Abstract
Background:
Though protective in secondary stroke prevention of intracranial arterial stenosis (ICAS), it is uncertain if the benefits of leisure-time physical activity (LTPA) extend to asymptomatic ICAS or extracranial carotid stenosis (ECAS). Therefore, we sought to determine LTPA’s relationship with ECAS and ICAS in a stroke-free, race-ethnically diverse cohort.
Methods:
This cross-sectional study included participants from the MRI substudy of the Northern Manhattan Study, of whom 1274 had LTPA assessments at enrollment. LTPA was represented continuously as metabolic equivalent score (MET-score) and ordinally as model-based cluster analysis (LTPA-cluster), both based on the same LTPA assessments. We evaluated ECAS sonographically using carotid intima-media thickening (cIMT) and number of carotid plaques. ICAS was assessed with time-of-flight MRA and defined as ≥50% or ≥70% stenosis. We applied regression analyses to evaluate the association between LTPA with ECAS and ICAS, adjusting for confounders.
Results:
Of 1274 included participants (mean age 71±9 years; 60% female; 65% Hispanic), the mean MET-score was 10±16 and 60% were in a LTPA-cluster with any activity. Among those with carotid ultrasound (n=1234), the mean cIMT was 0.97±0.09 mm and 56% of participants had at least one carotid plaque identified. Among those with MRA (n=1211), 8% had ≥50% ICAS and 5% had ≥70% ICAS. For ICAS, MET-score was associated with ≥70% ICAS (adjusted OR (aOR) per unit increase in MET-score [95% confidence interval (CI)]: 0.97 [0.94–0.99]) but not with ECAS measures (cIMT, adjusted β-estimate per unit increase in MET-score [95% CI]: 0.002 [−0.003–0.006] or number of plaques, adjusted β-estimate [95% CI]: 0.0001 [−0.0001–0.0003]). Substituting MET-score with LTPA-clusters replicated the association between ≥70% ICAS and LTPA (aOR per each increased LTPA-cluster [95% CI]: 0.83 [0.70–0.99]).
Conclusions:
In this diverse stroke-free population, we found LTPA most strongly associated with asymptomatic ≥70% ICAS. Given the high-risk nature of ≥70% ICAS, these findings may emphasize the role of LTPA in people at risk for ICAS.
Keywords: physical activity, extracranial carotid stenosis, intracranial artery stenosis
Introduction
Leisure time physical activity (LTPA) is associated with reduced risk of all-cause stroke, cardiovascular disease, and mortality [1–4], but less is known about whether LTPA is protective against the development of asymptomatic cervicocephalic atherosclerotic stenosis. The benefits of regular LTPA on the primary prevention of cardiac disease has been well described [5], while the benefit on asymptomatic carotid atherosclerosis has been mixed [6]. The Atherosclerosis Risk in Communities Study showed an inverse association between workplace physical activity and cervical carotid intima-media thickness (cIMT), a measure of early carotid atherosclerosis, but not LTPA [7]. The Tromsø study showed a benefit of LTPA in cIMT only in men [8], while the British Regional Heart Study did not detect a significant association between PA and cIMT [9].
Discordant results may be due to differences in LTPA measurements and study populations [10,11]. Notably fewer studies have included multi-ethnic populations which can be important given disparate prevalence of carotid atherosclerosis between race and ethnic groups. Further, the relationship between physical activity and asymptomatic intracranial artery stenosis (ICAS) is even less studied. In a subgroup analysis of the SAMMPRIS (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial medical arm, physical activity was the strongest predictor of fewer recurrent vascular events [12,13].
Given the literature gaps, we sought to study the relationship between LTPA and asymptomatic extracranial carotid artery stenosis (ECAS) and ICAS in the Northern Manhattan Study (NOMAS), a large, urban, multi-ethnic and multiracial cohort of stroke-free participants. We hypothesized that increased levels of LTPA are associated with lower prevalence of asymptomatic ECAS and ICAS, after adjusting for demographic characteristics and vascular risk factors.
Methods
Study Cohort
NOMAS is a prospective, population-based study of stroke risk factors in an urban, multi-ethnic and multiracial cohort based in northern Manhattan. Methods of participant recruitment and evaluation have been previously described [14]. A total of 3,298 stroke-free participants were recruited from 1993–2001. In 2003, standardized brain magnetic resonance imaging (MRI) protocols were introduced in NOMAS and 1091 surviving stroke-free participants aged 50 years or older and 199 household members, for a total of 1290 participants, were invited to participate and screened for eligibility (Figure 1). All participants gave written informed consent. The study was approved by the Columbia University Medical Center and University of Miami Institutional Review Boards. Study data is available upon reasonable request made to the corresponding author.
Figure 1.
