Abstract
Background:
Reports on outcomes following SARS-CoV-2 infection in lung transplant recipients remain limited.
Methods:
We performed a single-center, observational study of outcomes in lung transplant recipients diagnosed with SARS-CoV-2 between 5/1/2020 – 3/15/2022 that were followed for a median of 123 days. We analyzed changes in spirometry, ALAD incidence, hospitalization, mechanical ventilation needs, secondary infection, and survival.
Results:
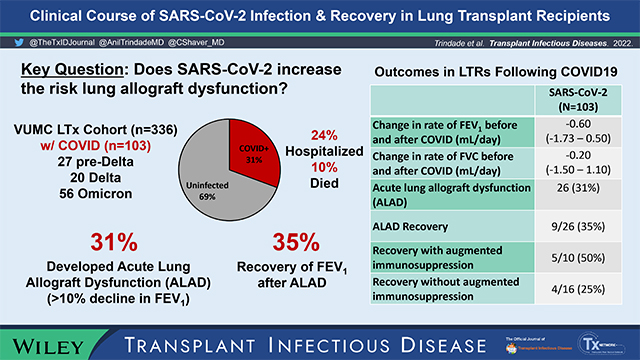
In our cohort of 336 patients, 103 developed COVID (27 pre-Delta, 20 Delta and 56 Omicron-era). Twenty-five patients (24%) required hospitalization and 10 patients ultimately died (10%). Among 85 survivors who completed ambulatory spirometry, COVID-19 did not alter change in FEV1 or FVC over time compared to the preceding 6 months. The pre-COVID FEV1 change was −0.05 mL/day (IQR −0.50 – 0.60) compared to −0.20 mL/day (IQR −1.40 – 0.70) post-COVID (p= 0.16). The pre-COVID change in FVC was 0.20 mL/day (IQR −0.60 – 0.70) compared to 0.05 mL/day (IQR −1.00 – 1.10) post-COVID (p= 0.76). While the cohort overall had stable lung function, 33 patients (39%) developed ALAD or accelerated CLAD (FEV1 decline >10% from pre-COVID baseline). Nine patients (35%) with ALAD recovered lung function. Within 3-months of acute COVID infection, 18 patients (17%) developed secondary infections, the majority being bacterial pneumonia. Finally, vaccination with at least 2 doses of mRNA vaccine was not associated with improved outcomes.
Conclusions:
This study describes the natural history of SARS-CoV-2 infection in a large cohort of lung transplant recipients. Although one-third of patients develop ALAD requiring augmented immunosuppression, infection with SARS-CoV-2 is not associated with worsening lung function.
Keywords: Lung transplantation, SARS-CoV-2, COVID-19, acute lung allograft dysfunction, ALAD, clinical outcomes
Graphical Abstract

INTRODUCTION:
The novel coronavirus, SARS-CoV-2, has emerged as a leading cause of morbidity and mortality throughout the general population worldwide.1 Hospitalization rates of those infected range from 0.5% to 9% (depending on age) and the case fatality rate is 1.2%.2 Solid-organ transplant recipients are at increased risk of severe COVID-19 illness, with mortality as high as 24%.3 In lung transplant recipients specifically, high hospitalization rates with mortality of 10–39% have been reported.4–7,31,32 In addition, co-infections and secondary infections are common in the immunosuppressed population.33,34 There are limited data on risk factors for severe disease in lung transplant patients with SARS-CoV-2 infection. One study showed that patients with pre-existing chronic lung allograft dysfunction (CLAD) had more rapid decline in lung function and higher mortality after COVID-19.8 Vaccination against SARS-CoV-2 has been shown to be protective for solid-organ transplant recipients; despite having a limited ability to generate an immune response,9–12 transplant recipients who received two-doses of mRNA vaccine had reduced mortality from COVID-19.13
The long-term effects of COVID-19 on lung allograft function and patient survival are not well understood. Mahan and colleagues reported frequent persistent lung parenchymal abnormalities on imaging (65%), mild to moderately impaired performance status, and a persistent decline in FEV1 or FVC >10% of baseline in a single-center analysis of 44 patients with median follow-up of 4.5 months post-COVID.14 In 24 lung transplant recipients with COVID-19, Permpalung et al. showed a high rate of hospital readmissions and secondary bacterial and fungal infections, though did not detect an increased incidence of acute cellular rejection (ACR), antibody-mediated rejection (AMR), de novo donor-specific antibodies (dnDSA), or lung function decline within 60–90 days of infection compared to matched controls.15
We report clinical outcomes following SARS-CoV-2 infection in an observational single-center study of 103 lung transplant patients across multiple SARS-CoV-2 variants. We focus specifically on the impact of COVID-19 on lung function, risk of acute lung allograft dysfunction (ALAD), and whether decline in lung function during COVID-19 illness is reversible. We hypothesize that acute SARS-CoV-2 infection will lead to an increased rate of lung function decline in lung transplant recipients.
