Abstract
Both global and local factors affect coral reefs worldwide, sometimes simultaneously. An interplay of these factors can lead to phase shifts from hard coral dominance to algae or other invertebrates, particularly soft corals. However, most studies have targeted the effects of single factors, leaving pronounced knowledge gaps regarding the effects of combined factors on soft corals. Here, we investigated the single and combined effects of phosphate enrichment (1, 2, and 8 μM) and seawater temperature increase (26 to 32 °C) on the soft coral Xenia umbellata by quantifying oxygen fluxes, protein content, and stable isotope signatures in a 5-week laboratory experiment. Findings revealed no significant effects of temperature increase, phosphate enrichment, and the combination of both factors on oxygen fluxes. However, regardless of the phosphate treatment, total protein content and carbon stable isotope ratios decreased significantly by 62% and 7% under temperature increase, respectively, suggesting an increased assimilation of their energy reserves. Therefore, we hypothesize that heterotrophic feeding may be important for X. umbellata to sustain their energy reserves under temperature increase, highlighting the advantages of a mixotrophic strategy. Overall, X. umbellata shows a high tolerance towards changes in global and local factors, which may explain their competitive advantage observed at many Indo-Pacific reef locations.
Subject terms: Biogeochemistry, Ecophysiology, Metabolism
Introduction
Coral reefs are highly diverse and complex ecosystems that play a crucial role for humankind as they provide a range of ecosystem services1,2. Although coral reefs are ecologically and economically important, they are in decline due to several global and local anthropogenic factors2,3. On a global scale, they are affected by increasing seawater temperatures4, ocean acidification5, and higher ultraviolet radiation6. While on a local scale, they may additionally experience factors such as eutrophication in coastal waters7,8. This often results in substantial ecological shifts, also termed phase shifts, due to the decline in coral cover, loss of diversity on coral reefs9, and general reef degradation10. As these global and local factors can impact a coral reef simultaneously, it is important to understand which factors are the main drivers of ecological degradation and possible phase shifts, and how they interact with each other11.
Over the last decades, many studies examined the effects of thermal stress12 or nutrient enrichment9,13 on corals. However, their main focus was on single factors and their impacts on hard coral species. Thermal stress can lead to bleaching of the corals and subsequently reduced photosynthetic capacity14 but also a higher rate of mortality15, decreased growth16, and an increased susceptibility to diseases17. Experiments that examined hard corals under nutrient-enriched conditions showed widely varying results, ranging from somewhat positive effects18–21 to clear negative impacts22–24.
Recently, research is increasingly focussed on the combination of different potential stressors11,25. Experiments with combined nutrient enrichment and thermal stress showed divergent results. Experiments by Wiedenmann et al.26 showed that hard corals exposed to increased concentrations of dissolved inorganic nitrogen (DIN) were more susceptible to bleaching than corals exposed to low DIN concentrations. This increased bleaching susceptibility may ultimately be caused by a change in the holobionts’ resource partitioning27. The endosymbiotic algae of the family Symbiodiniaceae28 may retain more photosynthates for its own growth as nitrogen (N) is no longer the limiting nutrient, which is essential for steady translocation of photosynthates from the Symbiodiniaceae to the coral host29–31. Consequently, an increase in DIN leads to increasing numbers of algae, which results in phosphorous (P) starvation and in the end to alterations in the thylakoid membranes. This increases the susceptibility of the coral holobiont to thermal and light stress26. As in this hypothesis P is the limiting factor, enriching the water with P can potentially prevent bleaching26,32. This interruption of N limitation for the Symbiodiniaceae may also be induced by increasing water temperatures27,32. As such, P may be of extreme importance for future coral reef health. Since the scientific focus was directed at hard corals, it remains primarily unknown how soft corals are affected by multiple stressors33.
In general, soft corals are more resilient to ocean acidification and warming compared to hard corals34,35. In addition, many soft corals can rapidly colonize new areas due to their high fecundity and different dispersal modes36,37. Given the decrease in hard coral cover on many reefs and reported shifts in benthic reef communities towards non-hard coral taxa38,39, it is crucial to understand the ecophysiology of soft corals under different factors33,35, and how they may alter the nutrient budgets on coral reefs40,41. Particularly successful spreaders are soft corals that belong to the family of Xeniidae. Xeniids are recently being considered as strong invasive species that dominate alternative states on many reefs after phase shifts, like in the Caribbean Sea42,43, Indonesia36,44, or the southwest Atlantic45,46. Few studies, conducted on the soft coral Xenia umbellata, showed that glucose enrichment47,48 increased, while nitrate enrichment49 decreased the corals’ tolerance to thermal stress. Yet, it remains unknown how phosphate (PO4) enrichment affects its tolerance to warming.
Therefore, we conducted a 5-week manipulative aquarium experiment to assess the effects of PO4 enrichment and warming as single and combined factors on (1) oxygen fluxes, (2) protein content, and (3) elemental and stable isotope composition of the soft coral X. umbellata. For this, coral fragments were exposed to three different, ecologically relevant50 concentrations of PO4 (1, 2, and 8 µM) in combination with warming (26 to 32 °C). The response variables were assessed at different timepoints and finally we put the results in the context of X. umbellata’s metabolism and hypothesize what the ecological effects may be.
Materials and methods
Experimental design
We conducted a 5-week manipulative aquarium experiment in the Marine Ecology Lab in the Centre for Environmental Research and Sustainable Technology (UFT) at the University of Bremen in Bremen, Germany. The specimens of X. umbellata that were used in this experiment are originally from the Red Sea and have been in steady culture in the main holding tank for more than 2 years on a day-night rhythm of 12:12 h (temperature ~ 27 °C, salinity ~ 35 ‰, light ~ 100 µmol photons m−2 s−1 photosynthetically active radiation (PAR)).
Clonal Xenia colonies from the main holding tank were fragmented into 260 smaller colonies of approximately 1–2 cm in width using a scalpel. All colony fragments, at the beginning of the experiment, were similarly sized and roughly contained between 20 and 60 polyps. We then attached these fragments to plugs made of calcium carbonate (AF Plug Rocks, Aquaforest, Poland) using rubber bands. For healing from the fragmentation process, colonies were kept under regular maintenance conditions for 2 weeks. After that, we evenly and randomly distributed the colonies among 12 individual tanks with each individual tank having a total volume of 60 L filled with 40 L of artificial seawater to let them acclimatize to the new environment for 2 weeks before the start of the experiment. Each tank consisted of 2 parts that were interconnected, i.e. a technical part and an experimental part. In the technical part we installed a heater (3613 aquarium heater; 75 W 220–240 V; EHEIM GmbH and Co. KG, Germany) and a recirculation pump (EHEIM CompactOn 1000 pump; EHEIM GmbH and Co. KG, Germany) to ensure constant temperatures and water flow. Each heater was connected to a separate temperature controller (TRD digital, SCHEGO GmbH and Co. KG, Germany) to allow for precise control of the water temperature. Each tank was filled with artificial seawater (Zoo Mix, Tropic Marin, Switzerland) and a layer of CaCO3 reef sand to ensure the development of healthy mesocosms. A HOBO Pendant Data Logger (HOBO pendant temp/light, Onset, USA) was placed at the bottom of the experimental part of the tank during the whole experiment to monitor temperature and light conditions (measurements were taken every hour). Coral fragments (n = 20 per tank) were then placed on a grid made from eggcrate. Above each experimental part of the tank, we placed two light-emitting diode (LED) lamps (one Royal Blue matrix module and one Ultra Blue White matrix module, WALTRON daytime LED light, Germany) so that light levels were similar to conditions in the maintenance aquarium (~ 100 µmol photons m−2 s−1 PAR) on a day-night rhythm of 12:12 h. Light intensities were tested twice a week with a LI-1400 Data Logger (LI-COR Biosciences GmbH, Germany) and adjusted when necessary.