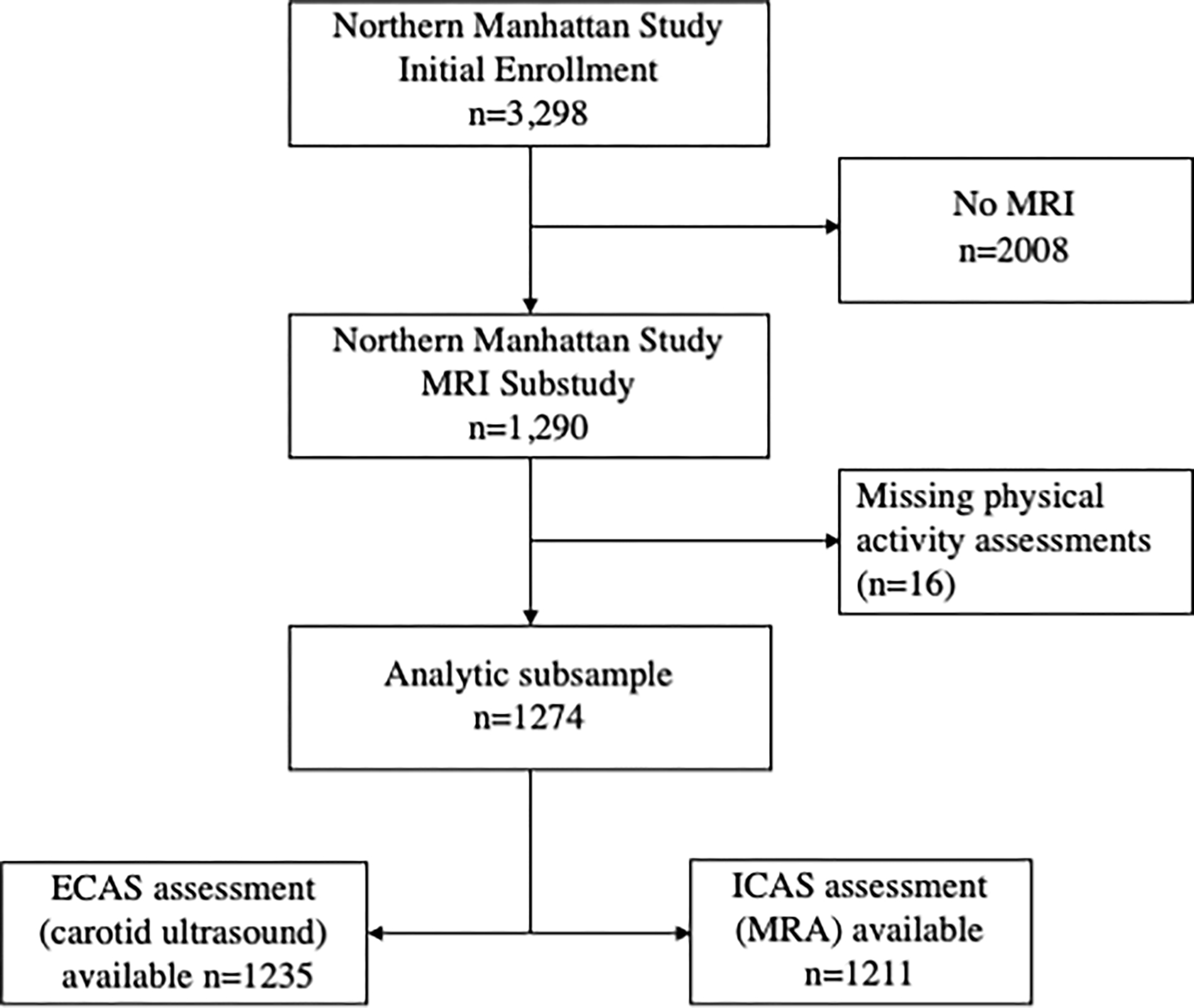
Northern Manhattan Study subsample
Baseline Characteristics
All NOMAS participants underwent a structured questionnaire for their baseline characteristics and medical history at time of enrollment. Age, sex, race, ethnicity, and education were self-reported at time of baseline enrollment. Race and ethnicity were based on self-identification through questions modeled after the US Census. Insurance status was classified as having Medicare or private insurance or having Medicaid or no insurance. All other baseline characteristics were additionally recorded at time of MRI assessment. We defined hypertension as a systolic blood pressure (SBP) ≥140 mm Hg or a diastolic blood pressure (DBP) ≥90 mm Hg, averaged from at least two separate blood pressure measurements, or a patient’s self-reported history of hypertension or use of antihypertensive medications. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the patient’s self-report of such a history or use of insulin or hypoglycemic medications. Hyperlipidemia was defined as fasting cholesterol levels ≥200 mg/dL or self-reported history of increased blood cholesterol levels or cholesterol-lowering medication use. Participants were also asked duration of their diagnoses, number of medications used, and class of medications used. We dichotomized smoking history as active smoking or not active smoking at time of MRI. Participants were asked their duration of smoking history of packs per day. Body mass index (BMI) was calculated as kg/m2. SBP, DBP, serum glucose and lipid profile were recorded at time of MRI assessment.
