Abstract
Background:
The global burden of sepsis is concentrated in high HIV-burden settings in sub-Saharan Africa (SSA). Despite this, little is known about the immunopathology of sepsis in persons living with HIV (PLWH) in the region. We sought to determine the influence of HIV on host immune responses and organ dysfunction among adults hospitalized with suspected sepsis in Uganda.
Design:
Prospective cohort study.
Methods:
We compared organ dysfunction and 30-day outcome profiles of PLWH and those without HIV. We quantified 14 soluble immune mediators, reflective of key domains of sepsis immunopathology, and performed whole-blood RNA-sequencing on samples from a subset of patients. We used propensity score methods to match PLWH and those without HIV by demographics, illness duration, and clinical severity, and compared immune mediator concentrations and gene expression profiles across propensity score-matched groups.
Results:
Among 299 patients, 157 (52.5%) were PLWH (clinical stage 3 or 4 in 80.3%, 67.7% with known HIV on antiretroviral therapy). PLWH presented with more severe physiologic derangement and shock, and had higher 30-day mortality (34.5% vs. 10.2%; p<0.001). Across propensity score-matched groups, PLWH exhibited greater pro-inflammatory immune activation, including upregulation of IL-6, IL-8, IL-15, IL-17 and HMGB1 signaling, with concomitant T-cell exhaustion, prothrombotic pathway activation, and angiopoeitin-2-related endothelial dysfunction.
Conclusions:
Sepsis-related organ dysfunction and mortality in Uganda disproportionately affect PLWH, who demonstrate exaggerated activation of multiple immunothrombotic and metabolic pathways implicated in sepsis pathogenesis. Further investigations are needed to refine understanding of sepsis immunopathology in PLWH, particularly mechanisms amenable to therapeutic manipulation.
Keywords: HIV, sepsis, biomarkers, high-throughput nucleotide sequencing, Uganda, Africa
Introduction
The global burden of sepsis is concentrated in sub-Saharan Africa (SSA), where regional estimates of sepsis incidence are the highest in the world [1]. In contrast to high-income countries (HICs), where sepsis typically affects older adults with severe bacterial infections, sepsis in SSA disproportionately affects young adults with HIV hospitalized with severe tuberculosis (TB), malaria, and other infections highly divergent from those prevalent in HICs [1-3]. In a recent systematic review and meta-analysis examining etiologies and outcomes of 2,800 adults hospitalized with sepsis in SSA, the median age of study participants ranged from 27-39 years and 66% were persons living with HIV (PLWH), of whom 20% had disseminated tuberculosis (TB) [3]. Across all included studies, HIV-infection was the major driver of in-hospital and post-discharge mortality [3].
Despite the disproportionate burden of sepsis-related morbidity and mortality among hospitalized PLWH in SSA, little is known about the immunopathological mechanisms that underlie this unique clinical phenotype. Although HIV-related immune dysregulation may contribute to sepsis pathogenesis, recent studies from high- and middle-income countries have identified few differences in the host response between PLWH and those without HIV who are critically ill with sepsis [4-7]. However, patients in these studies, many of whom were on stable anti-retroviral therapy (ART), were hospitalized primarily with severe bacterial respiratory infections and were older than PLWH hospitalized with sepsis in SSA, most of whom are young adults with advanced immunosuppression.
Directly addressing critical gaps in understanding of sepsis pathobiology in SSA, we sought to determine the influence of HIV on host immune responses and related organ dysfunction among a prospective cohort of adults hospitalized with suspected sepsis in Uganda.
Methods
Study setting, participants, outcomes, and design
In this study, we analyzed data and blood samples from a prospective observational cohort (Research in the Epidemiology of Severe and Emerging Infections in Uganda; RESERVE-U) of adults (age ≥18 years) hospitalized with severe, undifferentiated infection (suspected sepsis) at Entebbe General Referral Hospital (EGRH), a 200-bed public district referral hospital in central Uganda, from April 2017-August 2019 [8]. HIV prevalence in the hospital catchment area is approximately 6% [8]. The primary outcome of the RESERVE-U study was vital status at 30-days after hospital discharge. Secondary outcomes included a composite measure of in-hospital outcome (death in-hospital or transfer to Uganda’s national referral hospital due to progressive severity of illness) and functional status at discharge (among patients who survived and were not transferred). Further details of the RESERVE-U study and study site capacity have been published and are summarized in the supplement [8,9].
Among adults enrolled in the RESERVE-U study, we first determined the impact of HIV on infecting pathogens, organ dysfunction, and in-hospital and 30-day outcomes. We then determined the influence of HIV on host immune response by comparing soluble host immune mediators and gene expression profiles between PLWH and those without HIV, propensity score-matched by demographics, illness duration and clinical severity (Figure E1 in supplement). In an exploratory analysis, we used similar matching methods to compare soluble immune mediators between PLWH who survived versus those who died at 30-days (Figure E1 in supplement).
Clinical data collection and pathogen diagnostics
Study procedures for the RESERVE-U cohort have been described [8,9]. Briefly, enrollment occurred within 24 hours of hospitalization, at which time all patients underwent clinical assessments, blood samples were collected, and rapid testing was performed for HIV (serial diagnostic platforms for detection of HIV-1/2 antibodies and HIV-1 p24 antigen), malaria (qualitative detection of histidine-rich protein II and lactate dehydrogenase of P. falciparum in whole-blood), and influenza (polymerase chain reaction of naso-/oro-pharyngeal swab samples). For PLWH, testing for tuberculosis (TB) was also performed in urine and sputum samples (if obtainable) using the Determine™ TB-LAM Ag (Alere/Abbott) and Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, CA, USA) platforms, respectively. TB testing for HIV-uninfected patients was performed at the discretion of treating clinicians. Testing for these pathogens was informed by World Health Organization (WHO) Integrated Management of Adolescent and Adult Illness guidelines for sepsis and septic shock in resource-limited hospitals in SSA [10]. Further details are in the supplement.
