Abstract
T cell-independent (TI) B cell responses to non-protein antigens involve multiple cues from the innate immune system. Neutrophils express complement receptors and activated neutrophils can release B cell activating factor (BAFF), but mechanisms effectively linking neutrophil activation to TI B cell responses are incompletely understood. Using germline and conditional knockout mice, we found that TI humoral responses involve alternative pathway complement activation and neutrophil-expressed C3a- and C5a-receptors (C3aR1/C5aR1) that promote BAFF-dependent B1 cell expansion and TI antibody production. Conditional absence of C3aR1/C5aR1 on neutrophils lowered serum BAFF levels, led to fewer Peyer’s patch germinal center B-cells (GCB), reduced GCB IgA class switching and lower fecal IgA levels. Together, the results indicate that sequential activation of complement on neutrophils crucially supports humoral TI and mucosal IgA responses through upregulating neutrophil production of BAFF.
Introduction
T-cell-independent (TI) humoral immune responses to polysaccharide antigens involve B1 cell activation and differentiation in the absence of germinal centers (GC). B1 cells in secondary lymphoid organs and in close proximity to mucosal surfaces of lungs, gut and peritoneum, are responsible for early IgM response to pathogens and contribute to mucosal IgA immune responses (1, 2).
The complement system is intricately associated with the development of adaptive T cell and B cell immune responses (3). Complement activation initiates via the classical, the mannan binding lectin (MBL) and alternative pathways, which converge to form C3 convertases that cleave C3 into C3a, C3b and ultimately C3dg. C3 convertases also facilitate assembly of C5 convertases that cleave C5 into C5a and C5b, the latter required for C5b-9 membrane attack complex (MAC) formation. Surface-expressed and soluble complement regulators, including decay accelerating factor (DAF, CD55), complement receptor (CR) 1, murine Crry, and membrane inhibitor of reactive lysis (MIRL, CD59) (3), control complement activation.
C3 deficiency results in severe defects in TI and T cell-dependent (TD) immune responses (4–6). Mechanistically, C3dg-coated antigens co-ligate the B cell co-receptor and CR2-CD19-CD81, lowering B cell receptor activation thresholds (6, 7). C3b-opsonized antigen facilitates trafficking to follicular dendritic cells, which employ CR2/C3dg ligations to present the antigens to GC B cells during affinity maturation (8, 9). GC B cells undergo coordinate downregulation of surface-expressed CD55/DAF to permit local complement activation and autocrine C3aR1/C5aR1 signaling required for GC B cell positive selection and antibody affinity maturation (10). The unanticipated role for complement regulators and receptors during TD responses raised the possibility that C3aR1/C5aR1 signaling may also affect TI IgM and mucosal IgA B-cell responses, hypotheses that we addressed herein.
Materials and Methods
Mice
C57BL/6/J (B6), B6;129S4-C3tm1Crr/J (C3tm1Crr, C3−/−), C57BL/6-Tg(IghelMD4)4Ccg/J (MD4), B6.129P2-Lyz2tm1(cre)Ifo/J (LysM-Cre), B6.Cg-Tg(Cd4-cre)1Cwi/BfluJ (CD4-Cre) and B6.Cg-Tg(S100A8-cre,-EGFP)1Ilw/J (S100-Cre) mice were purchased from Jackson Laboratory (Bar Harbor, ME). We generated B6 C3aR1−/−C5aR1−/− mice, C3ar1fl/fl, C3ar1fl/fl and C3ar1fl/flC3ar1fl/fl (10, 11). We crossed C5ar1fl/fl and C3ar1fl/fl mice to CD4-Cre+, LySM-Cre+ or S100-Cre+ transgenic mice and intercrossed their progeny to produce C3ar1fl/fl/C5ar1fl/flxCD4-Cre+/− (C3aR1/C5aR1ΔCD4), C3ar1fl/fl/C5ar1fl/flxLysM-Cre+/− (C3aR1/C5aR1ΔLySM), C5ar1fl/flxS100-Cre+/− (C5aR1ΔS100) and C3ar1fl/fl/C5ar1fl/flxS100-Cre+/− (C3aR1/C5aR1ΔS100) mice. B6 factor B−/− (fB, M. Pekna, Gothenburg, Sweden) and CR1/2−/− mice (M Carroll, Harvard Medical School) were kind gifts. We housed all animals in the Center for Comparative Medicine and Surgery at Mount Sinai under Institutional Animal Care in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (approval IACUC-2018-0014). Experiments were performed with groups of age- (6–12 weeks) and sex- matched (both sexes) mice, using littermates or animals maintained in the same room and co-housed for >2 weeks within the same cages from the age of 4 weeks.