Leisure Time Physical Activity Assessment
At initial enrollment, reported LTPA was measured using an in-person questionnaire adapted from the National Health Interview Survey of the National Center for Health Statistics [15], which records the duration and frequency of 14 various recreational activities within two weeks before the interview. Participants were asked: “In the last 2 weeks, have you engaged in _______ for physical activity?” Each affirmative response was then followed by two other questions on frequency and duration of activity. Those who answered “no” to having performed any activity or those who reported less than 10 minutes of total activity were coded as inactive. The questionnaire has been previously validated in this population and correlated with body-mass index, activities of daily living, and quality of well-being activity scores [16]. We then correlated questionnaires with a validated compendia of physical activity to determine metabolic equivalents (MET, kilocalories/kg-hour) for the intensity and energy expenditure in kilocalories of each activity [17]. We used a total MET-score (MET-hours per week) to represent LTPA as a summated MET expenditure per activity duration each week, which is calculated as the product of the MET for each individual activity, frequency per week, and duration. MET-score was available for 1274 participants in the MRI substudy.
Given possible limitations in MET-score representing multidimensional aspects of LTPA, such as diversity of activities, we secondarily used an ordinal model-based cluster analysis as a measure of physical activity. As previously described [18], multivariate finite mixture modeling analysis inputs duration of any physical activity, frequency of any physical activity, number of activity types, and energy expenditure due to any activity to derive five LTPA groups, or LTPA-clusters, amongst those with any reported physical activity in the preceding 2 weeks. Among those with any physical activity, participants in the lowest LTPA-cluster, considered “rarely active”, averaged 0.5 LTPA sessions per week, while the highest LTPA-cluster, considered “highly active”, had a mean of 14 LTPA sessions per week (mean MET-score (sd) of each LTPA-cluster: II (rare activity): 2 (3); III (active weekly): 3 (3); IV (active every other day): 10 (13); V (active daily): 22 (19); VI (highly active): 39 (24)). Practically, 97% of participants in the highest LTPA-cluster met American Heart Association guidelines for physical activity (150–300 minutes of moderate intensity or 75–150 minutes of vigorous activity or combination of the two), while only 3% met the guidelines in the lowest LTPA-cluster. We previously showed LTPA-clusters to be associated with cardiovascular risk factors independent of METS [18]. There were 1034 participants in the MRI substudy with data for LTPA-cluster grouping.
Imaging Evaluation of ECAS
ECAS was assessed through plaque thickness, number of plaques, and cIMT using high-resolution B-mode carotid ultrasound imaging (GE LogIQ 700; 9- to 13- MHz linear-array transducer) by trained and certified sonographers, as previously described [19]. An automated computerized edge tracking software program M’Ath (Intelligence in Medical Technologies, Inc, Paris, France) was used to measure cIMT (mm) and quantify number of carotid plaques. M’Ath detects boundaries of plaque from multiple images using an automated plaque edge detection algorithm. Plaque was defined as an area of focal wall thickening or luminal protrusion at least 50% greater than surrounding total wall thickness. In accordance with the Mannheim consensus [20], cIMT was measured in areas without plaque and calculated as an average measure of near and far walls of the common carotid artery, bifurcation, and internal carotid artery, bilaterally. cIMT was examined continuously as a mean of the maximum measures of 12 carotid sites.
ICAS Assessment
The NOMAS study assessed ICAS through 3-dimensional, time-of-flight magnetic resonance angiography (MRA). From 2003–2008, surviving NOMAS participants and a cohort of household members were invited to undergo MRI if they remained stroke-free, were 50 years or older, and had no contraindications (Figure 1). Following a standardized protocol, this MRI substudy used 1.5-T MRI system (Philips Medical Systems, Best, Netherlands) at the Columbia University Medical Center with the following parameters for MRA: field of view of 15 cm; 1-mm effective slice thickness; acquisition matrix interpolated to a 256 × 228 matrix; flip angle of 25°; and repetition time/echo time of 20 and 2.7 ms, respectively. We inspected all major intracranial large arteries. If arterial stenosis was identified, the narrowest lumen area was measured to define stenosis, referencing the immediately preceding segment with normal lumen (or the next normal appearing lumen if the stenosis at the arterial origin) [21]. We categorized stenosis as no stenosis, <50% (or luminal irregularities), 50–69%, and ≥70% or flow gap. A trained neurologist and vascular neurologist, blinded to all clinical information and each other’s read, independently graded stenoses. Interrater reliability was high with a kappa = 0.93 for identifying >50% stenosis and intraclass correlation coefficient for the ordinal stenosis scale as >0.90 for single and average measures [21].
Statistical Analyses
Our primary analysis sought to determine the association between exposure of LTPA, measured as MET-score or LTPA-clusters, and imaging outcomes of ECAS and ICAS. Outcome variables of cIMT and number of carotid plaques were treated continuously, while ICAS groups were represented dichotomously (≥50% vs <50% ICAS and ≥70% ICAS vs <70% ICAS). We represented cIMT continuously given varied definitions of abnormal cIMT in research [22]. We chose ICAS stenosis severity cut-offs following widely accepted ICAS severity categories used clinically and in research [12], and grouped them dichotomously as the risk factors and mechanisms leading to ≥70% stenosis would likely be similar for ≥50% stenosis in our population and it would allow for greater analytic power. We conducted multivariate linear regression and logistic regression where appropriate. We used 3 models to account for potential confounders: 1) adjusted for age, sex, race and ethnicity, high school completion, and insurance status; 2) model 1 additionally adjusted for BMI, hypertension, diabetes mellitus, hyperlipidemia, current smoking; and 3) model 1 additionally adjusted for BMI, SBP, DBP, and years of hypertension, number of anti-hypertensive medications, blood glucose, years of diabetes mellitus, number of anti-glycemic medications, low-density lipoprotein, high-density lipoprotein, triglycerides, statin use, years smoked, packs per day.