METHODS:
Study population
We performed a single-center, observational study [VUMC Institutional Review Board #210566]. Adult (>18 years) lung transplant recipients who were at least 3 months post-transplant were included. A diagnosis of COVID-19 was based on positive quantitative polymerase chain reaction assays of nasopharyngeal swabs or a positive home test between 3/1/2020 and 3/15/2022. Patients were classified as “pre-Delta variant era” if infection occurred before 5/31/2021, “Delta variant era” if diagnosed between 6/1/2021 and 11/30/2021, and “Omicron variant era” if diagnosed after 12/1/2021, based on epidemiological data of variant prevalence in our geographic region; SARS-CoV-2 strains were not formally genotyped. Patients were considered vaccinated 2 weeks after the second dose of BNT162b2 (Pfizer) or mRNA-1273 (Moderna) vaccines or after 1 dose of JNJ-78436735 (Johnson & Johnson). Monoclonal antibody therapy was bamlanivimab during the pre-Delta era, casirivimab-imdevimab during the Delta era, and sotrovimab during the Omicron era, based on our hospital and regional public health guidelines. Patients were classified as diabetic if hemoglobin A1c level was >6.5% within 3 months of SARS-CoV-2 diagnosis or if they were prescribed glucose-lowering medications. ALAD was defined as a reduction in FEV1 >10% of pre-COVID-19 values. Secondary infections were diagnosed based on positive culture or PCR data within 90 days of SARS-CoV-2 diagnosis.
Immunosuppressive and antimicrobial protocols
At our center, standard maintenance immunosuppression included a calcineurin inhibitor (tacrolimus preferred, goal trough 10–14 ng/mL within the first-year post-transplant, followed by goal 8–12 ng/mL thereafter), an antiproliferative agent (mycophenolate mofetil preferred, 1000mg twice daily), and prednisone (tapered from 20mg daily to 5mg daily over the first 3 months post-transplant).16 The anti-proliferative was held if a patient had persistent or recurrent infection, malignancy, or cytopenias. Standard infection prophylaxis included trimethoprim-sulfamethoxazole, valganciclovir (for 6–12 months post-transplant depending on donor/recipient CMV serostatus), and an antifungal (posaconazole preferred through 2 months post-transplant). SARS-CoV-2 vaccination was recommended beginning in January 2021 with mRNA vaccination preferred. Monoclonal antibody therapy was readily available beginning in November 2020.
Management of SARS-CoV-2 infection and recovery
Patients diagnosed with SARS-CoV-2 were managed according to our center’s protocol. Patients were contacted daily for assessment of vital signs and symptoms. Anti-proliferative immunosuppression was held during acute infection. Patients were encouraged to receive monoclonal antibody infusion as soon as possible.17,18 Patients admitted to the hospital were treated with remdesevir, corticosteroids, and/or baricitinib per hospital protocols at the time of diagnosis.19 After completion of CDC-recommended isolation,6 patients were assessed in clinic with chest X-ray imaging, formal spirometry, and measurement of donor-specific anti-HLA antibodies using a solid-phase flow cytometry assay (Luminex)20. Bronchoscopy with bronchoalveolar lavage and transbronchial biopsies was performed if there was a >10% decline in FEV1 compared to pre-COVID baseline values or if there were chest imaging abnormalities that persisted beyond the first 4–6 weeks, unless contraindicated.21 Tissue specimens were assessed by two pulmonary pathologists and graded for ACR and other pathology according to ISHLT guidelines.22 Persistent non-infectious ALAD was treated with either corticosteroids (methylprednisolone 7.5mg/kg IV daily x 3 days, followed by prednisone taper), anti-thymocyte globulin, or plasmapheresis (PLEX) with IVIg.23 Probable antibody mediated rejection was treated with PLEX, IVIg, and rituximab.35
Primary and secondary clinical outcomes
The primary study endpoint was a change in rate of FEV1 before and after SARS-CoV-2 infection. Patients with at least two FEV1 measurements (separated by 30 days) before and after COVID infection were included, and the change in FEV1 or FVC (mL/day) was calculated by identifying the slope of trendlines pre-COVID and post-COVID. Spirometry was performed at the VUMC Pulmonary Function Lab according to ATS/ERS guidelines.36 Secondary outcomes included incidence of ALAD, AMR, dnDSA, hospitalization, intubation, length of hospital stay, secondary infections, and death.35
Statistics
Continuous variables were described as median with interquartile range and categorial variables as proportions. The rates of change in FEV1 and FVC (mL/day) before and after SARS-CoV-2 infection were compared using Wilcoxon ranked sum testing. Comparisons between SARS-CoV-2 eras (pre-Delta, Delta, or Omicron) were performed using Kruskal-Wallis testing for continuous variables and Chi-squared testing for categorical variables. Univariate analysis to assess for ALAD risk factors was performed using a Fisher’s exact test for categorical variables and Mann-Whitney U tests for continuous variables.
RESULTS
Study population
A total of 103 patients developed SARS-CoV-2 during the study period (Table 1), representing 30.7% of the VUMC living lung transplant recipient cohort (n=336). Of these, 27 patients were classified as having pre-Delta-era infections, 20 patients with Delta-era infections, and 56 patients with Omicron-era infections. The median follow- up time after SARS-CoV-2 diagnosis was 123 days (IQR 98–213). Patients acquired COVID-19 a median of 3.5 years post-transplant (IQR 1.6 – 6.5). Most patients (91%) had upper or lower respiratory tract infection symptoms. The majority of patients (77%) were CLAD-free when infected with COVID, while 14% had CLAD stage 1 and 8% of patients had CLAD stage 2 or greater. Patient demographics and maintenance immunosuppression were similar across SARS-CoV-2 eras (Table 1). Most patients (69%) received outpatient monoclonal antibody therapy, with increased administration to patients during the Delta and Omicron eras. In our cohort, 25 patients (24%) required hospitalization for acute SARS-CoV-2 infection, with 7 (7%) patients requiring mechanical ventilation (invasive or non-invasive). A total of 10 patients (10%) died, 6 of whom died during the initial hospitalization for acute COVID-19. Rates of hospitalization, intubation, and length of stay did not differ between SARS-CoV-2 variants. For patients in the Delta variant era, a greater concentration of supplemental oxygen was required.