Mouse immunizations
We immunized mice with 50 μg of 2,4,6, Tri-nitrophenyl-lipopolysaccharide (TNP-LPS; Innovative Research, Peary Court Novi, MI) i.p. (TI-responses) or with 100μg of NP-KLH in Alum (Immject Alum, Thermofischer) i.p (TD-responses). In some experiments, 4×107 enriched splenic IgMb+ WT or C3aR1−/−C5aR1−/− B-cells (Magnisort, Stem cell) were injected into IgMa+ MD4 BCR-transgenic recipients by retro-orbital injections and immunized with TNP-LPS as above.
ELISA
We quantified anti-TNP antibodies (10), fecal and serum IgA levels (12) murine BAFF, and anti-PC, anti-CPS 9 and 14 (13) by ELISA.
Immunofluorescence analysis
Peyer’s patches (PP) fixed in 1.6% PFA 20% sucrose at 4°C were washed in PBS, embedded OCT compound (Tissue Tek, Sakura Finetek USA, Torrance, CA, USA), cut into 8μm sections, washed with PBS, blocked with 5% Bovine serum albumin (BSA, Sigma Aldrich) and Fc-block (1:200), then sequentially stained with anti-Ly6G and anti-BAFF antibodies overnight. Sections were then stained with anti-rat F(ab’)2-FITC (for Ly6G) and anti-rat IgG-PE (for BAFF) (Jackson Immunology). We acquired images on a Zeiss LSM780 (confocal) and processed them with Zen 2.3 software (Zeiss).
Flow Cytometry
Single cell suspensions of spleen cells, lavaged peritoneal leukocytes (5 mL PBS-1% FCS), and excised PP cells (incubated at 37°C for 10min in 5mM EDTA, 15mM HEPES, FCS 20% in HBSS) were stained (see Supplemental Table 1 for antibody clones) as indicated. Preps were analyzed by flow cytometry (gating strategy Supplemental Fig 1) using FACS Lyric™ or Canto™ II cytometers (BD Biosciences, San Jose CA), and analysis performed with FlowJo software (FlowJo LLC, Ashland OR).
Statistical Analysis
We used GraphPad Prism Software (version 8.4.2) for all statistical analyses (see figure legends for details). Each figure panel represents ≥2–3 independent experiments with multiple biological replicates (single mice or tissues samples, n>3) and 3–5 technical replicates.
Results and Discussion
Upon immunization of WT and congenic B6 C3aR1−/−, C5aR1−/− and C3aR1−/−C5aR1−/− mice (intraperitoneally) i.p. with TNP-LPS (10 d; Fig 1A, Supplemental Fig 1A–B), we observed lower frequencies of total and TNP-reactive peritoneal B1 B cells, and reduced anti-TNP IgM antibody titers in mice lacking C3aR1, C5aR1 or both, without differences among the knockout strains. We also observed lower titers of natural IgM reactive to phosphatidyl-choline and capsular polysaccharides in unimmunized C3aR1−/−C5aR1−/− mice vs. WT controls (Supplemental Fig 1C). Reduced frequencies of B1 cells and lower antibody titers in immunized C3−/−, CR2−/− and, specifically, fB−/− mice (Fig 1B), implicated involvement of the alternative complement pathway.
Fig 1. T-cell independent responses require alternative complement pathway and C3aR/C5aR on neutrophils.