Post-hoc, we performed a causal model-based mediation analysis of hypertension, diabetes mellitus, and hyperlipidemia with MET-score and ≥70% ICAS. For each moderator of hypertension, diabetes mellitus, or hyperlipidemia, we estimated average causal mediation effects separately on exposure MET-score and outcome ≥70% ICAS using generalized linear models with quasi-Bayesian Monte Carlo method (100 simulations) and White’s heteroscedasticity-consistent estimator [23], while controlling for age, sex, race, ethnicity, high school completion, and insurance status.
We used SAS version 9.4 (SAS Institute Inc., Cary, NC) to conduct all regression analyses and the “mediation” package of R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) to conduct mediation analyses [23]. All p-values less than 0.05 were considered statistically significant. This manuscript follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [24].
Results
Baseline Characteristics
In this analysis, 1274 participants with LTPA assessments from the NOMAS MRI substudy were included (mean age 71±9 years; 60% female; 17% white, 17% black, and 65% Hispanic), of whom 1235 had ECAS evaluation with carotid ultrasound and 1211 had ICAS evaluation with MRA. Table 1 shows baseline characteristics. The mean MET-score was 10±16 (range 0–129, right-skewed). LTPA-cluster grouping was available for 1034 participants, of whom 60% had any reported physical activity. Among those with carotid ultrasound, the average cIMT was 0.93±0.09 mm and 56% of participants had at least one carotid plaque identified. Among those with MRA, 96 (8%) had ≥50% ICAS and 57 (5%) had ≥70% ICAS.
Table 1.
Baseline characteristics of the MRI substudy in NOMAS with PA assessment
| Characteristics | Study Population |
|---|---|
| Age | 71 (9) |
| Female | 768 (60) |
| Race and Ethnicity | |
| Non-Hispanic White | 220 (17) |
| Non-Hispanic Black | 222 (17) |
| Hispanic | 832 (65) |
| High School completed | 585 (46) |
| Insured | 1081 (85) |
| Body Mass Index, kg/m2 | 28 (5) |
| MET-score | 10 (16) |
| PA-clusters (n=1034) | |
| I: no activity | 416 (40) |
| II: rare activity | 23 (2) |
| III: active weekly | 60 (6) |
| IV: active every other day | 152 (15) |
| V: active daily | 316 (31) |
| VI: highly active | 67 (6) |
| Comorbidities | |
| Hypertension | 1001 (85) |
| Diabetes Mellitus | 326 (26) |
| Hyperlipidemia | 1034 (81) |
| Current Smoking | 149 (12) |
| ECAS/ICAS Imaging Measures | |
| Extracranial Carotid Plaque Identified | 687 (56) |
| cIMT, mm | 0.93 (0.09) |
| ≥50% ICAS | 96 (8) |
| ≥70% ICAS | 57 (5) |
NOMAS = Northern Manhattan Study; PA = physical activity; MET-score = metabolic equivalent score; cIMT = carotid intima-media thickness; ECAS = extracranial carotid artery stenosis; ICAS = intracranial artery stenosis
Age, body mass index, MET-score, and cIMT displayed as mean (sd); remainder of characteristics displayed as n (%).
LTPA and Imaging Outcomes
For ICAS, we found MET-score associated with both ≥50% and ≥70% ICAS in minimally adjusted models. After fully adjusting for vascular comorbidities, however, MET-score demonstrated a significant association with stenoses of ≥70% (fully adjusted OR (aOR) per unit increase in MET-score [95% confidence interval (CI)]: 0.97 [0.94–0.99]), but not ≥50% stenoses (aOR per unit increase in MET-score [95% CI]: 0.99 [0.97–1.00]) (Table 2). Similarly, more active LTPA-cluster groups were associated with reduced odds of having ≥70% ICAS (aOR per each increased LTPA-cluster [95% CI]: 0.83 [0.70–0.99]) but not ≥50% stenoses (aOR per each increased LTPA-cluster [95% CI]: 0.96 [0.84–1.10]) (Table 3).
Table 2.