To ensure stable conditions in the tanks, 10% daily water exchanges were done to mimic the high renewal rate of seawater that can be found on coral reefs. Due to the daily water exchanges we did not install additional protein skimmers. Water parameters were maintained at the following levels (mean ± SD): salinity 36.1 ± 0.3 ‰, nitrate < 0.5 mg L−1, ammonium < 0.05 mg L−1, nitrite < 0.01 mg L−1, calcium 435 ± 150 mg L−1, alkalinity 7.3 ± 2.5°dH, magnesium 1495 ± 514 mg L−1, and pH 8.6 ± 0.2. These water parameters are equal to the ones the corals previously experienced over the last 2 years in the main holding tank filled with artificial seawater, with the elevated pH value likely caused by a high animal to water ratio. Coral plugs were cleaned from biofouling twice per week. Also, we fed the corals with 0.1 g of dried marine plankton (Reef-Roids, Polyp Lab, USA) per tank twice a week to avoid stress from starvation and to mimic conditions from the main holding tank. Yet, in the main holding tank corals lived in an ecosystem also containing fish and other organisms, hence in the holding tank they may have benefited also indirectly from the fish feed entering the water column which was missing in our experimental setup.
Experimental phosphate and temperature treatments
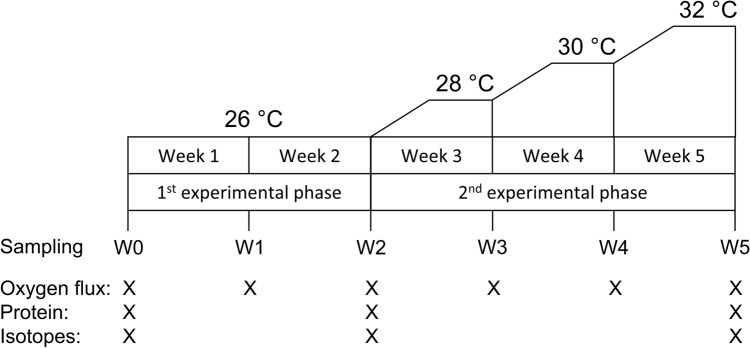
We created three different PO4 enrichment treatments (1, 2, and 8 µM), which are comparable to previously conducted experiments with corals in the lab and the field23,24,51–54 and a control treatment without PO4 addition (n = 3 tanks per treatment). For the first 14 days of the experiment, the corals were exposed to PO4 enrichment only (Fig. 1). The length of the pure eutrophication treatment was chosen (1) as a previous study could already detect an effect of PO4 and nitrate (NO3) after 2 weeks55, and (2) to allow for a better comparison due to similar experimental design of closely related studies conducted on X. umbellata47–49. To keep the PO4 enrichment treatments stable, we measured PO4 concentrations every day after the water exchange with an adjusted protocol of a commercially available PO43− test kit for salt water (TESTLAB MARIN, JBL, Germany) using a photometer (Turner Designs Trilogy Laboratory Fluorometer). For this we quantified weights and volumes of reagents and created a calibration curve (R2 = 0.967). Afterwards PO4 concentrations were manually adjusted in each tank using a 1 M stock solution from sodium phosphate dibasic Dihydrate (Na2HPO4 × 2 H2O).
Figure 1.
Experimental design with a 1st experimental phase of pure PO4 enrichment, followed by a 2nd experimental phase during which temperature was increased stepwise from 26 to 32 °C. Sampling of oxygen fluxes took place after each week (W0-5), while corals for protein content as well as elemental and stable isotope analysis were collected at the beginning, and at the end of each experimental phase.
On day 15, we started the second part of the experiment with the stepwise ramping of the temperature up to 32 °C (Fig. 1). For this, the temperature was increased by 1 °C day−1 on 2 consecutive days and then kept constant for 5 days. We conducted such a 7-day cycle three times until the temperature reached 32 °C. The temperature treatment in our laboratory experiment were synonymous to 2 degree heating weeks (DHW)56 in the first, 6 DHW in the second, and 12 DHW in the third week of the 2nd phase of the experiment.
Oxygen flux measurements
One fragment per tank (n = 3 per treatment) was labeled and used throughout the experiment to determine the rates of oxygen consumption in the dark (dark respiration, R) and oxygen production in the light (net photosynthesis, Pnet). The smallest colony tested for oxygen fluxes consisted of 28 polyps, while the biggest colony consisted of 70 polyps. To avoid stress, we transferred the colonies without air exposure to incubation chambers with a volume of 160 mL. We filled the incubation chambers with water from the respective tank and sealed them gas-tight without air bubbles inside. Then, we placed them in a temperature bath on a magnetic stirrer (Thermo Scientific, Variomag Poly) with 190 rotations per minute (rpm) for 1.5 to 2 h in the light and dark. The stirring bars allowed for mixing of the water column and ensured homogenous oxygen concentrations in the chambers throughout the measurement. The light used to measure oxygen production was emitted with approximately 100 µmol photons m−2 s−1 PAR by an LED light (Royal Blue—matrix module and Ultra Blue White 1:1—matrix module, WALTRON daytime LED light, Germany) to ensure equal light conditions as in the experimental tower.
We measured O2 concentrations within each chamber before and after each incubation by an optode sensor (HACH LDO, HACH HQ 40d, Hach Lange, Germany). For later analysis, we calculated Pnet in the light, and R in the dark, accounted for water background metabolism, and normalized the flux by incubation duration, chamber volume, and the corals’ surface area (SA), to account for the differences in coral fragment size. This is common procedure when calculating oxygen fluxes and has been done in many studies beforehand57–59, with studies conducted on X. umbellata reporting oxygen fluxes in the unit of mg O2 m−2 h−147,49. The calculations of the SA per polyp were based on the geometric method developed by Bednarz et al.52, multiplying the average polyp SA with the number of polyps. We additionally calculated gross photosynthesis (Pgross) rates, based on the assumption that respiration is constant during the day. Yet, as respiration rates may be significantly higher during active photosynthesis than in the dark60, the calculated Pgross rates can be seen as conservative estimates based on dark respiration. Running a linear regression on the log-transformed data, we analysed that both Pgross and R were negatively allometric to the number of polyps with a slope of 0.34 and 0.50, respectively. This indicates that larger colonies produce and consume more oxygen, but relatively less oxygen is produced and consumed per polyp. Therefore, two hypotheses arise: (1) the ratio of coral base to polyp number of coral fragments with many polyps may be smaller than the ratio of colonies with fewer polyps, and is not accounted for by the method of Bednarz et al.52, and (2) self-shading in bigger colonies could also potentially cause non-isometric scaling.
Protein content measurements
For measuring the total protein quantification, we used the Bradford assay61 following the Coomassie Protein Assay Kit (Thermo Scientific). For this, we took one X. umbellata fragment out of each tank (n = 3 per treatment) randomly each week, rinsed it in distilled water to remove salt, and stored it in a plastic bag in the freezer at − 20 °C until further processing. We lyophilized these colonies for 24 h at − 60 °C and stored them under dark and dry conditions pending analysis. During lyophilization the samples get freeze-dried by sublimating all the liquid from the sample, leaving only the dry compounds of the animal, e.g., protein and carbohydrates, behind. After that, we then ground those dried samples using mortar and pestle and measured the dry weight (DW) of each colony to use as a normalization metric. The DW of the colonies ranged from 12 to 97 mg (see Supplementary Material). We used this to standardize the protein content of each colony. Using a linear regression on the log-transformed data of protein content and DW, we found a strong correlation between protein content and DW (R2 = 0.80, see Supplementary Fig. S3). The slope above 1 indicates a positive allometry with protein content increasing faster than colony size.
After this, we homogenized the powder from every single sample in 5 mL of distilled water using a high-speed homogenizer (VEVOR, FSH-2A) for 2 min to extract the protein. For further quantification of the protein, we loaded 1 mL from each sample and mixed it with 1 mL of Bradford Dye Reagent 1× (Coomassie Brilliant Blue) in cuvettes. Lastly, we incubated the samples at room temperature for 10 min and read the absorbance in the spectrophotometer under 595 nm wavelength. To gain the resulting concentration of protein in the water we calculated it via the measured absorbance, using a diluted Bovine Serum Albumin (BSA) calibration curve, and then standardized it to water volume and coral dry weight.