Table 1:
Baseline characteristics of the study cohort
| Total SARS-CoV-2 (N=103) | SARS-CoV-2 Pre-Delta Era (N=27) | SARS-CoV-2 Delta Era (N=20) | SARS-CoV-2 Omicron Era (N=56) | p value | |
|---|---|---|---|---|---|
| Age (years) | 62 (52–68) | 58 (47–64) | 62 (55–68) | 63 (46–68) | 0.15 |
| Female Sex | 49 (48%) | 13 (48%) | 10 (50%) | 26 (46%) | 0.96 |
| White Race | 87 (84%) | 24 (89%) | 17 (85%) | 46 (82%) | 0.73 |
| LAS at Transplant | 39.5 (35.9–49) | 40.4 (35.2–49.8) | 39.4 (34.4–46.7) | 39.9 (37.1–48.0) | 0.75 |
| Bilateral Transplant | 80 (78%) | 21 (78%) | 14 (70%) | 45 (80%) | 0.63 |
| Transplant Indication | 0.49 | ||||
| Obstructive | 20 (19%) | 5 (19%) | 5 (25%) | 10 (18%) | |
| Pulmonary Vascular | 3 (3%) | 1 (4%) | 0 (0%) | 2 (4%) | |
| CF/ Bronchiectasis | 24 (23%) | 10 (37%) | 2 (10%) | 12 (21%) | |
| Diffuse Parenchymal | 56 (54%) | 11 (41%) | 13 (65%) | 32 (57%) | |
| Time from transplant to SARS-CoV-2 (years) | 3.5 (1.6–6.5) | 4.4 (2.6–7.1) | 3.0 (1.9–5.4) | 3.3 (1.6–6.5) | 0.34 |
| BMI* | 27.3 (23.7–30) | 27.3 (23.1–29.5) | 27.8 (25.4–29.5) | 26.5 (23.8–30.3) | 0.80 |
| Diabetes* | 37 (36%) | 13 (48%) | 6 (30%) | 18 (32%) | 0.30 |
| Calcineurin Inhibitor Type* | 0.76 | ||||
| Tacrolimus | 94 (91%) | 24 (89%) | 19 (95%) | 51 (91%) | |
| Cyclosporine A | 9 (9%) | 3 (11%) | 1 (5%) | 5 (9%) | |
| Anti-proliferative Type* | 0.44 | ||||
| Mycophenolate | 54 (52%) | 15 (56%) | 8 (40%) | 31 (55%) | |
| Azathioprine | 13 (13%) | 4 (15%) | 1 (5%) | 8 (14%) | |
| Sirolimus | 5 (5%) | 2 (7%) | 1 (5%) | 2 (4%) | |
| No Anti-proliferative | 31 (30%) | 6 (22%) | 10 (50%) | 15 (27%) | |
| Prednisone | 103 (100%) | 27 (100%) | 20 (100%) | 56 (100%) | 1.00 |
| CLAD Stage* | 0.43 | ||||
| 0 | 79 (77%) | 23 (85%) | 18 (90%) | 40 (71%) | |
| 1 | 14 (14%) | 1 (4%) | 1 (5%) | 11 (20%) | |
| 2 | 3 (3%) | 2 (7%) | 0 (0%) | 1 (2%) | |
| 3 or 4 | 6 (6%) | 1 (4%) | 1 (5%) | 4 (7%) | |
| Symptomatic SARS-CoV-2 | 94 (91%) | 24 (89%) | 18 (90%) | 52 (93%) | 0.81 |
| SARS-CoV-2 Vaccinated* | 69 (67%) | 0 | 17 (85%) | 53 (95%) | <0.01 |
| mRNA vaccine (2 doses) | 0 | 15 (88%) | 51 (96%) | 0.25 | |
| Non-mRNA vaccine | 0 | 2 (12%) | 2 (4%) | ||
| Time from Vaccine to COVID | 269 (175–298) | n/a | 156 (126–176) | 281 (261–310) | <0.01 |
| Tixagevimab / Cilgavimab | 4 (4%) | 0 | 0 | 4 (7%) | 0.89 |
| Monoclonal Antibody Therapy | 71 (69%) | 13 (48%) | 17 (85%) | 41 (73%) | 0.02 |
| Other Therapies | 22 (21%) | 5 (19%) | 6 (30%) | 11 (20%) | 0.57 |
| Remdesevir | 15 15%) | 2 (7%) | 5 (25%) | 8 (14%) | |
| Dexamethasone / Steroids | 19 (18%) | 3 (11%) | 6 (30%) | 10 (18%) | |
| Baricitinib | 3 (3%) | 0 | 2 (10%) | 1 (2%) | |
| Molnupiravir | 1 (1%) | 0 | 0 | 1 (2%) | |
| Time first follow-up appt (d) | 30 (27–39) | 27 (25–37) | 33 (28–40) | 32 (28–38) | 0.15 |
| Days follow-up after COVID | 123 (98 – 213) | 414 (376 – 439) | 147 (133 – 163) | 100 (72–114) | <0.01 |
At time of SARS-CoV-2 diagnosis
Table indicates n (%) for categorical variables or median (interquartile range) for continuous variables.
