BCC’s recommendations, to enhance fidelity of the intervention related to study design, researchers used theory (Andersen’s Model) to help guide the intervention, ensured the same dose of treatment across intervention groups, and established contingency plans in the case of setbacks. To address implementation fidelity related to training interventionists, patient-site facilitators underwent a multi-level training, and were required to meet performance criteria before engaging in service delivery. Details and CHW group results are published in a previous report (Coco et al., 2021). To evaluate fidelity regarding the delivery of the intervention, study procedures were video recorded when consent was given, and analyzed for length of appointment and adherence to study protocol. Time-stamped coding was done by a bilingual research assistant who was not involved in study procedures. Recordings were considered in analyses if they captured the entire appointment. We restricted our analyses to recordings from Study Visit 1, because the procedures involved in Visit 2 varied significantly based on participant needs.
Maintenance.
Individual-level maintenance outcomes (long-term effects of the intervention on study outcomes) are reported as part of the effectiveness construct (above). Organization-level maintenance refers to the factors that impact how the intervention would become institutionalized in the community. Given that the Conexiones trial is an early-stage short-term feasibility study, organization-level integration of the intervention was not directly assessed. However, based on experiences in this trial, we comment on the resources needed to maintain the service delivery model long-term. Because the sustainability of any intervention is affected by the cost of services, we asked participants to report the amount of money they would be willing to pay for one of their hearing aids.
Community-Based Participatory Research
This study was guided by the principles of Community Based Participatory Research (CBPR), an approach to research that involves an equitable and intentional collaboration of information, ideas, and decision-making power between academic researchers and other stakeholders, including representatives from the community (Wallerstein & Duran, 2006). Following a CBPR framework, the Conexiones trial was motivated by community members’ expressed need for hearing aid services locally (Marrone et al., 2017). In agreement with CBPR, community partners and researchers met to discuss project goals and study design, integrating community knowledge with clinical and research to build trust among the team and improve intervention relevance and appropriateness (Marrone, Nieman, & Coco, 2022). Our community-academic partnership emphasized an iterative, co-learning process in which community members and academic-clinicians collaborated to improve the cultural relevance, readability, and usability of study materials. Additionally, there was community-level engagement in research dissemination, and collaboration with the CBPR team to explore the implications of the research findings following the conclusion of the trial.
Setting
The Conexiones RCT intervention activities took place at two separate workplaces located approximately 70 miles apart. The participant community was identified based on long-standing and ongoing CBPR research collaborations with CHWs at the partnering health clinic. The CBPR partnerships helped us refine the intervention based on community context. The study community is located in Southern Arizona on the US/Mexico border between the Mexican state of Sonora, and Pima County, Arizona. Like many rural communities across the U.S., the area has a higher percentage of persons in poverty than the state average (county: 24%; Arizona: 14%). The county is 83% Hispanic/Latino, and 80% of individuals in the county speak a language other than English at home, with the most common language being Spanish (US Census, 2010). The clinician site was located in Pima County, Arizona, the second largest county in the state, with 1,047,279 people (US Census, 2010). The population of Pima County is 38% Hispanic/Latino, and 29% of individuals in the county speak a language other than English at home. Based on research from our lab, 77 audiologists were registered in Pima County, and zero audiologists were in the participant county (Coco, Sorlie Titlow, & Marrone, 2018; Ingram et al., 2016).
Participant engagement and determination of eligibility
CHWs recruited participants by phone call, flyer, and word of mouth. Individuals were invited to a local community center to learn more about the Conexiones trial and learn if they met study inclusion criteria. At the community informational event, a member of the research team explained the study in further detail, and if the individual agreed, a consenting document was signed.
Consented individuals then underwent the following study procedures: (a) demographic intake form; (b) paper-based version of the Mini-Mental State Evaluation, 2nd edition (MMSE-2 BV, Folstein, Folstein, White, & Messer, 2010); (c) clinical case history performed by an audiologist; otoscopy, and air and bone conduction audiometry, using a calibrated portable KUDUwave Type 2 clinical computer-based audiometer (GeoAxon, Pretoria, South Africa) at octave intervals from 500 Hz to 8000 Hz, plus 6000 Hz. After thresholds were obtained, a preliminary determination of eligibility was determined. For those who were preliminarily determined to be eligible, we obtained word-recognition scores using recorded Maryland CNC wordlists presented at 30 dB above Speech Reception Threshold (SRT). After the audiological evaluation, all individuals, regardless of study eligibility, were provided individualized counseling by an audiologist or an audiology doctoral student clinician under supervision.
Inclusion criteria were as follows: (1) age ≥ 50 years; (2) pure-tone air conduction thresholds within fitting criteria of study hearing aids (mild to moderately severe); (3) MMSE2-BV score >10 adjusted for education level (Folstein, Folstein, White, & Messer, 2010). Exclusion criteria were as follows: (1) currently using hearing aids; and (2) medical contraindications for use of hearing aids (e.g., ear drainage, recent sudden hearing loss, acute dizziness). Eligibility determination took place over the course of three days, and RCT intervention activities began no more than two weeks post-randomization.
Randomization
Individuals who qualified and were interested in participating were then randomized to one of two parallel intervention arms (experimental or control). Both intervention arms included a hearing aid fitting, verification, and follow-up services from a remote clinician via synchronous teleaudiology. Given that the Conexiones trial was designed to test the feasibility of CHWs as patient-site facilitators on patient outcomes, the key difference between the two intervention groups was at the level of the patient-site facilitator. Participants in the experimental (CHW) group were assisted locally by a trained CHW, and participants in the control group were assisted locally by a trained non-CHW (university student). Figure 2 illustrates the Conexiones study design including the two intervention arms. Randomization was by a computer-generated randomization sequence using block sizes of four. Individuals were randomly assigned to two groups at a 1:1 ratio using a REDCap randomization module. Group assignments were delivered by LC.
Figure 2.
Overall study design for the Conexiones Randomized Controlled Trial, including enrollment and randomization, hearing aid fitting, follow-up, and longitudinal surveys.
Equipment and Instrumentation
During teleaudiology-based intervention procedures, each site used a desktop computer connected to a high-definition tower web camera with embedded omnidirectional speaker. A HIPAA-compliant videoconferencing platform was used for real-time exchange of audio and video between sites. The videoconferencing software’s remote desktop control feature allowed the clinician site to view any actions taking place on the rural site’s computer screen, and remotely control the rural site’s computer system.
All participants were fit with digital Unitron Moxi T Stride M 500 mini-BTE hearing aids. Programming was conducted using Unitron TrueFit software v4.0.2 and NOAH v4.5™ (HIMSA, Copenhagen, Denmark). To verify the acoustic performance of the hearing aids, real-ear verification (probe microphone) measures were completed using an Aurical FreeFit controlled by OTOstuite™ software platform (v.4.84, GN Otometrics, Taastrup, Denmark).
Intervention procedures
Following baseline procedures, which included a hearing test, all participants underwent two intervention sessions and two longitudinal data collection sessions (Figure 2). The study’s intervention sessions involved a hearing aid fitting (Session 1) and a follow-up visit (Session 2) conducted via synchronous teleaudiology. Procedures for these sessions followed evidence-based guidelines and recommendations from the American Academy of Audiology Task Force for Guidelines for the Audiologic Management of Adult Hearing Impairment (Valente et al., 2006). Participants were given a paper copy of a hearing aid guide in English or Spanish, created by the research team.
To help minimize variability between subjects, the researchers selected the study hearing aid device. Although the researchers were thoughtful in selecting a hearing aid that would be appropriate for all individuals, in a real-world clinic setting, patients would actively participate in choosing their own devices.