Serum immunoassays
From cryopreserved serum samples, interleukin (IL)-6, IL-8, IL-10, interferon (IFN)-γ, IFN-γ-induced protein-10/C-X-C motif chemokine 10 (IP-10/CXCL10), macrophage inflammatory protein-1-alpha/chemokine (C-C motif) ligand 3 (MIP-1α/CCL3), macrophage inflammatory protein-1-beta/chemokine (C-C motif) ligand 4 (MIP-1β/CCL4), tumor necrosis factor-alpha (TNF-α), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), macrophage migration inhibitory factor (MIF), plasminogen activator inhibitor-1 (PAI-1), soluble TNF-receptor type 1 (sTNFR1), and soluble IL-2 receptor alpha/soluble CD25 (sIL-2RA/sCD25), were quantified using custom Luminex immunoassays (Luminex, Austin, TX, USA). Further details are in the supplement.
Whole-blood RNA isolation, library preparation, and sequencing
From cryopreserved whole-blood samples collected in PAXgene blood RNA tubes (PreAnalytiX, Qiagen/BD, Hombrechtikon, Switzerland), RNA was isolated and purified using PAXgene blood RNA kits (Qiagen, Hilden, Germany). RNA sequencing libraries were prepared using the NEBNext Ultra RNA Library Prep Kit (NEB, Ipswich, MA, USA). Sequencing libraries were multiplexed and analyzed using a 2x150 paired-end configuration on the Illumina HiSeq 4000 platform (Illumina, Inc., San Diego, CA, USA). Further details on RNA-sequencing methods including data processing, alignment, and transcript quantification are in the supplement [11-14].
Analyses of clinical, laboratory, and microbiological data
Continuous variables are expressed as medians (interquartile range [IQR]) or means (standard deviation [SD]) as appropriate. Categorical variables are summarized as counts and percentages with 95% Wilson score confidence intervals (CI) presented where relevant. Missing values for white blood cell count and hemoglobin were imputed using chained equations and predictive mean matching (mice R package). Where indicated, continuous and categorical clinical and laboratory variables were compared using Chi-squared, Fisher exact, Wilcoxon rank-sum, or t-tests as appropriate, with two-sided p-values ≤0.05 considered statistically significant. Univariable and multivariable logistic regression were used to determine the association between HIV and in-hospital and 30-day outcomes (finalfit R package). Principal component analysis (PCA; FactoMineR and factoextra R packages) was used to explore the relationship between HIV and sepsis severity using unsupervised methods [15].
Propensity score matching
To mitigate differences between PLWH and those without HIV in demographics, illness duration, and severity, factors that may affect the host response to severe infection, we applied propensity score matching (PSM) to balance relevant variables prior to immune mediator and differential gene expression analyses. Using this approach, individuals in the exposure group (PLWH) are matched to the unexposed (those without HIV) based on similar values of the propensity score, which is the predicted probability of exposure derived from a set of predictor variables [16]. Specifically, we performed PSM using the MatchIt R package, with individual propensity scores estimated using a multivariable logistic regression model including age, sex, illness duration prior to hospitalization, Modified Early Warning Score (MEWS), quick Sepsis-related Organ Failure Assessment (qSOFA) score, and the presence of shock (systolic blood pressure ≤90 mmHg despite administration of ≥1 liter of intravenous fluid) [17]. PLWH were matched 1:3 without replacement to those without HIV using nearest neighbor matching and a caliper width (the maximum allowable difference in propensity score between matched individuals) of 0.20. If less than three HIV-uninfected patients could be matched, fewer matches were allowed. Following PSM, between-group comparability in variables used for matching was assessed using standardized mean differences [18,19].
In a secondary analysis, we applied similar PSM methods to compare soluble immune mediators between PLWH who died versus those who survived at 30-days. Propensity scores in this analysis were estimated using a multivariable logistic regression model including the same variables as above, in addition to WHO-defined HIV clinical stage and exposure to ART and trimethoprim-sulfamethoxazole prophylaxis prior to admission. PLWH who died by day 30 were matched 1:3 without replacement to those who survived using nearest neighbor matching and a caliper width of 0.10.
Analyses of soluble immune mediators and differential gene expression
Following PSM, between-group comparisons of log10-transformed immune mediator concentrations were performed using Wilcoxon rank-sum tests. Differential gene expression analysis between PSM groups of PLWH and those without HIV was performed using the DESeq2 and DEvis R packages, with genes considered differentially expressed based on log2-fold change ≥∣1.2∣ and Benjamini-Hochberg (BH) adjusted p-value ≤0.05 [20,21]. Differentially expressed gene sets were selected for biological pathway analysis, results of which were examined to infer functional differences between groups.
All analyses were performed using R (v3.6.1, R Foundation for Statistical Computing, Vienna, Austria) with specific packages specified above and in the supplement. Biological pathway analysis was performed using Ingenuity Pathway Analysis (Qiagen, Hilden, Germany).
Ethics Statement
Each enrolled participant ≥18 years or their surrogate provided written informed consent. This study was approved by ethics committees at Columbia University (AAAR1450), Uganda Virus Research Institute (GC/127/17/02-06/582), and Uganda National Council for Science and Technology (HS2308).
Results
Participants and Microbiology
Of 301 adults enrolled in the RESERVE-U cohort, 299 (99.3%) had definitive assessment of HIV status and were included in this analysis (Figures E1-E2 in supplement). Clinical, laboratory, and microbiological characteristics and outcomes of these patients are presented in Table 1. Most PLWH had evidence of advanced immunosuppression (WHO clinical stage 3 or 4 in 80.3% [126/157]) and 67.7% (92/136) with known HIV prior to admission were on ART.