Regression analysis for MET-score and ECAS/ICAS
| Association of LTPA with ICAS | |||
| Characteristic | Model 1 | Model 2 | Model 3 |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| ≥50% | 0.98 (0.96–0.99) | 0.98 (0.97–1.00) | 0.99 (0.97–1.00) |
| ≥70% | 0.96 (0.94–0.99) | 0.97 (0.94–0.99) | 0.97 (0.94–0.99) |
| Association of LTPA with ECAS | |||
| Characteristic | Model 1 | Model 2 | Model 3 |
| β-Estimate (95% CI) | β-Estimate (95% CI) | β-Estimate (95% CI) | |
| Number of carotid plaques | 0.001 (−0.004–0.005) | 0.008 (−0.003–0.006) | 0.002 (−0.003–0.006) |
| cIMT | 0.0001 (−0.0001–0.0002) | 0.0001 (−0.0001–0.0002) | 0.0001 (−0.0001–0.0003) |
LTPA = leisure time physical activity; MET-score = metabolic equivalent score; OR = odds ratio; CI = confidence interval; ICAS = intracranial artery stenosis; ECAS = extracranial carotid artery stenosis; cIMT = carotid intima-media thickness
Model 1: adjusted for age, sex, race and ethnicity, high school completion, and insurance status
Model 2: model 1 additionally adjusted for body mass index, hypertension, diabetes mellitus, hyperlipidemia, current smoking
Model 3: model 1 additionally adjusted for body mass index, systolic and diastolic blood pressure, years of hypertension, number of anti-hypertensive medications, blood glucose, years of diabetes mellitus, number of anti-glycemic medications, low-density lipoprotein, high-density lipoprotein, triglycerides, statin use, years smoked, and packs per day.
Table 3.
Regression analysis for LTPA-clusters and ECAS/ICAS
| Association of LTPA with ICAS | |||
| Characteristic | Model 1 | Model 2 | Model 3 |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| ≥50% | 0.80 (0.45–1.41) | 0.92 (0.81–1.05) | 0.96 (0.84–1.10) |
| ≥70% | 0.78 (0.67–0.92) | 0.79 (0.67–0.93) | 0.83 (0.70–0.99) |
| Association of LTPA with ECAS | |||
| Characteristic | Model 1 | Model 2 | Model 3 |
| β-Estimate (95% CI) | β-Estimate (95% CI) | β-Estimate (95% CI) | |
| Number of carotid plaques | −0.02 (−0.06–0.02) | −0.01 (−0.03–0.45) | −0.01 (−0.05–0.04) |
| cIMT | 0.002 (0.001–0.003) | 0.003 (0.002–0.005) | 0.004 (0.002–0.005) |
LTPA = leisure time physical activity; OR = odds ratio; CI = confidence interval; ICAS = intracranial artery stenosis; ECAS = extracranial carotid artery stenosis; cIMT = carotid intima-media thickness
Model 1: adjusted for age, sex, race and ethnicity, high school completion, and insurance status
Model 2: model 1 additionally adjusted for body mass index, hypertension, diabetes mellitus, hyperlipidemia, current smoking
Model 3: model 1 additionally adjusted for body mass index, systolic and diastolic blood pressure, years of hypertension, number of anti-hypertensive medications, blood glucose, years of diabetes mellitus, number of anti-glycemic medications, low-density lipoprotein, high-density lipoprotein, triglycerides, statin use, years smoked, and packs per day.
For measures of ECAS, we did not find an association between MET-score and number of carotid plaques or cIMT (Table 2). Examining by LTPA-clusters had a positive statistical association between more active groups and cIMT (adjusted β-Estimate per each increased LTPA-cluster [95% CI]: 0.004 [0.002–0.005]) (Table 3).
Lastly, we did not find that hypertension, diabetes, or hyperlipidemia had a significant mediation (indirect) effect between MET-score and ≥70% ICAS (Figure 2).
Figure 2.
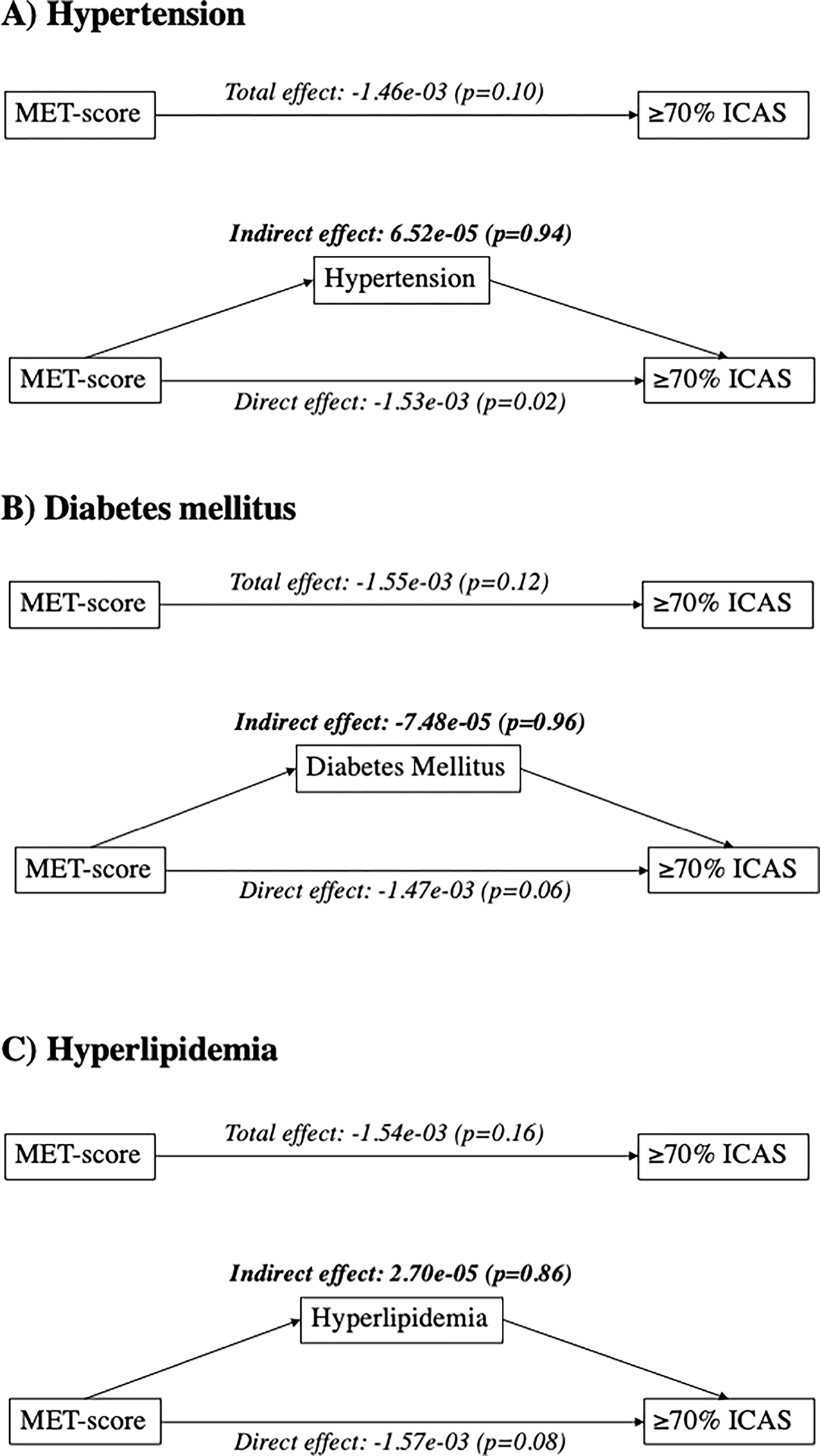
Mediation analysis of vascular risk factors between MET-score and ≥70% ICAS. Mediation models for A) hypertension, B) diabetes mellitus, and C) hyperlipidemia in the relationship between MET-score and ≥70% ICAS, adjusted for age, sex, race, ethnicity, high school completion, and insurance status. Values displayed are β-Estimate (p-value).