Hearing aid fitting, verification, and validation via teleaudiology (Session 1)
Participants completed baseline outcome measures. Then, the facilitator (CHW or student) connected to the remote (audiologist) site via real-time video and audio (videoconferencing), and desktop control applications. The participant underwent video otoscopy, with the facilitator manually operating the video otoscope and the remote clinician analyzing the video image in real-time. Next, the facilitator prepared the hearing aids for the participant (e.g., selected appropriate length of thin tube and size of dome). The facilitator placed the hearing aids in the participants’ ears, and any adjustments to the physical components of the hearing aids were made (dome size, thin tube length) based on discussion between the remote clinician and facilitator, and feedback from the participant. The clinician then remotely performed an initial programming and verification of the hearing aids with hands-on assistance by the facilitator. The remote clinician also discussed adjustment to hearing loss and use of hearing aids, communication help, goal setting, and management of expectations (i.e., rehabilitation counseling). The facilitator helped the participant practice placing the hearing aids in the ears, use the volume control, clean the devices, open and close the battery door, and insert and remove the hearing aid batteries. The remote clinician remained connected via videoconference to provide support when necessary. When the video conferencing session with the clinician ended, some participants continued to have questions, typically about care and maintenance, and these concerns were addressed by the facilitator.
Follow-up fitting via teleaudiology (Session 2)
Session 2 occurred typically three weeks following the initial hearing aid fitting (M = 23.7 days; SD = 9.9 days). There were considerable scheduling constraints surrounding Session 2, including participants’ availability, the availability of the consultation room at the community health center, and availability of study staff. Upon inspection of the data, we discovered that the average number of days between Session 1 and Session 2 differed between groups, t(25) = 2.84, p < 0.01. The average difference between Session 1 and Session 2 for the CHW group was 18.1 days, with a range of 12 to 34 days, and for the non-CHW group it was 30.8 days, with a range of 14 to 35 days. Since there is no known theoretical basis for the difference between two and four weeks impacting measured outcomes, we assume no systematic bias took place because of the difference in timing at Session 2.
In the second session, the remote clinician focused on the following topics: (1) rehabilitation counseling; (2) adjustments to the physical fit of the devices; (3) review of goals; and (4) expectation management. Based on feedback from the participant, the remote clinician made changes in the hearing aid programming (and conducted real-ear verification measures as needed) and adjusted physical fit aspects of the hearing aid. The facilitator was responsible for (a) placing the hearing aid programming loop around the participant’s neck (b) changing the size of dome or slim tube based on the remote clinician’s recommendations; (c) manually operating the video otoscope; (d) manual aspects of operating the real-ear verification system, including assisting with calibration, positioning the silicone probe tubes in the participant’s ear canals, and placing the participant’s hearing aids in their ears; and (e) under the remote clinician’s supervision, the facilitator also provided further instructions on device orientation. The remote clinician collected the hours of hearing aid use (datalogging) information from the hearing aids, and participants completed follow-up surveys.
Post-Intervention Outcomes (Session 3)
Session 3 occurred seven weeks after Session 2 (M = 48.1 days; SD = 7.1 days). The length of time between sessions was similar for each group, t(21) = 1.68, p = 0.10. Session 3 was a post-intervention outcomes visit. Researchers met individually with participants at a local community center to complete follow-up surveys and collect datalogging information from the hearing aids.
Post-Intervention Outcomes (Session 4)
The final longitudinal outcomes session occurred seven weeks after Session 3 (M = 51.6 days; SD = 5.0 days). The length of time between sessions was similar for each group, t(16) = 2.02, p = 0.06. Researchers planned to meet in-person with participants to complete surveys and conduct a post-program interview. However, this session coincided with the beginning of the COVID-19 global pandemic (March 2020), at a time when University personnel were not allowed to engage in face-to-face research. As such, a member of the research team called participants on the phone to collect survey data and conduct qualitative interviews. Because participants and researchers did not meet in person, datalogging information was not able to be collected.
Masking
Study staff were physically present during study procedures, and therefore it was not possible for researchers and clinicians to be masked to participants’ group assignments. Participants were not explicitly notified which group they were in, although similarly, since they were physically present with facilitators during study procedures, it was not possible to completely mask participants to their group assignment. At the conclusion of the intervention, participants were informed about the study hypothesis and their group assignment in a debriefing document.
Patient-Site Facilitators
CHW facilitators.
Experimental group (CHW) facilitators were employees at the partnering Federally Qualified Health Center. All were female, and native speakers of Mexican Spanish. The CHWs were selected due to their previous experience in community-based hearing health promotion through their participation in the Oyendo Bien trial (Marrone et al., 2017). Their experience included multiple years of delivering a hearing health promotion program following extensive trainings (Sánchez et al., 2017). The CHWs’ work on this project is supported by a community grant.
Non-CHW facilitators.
Control group facilitators were undergraduate students at the University of Arizona, and one recently graduated student. All were female. Each had completed at least one year of coursework in speech, language, and hearing sciences. None of the control facilitators were CHWs, none were from the participant community, and all spoke only English to the clients, communicating through an interpreter.
Training of facilitators.
The individuals (CHWs and university students) who served in the role of patient-site facilitator in Conexiones received the same series of trainings and were required to pass the same evaluations before engaging in trial procedures. Trainings were delivered separately for CHW and non-CHW groups due to scheduling and language differences. All individuals underwent a multi-level training across four days (14 hours total). A detailed description of the protocol used to train facilitators, along with CHW-group outcomes, is published in Coco, Piper, and Marrone (2021). In brief, the training covered the basics of age-related hearing loss, including its prevalence and the psychosocial impacts; an introduction to the roles on the teleaudiology team; technology used in teleaudiology hearing aid service delivery; and how to conduct the hands-on tasks needed to serve as a facilitator in synchronous hearing aid services for adults. The training also included four hours of in-person observation at a university-based in-person adult hearing aid clinic. Following training, individuals were evaluated on their knowledge and hands-on skills related to patient-site facilitator duties using a structured assessment. All CHWs (n = 3) and non-CHWs (n = 4) achieved the benchmark score (≥80%) on the knowledge-based survey, and all passed the performance-based assessment.
Data analyses
Sample size calculation
We estimated sample size using the suggested calculation from (Gupta, Attri, Singh, Kaur, & Kaur, 2016), based on the following parameters: (1) on the SESMQ, an expected average score of 84, a minimal difference considered real (MDR) score of 26 points, and standard deviation of 35.8 (Jennings et al. 2014); and (2) a conservative 20 percent loss to follow-up. A total enrollment of 30 participants (15 per group) was estimated to produce 80 percent power for a two-tailed test with an alpha error of 5 percent.
Analyses
To test for differences between groups on demographic data, analyses were done using independent sample t-tests the chi-square test or Fisher’s exact test. Linear mixed effect models were used to test differences between groups (CHW and non-CHW facilitator) across the timepoints. Fixed effects for time, group, and their interaction were included. A random intercept per subject was included to account for the possible correlation among data points for the same subject. Models were fitted using the lmer function in the statistical software R (R Core Team, 2017). P-values were obtained using Satterthwaite approximations for degrees of freedom, and were considered statistically significant at p < 0.05 (Bates et al., 2015). An intent-to-treat (ITT) approach was used, meaning all randomized participants were included in the analyses (Fisher et al., 1990). Post hoc analyses were carried out if significant main effects were found, using Bonferroni corrections for multiple testing. We used Cook’s Distance to compute the estimated degree of influence exerted by each data point on each of the predicted outcomes (Cook, 1977). Data points with large Cook’s Distance (a percentile greater than 50 using the F-distribution) were investigated further for validity, and models were fit without those points to identify if there was a change in significance (Foley, 2019). A linear mixed effect model is robust in the presence of data that are missing completely at random (MCAR) due to likelihood-based estimation (Ibrahim et al., 2005). To investigate missingness, potential relationships between missing data and other variables (age, degree of hearing loss, sex) were conducted using t-tests and Fisher’s exact test.