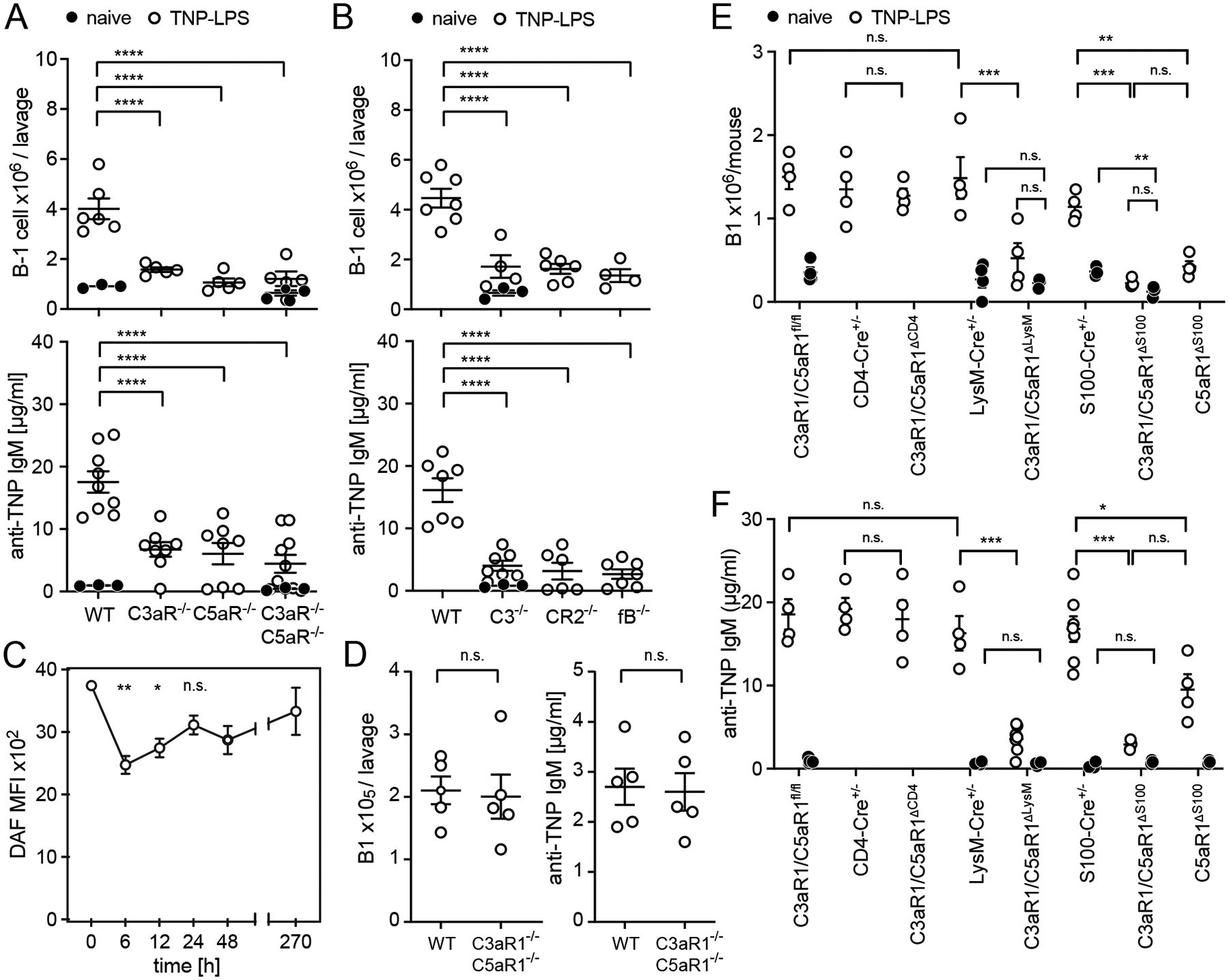
Mice were injected with 50μg TNP-LPS i.p, sacrificed on d10 after immunization. A-B. Peritoneal B1 cell counts (top) and serum concentration of anti-TNP IgM antibodies (bottom) were analyzed in (A) WT, C3aR1−/−, C5aR1−/−, C3aR1−/−C5aR1−/− and (B) WT, C3−/−, CR2−/−, fB−/− mice. D. Kinetics of DAF expression on peritoneal B1 cells (MFI, mean fluorescence intensity via flow cytometry); n=4/experiment. D. Donor peritoneal B1 cell count (IgMa−CD19+B220lowCD11b+) and concentration of anti-TNP IgM in MD4+ mice d10 after transfer of 4×107 WT or C3aR1−/−C5aR1−/− B-cells and TNP-LPS immunization. E-F. Peritoneal B1 cell count (E) and serum anti-TNP IgM concentration (F) in naïve and TNP-LPS immunized groups of mice as indicated. Graphs show mean ± SEM from ≥2 independent experiments with *p<0.05, **p<0.01, ***p<0.005, ****p<0.001, and n.s.; not significant, by non-parametric ANOVA with Bonferroni post-test (A-C, E) or Student’s t-test (D). In E-F, responses in immunized mice greater than in naïve controls (p<0.001 for each) unless otherwise indicated.
To determine whether complement regulator and/or receptor levels change on B1 cells upon activation or class switching after immunization [as observed on TD-induced GC B cells (10)], we phenotyped peritoneal B1 cells after TNP-LPS immunization. B1 cells in naïve mice expressed high levels of DAF (Fig 1C), which underwent only modest and transient reduction 6–12h following immunization. At 24h post-immunization, analyses showed higher expression of C3aR1, C5aR1, CR1/2, and CD59 on B1 cells (Supplemental Fig 1D), all of which returned to baseline levels by day 7.