Carbon and nitrogen elemental, and stable isotope analysis
To assess carbon (C) and nitrogen (N) isotope signatures and C:N ratios of X. umbellata, we conducted an elemental and stable isotope analysis on the entire holobiont. Every week on the first day we randomly selected one fragment of each tank (n = 3 per treatment), counted polyps, removed the colony from the plug, washed it in distilled water to remove salt, stored it in a plastic bag, and froze the sample at − 20 °C until further processing. Upon processing, X. umbellata colonies were dried in sterile glass petri dishes at 40 °C until weight consistency was reached (~ 48 h). Then, we ground the dried colonies into a fine powder using mortar and pestle, weighed the tissue powder, and transferred 1–2 mg into tin cups. Samples were analysed for C and N quantities as well as stable isotope ratios as described in Karcher et al.40. Isotopic ratios (r) are given as the ratio of the heavier to the lighter isotope (15N:14N or 13C:12C) and notated as either δ15N or δ13C (‰) using
| 1 |
where rreference for δ15N is atmospheric N (0.00368) and Vienna Pee Dee Belemnite (0.01118) for δ13C.
By analysing the corals’ elemental and stable isotope composition, it is possible to infer the sources of C and N in the coral holobiont. While carbon stable isotope ratios (δ13C) are often used to understand the corals’ C-metabolism55, the N stable isotope composition (δ15N) can be used as a bioindicator to determine the N source of the holobiont62. This can be done as different sources for C and N result in different stable isotope ratios. While C fixed by the Symbiodiniaceae is mostly comprised out of dissolved inorganic carbon (DIC) from the surrounding seawater (δ13C DIC = 0‰)63,64, C acquired via heterotrophic feeding on plankton has a different δ13C value (e.g. δ13C zoop = − 19‰ or more negative65,66). Therefore, δ13C values vary proportionately to the contribution of either photosynthates or heterotrophic feeding as the main C source for the coral67–72. The same applies to δ15N values, which are used to determine whether the corals are getting their N mainly from dinitrogen (N2) fixation of atmospheric N2 (δ15N = 0‰)73, which decreases the values74. Or if the coral is getting its N from other sources like sewage- and tourism-derived nutrient loading75, which increases the value with increasing uptake of anthropogenic N62,76.
Statistical analysis
We carried out the statistical analysis using RStudio (Version 1.4.1106)77 with the packages tidyverse78, ggpubr79, and rstatix80. As we used three separate tanks per treatment, we did not have a nested design and assume the tank effect to be zero for all statistical analyses. Additionally, all data was tested for possible tank effects by inspecting it for significant differences between tanks in all measured water parameters, but none were detected. To test for significant effects of treatments over time in invasive parameters (protein content and elemental stoichiometry) we used the 2-way analyses of variance (ANOVA). Log-transformation of ratios was not conducted as the data was normally distributed. We tested repeated measurements (Pgross, and R) using a 2-way mixed ANOVA with ‘Time’ as within-subject factor and ‘Treatment’ as between-subject factor. First, we tested data sets for outliers and then tested normal distribution of the data using the Shapiro Wilk test and additionally qqplots for visual confirmation. We conducted Levene’s test to test for homogeneity of variances, while we used Box’s M-test for homogeneity of covariances. Thereby, sphericity was automatically tested with Mauchly’s test and corrected when violated using the Greenhouse–Geisser sphericity correction. Lastly, we did a post-hoc analysis using pairwise comparison tests with Bonferroni adjustment with t-tests, and Dunn’s test for non-parametric data. We considered results to be significant with a p-value lower than 0.05 (p < 0.05) and display them as mean ± standard deviation. All raw data is given in the Supplementary Material online.
Results
Effects on oxygen fluxes
Compared to baseline values both, gross photosynthesis (Pgross) values and respiration (R), remained stable under PO4 enrichment and temperature increase, alone and combined. The growth of investigated coral colonies was around 0.14–0.30 polyps per day without any significant differences between treatments.
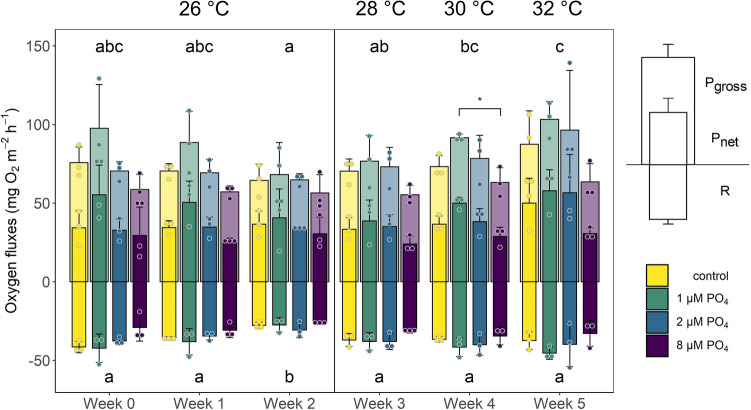
Under ambient conditions, X. umbellata colonies exhibited stable oxygen fluxes with Pgross and R values averaging 74 and − 36 mg O2 m−2 h−1, respectively. Though the overall effect of PO4 enrichment on Pgross values (Fig. 2) was not significant (2-way mixed ANOVA, F(3, 8) = 2.987, p = 0.096), there was a significant difference between the Pgross values of colonies in the low and high PO4 treatment in week 4 (pwc, Bonferroni adjustment, t-test, p = 0.0386). Additionally, Pgross values for all treatments varied significantly over time (2-way mixed ANOVA, F(5, 40) = 8.538, p < 0.001) with highest values after 5 and lowest values after 2 weeks. When temperatures increased to 30 °C, colonies from the low PO4 treatment had a Pgross rate 31% higher than colonies from the high PO4 treatment (91.5 and 63.2 mg O2 m−2 h−1, respectively). During the remaining days of the experiment, no significant treatment effects were observed, and all Pgross values were stable compared to baseline measurements throughout the experiment.
Figure 2.
Gross- (Pgross), net photosynthesis (Pnet), and respiration (R) of Xenia umbellata under experimental conditions: only temperature increase without PO4 addition (control), 1 µM PO4 + temperature increased from week 3 onwards (1 µM PO4), 2 µM PO4 + temperature increased (2 µM PO4), and 8 µM PO4 + temperature increased (8 µM PO4). Letters indicate significant differences for Pgross and R between weeks and asterisks indicate significant differences between treatments per time (p < 0.05, pairwise comparison t-test, Bonferroni adjustment). Error bars represent standard deviations. Dots represent the individually measured data points. The vertical line indicates the start of the temperature treatment and the average temperature for all tanks for the different time points is given on top of the graph.
Respiration, just like Pgross values, was unaffected by PO4 enrichment, as controls were not significantly different from PO4 treated colonies, but showed significant differences over time (2-way mixed ANOVA, F(1.34, 10.69) = 9.308, p = 0.008) with respiration rates being the lowest after 2 weeks of pure PO4 enrichment (Fig. 2). However, while respiration increased during the stepwise temperature increase compared to values from week 2, with the highest values in weeks 4 and 5 (− 38.215 and − 38.899 mg O2 m−2 h−1, respectively), it did not significantly change compared to baseline values (− 37.648 mg O2 m−2 h−1).
Effects on protein content
Protein content did not change between different PO4 enrichment treatments but changed over time and with increasing temperatures.
