Abstract
Background:
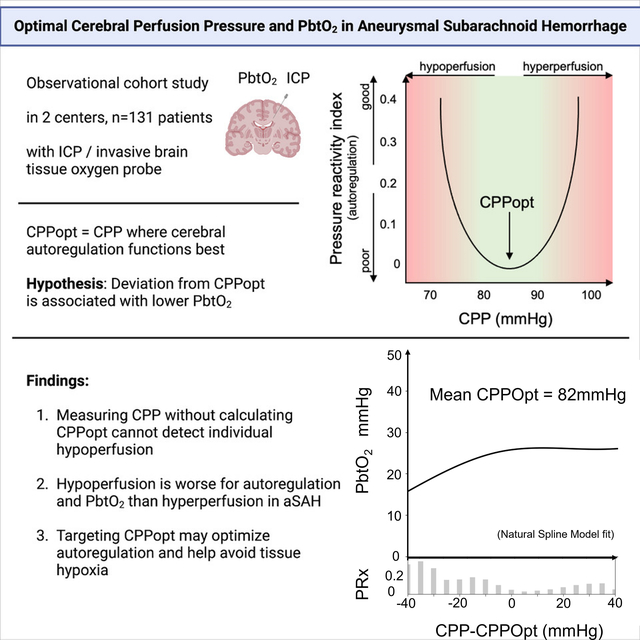
Targeting a cerebral perfusion pressure optimal for cerebral autoregulation (CPPopt) has been gaining more attention to prevent secondary damage after acute neurological injury. Brain tissue oxygenation (PbtO2) can identify insufficient cerebral blood flow and secondary brain injury. Defining the relationship between CPPopt and PbtO2 after aneurysmal subarachnoid hemorrhage (aSAH) may result in (1) mechanistic insights into whether and how CPPopt-based strategies might be beneficial, and (2) establishing support for the use of PbtO2 as an adjunctive monitor for adequate or ‘optimal’ local perfusion.
Methods:
We performed a retrospective analysis of a prospectively collected two-center dataset of patients with aSAH with or without later diagnosis of delayed cerebral ischemia (DCI). CPPopt was calculated as the CPP value corresponding to the lowest Pressure Reactivity index (PRx) (moving correlation coefficient of mean arterial and intracranial pressure). The relationship of (hourly) deltaCPP (CPP – CPPopt) and PbtO2 was investigated using natural spline regression analysis. Data after DCI diagnosis were excluded. Brain tissue hypoxia was defined as PbtO2 <20mmHg.
Results:
131 patients were included with a median of 44.0 [IQR 20.8–78.3] hourly CPPopt/PbtO2 datapoints. The regression plot revealed a non-linear relationship between PbtO2 and deltaCPP (p<0.001) with PbtO2 decrease with deltaCPP <0mmHg and stable PbtO2 with deltaCPP ≥0mmHg, although there was substantial individual variation. Brain tissue hypoxia (34.6% of all measurements) was more frequent with deltaCPP <0mmHg. These dynamics were similar in patients with or without DCI.
Conclusions:
We found a non-linear relationship between PbtO2 and deviation of patients’ CPP from CPPopt in aSAH patients in the pre-DCI period. CPP values below calculated CPPopt were associated with lower PbtO2. Nevertheless, the nature of PbtO2 measurements is complex and the variability is high. Combined multimodality monitoring with CPP/CPPopt and PbtO2 should be recommended to redefine individual pressure targets (CPP/CPPopt) and retain the option to detect local perfusion deficits during DCI (PbtO2), which cannot be fulfilled by both measurements interchangeably.
Graphical Abstract
Introduction
Functional cerebral autoregulation is paramount to compensate for the pathological cascades occurring after aSAH. Impairment of cerebral autoregulation typically precedes delayed cerebral ischemia (DCI) in patients with aneurysmal subarachnoid hemorrhage (aSAH)1–3 and information on autoregulation may be used to predict DCI4. It is yet unclear whether it can be used to direct therapy. Optimal cerebral perfusion pressure (CPPopt) is the value of cerebral perfusion pressure (CPP) at which autoregulation is best5–8. In studies on traumatic brain injury (TBI) patients, from where the concept of CPPopt was derived, the difference of CPP and CPPopt (deltaCPP) is an even better predictor of outcome than deviation from a universal guideline-driven CPP target range8. Targeting CPPopt may also be beneficial in aSAH patients to improve autoregulation and avoid both episodes of too low (hypoperfusion) and too high (hyperemia) cerebral blood flow (CBF). However, basic clinical research on the utility of CPPopt in aSAH is scarce.
Monitoring for complications such as DCI in comatose aSAH patients is currently based on several options of neuromonitoring, including the invasive measurement of partial pressure of brain tissue oxygenation (PbtO2). Measuring PbtO2 is recommended in the current consensus statement for multimodality neuromonitoring and its implementation has been associated with improved clinical outcomes9–11. Episodes with poor oxygenation can be associated with complications such as cerebral ischemia/infarction, breakdown of cellular metabolism, worsening of neurological outcome12,13. The relationship of CPPopt and PbtO2 has not been documented so far. Characterizing their interplay is interesting to better understand the impact of deviations from CPPopt on cerebral physiology and, in turn, the potential benefit of CPPopt based management strategies. We hypothesized that PbtO2 correlates with the deviation from CPPopt, with (1) lowest PbtO2 values (focal cerebral hypoxia) with large negative deviation, (2) higher PbtO2 values with actual CPP close to CPPopt, and (3) highest PbtO2 values (focal cerebral hyperoxia) with large positive deviation, in correspondence to the suspected autoregulation curve. This relationship may differ between patients with and without DCI. In patients with DCI, where autoregulation is expected to be worse than in patients without DCI2, PbtO2 may react more strongly to deviations from CPPopt.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
Two patient cohorts were combined (NewYork - Presbyterian Hospital - Columbia University Irving Medical Center (CUIMC), USA, from 2007 to 202114 and Rheinisch-Westfälische Technische Hochschule Aachen (AU), Germany, from 2014–2021). Data collection for the prospective Subarachnoid Hemorrhage Outcomes Project (SHOP) at CUIMC has been described previously14. Relevant data on demography, clinical course and high-frequency vital signs from patients at AU were included into a prospective databank, including scheduled follow-up visits or telephone assessments of clinical outcome at 3, 6 and 12 months after discharge15. Prospective data collection was approved by institutional review boards in both centers and informed consent was given by all patients or their representatives before study inclusion. Patients with non-aneurysmal SAH, arteriovenous malformation-associated aneurysms and patients < 18 years of age were excluded. All patients with at least 12 hours of corresponding PbtO2 and CPPopt measurements were included in analysis. The study was reported according to the STROBE criteria16.
