Abstract
Functional hyperemia occurs when enhanced neuronal activity signals to increase local cerebral blood flow (CBF) to satisfy regional energy demand. Ca2+ elevation in astrocytes can drive arteriole dilation to increase CBF, yet affirmative evidence for the necessity of astrocytes in functional hyperemia in vivo is lacking. In awake mice, we discovered that functional hyperemia is bimodal with a distinct early and late component whereby arteriole dilation progresses as sensory stimulation is sustained. Clamping astrocyte Ca2+ signaling in vivo by expressing a plasma membrane Ca2+ ATPase (CalEx) reduces sustained but not brief sensory-evoked arteriole dilation. Elevating astrocyte free Ca2+ using chemogenetics selectively augments sustained hyperemia. Antagonizing NMDA-receptors or epoxyeicosatrienoic acid production reduces only the late component of functional hyperemia, leaving brief increases in CBF to sensory stimulation intact. We propose that a fundamental role of astrocyte Ca2+ is to amplify functional hyperemia when neuronal activation is prolonged.
Subject terms: Astrocyte, Neuro-vascular interactions
Neuronal activity increases local cerebral blood flow (CBF) to satisfy metabolic demand, yet the role of astrocytes in this phenomenon is controversial. Here, the authors show that astrocytes amplify CBF only when neuronal activity is sustained.
Introduction
Neuronal function requires tight and uninterrupted access to O2 and glucose via the blood supply because neurons have neither sufficient energy stores nor capacity for anaerobic metabolism. Thus, neural processing of sensory, motor, and cognitive information drives increased regional cerebral blood flow (CBF) for seconds to minutes as needed. This phenomenon—functional hyperemia—is essential for brain metabolism and also serves as the basis of the blood oxygen level-dependent (BOLD) signal of functional magnetic resonance imaging (fMRI)1,2.
Functional hyperemia occurs, in part, by large diameter changes in penetrating arterioles3–6 as a result of multiple parallel neurovascular coupling mechanisms thought to be governed by neurons, astrocytes, and endothelial cells that actuate vascular smooth muscle. Astrocytes respond to neural activity and their peri-vascular processes/endfeet can release Ca2+ dependent messengers to regulate arteriole diameter, both ex vivo (reviewed in refs. 7, 8) and in vivo9,10 when artificially stimulated. We have previously described that astrocytes sense behavioral-state and vascular signals as brief functional hyperemia evolves in awake mice11, yet their capacity to control CBF to a natural stimulus in vivo has not been demonstrated. It remains possible that astrocytes contribute to functional hyperemia only under distinct durations of neural activity. Indeed, astrocyte Ca2+ correlates to CBF when sensory stimulation is prolonged12. Under anesthesia, astrocyte Ca2+ signals can be suppressed13, and arteriole/CBF responses to sustained sensory stimulation consist of an initial rise followed by a plateau14. The latter has prompted many to solely explore initiating mechanisms or assume that there are no temporally distinct components to functional hyperemia. More recent studies, some conducted in awake animals, display CBF/arteriole response curves that appear bimodal15–17, yet these temporally distinct phases are rarely mechanistically explored or even described. We hypothesized that delayed astrocyte Ca2+ signals (>3 s) occurring in the awake state regulate CBF when sensory activation is sustained, therefore comprising an important and unrecognized mechanism of functional hyperemia12,18. Thus far, no causal ‘necessity’ and ‘sufficiency’ experiments support this hypothesis. The main framework of astrocyte Ca2+ in functional hyperemia in vivo relies on correlational studies12,18–23, none of which have clearly elucidated the physiological context of astrocytic contributions at arterioles. Though recent work suggests a Ca2+ independent astrocytic mechanism in CBF regulation involving CO224, the foundational work of this field manipulated and measured astrocyte free Ca2+7,8, highlighting the need to resolve the Ca2+ signaling hypothesis.
Using 2-photon fluorescence imaging in awake mice and tools to selectively clamp or augment astrocyte Ca2+ in vivo, we discovered that during sustained bouts of sensory activation (1) functional hyperemia increases bimodally and (2) astrocyte Ca2+ is causal for augmenting the second phase. These findings are important because impairments in sustained functional hyperemia occur in attention deficit25, age-related cognitive impairment26, dementia27, and stroke28. Our results help clarify neuro-glio-vascular coupling and will facilitate future work aimed at understanding how astrocytes impact the (patho)physiology of the cerebrovasculature.