SARS-CoV-2 infection did not change the rate of decline of FEV1 or FVC in lung transplant recipients
A minimum of two spirometric assessments (separated by at least 30 days) both pre-COVID and post-COVID were available for 85 patients (23 patients pre-Delta-era, 18 patients in the Delta-era, and 44 patients in the Omicron-era). There were 18 patients who did not meet the criteria for inclusion in spirometry analysis (6 died during the index hospital stay, 2 discharged to hospice, 1 unable to perform spirometry due to pre-existing chronic comorbidity, 1 without post-transplant FEV1 baseline, and 8 who did not have two spirometric assessments 30 days apart). Overall, the rate of FEV1 and FVC change did not differ pre- and post- COVID-19 disease. The median rate of FEV1 change pre-COVID was −0.05 mL/day (IQR −0.50 – 0.60) compared to −0.20mL/day (IQR −1.40 – 0.70) post-COVID (p= 0.16) (Figure 1, Table 2). The median rate of change in FVC pre-COVID was 0.20 mL/day (IQR −0.60 – 0.70) compared to 0.05mL/day (IQR −1.00 – 1.10) post-COVID (p= 0.76). During the Delta era, there was a significant difference in FEV1 change pre-COVID compared to post-COVID [median FEV1 change pre-COVID 0.25 mL/day (IQR −0.06 – 0.60) compared to −0.20 mL/day (IQR −0.58 – 0.15 mL/day) post-COVID, p=0.03]; in contrast, there was no significant change in rate of FEV1 decline in the pre-Delta or Omicron eras. There was no significant impact of SARS-CoV-2 variant on FVC change over time.
Figure 1. The effect of SARS-CoV-2 infection on rate of change of FEV1 in lung transplant recipients.

FEV1 rate of change was calculated before and after SARS-CoV-2 infection for 85 lung transplant recipients with two spirometric measurements at least 30 days apart after completion of isolation after acute SARS-CoV-2 infection. The slope of FEV1 change over time was calculated over 3–6 months before SARS-CoV-2 infection and for a minimum of 3 months after SARS-CoV-2 infection. Change in the slope of FEV1 decline before and after SARS-CoV-2 were compared by Wilcoxon ranked sum testing. A) Pre-Delta era, n=23. B) Delta era, n=18. C) Omicron era, n=44.
Table 2:
Clinical Outcomes in Lung allograft Recipients with COVID
| Total SARS-CoV-2 (N=103) | SARS-CoV-2 Pre-Delta Era (N=27) | SARS-CoV-2 Delta Era (N=20) | SARS-CoV-2 Omicron Era (N=56) | p value | |
| Hospitalized for SARS-CoV-2 | 25 (24%) | 8 (30%) | 7 (35%) | 10 (18%) | 0.23 |
| Peak FiO2 | 0.30 (0.21 – 1.0) | 0.21 (0.21 – 0.24) | 0.66 (0.27 – 1.0) | 0.35 (0.23 – 0.93) | 0.39 |
| Mechanical Ventilation | 7 (7%) | 1 (4%) | 3 (15%) | 3 (5%) | 0.26 |
| Hospital Length of Stay (d) | 6 (5–11) | 5 (4 – 7) | 5 (5 – 14) | 8 (6 – 17) | 0.33 |
| Death within 6 months of COVID | 10 (10%) | 3 (4%) | 3 (6%) | 4 (7%) | 0.57 |
| In-hospital / acute COVID | 6 (6%) | 1 (4%) | 2 (10%) | 3 (5%) | 0.64 |
| Post-acute infection | 4 (4%) | 2 (7%) | 1 (5%) | 1 (2%) | 0.44 |
| CXR changes >4 wk post-COVID diagnosis | 15 (15%) | 6 (22%) | 5 (25%) | 4 (7%) | 0.06 |
| Accelerated CLAD or death In patients with prior CLAD | 11/22 (50%) | 3/4 (75%) | 1/3 (33%) | 7/15 (47%) | 0.50 |
| Probable AMR (< 6 mo post-COVID) | 3 (3%) | 0 | 1 (5%) | 2 (4%) | 0.98 |
| Secondary infections within 90 d* | 14 (14%) | 1 (4%) | 2 (10%) | 11 (20%) | 0.12 |
| Cytomegalovirus viremia | 4 (4%) | 0 | 0 | 4 (7%) | |
| Community acquired viral infection | 5 (5%) | 0 | 1 (5%) | 4 (7%) | |
| Bacterial pneumonia | 7 (7%) | 0 | 1 (5%) | 5 (9%) | |
| Staphylococcus species | 3 (3%) | 0 | 0 | 2 (4%) | |
| Gram negative rods | 4 (4%) | 0 | 1 (5%) | 4 (8%) | |
| Bacteremia (MRSA) | 0 | 1 (4%) | 0 | 0 | |
| Total SARS-CoV-2 (N=85) | SARS-CoV-2 Pre-Delta Era (N=23) | SARS-CoV-2 Delta Era (N=18) | SARS-CoV-2 Omicron Era (N=44) | p value | |
| Change in rate of FEV1 decline before and after COVID (mL/d) | −0.60 (−1.73 – 0.50) | 0.16 (−1.11 – 1.10) | −0.69 (−1.35 to −0.15) | −1.05 (−2.53 – 0.40) | 0.19 |
| Change in rate of FVC decline before and after COVID (mL/d) | −0.20 (−1.50 – 1.10) | 0.60 (−0.45 – 1.20) | −0.54 (−0.80 – 0.33) | −0.75 (−2.63 – 1.38) | 0.07 |
| Acute decline in FEV1 >10% baseline values (ALAD) | 26 (31%) | 7 (30%) | 5 (28%) | 14 (32%) | 0.95 |
| For-cause bronchoscopy (ALAD) | 16 /26 (62%) | 5/7 (71%) | 3/5 (60%) | 8/14 (57%) | 0.31 |
| Etiology of ALAD | |||||
| ACR | 3 (4%) | 1 (4%) | 0 | 2 (5%) | |
| Organizing pneumonia | 4 (5%) | 0 | 2 (11%) | 2 (5%) | |
| Super-imposed infection | 1 (1%) | 0 | 1 (6%) | 0 | |
| Inflammation, non-specific | 8 (9%) | 4 (17%) | 0 | 4 (9%) | |
| Treatment of Post-COVID ALAD# | 9 /26 (35%) | 3 /7 (43%) | 2/5 (40%) | 4/14 (29%) | 0.60 |
| Steroids | 9 (35%) | 3 (43%) | 2 (40%) | 4 (29%) | |
| Anti-thymocyte globulin | 1 (4%) | 0 | 0 | 1 (7%) | |
| Plasma Exchange and IVIg | 2 (8%) | 0 | 0 | 2 (14%) | |
| ALAD Recovery | 9/26 (35%) | 3/7 (43%) | 2/5 (40%) | 4/14 (29%) | 0.59 |
| Recovery following treatment | 5/10 (50%) | 2/3 (66%) | 1/3 (33%) | 2/4 (50%) | 0.72 |
| Recovery without treatment | 4/16 (25%) | 1/4 (25%) | 1/2 (50%) | 2/10 (20%) | 0.67 |
Table indicates n (%) for categorical variables or median (interquartile range) for continuous variables.