Treatment algorithm
Treatment followed the American Heart Association guidelines17 at CUIMC, while AU followed the European Stroke Organization guidelines18, without major differences in standard operating procedures. All patients were treated in neurological or neurosurgical intensive care units. Patients with Hunt and Hess grades (HH) 3–5 and Glasgow Coma Scale ≤ 8 at CUIMC and patients with HH grades 3–5 or modified Fisher scale (mFS) 3–4 at AU were considered at high risk for DCI and received invasive neuromonitoring, unless early mortality was anticipated. At CUIMC, separate ICP and PbtO2 probes (ICP: Camino System, Integra Neurosciences, Plainsboro, NJ, USA; PbtO2: Licox, Integra, Plainsboro, NJ) were placed according to a previously published institutional protocol,19 while in patients at AU, a combined ICP-PbtO2-probe was placed (Neurovent PTO, Raumedic, Helmbrechts, Germany). Probes were placed unilaterally into the watershed zone between anterior and middle cerebral artery territories in the frontal lobe expected to be affected most (side of aneurysm or dominant blood distribution) or on the right side for midline aneurysms and symmetrical blood distribution. Monitoring was aligned to recommendations from the Neurocritical Care Society and the European Society of Intensive Care Medicine, and local guidelines9,20,21. All patients received arterial lines zeroed at the phlebostatic axis for ABP monitoring. Digital physiologic data were from General Electric Solar 8000i monitors (Milwaukee, WI) and acquired at a sampling frequency of 240 Hz using data acquisition system (CUIMC: BedmasterEX (Excel Medical Electronics, Jupiter, FL), AU: Moberg Component Neuromonitoring Systems (Moberg Research, Inc, Ambler, PA)). From 2012 until 2021 at CUIMC, digital physiologic data were from Philips Intellivue monitors (Amsterdam, Netherlands) and acquired at a sampling frequency of 125 Hz using data acquisition systems (BedmasterEX from 2012 until 2014, ICM+ (Cambridge Enterprise, UK) from 2014 until 2019, Philips Data Warehouse Connect from 2019 until 2021).
Outcome definition
The goal of the study was to assess the relationship between PbtO2 and deltaCPP which was additionally split between patients with and without DCI. Brain tissue hypoxia (BTH) was defined as hourly measurements with PbtO2 < 20mmHg. DCI was defined in both centers as a ≥2-point change in Glasgow Coma Scale or new focal neurological deficit lasting for >1 hour and not associated with surgical treatment, or a new cerebral infarct on brain imaging that is not attributable to any other causes17. AU additionally defined a territorial or watershed deficit on CT perfusion as DCI. CT perfusion was conducted when triggered by a worsening in neuromonitoring results or at individual elective time points in comatose patients. The large window for DCI onset or presentation makes direct comparison across patients over time challenging. To address this, we used the day of bleed as the temporal anchor to align the monitoring data and removed the data post DCI diagnosis to compare the physiologic monitoring values of two outcomes while avoiding potential influences of DCI treatment on the physiology. To avoid unbalanced or disparate data time frames of patients with and without DCI, data of patients without DCI were included only until the mean time of DCI diagnosis (i.e., until day 7).
Data Processing and Analysis
The following baseline characteristics and grading scales were prospectively recorded at admission: age, sex, history of hypertension, HH grade and mFS. Clinical outcome was assessed six months after discharge using the modified Rankin Scale (mRS) via in-person and/or telephone interviews. Artifact-free segments of PbtO2, ABP, and ICP data were manually identified at the two centers. The immediate time period following PbtO2 probe placement up until completed calibration was evaluated by two reviewers independently from each other, and excluded from analysis. Later artifacts were noted to typically result from patient monitor disconnections for nursing care, malfunctioning equipment, or transport off the unit.
Calculation of measures of autoregulation
Cerebrovascular pressure reactivity was calculated using the pressure reactivity index (PRx)6,22. ICP and ABP were time-averaged over 10-second intervals. The PRx was then computed as a Pearson correlation coefficient calculated over a 5-minute moving window (with 80% overlap) between slow changes in ICP and spontaneous fluctuations in ABP5,22. The CPP value at which the lowest value of PRx is experienced in a period of time is considered the ‘optimal’ CPP (CPPopt)5. To calculate CPPopt, 5-minute median CPP values were divided into 16 bins spanning 5mmHg. A parabolic curve was applied and the CPP bin with the lowest PRx value was recorded as the CPPopt. This value was updated every minute and trended based on a moving 4-hour window. A threshold of 50% was set in order to generate a curve to calculate CPPopt (≥50% PRx values within time window must be present). DeltaCPP was calculated as the patients’ actual CPP minus CPPopt every minute.
Statistical Analysis
We plotted PbtO2, CPP, PRx and BTH over binned deltaCPP for all patients and patients with and without diagnosis of DCI in follow-up (Figure 1B–D). Because of the non-linear relationship between PbtO2 and deltaCPP we modelled this relationship using nonlinear regression using natural splines (Figure 1A) 23. For this, we used the generalized linear model and fit the natural splines using python statsmodel library 24. Wilcoxon rank sum test was used for comparing continuous variable and Chi square test was used for comparing categorical variables. Statistical significance was assumed at p < 0.05. Data processing and analysis were performed using MATLAB (MATLAB and Statistics Toolbox Release 2015a, The Mathworks, Inc., Natick, MA, USA), Python (Python Software Foundation, https://www.python.org/), and ICM+® (Cambridge Enterprise, Cambridge, UK).