Results
Sustained functional hyperemia escalates and is associated with astrocyte Ca2+ transients
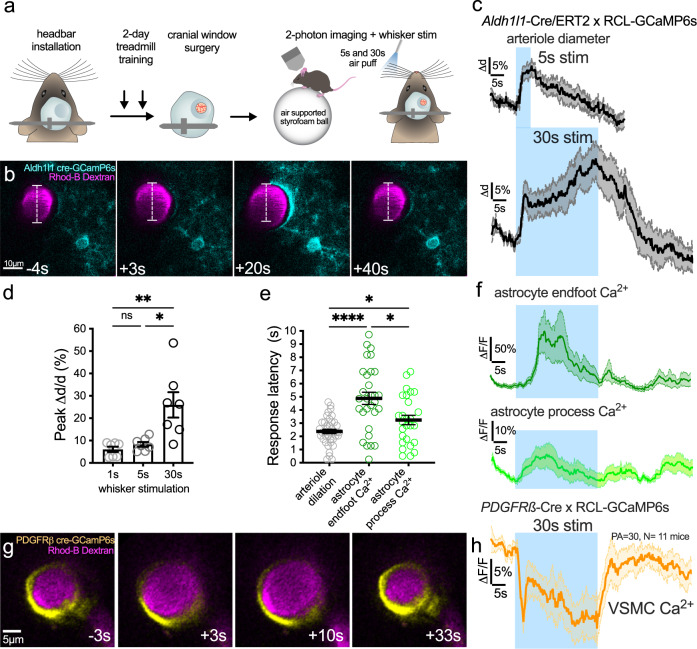
We investigated the temporal dynamics of penetrating arteriole diameter and astrocyte Ca2+ during brief (5 s) and sustained (30 s) whisker stimulation using 2-photon microscopy through an acutely installed cranial window over the barrel cortex of awake mice (Fig. 1a). First, imaging Aldh1l1-Cre/ERT2 × RCL-GCaMP6s mice, arteriole responses to whisker stimulation with air puff elicited rapid arteriole dilation that peaked within the first 5 s of stimulation, followed by progressively increasing diameter only when stimulation was maintained for 30 s revealing a bimodal response (Fig. 1b, c). Interestingly, peak dilations to 1 and 5 s stimulation were not different, but dilation to 30 s stimulation was significantly larger due to the putative secondary phase (Fig. 1d). Astrocyte fine processes, followed by endfeet, had Ca2+ signals that both increased after the onset of vasodilation (Fig. 1e, f)11. Arteriole responses were similar in c57bl/6 mice imaged through thinned skull (Supplementary Fig. 1a–c), confirming that acute craniotomy did not impair functional hyperemia. We found similar arteriole response dynamics and delayed elevation in Ca2+ within endfeet of membrane-tethered GCaMP6 expressing Aldh1l1-Cre/ERT2 x R26-Lck-GCaMP6f mice implanted with a chronic cranial window over an intact dura (>4weeks recovery) (Supplementary Fig. 2a–f). However, in this mouse line, we identified two distinct populations of fine process Ca2+ signals: 47% of signals started in the first sec of stimulation (ultrafast)19,21 before initiation of vasodilation and 45% appeared 3–5 s after stimulation onset (delayed) (Supplementary Fig. 2d, e). Ultrafast Ca2+ signals were much smaller in amplitude compared to slower Ca2+ signals (~4% vs 48%, Supplementary Fig. 2f). Astrocytes labeled with Rhod-2/AM in acute cranial windows had Ca2+ signals that increased after the onset of vasodilation but that remained elevated until stimulation ended (Supplementary Fig. 3a–c), unlike GCaMP6 generated signals that displayed a shorter duration. In further support of a bimodal arteriole response, imaging vascular smooth muscle cell Ca2+ changes in PDGFRß-Cre × RCL-GCaMP6s mice during 30 s whisker stimulation showed two separate peaks of Ca2+ drop at ~2.5 s and between 20 and 30 s (Fig. 1g, h). This suggests separate processes mediate smooth muscle relaxation in the early and late phase of sustained functional hyperemia.
Fig. 1. Sustained functional hyperemia escalates and is associated with delayed astrocyte Ca2+ transients.
a Cartoon and timeline of the awake mouse, acute cranial window (skull + dura removal), 2-photon imaging experiment in barrel cortex. b Time series images showing dilation of a Rhodamine (Rhod)-B-dextran labeled penetrating arteriole (magenta, median filtered image) and Ca2+ responses (cyan) of a GCaMP6s-expressing astrocyte in response to 30 s whisker stimulation at 3, 20 and 40 s in an Aldh1l1-CreERT2 × RCL-GCaMP6s mouse (representative of n = 9 mice). Time stamps refer to stimulation onset as 0 s. c Traces of arteriole dilation (black) to 5 and 30 s whisker stimulation. N = 28 trials (T) of 12 penetrating arterioles (PA) from 9 mice. d Summary data of peak arteriole dilation to 1, 5, and 30 s whisker stimulation. Friedman test (one-sided). N = 7 PA from 6 mice. e Summary of response onset (calculated from each trial as 3 × SD above baseline) for dilation and astrocyte Ca2+ from 13 PA of 9 mice. Arteriole dilation T = 39, astrocyte endfoot Ca2+:T = 30 astrocyte process Ca2+: T = 27. Mixed effect analysis (two-sided) with Holm–Sidak’s multiple comparison. f Astrocyte endfoot (dark green) and astrocyte arbor (light green) Ca2+ traces to 30 s whisker stimulation. g Time series images of GcaMP6s expressing vascular smooth muscle cells (VSMC)(yellow) within a penetrating arteriole loaded with Rhod-B-Dextran (magenta). Each image is an average projection of 4 raw images. h VSMC Ca2+ trace during 30 s whisker stimulation. N = 30 PA from 11 mice. All average trace and summary dot plot data show mean ± SEM. For further statistical details see Supplementary Table 1. Source data are provided as a Source Data file.