Table 1:
Patient characteristics in the unmatched cohort stratified by HIV status
| Patient characteristic | All patients (N=299) |
HIV-uninfected (N=142) |
Patients living with HIV (N=157) |
p-valuea |
|---|---|---|---|---|
| Demographics | ||||
| Female sex, n (%) | 177/299 (59.2) | 85/142 (59.9) | 92/157 (58.6) | 0.917 |
| Age, years, mean (SD) | 35 (12.2) | 34 (14.1) | 36 (10.2) | 0.099 |
| Presenting symptoms | ||||
| Duration of illness prior to hospitalization, days, median [IQR]b | 4 [3, 7] | 3 [2, 6] | 5 [3, 7] | <0.001 |
| Night sweats, n (%) | 234/299 (78.3) | 105/142 (73.9) | 129/157 (82.2) | 0.114 |
| Headache, n (%) | 236/299 (78.9) | 121/142 (85.2) | 115/157 (73.2) | 0.017 |
| Cough, n (%) | 187/299 (62.5) | 71/142 (50.0) | 116/157 (73.9) | <0.001 |
| Diarrhea, n (%) | 101/299 (33.8) | 35/142 (24.6) | 66/157 (42.0) | 0.002 |
| Shortness of breath, n (%) | 67/299 (22.4) | 25/142 (17.6) | 42/157 (26.8) | 0.079 |
| Dysuria, n (%) | 40/299 (13.4) | 22/142 (15.5) | 18/157 (11.5) | 0.394 |
| Pre-hospital management | ||||
| Received antimalarial or antibacterial agent prior to hospitalization, n (%) | 107/299 (35.8) | 57/142 (40.1) | 50/157 (31.8) | 0.170 |
| Vital signs | ||||
| Temperature ≥38°C, n (%) | 107/299 (35.8) | 49/142 (34.5) | 58/157 (36.9) | 0.751 |
| Temperature <36°C, n (%) | 86/299 (28.8) | 43/142 (30.3) | 43/157 (27.4) | 0.672 |
| Heart rate, beats/min, median [IQR] | 98 [87, 109] | 96 [84, 103] | 101 [90, 114] | <0.001 |
| Respiratory rate, breaths/min, median [IQR] | 22 [21, 26] | 22 [20, 24] | 23 [22, 26] | 0.010 |
| Systolic blood pressure, mmHg, median [IQR] | 103 [91, 117] | 107 [99, 123] | 98 [88, 110] | <0.001 |
| Oxygen saturation, %, median [IQR] | 97 [95, 98] | 97 [96, 98] | 97 [95, 98] | 0.898 |
| Encephalopathy, n (%)c | 58/299 (19.4) | 22/142 (15.5) | 36/157 (22.9) | 0.140 |
| Illness severity scores | ||||
| qSOFA score, median, IQR | 1 [1-2] | 1 [1-2 | 2 [1-2] | <0.001 |
| qSOFA score ≥2, n (%)d | 134/299 (44.8) | 41/142 (28.9) | 93/157 (59.2) | <0.001 |
| qSOFA score ≥1, n (%)d | 260/299 (87.0) | 119/142 (83.8) | 141/157 (89.8) | 0.171 |
| Modified SIRS score ≥2, n (%)e | 256/299 (85.6) | 117/142 (82.4) | 139/157 (88.5) | 0.178 |
| MEWS, median [IQR] | 3 [2, 5] | 3 [2, 3] | 4 [3, 5] | <0.001 |
| Cardiopulmonary organ dysfunctions | ||||
| Shock, n (%)f | 41/299 (13.7) | 9/142 (6.3) | 32/157 (20.4) | 0.001 |
| Acute respiratory failure, n (%)g | 64/299 (21.4) | 25/142 (17.6) | 39/157 (24.8) | 0.167 |
| HIV-related variables | ||||
| WHO HIV clinical stage 3 or 4, n (%) | -- | -- | 126/157 (80.3) | -- |
| New diagnosis of HIV, n (%) | -- | -- | 21/157 (13.4) | -- |
| Receiving ART prior to hospitalization, n (%) | -- | -- | 92/157 (58.6) | -- |
| Receiving ART prior to hospitalization if known HIV, n (%) | -- | -- | 92/136 (67.7) | -- |
| Receiving TMP-SMX prophylaxis prior to hospitalization, n (%) | -- | -- | 96/157 (61.1) | -- |
| Receiving TMP-SMX prophylaxis prior to hospitalization if known HIV, n (%) | -- | -- | 96/136 (70.6) | -- |
| Pathogen diagnostics | ||||
| Malaria RDT positive, n (%) | 61/294 (20.7) | 36/141 (25.5) | 25/153 (16.3) | 0.052 |
| Microbiological TB positive, n (%)h | 52/299 (17.4) | 3/142 (2.1) | 49/157 (31.2) | <0.001 |
| Urine TB-LAM positive, n (%) | -- | -- | 41/124 (33.1) | -- |
| Influenza PCR positive, n (%) | 17/270 (6.3) | 10/127 (7.9) | 7/143 (4.9) | 0.329 |
| Hematologic parameters | ||||
| White blood cell count, x109/L, median [IQR]i | 5.7 [3.7, 8.9] | 6.3 [4.8, 9.0] | 5.5 [3.1, 8.2] | 0.009 |
| Hemoglobin, g/dl, median [IQR]j | 10.9 [8.5, 13.5] | 12.4 [10.4, 14.8] | 10.0 [7.5, 12.0] | <0.001 |
| Inpatient management | ||||
| Received antibacterial agent during first 24h of hospitalization, n (%) | 283/299 (94.6) | 133/142 (93.7) | 150/157 (95.5) | 0.643 |