Figure 1.
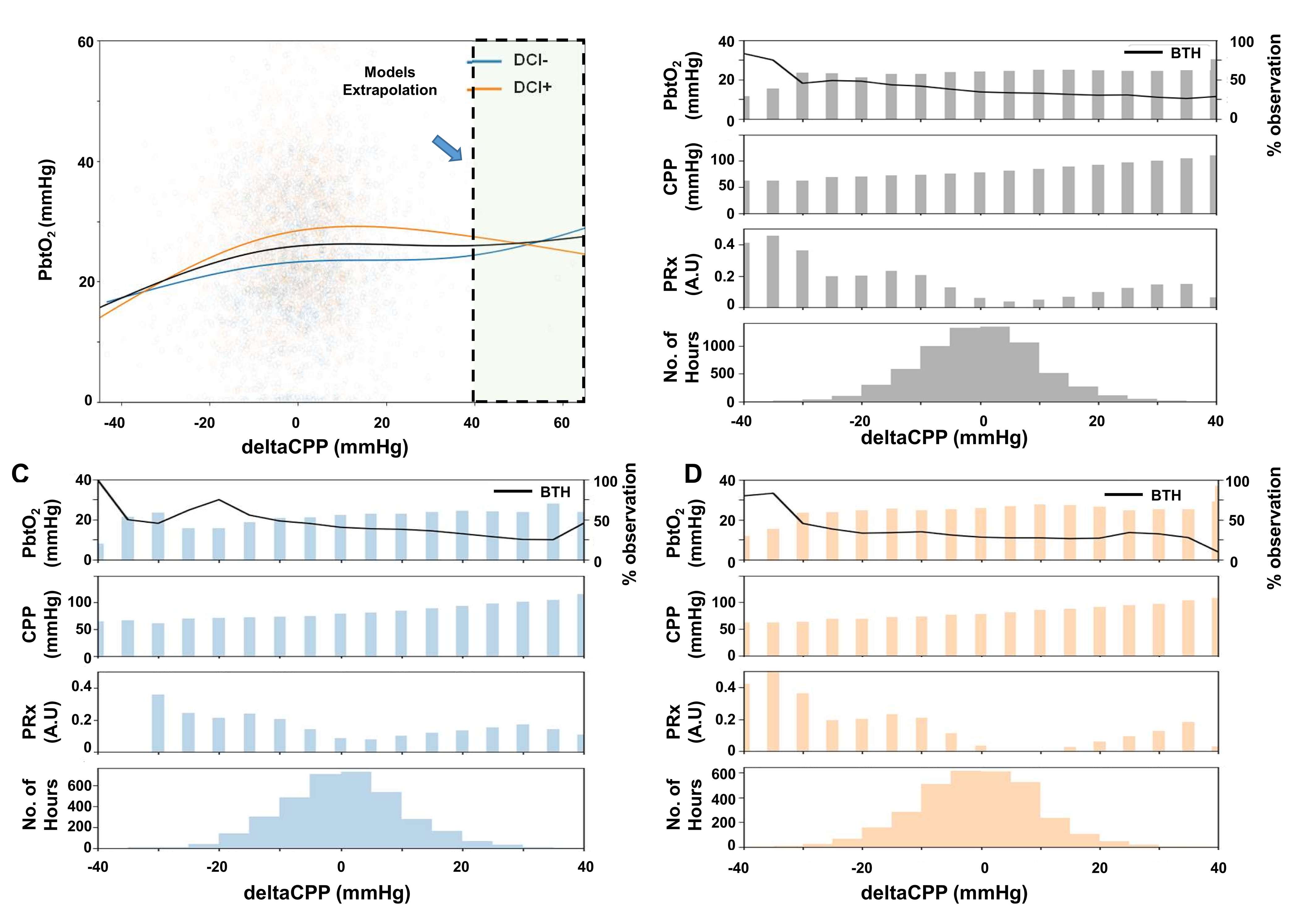
The relationship between PbtO2 and deltaCPP. A: Natural spline regression analysis of hourly deltaCPP and PbtO2 pairs. B: PbtO2, CPP, PRx values and data availability separated by 5mmHg deltaCPP bins. Black line indicates the proportion of brain tissue hypoxia (BTH) from all measurements within a bin. C+D: Data separated by patients without DCI (C) or with (D) DCI.
Results
There were 1233 (CUIMC: n=990; AU: n=243) aSAH patients of which 265 (CUIMC: n=140; AU: n=125) had continuous neuromonitoring data available. 131 patients (CUIMC: n=52, AU: n=79) fit the inclusion criteria (n=134 excluded without data before DCI before day 7 (mean time of DCI occurrence) or with <12 combined CPPopt / PbtO2 datapoints). The median age was 54 [IQR 46.5–64.0] years, n=94 (71.8%) female, median HH 4 [IQR 3–5] and median mFS 3 [IQR 2–4] (Table 1). DCI was diagnosed in n=64 (48.9%) patients with invasive neuromonitoring in total, with n=16 (30.8%) patients at CUIMC and n=48 (60.8%) patients at AU. DCI was diagnosed 7.2±3.3 days after hemorrhage. In 26 of 45 (57.8%) patients in the AU cohort who were diagnosed with DCI via CT perfusion, the ICP-PbtO2-probe was located within the hypoperfused territory at time of DCI diagnosis, according to previously established cut-offs25. Age and mFS were signficantly different (p<0.05) between patients with and without DCI (Table 1). There were no statistical differences in physiological data values between patients with and without DCI (Table 2). Out of 16.614 hours of physiological data, both deltaCPP and PbtO2 were present simulateously in 7806 (47.0%) hours, of which 4449 (57.0%) hours were recorded within the first seven days after hemorrhage and included for analysis (44.0 [20.6–78.3] per patient).