RESULTS
Reach
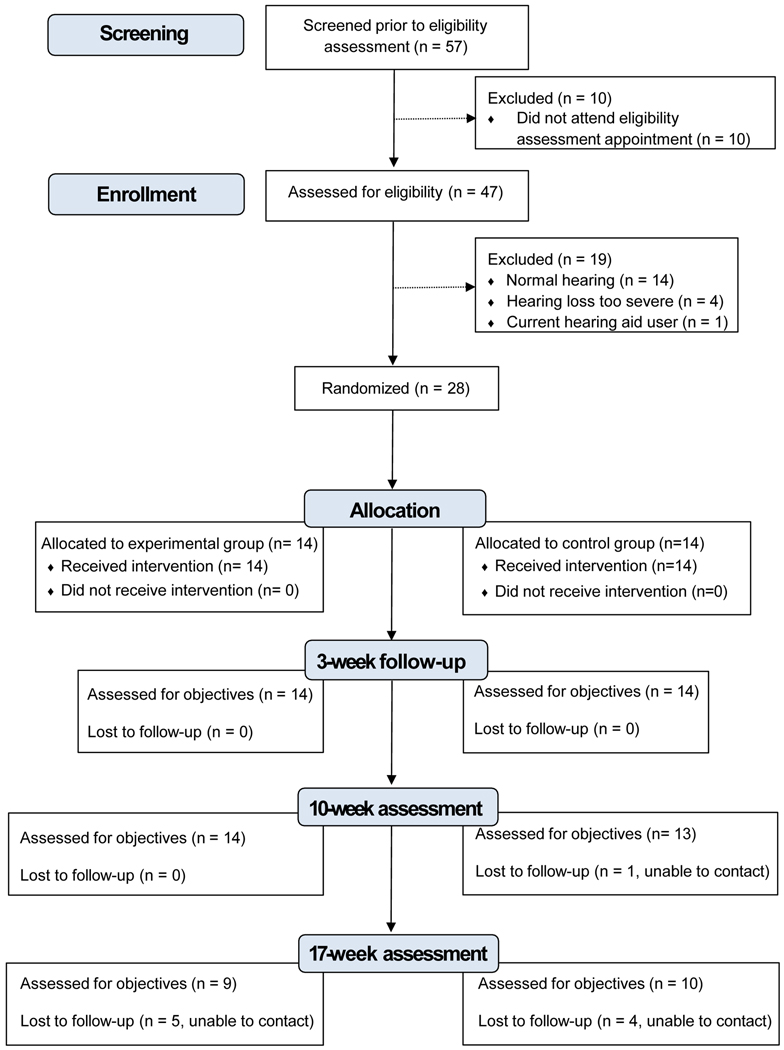
During the recruitment period, CHWs recruited 57 individuals, and 47 were consented and assessed for eligibility. Approximately forty percent (n = 19) did not meet study inclusion criteria, mainly because their hearing thresholds were measured at < 25 dB HL. Of 28 randomized individuals, all completed baseline surveys and 96% (27 of 28) completed at least one follow-up survey. Figure 3 is a flow diagram showing reasons for exclusion and loss to follow-up according to CONSORT guidelines for feasibility trials (Eldridge et al., 2016).
Figure 3.
CONSORT flow diagram showing study screening, enrollment, allocation, randomization, and loss to follow-up.
The average age of participants was 73.9 years, (range: 55 to 89 years) and most individuals were female (75%). The average education level was equivalent to high school (M = 9.9 years, SD = 4.1). The average amount of time participants reported having hearing loss was 5.3 years (SD = 3.8). Participants were asked to report on their vision by responding to the question “How well can you see in your daily life?” (Uchino et al., 2017). The mean score was 3.93 (SD = 0.94), equivalent to the response item “a little good” (possible range of responses: 1 = very poor; 5 = very good).
Participants’ health literacy levels were evaluated using two methods, subjective (self-report) and objective (assessment). According to the subjective single-item health literacy screening question (“How confident are you filling out medical forms by yourself?”), 25% of participants (7 of 28) had inadequate health literacy (response: “Somewhat” or less). Based on the objective 18-item Short Assessment of Health Literacy (SAHL), only 11% of participants (3 of 28) were classified as having inadequate health literacy (score ≤ 14). There were no demographic or hearing differences between study groups. See Table 1 for participant characteristics.
Table 1.
Participant characteristics.
| Demographic | Total sample (n = 28) | Experimental (CHW facilitator) group (n = 14) | Control (Non-CHW facilitator) group (n = 14) |
|---|---|---|---|
|
| |||
| Age in years, mean ± SD | 73.86 (8.41) | 73.79 (8.58) | 73.93 (8.57) |
| Female sex, n (%) | 21 (75) | 9 (75) | 12 (86) |
| Education in years, mean ± SD | 10.03 (4.32) | 9.57 (4.91) | 10.50 (3.76) |
| Hispanic and/or Latino, n (%) | 28 (100) | 14 (100) | 14 (100) |
| MMSE2-BV mean ± SD (range 0–16) | 14.07 (1.72) | 14.62 (1.50) | 13.50 (1.79) |
| HFPTA (dB HL) | 43.39 (12.19) | 43.71 (13.01) | 43.07 (11.81) |
| Self-reported duration of HL in years, mean ± SD | 5.3 (3.8) | 5.1 (3.7) | 5.5 (4.1) |
| Objective health literacy survey SAHL, mean ± SD (range 0 – 18) |
16.9 (1.8) | 16.5 (1.9) | 17.2 (1.8) |
| Subjective health literacy screening, mean ± SD (range 1 −5) |
3.85 (1.29) | 3.71 (1.44) | 4.0 (1.11) |
| Subjective visual acuity question, mean ± SD (range 1–5) |
3.93 (0.94) | 3.93 (0.83) | 3.93 (1.07) |
| Annual income in USD, n (%) | |||
| < $10k | 14 (50) | 6 (43) | 8 (57) |
| $10k - 20k | 10 (36) | 6 (43) | 4 (29) |
| $20k - $40k | 3 (11) | 2 (14) | 1 (0.1) |
| > $40k | 1 (.04) | 0 | 1 (0.1) |
Note. MMSE-2 BV = Mini-mental State Exam −2nd edition Brief Version (Folstein et al., 2010); HFPTA = high-frequency pure-tone average in the better ear (average at 1kHz, 2kHz, and 4kHz); HL = hearing loss; SAHL = Short Assessment of Health Literacy (Lee et al., 2010); subjective health literacy screening = validated single item question: “How confident are you filling out medical forms on your own?” Response options: 5 = extremely, 4 = quite a bit, 3 = somewhat, 2 = a little bit, or 1 = not at all (Chew et al., 2004; Wallace et al., 2006). Vision question: “How well can you see in your daily life (glasses or contacts on)?”; response options: 5 = very good, 4 = a little good, 3 = normal, 2 = little poor, 1 = very poor (Uchino et al., 2017).
All of the eligible participants presented with bilateral sensorineural hearing loss to different degrees, and four participants had an additional conductive component. The right ear high-frequency pure-tone threshold average (1, 2 and 4k Hz) was 46.2 dB HL (range: 23 – 93 dB HL) and the left ear high-frequency pure-tone threshold average was 47.1 dB HL (range: 12 – 83 dB HL). Participants were fit bilaterally with digital mini behind-the-ear (BTE) thin tube hearing aids with a dome fitting. There were two exceptions. One participant (CHW group) required a custom ear mold with a tubing attachment in one ear due to excessive feedback, and one participant (control group) was fit unilaterally (with a thin tube and dome fitting) due to feedback issues encountered with a dome, and inability to use an earmold due to a longstanding middle-ear issue.
Effectiveness
Primary outcome.
On the SESMQ, total scores on each of the two scales range from 0 to 200 in five-point increments, with higher scores indicating better hearing ability (SESMQ-H) or better self-efficacy for communication (SESMQ-C). Figures 4a and 4b show the means and interquartile ranges for SESMQ-H and SESMQ-C, respectively, used in the analyses. Linear mixed model analyses for the SESMQ-H revealed that there was a significant main effect for time, F(1, 42), 6.54, p < 0.0001, but the main effect for group was not significant, F(1, 66), 0.47, p = 0.64, and there was no significant interaction, F(1, 42), 0.16, p = 0.87. Regarding the SESMQ-C, there was a significant main effect for time, F(1, 43), 4.34, p < 0.0001, but the main effect for group was not significant, F(1, 64), 0.71, p = 0.48, and there was no significant interaction, F(1, 43), 0.69, p = 0.50. Post-hoc Bonferroni-adjusted comparisons pooling all participants indicated that scores on the SESMQ-H and SESMQ-C scales significantly improved over time (all ps< 0.0001).