Some patients had multiple infections
Some patients had combination therapies
Clinical course after SARS-CoV-2
Among the cohort of COVID-19 survivors (both hospitalized and non-hospitalized) that performed follow-up lung function testing (N=85), 33 patients developed ALAD or accelerated CLAD, defined as FEV1 decline >10% of baseline pre-COVID values. Of these patients, 26 patients (31%) met criteria for ALAD, while the remaining 7 had accelerated CLAD. A total of 19 patients (16 with ALAD and 3 with accelerated CLAD) underwent diagnostic bronchoscopy (Table 2). Common pathologic findings for the patients with ALAD included non-specific inflammation (11 patients), organizing pneumonia (5 patients), and biopsy-proven ACR (3 patients). For the patients with accelerated CLAD, diagnostic bronchoscopy revealed non-specific inflammation in 2 patients and was non-diagnostic in the other. Nine of the 26 patients (35%) with ALAD underwent treatment with either corticosteroids, anti-thymocyte globulin, or PLEX with IVIg. After 3 months of follow up, 9/26 (35%) patients with ALAD had recovery of FEV1 to within 10% of their pre-COVID values. There was a trend towards improved FEV1 in patients that received treatment for ALAD, though this was not statistically significant (50% vs 25%, p= 0.23). Twenty-two patients in the cohort met criteria for CLAD prior to acquiring COVID-19. Four (18%) of those patients died. As described above, 7 patients with CLAD experienced a >10% decline in FEV1 compared to pre-COVID values within 3 months of contracting SARS-CoV-2. A univariate analysis was performed to assess for risk factors for ALAD or accelerated CLAD after SARS-CoV-2. Lung allocation score was associated with increased ALAD risk, whereas azathioprine was associated with reduced ALAD risk. Diabetes was associated with increased risk for ALAD, but not progressive CLAD. Pre-existing CLAD, non-vaccinated status, and diffuse parenchymal lung disease as the underlying indication for transplant did not reach statistical significance as risk factors for ALAD (See Table 3). Secondary infection occurred in 14% of the cohort and was not associated with SARS-CoV-2 variant era type. Bacterial pneumonia was the most common infection, followed by community acquired viruses and CMV viremia.
Table 3:
Univariate Analysis Risk Factors for Post-COVID ALAD or Accelerated CLAD*
| ALAD or Accelerated CLAD (N= 33) | No Allograft Dysfunction (N=52) | P value | |
|---|---|---|---|
| ALAD | 26 (79%) | 0 | |
| Accelerated CLAD | 7 (21%) | 0 | |
| COVID Variant Era | |||
| Pre-delta | 10 (30%) | 13 (25%) | 0.62 |
| Delta | 5 (15%) | 13 (25%) | 0.41 |
| Omicron | 18 (55%) | 26 (50%) | 0.82 |
| Symptomatic COVID | 32 (97%) | 46 (88%) | 0.24 |
| Need for Hospitalization | 8 (24%) | 8 (15%) | 0.40 |
| COVID Vaccination | 18 (55%) | 38 (73%) | 0.10 |
| Receipt of Monoclonal Ab | 22 (66%) | 38 (73%) | 0.63 |
| Time from Transplant to COVID Diagnosis (years) | 3.49 (2.12 – 6.60) | 3.39 (1.57 – 6.40) | 0.75 |
| Age | 62 (52 – 67) | 62 (54 – 67) | 0.99 |
| Female sex | 15 (45%) | 28 (54%) | 0.51 |
| White Race | 27 (82%) | 45 (87%) | 0.55 |
| Bilateral Lung Transplant | 25 (76%) | 44 (85%) | 0.40 |
| Lung Allocation Score | 42.24 (38.36 – 52.02) | 38.71 (35.23 – 46.41) | 0.03 |
| Indication for Lung Transplant | |||
| Obstructive | 2 (6%) | 13 (25%) | 0.04 |
| Pulmonary Vascular | 0 | 3 (6%) | 0.28 |
| CF/ Bronchiectasis | 9 (27%) | 11 (21%) | 0.60 |
| Diffuse Parenchymal | 22 (66%) | 25 (48%) | 0.12 |
| Pre-existing CLAD | 11 (33%) | 8 (15%) | 0.07 |
| Diabetes | 13 (39%) | 16 (31%) | 0.48 |
| Diabetes as a risk factor for ALAD only | 12/26 (46%) | 16/59 (27%) | 0.13 |
| Last known estimated glomerular filtration rate pre-COVID (mL/min) | 43 (29 – 56) | 43 (30 – 56) | 0.85 |
| Body Mass Index | 26.7 (23.6 – 29.9) | 27.8 (24.3 – 30.1) | 0.79 |
| Antiproliferative Type | |||
| Mycophenolate | 19 (58%) | 24 (46%) | 0.38 |
| Azathioprine | 1 (3%) | 10 (19%) | 0.04 |
| Sirolimus | 2 (6%) | 3 (6%) | 1.0 |
| None | 11 (33%) | 15 (29%) | 0.81 |
For patients able to complete >2 spirometry assessments (>30 days apart) pre- and post-COVID
Impact of Vaccination Status on Clinical Outcomes