Clamping astrocyte Ca2+ reduces the late phase of functional hyperemia
Given the stronger association between astrocyte process/endfoot Ca2+ and sustained functional hyperemia, and the appearance of a distinct late component in smooth muscle Ca2+ traces, we tested for a causal role of astrocyte Ca2+ primarily in the late phase of functional hyperemia. Astrocyte Ca2+ transients mediate activity-dependent capillary but not arteriole dilation to 5 s stimulation in acute cortical brain slices29. Thus, we tested for a causal relationship between long (30 s) but not short (5 s) stimulation-evoked astrocyte Ca2+ and penetrating arteriole dilation in acute rat brain slices, by whole-cell patch infusing the Ca2+ chelator BAPTA (10 mM) into a network of peri-arteriolar astrocytes. We clamped intracellular free Ca2+ close to the resting concentration in astrocytes (~100 nM)30,31. We allowed 15 mins for the internal solution to equilibrate in the astrocyte network32 and for arteriole tone to stabilize. Rhod-2/AM was used to label large astrocyte and neuronal compartments, as well as the neuropil. Before patching an astrocyte, 30 s of high frequency electrical stimulation elicited Ca2+ transients in neurons, the neuropil, astrocyte somata, and endfeet, as well as caused arteriole dilation (Supplementary Fig. 4a–e). This was then repeated when the astrocyte network was filled with either control internal or Ca2+ clamp internal solution. We found that only the Ca2+ clamp solution reduced arteriole dilation to 30 s afferent stimulation (Supplementary Fig. 4d, e). While the astrocyte network filling itself caused reductions in evoked Ca2+ from all cell compartments, comparing to the Ca2+ clamp solution revealed that the only effect to explain the loss of dilation was the clamp on astrocyte Ca2+ (Supplementary Fig. 4d, e). Importantly, neither clamping astrocyte Ca2+, nor the control patch, affected arteriole dilation to 5 s stimulation, and patching itself (in any condition) did not affect baseline arteriole tone after whole-cell equilibration (Supplementary Fig. 5a–d). These data show that longer neural stimulation is required to recruit Ca2+ dependent astrocyte contributions to arteriole dilation in neocortical brain slices.
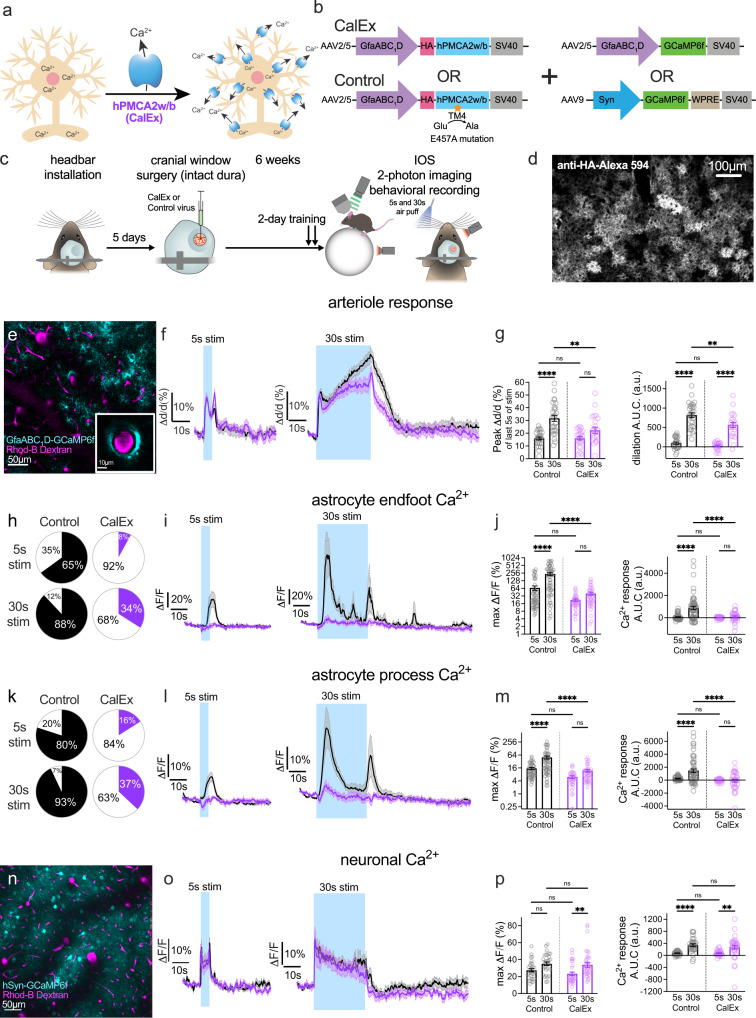
Previous attempts to connect astrocyte Ca2+ to functional hyperemia in vivo focused on IP3R2 knockout33–35, but to our knowledge there have been no in vivo manipulations to clamp astrocyte Ca2+ more generally for CBF control; thereby affecting multiple Ca2+ dependent pathways. We made use of the astrocyte Ca2+ silencing tool CalEx23. Here, astrocyte-selective AAV (AAV2/5-gfaABC1D-CalEx-HA) delivers a high-affinity Ca2+ ATPase pump (hPMCA2w/b) to the plasma membrane (Fig. 2a, b), which reduces evoked and spontaneous elevations in free Ca2+ both in vitro and in vivo23. As a control, we made a single point mutation in the CalEx sequence (E457A) to prevent Ca2+ transport across the cell membrane36,37. We validated both tools in awake c57bl/6 mice via intracortical viral injection followed by a chronic cranial window implantation (Fig. 2c). First, we compared astrocyte AAVs for CalEx and GCaMP6f (AAV2/5-gfaABC1D-GCaMP6f) vs the mutated CalEx control virus and GCaMP6f, by examining startle evoked bulk astrocyte Ca2+ signals in the barrel cortex (untrained body air puff, Supplementary Fig. 6a, b). Astrocytes expressing functional CalEx showed markedly reduced startle-evoked Ca2+ transients compared to control (Supplementary Fig. 6c, d). Post hoc CalEx expression was confirmed by immunostaining against the reporter hemagglutinin (HA) (Fig. 2d).