Figure 4a-b.
Box and whisker plots for the effect of time on the x-axes for average ratings of the Self-Efficacy for Situational Communication Management Questionnaire Hearing (SESMQ-H, 4a) and Confidence (SESMQ-C, 4b) on the y axes. The grey bars represent the CHW facilitator group and the white bars represent the non-CHW facilitator control group at the timepoints collected. The x represents the mean and the horizontal bars represent the median and upper and lower (25%−75%) quartiles. Dots indicate outlier data points.
Secondary outcome.
Datalogging for the left and right hearing aids were strongly correlated, r = 0.97, p < 0.01. Therefore, the two values were averaged to represent the mean hours of hearing aid use per day as per Humes et al. (2017). Two data points were identified as influential in the model (i.e., Cook’s Distance). These two participants had datalogging greater than 12 hours per day. The individuals were both in the CHW facilitator group, and data was from Session 2 and Session 3. Parameter estimates remained consistent whether or not these influential data points were included or excluded, so they remained in the analyses.
Figure 5 shows the means and interquartile ranges for hours of datalogging used in the linear mixed model analyses. There were significant effects of group, time, and the group by time interaction on hours of hearing aid usage (group: F(1,41) = −2.59, p = 0.014; time: F(1,23) = −2.38, p = 0.026; group by time: F(1,23) = 2.37, p = 0.027). Post hoc Bonferroni-adjusted contrasts indicated the CHW group wore their hearing aids significantly longer on average than the control group at Session 2 (p = 0.003). At Session 3, hearing aid usage was similar for the two groups (p = 0.621). Mean scores and change in scores with associated sensitivity analyses are shown in Appendix A, Supplemental Digital Content I.
Figure 5.
Box-and-whisker plots for the effect of group (CHW facilitator vs non-CHW facilitator) on the X axis for average hours of hearing aid usage per day (datalogging) across participants at the two data collection points (Session 2 and Session 3). The x represents the mean and the horizontal bars represent the median and upper and lower (25%−75%) quartiles. The dot indicates an outlier data point. The star indicates significant (p < 0.05).
Missing data.
Missing data were investigated for potential relationships with other measured variables. Results of t-tests and Fisher’s exact test revealed the missing variables were not significantly related to age, sex, or audiometric hearing loss as measured by high-frequency pure tone average in the better ear (all ps > 0.05).
Implementation fidelity.
Fourteen Visit 1 participants’ recordings were available for intervention fidelity analysis (CHW group: n = 7; control group: n = 7), and seven Session 2 recordings were available (CHW group: n = 4; control group: n = 3). The average length of CHW group recordings from Visit 1 (M = 41 minutes; SD = 7.2 minutes) and Visit 2 (M = 26 minutes; SD = 4.8 minutes) were shorter than control group recordings for Visit 1 (M = 48 minutes; SD = 14.9 minutes) and Visit 2 (M = 31 minutes; SD = 5.8 minutes) but the differences were not statistically significant (all ps > 0.05).
Recall that adherence to study protocol was examined according to the completion (or omission) of study procedures: greetings, establishing video conferencing, assisting with video otoscopy, assisting with hearing aid programming, verification, validation, and hearing aid orientation. Overall, the analyzed recordings indicated good fidelity; the control group yielded 97.6% adherence to protocol (82 out of 84 tasks completed) and the CHW group had 100% adherence to protocol (84 out of 84 tasks completed).
Maintenance.
As an indicator of potential long-term sustainability of teleaudiology with CHWs, participants were asked, at the final study visit, the price that they would be willing to pay for one of their hearing aids. There was a higher average US dollar amount for the CHW group $603.75 (SD = 395.11) compared with the non-CHW facilitator group $315.91 (SD = 277.10), although the difference was not statistically significant, p > 0.05. It is important to note that this comparison was underpowered due to a smaller total sample size at that timepoint (n = 18).
IV. DISCUSSION
The Conexiones trial is the first known RCT to test the feasibility of a novel service delivery model, CHWs as patient-site facilitators in teleaudiology, among rural, mostly low-income Hispanic/Latino, older adults with hearing loss. The experimental arm of the Conexiones RCT combines teleaudiology, a well-validated service delivery model that reduces geographic barriers, with local support from CHWs, an established public health workforce with a 60-year history of minimizing access barriers for vulnerable populations. Feasibility outcomes were operationalized in the RE-AIM framework and demonstrated that the delivery of hearing aid services via teleaudiology facilitated locally by CHWs is feasible for improving hearing aid-related outcomes for hard of hearing older adults in an area with limited providers.
Reach.
The characteristics of the population reached by this study (mostly low income, all Hispanic/Latino) are historically underrepresented in clinical trials (Pittman et al., 2020; Coakley et al., 2012). Research with these sub-groups is crucial for representativeness and to help inform culturally appropriate interventions. Investigators have documented potential challenges to conducting research and collecting data among historically underrepresented groups, including confidentiality concerns and language barriers. Community collaboration, including with CHWs, has been emphasized as an important strategy for reaching and maintaining trust among such populations (O’Hegarty et al., 2010; Parrado et al., 2005). In the Conexiones trial, CHWs were crucial to the success of the research, including for recruiting participants.
Interestingly, over one-fourth of individuals assessed for eligibility did not have enough hearing loss according to the audiogram to meet the inclusion criteria for a hearing aid fitting for this study. It is possible that individuals were pursuing a need to learn about the status of their hearing, rather than necessarily an interest in enrolling in a hearing aid study. However, it should also be noted that we did not evaluate binaural sensitivity, spatial hearing, speech in noise, or other measures of auditory processing, and therefore there may be a portion of individuals who did not qualify for this study who still experienced some degree of auditory difficulties. Given the documented lack of local hearing health care providers in that area (Coco et al., 2018), these data emphasize the need for local hearing services in that community.
The population reached in this study had a greater proportion of low health literacy compared to participants in other studies using the same assessments (Chew et al., 2004; Wallace et al., 2006; Penaranda et al., 2012). Results from the Conexiones trial contribute to the small cohort of studies demonstrating how US-based CHWs can support hard-of-hearing individuals with low health literacy in their community. Future studies will focus more explicitly on how CHWs can support individuals with low health literacy across the hearing healthcare journey, including in remote service delivery.
Effectiveness.
Results indicated that teleaudiology-delivered hearing aid services improved hearing-related outcomes from baseline for both groups. Additionally, we observed an apparent CHW group benefit for hours of hearing aid use, with CHW group wearing their hearing aids for longer than control group at the shorter data collection timepoint. Although we expected the data to reveal that the CHW facilitator group would have better subjective listening self-efficacy (SESMQ) relative to the non-CHW facilitator group, average scores for both groups were not significantly different. We speculate that the following elements of the study context may have modified participants’ experience in the Conexiones trial, potentially diminishing the effect of facilitator type.
In this study, participants’ post-fitting listening self-efficacy outcomes were near the highest possible scores in the range, leaving little room for variation between groups. Participants in both groups received teleaudiology-delivered hearing aid devices and services purchased by research grant funds. Therefore, because individuals in both groups were not purchasing on their own behalf, cost was removed as a barrier for all participants. As outlined in Andersen’s Model, health service utilization is dependent on enabling factors. In this study, receiving hearing aid devices and services at no cost may have been such a strong “enabling factor” that group-level comparisons of facilitator type were minimized. Past studies have illustrated that there is a relationship between income and hearing aid uptake, yet few studies have examined the impact of purchase price on outcomes. Humes et al. (2017) conducted an RCT to evaluate the efficacy of an “over-the-counter” intervention model on hearing aid outcomes. In that study, hearing aid purchase price (either $600 or $3600 per pair) did not impact outcomes, although individuals who purchased devices at the lower price were more likely to keep their hearing aids after the trial. According to Humes and colleagues, 80% of participants reported an annual income greater than US$45,000, leading the authors to speculate that the device prices used in that study may have been too low to influence outcomes. Further research is warranted to explore the effects of device price on outcomes, particularly among low-income populations. Interestingly, research has documented that even in countries where hearing aids and services are covered by public health insurance (e.g., Germany, Switzerland, Finland), device use is still low (Bisgaard & Ruf, 2017; Hartley et al., 2010).
