Abstract
Encephalitozoon cuniculi is an obligate intracellular, spore-forming parasite belonging to the microsporidia that can cause disseminated infection in immunocompromised persons. E. cuniculi spores infect host cells by germination, i.e., by explosively everting the polar filament, through which the spore contents (sporoplasms) are subsequently injected into the cytoplasm. In addition, we observed intracellular, nongerminated spores in various nonprofessional phagocytes. In MRC5 cells, the number of internalized spores was approximately 10-fold higher than the number of injected sporoplasms. Compared to the rate of uptake by human monocyte-derived macrophages, internalization rates by A549 cells, MRC5 cells, and 293 cells were 0.6, 4.4, and 22.2%, respectively. The mechanism of uptake was studied in MRC5 cells. Killed spores were internalized at the same rate as live spores, indicating that nongerminated parasites do not actively participate in cell entry. Cytochalasin D inhibited uptake of spores by 95%, demonstrating an actin-dependent process. By electron and epifluorescence microscopy, intracellular spores were found in a tightly fitting membrane-bound compartment. The vacuole containing the spores was positive for the lysosomal membrane protein LAMP-1 and colocalized with the late endosomal-lysosomal content marker rhodamine dextran. Our results show that, in addition to the unique way in which microsporidia infect cells, E. cuniculi spores enter nonprofessional phagocytes by phagocytosis and traffic into a late endosomal-lysosomal compartment.
Microsporidia are obligate intracellular protozoan parasites that are capable of infecting a wide variety of both vertebrate and invertebrate hosts. In humans, microsporidia have been recognized as emerging opportunistic parasites, causing infections mainly in severely immunocompromised patients with AIDS (7, 21, 23). In contrast to the microsporidian most frequently infecting humans, Enterocytozoon bieneusi, which causes infections limited to epithelial cells, most commonly of the gastrointestinal tract, Encephalitozoon cuniculi can cause disseminated disease in humans, including infections of the central nervous system, heart, kidneys, spleen, lymph nodes, and adrenal glands (17, 21, 22, 23). The portal of entry of this parasite remains unknown. Ingestion and possibly inhalation of viable spores seem to constitute the most likely routes of infection (17). How E. cuniculi penetrates epithelial surfaces to gain access to the vascular system has not been studied. The infectious stage of microsporidia is an environmentally resistant spore containing a polar filament which coils around the infectious sporoplasm. A distinguishing characteristic of microsporidia is their unique way of gaining access to their host cells by spore germination. The infection of target cells involves the explosive extrusion of the polar filament, causing this tubular structure to shoot out of the spore, impaling any cell in its path (9, 24). Subsequently, the infectious sporoplasm is injected through the polar filament into the cytoplasm of the penetrated cell, where replication takes place (4). The empty spore remains extracellular. Infection by E. cuniculi has been documented for a wide range of human cell types, including macrophages, epithelial cells, vascular endothelial cells, kidney tubule cells, and cardiac myocytes (3, 17).
Phagocytosis is the uptake of particulate ligands, usually measuring >0.5 μm, into cytoplasmic vacuoles by mechanisms that require actin polymerization. The vacuole, or phagosome, containing the ingested material matures into phagolysosomes by a series of fusion events with endosomal vesicles. Two distinct mechanisms, both inhibitable by blockers of actin polymerization, may lead to the transfer of microorganisms to the cytoplasm. In conventional, or zipper-type, phagocytosis, ingestion occurs by sequential engagement of a phagocyte's membrane with the particle surface, and pseudopod advance proceeds no further than receptor-ligand interaction permits. In macropinocytosis, or trigger-type phagocytosis, in contrast, the host cell forms large surface ruffles or pseudopods in the vicinity of a bound microorganism. This process can be induced by the microorganism by secretion and intracellular transfer of proteins that subvert the host cell's phagocytic machinery, resulting in the uptake of the parasite in a large vesicle (19). Apoptotic cells and an increasing number of organisms have been shown to be phagocytosed by cells other than macrophages and neutrophils. These nonmyeloid cells, including epithelial, endothelial, and mesenchymal cells, have been designated nonprofessional phagocytes (NPP), emphasizing that, in contrast to macrophages and neutrophils, phagocytosis is not their principal function (16).
When studying the interaction of E. cuniculi with NPP, we observed germinated microsporidia with polar tubes inserted into host cells and empty spore walls remaining extracellular, and we also observed a much larger number of intracellular, nongerminated spores. The unexpected finding of intracellular spores was further investigated. We show here that several NPP cell lines are capable of internalizing entire spores and that, in human lung fibroblasts, entire spores are taken up by conventional phagocytosis and trafficked to a late endosomal-lysosomal compartment.