Discussion
In this study of stroke-free participants from NOMAS, we found LTPA had the strongest protective association with ≥70% ICAS rather than milder forms of ICAS or ECAS. This relationship was not clearly mediated by vascular risk factors. This analysis adds to the evidence on cerebrovascular benefits of LTPA by studying the relationship in a stroke-free cohort with a high proportion of African-American and Hispanic participants and demonstrating an association between LTPA and ≥70% asymptomatic ICAS, which confers high risk for a future vascular event [21].
Larger studies have identified LTPA as protective of incident stroke, but these did not subtype stroke etiology [1,25]. Investigations of LTPA and asymptomatic ICAS have been limited. In a stroke-free Chinese cohort, an increasing number of ideal cardiovascular health metrics, which included a physical activity threshold of more than 80 minutes per week, was inversely associated with asymptomatic ICAS assessed by transcranial Doppler [26]. A smaller study from Pakistan reported multiple modifiable vascular risk factors associated with asymptomatic ICAS on MRA, but not LTPA, as most of their participants were classified as inactive [27].
Post-hoc analyses from both large randomized controlled trials on symptomatic ICAS, WASID (Warfarin-Aspirin Symptomatic Intracranial Disease) in 2005 and SAMMPRIS in 2011, have shown aggressive control of vascular risk factors and lifestyle modifications lowered risk of recurrent stroke from symptomatic ICAS [12,28]. Though WASID included stenoses of 50–69% and a quarter of its participants were identified as sedentary, LTPA was not specifically examined in this stenosis group [29]. In the medical arm of SAMMPRIS, low LTPA was the strongest risk factor for the composite outcome of stroke, myocardial infarction, or vascular death. Further, low LTPA was the only risk factor associated with increased ischemic stroke risk alone and increasing activity levels conferred more protective association for future vascular events [13]. Similarly, we found the association between LTPA and ICAS strongest at stenoses ≥70%. LTPA and vascular risk factor management may have different relative protective effects depending on ICAS severity stage. The lack of clear mediation through vascular risk factors in our study may suggest LTPA has more direct beneficial effects on ICAS such as preventing proinflammatory states or improving vessel wall homeostasis that protects against impaired hemodynamics [30,31].
Several prior physical activity and ECAS studies were unable to show a strong independent association between LTPA and cIMT when adjusted for vascular comorbidities [11, 32], similar to our findings. Other analyses that have reported associations between physical activity and cIMT consisted of primarily healthy participants with favorable risk factor profiles [33,34], therefore comorbidities like hypertension and hyperlipidemia may have stronger relationships with subclinical ECAS than LTPA. It is also possible LTPA or carotid ultrasound measurements have been too imprecise to detect a relationship with subclinical ECAS. When using LTPA-clusters, we found a weakly positive association between activity levels and cIMT, but it is not readily evident if the effect size has clinical significance. Lastly, ECAS and ICAS may differ in their association with vascular risk factors, with ECAS more closely linked to hyperlipidemia and ICAS associated with hypertension and diabetes mellitus, suggesting varied pathways towards atherosclerosis formation [35,36]. In this context, LTPA may be considered another factor that potentially has different beneficial effects on subclinical ECAS and ICAS.