Second, our field notes documented two instances in which CHWs and control group participants interacted outside of the study. Because field notes do not include participants’ names, we were not able to identify if these subjects had significantly different outcomes than other control group subjects. However, we acknowledge that their interactions may have impacted group-level comparisons. Also, all participants (regardless of group) interacted with CHWs before the trial during recruitment, evaluation for study candidacy, and consenting, and we recognize that these interactions may have potentially mediated the control group participants’ experience in the trial and diminished the effect of the facilitator on group-level comparisons. Future research will consider strategies for isolating and measuring the impacts of CHWs as facilitators. However, we acknowledge that this will be challenging, particularly in closely-knit communities, areas where CHWs are deeply ingrained in the social fabric of the community, areas where CHWs are involved in multiple health promotion initiatives, and where CHWs are an important part of the CBPR research process. We also acknowledge that bias can be introduced when researchers control aspects of the study environment to the extent that it is an unnatural setting for participants.
Datalogging results revealed that the CHW facilitator group used their hearing aids more on average than the control group at the short-term follow-up visit (Session 2), yet this difference was no longer evident as Session 3. Although further research is warranted to explain this relationship, we speculate that dose (i.e., amount of time with the facilitator) may have contributed to hearing aid use. The nature of the hearing aid fitting visit (Session 1) involved more interaction between the participant and the facilitator, including verification of the hearing aids and orientation to hearing aid maintenance, when compared with the follow-up visit (Session 2), which typically involved a conversation between the remote clinician and the participant. Comparing the average session length among all participants, Session 1 was significantly longer (M = 44.17, SD = 3.11) than Session 2 (M = 29.79, SD = 2.08), t(19) = 3.3, p < 0.01. While it is possible that the amount of facilitator involvement may mediate hearing aid use, the results of this study indicate that this topic should be studied further, including with larger participant populations.
Implementation fidelity.
In this study, facilitators had high levels of adherence to the protocol, as indicated by an analysis of video-recorded study sessions. We have identified key elements that helped ensure good fidelity to the protocol: facilitator training and remote supervision of facilitators. Conexiones facilitators completed a multi-level training program and were required to pass minimum benchmarks to demonstrate their knowledge and skills (Coco et al., 2021). Outside of this study, a teleaudiology facilitator’s training is typically the responsibility of the supervising audiologist. However, if a clinician is not prepared to train a facilitator, this may be a barrier to adopting teleaudiology. At the VA, telehealth facilitators undergo general facilitator training as well as on-the-job training by the supervising clinician in their assigned area, such as audiology. The VA facilitator model, as well as facilitator trainings created by our team (Coco et al., 2021), are a step towards improving the scalable sustainability of teleaudiology in clinical settings.
In the Conexiones trial, facilitators were supervised in real-time by a remote clinician via videoconferencing. With appropriate training, facilitators may also be able to assist with store-and-forward (asynchronous) teleaudiology services. Past research has demonstrated the success of store-and-forward video-otoscopy with a telehealth facilitator (Biagio et al, 2013). Store-and-forward audiology services may be particularly relevant in rural communities that lack high-speed internet connectivity. Additionally, store-and-forward service delivery reduces the need to coordinate schedules across sites. In Conexiones, as mentioned one challenge was coordinating the availability of study participants, remote clinicians, facilitators, interpreters, and two clinic spaces, and these scheduling constraints impacted the timing for each group’s study visits. Store-and-forward teleaudiology allows more flexibility in scheduling because the patient site and provider site do not need to connect at the same time. However, research is needed to evaluate if good treatment fidelity is also possible with store-and-forward teleaudiology when collaborating with a facilitator.
Maintenance.
The long-term success of CHW facilitators in teleaudiology service delivery is promising, given the results from this study. Participants in both facilitator groups improved hearing-related outcomes from baseline, and high scores were maintained over time. Furthermore, results showed that individuals who are supported by CHWs may be willing to pay more for hearing aids. If patients with CHWs value hearing services more, that may make the model easier to sustain. However, in this study, services were provided free of cost, and it is not known if the participants would have been willing or able to purchase the hearing aids on their own. One factor that may affect the sustainability of this service delivery model is the high cost of hearing aids and related services (Jilla et al. 2020).
At least three potential pathways exist for individuals in this community to access lower-cost hearing aids outside of this study. First, patients may pursue hearing aids at a clinic with a humanitarian arm, also known as a “hearing aid bank” (often refurbished lower-cost devices for those with qualified income levels). However, hearing aid banks typically have a high demand and a limited supply, resulting in long waiting lists. Additionally, given supply limitations, hearing aid bank clients may only be eligible to receive one hearing aid when two devices are needed. In the context of the study community, at the time of writing, the nearest hearing aid bank to this community was over 70 miles away.
A second option is to use health insurance to pay for hearing care. However, Medicare and most other insurers do not currently cover the cost of devices and related services (Jilla et al., 2020), although some Medicare (Part B) Advantage plans offer benefits for hearing aids. Arizona’s version of Medicaid does not cover hearing aids for adults (Arnold et al., 2017). Recently, legislation was introduced that proposes to expand hearing care coverage for Medicare patients (Stephenson, 2021). The Medicare Hearing Act of 2019 (HR 4618), if passed, would potentially benefit Medicare patients in this community who are interested in pursuing amplification. Importantly, like many rural areas across the US, the study community lacks permanent audiology practices (Coco et al., 2018). Teleaudiology may be used to help connect patients with audiologists in another location. As illustrated in this study, local CHWs, who are already part of the public health workforce in this community, may serve an important role in connecting patients with remote clinicians.
A third option for lower-cost devices is direct-to-consumer amplification or over-the-counter (OTC) hearing aids. Recently, the US Food and Drug Administration (FDA) introduced a regulatory category for OTC hearing aids, allowing consumers to purchase devices at retail stores without undergoing a hearing test or a fitting by a licensed provider (US Food & Drug Administration, 2018). OTC hearing aids are expected to be lower in cost and available closer to home, but it is still unclear the extent to which such self-fitting devices are suitable for older adults, adults with lower health and/or technical literacy, and adults with limited English.
Strengths and limitations.
The Conexiones RCT is the first study to test the feasibility of trained CHWs as patient-site facilitators in hearing aid services (fitting, verification, troubleshooting, and counseling) via synchronous teleaudiology. This study also represents a strong academic-community collaboration to improve hearing health and address structural inequities. This study also has limitations. The characteristics of our sample (Hispanic/Latino, mostly women, most with an annual income less than US$20,000, average high school education), were relatively homogeneous, and results may not be generalizable to all individuals. However, as mentioned, these demographic characteristics are historically underrepresented in the scientific literature, and therefore this study is considered an important step in improving diversity, inclusion, and representativeness in health-related human subjects research. Our research team translated surveys when a published Spanish-language version was not available, using a functional, team-based translation approach that took into account readability (Coco et al., 2017; Colina et al., 2016). Therefore, comparison with studies using the original version of surveys should be taken with caution. Additionally, all participants were fit with the same digital mini-BTE hearing aids. Studies using this design with different devices cannot directly compare results.
As mentioned, the study’s effectiveness outcomes may have been impacted by control group participants and CHWs interacting. Future research will attempt to mitigate control group interactions with CHWs without sacrificing the benefits of conducting research in real-world environments, such as by using a multi-site study design.