MATERIALS AND METHODS
Cell culture.
MRC5 cells (human lung fibroblasts; ATCC CCL 171), A549 cells (human respiratory epithelial cells; ATCC CCL 185), 293 cells (human embryonic kidney cells), MDCK cells (Madin-Darby canine kidney cells; ATCC CCL 34), and RK13 cells (rabbit kidney cells; ATCC CCL 37) were maintained under a 5% CO2 atmosphere in minimum essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, 100 mg of streptomycin per ml, and 0.25 mg of amphotericin B per ml. The culture medium was replaced twice weekly. The cultivation process was visually monitored using an inverted microscope. All culture supplies were purchased from Gibco-BRL.
For the experiments, approximately 1 × 105 A549 cells, 1 × 105 293 cells, and 5 × 104 MRC5 cells were seeded in wells of 24-well plates (TPP, Trasadingen, Switzerland) and grown at 37°C in 5% CO2 for 3 (A549 and 293 cells) or 7 (MRC5 cells) days.
Human monocytes were isolated from peripheral blood. Freshly drawn blood supplemented with 4% sodium citrate was centrifuged at 1,250 × g for 15 min to separate the plasma from the buffy coat. Autologous serum was recovered by clotting the plasma after adding CaCl2 (10 mM final concentration). The method of Böyum (2) was used to separate mononuclear cells from granulocytes and erythrocytes. In a subsequent step, monocytes were separated from lymphocytes by adhesion. Briefly, monocytes were suspended in RPMI 1640 medium with 2.5% autologous serum (4 × 106 cells/ml) and incubated for 1 h in 75-cm2 culture flasks (TPP) at 37°C in 5% CO2 to allow for adherence of the monocytes. Cells were then washed twice in RPMI 1640, resuspended in RPMI 1640 supplemented with 10% autologous serum (culture medium), and incubated overnight at 37°C in 5% CO2. After transfer of the culture flasks to 4°C for 30 min, supernatants were replaced by phosphate-buffered saline (PBS) at 4°C. After 15 min, adherent cells were scraped off and centrifuged at 300 × g at 4°C for 10 min. Monocytes were then resuspended in culture medium and cultured for 8 days at 37°C in 5% CO2 to allow for differentiation into monocyte-derived macrophages (MDM).
Cells were grown on 14-mm-diameter round glass coverslips (Assistent, Sondheim, Germany) in 24-well plates for immunofluorescence assays or, for electron microscopy, on the bottoms of the wells.
Microorganisms.
A human-derived isolate of E. cuniculi (IPZ:CH-H10 [14]) was grown in vitro in either MDCK or RK13 cell cultures in MEM supplemented with 10% fetal calf serum.
To recover E. cuniculi spores, culture supernatants were centrifuged at 900 × g at 4°C for 15 min, washed in PBS, filtered through 5-μm-pore-size Minisart filter units (Sartorius) to eliminate clumps and cellular debris, counted in a hemocytometer, and resuspended in MEM supplemented with 1% human serum albumin and 10 mM HEPES (pH 7.0) (inoculation medium).
For experiments comparing the internalization of live versus dead microsporidia, equal amounts of freshly recovered spores were left untreated or were fixed in 4% formaldehyde in PBS for 15 min and washed three times with PBS. Live and fixed parasites were then counted in a hemocytometer and resuspended in inoculation medium at the desired concentration.
Internalization assay.
Confluent monolayers of A549 cells, MRC5 cells, 293 cells, and MDM were washed twice with fresh MEM before inoculation medium was added. After 1 h at 37°C, the supernatant was replaced by 300 μl of a suspension of 2 × 106 E. cuniculi spores in inoculation medium. Plates were then centrifuged for 10 min at 460 × g at 4°C to promote sedimentation of microsporidia onto the cell layer. Plates were warmed to 37°C in 5% CO2 to initiate internalization. At the indicated time, supernatant was removed and the monolayers were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 min.
In preliminary experiments the influence of centrifugation on spore uptake was studied in MRC5 cells and 293 cells. Internalization rates after 2 h of coincubation did not significantly differ between spores that were centrifuged onto the monolayers and controls without centrifugation. The linear increase of spore uptake over time observed in all NPP cell lines studied (see Fig. 2) also argues against a notable influence of centrifugation on particle uptake, which might be expected to be strongest at early time points.