We tested for a functional role of B cell-expressed C3aR1/C5aR1 in TI humoral immunity by adoptively transferring splenic C3aR1−/−C5aR1−/− or WT B cells into MD4 B cell receptor-(BCR)-transgenic recipients (IgMa+IgDa+ B cells, specific for HEL; do not respond to TNP) and subsequent immunization with TNP-LPS (Fig 1D). Donor B1 cell count and concentration of anti-TNP IgM showed no differences between groups.
To discern which cells require C3aR1/C5aR1 signaling to support TI responses, we immunized mice lacking C3aR1/C5aR1 in myeloid cells (C3aR1/C5aR1ΔLysM) or neutrophils (C5aR1ΔS100 and C3aR1/C5aR1ΔS100). These animals all showed significant defects in B1 responses (Fig 1E) and anti-TNP antibody titers (Fig 1F). Controls showed no differences between mice lacking C3aR1/C5aR1 on T-cells (C3aR1/C5aR1ΔCD4) and WT. Marginal zone B cells and follicular B cells in naïve C3aR1/C5aR1ΔLySM and C3aR1/C5aR1ΔS100 did not differ from controls (Supplemental Fig. 1E–F). In contrast to TI immunization, TD immunization with TNP-KLH induced equivalent anti-TNP IgG titers in C3aR1/C5aR1ΔLySM and control C3aR1/C5aR1fl/fl mice (Supplemental Fig 1G). While frequencies of splenic B1 did not differ among the knockout and control strains (Supplemental Fig. 1H), we observed fewer peritoneal B1 cells in naïve C3aR1/C5aR1ΔS100 vs. naïve S100-Cre+/− mice (Fig 1E), suggesting a migratory defect (14). Additional controls showed that the neutrophils (gating strategy Supplemental Fig. 2A) from C3aR1/C5aR1ΔLysM, C5aR1ΔS100 and C3aR1/C5aR1ΔS100 mice lacked C3aR1/C5aR1 while neutrophil-expressed DAF, CD59, CR1/2 and Crry did not differ among strains (Supplemental Fig. 2B–C). Splenic neutrophil counts did not differ among naïve C3aR1/C5aR1ΔLysM, C3aR1/C5aR1ΔS100 and controls (Supplemental Fig. 2D).
Since B-cell Activating Factor of the TNF family (BAFF) can amplify TI responses, and neutrophils can produce BAFF (15, 16), we tested for potential mechanistic links among neutrophils, C5aR1 and BAFF-dependent antibody production. After TNP-LPS immunization (Fig 2A), intraperitoneal C5a rapidly increased followed by neutrophil influx and an associated increase in serum BAFF. Twelve hours after TNP-LPS immunization, we observed lower neutrophil (Fig 2B, C top) and serum (Fig 2C bottom) BAFF concentration in C3aR1/C5aR1ΔLySM and C5aR1ΔS100 mice. Conversely, C3a or C5a i.p. injections increased neutrophil BAFF (Fig 2D, E top) and serum BAFF (Fig 2E bottom) in control, but not C3aR1/C5aR1ΔLySM mice. Administration of exogenous recombinant BAFF (100 ng/day i.p. for 5 days) to TNP-LPS-immunized (Fig 2F) rescued the antibody defects observed in the C3aR1/C5aR1ΔLySM and C5aR1ΔS100 mice. These findings mechanistically link C3aR1 and/or C5aR1 signaling on granulocytes/neutrophils to BAFF release, which in turn drives TI B1-cell responses. Previous studies by others (17, 18) had connected neutrophil C5aR1 signaling to NF-kB mediated gene transcription, which is a crucial transcription factor for BAFF production.
Fig 2. Neutrophil C3aR1/C5aR1 ligations drive BAFF-dependent IgM responses to TNP-LPS.