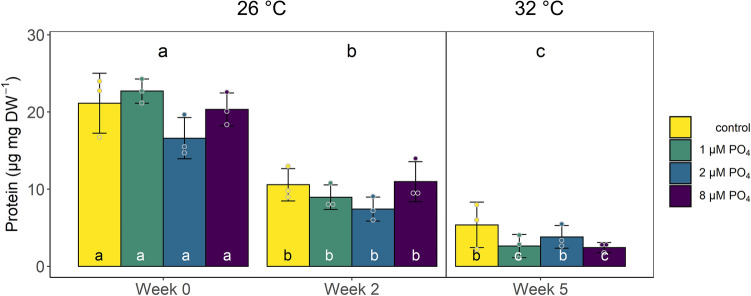
Protein content in X. umbellata colonies (Fig. 3, Supplementary Fig. S3) changed significantly between PO4 enrichment (2-way mixed ANOVA, F(3,24) = 3.076, p = 0.047) and over time (2-way mixed ANOVA, F(2,24) = 174.187, p < 0.001). The highest values were found in the baseline measurement with on average 20.2 µg protein mg−1 coral DW. After 2 weeks of PO4 enrichment alone, protein content significantly decreased by more than 50% to 9.48 µg protein mg−1 coral DW (pwc, Bonferroni adjustment, t-test, p < 0.001), and after the additional temperature increase, protein content dropped further down to 3.56 µg protein mg−1 coral DW, which differed significantly from the two previous measurements (pwc, Bonferroni adjustment, t-test, p < 0.001). While there were no significant differences between PO4 treatments at each time point, there were highly significant differences in each treatment over time, with the highest values always at the start and lowest values at the end of the experiment. Only the control and medium PO4 treatment corals showed no further significant decline in protein content during the additional temperature increase in the last 3 weeks of the experiment.
Figure 3.
Protein content of Xenia umbellata colonies under experimental conditions: only temperature increase without PO4 addition (control), 1 µM PO4 + temperature increased from week 3 onwards (1 µM PO4), 2 µM PO4 + temperature increased (2 µM PO4), and 8 µM PO4 + temperature increased (8 µM PO4). Letters above bars indicate significant differences between weeks and letters within bars indicate significant differences within the treatment over time (p < 0.05, pairwise comparison t-test, Bonferroni adjustment). Error bars represent standard deviations. Dots represent the individually measured data points. The vertical line indicates the start of the temperature treatment and the average temperature for all tanks for the different time points is given on top of the graph.
Effects on stable isotope signatures
All tested stable isotope signatures remained stable between different PO4 enrichment treatments throughout the experiment and were only significantly affected by time and the stepwise temperature increase.
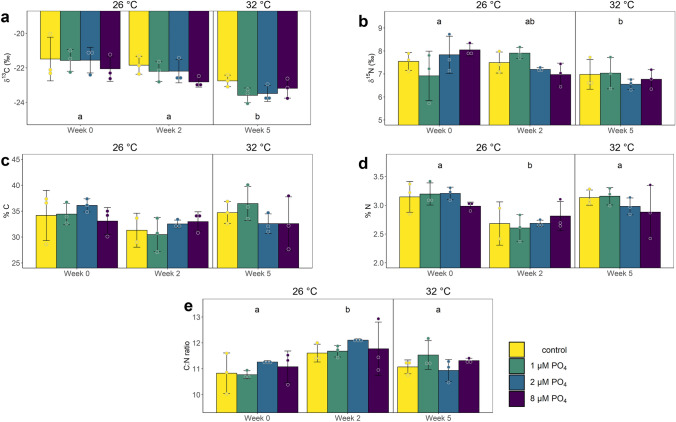
The δ15N, as well as δ13C values, changed significantly over time (2-way ANOVA, δ15N: F(2,24) = 5.957, p = 0.008; δ13C: F(2,24) = 18.212, p < 0.001) (Fig. 4). While δ15N values (Fig. 4A) stayed constant at a level of 7.5 ‰ within the first 2 weeks of pure PO4 enrichment, they decreased significantly by 10% over the stepwise temperature increase compared to the baseline (pwc, Bonferroni adjustment, Dunn’s test, p = 0.0112). The δ13C values (Fig. 4C) also decreased significantly during the temperature increase by 6.9 and 4.3% compared to baseline and the values after 2 weeks of PO4 treatment (p < 0.001 and p = 0.0018, respectively).
Figure 4.
Carbon (a), and Nitrogen (b) stable isotope ratio, percent carbon (c), percent nitrogen (d), and carbon to nitrogen ratio (e) of Xenia umbellata colonies under experimental phosphate conditions: only temperature increase without PO4 addition (control), 1 µM PO4 + temperature increased from week 3 onwards (1 µM PO4), 2 µM PO4 + temperature increased (2 µM PO4), and 8 µM PO4 + temperature increased (8 µM PO4). Letters indicate significant differences between weeks (p < 0.05, pairwise comparison t-test, Bonferroni adjustment). Error bars represent standard errors. Dots represent the individually measured data points. The vertical line indicates the start of the temperature treatment and the average temperature for all tanks for the different time points is given on top of the graph.
The C content of the coral colonies showed no significant differences, while percent N content significantly changed over time (2-way ANOVA, F(2,23) = 11.212, p < 0.001) (Fig. 4B,D). The values decreased by 14% within the first 2 weeks of the experiment compared to the baselines (p < 0.001) and returned close to starting values of 3.1% N again after the stepwise temperature increase. These differences in percent N content then lead to a significant difference in the total C:N ratio over time (2-way ANOVA, F(2,24) = 8.863, p = 0.001) (Fig. 4E). The C:N ratio after 2 weeks of PO4 enrichment was on average 6.9% higher and significantly different from the ratios at the start and end of the experiment (p < 0.001 and p = 0.0137, respectively).
Discussion
While hard coral cover decreases on reefs worldwide81–85, a shift to other benthic organisms, such as soft corals, can be observed38,39. As such, we tested the (combined) effects of two potentially stressful factors, i.e., PO4 enrichment and warming, on the pulsating soft coral X. umbellata. Our results suggest a high tolerance of X. umbellata towards increasing temperatures, regardless of PO4 enrichment.
How does PO4 enrichment affect X. umbellata?
In general, no significant differences between X. umbellata in the control and PO4 treatment groups were observed throughout the experiment, showing that PO4 had no measurable effect on the coral in the observed parameters. Compared to hard corals, soft corals have lower photosynthetic productivity, observable in their respective Pgross/R ratio of 1.0–1.3, while hard corals range within a Pgross/R ratio of 2–460,86. Light intensity may have played a role, too. Mergner and Svoboda86 measured oxygen fluxes of soft and hard corals from the same location, thereby reducing the effect of light intensity as far as possible. Also, Fabricius and Klumpp60 acknowledged this possible limitation when comparing oxygen fluxes from different studies in their discussion. When comparing the absolute values for Pgross and R of studies on X. umbellata, Pgross values reach 22 up to 140 mg O2 m−2 h−1, while respiration ranges between − 11 and − 60 mg O2 m−2 h−1. Despite this range, with a Pgross/R ratio of around 2 measured in this study and previously47,49,52, X. umbellata has higher photosynthetic productivity compared to other soft corals but is still at the lower edge of productivity compared to hard corals. The measured oxygen fluxes in our experiment remained stable under all added PO4 concentrations. These results are in contrast with a study by Bednarz et al.52, which showed increased gross photosynthesis in xeniids under PO4 enrichment, most likely due to increased chlorophyll a (chl a) concentrations. Our results also show that throughout the experiment the protein content significantly decreased in all colonies, regardless of PO4 enrichment. Particularly the decrease in protein content over the first 2 weeks without warming was surprising, as the corals have not faced a specific stressor and did not show increased respiration rates, which would indicate an increased energy demand. While X. umbellata can live autotrophically87, it is a heterotrophic suspension feeder88. So, this reduction in protein content indicates that X. umbellata metabolised its protein energy reserves, probably as a result of reduced heterotrophy during the experiment, as they were only fed twice a week with dried zooplankton.
How does PO4 enrichment affect the response of X. umbellata to a temperature increase?