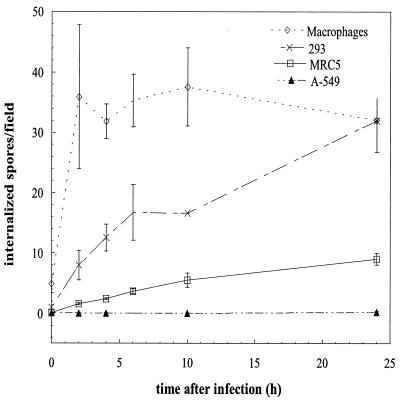
FIG. 2.
Kinetics of the internalization of nongerminated E. cuniculi spores by various NPP cell lines and human MDM. Two million E. cuniculi spores were added to each well of confluent cells, and the monolayers were fixed after 0, 2, 4, 6, 10, and 24 h. Intracellular spores were identified using differential immunofluorescence staining. To account for the size difference between the cells studied, internalization rates are given as the number of intracellular spores per high-power field of view (magnification, ×1,000). In confluent monolayers every field covers approximately 11.5 MRC5 cells, 50 293 cells, 60 A549 cells, and 30 macrophages. Data shown are means ± SD for three independent experiments, except for time points of >6 h for 293 cells, where the results of a single experiment are given (monolayers of 293 cells tended to detach from the coverslip after >6 h). Differences between cell lines were significant at a P value of <0.0001 (two-way analysis of variance).
Inhibition of internalization.
To study the effect of the inhibition of actin microfilament aggregation on invasion, MRC5 cells were treated with cytochalasin D, an inhibitor of actin polymerization (Sigma). Monolayers were treated exactly as described above, except that cells were preincubated with cytochalasin D (10 μg/ml) in invasion medium for 30 min and coincubated with E. cuniculi in the presence of the inhibitor at the same concentration for 90 min. The internalization rate was determined by immunofluorescence microscopy using differential staining of intra- and extracellular spores (see below).
Data are expressed as percent internalization relative to a control assay performed concurrently without the addition of cytochalasin D.
Immunofluorescence.
For immunofluorescence, formaldehyde-fixed cells were washed three times with PBS. To analyze the internalization of E. cuniculi spores, a differential fluorescence labeling technique (11) was used to distinguish between intracellular and extracellular microsporidia, as well as intra- and extracellular portions of discharged polar filaments. Extracellular spores and filaments were labeled by incubation with an anti-E. cuniculi polyclonal antibody (from a rabbit experimentally infected with E. cuniculi) diluted 1:1,000 in RPMI 1640. After 15 min, unbound primary antibody was removed in three washes with PBS and followed by a second incubation with a Cy3-conjugated affinity-purified goat anti-rabbit immunoglobulin G (IgG) (5-μg/ml final concentration; Jackson ImmunoResearch) in PBS containing 5% goat serum for 15 min. Cells were then permeabilized with 0.01% saponin in RPMI 1640 for 15 min and incubated with the same anti-E. cuniculi antibody for 15 min in the presence of 0.01% saponin. Monolayers were then washed three times and incubated with a fluorescein isothiocyanate (FITC)-labeled affinity-purified goat anti-rabbit secondary antibody (7-μg/ml final concentration; Jackson ImmunoResearch) diluted in PBS containing 5% goat serum, 0.1% Triton X-100, and 0.02% sodium dodecyl sulfate and 0.5 μg of 4′,6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes). After 15 min, cells were washed three times with PBS and twice with distilled water, air dried, and mounted on glass slides using ProLong Antifade (Molecular Probes). Intracellular parasites were counted using a Zeiss Axiophot epifluorescence microscope equipped with single-, dual-, and triple-band-pass filter sets (Omega Optical, Inc., Brattleboro, Vt.). Intracellular deposition of the sporoplasm was indicated by polar filaments originating from extracellular spores ending intracellularly. For every experimental condition, 50 consecutive fields on each of three coverslips were evaluated using a ×100 oil immersion objective. Data represent the means of three separate experiments ± the standard deviations (SD).
To simultaneously localize lysosome-associated membrane protein (LAMP-1) and E. cuniculi spores, 0.4 ml of RPMI 1640 with 10% H4A3 hybridoma supernatant (Developmental Hybridoma Bank, Iowa City, Iowa), a polyclonal rabbit anti-E. cuniculi antibody diluted 1:1,000, and 0.1% saponin was added to each well. After 15 min, unbound primary antibodies were removed in three washes with PBS. Cells were then incubated with 0.4 ml of a Cy3-conjugated affinity-purified goat anti-mouse IgG (5-μg/ml final concentration; Jackson ImmunoResearch) and a FITC-conjugated affinity-purified goat anti-rabbit IgG (7-μg/ml final concentration; Jackson ImmunoResearch) in PBS containing 5% goat serum, 0.1% Triton X-100, and 0.02% sodium dodecyl sulfate for 15 min. Infected monolayers were then washed, dried, and mounted on slides as described above.