Because of the timing of vaccine availability, none of the lung transplant recipients that acquired pre-Delta SARS-CoV-2 variants were vaccinated. Two doses of mRNA vaccine were completed in 15 patients in the Delta era and 51 patients in the Omicron era, with 2 additional patients in each group completing non-mRNA vaccination (Table 1). The median time between completion of the primary vaccination series and SARS-CoV-2 diagnosis was 269 days (156 days for Delta, 281 for Omicron). Vaccination status had no significant impact on FEV1 trajectory pre- and post-COVID infection; the median change in FEV1 in vaccinated patients was −0.85 mL/day (IQR −2.14 to 0.25) compared to 0 mL/day (IQR −1.42 to +1.10) in unvaccinated patients. There was an association between vaccination status and change in FVC [−0.71 mL/day (IQR −2.30 to −.90) in vaccinated patients vs. 0.50 ml/day (IQR −0.70 to 1.10) in unvaccinated patients, p= 0.02]. Vaccination status had no detectable impact on rates of hospitalization, intubation, or death from acute COVID-19 (Supplemental Table 1, p>0.1 for each). Vaccination status was not associated with AMR or acceleration of CLAD. There was a non-significant trend towards higher incidence of persistent chest radiograph abnormalities and ALAD in vaccinated patients (p=0.07 and 0.14, respectively).
DISCUSSION
In this single-center, observational study, we report the clinical course of lung transplant recipients infected by SARS-CoV-2 from initial illness through 3 months of follow-up. Overall, SARS-CoV-2 infection was not associated with a change in the rate of change of FEV1 or FVC. However, 39% of lung transplant recipients developed ALAD or accelerated CLAD after SARS-CoV-2 infection, with approximately one-third recovering lung function back to baseline during the follow up period. Non-specific inflammation was the most common pathology found on lung biopsy during recovery. We identified potential risk factors for ALAD including higher LAS at the time of transplant, lack of azathioprine use, and diabetes.
Our findings build on a small cohort of patients described by Permpalung et al., who had an acute FEV1 decline of >10% (ALAD) in 20% of their cohort. Rates of biopsy-proven ACR, dnDSA, and AMR were similarly low15. Mahan et al. found a higher rate of ALAD in their cohort, with almost half of patients having a sustained decline in FEV1 or FVC >10%.14 This may be due to higher rates of hospitalization (88%), lower rates of monoclonal antibody infusion (13.6%), or infection with pre-Delta variants. Our data also build on these previous studies by specifically assessing for differential impact of SARS-CoV-2 variants on clinical outcomes. We showed that infection with the Delta variant was associated with a change in the rate of FEV1 decline, but we did not find spirometric differences in pre-Delta or Omicron-era infections. We found that the rate of secondary bacterial infections was greatest during the Omicron era, despite an overwhelming majority of patients being vaccinated and receiving appropriate monoclonal antibody therapy within the first few days of their illness.
Seventy three percent of the patients that developed ALAD did not require hospitalization for their acute SARS-CoV-2 infection, suggesting that minimal disease from SARS-CoV-2 is sufficient to trigger allograft injury. Our center chose to treat patients surviving COVD-19 who had persistent lung dysfunction for presumed immune-mediated lung injury with augmentation of immunosuppression with steroids, ATG, or PLEX with IVIg, depending on the clinical context. Augmented immunosuppression for COVID-related ALAD was associated with increased recovery of FEV1, suggesting that COVID-induced lung inflammation in lung transplant recipients may be potentially reversible. In addition, we identified a trend that patients with diffuse parenchyma lung disease may have increased ALAD risk. Others have previously reported an association between telomere length and COVID-19 severity.37,38 One possible explanation of these findings could be that genetic telomeropathies in the setting of diffuse parenchymal lung disease may alter immune responses during post-COVID ALAD. The potential protective benefit of azathioprine is also interesting. Multiple groups have identified a decreased humoral immune response with mycophenolate in the setting of vaccine responsiveness.39 It is possible that the antiproliferative immunosuppressants may potentiate early pro-inflammatory responses following infection with SARS-CoV-2. Further mechanistic work is needed to define the specific inflammatory pathways triggered by SARS-CoV-2 and whether targeted therapy is possible in the future to reduce the risk of ALAD. The finding that SARS-CoV-2 pneumonia can precipitate persistent inflammation may be similar to allograft injury triggered by other community-acquired respiratory viral infections, which is well-described.25–28 Longer follow-up of this patient cohort is needed to determine whether SARS-CoV-2 infection increases risk of CLAD.