Table 1.
Comparison of patient characteristics
| n=131 | DCI+ n=64 | DCI− n=67 | p value* | |
|---|---|---|---|---|
| Age, median (IQR) | 54 [46.5–64.0] | 44.0[51.0–60.0] | 49.0[59.0–65.0] | 0.008 |
| Female Sex, n (%) | 94(71.76) | 44(72.1) | 47(72.3) | 0.869 |
| MFS, n (%) | 0.047 | |||
| 1 | 29(23.02) | 11(17.19) | 18(26.87) | |
| 2 | 26(20.63) | 9(14.06) | 17(25.37) | |
| 3 | 33(26.19) | 17(26.56) | 18(26.87) | |
| 4 | 35(27.78) | 26(40.63) | 12(17.91) | |
| HH, n (%) | 0.589 | |||
| 1 | 5(3.82) | 3(4.92) | 2(2.99) | |
| 2 | 14(10.69) | 8(13.11) | 6(8.96) | |
| 3 | 31(23.66) | 13(21.31) | 18(26.87) | |
| 4 | 47(35.88) | 25(40.98) | 21(31.34) | |
| 5 | 34(25.95) | 12(19.67) | 20(29.85) | |
| MRS (3 months), n (%) | 0.075 | |||
| 1 | 7(5.34) | 5(7.46) | 2(3.13) | |
| 2 | 16(12.21) | 10(14.93) | 6(9.38) | |
| 3 | 15(11.45) | 10(14.93) | 5(7.81) | |
| 4 | 19(14.5) | 6(8.96) | 13(20.31) | |
| 5 | 21(16.03) | 7(10.45) | 14(21.88) | |
| 6 | 41(31.3) | 23(34.33) | 18(28.13) |
considered significant if p<0.05; DCI+: patients with DCI; DCI-: patients without DCI; IQR: Interquartile range; MFS: Modified Fisher Scale; HH: Hunt and Hess grade; MRS: Modified Rankin Scale
Table 2.
Comparison of physiological data within the first seven days after hemorrhage
| All | DCI+ | DCI− | p value | |
|---|---|---|---|---|
| PbtO2 | 24.12[18.16–32.35] | 23.41[16.64–29.6] | 24.62[18.61–33.97] | 0.325 |
| ABP | 92.53[86.41–101.7] | 93.99[86.69–104.57] | 90.33[85.87–99.02] | 0.263 |
| ICP | 7.89[5.88–11.26] | 8.66[5.8–12.78] | 7.61[6.0–9.78] | 0.338 |
| CPP | 82.69[75.55–94.67] | 82.62[75.36–96.93] | 83.03[76.48–94.03] | 0.680 |
| CPPopt | 82.69[76.97–91.99] | 81.85[76.21–91.99] | 83.73[78.6–91.8] | 0.667 |
| deltaCPP | −0.13[−3.03–3.0] | −0.13[−3.04–3.83] | −0.16[−3.03–1.8] | 0.474 |
| PRx | 0.07[−0.06–0.21] | 0.12[0.0–0.24] | 0.04[−0.09–0.16] | 0.086 |
DCI+: patients with DCI; DCI−: patients without DCI; ABP: Arterial blood pressure; ICP: Intracranial pressure; CPP: Cerebral perfusion pressure; CPPopt: Optimal cerebral perfusion pressure; deltaCPP: CPP-CPPopt; PRx: Pressure reactivity index
Median CPPopt was 81.8 [76.8–90.5] mmHg and median PbtO2 was 23.6 [18.7–31.8] mmHg. Spline regression demonstrated that there was a non-linear relationship between PbtO2 and deltaCPP (p<0.001) (Table S1). Visual inspection of the regression plot (Figure 1A) showed that the curve may be divided into (at least) two segments, with increase of PbtO2 with increase of deltaCPP until deltaCPP approximates 0mmHg and with relatively stable PbtO2 values with deltaCPP ≥0mmHg. This dynamic was observed regardless of the occurrence of DCI later on. PbtO2 with deltaCPP <0mmHg (23.6 [16.5–32.7] mmHg) was significantly different to deltaCPP ≥0mmHg (25.0 [17.9–33.84] mmHg, p<0.001). Mean PRx was significantly higher with deltaCPP <0mmHg (0.11 [−0.09–0.33]) than with ≥0mmHg (0.06 [−0.13–0.26], p<0.0001).
BTH (PbtO2 <20mmHg) was noted in n=1542 (34.7%) measurements in total. Of these, n=843 (54.7%) and n=699 (45.3%) were observed with deltaCPP <0mmHg or ≥0mmHg, respectively. When separating the proportion of BTH by 5mmHg deltaCPP bins, BTH episodes were more frequent with decreasing deltaCPP (Figure 1B).
Discussion
Cerebral autoregulation has historically been described as a triphasic relationship (Lassen’s curve), in which CBF is maintained stable between certain blood pressure limits, but this concept has been challenged recently26,27. A quadriphasic theory of autoregulation was recently proposed by Klein et al. from experimental data, who documented two upper limits of autoregulation, owing to the separate reactions of smaller and larger arterioles27. Our hypothesis was that the reaction of cohort PbtO2 could delinate the functionality of autoregulation. Our results support a non-linear relationship between PbtO2 and the deviation of actual patients’ CPP from CPPopt (deltaCPP) in aSAH patients. However, mainly two phases can be observed in our data: PbtO2 appears to be most stable when CPP is close to or above CPPopt, while CPP below CPPopt corresponds to decreasing PbtO2 values. Mean PbtO2 was slightly but significantly lower with deltaCPP <0mmHg. With very low deltaCPP (< −30mmHg), episodes of brain tissue hypoxia become more frequent. Conversely, positive deltaCPP does not lead to further increase of PbtO2. The natural spline regression model predicts this upwards trend for deltaCPP values > +40mmHg. However, deltaCPP values in this range were rarely reached in our cohort, therefore this trend must be interpreted as a purely mathematical extrapolation. A third (or fourth) phase of autoregulation therefore cannot be observed in our data.