Lastly, as mentioned, masking of participants and facilitators to their group assignment was not possible due to obvious differences between intervention groups. Traditional RCTs prioritize masking as a strategy to reduce reporting bias. However, in pragmatic RCTs, which take place in real-world settings, it can be unethical or impractical to accomplish masking (Christian et al., 2020). This research leveraged strategies that have been recommended in the literature to reduce bias when masking is not possible, including utilizing objective outcomes and subject randomization (Christian et al., 2020). In future expansions of this work, we will continue to explore strategies for minimizing potential sources of bias while maintaining the benefit of conducting research in real-world settings.
Future directions
The long-term goal of this line of research is to improve hearing healthcare equity through improvements in hearing aid service delivery. Given the feasibility of trained CHWs as patient-site facilitators in teleaudiology-delivered hearing aid services for older adults in this study, additional larger-scale trials are warranted to investigate the efficacy and effectiveness of this approach. As discussed, there is a lack of teleaudiology implementation research in the US. Future work will include teleaudiology intervention studies, with particular focus on sub-groups who may benefit from a remote service delivery model due to access issues (e.g., racial/ethnic minorities, low-income individuals, and rural residents). Additionally, future studies are planned that continue to explore CHWs’ role on the hearing healthcare team, including within a teleaudiology service delivery model. Such implementation research is crucial for understanding the effectiveness of study outcomes in real-world contexts, translating evidence to practice, and for advancing equity in hearing healthcare.
V. CONCLUSION
Low-income, minority older adults in rural communities can face overlapping access barriers impacting their use of hearing aid services. Teleaudiology can minimize the geographic distance from clinician to patient. The Conexiones feasibility trial showed that CHWs, as patient-site facilitators, can help improve patient-related outcomes within teleaudiology hearing aid service delivery. Given that CHWs are an established public health workforce who work in disparity communities, combining teleaudiology with CHW support is a pragmatic and potentially scalable approach to expanding access to hearing care. Additional research is warranted to investigate how this approach can become institutionalized as part of routine practice (e.g., embedded in a community health clinic), as well as the efficacy of this model among different settings and among different populations. As this project has helped illustrate, CHW-supported teleaudiology-delivered hearing aid services were feasible and well-accepted by participants. Such strategies are needed to increase the number of older, rural, minority adults who obtain and use hearing aids, helping to improve hearing healthcare equity.
Supplementary Material
Acknowledgments
Community Health Workers were paid using SERTOMA grant funding, and student workers were paid using grant funding from the GPSC. The authors would like to thank the Community Health Workers for their contributions to this project, and more broadly for their dedication to the health and wellbeing of their community. We would also like to thank the undergraduate and graduate students who contributed to this research. This work is dedicated to the memory of Conchita Somoza.
Sources of Funding:
Research was supported by the National Institute on Deafness and Other Communication Disorders/National Institutes of Health (Coco: F32DC017081). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also obtained from the Arizona Community Foundation (ACF) and the University of Arizona Graduate & Professional Student Council (GPSC) and SERTOMA. No funding was received from Unitron for this trial. Hearing aids were purchased from the manufacturer at a bulk price and were provided to participants at no cost. Hearing aids were purchased using grant funding from ACF. Community Health Workers were paid using SERTOMA grant funding, and student workers were paid using grant funding from the GPSC.
Footnotes
Conflicts of Interest:
There are no conflicts of interest, financial, or otherwise.
REFERENCES
- Akilan R, Vidya R, & Roopa N. (2014). Perception of ‘mothers of beneficiaries’ regarding a rural community based hearing screening service. International Journal of Pediatric Otorhinolaryngology, 78(12), 2083–2088. 10.1016/j.ijporl.2014.09.009 [DOI] [PubMed] [Google Scholar]
- American Public Health Association (APHA): Community Health Workers. (2020). Community Health Workers. https://www.apha.org/apha-communities/member-sections/community-health-workers [DOI] [PubMed]
- Amieva H, Ouvrard C, Giulioli C, Meillon C, Rullier L, & Dartigues J-F (2015). Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: A 25-year study. Journal of the American Geriatrics Society, 63(10), 2099–2104. 10.1111/jgs.13649 [DOI] [PubMed] [Google Scholar]
- Andersen RM (1968). A behavioral model of families’s use of health services. In Research series: Center for Health Administration Studies. (p. 25). [Google Scholar]
- Andersen RM, Davidson PL, & Baumeister SE (2007). Changing the US health system: Key issues in health services policy and management (Andersen RM, Davidson PL, & Baumeister SE, Eds.; 3rd ed.). Jossey-Bass. [Google Scholar]
- Arnold ML, Hyer K, & Chisolm T.(2017). Medicaid Hearing Aid Coverage For Older Adult Beneficiaries: A State-By-State Comparison. Health Affairs, 36(8), 1476–1484. 10.1377/hlthaff.2016.1610 [DOI] [PubMed] [Google Scholar]
- Baciu A, Negussie Y, & Geller A.(2017). National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to health equity (Weinstein JN, Geller A, Negussie Y, & Baciu A, Eds.). National Academies Press. 10.17226/24624 [DOI] [PubMed] [Google Scholar]
- Bainbridge KE, & Ramachandran V.(2014). Hearing aid use among older United States adults: The National Health and Nutrition Examination Survey, 2005–2006 and 2009– 2010. Ear and Hearing, 35(3), 289–294. 10.1097/01.aud.0000441036.40169.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar H, Lee Rosenthal E, Nell Brownstein J, Rush CH, Matos S, & Hernandez L.(2011). Community Health Workers can be a public health force for change in the united states: Three actions for a new paradigm. American Journal of Public Health, 101(12), 2199–2203. 10.2105/AJPH.2011.300386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A.(1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- Bandura A.(1986). Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall. [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, & Czajkowski S.(2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH behavior change consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Biagio L, Swanepoel DW, Laurent C, & Lundberg T.(2014). Video-otoscopy recordings for diagnosis of childhood ear disease using telehealth at primary health care level. Journal of Telemedicine and Telecare, 20(6), 300–306. 10.1177/1357633X14541038 [DOI] [PubMed] [Google Scholar]
- Bisgaard N, & Ruf S.(2017). Findings From EuroTrak Surveys From 2009 to 2015: Hearing Loss Prevalence, Hearing Aid Adoption, and Benefits of Hearing Aid Use. American Journal of Audiology, 26(3S), 451–461. 10.1044/2017_AJA-16-0135 [DOI] [PubMed] [Google Scholar]
- Braveman P, Arkin E, Orleans T, Proctor D, Acker J, & Plough A.(2018). What is health equity? Behavioral Science & Policy, 4(1), 1–14. 10.1353/bsp.2018.0000 [DOI] [Google Scholar]
- Campos PD, & Ferrari DV (2012). Teleaudiology: Evaluation of teleconsultation efficacy for hearing aid fitting. J Soc Bras Fonoaudiol., 24(4), 301–308. 10.1590/S2179-64912012000400003 [DOI] [PubMed] [Google Scholar]
- Chan S, Hixon B, Adkins M, Shinn JB, & Bush ML (2017). Rurality and determinants of hearing healthcare in adult hearing aid recipients. The Laryngoscope, 127(10), 2362–2367. 10.1002/lary.26490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, & Vanryn M.(2008). Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of General Internal Medicine, 23(5), 561–566. 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W, & Lin FR (2012). Prevalence of hearing aid use among older adults in the United States. Archives of Internal Medicine, 172(3), 292–293. 10.1001/archinternmed.2011.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm TH, Johnson CE, Danhauer JL, Portz LJP, Abrams HB, Lesner S, McCarthy PA, & Newman CW (2007). A systematic review of health-related quality of life and hearing aids: Final report of the American Academy of Audiology task force on the health-related quality of life benefits of amplification in adults. Journal of the American Academy of Audiology, 18(02), 151–183. 10.3766/jaaa.18.2.7 [DOI] [PubMed] [Google Scholar]
- Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, & Carter C.(2012). Dialogues on diversifying clinical trials: Successful strategies for engaging women and minorities in clinical trials. Journal of Women’s Health, 21(7), 713–716. 10.1089/jwh.2012.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco L, Colina S, Atcherson SR, & Marrone N.(2017). Readability level of Spanish-language patient-reported outcome measures in audiology and otolaryngology. American Journal of Audiology, 26(3), 309–317. 10.1044/2017_AJA-17-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco L, Davidson A, & Marrone N.(2020). The role of patient-site facilitators in teleaudiology: A scoping review. American Journal of Audiology, 29(3S), 661–675. 10.1044/2020_AJA-19-00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco L, Piper R, & Marrone N.(2021). Feasibility of community health workers as teleaudiology patient-site facilitators: a multilevel training study. International Journal of Audiology, 60(9), 663–676. 10.1080/14992027.2020.1864487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco L, Sorlie Titlow K, & Marrone N.(2018). Geographic distribution of the hearing aid dispensing workforce: A teleaudiology planning assessment for Arizona. American Journal of Audiology, 27(3), 462–473. 10.1044/2018_AJA-IMIA3-18-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colina S, Marrone N, Ingram M, Sánchez D, & Sanchez D.(2016). Translation quality assessment in health research: A functionalist alternative to back-translation. Evaluation & the Health Professions, 40(3), 1–27. 10.1177/0163278716648191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD (1977). Detection of influential observation in linear regression. Technometrics, 19(1), 15–18. 10.1080/00401706.1977.10489493 [DOI] [Google Scholar]
- Cosh S, Helmer C, Delcourt C, Robins TG, & Tully PJ (2019). Depression in elderly patients with hearing loss: current perspectives. Clinical Interventions in Aging, 14, 1471–1480. 10.2147/CIA.S195824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzos S, Lea T, Mueller R, Murray R, & Culbong M.(2003). Effectiveness of ototopical antibiotics for chronic suppurative otitis media in Aboriginal children: a community-based, multicentre, double-blind randomised controlled trial. The Medical Journal of Australia, 179(4), 185–190. [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, & Nondahl DM (2003). The impact of hearing loss on quality of life in older adults. The Gerontologist, 43(5), 661–668. 10.1093/geront/43.5.661 [DOI] [PubMed] [Google Scholar]
- Eikelboom RH, Bennett RJ, Manchaiah V, Parmar B, Beukes E, Rajasingam SL, & Swanepoel DW (2022). International survey of audiologists during the COVID-19 pandemic: Use of and attitudes to telehealth. International Journal of Audiology, 61(4), 283–292. [DOI] [PubMed] [Google Scholar]
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, & Lancaster GA (2016). CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot and Feasibility Studies, 2(1), 64. 10.1186/s40814-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson LP, Job A, & Abraham V.(2013). Pilot study to evaluate hearing aid service delivery model and measure benefit using self-report outcome measures using community hearing workers in a developing country. ISRN Otolaryngology, 2013, 1–6. 10.1155/2013/973401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Robler SK, Gallo JJ, Wang N-Y, Labrique A, & Hofstetter P.(2019). Hearing Norton Sound: mixed methods protocol of a community randomised trial to address childhood hearing loss in rural Alaska. BMJ Open, 9(1), e023081. 10.1136/bmjopen-2018-023081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Robler SK, Wang N-Y, Labrique A, Gallo JJ, & Hofstetter P.(2019). Hearing Norton Sound: a community randomised trial protocol to address childhood hearing loss in rural Alaska. BMJ Open, 9(1), e023078. 10.1136/bmjopen-2018-023078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari DV, & Bernardez-Braga GRA (2009). Remote probe microphone measurement to verify hearing aid performance. Journal of Telemedicine and Telecare, 15(3), 122–124. 10.1258/jtt.2009.003005 [DOI] [PubMed] [Google Scholar]
- Fisher LD, Dixon DO, Herson J, Frankowski RF, Hearron MS, & Peace KE (1990). Intention to treat in clinical trials. In Dekker M.(Ed.), Statistical Issues in Drug Research and Development (pp. 331–350). 10.1201/9780203738610 [DOI] [Google Scholar]
- Foley M.(2019). Influential Points. R Pubs by Rstudio. https://rpubs.com/mpfoley73/501093 [Google Scholar]
- Folstein MF, Folstein SE, White T, & Messer MA (2010). Mini-Mental State Examination, 2nd edition. Psychological Assessment Resources. [Google Scholar]
- Glasgow RE, Vogt TM, & Boles SM (1999). Evaluating the public health impact of health promotion interventions: the RE-AIM framework. American Journal of Public Health, 89(9), 1322–1327. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goman AM, & Lin FR (2016). Prevalence of hearing loss by severity in the United States. American Journal of Public Health, 106(10), 1820–1822. 10.2105/AJPH.2016.303299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Attri J, Singh A, Kaur H, & Kaur G.(2016). Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi Journal of Anaesthesia, 10(3), 328. 10.4103/1658-354X.174918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden SM, Gaglio B, Shoup JA, Kinney KA, Johnson SB, Brito F, Blackman KCA, Zoellner JM, Hill JL, Almeida FA, Glasgow RE, & Estabrooks PA (2015). Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: A systematic review. Systematic Reviews. 10.1186/s13643-015-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D, Rochtchina E, Newall P, Golding M, & Mitchell P.(2010). Use of Hearing Aids and Assistive Listening Devices in an Older Australian Population. Journal of the American Academy of Audiology, 21(10), 642–653. 10.3766/jaaa.21.10.4 [DOI] [PubMed] [Google Scholar]
- Humes LE, Halling D, & Coughlin M.(1996). Reliability and stability of various hearing-aid outcome measures in a group of elderly hearing-aid wearers. Journal of Speech, Language, and Hearing Research, 39(5), 923–935. 10.1044/jshr.3905.923 [DOI] [PubMed] [Google Scholar]
- Humes LE, Kinney DL, Main AK, & Rogers SE (2019). A follow-up clinical trial evaluating the consumer-decides service delivery model. American Journal of Audiology. 10.1044/2018_AJA-18-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Rogers SE, Quigley TM, Main AK, Kinney DL, & Herring C.(2017). The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: A randomized double-blind placebo-controlled clinical trial. American Journal of Audiology, 26(1), 53–79. 10.1044/2017_AJA-16-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim JG, Chen M-H, Lipsitz SR, & Herring AH (2005). Missing-data methods for Generalized Linear Models. Journal of the American Statistical Association, 100(469), 332–346. 10.1198/016214504000001844 [DOI] [Google Scholar]
- Ingram M, Marrone N, Sanchez DT, Sander A, Navarro C, de Zapien JG, Colina S, Harris F, Sánchez DT, Sander A, Navarro C, de Zapien JG, Colina S, & Harris F.(2016). Addressing hearing health care disparities among older adults in a US-Mexico border community. Frontiers in Public Health, 4(169), 1–8. 10.3389/fpubh.2016.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MB (2005). Factors that influence outcomes from aural rehabilitation of older adults: The role of perceived self-efficacy (unpublished doctoral dissertation). University of Western Ontario, London, Canada. [Google Scholar]
- Jennings MB, Cheesman MF, & Laplante-Lévesque A.(2014). Psychometric properties of the Self-Efficacy for Situational Communication Management Questionnaire (SESMQ). Ear and Hearing, 35(2), 221–229. 10.1097/01.aud.0000441081.64281.b9 [DOI] [PubMed] [Google Scholar]
- Jilla AM, Johnson CE, & Huntington-Klein N.(2020). Hearing aid affordability in the United States. Disability and Rehabilitation: Assistive Technology, 1–7. 10.1080/17483107.2020.1822449 [DOI] [PubMed] [Google Scholar]