| Received antimalarial agent during first 24h of hospitalization, n (%) | 142/299 (47.5) | 93/142 (65.5) | 49/157 (31.2) | <0.001 |
| Received IV fluid during first 24h of hospitalization, n (%) | 272/299 (91.0) | 127/142 (89.4) | 145/157 (92.4) | 0.498 |
| Volume of IV fluid received, ml, median [IQR] | 1500 [1000, 2000] | 1000 [1000, 2000] | 1500 [1000, 2000] | 0.007 |
| Received red cell transfusion during hospitalization, n (%) | 25/299 (8.4) | 5/142 (3.5) | 20/157 (12.7) | 0.004 |
| Received anti-TB drugs during hospitalization, n (%) | 57/299 (19.1) | 8/142 (5.6) | 49/157 (31.2) | <0.001 |
| In-hospital and 30-day vital status | ||||
| Death in-hospital or transfer, n (%) | 42/299 (14.0) | 8/142 (5.6) | 34/157 (21.7) | <0.001 |
| Duration of hospitalization, days, median [IQR]k | 5 [3, 7] | 4 [3, 6] | 6 [4, 9] | <0.001 |
| KPS ≤70 at alive discharge, n (%) | 20/255 (7.8) | 4/133 (3.0) | 16/122 (13.1) | <0.001 |
| Death at 30-days post-discharge, n (%)l | 62/270 (23.0) | 13/128 (10.2) | 49/142 (34.5) | <0.001 |
| Indeterminate 30-day vital status, n (%) | 29/299 (9.7) | 14/142 (9.9) | 15/157 (9.6) | 0.929 |
Abbrevations: SD: standard deviation, IQR: interquartile range, qSOFA: quick Sequential (Sepsis-related) Organ Failure Assessment, SIRS: Systemic Inflammatory Response Syndrome, MEWS: Modified Early Warning Score, UVA: Universal Vital Assessment, HIV: human immunodeficiency virus, WHO: World Health Organization, ART: anti-retroviral therapy, TMP-SMX: trimethoprim-sulfamethoxazole, RDT: rapid diagnostic test, TB: tuberculosis, LAM: lipoarabinomannan, PCR: polymerase chain reaction
t-test, Wilcoxon rank-sum, Chi-squared, or Fisher exact test
Unknown for 1 patient
Anything other than “Alert” on AVPU (alert, responsive to voice, responsive to pain, unresponsive) mental status assessment
Systolic blood pressure ≤100mmHg, respiratory rate ≥22 breaths/min, and encephalopathy, latter defined using AVPU scale
Temperature ≥38°C or <36°C, heart rate ≥90 beats/min, respiratory rate ≥20 breaths/min
Systolic blood pressure ≤90 mmHg despite administration of ≥1 liter of intravenous fluid
Oxygen saturation ≤90% or respiratory rate ≥30 breaths/min
Positive result by sputum Xpert Ultra or smear or urine TB-LAM
Imputed using chained equations for 129 patients
Imputed using chained equations for 125 patients
Unknown for 11 patients
Denominator excludes patients with indeterminate 30-day vital status.
Over a third of PLWH had microbiological evidence of TB (31.2% [49/157] in PLWH vs. 2.1% [3/142] in HIV-uninfected; p<0.001) including 33.1% (41/124) with disseminated TB as indicated by positive urine TB-LAM testing. Malaria was more common in HIV-uninfected patients (16.3% [25/153] in PLWH vs. 25.5% [36/141] in HIV-uninfected; p=0.052). There was no significant difference in influenza frequency by HIV status (4.9% [7/143] in PLWH vs. 7.9% [10/127] in HIV-uninfected; p=0.329) (Table 1).
Sepsis in PLWH in Uganda is defined by severe physiologic derangement, multiple organ dysfunction, and high mortality
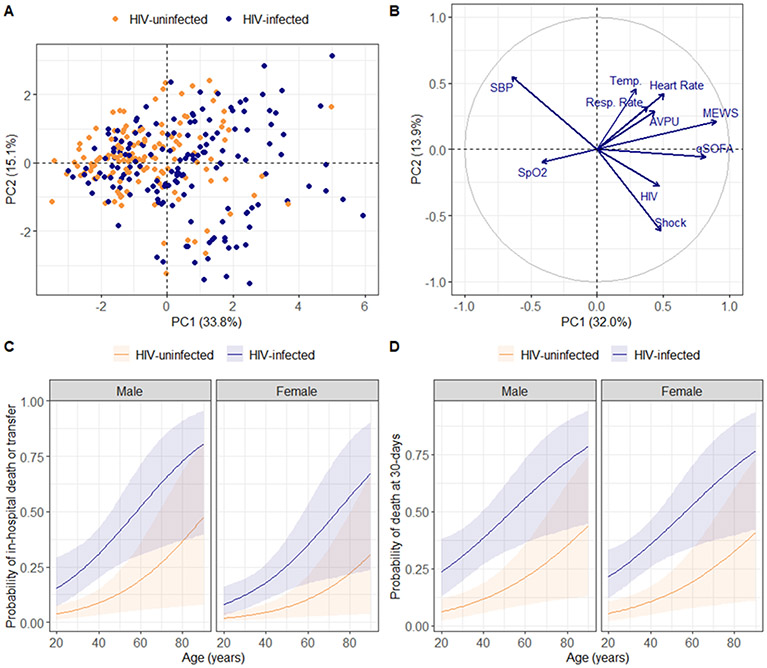
Clinically, PLWH exhibited more severe physiologic derangement, with significantly higher MEWS, Universal Vital Assessment (UVA) and qSOFA scores, and a significantly higher prevalence of shock (Table 1). Although not statistically significant, acute respiratory failure and encephalopathy were also more frequent in PLWH. PCA applied to vital signs and indices of physiologic severity revealed inter-individual separation by HIV status across the first PC of clinical variance (Figure 1A). When HIV status was added to the PCA model, PC1 and PC2 identified an axis of HIV, hypotension, and shock (Figure 1B).
Figure 1: Relationship between HIV, sepsis severity, and clinical outcomes.