Warming does not disrupt oxygen fluxes in X. umbellata
While direct evidence showing that X. umbellata experiences PO4 starvation under warming scenarios, as hypothesized for hard corals by Wiedenmann et al.26, is missing in our study, we here show that PO4 enrichment does not affect the corals tolerance towards warming. Overall, the measured oxygen fluxes in our experiment and respectively the Pgross/R ratio remained stable around 2 in response to warming, regardless of PO4 enrichment. The Pgross/R ratio above 1 indicates that X. umbellata can sustain its energy needs via net autotrophy and does not necessarily rely on heterotrophic feeding to meet their daily metabolic demands89. These results are surprising at first, as warming increases the respiration of hard corals as a sign of stress and increased energy demand27,90. At the same time, a general decrease in photosynthesis was often observed when temperatures exceeded 31 °C, due to a damaged photosystem II during temperature stress14,91,92 or as a result of decreased Symbiodiniaceae density or their chl a concentrations93. A possible explanation for the constant photosynthesis rates observed in the present study may be the special characteristic of X. umbellata being a pulsating coral94. Pulsation is known to have a positive effect on the corals’ photosynthetic activity, as it enhances gas exchange, leading to a greater efflux of oxygen from the coral tissues and increases the photosynthetic activity35,94. Additionally, the constant water movement may alleviate the coral from high temperature stress as fresh seawater is constantly circulating around the polyps, avoiding the build-up of a heated boundary layer. This may have enabled the corals used in the present study to sustain their photosynthetic efficiency even under warming scenarios. An experiment observing the effects of NO3 enrichment under warming on X. umbellata49 found that NO3 enrichment decreased Pgross rates, possibly due to the reduced pulsation of the polyps. Lastly, Simancas-Giraldo et al.47 found that X. umbellata colonies exhibit both reduced Pgross and R under warming, regardless of additional glucose enrichment. The authors argued that the reduced respiration rates may be a result of an explicit metabolic depression.
Warming leads to a significant decrease in protein content, δ15N, and δ13C
The protein content continued to significantly decrease over time with increasing temperature. This may contradict other studies, which showed that water temperature did not affect the protein content of the hard corals Stylophora pistillata95 and Turbinaria reniformis93. Yet, it could also be an effect of time and not temperature specifically, as protein content already significantly declined without increased temperatures, showing that X. umbellata metabolizes its energy reserves in the experimental setup. Additionally, total N content went back to baseline values under increased temperatures, regardless of PO4 treatment. A possible explanation could be that the higher temperatures led to increased N2 fixation, as shown in several studies on hard corals96–99, and therefore higher N availability and incorporation of it in the tissue of the holobiont. This hypothesis is supported by the decrease in δ15N as this could be a sign of increased N2 fixation74. Increased N2 fixation may relieve the Symbiodiniaceae from N-limitation. In combination with retaining their photosynthates for themselves, these conditions may allow the Symbiodiniaceae to grow, leaving the host with less organic C, as demonstrated for the hard coral species Stylophora pistillata27. This hypothesis is supported by the parallel study of Klinke et al.100, who showed increased algal cell densities under higher temperatures. However, a recent study by Rädecker et al.101 shows that diazotrophs derived N may not be utilized by the Symbiodiniaceae under heat stress. Yet, as the coral is catabolizing its protein reserves, N is also released and becomes available. As X. umbellata seems to be rather tolerant toward increased temperatures, the alleviation from N-limitation may be due to N2 fixation, or N from the proteins, or a combination of both.
In the present study, both δ15N and δ13C significantly decreased from the start of the experiment until the end after 5 weeks of PO4 enrichment and additional warming, regardless of the PO4 treatment. A decrease in δ13C is often a sign that less photosynthates are shared with the host organism who then, in turn, relies more on heterotrophic feeding, as zooplankton is depleted of the heavier 13C isotope102,103. However, in our study, we did not observe a change in oxygen fluxes, therefore fewer photosynthates are unlikely to be the reason for this decrease. A possible explanation could be that (1) the corals preferentially catabolized isotopically heavier organic matter, therefore reducing the δ13C value55. For example, bleached corals have reduced photosynthesis and catabolize heavier-isotope lipids, which overall depletes the lipid δ13C70. In the present study, the corals gradually lost their protein content, suggesting that at the same time isotopically heavier lipids may have been catabolized. Tanaka et al.55 suggest the same pathway as an explanation for the depletion of δ13C in their corals experiencing pure NO3 enrichment, implying that corals under imbalanced N:P ratios consume more lipids than corals under balanced N:P ratios. Another possible explanation for a decrease in δ13C may be (2) a net release of Symbiodiniaceae derived photosynthates into the water column, thereby shifting the δ13C value. While the results of measured total organic carbon (TOC) concentrations in the water during the incubation for oxygen fluxes were obscured by high standard deviation and background fluxes, a net release of TOC was observed for colonies under PO4 enrichment (see Supplementary Fig. S1). Lastly, an alternative explanation could be (3) the metabolization of the added marine zooplankton (reef roids) in the tanks’ sediment. Thereby, the reef roids may have altered the isotopic composition of the available CO2 in the tank which was then used by the corals for photosynthesis thereby causing the more negative δ13C through a secondary pathway.
Ecological implications
Despite soft corals’ growing importance due to phase shifts and their increasing abundance on reefs worldwide, there is a knowledge gap on how the simultaneously occurring alterations in the seawater N:P ratio and ocean warming scenarios affect soft coral species. Since 64% of all reefs are located near the shoreline of densely populated areas, they are more likely to be exposed to high inputs of inorganic nutrients in the water from human activities26,104,105. Groundwater percolation and submarine groundwater discharge is likely a major source of nutrient input on reefs50,106,107. Combined with ocean warming, this can have detrimental effects on hard corals26,108.
Compared to studies conducted with hard corals looking at either nutrient enrichment or temperature increase (Supplementary Material Table S1), it becomes clear that X. umbellata is relatively unaffected by these factors. In Table S1 it is shown that warming above 2.5 °C led to lowered Pgross, increased R and consequently reduced P/R ratios in 85% of species observed. Also, Fv/Fm, a proxy of photosynthetic activity, decreased in 6 of 7 observed species. Additionally, protein content was reduced in half of the observed species, indicating a need for using energy reserves under increased temperatures. While generally less studies focus on the effects of PO4 enrichment compared to temperature increase, only two studies combined these two factors. One of these two studies showed that Stylophora pistillata had reduced Fv/Fm and Pnet under temperature increase and nutrient enrichment, respectively, leaving the authors to hypothesize that eutrophication may compromise the corals resilience to global change109. While the other study showed that Pocillopora damicornis had increased R under warming, even more pronounced when combined with PO4 enrichment, and decreased Pgross rates54.
With X. umbellata being able to live autotrophically87, and not showing any changes in Pgross and R values under warming and PO4 enriched conditions, this would imply their resistance towards these two factors, even under extended periods. Nevertheless, our study indicates that X. umbellata had to utilize its energy reserves during the course of the experiment. Thobor et al.49 found a significant decrease in Pgross of X. umbellata under nitrate enrichment, but no significant changes under temperature increase. The combination of both factors led to severe declines in polyp pulsation, increased tissue loss, and mortality. This indicates that while X. umbellata is resistant towards warming, nitrate enrichment may reduce this resistance, potentially by disrupting the symbiosis between the coral host and its Symbiodiniaceae, as was found for hard corals26. This scenario would lead to a higher dependence of the coral on heterotrophic feeding.
While hard corals from the northern Red Sea show a net release of particulate organic matter (POM)110, X. umbellata showed net POM uptake52. Such coral-derived POM plays an essential role in the recycling of nutrients in reef ecosystems111. With X. umbellata not contributing to POM release, but instead actively taking up the organic matter, this may have implications for reef C cycling under shifts towards soft coral dominance due to their invasiveness. But not only C cycling may change in soft coral dominated reefs, but also N cycling. In this context, a study of El-Khaled et al.41 speculated that soft corals, i.e. Xenia sp., can help alleviate reefs from excessive N, as they may play a key role in reef-wide denitrification. Future studies should therefore conduct further investigations into the metabolism of X. umbellata to help clarify under which scenarios this functional autotroph changes to a mixotrophic strategy, to what extent, and how this may affect overall energy and nutrient budgets on future reefs.
Supplementary Information
Acknowledgements
The authors want to thank Ulrich Struck from the stable isotope facility at Free University of Berlin, Germany, for his sample preparation and analysis support. The authors also want to thank Daisy Ruhlmann for her support in daily maintenance activities during the experiment.