A possible explanation is the effort to maintain physiological CPP values in patients (observational study) while experimental settings can actively explore the reaction to more extreme pressure values 27. A CPP challenge would be necessary to determine individual limits of autoregulation and therefore, the individual range that CPP may deviate from CPPopt before PbtO2 begins to decrease or increase more passively. Overall, the autoregulation curve may be shifted towards higher CPP values in aSAH patients but with lower total CBF, increasing the chance to encounter hypoxia at lower CPP values and to observe the lower limit of autoregulation in the PbtO2/deltaCPP relationship, but not the upper limit of autoregulation 25,28,29. Treatment phases with induced hypertension may depict a third phase more clearly, but were excluded from this analysis to avoid iatrogenic influence on CPPopt calculations. In theory, CBF (and PbtO2 to some extent) may increase further during hypertension, if the upper limit of autoregulation is exceeded. This represents the rationale for treating DCI with induced hypertension, for which conflicting data have been reported. Gathier et al. found that the overall CBF in the HIMALAIA trial (Hypertension induction in the management of aneurysmal subarachnioid haemorrhage with secondary ischaemia) did not increase during induced hypertension, but the region of interest with the lowest pre-treatment CBF increased substantially, albeit not significantly (p=0.05) 30,31. In contrast, in a setting with induced hypertension and vasodilation together, we found that a higher pressure target (>180mmHg systolic) corresponded to even lower PbtO2 values than a lower pressure target (>120mmHg systolic)32. As a future prospect, analyzing the relationship of deltaCPP and PbtO2 during phases with induced hypertension may delineate an upper limit of autoregulation, and give insight into the oxygen-plus that can or cannot be expected by hypertensive treatment.
It must also be acknowledged that autoregulation refers to the relationship of perfusion pressure and cerebral flow, for which PbtO2 is only an imperfect surrogate and may therefore not reflect the autoregulation curve entirely. PbtO2 measurements are influenced by other factors beyond insufficient CBF such as oxygen diffusion depending on arterial and venous oxygen tensions, oxygen consumption or capillary blood distribution, which may be severely disturbed after aSAH 33–37. Furthermore, PbtO2 is a local marker and may be noninformative for distal areas of impaired autoregulation or mismatched perfusion. Regional differences in autoregulation have recently been found in patients with malignant stroke, therefore it is conceivable that both PbtO2 and CPPopt values (by the current method of calculation) may differ in areas with intact or disturbed autoregulation38. These may be reasons that limits of autoregulation could not be detected as clearly in our data. Nevertheless, detecting episodes of brain tissue hypoxia remains interesting as an adjunctive parameter to monitoring of CPP/CPPopt, as it can aid the diagnosis of DCI and estimate the efficacy of DCI treatment32. Active measures to improve PbtO2 such as increasing CPP are also under investigation as they may have a positive impact on long-term neurological outcome39,40.
Generally poorer autoregulation has been described in patients with DCI versus those without DCI and autoregulation may be used to predict DCI 41. We hypothesized that patients with DCI could therefore be more strongly dependent on a deltaCPP close to zero to maintain good PbtO2. Our data did not support this hypothesis. The two-phasic PbtO2 development with increasing deltaCPP was similar between groups. Mean PbtO2 and PRx were comparable during the analyzed time frame. We have recently demonstrated that autoregulation may deteriorate significantly only several hours before DCI, similarly to PbtO225,42. Such short-term differences may not be captured in our comparatively longer data time frame, while comparison of selected time frames around DCI to patients without DCI is difficult as there is no corresponding DCI event to match data to.
The range of CPP values which is optimal as determined by PRx values has been discussed primarily in TBI patients with ICP monitoring. There is most consensus for a recommendation of maintaining deltaCPP +/−5mmHg which was the target of the COGITATE trial (feasibility and safety trial to guide cerebral perfusion pressure according to CPPopt goals in traumatic brain injury)43. It is not yet proven that CPPopt can be used for this purpose in aSAH. CPPopt may be too blunt a target when regional differences in autoregulation impairment and/or perfusion exist with DCI. Differences between TBI and aSAH may require a different strategy for CPPopt calculation and the resulting management, including a reinvestigation of the +/−5mmHg optimal range around the target in aSAH patients 44. If interpreting PbtO2 as a surrogate for adequate CBF and considering only PbtO2, our results would suggest that the range for CPPopt may be much wider in aSAH, as decrease of PbtO2 <20mmHg on cohort level was only observed with deltaCPP < −20mmHg. However, our data also indicate that hyperperfusion with positive deltaCPP may be tolerated better than hypoperfusion in aSAH patients. Besides a progressive decrease of PbtO2 with negative deltaCPP, worsening of PRx values was also more progressive with negative deltaCPP as compared to positive deltaCPP values. The U-shaped curve in TBI patients similarly depicts a steeper increase of PRx with deltaCPP below CPPopt than with positive deltaCPP8, but the overall CPPopt recommendation in TBI patients is significantly lower and complications from hyperperfusion such as cerebral edema or seizures are feared more quickly in patients with TBI than with aSAH43,45. Avoiding negative deltaCPP could be more important than avoiding positive deltaCPP in aSAH, which may have to be considered when a CPPopt target is defined in aSAH patients.
The CPP values that corresponded to low deltaCPP values and hypoxia were not necessarily pathological according to the current empirical recommendation that only defines a treatment floor of CPP >60–70mmHg46,47. Thus, if CPPopt is not calculated, many of these CPPs are accepted as normal and not intervened for despite the potential of improving PbtO240. Similar relationships of CPPopt or optimal ABP and PbtO2 were determined in patients with traumatic brain injury and hypoxic-ischemic brain injury48,49. As we observed a large variability of absolute PbtO2 values in relation to deltaCPP, we recommend monitoring both CPP/CPPopt and PbtO2, as they give adjunctive information: CPP/CPPopt may help determine an individual pressure target (range or lower limit), while PbtO2 is most valuable to detect hypoperfusion during DCI.