The rate of infection in vaccinated individuals in our cohort (16/1000 patient months) is similar to other reports in solid organ transplant recipients29. Our lack of mortality benefit from vaccination differs from one previous registry study in the United Kingdom that showed reduced mortality from COVID-19 in solid-organ transplant recipients who were fully vaccinated,13 though the number of lung transplant recipients included in the registry was not stated. Alternatively, differences in compliance with public health measures may differ between the UK and the southeastern United States. Our study suggests that vaccinated lung transplant patients remain at risk for SARS-CoV-2-induced ALAD, a finding that will require additional follow-up in larger study cohorts. We were unable to statistically test the impact of supplemental booster doses of vaccination in our cohort, because the majority of Delta infections occurred prior to booster dose administration and the majority of Omicron infections occurred despite booster dosing. Additional doses of mRNA vaccines and prophylactic monoclonal antibodies like tixagevimab/cilgavimab may provide additional protection from poor clinical outcomes after SARS-CoV-2.
Our study has several strengths. This is a large cohort study specifically focused on lung transplant recipients in a real-world management setting. Comparison of changes in FEV1 over time pre-and post-COVID infection is more robust than comparison of singular spirometric values. Changes in spirometry provide more significant prognostic value than absolute values.30 In addition, use of each patient’s previous spirometry over time strengthened the statistical power of our analysis. We show that the impact of SARS-CoV-2 changes with different variants and that increased immunosuppression after initial infection may facilitate lung function recovery. Our study also has some important limitations. This is a retrospective single-center study. Diagnostic assays for COVID varied widely from hospital-based PCR tests to home kits, raising the possibility that some false-positive tests were included; since 91% of patients were symptomatic at the time of COVID diagnosis, the false positive rate was likely low. We did not collect data on specific symptoms at the time of COVID diagnosis to allow for delineation of upper versus lower respiratory tract disease. Several patients were unable to complete the required spirometric follow up to be included in the analysis, potentially introducing selection bias in our conclusions. Differences in local public health compliance and vaccination adherence may impact the generalizability of our findings. None of our patients with pre-Delta infections had been vaccinated prior to SARS-CoV-2 diagnosis, so we were unable to assess vaccine efficacy in this group.
In conclusion, in an observational cohort of lung transplant recipients, we demonstrate that SARS-CoV-2 infection has limited impact on trajectory of lung function. While many survivors developed transient ALAD, augmentation of immunosuppression promoted recovery of lung function in a minority of patients. This study provides key information about short-term clinical outcomes after COVID infection in lung transplant recipients and provides data that can guide design of future interventional studies to improve clinical outcomes.
Supplementary Material
Funding:
This work was supported by NIH HL136888 (CMS).
Abbreviations:
- ACR
acute cellular rejection
- AFOP
acute fibrinous organizing pneumonia
- AMR
antibody-mediated rejection
- ALAD
acute lung allograft dysfunction
- CARV
Community-acquired respiratory viruses
- CLAD
chronic lung allograft dysfunction
- CMV
cytomegalovirus
- COVID
coronavirus disease
- dnDSA
de novo donor specific antibodies
- FEV1
Forced Expiratory Volume in 1 second
- FiO2
fraction of inspired oxygen
- FVC
Forced Vital Capacity
- ISHLT
International Society of Heart and Lung Transplantation
- IVIg
intravenous immunoglobulin
- LAS
Lung allocation score
- LOS
length of stay
- OP
organizing pneumonia
- PLEX
Plasma exchange
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplant
- VUMC
Vanderbilt University Medical Center
Footnotes
Disclosures:
The authors of this manuscript have no conflicts of interest to disclose.
COVID19 in lung transplant recipients is associated with acute lung allograft dysfunction, but long-term lung function remains stable.
Data Sharing and Data Accessibility
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. Any other reasonable information is available from the corresponding author (AJT) upon request.