Author contributions
All authors conceived and designed the experiment. S.D.M. and A.K. conducted the experiment. S.D.M. processed samples, analysed and visualized the data, and wrote the manuscript with significant contributions of all authors. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by baseline funds of Marine Ecology Department of University of Bremen, Germany, and DFG Grant Wi 2677/16-1.
Data availability
Raw data of the current study is available in the Supplementary Material online.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-26325-5.
References
- 1.Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999;29:215–233. doi: 10.1016/S0921-8009(99)00009-9. [DOI] [Google Scholar]
- 2.Woodhead AJ, Hicks CC, Norström AV, Williams GJ, Graham NAJ. Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 2019;33:1023–1034. [Google Scholar]
- 3.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 4.Hughes TP, Kerry JT, Simpson T. Large-scale bleaching of corals on the Great Barrier Reef. Ecology. 2017;99:501. doi: 10.1002/ecy.2092. [DOI] [PubMed] [Google Scholar]
- 5.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. PNAS. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtial L, Roberty S, Shick JM, Houlbrèque F, Ferrier-Pagès C. Interactive effects of ultraviolet radiation and thermal stress on two reef-building corals. Limnol. Oceanogr. 2017;62:1000–1013. doi: 10.1002/lno.10481. [DOI] [Google Scholar]
- 7.Jessen C, et al. In-situ effects of eutrophication and overfishing on physiology and bacterial diversity of the Red Sea Coral Acropora hemprichii. PLoS ONE. 2013;8:e62091. doi: 10.1371/journal.pone.0062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessen C, Roder C, Villa Lizcano JF, Voolstra CR, Wild C. In-situ effects of simulated overfishing and eutrophication on benthic coral reef algae growth, succession, and composition in the Central Red Sea. PLoS ONE. 2013;8:e66992. doi: 10.1371/journal.pone.0066992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005;50:125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 11.Fabricius KE, Cséke S, Humphrey C, De’ath G. Does trophic status enhance or reduce the thermal tolerance of scleractinian corals? A review, experiment and conceptual framework. PLoS ONE. 2013;8:e54399. doi: 10.1371/journal.pone.0054399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLachlan RH, Price JT, Solomon SL, Grottoli AG. Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs. 2020;39:885–902. doi: 10.1007/s00338-020-01931-9. [DOI] [Google Scholar]
- 13.Fabricius KE. Factors determining the resilience of coral reefs to eutrophication: A review and conceptual model. In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. Springer; 2011. [Google Scholar]
- 14.Tilstra A, et al. Light induced intraspecific variability in response to thermal stress in the hard coral Stylophora pistillata. PeerJ. 2017 doi: 10.7717/PEERJ.3802/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly SR, Lopez-Yglesias MA, Anthony KRN. Food availability promotes rapid recovery from thermal stress in a scleractinian coral. Coral Reefs. 2012;31:951–960. doi: 10.1007/s00338-012-0925-9. [DOI] [Google Scholar]
- 16.Coles SL, Brown BE. Coral bleaching—Capacity for acclimatization and adaptation. Adv. Mar. Biol. 2003;46:183. doi: 10.1016/S0065-2881(03)46004-5. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- 18.Szmant AM. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries. 2002;25:743–766. doi: 10.1007/BF02804903. [DOI] [Google Scholar]
- 19.Atkinson MJ, Carlson B, Crow GL. Coral growth in high-nutrient, low-pH seawater: A case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs. 1995;14:215–223. doi: 10.1007/BF00334344. [DOI] [Google Scholar]
- 20.Bongiorni L, Shafir S, Angel D, Rinkevich B. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Mar. Ecol. Prog. Ser. 2003;253:137–144. doi: 10.3354/meps253137. [DOI] [Google Scholar]
- 21.Grigg RW. Coral reefs in an urban embayment in Hawaii: A complex case history controlled by natural and anthropogenic stress. Coral Reefs. 1995;14:253–266. doi: 10.1007/BF00334349. [DOI] [Google Scholar]
- 22.Fabricius KE, De’ath G. Identifying ecological change and its causes: A case study on coral reefs. Ecol. Appl. 2004;14:1448–1465. doi: 10.1890/03-5320. [DOI] [Google Scholar]
- 23.Ferrier-Pagès C, Gattuso JP, Dallot S, Jaubert J. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs. 2000;19:103–113. doi: 10.1007/s003380000078. [DOI] [Google Scholar]
- 24.Rosset S, Wiedenmann J, Reed AJ, D’Angelo C. Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar. Pollut. Bull. 2017;118:180–187. doi: 10.1016/j.marpolbul.2017.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ban SS, Graham NAJ, Connolly SR. Evidence for multiple stressor interactions and effects on coral reefs. Glob. Change Biol. 2014;20:681–697. doi: 10.1111/gcb.12453. [DOI] [PubMed] [Google Scholar]
- 26.Wiedenmann J, et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change. 2012;3:160–164. doi: 10.1038/nclimate1661. [DOI] [Google Scholar]
- 27.Rädecker N, et al. Heat stress destabilizes symbiotic nutrient cycling in corals. PNAS. 2021 doi: 10.1073/pnas.2022653118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaJeunesse TC, et al. Systematic revision of symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018;28:2570–2580. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. Population control in symbiotic corals—Ammonium ions and organic materials maintain the density of zooxanthellae. Bioscience. 1993;43:606–611. doi: 10.2307/1312147. [DOI] [Google Scholar]
- 30.Muscatine L, Pool RR. Regulation of numbers of intracellular algae. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1979;204:131–139. doi: 10.1098/rspb.1979.0018. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Parker G, D’Elia CF, Cook CB. Interactions between corals and their symbiotic algae. Coral Reefs Anthr. 2015 doi: 10.1007/978-94-017-7249-5_5. [DOI] [Google Scholar]
- 32.Rädecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C. Nitrogen cycling in corals: The key to understanding holobiont functioning? Trends Microbiol. 2015;23:490–497. doi: 10.1016/j.tim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg RK, Dafforn KA, Ainsworth T, Johnston EL. Know thy anemone: A review of threats to octocorals and anemones and opportunities for their restoration. Front. Mar. Sci. 2020;7:590. doi: 10.3389/fmars.2020.00590. [DOI] [Google Scholar]
- 34.Inoue S, Kayanne H, Yamamoto S, Kurihara H. Spatial community shift from hard to soft corals in acidified water. Nat. Clim. Change. 2013;3:683–687. doi: 10.1038/nclimate1855. [DOI] [Google Scholar]
- 35.Wild C, Naumann MS. Effect of active water movement on energy and nutrient acquisition in coral reef-associated benthic organisms. PNAS. 2013;110:8767–8768. doi: 10.1073/pnas.1306839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox HE, Pet JS, Dahuri R, Caldwell RL. Recovery in rubble fields: Long-term impacts of blast fishing. Mar. Pollut. Bull. 2003;46:1024–1031. doi: 10.1016/S0025-326X(03)00246-7. [DOI] [PubMed] [Google Scholar]
- 37.Benayahu Y, Loya Y. Settlement and recruitment of a soft coral: Why is Xenia macrospiculata a successful colonizer? Bull. Mar. Sci. 1985;36:177–188. [Google Scholar]
- 38.Norström AV, Nyström M, Lokrantz J, Folke C. Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009;376:293–306. doi: 10.3354/meps07815. [DOI] [Google Scholar]
- 39.Reverter M, Helber SB, Rohde S, De Goeij JM, Schupp PJ. Coral reef benthic community changes in the Anthropocene: Biogeographic heterogeneity, overlooked configurations, and methodology. Glob. Change Biol. 2022;28:1956–1971. doi: 10.1111/gcb.16034. [DOI] [PubMed] [Google Scholar]
- 40.Karcher DB, et al. Nitrogen eutrophication particularly promotes turf algae in coral reefs of the central Red Sea. PeerJ. 2020;2020:1–25. doi: 10.7717/peerj.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Khaled YC, et al. Nitrogen fixation and denitrification activity differ between coral- and algae-dominated Red Sea reefs. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-90204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Allais JP, Benayahu Y, Lasso-Alcalá OM. The invasive octocoral Unomia stolonifera (Alcyonacea, Xeniidae) is dominating the benthos in the Southeastern Caribbean Sea. Mem. la Fund La Salle Ciencias Nat. 2021;79:63–80. [Google Scholar]