Limitations
The advantage of a larger case number achieved by combining two cohorts was weighted against the disadvantage of potential confounders. We are not aware of major differences in diagnosis and treatment procedures between both centers but unknown differences cannot be ruled out completely. DCI was defined differently in the two centers owing to the limitations of the current DCI definition which is based primarily on the clinical diagnosis in awake patients50. Both centers defined DCI according to Vergouwen et al. as clinical deterioration with an additional imaging basis (cerebral infarction at CU; perfusion delay at AU). CT perfusion is frequently used as another basis for DCI diagnosis as most pathomechanisms assumed to be involved in DCI may result in hypoperfusion measurable by CT perfusion51. Assessment of brain tissue oxygen relied on local, invasive measurements which are dependent on type of probe, location, and site of occurrence of DCI. CUIMC used Licox probes while AU placed Raumedic probes which can be associated with slightly deviating measurements52–54. Independent of probe type, potential differences between patients with and without DCI may be diluted by measurements from DCI patients in whom DCI occurred in territories not covered by the probe. Alternative methods of calculating autoregulation by transcranial doppler velocities or near-infrared spectroscopy are under investigation but are not available for our cohort55,56. Finally, the detection of autoregulatory thresholds were made on cohort level, which does not vice versa allow conclusions on an individual patient level.
Conclusion
PbtO2 and deltaCPP have a non-linear relationship with CPP close to or above CPPopt associated with stable PbtO2 values while CPP below CPPopt exhibits PbtO2 decrease. Calculating CPPopt may be more informative than CPP alone, as it helps identify these alterations with CPP in normal boundaries. Owing to the high variability and the manifold influences on PbtO2, additional PbtO2 monitoring may yield complementary information to detect hypoxia as part of DCI and guide rescue treatment.
Supplementary Material
Acknowledgements
The graphical abstract was created with biorender.com.
Disclosures
Marcel Aries is supported by the non-profit organization BrainBattle Foundation (HersenStrijd). Dr. Park discloses a grant by the National Institutes of Health (R21NS113055), which was not used for the funding of this study. Dr. Megjhani discloses a grant by American Heart Association grant number (20POST35210653). Dr. Agarwal discloses a grant from the National Institutes of Health (R01 HL153311), which was not used for the funding of this study. Dr. Claassen discloses stock in iCE Neurosystems and consulting for Marinus Pharmaceuticals, products that are not related to the study. Dr. Claassen discloses grants from the James S. McDonnell Foundation and the National Institutes of Health (R01 NS106014), which were not used for the funding of this study. Dr. Roh discloses grants from the National Institutes of Health (K23 HL151901) and the National Blood Foundation, which were not used for the funding of this study.
Sources of funding
This study was funded by American Heart Association (Grant Number 20POST35210653 [MM]).
List of abbreviations
- ABP
arterial blood pressure
- AU
RWTH Aachen University (Rheinisch-Westfälische Technische Hochschule Aachen)
- aSAH
aneurysmal subarachnoid hemorrhage
- CPP
cerebral perfusion pressure
- CPPopt
optimal cerebral perfusion pressure
- CUIMC
Columbia University Irving Medical Center
- DCI
delayed cerebral ischemia
- HH
Hunt and Hess
- ICP
intracranial pressure
- iHTN
induced hypertension
- mFS
modified Fisher Scale
- mRS
modified Rankin Scale
- PbtO2
brain tissue oxygenation
- PRx
pressure reactivity index
- SHOP
Subarachnoid Hemorrhage Outcomes Project
Footnotes
Supplemental Material
References
- 1.Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29:158–163. [DOI] [PubMed] [Google Scholar]
- 2.Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, Pickard JD, Kirkpatrick PJ. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788 [DOI] [PubMed] [Google Scholar]
- 3.Jaeger M, Soehle M, Schuhmann MU, Meixensberger J. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:2097–2101. doi: 10.1161/STROKEAHA.112.659888 [DOI] [PubMed] [Google Scholar]
- 4.Megjhani M, Weiss M, Kwon SB, Ford J, Nametz D, Kastenholz N, Fogel H, Velazquez A, Roh D, Agarwal S, et al. Vector Angle Analysis of Multimodal Neuromonitoring Data for Continuous Prediction of Delayed Cerebral Ischemia. Neurocrit Care. 2022. doi: 10.1007/s12028-022-01481-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17; discussion 17–19. doi: 10.1097/00006123-199707000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, Hiler M, Balestreri M, Kirkpatrick PJ, Pickard JD, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurgical focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.10.E2 [DOI] [PubMed] [Google Scholar]
- 7.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–738. [DOI] [PubMed] [Google Scholar]
- 8.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6 [DOI] [PubMed] [Google Scholar]
- 9.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care : a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1189–1209. doi: 10.1007/s00134-014-3369-6 [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishna R, Stiefel M, Udoetuk J, Spiotta A, Levine JM, Kofke WA, Zager E, Yang W, Leroux P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109:1075–1082. doi: 10.3171/JNS.2008.109.12.1075 [DOI] [PubMed] [Google Scholar]