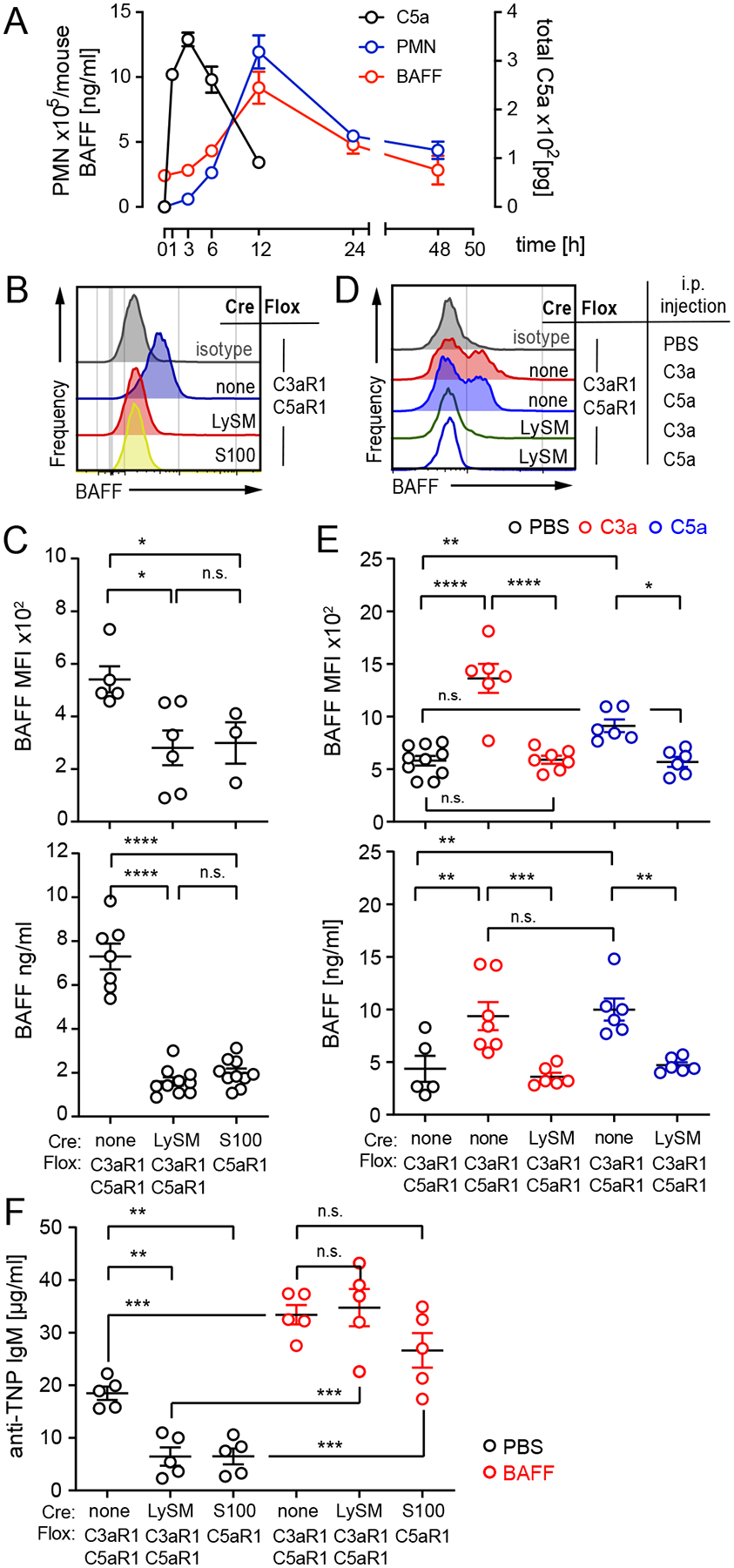
A. Kinetics of intra-peritoneal C5a (pg/ml × ml per lavage), neutrophil (CD11b+Ly6G+) influx and serum BAFF levels following TNP-LPS immunization of WT mice (n=3–4/experiment). B-C. Intracellular BAFF expression in neutrophils (MFI, flow cytometry), representative histograms, quantification (B, top) and serum BAFF concentration (C, bottom) 12h after TNP-LPS immunization. D-E. Neutrophil intracellular BAFF MFI, representative histogram (D), quantification (E, top) and serum BAFF levels (E, bottom) 12h after 5μg rmC3a and rmC5a, i.p. (F) Concentration of anti-TNP IgM on d10 after TNP-LPS immunization of groups of mice injected with 100ng recombinant mBAFF daily for 5 days. Graphs show mean ± SEM from ≥2 independent experiments with *p<0.05, **p<0.01, ***p<0.005, ****p<0.001 and n.s.: not significant, by non-parametric ANOVA with Bonferroni post-test (B-F).
Mucosal IgA production in the gastrointestinal tract requires T-dependent (TD) and TI mechanisms involving BAFF and TACI (19–21). Prior evidence also indicated roles for granulocytes: eosinophils support the survival of IgA-secreting cells in the lamina propria (22); and Peyer’s patches (PP), which typically support TD IgA production, can contain neutrophils (23). To test whether neutrophil-expressed C3aR1/C5aR1 contributed to steady state mucosal IgA responses, we quantified frequencies of IgA+ GC B cells (gating strategy Supplemental Fig. 3A) and total GC B cell numbers in PPs, and measured concentrations of fecal IgA in WT and various germline knockout mice (Fig 3A–B). These analyses showed that all readouts were reduced in C3−/− and fB−/− animals, and revealed similar defects in C3aR1−/−, C5aR1−/− and C3aR1−/−C5aR1−/− mice. Antibiotic treatment to limit potential microbiome effects (Supplemental Fig. 2B) reduced PP GCB by ~10-fold while maintaining IgA+ GCB differences between WT and C3aR1−/−C5aR1−/− mice, indicating the C3aR1/C5aR1 effects are microbiome-independent.