- 43.Ruiz Allais JP, Amaro ME, McFadden CS, Halász A, Benayahu Y. The first incidence of an alien soft coral of the family Xeniidae in the Caribbean, an invasion in eastern Venezuelan coral communities. Coral Reefs. 2014;33:287. doi: 10.1007/s00338-013-1122-1. [DOI] [Google Scholar]
- 44.Baum G, Januar I, Ferse SCA, Wild C, Kunzmann A. Abundance and physiology of dominant soft corals linked to water quality in Jakarta Bay, Indonesia. PeerJ. 2016;2016:1–29. doi: 10.7717/peerj.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menezes NM, et al. New non-native ornamental octocorals threatening a South-west Atlantic reef. J. Mar. Biol. Assoc. U.K. 2022 doi: 10.1017/S0025315421000849. [DOI] [Google Scholar]
- 46.Mantelatto MC, da Silva AG, dos Louzada TS, McFadden CS, Creed JC. Invasion of aquarium origin soft corals on a tropical rocky reef in the southwest Atlantic. Brazil. Mar. Pollut. Bull. 2018;130:84–94. doi: 10.1016/j.marpolbul.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Simancas-Giraldo SM, et al. Photosynthesis and respiration of the soft coral Xenia umbellata respond to warming but not to organic carbon eutrophication. PeerJ. 2021;9:e11663. doi: 10.7717/peerj.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollstedt S, Xiang N, Simancas-Giraldo SM, Wild C. Organic eutrophication increases resistance of the pulsating soft coral Xenia umbellata to warming. PeerJ. 2020;2020:1–16. doi: 10.7717/peerj.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thobor B, et al. The pulsating soft coral Xenia umbellata shows high resistance to warming when nitrate concentrations are low. Sci. Rep. 2022 doi: 10.1038/s41598-022-21110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa OS, Leão ZMAN, Nimmo M, Attrill MJ. Nutrification impacts on coral reefs from northern Bahia, Brazil. Hydrobiologia. 2000;440:307–315. doi: 10.1023/A:1004104118208. [DOI] [Google Scholar]
- 51.Fleury BG, Coll JC, Tentori E, Duquesne S, Figueiredo L. Effect of nutrient enrichment on the complementary (secondary) metabolite composition of the soft coral Sarcophyton ebrenbergi (Cnidaria: Octocorallia: Alcyonaceae) of the Great Barrier Reef. Mar. Biol. 2000;136:63–68. doi: 10.1007/s002270050009. [DOI] [Google Scholar]
- 52.Bednarz VN, Naumann MS, Niggl W, Wild C. Inorganic nutrient availability affects organic matter fluxes and metabolic activity in the soft coral genus Xenia. J. Exp. Biol. 2012;215:3672–3679. doi: 10.1242/jeb.072884. [DOI] [PubMed] [Google Scholar]
- 53.Bruno JF, Petes LE, Harvell CD, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003;6:1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x. [DOI] [Google Scholar]
- 54.Ezzat L, Maguer J-FF, Grover R, Ferrier-Pagès C. Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep31768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka Y, Grottoli AG, Matsui Y, Suzuki A, Sakai K. Effects of nitrate and phosphate availability on the tissues and carbonate skeleton of scleractinian corals. Mar. Ecol. Prog. Ser. 2017;570:101–112. doi: 10.3354/meps12079. [DOI] [Google Scholar]
- 56.Liu, G., Strong, A. E., Skirving, W. & Arzayus, L. F. Overview of NOAA coral reef watch program’s near-real time satellite global coral bleaching monitoring activities. In Proc. 10th International Coral Reef Symposium, 1783–1793 (2006).
- 57.Bellworthy J, Fine M. Beyond peak summer temperatures, branching corals in the Gulf of Aqaba are resilient to thermal stress but sensitive to high light. Coral Reefs. 2017;36:1071–1082. doi: 10.1007/s00338-017-1598-1. [DOI] [Google Scholar]
- 58.Rex A, Montebon F, Yap HT. Metabolic responses of the scleractinian coral Porites cylindrica Dana to water motion. I. Oxygen flux studies. J. Exp. Mar. Biol. Ecol. 1995;186:33–52. doi: 10.1016/0022-0981(95)00150-P. [DOI] [Google Scholar]
- 59.Long MH, Berg P, de Beer D, Zieman JC. In situ coral reef oxygen metabolism: An eddy correlation study. PLoS ONE. 2013;8:e58581. doi: 10.1371/journal.pone.0058581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fabricius KE, Klumpp DW. Widespread mixotrophy in reef-inhabiting soft corals: The influence of depth, and colony expansion and contraction on photosynthesis. Mar. Ecol. Prog. Ser. 1995;125:195–204. doi: 10.3354/meps125195. [DOI] [Google Scholar]
- 61.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 62.Raimonet M, Guillou G, Mornet F, Richard P. Macroalgae δ15N values in well-mixed estuaries: Indicator of anthropogenic nitrogen input or macroalgae metabolism? Estuar. Coast. Shelf Sci. 2013;119:126–138. doi: 10.1016/j.ecss.2013.01.011. [DOI] [Google Scholar]
- 63.Furla P, Galgani I, Durand I, Allemand D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 2000;203:3445–3457. doi: 10.1242/jeb.203.22.3445. [DOI] [PubMed] [Google Scholar]
- 64.Hughes AD, Grottoli AG, Pease TK, Matsui Y. Acquisition and assimilation of carbon in non-bleached and bleached corals. Mar. Ecol. Prog. Ser. 2010;420:91–101. doi: 10.3354/meps08866. [DOI] [Google Scholar]
- 65.Rau GH, Takahashi T, Des Marais DJ. Latitudinal variations in plankton delta C-13—Implications for CO2 and productivity in past oceans. Nature. 1989;341:516–518. doi: 10.1038/341516a0. [DOI] [PubMed] [Google Scholar]
- 66.McMahon KW, Hamady LL, Thorrold SR. A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol. Oceanogr. 2013;58:697–714. doi: 10.4319/lo.2013.58.2.0697. [DOI] [Google Scholar]
- 67.Muscatine L, Porter JW, Kaplan IR. Resource partitioning by reef corals as determined from stable isotope composition. Mar. Biol. 1989;100:185–193. doi: 10.1007/BF00391957. [DOI] [Google Scholar]
- 68.Swart PK, et al. The isotopic composition of respired carbon dioxide in scleractinian corals: Implications for cycling of organic carbon in corals. Geochim. Cosmochim. Acta. 2005;69:1495–1509. doi: 10.1016/j.gca.2004.09.004. [DOI] [Google Scholar]
- 69.Rodrigues LJ, Grottoli AG. Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta. 2006;70:2781–2789. doi: 10.1016/j.gca.2006.02.014. [DOI] [Google Scholar]
- 70.Grottoli AG, Rodrigues LJ. Bleached Porites compressa and Montipora capitata corals catabolize δ13C-enriched lipids. Coral Reefs. 2011;30:687–692. doi: 10.1007/s00338-011-0756-0. [DOI] [Google Scholar]
- 71.Levas SJ, Grottoli AG, Hughes A, Osburn CL, Matsui Y. Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral porites lobata: Implications for resilience in mounding corals. PLoS ONE. 2013;8:32–35. doi: 10.1371/journal.pone.0063267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoepf V, et al. Annual coral bleaching and the long-term recovery capacity of coral. Proc. R. Soc. B Biol. Sci. 2015;282:20151887. doi: 10.1098/rspb.2015.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lesser MP, et al. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 2007;346:143–152. doi: 10.3354/meps07008. [DOI] [Google Scholar]
- 74.Carpenter EJ, Harvey HR, Brian F, Capone DG. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep Sea Res. I Oceanogr. Res. Pap. 1997;44:27–38. doi: 10.1016/S0967-0637(96)00091-X. [DOI] [Google Scholar]
- 75.Lachs L, et al. Effects of tourism-derived sewage on coral reefs: Isotopic assessments identify effective bioindicators. Mar. Pollut. Bull. 2019;148:85–96. doi: 10.1016/j.marpolbul.2019.07.059. [DOI] [PubMed] [Google Scholar]
- 76.Kürten B, et al. Influence of environmental gradients on C and N stable isotope ratios in coral reef biota of the Red Sea, Saudi Arabia. J. Sea Res. 2014;85:379–394. doi: 10.1016/j.seares.2013.07.008. [DOI] [Google Scholar]
- 77.Core Team, R. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
- 78.Wickham H, et al. Welcome to the Tidyverse. J. Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 79.Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0 (2020).