Strengths of the study include the large sample size and its racial/ethnic composition that allows for extrapolation of the results to their respective population subgroups. We also used well-validated and reproducible measures of ECAS and ICAS. The results in this study should be evaluated in the context of its limitations. With any observational study, the cross-sectional design limits conclusions of causality and there is potential for residual confounding. The compendium and construct used to quantify LTPA would be outdated by today’s standards, but it was well validated for our population at the time of enrollment [17]. Though reasonably reliable with a crude concordance rate of 0.69 when obtained from proxies [17], the self-reported nature of our 2-week LTPA assessment may be subject to misclassification bias. Some constructs for vascular risk factors were also based on self-report.
Additionally, LTPA assessments were made on average 5 years before imaging evaluation. ICAS severity would likely remain unchanged, as a study on the natural history of ICAS-related stroke found 70–80% of concomitant asymptomatic ICAS had static stenosis severity after 5 years [37]. However, it is unknown in our cohort how LTPA would change, if at all, during follow-up. Therefore, clinical interpretation of our results may be limited due to possibility of differential misclassification. Further, we were unable to capture potential longitudinal effects of LTPA changes, and it is possible interval changes in vascular risk factor status would affect LTPA levels, which we attempted to consider analytically. We did not have direct measures of LTPA, such as those made through accelerometry, but prior studies showed the LTPA questionnaires correlate well with cardiorespiratory fitness measured by maximal oxygen consumption [38]. Lastly, we did not examine other measures that may be relevant to subclinical ECAS such as common carotid inter-adventitial or intraluminal diameters [39].
In conclusion, we found LTPA most strongly associated with asymptomatic ICAS ≥70% stenosis than with less severe ICAS or cIMT in an elderly, urban-dwelling, and racially and ethnically diverse stroke-free cohort. Given the high-risk nature of asymptomatic ≥70% ICAS [22], physical activity may be especially beneficial in populations at risk for ICAS. As ICAS prevalence differs by race-ethnic groups [40], further investigations may seek to replicate these results in different populations. Other important upstream factors, such as structural health inequities that may interact with lifestyle and traditional risk factors, should also be considered in the context of physical activity and asymptomatic ICAS [41].
Supplementary Material
Acknowledgments
Sources of Funding
NOMAS is funded by the National Institutes of Health/National Institutes of Neurologic Disorders and Stroke (NIH/NINDS) (R01NS029993). Dr. Yang is supported by NIH/NINDS (T32NS007153).
Non-standard Abbreviations and Acronyms:
- LTPA
leisure time physical activity
- ICAS
intracranial arterial stenosis
- ECAS
extracranial carotid artery stenosis
- cIMT
carotid intima-media thickening
- MET-score
metabolic equivalent score
- NOMAS
Northern Manhattan Study
Footnotes
Disclosures
Dr. Willey reported receiving grant funding from NIH/NINDS outside the submitted work. Dr. Tom reported receiving grant funding from NIH/National Institute on Aging (NIA) outside the submitted work. Dr Wright reported other intellectual property and employment by NINDS. Dr. Sacco reported receiving grant funding from NIH, grant funding from the Florida Department of Health, and compensation as editor-in-chief of the American Heart Association’s Stroke outside the submitted work. Dr. Gutierrez reported receiving grant funding from the NIH/NIA.