(A) First two principal components of clinical variance plotted with the proportion of variance explained by each component; individuals stratified by HIV status (N=299); analyzed variables include temperature (degrees celsius), heart rate, respiratory rate, systolic blood pressure, oxygen saturation, mental status (AVPU) assessment, quick Sepsis-related Organ Failure Assessment score, Modified Early Warning Score, and presence of shock. (B) Variable correlation plot used to explore relationships between clinical variables and HIV status; positively correlated variables are located in the same quadrant while negatively correlated variables are in opposite quadrants. (C-D) Predicted probabilities of in-hospital death or transfer (N=299) and 30-day mortality (N=270) by HIV status over age and sex; probabilities derived from 10,000 bootstrap simulations of logistic model predictions.
Consistent with disproportionate physiologic derangement and more frequent shock, PLWH had significantly worse in-hospital and 30-day outcomes, including in multivariable models adjusted for age and sex (Table 1, Figures 1C-1D, Table E1 in supplement). The relationship between HIV-infection and 30-day survival persisted in multivariable models additionally adjusted for illness duration, severity, and microbiologically-diagnosed TB or malaria (Table E2 in supplement). Results were also consistent when patients with indeterminate 30-day vital status were considered deceased (Table E2 in supplement). Among PLWH with known 30-day vital status, those who died more frequently had shock and were more likely to be on ART prior to hospitalization (Tables E3-E4 in supplement).
PLWH exhibit disproportionate pro-inflammatory innate and adaptive immune activation and endothelial dysfunction
PSM generated a matched cohort of 229 patients (97 [42.4%] PLWH, 132 [57.6%] uninfected] (Table E5 and Figures E1 and E3 in supplement). Out of this matched cohort of 229 patients, serum immune mediators and whole-blood RNA-sequencing data were analyzed for 216 (94 [43.5%] PLWH, 122 [56.5%] HIV-uninfected) and 90 (38 [42.2%] PLWH, 52 [57.8%] HIV-uninfected) patients, respectively. Characteristics of patients who had RNA samples analyzed were similar to those in the larger matched cohort (Tables E6-E7 in supplement).
Between-group comparisons of serum immune mediators indicated greater pro-inflammatory innate and adaptive immune activation among PLWH, with significantly higher concentrations of mediators integral to acute phase response signaling (IL-6), monocyte/macrophage, NK-, and T-cell activation and chemotaxis (MIF, IP-10/CXCL10) and T-cell activation and exhaustion (sIL-2Ra/sCD25) (Figure 2, Table E8 in supplement). PLWH also had higher concentrations of Ang-2 and a higher ratio of Ang-2/Ang-1, indicative of pro-inflammatory endothelial activation and destabilization. Results were consistent in multiple sensitivity analyses, including when PSM was repeated after excluding PLWH with stage 3 of 4 disease and patients with microbiologically-diagnosed TB or malaria, and when ART-naïve PLWH were analyzed separately (Tables E9-E11 in supplement).
Figure 2: Soluble immune mediator concentrations by HIV status in propensity score-matched cohort.
N=216.
PLWH demonstrate exaggerated activation of multiple immunothrombotic and metabolic pathways implicated in sepsis pathogenesis
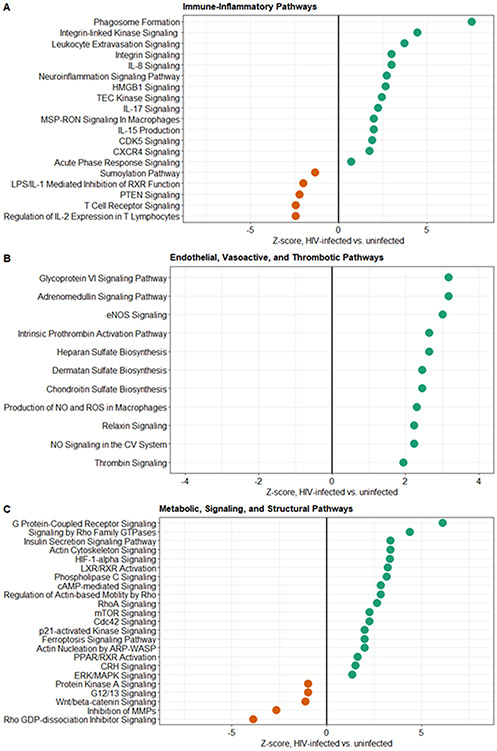
Analysis of whole-blood RNA-sequencing data in the matched cohort identified 1,669 differentially expressed genes across PLWH and those without HIV (Figure E4 in supplement). Similar to results of analysis of soluble mediators, PLWH exhibited enhanced activation of pro-inflammatory innate immune pathways, including monocyte/macrophage and neutrophil recruitment, extravasation, and phagocytosis and NK-cell activation, the latter indicated by increased IL-15 signaling. PLWH also showed increased activation of high-mobility-group box-1 (HMGB1), an endogenous damage-associated molecular pattern nucleoprotein (i.e. alarmin) that facilitates pro-inflammatory cytokine release and activation of NF-κB (Figure 3A). Activation of these pathways occurred alongside downregulation of sumolyation, a process of post-translational modification that may dampen NF-κB and toll-like receptor-driven inflammation. We also observed increased IL-17 signaling, which in addition to inducing broad pro-inflammatory responses, innate immune cell chemotaxis, and maintenance of mucosal barrier protection, may play a central role in mediating severe HIV-associated TB immunopathology [22]. Lastly, PLWH showed evidence of T-cell exhaustion, with downregulation of IL-2 expression, T-cell receptor and PTEN signaling, and upregulation of CDK5, a protein kinase that facilitates activation of PD-1/PD-L1 checkpoint inhibitor pathways [23] (Figure 3A).
Figure 3: Ingenuity Pathway Analysis of canonical signaling gene sets differentially expressed across HIV status in propensity score-matched cohort.
Differential expression defined by log2-fold change ≥∣1.2∣ and Benjamini-Hochberg adjusted p-value ≤0.05; Z-score indicates up- versus down-regulation of signaling gene sets in patients with HIV versus those without HIV (N=90).