Fig 3. Normal IgA mucosal responses require neutrophil C3aR1/C5aR1 activation and can be rescued by exogenous BAFF.
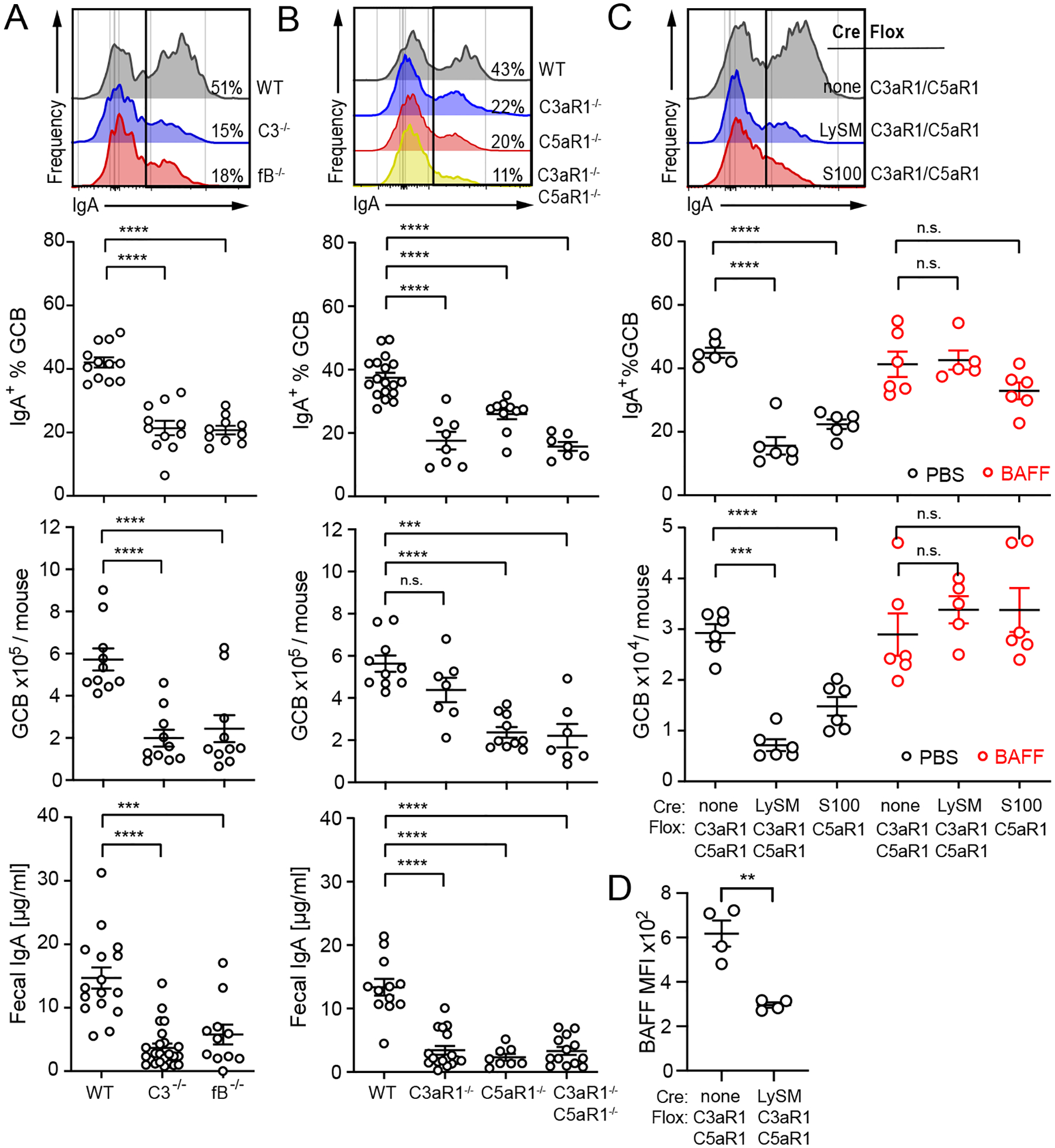
A-C. %IgA+ GCB cells (top row), absolute GCB cell count in Peyer’s patches (middle row) and concentration of fecal IgA (bottom row) in (A) WT, C3−/−, fB−/− mice, (B) WT, C3aR1−/−, C5aR1−/−, C3aR1−/−C5aR1−/− mice and (C) C3aR1/C5aR1fl/fl, C3aR1/C5aR1ΔLySM, C5aR1ΔS100 mice. Mice in (C) were injected daily for 5 days with PBS or 100ng mBAFF as indicated and PP % IgA+ GCB (top) and GCB number (bottom) were quantified on d6. D. Intracellular BAFF expression in neutrophils from Peyer’s patches of C3aR1/C5aR1fl/fl and C3aR1/C5aR1ΔLySM mice quantified as MFI by flow cytometry, representative histograms. All graphs show mean ± SEM from ≥3 independent experiments with ***p<0.005, ****p<0.001 and n.s.: not significant, by non-parametric ANOVA with Bonferroni post-test (A-C).