Labeling NPP with RD.
To visualize the lysosomal contents of NPP, MRC5 cells were labeled by incubation with lysine-fixable tetramethylrhodamine-conjugated dextran (2 mg/ml) in culture medium for 24 h. After the noninternalized marker was washed off, cells were incubated in culture medium for another 2 h to ensure the transfer of rhodamine dextran (RD) to a late endosomal-lysosomal compartment (26). The invasion assay was then performed as described above.
Electron microscopy.
Cells were fixed with 2.5% glutaraldehyde followed by 1% OsO4, dehydrated with ethanol, and embedded in Epon as previously described (6). Ultrathin sections were stained with uranyl acetate and lead citrate and were observed with a Philips 300 electron microscope.
Statistical analysis.
Statistical significance of the data was determined by Student's t test, except for the data on uptake kinetics, which were analyzed by two-way analysis of variance. Values were considered significantly different if P was <0.05.
RESULTS
Uptake of E. cuniculi spores by NPP. (i) MRC5 cells internalize nongerminated E. cuniculi spores.
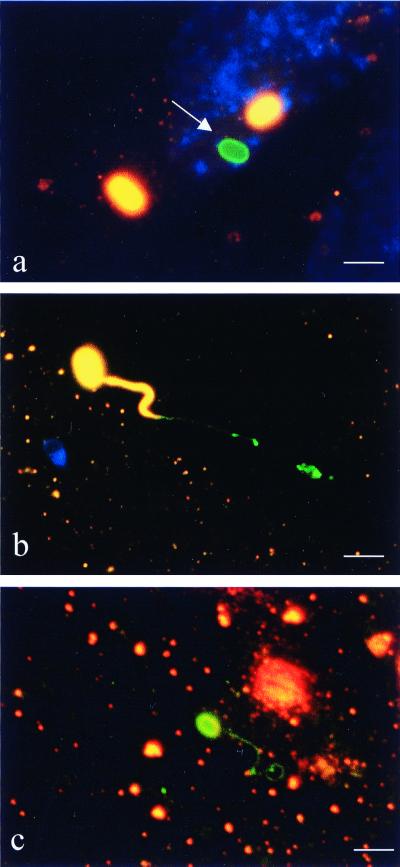
Using differential immunofluorescence staining to distinguish between intracellular and extracellular E. cuniculi spores, we observed nongerminated spores inside MRC5 cells (Fig. 1A). When spores were allowed to interact with cells for 2 h at spore/cell ratios of 10:1 and 25:1, the means ± SD were 17.5 ± 8.6 and 33.6 ± 24.2 intracellular spores found per 100 MRC5 cells, respectively. In contrast, the unique invasion process of microsporidia, in which the polar filament originating from an empty extracellular spore penetrates the cell membrane to terminate in the cytoplasm, was observed at an approximately 10-fold-lower rate, e.g., in 1.8 ± 0.5 and 2.9 ± 1.4 spores per 100 cells at spore-to-cell ratios of 10:1 and 25:1, respectively (Fig. 1B) (P = 0.001 and 0.005, respectively). Occasionally, germinated spores, including their entire polar filament, were found intracellularly (Fig. 1C).
FIG. 1.
Differential immunofluorescence staining of MRC5 cells coincubated with E. cuniculi. The first labeling, using an anti-E. cuniculi antibody and a Cy3-conjugated secondary antibody (orange fluorescence), was performed without permeabilizing the host cells in order to label only extracellular spores. Host cells were then permeabilized with saponin followed by labeling with the same anti-E. cuniculi antibody visualized by a FITC-conjugated secondary antibody (green fluorescence). Extracellular microsporidia (spore walls and polar filaments) appear orange and intracellular ones appear green. Nuclei of MRC5 cells are stained blue (revealed by DAPI). (a) Intracellular, nongerminated spore (arrow). (b) Germinated extracellular spore inserting terminal portion of polar filament into host cell. (c) Germinated intracellular spore. Spore wall and entire polar filament are intracellular. Bar = 3 μm.
(ii) Various types of NPP internalize nongerminated E. cuniculi.