PLWH exhibited a relatively pro-thrombotic state, including upregulation of the intrinsic prothrombin activation pathway and thrombin signaling, as well as increased signaling related to glycoprotein VI, a platelet glycoprotein and collagen receptor integral to platelet activation, adhesion, and thrombus formation (Figure 3B). Suggestive of changes to endothelial barrier integrity, we also observed increased expression of genes essential for restoration and maintenance of the glycocalyx (heparan, dermatan, chondroitin sulfates) among PLWH. Moreover, PLWH, for whom shock was a common clinical feature, demonstrated upregulation of endothelial nitric oxide (NO), adrenomedullin, and relaxin signaling pathways, activation of which leads to systemic vasodilation, as well as increased macrophage-driven production of NO and reactive oxygen species (ROS) [24,25] (Figure 3B).
Metabolically, PLWH demonstrated broad upregulation of G protein-coupled receptor pathways (Figure 3C). These pathways, in addition to playing a role in HIV replication and immunopathogenesis, are integral to multiple pro-inflammatory immune responses to severe infection, including cell migration, phagocytosis and ROS production, as well as endothelial destabilization and permeability [26]. PLWH also demonstrated upregulation of multiple pathways integral to control of the cellular cytoskeleton, rearrangements of which are manipulated by HIV to enable host cell infection, replication, and immune evasion, and may be used by intracellular and extracellular bacterial pathogens to facilitate cell invasion and mitigation of phagolysosome-driven killing, respectively [27,28] (Figure 3C). PLWH also showed evidence of a switch to hypoxia-mediated cellular metabolism, with upregulated HIF-1α signaling, as well as increased signaling of mTOR pathways.
Early elevations of IL-6, IL-10, sTNFR1, and sIL-2Ra/sCD25 are associated with 30-day mortality among PLWH
PSM generated a matched cohort of 117 PLWH (41 [35.0%] 30-day non-survivors, 76 [65.0%] survivors), 116 (99.1%) of whom had serum mediators analyzed (Table E12 and Figures E1 and E5 in supplement). Between-group comparisons showed significantly higher concentrations of IL-6, IL-10, sTNFR1, and sIL-2Ra/sCD25 at enrollment among patients who died (Figure 4, Table E13 in supplement). These results were consistent when PSM was repeated and patients with indeterminate 30-day vital status were considered deceased (Table E14 in supplement).
Figure 4: Soluble immune mediator concentrations by 30-day vital status in HIV-restricted propensity score-matched cohort.
N=116.
Discussion
In a prospective cohort of adults hospitalized with suspected sepsis in Uganda, we show that sepsis-related organ dysfunction and mortality remain concentrated among young adult PLWH, most of whom had evidence of advanced immunosuppression. After matching for clinically and biologically-relevant variables, PLWH demonstrated exaggerated activation of multiple immunothrombotic and metabolic pathways implicated in sepsis pathogenesis. Our results reinforce the importance of HIV-infection as a key risk factor for sepsis-related organ dysfunction and poor outcomes in SSA, and provide novel insights into biological mechanisms underlying these clinical sequelae, some of which may be amenable to therapeutic manipulation.
In contrast to studies from the ART era in HICs, where sepsis etiologies and host responses are largely comparable across HIV status, our findings emphasize the distinct clinical, microbiological, and immunopathological profile of sepsis in PLWH in SSA. Clinically, this unique phenotype is characterized by advanced immunosuppression with a high incidence of severe and often disseminated HIV-associated TB, frequent shock, and high mortality [2,3,29-32]. Biologically, we show that sepsis in PLWH in SSA is defined by disproportionate and multidimensional immune dysregulation, including amplified pro-inflammatory innate immune activation, T-cell exhaustion, endothelial dysfunction, metabolic reprogramming, and prothrombotic pathway activation. Independent of initial severity, mortality among PLWH critically ill with sepsis is associated with early elevations of IL-6, IL-10, sTNFR1, and sIL-2Ra/sCD25, suggesting concomitant activation of pro-inflammatory and immunosuppressive pathways, as well as T-cell activation and exhaustion, in patients at high risk for death.
Our immunological findings are consistent with those from outpatient studies from SSA, in which ART-naïve PLWH exhibit exaggerated pro-inflammatory innate immune activation, T-cell activation and exhaustion, endotheliopathy, and coagulopathy [33-37]. This immune dysregulation, likely multifactorial and due to opportunistic co-infections, microbial translocation, and viral replication, is only partly mitigated by suppressive ART, suggesting residual HIV-driven immune activation [36-39]. In the setting of acute infection, in vitro data suggest that HIV-related activation of monocytes/macrophages may induce a state of hyperresponsive innate immune priming that precipitates disproportionate release of pro-inflammatory cytokines following pathogen stimulation [40]. However, a recent study of adults with HIV-associated mycobacterial sepsis in South Africa identified impaired monocyte and neutrophil production of pro-inflammatory cytokines following stimulation with lipopolysaccharide and heat-killed S. pneumoniae, and an ex-vivo study revealed no difference by HIV status in lipopolysaccharide-induced cytokine production in patients with bacteremia in Gabon [41,42]. While functional studies in broader populations are needed, it seems plausible that in the context of infection-related organ dysfunction, underlying HIV-related immune dysregulation persists and may potentiate key domains of sepsis pathogenesis, thereby contributing to a severe clinical phenotype.