Fig. 2. Clamping astrocyte Ca2+ reduces the late phase of functional hyperemia.
a Cartoon of astrocyte Ca2+ extrusion tool: a high-affinity plasma membrane Ca2+ ATPase hPMCA2w/b (CalEx). b Viral vector strategy to express CalEx (Top Left) in astrocyte (or control virus- Bottom Left) plus GCaMP6f targeting astrocytes (Top Right) or neurons (Bottom Right). c Timeline from viral vector intracortical injection to the imaging experiment. d Representative post hoc immunofluorescence of CalEx expression against the fused Hemagglutinin (HA) reporter completed in 7 CalEx and 5 Control mice. e Representative image of gfaABC1D-GCaMP6f expressing astrocytes (cyan, median filtered) and Rhodamine (Rhod)-B-Dextran loaded vasculature (magenta, median filtered) during startle in layer 2/3 of the barrel cortex in a control mouse (similar in 5 more mice) (channels were merged from separate acquisitions as startle experiments were performed before vascular dye loading). f Average arteriole diameter traces in CalEx and Control for 5 s (Left) and 30 s (Right) whisker stimulation. g Left: summary data showing peak arteriole dilation of the last 5 s of the stimulation period. Right: net Area Under the Curve (AUC: 40 s from stimulation onset) in the four conditions. Control, 5 s stim: n = 26 penetrating arterioles (PA), Control 30 s stim: n = 28 PA from 11 mice; CalEx, 5 s and 30 s stim: n = 23 PA from 10 mice. h Evoked astrocyte endfoot Ca2+ event occurrence. Black (control) and purple (CalEx) slices are events, white is no event detected. An event is >3 standard deviation of baseline. i Summary time series data of astrocyte endfoot Ca2+ in CalEx vs control for both 5 s (Left) and 30 s (Right) whisker stimulation. j Left: Summary bar graph of peak astrocyte endfoot Ca2+ signal. Right: net AUC (stimulation + 10 s) data in the four conditions. Control, 5s stim: n = 51 trials, penetrating arterioles (PA), Control 30 s stim: n = 57 trials, 18 PA from 6 mice; CalEx, 5 s stim: n = 36 trials, and 30 s stim: n = 38 trials 12 PA from 5 mice. k–m Same as h–j but for astrocyte arbor Ca2+. Control, 5 s stim: n = 48 trials, 16 PA, Control 30 s stim: n = 54 trials, 17 PA from 6 mice; CalEx, 5 s stim: n = 36 trials, and 30 s stim: n = 37 trials 12 PA from 5 mice. n AAV9.hSyn.GCaMP6f expressing neurons (cyan) and Rhodamine-B-Dextran loaded vasculature (magenta, median filtered image) in layer 2/3 of the barrel cortex. Representative example of 10 experiments. o Average neuronal Ca2+ traces in CalEx and Control for 5 s (Left) and 30 s (Right) whisker stimulation. p Left: summary data showing peak neuronal Ca2+ during the stimulation period. Right: net AUC of the stimulation period in the four conditions. Control, 5 s stim: n = 30 trials, 10 PA; Control 30 s stim: n = 30 trials, 10 PA from 5 mice; CalEx, 5 s stim: n = 32 trials, and 30 s stim: n = 38 trials 11 PA from 5 mice. All average trace and summary dot plot data show mean ± SEM. All statistical tests are Two-way ANOVA with Tukey’s multiple comparisons (two-sided). For further statistical details see Supplementary Table 2. Source data are provided as a Source Data file.
In response to 5 s whisker stimulation, we found that astrocyte CalEx had no impact on evoked arteriole dilation compared to control AAV (Fig. 2f, g). Yet remarkably, astrocytic CalEx reduced only the late component (last 5 s of stim) of arteriole dilation to 30 s whisker stimulation compared to control (Fig. 2f, g). These results could not be explained by differences in baseline arteriole tone caused by CalEx expression (Supplementary Fig. 7), and instead suggest that astrocyte Ca2+ is important for amplifying arteriole dilation during sustained, but not brief, functional hyperemia.
Confirming a clamp on astrocyte Ca2+, CalEx reduced the delayed astrocyte endfoot Ca2+ signal occurrence (Fig. 2h) and peak amplitude caused by 5 s (Fig. 2i, j) and 30 s whisker stimulation, as well as the integrated Ca2+ response with an overall significance of PCalEx < 0.0001 (Fig. 2j). CalEx also diminished astrocyte process Ca2+ transient occurrence (Fig. 2k), Ca2+ peaks and Area Under the Curve (AUC) Ca2+ responses when stimulating for 5 s and for 30 s (Fig. 2l, m). These data demonstrate that CalEx is an effective tool for clamping astrocyte Ca2+ signals in vivo.