Analyses of PP IgA+ GCB cells (Fig 3C) and fecal IgA (Supplemental Fig. 3C) from groups of C3aR1/C5aR1ΔLySM and C5aR1ΔS100 mice phenocopied the findings from the germline C3aR1−/−C5aR1−/− animals, confirming a role for granulocyte-expressed C5aR1 in mucosal IgA production. Serum IgA levels, proportion of IgM+ and IgG+ GC B-cells in PP of C3aR1/C5aR1ΔLySM and C5aR1ΔS100 mice did not differ from controls (Supplemental Fig. 3D–F). BAFF administration rescued PP responses in the C3aR1/C5aR1ΔLySM and C5aR1ΔS100 mice to levels observed in control animals (Fig 3C). Immunofluorescence microscopy of PP in WT mice (Supplemental Fig. 3G) showed BAFF co-localizing with Ly6G+ cells in the subepithelial PP dome. The IF staining suggested reduced BAFF within the Ly6G+ neutrophils of the C3aR1/C5aR1ΔLySM animals, a result confirmed by flow cytometry (Fig 3D, Supplemental Fig. 3H). Thus, C3aR1/C5aR1 signaling on intestinal lamina propria neutrophils is critical for optimal production of BAFF, which Supplementalorts mucosal IgA production and PP responses.
Our new observations are distinct from known mechanisms linking complement to B cell immunity (10). While previous studies suggested that C5aR1 signaling can modulate B1 cell function (14) and neutrophils express C5aR1 and can produce BAFF (24), mechanistic links were not reported previously for B1 responses or for mucosal IgA production. Our findings are also distinct from prior work showing macrophage C3aR1/C5aR1 ligations release GCSF (25), which stimulates neutrophil BAFF production (16).
BAFF regulates B cells (26) by ligating BAFF-R, TACI and BCMA (27, 28) to activate canonical (via TACI) and alternative (via BAFF-R causing class switch recombination) NF-κB-induced survival signals (20, 26, 29). Elevated plasma BAFF levels in TACI−/− mice also associate with an increase in IgA+ GC B cells and GC size in PP (19), consistent with our results. Since IgA switching is not dependent on TACI in PP, we think that neutrophil-derived BAFF may be mediating its effect through BAFF-R in TD responses in PP. BAFF could also drive TI IgA outside of PP, contributing to the production of fecal IgA.
In sum, our findings newly identify a neutrophil-C5aR1-BAFF axis that crucially Supplementalorts humoral TI and mucosal TD responses. This fundamental mechanism has implications for designing strategies to improve polysaccharide and mucosal vaccines, and/or for therapy of antibody-mediated diseases.
Supplementary Material
Key Points.
Granulocyte-expressed C3aR1 and C5aR1 drive T-independent humoral responses
The effects are mediated through upregulation of neutrophil BAFF.
Analogous mechanisms are required for optimal mucosal IgA production
Acknowledgements
The authors thank the mouse genetics core at the Icahn School of Medicine at Mount Sinai (ISMMS) for their assistance in generating the conditional knockout animals.
The work was funded by R01 AI141434 awarded to PSH and DDS. EC received support by NIH T32 AI078892, EG by Crohn’s and Colitis Foundation career development award 877970, and AC received a fellowship grant from the American Society of Transplantation. SMY received support from NIH/NIDDK T32DK007757 and Agena Physician Scientist Training Program at ISMMS. The authors declare no competing financial interests.