Our findings have several implications relevant to current and future therapeutic strategies for sepsis in PLWH in SSA. First, the high incidence of disseminated TB in our cohort reinforces the importance of rapid diagnostics for severe HIV-associated TB and of clinical trials evaluating the role of urine TB-LAM in guiding empiric TB treatment for critically ill PLWH in SSA [43,44]. Second, the high prevalence of ART-experienced PLWH with stage 3 and 4 disease in our cohort is likely a reflection of virological failure. As has been highlighted in studies from South Africa and Malawi, PLWH hospitalized with sepsis in SSA should be evaluated for ART failure and HIV drug resistance [30,45-47]. In terms of clinical trial design, the disproportionate burden of organ dysfunction, shock, and mortality among PLWH suggests that future trials of sepsis management in SSA should focus on this high-risk population, as doing so is likely to improve power and enrollment efficiency (i.e. prognostic enrichment) [48,49]. Similarly, in the context of repeated clinical trials of protocol-driven fluid resuscitation for sepsis and septic shock in SSA showing harm, including among PLWH, development and evaluation of biologically-informed sepsis management strategies in the region are imperative [50]. Considering closely linked and potentially causal relationships between pro-inflammatory responses and microcirculatory dysfunction, organ failure, and mortality, future trials in the region should evaluate the efficacy and safety of low-cost immunomodulatory therapies among PLWH hospitalized with sepsis. Corticosteroids are one such candidate therapeutic, as these agents inhibit production of key pro-inflammatory mediators upregulated in PLWH in our study, including IL-6, IL-8, phospholipase C, and MAPK [51-53].
Our study has limitations. First, our findings are derived from a single-center and require validation in other settings in SSA. Second, while we determined WHO clinical stage and reported ART exposure for all PLWH, we were unable to quantify viral loads and CD4 T-cell counts due to resource limitations at our study site. Although the majority of PLWH had advanced immunosuppression based on clinical stage, this reflects the population in whom the burden of sepsis in SSA is concentrated. However, results from immune mediator analyses were consistent when PLWH with stage 3 or 4 disease were excluded, suggesting that such findings are not likely to be driven entirely by patients with advanced immunosuppression. Third, although rapid diagnostics for key pathogens were performed in the parent study based on WHO sepsis guidelines, we did not perform bacterial or mycobacterial cultures or testing for fungal pathogens or non-malarial parasitic infections, also due to study site resource limitations. Fourth, although serum samples were available for nearly all patients (94%), whole-blood RNA samples were available from approximately 40%. Despite this, findings derived from each sample type were broadly consistent, and patients without RNA samples were similar to those analyzed. Next, although we included well-established variables reflective of clinical sepsis severity in our PSM models, we cannot exclude residual effects of clinical severity on host response differences. Lastly, we did not enroll outpatients, and thus cannot precisely determine which elements of the host response in ambulatory PLWH persist or are dynamic during critical illness.
Sepsis-related organ dysfunction and mortality in Uganda disproportionately affect PLWH, who demonstrate exaggerated activation of multiple immunothrombotic and metabolic pathways implicated in sepsis pathogenesis. Further studies are needed to refine understanding of the immunopathology of sepsis in PLWH, with the goal of developing biologically-informed treatment strategies that can be tested in locally-relevant clinical trials.
Supplementary Material
Acknowledgements and Author contributions:
The authors would like to acknowledge and thank the patients enrolled in this study and their families, clinicians at Entebbe General Referral Hospital, and the staff of the UVRI Arbovirology and Emerging and Reemerging Infections Laboratory for their assistance with data and sample collection and laboratory testing. MJC, BB, and MRO’D conceived the study and its design. BB, JJL, JK, TB, JN, NO, IN, SK, CN, and MM collected, organized and entered clinical data and blood samples and contributed to pathogen diagnostics. SS, WW, ML, MJC, and RT contributed to laboratory work. MJC, KJ, and AP performed statistical analyses. MJC, BB, AP, XC, TP, SSS, WIL, and MRO’D contributed to data analysis and interpretation. MJC drafted the manuscript. All authors critically revised the drafted manuscript and approved of the final manuscript.
Conflicts of Interest and Funding:
The authors declare that they have no competing interests. This work was supported by the National Center for Advancing Translational Sciences [UL1TR001873, sub-award to M.R.O.], the National Institute of Allergy and Infectious Diseases [K23AI163364 to M.J.C], and the MakCHS-Berkeley-Yale Pulmonary Complications of AIDS Research Training (PART) Program (D43TW009607, sub-award to B.B.) from the Fogarty International Center, National Institutes of Health. Additional support was provided by the Stony Wold-Herbert Fund [M.J.C.], Potts Memorial Foundation [M.J.C.], Thrasher Research Fund [M.J.C.], Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene [M.J.C.], and DELTAS Africa Initiative [107743, sub-award to M.J.C., B.B]. The funders had no role in study design, data analysis and interpretation, manuscript preparation, or decision to publish.
Data availability:
RNA-sequencing data analyzed in this study are available in the NIH National Center for Biotechnology Information Sequence Read Archive under BioProject accession number PRJNA794277. Other de-identified data will be made available to researchers affiliated with an appropriate institution following mutual signing of a data access agreement and obtainment of necessary ethics approvals. All code is available on request from Drs. Cummings and Price.
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford N, Shubber Z, Meintjes G, et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–e444. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JM, Feasey NA, Rylance J. Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Crit Care 2019;23:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huson MA, Scicluna BP, van Vught LA, et al. The Impact of HIV Co-Infection on the Genomic Response to Sepsis. PLoS One 2016;11:e0148955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva JM Jr., dos Santos Sde S. Sepsis in AIDS patients: clinical, etiological and inflammatory characteristics. J Int AIDS Soc 2013;16:17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiewel MA, Huson MA, van Vught LA, et al. Impact of HIV infection on the presentation, outcome and host response in patients admitted to the intensive care unit with sepsis; a case control study. Crit Care 2016;20:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amancio RT, Japiassu AM, Gomes RN, et al. The innate immune response in HIV/AIDS septic shock patients: a comparative study. PLoS One. 2013;8:e68730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings MJ, Bakamutumaho B, Owor N, et al. Stratifying Sepsis in Uganda Using Rapid Pathogen Diagnostics and Clinical Data: A Prospective Cohort Study. Am J Trop Med Hyg 2021;105:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings MJ, Bakamutumaho B, Price A, et al. Multidimensional analysis of the host response reveals prognostic and pathogen-driven immune subtypes among adults with sepsis in Uganda. Crit Care 2022;26:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. IMAI district clinician manual: hospital care for adolescents and adults. 2011. Available at: https://www.who.int/hiv/pub/imai/imai2011/en/. Accessed 8 June 2022.