It is well established that astrocyte Ca2+ signals and subsequent gliotransmission can shape synaptic transmission and neuronal excitability23,30,38,39. For example, astrocyte-targeted CalEx in the striatum caused a loss of tonic inhibition in medium spiny inhibitory neurons, yet excitatory inputs onto these cells were unaltered23. This prompted us to explore the effect of astrocyte CalEx on neuronal Ca2+ signals in the barrel cortex during brief and prolonged whisker stimulation by co-injecting CalEx or its control virus with a pan-neuronal GCaMP6f encoding virus (AAV9.hSyn.GCaMP6f). We recorded bulk neuronal Ca2+ signals over a set 5000 µm2 area in layer2/3 around a responsive penetrating arteriole (Fig. 2n) and observed that neuronal Ca2+ peaked immediately after stimulation onset and gradually declined during 30s stimulation in both control and CalEx mice (Fig. 2o, p). This showed that the overall neural Ca2+ response was unaffected by attenuating astrocyte Ca2+ with CalEx. We also performed a more elaborate analysis of discrete Ca2+ transients occurring in various neuronal compartments in our field of view using an event-based toolkit40. We calculated absolute event frequency, peak Ca2+ responses, AUC, absolute spatial area, and duration of many thousands of Ca2+ events across the dataset (Supplementary Fig. 8a–f). Some baseline differences between the groups were noted, which may be due to either a direct effect of astrocyte CalEx on local baseline neuronal activity or small differences in mouse locomotor activity during this time (Supplementary Fig. 8g). The strongest effect of astrocyte CalEx on evoked neuronal Ca2+ transients was the reduction in peak amplitudes, which was seen in both 5 and 30 s stimulations (Supplementary Fig. 8b). However, both groups showed no effect on the initial vasodilation early in the response (see Fig. 2f), suggesting this change had no impact on early functional hyperemia. Additionally, during 30 s whisker stimulation, neuronal Ca2+ transients exhibited a small increase in AUC (Supplementary Fig. 8c), yet both the AUC and peak effects on neuronal Ca2+ signals were observed uniformly across the entire 30 s stimulation period—not selectively on the late phase. Collectively, these data exploring an indirect role for neuronal activity did not adequately explain the preferential effect of astrocyte CalEx on the late component of functional hyperemia.
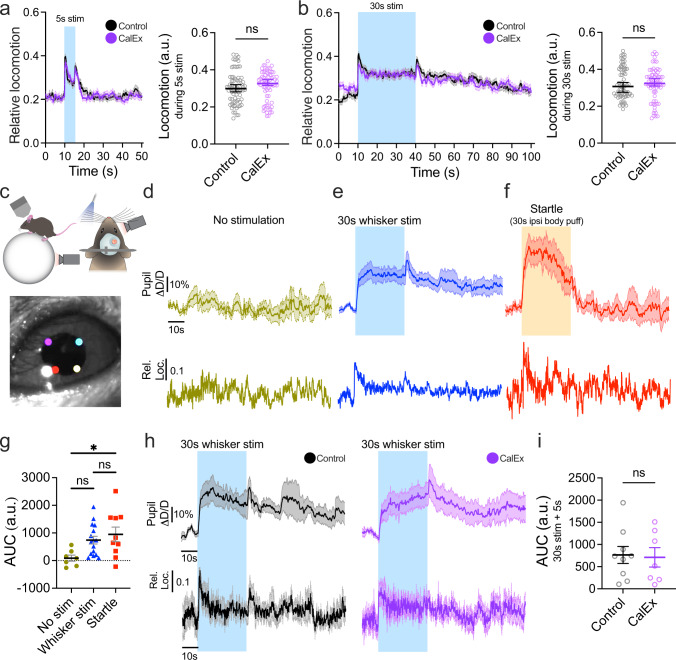
Cortical astrocytes integrate local sensory information and behavioral state11,41. Locomotion, alone42,43 or concomitantly with tactile stimulation of the whiskers, was shown to trigger astrocyte Ca2+ events42,44, as well as to modulate CBF in the neocortex17,45. Notably, two-thirds of barrel cortex excitatory neurons respond predominantly to the combination of vibrissa deflection and running46. Therefore, we assessed whether differences in relative locomotion during the stimulation period were confounding the differences in arteriole response and astrocyte Ca2+ between CalEx and control mice. Whisker stimulation prompted running at the onset and offset of whisker stimulation (Fig. 3a, b). Mice ran more during and after stimulation than at baseline. We found no difference in average locomotion during the 5 and 30 s stimulation period between CalEx and the control group (Fig. 3a, b). Importantly, arteriole dilation and astrocyte Ca2+ signals returned to baseline after the stimulus (see Fig. 2f, i, l). This was despite mice running more after the stimulus than during the pre-stimulus period, showing that running per se did not drive a prolonged increase in arteriole diameter or astrocyte calcium elevation in the barrel. Co-variate analysis of locomotion during the stimulation period in a general linear model confirmed that running does not account for differences in arteriole and neuronal Ca2+ responses, as well as in astrocyte process Ca2+ caused by CalEx. Interestingly, locomotion influenced the size of endfoot Ca2+ signals, but it did not negate the effect of CalEx on endfoot Ca2+ transients (statistical analysis in Supplementary Table 3). Collectively, these results suggest that differences in locomotion are unlikely to explain the differences in astrocyte Ca2+ and arteriole responses in the late phase of functional hyperemia between CalEx and control.