Footnotes
References
- 1.Bunker JJ, and Bendelac A. 2018. IgA Responses to Microbiota. Immunity 49: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith FL, and Baumgarth N. 2019. B-1 cell responses to infections. Curr Opin Immunol 57: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis ES, Mastellos DC, Hajishengallis G, and Lambris JD. 2019. New insights into the immune functions of complement. Nat Rev Immunol 19: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, and Carroll MC. 1996. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity 4: 251–262. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, and Fearon DT. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271: 348–350. [DOI] [PubMed] [Google Scholar]
- 6.Haas KM, Hasegawa M, Steeber DA, Poe JC, Zabel MD, Bock CB, Karp DR, Briles DE, Weis JH, and Tedder TF. 2002. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity 17: 713–723. [DOI] [PubMed] [Google Scholar]
- 7.Carroll MC, and Isenman DE. 2012. Regulation of humoral immunity by complement. Immunity 37: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, and Carroll MC. 1998. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science 280: 582–585. [DOI] [PubMed] [Google Scholar]
- 9.Phan TG, Green JA, Gray EE, Xu Y, and Cyster JG. 2009. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 10: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumpelik A, Heja D, Hu Y, Varano G, Ordikhani F, Roberto MP, He Z, Homann D, Lira SA, Dominguez-Sola D, and Heeger PS. 2021. Dynamic regulation of B cell complement signaling is integral to germinal center responses. Nat Immunol 22: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan WH, van der Touw W, Paz-Artal E, Li MO, and Heeger PS. 2013. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med 210: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C, Mogno I, Contijoch EJ, Borgerding JN, Aggarwala V, Li Z, Siu S, Grasset EK, Helmus DS, Dubinsky MC, Mehandru S, Cerutti A, and Faith JJ. 2020. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host Microbe 27: 467–475 e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chorny A, Casas-Recasens S, Sintes J, Shan M, Polentarutti N, Garcia-Escudero R, Walland AC, Yeiser JR, Cassis L, Carrillo J, Puga I, Cunha C, Bastos H, Rodrigues F, Lacerda JF, Morais A, Dieguez-Gonzalez R, Heeger PS, Salvatori G, Carvalho A, Garcia-Sastre A, Blander JM, Mantovani A, Garlanda C, and Cerutti A. 2016. The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells. J Exp Med 213: 2167–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broker K, Figge J, Magnusen AF, Manz RA, Kohl J, and Karsten CM. 2018. A Novel Role for C5a in B-1 Cell Homeostasis. Front Immunol 9: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Arostegui JI, Juan M, Yague J, Merad M, Fagarasan S, and Cerutti A. 2014. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 15: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, and Cassatella MA. 2003. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med 197: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu MH, Wang M, Browning DD, Mukaida N, and Ye RD. 1999. NF-kappaB activation is required for C5a-induced interleukin-8 gene expression in mononuclear cells. Blood 93: 3241–3249. [PubMed] [Google Scholar]
- 18.Moon EY, and Park H. 2007. B cell activating factor (BAFF) gene promoter activity depends upon co-activator, p300. Immunobiology 212: 637–645. [DOI] [PubMed] [Google Scholar]
- 19.Grasset EK, Chorny A, Casas-Recasens S, Gutzeit C, Bongers G, Thomsen I, Chen L, He Z, Matthews DB, Oropallo MA, Veeramreddy P, Uzzan M, Mortha A, Carrillo J, Reis BS, Ramanujam M, Sintes J, Magri G, Maglione PJ, Cunningham-Rundles C, Bram RJ, Faith J, Mehandru S, Pabst O, and Cerutti A. 2020. Gut T cell-independent IgA responses to commensal bacteria require engagement of the TACI receptor on B cells. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, and Geha RS. 2005. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Magri G, Grasset EK, and Cerutti A. 2020. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat Rev Immunol 20: 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, and Berek C. 2014. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40: 582–593. [DOI] [PubMed] [Google Scholar]
- 23.Casanova-Acebes M, Nicolas-Avila JA, Li JL, Garcia-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, N AG, Ballesteros I, Devi S, Quintana JA, Crainiciuc G, Leiva M, Gunzer M, Weber C, Nagasawa T, Soehnlein O, Merad M, Mortha A, Ng LG, Peinado H, and Hidalgo A. 2018. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med 215: 2778–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, and Cerutti A. 2011. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosmann M, Haggadone MD, Zetoune FS, Sarma JV, and Ward PA. 2013. The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur J Immunol 43: 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, and Schneider P. 2009. Cracking the BAFF code. Nat Rev Immunol 9: 491–502. [DOI] [PubMed] [Google Scholar]
- 27.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, and Browning JL. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190: 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, and Scott ML. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293: 2111–2114. [DOI] [PubMed] [Google Scholar]
- 29.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, and Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 3: 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