To determine if NPP other than MRC5 cells are also able to internalize microsporidial spores and to assess the relative efficiency of the process, we compared the rates of uptake of E. cuniculi spores of several nonmyeloid cell lines (MRC5, A549, and 293 cells). Nongerminated E. cuniculi spores can enter all NPP cell lines studied, albeit at different rates (Fig. 2). After 2 h of coincubation with E. cuniculi spores, the following numbers of intracellular spores (means ± SD) per high-power field of view were found: 0.21 ± 0.12 in A549 cells, 1.6 ± 0.23 in MRC5 cells, and 7.97 ± 2.43 in 293 cells. Internalization increased linearly over 24 h in all NPP cell lines. Efficacy of uptake was lowest in A549 and highest in 293 cells. Differences between cell lines were significant at P values of <0.0001.
(iii) NPP internalize nongerminated E. cuniculi spores at a lower rate than that for MDM.
To compare the rate of uptake of spores by NPP to that of professional phagocytes, we studied the internalization of spores by human MDM. Uptake of nongerminated E. cuniculi spores was more efficient in macrophages than in all NPP cell lines studied (Fig. 2). After 2 h of coincubation, 35.9 ± 11.9 spores per field had been taken up by this professional phagocyte. Compared to the rate of internalization by MDM, rates of uptake by A549 cells, MRC5 cells, and 293 cells were 0.6, 4.4, and 22.2%, respectively. Nearly all the E. cuniculi spores added to the wells were phagocytosed by MDM after 2 h of coincubation, as evidenced by the plateau phase observed between 2 and 24 h.
Characterization of the mechanism of uptake. (i) Parasites do not actively contribute to internalization of spores.
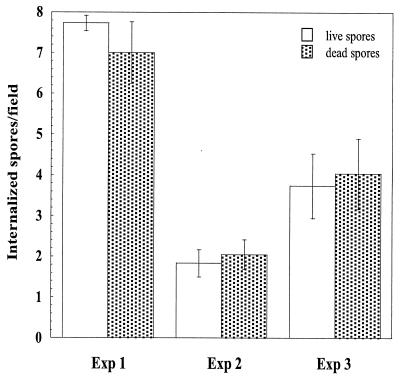
To study whether internalization of nongerminated spores is the result of active invasion by the parasite or if it represents uptake by the host cell, we compared the internalization rates of both live and formaldehyde-fixed spores. The rate of uptake of spores differed markedly between experiments. However, there was no statistically significant difference in the rate of uptake between live and fixed spores (P ≫ 0.05) (Fig. 3). This indicates that an active contribution by the parasites is not needed for internalization.
FIG. 3.
Internalization of live and dead E. cuniculi spores by MRC5 cells. Two million either live or formaldehyde-fixed spores were added to each well of confluent cells and coincubated for 2 h. Intracellular spores were identified using differential immunofluorescence staining. The rate of uptake of spores differed markedly between experiments. However, there was no statistically significant difference in the rate of uptake between live and formaldehyde-fixed spores (P ≫ 0.05 by Student's t test). Exp, experiment.
(ii) Uptake is an actin-dependent process.
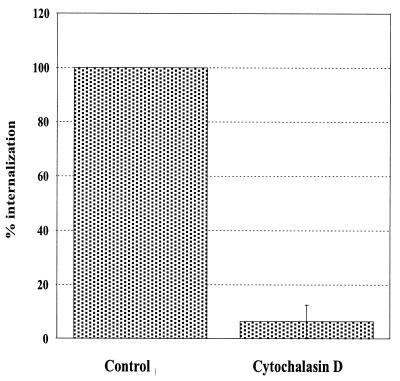
To study whether polymerization of the actin cytoskeleton contributes to the internalization of E. cuniculi spores, we determined the rate of parasite uptake in the presence of cytochalasin D. Treatment of MRC5 cells with cytochalasin D inhibited uptake by 95% (P = 0.03), demonstrating that internalization is an actin-dependent process (Fig. 4).
FIG. 4.
The role of actin polymerization in the internalization of E. cuniculi spores. The uptake of nongerminated spores by MRC5 cells in the presence of 10 μg of cytochalasin D per ml is compared to that for concurrently performed control assays in the absence of cytochalasin D. Data are expressed as the means ± SD of three experiments (P = 0.03 by paired Student's t test).
(iii) Internalized spores are contained in a membrane-bound vacuole.