Fig. 3. Locomotion and arousal changes do not underlie CalEx effect on sustained functional hyperemia.
a Left: Average traces and (Right) summary data of relative locomotion during 5 s whisker stimulation for Control and CalEx mice. Mann-Whitney test (two-tailed). Control, 5 s stim: n = 73 trials from 11 mice. CalEx, 5 s stim: n = 67 trials from 10 mice. b Same as a but for 30 s whisker stimulation. Control, 30s stim: n = 75 trials from 11 mice. CalEx, 30 s stim: n = 69 trials from 10 mice. c Cartoon of locomotion (Top Left) and pupil (Top Right) recording with infrared cameras. Bottom: Pupil diameter tracking in two axes with the DeepLabCut tracking toolbox performed in n = 16 mice. d Average traces of relative pupil diameter and relative locomotion (mean only) during no stimulation recording, n = 28 trials, 7 mice; during e 30 s whisker stimulation, n = 68 trials, 16 mice; and during f startle evoked by 30 s ipsilateral neck air puff, n = 22 trials, 10 mice (d–f: CalEx and Control combined). g Summary data of net Area Under the Curve (AUC) for pupil diameter for 35 s of no stimulation (n = 7 mice), for 30 s whisker stimulation + 5 s post-stimulation (n = 16 mice) and 30 s startle + 5 s post-startle period (n = 10 mice). One-way ANOVA with Tukey’s multiple comparisons. h Average traces of relative pupil diameter and relative locomotion during 30 s whisker stimulation in Control (Left) and CalEx mice (Right). Control: n = 38 trials from 9 mice. CalEx: n = 30 trials from 7 mice. i Summary data of net AUC for relative pupil diameter changes (stimulation + 5 s) in the Control (n = 9 mice) and CalEx (n = 7 mice) groups. Mann-Whitney test (two-tailed). All average trace and summary dot plot data show mean ± SEM. For further statistical details see Supplementary Table 3. Source data are provided as a Source Data file.
Arousal provides a robust non-sensory modulation of brain activity across several sensory areas41,47,48 and has been linked to astrocyte Ca2+ transients. The measurement of pupil diameter allows a good estimation of arousal state49. We assessed and compared 30 s whisker stimulation-induced pupil diameter changes to a stimulus-free period and to a startle response elicited by 30 s ipsilateral air puff to the neck in a separate set of trials (Fig. 3c–i). To establish a bidirectionally responsive mid-size pupil, we used ambient green light. In trials with no stimulation, subtle changes in pupil diameter around the baseline corresponded to simultaneous changes in locomotion50 (Fig. 3c, d). Whisker stimulation for 30 s showed a non-significant pupil dilation that was at least partly reflected by an increase in locomotion (Fig. 3e, g). In contrast, the pupil dilated more robustly in response to startle which was uncoupled from locomotion passed the initial onset period (Fig. 3f, g). These results indicate potentially heightened arousal to whisker air puff which was not as pronounced as a startle reaction. Finally, we confirmed that the pupil reaction in the CalEx group was not different from control (Fig. 3h, i), indicating a similar influence of arousal on functional hyperemia.
Chemogenetic stimulation of astrocytes enhances the late phase of functional hyperemia
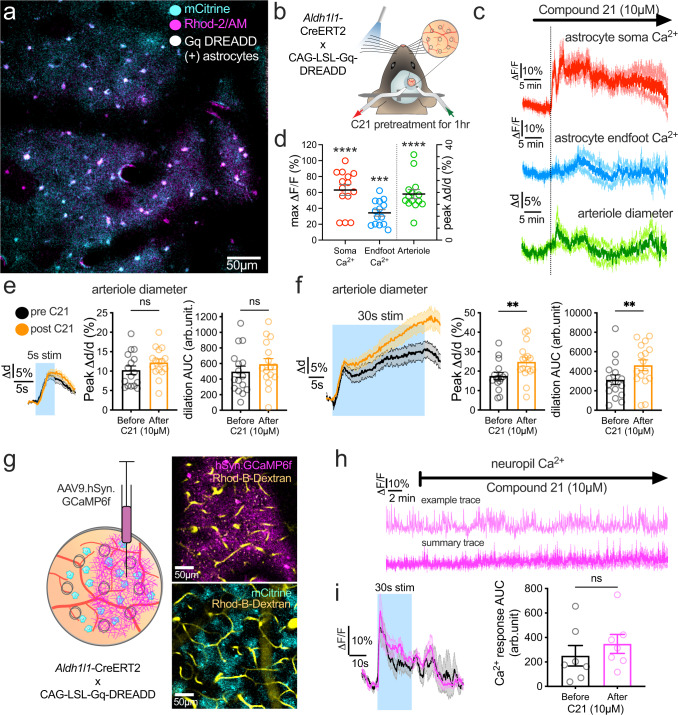
Large astrocyte Ca2+ signals occur when synaptic glutamate or neuromodulators stimulate Gq-coupled receptors through inositol triphosphate (IP3) signaling22,51,52. However, functional hyperemia is intact in anesthetized, sedated, and awake mice lacking IP3-receptor 233–35,53,54, the predominant subtype in astrocytes. Nevertheless, Gq-chemogenetics permits manipulation of cytosolic astrocyte Ca2+, which likely impacts signaling cascades outside of IP3R2 as a non-native receptor, allowing us to explore the contribution of general Gq signaling in awake mice. We aimed to test if (1) Gq-DREADD activation in astrocytes directly affects arteriole diameter, and if (2) continuous activation of astrocytic Gq either occludes or amplifies functional hyperemia to short vs long whisker stimulation. We crossed Aldh1l1-Cre/ERT2 with CAG-LSL-Gq-DREADD mice to selectively express hM3Dq receptors on astrocytes, which was confirmed by visualizing the fused reporter mCitrine (Fig. 4a). A perforated coverglass was applied to the brain surface to permit stable imaging while allowing topical superfusion of the DREADD agonist