- Kokesh J, Ferguson AS, Patricoski C, Koller K, Zwack G, Provost E, & Holck P.(2008). Digital images for postsurgical follow-up of tympanostomy tubes in remote Alaska. Otolaryngology–Head and Neck Surgery, 139(1), 87–93. 10.1016/j.otohns.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Lancaster P, Krumm M, Ribera J, & Klich R.(2008). Remote hearing screenings via telehealth in a rural elementary school. American Journal of Audiology, 17(2), 114–122. 10.1044/1059-0889(2008/07-0008) [DOI] [PubMed] [Google Scholar]
- Lee S-YD, Stucky BD, Lee JY, Rozier RG, & Bender DE (2010). Short assessment of health literacy-Spanish and English: A comparable test of health literacy for Spanish and English speakers. Health Services Research, 45(4), 1105–1120. 10.1111/j.1475-6773.2010.01119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhemann U, & Sanders D.(2007). Community health workers: What do we know about them? https://www.who.int/hrh/documents/community_health_workers.pdf [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, & Ferrucci L.(2011). Hearing loss prevalence and risk factors among older adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 66A(5), 582–590. 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue Q-L, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM, & Health ABC Study Group, for the. (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173(4), 293. 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan J, Ostwald SK, & Ortiz M.(2007). Promotora diabetes intervention for Mexican Americans. The Diabetes Educator, 33(4), 660–670. 10.1177/0145721707304080 [DOI] [PubMed] [Google Scholar]
- Mamo SK, Nieman CL, & Lin FR (2016). Prevalence of untreated hearing loss by income among older adults in the United States. Journal of Health Care for the Poor and Underserved, 27(4), 1812–1818. 10.1353/hpu.2016.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone N, Ingram M, Somoza M, Jacob DS, Sánchez A, Adamovich S, & Harris FP (2017). Interventional audiology to address hearing health care disparities: Oyendo Bien pilot study. Seminars in Hearing, 38(2), 198–211. 10.1055/s-0037-1601575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone N, Nieman CL, & Coco L.(2022). Community-Based Participatory Research and Human-Centered Design Principles to Advance Hearing Health Equity. Ear & Hearing, 43(Suppl 1), 33S–44S. 10.1097/AUD.0000000000001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz K, Kibbe K, Preston E, Caballero A, Nelson L, White K, & Twohig M.(2017). Paediatric hearing aid management: a demonstration project for using virtual visits to enhance parent support. International Journal of Audiology, 56(2), 77–84. 10.1080/14992027.2016.1226521 [DOI] [PubMed] [Google Scholar]
- National Academies of Science Engineering and Medicine (NASEM). (2016). Hearing health care for adults: priorities for improving access and affordability (Blazer DG, Domnitz S, & Liverman CT, Eds.). National Academies Press. 10.17226/23446 [DOI] [PubMed] [Google Scholar]
- NIDCD. (2017). National Institute on Deafness and Other Communication Disorders (NIDCD) Strategic Plan 2017 – 2021. 2017–2021 Strategic Plan. https://www.nidcd.nih.gov/about/strategic-plan/2017-2021 [Google Scholar]
- Nieman CL, Marrone N, Mamo SK, Betz J, Choi JS, Contrera KJ, Thorpe RJ, Gitlin LN, Tanner EK, Han H-R, Szanton SL, & Lin FR (2017). The Baltimore HEARS pilot study: An affordable, accessible, community-delivered hearing care intervention. The Gerontologist, 57(6), 1173–1186. 10.1093/geront/gnw153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan J, Verkerk M, Winters N, Chadha S, & Bhutta MF (2019). The role of community health workers in addressing the global burden of ear disease and hearing loss: A systematic scoping review of the literature. BMJ Global Health, 4(2), 1–10. 10.1136/bmjgh-2018-001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hegarty M, Pederson LL, Thorne SL, Caraballo RS, Evans B, Athey L, & McMichael J.(2010). Customizing survey instruments and data collection to reach Hispanic/Latino adults in border communities in Texas. American Journal of Public Health, 100(S1), S159–S164. 10.2105/AJPH.2009.167338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusanya BO, & Akinyemi OO (2009). Community-based infant hearing screening in a developing country: Parental uptake of follow-up services. BMC Public Health, 9(1), 66. 10.1186/1471-2458-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrado EA, McQuiston C, & Flippen CA (2005). Participatory survey research. Sociological Methods & Research, 34(2), 204–239. 10.1177/0049124105280202 [DOI] [Google Scholar]
- Pearce W, Ching TYC, & Dillon H.(2009). A pilot investigation into the provision of hearing services using tele-audiology to remote areas. The Australian and New Zealand Journal of Audiology, 31(2), 96–100. [Google Scholar]
- Penteado S, Ramos S, Battistella L, Marone S, & Bento R.(2012). Remote hearing aid fitting: Tele-audiology in the context of Brazilian public policy. International Archives of Otorhinolaryngology, 16(03), 371–381. 10.7162/S1809-97772012000300012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry HB, Zulliger R, & Rogers MM (2014). Community health workers in low-, middle-, and high-income countries: An overview of their history, recent evolution, and current effectiveness. Annual Review of Public Health. 10.1146/annurev-publhealth-032013-182354 [DOI] [PubMed] [Google Scholar]
- President’s Council Advisors on Science and Technology (PCAST). (2015). Aging America & hearing loss: Imperative of improved hearing technologies. https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/PCAST/PCASThearingletterreport.pdf
- Pross SE, Bourne AL, & Cheung SW (2016). TeleAudiology in the Veterans Health Administration. Otology & Neurotology, 37(7), 847–850. 10.1097/MAO.0000000000001058 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Robler SK, Inglis SM, Gallo JJ, Parnell HE, Ivanoff P, Ryan S, Jenson CD, Ross A, Labrique A, Wang N-Y, & Emmett SD (2020). Hearing Norton Sound: Community involvement in the design of a mixed methods community randomized trial in 15 Alaska Native communities. Research Involvement and Engagement, 6(1), 67. 10.1186/s40900-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild SK, Martin MA, Swider SM, Tumialán Lynas CM, Janssen I, Avery EF, & Powell LH (2014). Mexican American trial of community health workers: A randomized controlled trial of a community health worker intervention for Mexican Americans with ttype 2 diabetes mellitus. American Journal of Public Health, 104(8), 1540–1548. 10.2105/AJPH.2013.301439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez D, Adamovich S, Ingram M, Harris FP, de Zapien J, Sánchez A, Colina S, & Marrone N.(2017). The potential in preparing Community Health Workers to address hearing loss. Journal of the American Academy of Audiology, 28(6), 562–574. 10.3766/jaaa.16045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GH, & Roughley A.(2020). Audiology in the time of COVID-19: practices and opinions of audiologists in the UK. International Journal of Audiology, September 2020, 1–8. 10.1080/14992027.2020.1814432 [DOI] [PubMed] [Google Scholar]
- Shrestha R, Baral K, & Weir N.(2001). Community ear care delivery by community ear assistants and volunteers: a pilot programme. The Journal of Laryngology & Otology, 115(11), 869–873. 10.1258/0022215011909314 [DOI] [PubMed] [Google Scholar]
- Stephenson J.(2021). Adding hearing, vision coverage to Medicare could address unmet needs at a modest cost. JAMA Health Forum, 2(12), e214858. 10.1001/jamahealthforum.2021.4858 [DOI] [PubMed] [Google Scholar]
- Suen JJ, Han H-R, Peoples CY, Weikert M, Marrone N, Lin FR, & Nieman CL (2021). A Community Health Worker Training Program to Deliver Accessible and Affordable Hearing Care to Older Adults. Journal of Health Care for the Poor and Underserved, 32(1), 37–49. 10.1353/hpu.2021.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States (US) Census: QuickFacts. (2010). https://www.census.gov/quickfacts/santacruzcountyarizona
- US Food & Drug Administration. (2018). FDA Reauthorization Act of 2017. [Google Scholar]
- Valente M, Abrams H, Benson D, Chisolm T, Citron D, & Hampton D.(2006). Guidelines for the audiologic management of adult hearing impairment. American Academy of Audiology. https://audiology-web.s3.amazonaws.com/migrated/haguidelines.pdf_53994876e92e42.70908344.pdf [Google Scholar]
- Wallerstein NB, & Duran B.(2006). Using community-based participatory research to address health disparities. Health Promotion Practice. 10.1177/1524839906289376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.