- 11.Veldeman M, Albanna W, Weiss M, Conzen C, Schmidt TP, Schulze-Steinen H, Wiesmann M, Clusmann H, Schubert GA. Invasive neuromonitoring with an extended definition of delayed cerebral ischemia is associated with improved outcome after poor-grade subarachnoid hemorrhage. J Neurosurg. 2020:1–8. doi: 10.3171/2020.3.JNS20375 [DOI] [PubMed] [Google Scholar]
- 12.Burzynska M, Uryga A, Kasprowicz M, Czosnyka M, Dragan B, Kubler A. The relationship between the time of cerebral desaturation episodes and outcome in aneurysmal subarachnoid haemorrhage: a preliminary study. J Clin Monit Comput. 2019. doi: 10.1007/s10877-019-00377-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Clinical cerebral microdialysis: brain metabolism and brain tissue oxygenation after acute brain injury. Neurol Res. 2001;23:801–806. doi: 10.1179/016164101101199379 [DOI] [PubMed] [Google Scholar]
- 14.Witsch J, Frey HP, Patel S, Park S, Lahiri S, Schmidt JM, Agarwal S, Falo MC, Velazquez A, Jaja B, et al. Prognostication of long-term outcomes after subarachnoid hemorrhage: The FRESH score. Ann Neurol. 2016;80:46–58. doi: 10.1002/ana.24675 [DOI] [PubMed] [Google Scholar]
- 15.Weiss M, Conzen C, Mueller M, Wiesmann M, Clusmann H, Albanna W, Schubert GA. Endovascular Rescue Treatment for Delayed Cerebral Ischemia After Subarachnoid Hemorrhage Is Safe and Effective. Front Neurol. 2019;10:136. doi: 10.3389/fneur.2019.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke; a journal of cerebral circulation. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 18.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, Organization ES. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112. doi: 10.1159/000346087 [DOI] [PubMed] [Google Scholar]
- 19.Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, Lee K, Badjatia N, Hirsch LJ, Connolly ES, et al. Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care. 2010;12:188–198. doi: 10.1007/s12028-010-9330-9 [DOI] [PubMed] [Google Scholar]
- 20.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy G, Diringer MN, Stocchetti N, Videtta W, Armonda R, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21 Suppl 2:S282–296. doi: 10.1007/s12028-014-0077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE, Lee K, Badjatia N, Connolly ES, Mayer SA. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397–410; discussion 410–391. doi: 10.1227/01.NEU.0000338946.42939.C7 [DOI] [PubMed] [Google Scholar]
- 22.Megjhani M, Terilli K, Martin A, Velazquez A, Claassen J, Roh D, Agarwal S, Smieleweski P, Boehme A, Schmidt JM, et al. Deriving PRx and CPPopt from 0.2 Hz data: Establishing Generalizability to Bedmaster Users. Acta Neurochir Suppl. 2017;Proceedings of Intracranial Pressure & Neuromonitoring XVI. [DOI] [PubMed] [Google Scholar]
- 23.Hastie T, Tibshirani R, Friedman J, Franklin J. The elements of statistical learning: data mining, inference and prediction. The Mathematical Intelligencer. 2005;27:83–85. [Google Scholar]
- 24.Seabold S, Perktold J. Statsmodels: Econometric and statistical modeling with python. Paper/Poster presented at: Proceedings of the 9th Python in Science Conference; 2010; [Google Scholar]
- 25.Weiss M, Albanna W, Conzen C, Megjhani M, Tas J, Seyfried K, Kastenholz N, Veldeman M, Schmidt TP, Schulze-Steinen H, et al. Optimal Cerebral Perfusion Pressure During Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Crit Care Med. 2022;50:183–191. doi: 10.1097/CCM.0000000000005396 [DOI] [PubMed] [Google Scholar]
- 26.Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein SP, De Sloovere V, Meyfroidt G, Depreitere B. Differential Hemodynamic Response of Pial Arterioles Contributes to a Quadriphasic Cerebral Autoregulation Physiology. J Am Heart Assoc. 2021:e022943. doi: 10.1161/JAHA.121.022943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svedung Wettervik T, Howells T, Lewen A, Ronne-Engstrom E, Enblad P. Temporal Dynamics of ICP, CPP, PRx, and CPPopt in High-Grade Aneurysmal Subarachnoid Hemorrhage and the Relation to Clinical Outcome. Neurocrit Care. 2021. doi: 10.1007/s12028-020-01162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lidington D, Wan H, Bolz SS. Cerebral Autoregulation in Subarachnoid Hemorrhage. Front Neurol. 2021;12:688362. doi: 10.3389/fneur.2021.688362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gathier CS, Dankbaar JW, van der Jagt M, Verweij BH, Oldenbeuving AW, Rinkel GJ, van den Bergh WM, Slooter AJ, Group HS. Effects of Induced Hypertension on Cerebral Perfusion in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. Stroke. 2015;46:3277–3281. doi: 10.1161/STROKEAHA.115.010537 [DOI] [PubMed] [Google Scholar]
- 31.Gathier CS, van den Bergh WM, van der Jagt M, Verweij BH, Dankbaar JW, Muller MC, Oldenbeuving AW, Rinkel GJE, Slooter AJC, Group HS. Induced Hypertension for Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. Stroke. 2017. doi: 10.1161/STROKEAHA.117.017956 [DOI] [PubMed] [Google Scholar]
- 32.Weiss M, Albanna W, Conzen-Dilger C, Kastenholz N, Seyfried K, Ridwan H, Wiesmann M, Veldeman M, Schmidt TP, Megjhani M, et al. Intraarterial Nimodipine Versus Induced Hypertension for Delayed Cerebral Ischemia: A Modified Treatment Protocol. Stroke. 2022:101161STROKEAHA121038216. doi: 10.1161/STROKEAHA.121.038216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal G, Hemphill JC 3rd, Sorani M, Martin C, Morabito D, Obrist WD, Manley GT. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008;36:1917–1924. doi: 10.1097/CCM.0b013e3181743d77 [DOI] [PubMed] [Google Scholar]
- 34.Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anzabi M, Angleys H, Aamand R, Ardalan M, Mouridsen K, Rasmussen PM, Sorensen JCH, Plesnila N, Ostergaard L, Iversen NK. Capillary flow disturbances after experimental subarachnoid hemorrhage: A contributor to delayed cerebral ischemia? Microcirculation. 2019;26:e12516. doi: 10.1111/micc.12516 [DOI] [PubMed] [Google Scholar]