compound 21 (C21, 10 µM, Fig. 4b). C21 triggered a prolonged (20–40 min) astrocyte soma Ca2+ and endfoot Ca2+ elevation, accompanied by transient arteriole dilation (Fig. 4c, d). After an hour of continuous C21, astrocyte Ca2+ levels and arteriole diameter returned to baseline (Supplementary Fig. 9). Notably, arteriole dilation to 5 s whisker stimulation remained unchanged in the presence of C21 compared to pre-drug control (Fig. 4e), whereas 30 s stimulation showed a significant augmentation in only the late phase of functional hyperemia in C21 relative to before drug (Fig. 4f). Due to unexplained poor loading of Gq-DREADD positive astrocytes with Rhod-2/AM, we could not adequately capture endfoot Ca2+ signals in response to whisker stimulation before and during C21 application. To test whether the amplification of the late phase of sustained functional hyperemia was indirectly mediated via boosting neuronal activity, another group of Aldh1l1-Cre/ERT2 × CAG-LSL-Gq-DREADD mice received an AAV9.hSyn.GCaMP6f injection in the barrel cortex 4 weeks prior to imaging neuronal Ca2+ responses (Fig. 4g). Topical superfusion of C21 for 30 min did not change bulk neuronal GCaMP6 signals at rest (Fig. 4h) and failed to facilitate neuronal Ca2+ responses during 30 s whisker stimulation (Fig. 4i). Our results demonstrate that selectively driving the astrocyte Gq-pathway causes arteriole dilation and only influences the late phase of sustained functional hyperemia.
Fig. 4. Chemogenetic stimulation of astrocytes enhances the late phase of sustain functional hyperemia.
a Representative 2-photon image in barrel cortex (one from seven animals) of a Aldh1l1-Cre x CAG-LSL-Gq-DREADD mouse. Astrocytes are loaded with Rhod-2/AM (magenta, median filtered) and express Gq-DREADD-mCitrine (cyan, median filtered). b Cartoon of awake mouse sensory stimulation experiment in the presence of C21 delivered via a perforated window. c Average traces of astrocyte soma and endfoot Ca2+ and penetrating arteriole (PA) diameter in response to the local superfusion of the DREADD agonist C21 into the perforated window (n = 7 mice). d Summary data of peak responses to C21 application. All comparisons were Paired t tests (two-sided). Astrocyte soma Ca2+: n = 14 astrocyte somata and endfeet (average of 38 cells), and n = 14 PA. All data are from 7 mice. e Left: Average arteriole diameter traces in pre-drug control (black) and in the presence of C21 (orange), in response to 5 s whisker stimulation (n = 16 PA from 6 mice). Right: summary data showing peak dilation and Area Under the Curve (AUC) for 5 s in control and C21. Peak dilation and AUC of dilation: Paired t test (two-sided). N = 16 PA, 6 mice. f Same as e but for 30 s whisker stimulation. g Left: Cartoon showing the site of AAV9.hSyn.GCaMP6f injection 4 weeks prior to acute cranial window experiment. Top Right: Representative image from experiments in 5 mice of neuronal AAV9.hSyn.GCaMP6f expression (magenta) and Rhodamine-B-Dextran (yellow, median filtered image) labeled vasculature in the lateral side of the window imaged at 920 nm. Bottom Right: image of astrocytic mCitrine expression (cyan) in the medial side of the window imaged at 980 nm. h Time series measurements of neuronal Ca2+ in response to the local superfusion of the DREADD agonist C21 into the perforated window. Upper trace shows a representative region of interest (ROI). Lower trace shows averaged data, n = 5 ROI from 5 mice. i Average neuronal Ca2+ traces in pre-drug control (black) and in the presence of C21 (magenta), in response to 30 s whisker stimulation. n = 7 perivascular ROI from 5 mice. Right: summary data showing AUC for 30 s stimulation before and after C21. Paired t test (two-sided). All average trace and summary dot plot data show mean ± SEM. For further statistical details see Supplementary Table 4. Source data are provided as a Source Data file.
NMDA receptors and EETs are involved in sustained functional hyperemia
N-methyl-D-aspartate receptors (NMDAR) are key participants in functional hyperemia. In the canonical model, synapses generate NMDAR-dependent nitric oxide, which causes rapid vasodilation (reviewed in refs. 55–57). More recent work implicates NMDAR on astrocytes58 and endothelium59 in CBF control. Little is known about the contribution of NMDARs to different durations of functional hyperemia in awake animals.