References
- 1.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. (In eng). DOI: 10.1136/bmj.n436. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and Mortality Among Adults Hospitalized With COVID-19 at US Medical Centers. JAMA Netw Open 2021;4(3):e210417. (In eng). DOI: 10.1001/jamanetworkopen.2021.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heldman MR, Kates OS, Safa K, et al. COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: A comparative analysis from a multicenter study. Am J Transplant 2021;21(8):2774–2784. DOI: 10.1111/ajt.16692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messika J, Eloy P, Roux A, et al. COVID-19 in Lung Transplant Recipients. Transplantation 2021;105(1):177–186. (In eng). DOI: 10.1097/tp.0000000000003508. [DOI] [PubMed] [Google Scholar]
- 5.Verleden GM, Godinas L, Lorent N, et al. COVID-19 in lung transplant patients: A case series. Am J Transplant 2020;20(11):3234–3238. (In eng). DOI: 10.1111/ajt.16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanka MR, Mahan LD, Joerns J, et al. Clinical characteristics, management practices, and outcomes among lung transplant patients with COVID-19. J Heart Lung Transplant 2021;40(9):936–947. (In eng). DOI: 10.1016/j.healun.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence A, Mahan LD, Mohanka MR, et al. Predictors and outcomes of respiratory failure among lung transplant patients with COVID-19. Clin Transplant 2021:e14540. (In eng). DOI: 10.1111/ctr.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients-Risk prediction and outcomes. PLoS One 2021;16(10):e0257807. (In eng). DOI: 10.1371/journal.pone.0257807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021;325(21):2204–2206. DOI: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant 2021;40(8):763–766. DOI: 10.1016/j.healun.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shostak Y, Shafran N, Heching M, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med 2021;9(6):e52–e53. DOI: 10.1016/S2213-2600(21)00184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett AM, Greenberg RS, Boyarsky BJ, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant 2021;40(12):1579–1588. DOI: 10.1016/j.healun.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two Doses of SARS-CoV-2 Vaccines Reduce Risk of Death Due to COVID-19 in Solid Organ Transplant Recipients: Preliminary Outcomes From a UK Registry Linkage Analysis. Transplantation 2021;105(11):e263–e264. DOI: 10.1097/TP.0000000000003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahan LD, Lill I, Halverson Q, et al. Post-infection pulmonary sequelae after COVID-19 among patients with lung transplantation. Transpl Infect Dis 2021;23(6):e13739. (In eng). DOI: 10.1111/tid.13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Permpalung N, Bazemore K, Chiang TP, et al. Impact of COVID-19 on Lung Allograft and Clinical Outcomes in Lung Transplant Recipients: A Case-control Study. Transplantation 2021;105(9):2072–2079. (In eng). DOI: 10.1097/tp.0000000000003839. [DOI] [PubMed] [Google Scholar]
- 16.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis 2014;6(8):1039–53. (In eng). DOI: 10.3978/j.issn.2072-1439.2014.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol 2021;21(6):382–393. (In eng). DOI: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarrell BA, Bloch K, El Chediak A, et al. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transpl Infect Dis 2022;24(1):e13759. (In eng). DOI: 10.1111/tid.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383(19):1813–1826. (In eng). DOI: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westall GP, Paraskeva MA, Snell GI. Antibody-mediated rejection. Curr Opin Organ Transplant 2015;20(5):492–7. (In eng). DOI: 10.1097/mot.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 21.Martinu T, Koutsokera A, Benden C, et al. International Society for Heart and Lung Transplantation consensus statement for the standardization of bronchoalveolar lavage in lung transplantation. J Heart Lung Transplant 2020. (In eng). DOI: 10.1016/j.healun.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26(12):1229–42. (In eng). DOI: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Hage CA, Klesney-Tait J, Wille K, et al. Extracorporeal photopheresis to attenuate decline in lung function due to refractory obstructive allograft dysfunction. Transfus Med 2021;31(4):292–302. (In eng). DOI: 10.1111/tme.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roux A, Levine DJ, Zeevi A, et al. Banff Lung Report: Current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR). Am J Transplant 2019;19(1):21–31. (In eng). DOI: 10.1111/ajt.14990. [DOI] [PubMed] [Google Scholar]
- 25.Sayah DM, Koff JL, Leard LE, Hays SR, Golden JA, Singer JP. Rhinovirus and other respiratory viruses exert different effects on lung allograft function that are not mediated through acute rejection. Clin Transplant 2013;27(1):E64–71. (In eng). DOI: 10.1111/ctr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shino MY, Weigt SS, Li N, et al. Impact of Allograft Injury Time of Onset on the Development of Chronic Lung Allograft Dysfunction After Lung Transplantation. Am J Transplant 2017;17(5):1294–1303. (In eng). DOI: 10.1111/ajt.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigt SS, Derhovanessian A, Liao E, et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. Am J Transplant 2012;12(2):477–84. (In eng). DOI: 10.1111/j.1600-6143.2011.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunasekaran M, Bansal S, Ravichandran R, et al. Respiratory viral infection in lung transplantation induces exosomes that trigger chronic rejection. J Heart Lung Transplant 2020;39(4):379–388. (In eng). DOI: 10.1016/j.healun.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Zheng Q, Madhira V, et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern Med 2021. DOI: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usmani OS. Calling Time on Spirometry: Unlocking the Silent Zone in Acute Rejection after Lung Transplantation. Am J Respir Crit Care Med 2020;201(12):1468–1470. (In eng). DOI: 10.1164/rccm.202003-0581ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez-Giménez B, Berastegui C, Barrecheguren M, et al. COVID-19 in lung transplant recipients: A multicenter study. Am J Transplant. May 2021;21(5):1816–1824. doi: 10.1111/ajt.16364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An W, Wang Q, Kim TE, Kang JS. Clinical characteristics and outcome of coronavirus disease 2019 infection in patients with solid organ transplants: A systematic review and meta-analysis. J Infect Public Health. Mar 2022;15(3):365–372. doi: 10.1016/j.jiph.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fekkar A, Lampros A, Mayaux J, et al. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am J Respir Crit Care Med. Feb 1 2021;203(3):307–317. doi: 10.1164/rccm.202009-3400OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripa M, Galli L, Poli A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. Mar 2021;27(3):451–457. doi: 10.1016/j.cmi.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant Apr 2016;35(4):397–406. doi: 10.1016/j.healun.2016.01.1223 [DOI] [PubMed] [Google Scholar]
- 36.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. Oct 15 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGroder CF, Zhang D, Choudhury MA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax Dec 2021;76(12):1242–1245. doi: 10.1136/thoraxjnl-2021-217031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsilingiris D, Tentolouris A, Eleftheriadou I, Tentolouris N. Telomere length, epidemiology and pathogenesis of severe COVID-19. Eur J Clin Invest. 2020:e13376. vol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. Feb 2022;22(2):634–639. doi: 10.1111/ajt.16851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. Any other reasonable information is available from the corresponding author (AJT) upon request.