- 80.Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0 (2021).
- 81.Contreras-Silva AI, et al. A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macroalgae cover in the Mexican Caribbean. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-65801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ledlie MH, et al. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs. 2007;26:641–653. doi: 10.1007/s00338-007-0230-1. [DOI] [Google Scholar]
- 83.Kuffner IB, Toth LT. A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conserv. Biol. 2016;30:706–715. doi: 10.1111/cobi.12725. [DOI] [PubMed] [Google Scholar]
- 84.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean Coral Reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 85.de Bakker DM, Meesters EH, Bak RPM, Nieuwland G, van Duyl FC. Long-term shifts in coral communities on shallow to deep reef slopes of Curaçao and Bonaire: Are there any winners? Front. Mar. Sci. 2016;3:247. doi: 10.3389/fmars.2016.00247. [DOI] [Google Scholar]
- 86.Mergner H, Svoboda A. Productivity and seasonal changes in selected reef areas in the Gulf of Aqaba (Red Sea) Helgoländer Meeresun. 1977;30:383–399. doi: 10.1007/BF02207849. [DOI] [Google Scholar]
- 87.Schlichter D, Svoboda A, Kremer BP. Functional autotrophy of Heteroxenia fuscescens (Anthozoa: Alcyonaria): Carbon assimilation and translocation of photosynthates from symbionts to host. Mar. Biol. 1983;78:29–38. doi: 10.1007/BF00392968. [DOI] [Google Scholar]
- 88.Al-Sofyani AA, Niaz GR. A comparative study of the components of the hard coral Seriatopora hystrix and the soft coral Xenia umbellata along the Jeddah coast, Saudi Arabia. Rev. Biol. Mar. Oceanogr. 2007;42:207–219. doi: 10.4067/S0718-19572007000300001. [DOI] [Google Scholar]
- 89.McCloskey LR, Wethey DS, Porter JW. Measurement and interpretation of photosynthesis and respiration in reef corals. In: Stoddart DR, Johannes RE, editors. Coral Reefs: Research Methods. United Nations Educational, Scientific and Cultural Organization; 1978. pp. 379–396. [Google Scholar]
- 90.Baker DM, Freeman CJ, Wong JCY, Fogel ML, Knowlton N. Climate change promotes parasitism in a coral symbiosis. ISME J. 2018;12:921–930. doi: 10.1038/s41396-018-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoegh-Guldberg O, Smith GJ. The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 1989;129:279–303. doi: 10.1016/0022-0981(89)90109-3. [DOI] [Google Scholar]
- 92.Iglesias-Prieto R, Matta JL, Robins WA, Trench RK. Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. 1992;89:10302–10305. doi: 10.1073/pnas.89.21.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Béraud E, Gevaert F, Rottier C, Ferrier-Pagès C. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J. Exp. Biol. 2013;216:2665–2674. doi: 10.1242/jeb.085183. [DOI] [PubMed] [Google Scholar]
- 94.Kremien M, Shavit U, Mass T, Genin A. Benefit of pulsation in soft corals. Proc. Natl. Acad. Sci. U.S.A. 2013;110:8978–8983. doi: 10.1073/pnas.1301826110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grover R, et al. Coral uptake of inorganic phosphorus and nitrogen negatively affected by simultaneous changes in temperature and pH. PLoS ONE. 2011;6:1–10. doi: 10.1371/journal.pone.0025024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cardini U, et al. Microbial dinitrogen fixation in coral holobionts exposed to thermal stress and bleaching. Environ. Microbiol. 2016;18:2620–2633. doi: 10.1111/1462-2920.13385. [DOI] [PubMed] [Google Scholar]
- 97.Cardini U, et al. Functional significance of dinitrogen fixation in sustaining coral productivity under oligotrophic conditions. Proc. R. Soc. B Biol. Sci. 2015;282:20152257. doi: 10.1098/rspb.2015.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santos HF, et al. Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 2014;8:2272–2279. doi: 10.1038/ismej.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tilstra A, et al. Relative diazotroph abundance in symbiotic red sea corals decreases with water depth. Front. Mar. Sci. 2019;6:372. doi: 10.3389/fmars.2019.00372. [DOI] [Google Scholar]
- 100.Klinke A, Mezger S. D., Thobor B, Tilstra A, El-Khaled Y. C., Wild C, et al. Impact of phosphate enrichment on the susceptibility of the pulsating soft coral Xenia umbellata to ocean warming. Front. Mar. Sci. 2022;9:1026321. doi: 10.3389/fmars.2022.1026321. [DOI] [Google Scholar]
- 101.Rädecker N, et al. Heat stress reduces the contribution of diazotrophs to coral holobiont nitrogen cycling. ISME J. 2021 doi: 10.1038/s41396-021-01158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swart PK, Saied A, Lamb K. Temporal and spatial variation in the δ15N and δ13C of coral tissue and zooxanthellae in Montastraea faveolata collected from the Florida reef tract. Limnol. Oceanogr. 2005;50:1049–1058. doi: 10.4319/lo.2005.50.4.1049. [DOI] [Google Scholar]
- 103.Grottoli AG, Tchernov D, Winters G. Physiological and biogeochemical responses of super-corals to thermal stress from the Northern Gulf of Aqaba, Red Sea. Front. Mar. Sci. 2017;4:215. doi: 10.3389/fmars.2017.00215. [DOI] [Google Scholar]
- 104.Dubinsky Z, Stambler N. Marine pollution and coral reefs. Glob. Change Biol. 1996;2:511–526. doi: 10.1111/j.1365-2486.1996.tb00064.x. [DOI] [Google Scholar]
- 105.Loya Y, Lubinevsky H, Rosenfeld M, Kramarsky-Winter E. Nutrient enrichment caused by in situ fish farms at Eilat, Red Sea is detrimental to coral reproduction. Mar. Pollut. Bull. 2004;49:344–353. doi: 10.1016/j.marpolbul.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Costa OS, Nimmo M, Attrill MJ. Coastal nutrification in Brazil: A review of the role of nutrient excess on coral reef demise. J. S. Am. Earth Sci. 2008;25:257–270. doi: 10.1016/j.jsames.2007.10.002. [DOI] [Google Scholar]
- 107.Tait DR, et al. The influence of groundwater inputs and age on nutrient dynamics in a coral reef lagoon. Mar. Chem. 2014;166:36–47. doi: 10.1016/j.marchem.2014.08.004. [DOI] [Google Scholar]
- 108.Guan Y, Hohn S, Wild C, Merico A. Vulnerability of global coral reef habitat suitability to ocean warming, acidification and eutrophication. Glob. Change Biol. 2020;26:5646–5660. doi: 10.1111/gcb.15293. [DOI] [PubMed] [Google Scholar]
- 109.Hall ER, et al. Eutrophication may compromise the resilience of the Red Sea coral Stylophora pistillata to global change. Mar. Pollut. Bull. 2018;131:701–711. doi: 10.1016/j.marpolbul.2018.04.067. [DOI] [PubMed] [Google Scholar]
- 110.Naumann MS, et al. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs. 2010;29:649–659. doi: 10.1007/s00338-010-0612-7. [DOI] [Google Scholar]
- 111.Wild C, et al. Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature. 2004;428:66–70. doi: 10.1038/nature02344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data of the current study is available in the Supplementary Material online.