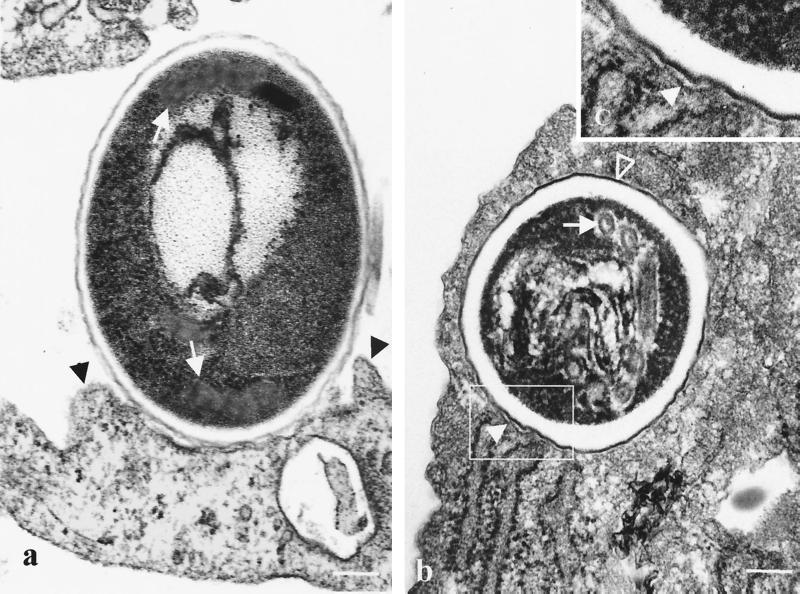
As observed by transmission electron microscopy, spores bound to the surface of MRC5 cells were associated with membrane extensions resembling pseudopods closely apposed to the spore wall (Fig. 5a). Fully internalized spores were surrounded by a membrane tightly apposed to the surface of the parasite (Fig. 5b). The ultrastructure of internalized parasites was typical of mature nongerminated spores; it consisted of a thick coat composed of an electron-dense outer layer (the exospore) and an electron-lucent inner layer, as well as the profiles of the polar filament coiled up inside the spore wall.
FIG. 5.
Electron micrographs of MRC5 cells coincubated with E. cuniculi spores for 2 h. (a) Tight association of the bound spore with the cell membrane and membrane protrusions resembling pseudopods extended along the spore wall (black arrowheads) are observed. Cross sections of coiled polar filament can also be seen (white arrows). (b) Characteristic electron-dense outer (open arrowhead) and electron-lucent inner layers of the wall of a mature E. cuniculi spore, as well as various sections of coils of the polar tube (white arrow), are shown. (c) Detail of the enclosed area from panel b showing a membrane tightly apposed to the contour of the internalized spore (closed arrowhead). Bar = 0.2 μm.
(iv) The spore-containing vacuole fuses with late endosomes and lysosomes.
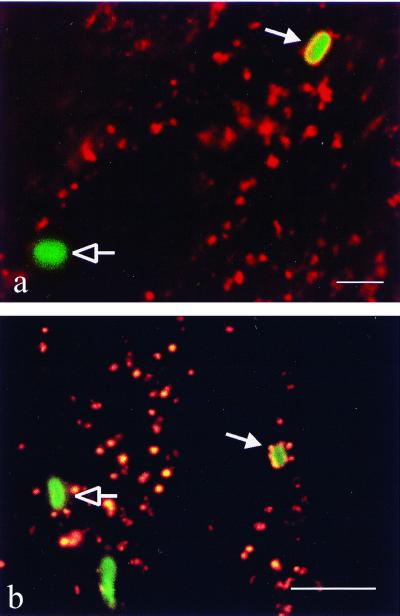
In professional phagocytes, newly formed phagosomes undergo a maturation process involving a series of fusion and fission events with endosomal and possibly lysosomal vesicles. Thereby the composition of the phagosomal membrane and its contents gradually acquire the characteristics of a phagolysosome. To study whether uptake by NPP was followed by comparable maturation steps that would result in phagolysosomal characteristics of the vacuole containing spores, we characterized the nature of this compartment. By epifluorescence microscopy, we found that the membrane surrounding intracellular spores stained strongly positive for the late endosomal-lysosomal membrane protein LAMP-1 (Fig. 6a). The membrane was always tightly apposed to the spore wall, corroborating the observations by electron microscopy. The late endosomal-lysosomal content marker RD was used to further characterize the nature of the vacuole. Spores internalized after MRC5 cells had been prelabeled with RD were found in vacuoles that stained strongly positive for RD (Fig. 6b). This finding offers additional evidence for the late endosomal-phagolysosomal trafficking of internalized E. cuniculi spores.
FIG. 6.