Supplemental Material
STROBE checklist
References
- 1.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. [DOI] [PubMed] [Google Scholar]
- 2.Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654 [DOI] [PubMed] [Google Scholar]
- 3.Smith TC, Wingard DL, Smith B, Kritz-Silverstein D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. J Clin Epidemiol. 2007;60:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: a systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40:121–138. [DOI] [PubMed] [Google Scholar]
- 5.Winzer EB, Woitek F, Linke A. Physical activity in the prevention and treatment of coronary artery disease. J Am Heart Assoc. 2018;7:e007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadoglou NPE, Iliadis F, Liapis CD. Exercise and carotid atherosclerosis. Eur J Vasc Endovasc Surg. 2008;35:264–72. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. [DOI] [PubMed] [Google Scholar]
- 8.Stensland-Bugge E, Bonaa KH, Joakimsen O, Njolstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromsø study. Stroke. 2000;31:574–581. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Bi Y, Su J, Cui L, Han R, Tao R, Zhou J, Wu M, Qin Y. Physical activity and carotid atherosclerosis risk reduction in population with high risk for cardiovascular diseases: a cross-sectional study. BMC Public Health. 2022;22:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boss HM, van der Graaf Y, Visseren FLJ, Berg-Vos RMV, Bots ML, Borst GJ, Cramer MJ, Kappelle LJ, Geerlings MI. Physical activity and characteristics of the carotid artery wall in high-risk patients – the SMART (Second Manifestations of Arterial Disease) Study. J Am Heart Assoc. 2017;6:e005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turan TN, Nizam A, Lynn MJ, Egan BM, Le N, Lopes-Virella MF, Hermayer KL, Harrell J, Derdeyn CP, Fiorella D, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and hispanic residents of an urban community: The Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 15.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey. United States, 1985; (160):i–iv. 1–182. [PubMed] [Google Scholar]
- 16.Sacco RL, Gan R, Boden-Albala B, Fin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. [DOI] [PubMed] [Google Scholar]
- 18.Cheung YK, Yu G, Wall MM, Sacco RL, Elkind MSV, Willey JZ. Patterns of leisure-time physical activity using multivariate finite mixture modeling and cardiovascular risk factors in the Northern Manhattan Study. Ann Epidemiol. 2015;25:496–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Juo SH, Sacco RL. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, et al. Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc Dis. 2007;23:75–80. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez J, Khasiyev F, Liu M, DeRosa JT, Tom SE, Rundek T, Cheung K, Wright CB, Sacco RL, Elkind MSV. Determinants and outcomes of asymptomatic intracranial atherosclerotic stenosis. J Am Coll Cardiol. 2021;78:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2004;7:1025–1038. [DOI] [PubMed] [Google Scholar]
- 23.Tingly D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59:1–38.26917999 [Google Scholar]
- 24.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 25.Kubota Y, Iso H, Yamagishi K, Sawada N, Tsugane S, JPHC Study Group. Daily total physical activity and incident stroke: the Japan Public Health Center-based prospective study. Stroke. 2017;48:1730–1736. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Zhang S, Wang C, Gao X, Zhou Y, Zhou H, Wang A, Wu J, Bian L, Wu S, et al. Ideal cardiovascular health metrics on the prevalence of asymptomatic intracranial artery stenosis: a cross-sectional study. PLoS One. 2013;8:e58923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamal AK, Majeed F, Pasha O, Rehman H, Islam M, Azam I, Ilyas MS, Hussain M, Masood K, Ahmed B, et al. Clinical, lifestyle, socioeconomic determinants and rate of asymptomatic intracranial atherosclerosis in stroke free Pakistanis. BMC Neurol. 2014;14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi S, Turan TN, Lynn MJ, Derdeyn CP, Fiorella D, Janis LS, Chimowitz MI, SAMPPRIS Trial Investigators. Do patient characteristics explain the differences in outcome between medically treated patients in SAMMPRIS and WASID? Stroke. 2015;46:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamer M, Sabia S, Batty GB, Shipley MJ, Tabak AG, Singh-Manoux A, Kivimaki M. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2021;126:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandercappellen EJ, Henry RMA, Savelberg HHCM, van der Berg JD, Reesink KD, Schaper NC, Eussen SJPM, van Dongen MCJM, Dagnelie PC, Schram MT, et al. Association of the amount and pattern of physical activity with arterial stiffness: the Maastricht Study. J Am Heart Assoc. 2020;9:e017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Chen B, Kohl HW, Barlow CE, Lee CD, Radford NB, DeFina LF, Gabriel KP. The association of physical activity with carotid intima media thickening in a healthy older population: Cooper Center Longitudinal Study. J Aging Phys Act. 2019;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozakova M, Palombo C, Morizzo C, Nolan HH, Konrad T, Balkau B, RISC Investigators. Effect of sedentary behaviour and vigorous physical activity on segment-specific carotid wall thickness and its progression in a healthy population. Eur Heart J. 2010;31:1511–1519. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Cai Y, Zhao M, Sun J. Risk factors between intracranial-extracranial atherosclerosis and anterior-posterior circulation stroke in ischaemic stroke. Neurol Res. 2017;39:30–35. [DOI] [PubMed] [Google Scholar]
- 36.Kim JS, Nah HW, Park SM, Kim SK, Cho KJ, Lee J, Lee YS, Kim J, Ha SW, Kim EG, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–3318. [DOI] [PubMed] [Google Scholar]
- 37.Ryu WS, Park SS, Kim YS, Lee SH, Kang K, Kim C, Sohn CH, Lee SH, Yoon BW. Long-term natural history of intracranial arterial stenosis: an MRA follow-up study. Cerebrovasc Dis. 2014;38:290–296. [DOI] [PubMed] [Google Scholar]
- 38.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14;422–428. [DOI] [PubMed] [Google Scholar]
- 39.Polak JF, Sacco RL, Post WS, Vaidya D, Arnan MK, O’Leary DH. Incident stroke is associated with common carotid artery diameter and not common carotid artery intima-media thickness. Stroke. 2014;45:1442–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner H, Sacco RL, Rundek T, Battistella V, Cheung YK, Elkind MSV. Race and ethnic disparities in stroke incidence in the Northern Manhattan Study. Stroke. 2020;51:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Z, Liu W, Lu Y, Sun N, Chu Y, Chen H. The influence mechanism of community-built environment on the health of older adults: from the perspective of low-income groups. BMC Geriatr. 2022;22:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