- 11.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. [Google Scholar]
- 13.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014; 30:923–30. [DOI] [PubMed] [Google Scholar]
- 15.Lê S, Josse J, Husson F FactoMineR: An R Package for Multivariate Analysis. J Stat Softw. 2008; 25, 1 – 18. [Google Scholar]
- 16.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho DE IK, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 18.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Ann Transl Med 2019;7:16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price A, Caciula A, Guo C, et al. DEvis: an R package for aggregation and visualization of differential expression data. BMC Bioinformatics 2019;20:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruyn ED, Fukutani KF, Rockwood N, et al. Inflammatory profile of patients with tuberculosis with or without HIV-1 co-infection: a prospective cohort study and immunological network analysis. Lancet Microbe 2021;2:e375–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geven C, Kox M, Pickkers P. Adrenomedullin and Adrenomedullin-Targeted Therapy As Treatment Strategies Relevant for Sepsis. Front Immunol. 2018;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarwar M, Du XJ, Dschietzig TB, Summers RJ. The actions of relaxin on the human cardiovascular system. Br J Pharmacol. 2017;174:933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor MP, Koyuncu OO, Enquist LW. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol. 2011;9:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haglund CM, Welch MD. Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton. J Cell Biol. 2011. Oct 3;195:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob ST, Moore CC, Banura P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One 2009;4:e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis JM, Mphasa M, Keyala L, et al. A longitudinal observational study of aetiology and long-term outcomes of sepsis in Malawi revealing the key role of disseminated tuberculosis. Clin Infect Dis 2021. (In eng). Epub ahead of print: DOI: 10.1093/cid/ciab710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings MJ, O'Donnell MR. Inverting the pyramid: increasing awareness of mycobacterial sepsis in sub-Saharan Africa. Int J Tuberc Lung Dis 2015;19:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezoh G, Lutchman N, Worsley C, et al. Biomarkers of Endothelial Activation in Black South African HIV-Positive Subjects are Associated with Both High Viral Load and Low CD4 Counts. AIDS Res Hum Retroviruses. 2022;38:152–161. [DOI] [PubMed] [Google Scholar]
- 36.Kroeze S, Wit FW, Rossouw TM, et al. Plasma Biomarkers of Human Immunodeficiency Virus-Related Systemic Inflammation and Immune Activation in Sub-Saharan Africa Before and During Suppressive Antiretroviral Therapy. J Infect Dis 2019;220:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siedner MJ, Bwana MB, Asiimwe S, et al. Inflammatory biomarkers prior to antiretroviral therapy as prognostic markers of 12-month mortality in South Africa and Uganda. AIDS 2019;33:2043–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010;202(5):723–33. [DOI] [PubMed] [Google Scholar]
- 39.Janssen S, Huson MA, Osbak KK, et al. HIV infection rather than concurrent opportunistic infections drives most systemic procoagulant, vascular and damage responses - a prospective cohort study in central Africa. Antivir Ther 2017;22:153–161. [DOI] [PubMed] [Google Scholar]
- 40.Huson MA, Grobusch MP, van der Poll T. The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect Dis 2015;15:95–108. [DOI] [PubMed] [Google Scholar]
- 41.Janssen S, Schutz C, Ward A, et al. Mortality in Severe Human Immunodeficiency Virus-Tuberculosis Associates With Innate Immune Activation and Dysfunction of Monocytes. Clin Infect Dis 2017;65:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huson MAM, Hoogendijk AJ, de Vos AF, Grobusch MP, van der Poll T. The impact of HIV infection on blood leukocyte responsiveness to bacterial stimulation in asymptomatic patients and patients with bloodstream infection. J Int AIDS Soc 2016;19:20759–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy KP, Gupta-Wright A, Fielding KL, et al. Cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV in Africa: a microsimulation modelling study. Lancet Glob Health. 2019;7:e200–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Said B, Nuwagira E, Liyoyo A, et al. Early empiric anti-Mycobacterium tuberculosis therapy for sepsis in sub-Saharan Africa: a protocol of a randomised clinical trial. BMJ Open. 2022;12:e061953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta-Wright A, Fielding K, van Oosterhout JJ, et al. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. Lancet HIV 2020;7:e620–e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta-Wright A, Fielding K, Wilson D, et al. Tuberculosis in Hospitalized Patients With Human Immunodeficiency Virus: Clinical Characteristics, Mortality, and Implications From the Rapid Urine-based Screening for Tuberculosis to Reduce AIDS Related Mortality in Hospitalized Patients in Africa. Clin Infect Dis. 2020;71:2618–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griesel R, Stewart A, van der Plas H, Sikhondze W, Mendelson M, Maartens G. Prognostic indicators in the World Health Organization's algorithm for seriously ill HIV-infected inpatients with suspected tuberculosis. AIDS Res Ther. 2018;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med 2016;194:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 2020;16:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morton B, Stolbrink M, Kagima W, Rylance J, Mortimer K. The Early Recognition and Management of Sepsis in Sub-Saharan African Adults: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2018;15:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pool R, Gomez H, Kellum JA. Mechanisms of Organ Dysfunction in Sepsis. Crit Care Clin 2018;34:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416 [DOI] [PubMed] [Google Scholar]
- 53.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-sequencing data analyzed in this study are available in the NIH National Center for Biotechnology Information Sequence Read Archive under BioProject accession number PRJNA794277. Other de-identified data will be made available to researchers affiliated with an appropriate institution following mutual signing of a data access agreement and obtainment of necessary ethics approvals. All code is available on request from Drs. Cummings and Price.