Colocalization of late endosomal-lysosomal markers with internalized E. cuniculi spores. (a) Lysosomal membrane protein LAMP-1 revealed by immunofluorescence. MRC5 cells were coincubated with spores for 2 h, fixed, and simultaneously labeled with a rabbit anti-E. cuniculi antibody and a mouse anti-LAMP-1 monoclonal antibody visualized by a FITC-conjugated and a Cy3-conjugated secondary antibody, respectively. The internalized spore (closed arrow) is surrounded by a ring of orange fluorescence (LAMP-1), indicating fusion of the vesicle containing the spore with late endosomes and lysosomes. LAMP-1 does not colocalize with the extracellular spore (open arrow). (b) RD localization by fluorescence. Cells were incubated with RD for 24 h, washed, and kept in RD-free medium for 2 h to allow passage of the marker to the late endosomal-lysosomal compartment. The RD-labeled cells were then coincubated with spores for 30 min, fixed, and labeled with an anti-E. cuniculi antibody visualized by a FITC-conjugated secondary antibody. The phagocytosed spore (closed arrow) surrounded by red fluorescent marker indicates phagosome-lysosome fusion. Extracellular spores are not associated with RD (open arrow). Bar = 3 μm.
DISCUSSION
To enter host cells, the obligate intracellular parasite E. cuniculi uses a unique mechanism of active invasion shared among all microsporidia. Here we show that, in addition, a remarkable proportion of E. cuniculi spores gain access to NPP by conventional phagocytosis and that particle internalization is followed by phagosome maturation.
All the NPP cell lines studied (representing fibroblasts, epithelial cells, and embryonic kidney cells) were able to internalize E. cuniculi spores. The rate of uptake, however, differed markedly between the NPP. It was approximately 40 times higher in 293 (embryonic kidney) cells than in A549 (respiratory epithelial) cells. Differential expression of receptors recognizing ligands on microsporidia and mediating phagocytosis may be partly responsible for the observed difference in the rate of internalization. Work with fish microsporidia suggests that glycoproteins of the exospore are important ligands on microsporidia (13). Compared to that of professional phagocytes, the efficacy of uptake by NPP was low and, for the phagocytically most active 293 cells, narrowly exceeded 20% of the rate observed in macrophages. NPP lack dedicated phagocytic receptors such as complement receptors or receptors for the Fc fragment of immunoglobulins, which may explain why they take up a more limited range of particles at a lower rate than macrophages and neutrophils (1, 16). Our observation that the rate of uptake in MRC5 cells was proportional to the number of spores coincubated with the cells argues against the possibility that the rate of internalization is limited by the number of receptors capable of mediating phagocytosis and suggests that events downstream of receptor binding may be rate limiting.
NPP have been reported to internalize apoptotic cells (10), as well as an increasing number of bacteria that are commonly extracellular, like group A Streptococcus spp. (12), Streptococcus pneumoniae (20), Staphylococcus aureus (18), Pseudomonas aeruginosa (5), and Neisseria gonorrhoeae (25). The mechanism of the uptake, however, has received little attention in research. The demonstration that the process is actin dependent has commonly been taken as proof of phagocytosis. Nevertheless, this does not allow for differentiation between zipper-type and trigger-type (also called macropinocytosis) phagocytosis, which are both dependent on actin polymerization. In addition, the events following actin polymerization and particle uptake by NPP are poorly characterized. Our results show that internalization of E. cuniculi spores by NPP is the exclusive result of cellular activity in which the parasites' role is that of a passive object. The identical rates of uptake of live and killed spores, the cytochalasin sensitivity of the internalization, and the close contact between microsporidia and both the cell surface and the phagosomal membrane can be taken as evidence that microsporidia are internalized by conventional, or zipper-type, phagocytosis. Zipper-type phagocytosis has also been reported to mediate the uptake of S. aureus by 293 cells (18).
By using markers of late endosomes and/or lysosomes, we demonstrated that phagosomes containing E. cuniculi spores acquire phagolysosomal characteristics. To our knowledge, phagocytosis followed by trafficking of the internalized microorganism to a phagolysosomal compartment has not been previously reported for NPP. The fate of apoptotic cells and bacteria phagocytosed by NPP has not been well characterized. It is unknown whether phagocytosis by NPP of nongerminated spores is beneficial or detrimental to the parasite. The finding that phagocytosed nongerminated spores outnumbered injected sporoplasms by a factor of 10 suggests that, irrespective of the fate of phagocytosed spores, phagocytic uptake has important consequences for the efficiency of this parasite to infect NPP. If uptake resulted in degradation of spores, phagocytosis might represent a defense mechanism that efficiently reduces the number of spores able to infect host cells by germination. Phagocytosis, on the other hand, may not lead to killing and digestion of the resistant spores. Indeed, in contrast to macrophages and neutrophils, NPP do not produce microbicidal oxygen or nitrogen intermediates (16). Similar to the mechanism used by N. gonorrhoeae and Neisseria meningitidis, which cross epithelial barriers to gain access to the subepithelial matrix or the bloodstream (15), phagocytosis may be the first step of transcytosis. The observation of germinated spores, including their entire polar filament, inside MRC5 cells suggests that spores may germinate after phagocytosis and thus escape from the phagolysosome. In this case, phagocytic uptake might add to the infectivity of E. cuniculi.