- 36.Wang KC, Tang SC, Lee JE, Tsai JC, Lai DM, Lin WC, Lin CP, Tu YK, Hsieh ST. Impaired microcirculation after subarachnoid hemorrhage in an in vivo animal model. Sci Rep. 2018;8:13315. doi: 10.1038/s41598-018-31709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, Iversen NK, Secher N, Engedal TS, Anzabi M, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–1837. doi: 10.1038/jcbfm.2013.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hecht N, Schrammel M, Neumann K, Muller MM, Dreier JP, Vajkoczy P, Woitzik J. Perfusion-dependent cerebral autoregulation impairment in hemispheric stroke. Ann Neurol. 2020. doi: 10.1002/ana.25963 [DOI] [PubMed] [Google Scholar]
- 39.Gouvea Bogossian E, Diaferia D, Ndieugnou Djangang N, Menozzi M, Vincent JL, Talamonti M, Dewitte O, Peluso L, Barrit S, Al Barajraji M, et al. Brain tissue oxygenation guided therapy and outcome in non-traumatic subarachnoid hemorrhage. Sci Rep. 2021;11:16235. doi: 10.1038/s41598-021-95602-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovacs M, Peluso L, Njimi H, De Witte O, Gouvea Bogossian E, Quispe Cornejo A, Creteur J, Schuind S, Taccone FS. Optimal Cerebral Perfusion Pressure Guided by Brain Oxygen Pressure Measurement. Front Neurol. 2021;12:732830. doi: 10.3389/fneur.2021.732830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Z, Zheng J, Ma L, Li H, You C, Jiang Y. Predictive Value of Cerebral Autoregulation Impairment for Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. World Neurosurg. 2019. doi: 10.1016/j.wneu.2019.02.188 [DOI] [PubMed] [Google Scholar]
- 42.Khatibi K, Szeder V, Blanco MB, Tateshima S, Jahan R, Duckwiler G, Vespa P. Role of Bedside Multimodality Monitoring in the Detection of Cerebral Vasospasm Following Subarachnoid Hemorrhage. Acta Neurochir Suppl. 2020;127:141–144. doi: 10.1007/978-3-030-04615-6_20 [DOI] [PubMed] [Google Scholar]
- 43.Tas J, Beqiri E, van Kaam RC, Czosnyka M, Donnelly J, Haeren RH, van der Horst ICC, Hutchinson PJ, van Kuijk SMJ, Liberti AL, et al. Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury (COGiTATE): A Feasibility Randomized Controlled Clinical Trial. J Neurotrauma. 2021. doi: 10.1089/neu.2021.0197 [DOI] [PubMed] [Google Scholar]
- 44.Weiss M, Meyfroidt G, Aries MJH. Individualized cerebral perfusion pressure in acute neurological injury: are we ready for clinical use? Curr Opin Crit Care. 2022. doi: 10.1097/MCC.0000000000000919 [DOI] [PubMed] [Google Scholar]
- 45.Svedung Wettervik T, Hanell A, Howells T, Ronne Engstrom E, Lewen A, Enblad P. ICP, CPP, and PRx in traumatic brain injury and aneurysmal subarachnoid hemorrhage: association of insult intensity and duration with clinical outcome. J Neurosurg. 2022:1–8. doi: 10.3171/2022.5.JNS22560 [DOI] [PubMed] [Google Scholar]
- 46.Schmidt JM, Ko SB, Helbok R, Kurtz P, Stuart RM, Presciutti M, Fernandez L, Lee K, Badjatia N, Connolly ES, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke. 2011;42:1351–1356. doi: 10.1161/STROKEAHA.110.596874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wartenberg KE. Critical care of poor-grade subarachnoid hemorrhage. Curr Opin Crit Care. 2011;17:85–93. doi: 10.1097/MCC.0b013e328342f83d [DOI] [PubMed] [Google Scholar]
- 48.Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34:1783–1788. doi: 10.1097/01.CCM.0000218413.51546.9E [DOI] [PubMed] [Google Scholar]
- 49.Sekhon MS, Gooderham P, Menon DK, Brasher PMA, Foster D, Cardim D, Czosnyka M, Smielewski P, Gupta AK, Ainslie PN, et al. The Burden of Brain Hypoxia and Optimal Mean Arterial Pressure in Patients With Hypoxic Ischemic Brain Injury After Cardiac Arrest. Crit Care Med. 2019;47:960–969. doi: 10.1097/CCM.0000000000003745 [DOI] [PubMed] [Google Scholar]
- 50.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 51.Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK, Vergouwen MD. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2014;34:200–207. doi: 10.1038/jcbfm.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dengler J, Frenzel C, Vajkoczy P, Wolf S, Horn P. Cerebral tissue oxygenation measured by two different probes: challenges and interpretation. Intensive Care Med. 2011;37:1809–1815. doi: 10.1007/s00134-011-2316-z [DOI] [PubMed] [Google Scholar]
- 53.Morgalla MH, Haas R, Grozinger G, Thiel C, Thiel K, Schuhmann MU, Schenk M. Experimental comparison of the measurement accuracy of the Licox((R)) and Raumedic ((R)) Neurovent-PTO brain tissue oxygen monitors. Acta Neurochir Suppl. 2012;114:169–172. doi: 10.1007/978-3-7091-0956-4_32 [DOI] [PubMed] [Google Scholar]
- 54.Huschak G, Hoell T, Hohaus C, Kern C, Minkus Y, Meisel HJ. Clinical evaluation of a new multiparameter neuromonitoring device: measurement of brain tissue oxygen, brain temperature, and intracranial pressure. J Neurosurg Anesthesiol. 2009;21:155–160. doi: 10.1097/ANA.0b013e31818f2eac [DOI] [PubMed] [Google Scholar]
- 55.Silverman A, Kodali S, Strander S, Gilmore EJ, Kimmel A, Wang A, Cord B, Falcone G, Hebert R, Matouk C, et al. Deviation From Personalized Blood Pressure Targets Is Associated With Worse Outcome After Subarachnoid Hemorrhage. Stroke. 2019:STROKEAHA119026282. doi: 10.1161/STROKEAHA.119.026282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeiler FA, Czosnyka M, Smielewski P. Optimal cerebral perfusion pressure via transcranial Doppler in TBI: application of robotic technology. Acta Neurochir (Wien). 2018;160:2149–2157. doi: 10.1007/s00701-018-3687-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