Phagocytosis by NPP of protozoan parasites that have developed efficient mechanisms to actively invade host cells, like Toxoplasma gondii or Trypanosoma cruzi, has not been reported. However, actin-mediated internalization of nongerminated spores by NPP has been demonstrated for Encephalitozoon intestinalis (8). Our findings show that, in addition to the unique way in which microsporidia infect host cells, nongerminated E. cuniculi spores can gain access to host cells by phagocytosis. We are currently planning to investigate the intracellular fate of phagocytosed spores.
ACKNOWLEDGMENT
This study was supported by a grant from the Swiss National Science Foundation (31-50′723.97).
REFERENCES
- 1.Aderem A, Underhill D M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;7:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 3.Canning E, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986. [Google Scholar]
- 4.Canning E U. Microsporidia. In: Kreier J P, Vaker J R, editors. Parasitic protozoa. 2nd ed. Vol. 6. New York, N.Y: Academic Press; 1993. pp. 299–385. [Google Scholar]
- 5.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couzinet S, Dubremetz J F, David L, Prensier G. Toxoplasma gondii: activity of the polyether ionophorous antibiotic nigericin on tachyzoites in cell culture. Exp Parasitol. 1994;78:341–351. doi: 10.1006/expr.1994.1037. [DOI] [PubMed] [Google Scholar]
- 7.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 8.Foucault C, Drancourt M. Actin mediates Encephalitozoon intestinalis entry into the human enterocyte-like cell line, Caco-2. Microb Pathog. 2000;28:51–58. doi: 10.1006/mpat.1999.0329. [DOI] [PubMed] [Google Scholar]
- 9.Frixione E, Ruiz L, Santillan M, De Vargas L, Tejero J, Undeen A. Dynamics of polar filament discharge and sporoplasm expulsion by microsporidian spores. Cell Motil Cytoskelet. 1992;22:38–50. [Google Scholar]
- 10.Hall S E, Savill J S, Henson P M, Haslett C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J Immunol. 1994;153:3218–3227. [PubMed] [Google Scholar]
- 11.Heesemann J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985;22:168–175. doi: 10.1128/jcm.22.2.168-175.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPenta D, Rubens C, Chi E, Cleary P P. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiro J, Ortega M, Siso M I, Sanmartin M L, Ubeira F M. Effects of chitinolytic and proteolytic enzymes on in vitro phagocytosis of microsporidians by spleen macrophages of turbot, Scophthalmus maximus L. Vet Immunol Immunopathol. 1997;59:171–180. doi: 10.1016/s0165-2427(97)00052-4. [DOI] [PubMed] [Google Scholar]
- 14.Mathis A, Michel M, Kuster H, Muller C, Weber R, Deplazes P. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology. 1997;114:29–35. doi: 10.1017/s0031182096008177. [DOI] [PubMed] [Google Scholar]
- 15.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinovitch M R. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz D A, Bryan R T, Hewan-Lowe K O, Visvesvara G S, Weber R, Cali A, Angritt P. Disseminated microsporidiosis (Encephalitozoon hellem) and acquired immunodeficiency syndrome. Autopsy evidence for respiratory acquisition. Arch Pathol Lab Med. 1992;116:660–668. [PubMed] [Google Scholar]
- 18.Sinha B, Francois P P, Nusse O, Foti M, Hartford O M, Vaudaux P, Foster T J, Lew D P, Herrmann M, Krause K-H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 19.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 20.Talbot U M, Paton A W, Paton J C. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 23.Weber R, Schwartz D, Bryan R. Microsporidia. In: Mandell G, Bennett J, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2920–2933. [Google Scholar]
- 24.Weidner E. Ultrastructural study of microsporidian invasion into cells. Z Parasitenkd. 1972;40:227–242. doi: 10.1007/BF00329623. [DOI] [PubMed] [Google Scholar]
- 25.Zenni M K, Giardina P C, Harvey H A, Shao J, Ketterer M R, Lubaroff D M, Williams R D, Apicella M A. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect Immun. 2000;68:1696–1699. doi: 10.1128/iai.68.3.1696-1699.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerli S, Majeed M, Gustavsson M, Stendahl O, Sanan D A, Ernst J D. Phagosome-lysosome fusion is a calcium-independent event in macrophages. J Cell Biol. 1996;132:49–61. doi: 10.1083/jcb.132.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]