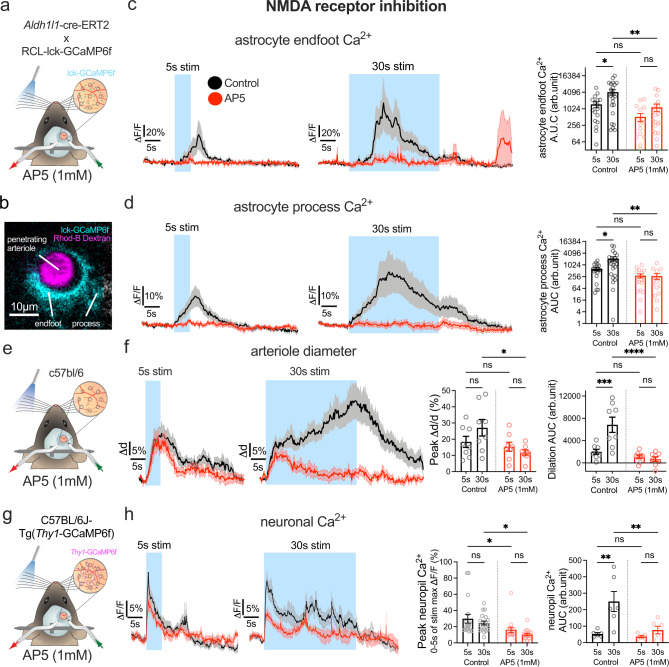
As NMDARs localize to the cell surface, we used Aldh1l1-Cre/ERT2 × R26-Lck-GCaMP6f mice (Fig. 5a) to express a plasma membrane-tethered GCaMP in astrocytes and imaged endfeet and processes surrounding a penetrating arteriole (Fig. 5b) or surrounding a precapillary sphincter—a control point for capillary access to blood (Supplementary Fig. 10b, c)4. We again applied short (5 s) and long (30 s) duration whisker stimulation before and during topical NMDAR antagonist D,L-AP5 (1 mM) via a perforated coverglass window. The lck-GCaMP6f showed similar astrocyte Ca2+ responses to the cytosolic GCaMP6s under control conditions (Fig. 5c, d and see Fig. 1f). Notably, in response to 5 and 30 s whisker stimulation, D,L-AP5 eliminated both astrocyte endfoot and process Ca2+ transients around penetrators (Fig. 5c, d), and a similar effect was observed in astrocytes around sphincters but failed to reach significance (Supplementary Fig. 10d, e). In a separate set of experiments using c57bl/6 mice (Fig. 5e), arteriole dilation to 5 s whisker stimulation remained surprisingly unchanged in the presence of D,L-AP5 from control (Fig. 5f). Before NMDAR antagonism, vasodilation was again larger during 30 s stimulation compared to 5 s (Fig. 5f), but in the presence of D,L-AP5, the late component of functional hyperemia was completely blocked, as arteriole diameter returned to baseline quickly after the initial peak (Fig. 5f). These data show NMDARs are critical for astrocyte Ca2+ elevation and for the late phase of functional hyperemia.
Fig. 5. NMDA receptor inhibition abolishes astrocyte activation and reduces the late component of sustained functional hyperemia.
a Cartoon of awake mouse 2-photon imaging experiment with sensory stimulation and perforated window for AP5 superfusion in Aldh1l1-CreERT2 × R26-lck-GCaMP6f mice. b 2-photon image of a penetrating arteriole (PA) (magenta, median filtered image) and surrounding astrocyte expressing membrane targeted lck-GCaMP6f (cyan). One example from 8 experiments from 8 mice. c Left: Average time series trace data of astrocyte endfoot Ca2+ in pre-drug control (black) and in the presence of AP5 (red) surrounding a PA in response to 5 s (n = 18 trials at 7 PA from 7 mice) and 30 s (n = 25 trials at 8 PA from 8 mice) whisker stimulation. Right: summary data of Area Under the Curve (AUC). d Same as for c but for astrocyte arbor Ca2+. 5 s stimulation: n = 18 trials at 7 PA from 7 mice 30 s stimulation: n = 24 trials at 8 PA from 8 mice. e Cartoon of a C57Bl/6 mouse with whisker puffer and perforated cranial window for superfusion of AP5. f Left: Average traces of penetrating arteriole diameter in pre drug control (black) or in the presence of AP5 (red), in response to 5 s or 30 s (n = 8 PA from 7 mice) whisker stimulation. Right: summary data of dilation peak and AUC (average of 3–4 trials per vessel). Peak dilation and AUC. g Cartoon of a Thy1-GCaMP6f mouse with whisker air puffer and perforated cranial window for superfusion of AP5. h Left: Average traces of neuronal Ca2+ in pre drug control (black) or in the presence of AP5 (red), in response to 5 s (control n = 18 trials, AP5 n = 15 trials) or 30 s whisker stimulation (control n = 19 trials, AP5 n = 18 trials) recorded from 6 perivascular ROIs from 6 mice. Right: summary data of maximum neuronal Ca2+ signal in the first 5 s of stimulation and neuropil Ca2+ signal AUC of stimulation period. Max ΔF/F and AUC. Control 5 s and 30 s: n = 6 ROIs, AP5 5 s: n = 5 ROIs; 30 s: n = 6 ROIs. All average trace and summary dot plot data show mean ± SEM. All statistical tests were Two-way ANOVA with Tukey’s multiple comparisons (two-sided). For further statistical details see Supplementary Table 5. Source data are provided as a Source Data file.
To test if NMDA inhibition abolished sustained neural activity as a potential explanation for the loss of evoked astrocyte Ca2+ elevation and late-phase functional hyperemia, we examined the temporal components of neuronal GCaMP6f during short and long duration whisker stimulation. We used C57BL/6J-Tg(Thy1-GCaMP6f) mice to measure neuronal Ca2+ signals before and during topical AP5 via a perforated coverglass window (Fig. 5g). We found that AP5 caused a reduction in the peak of the bulk neural Ca2+ measurements around the arteriole to 5 s whisker stimulation (Fig. 5h) and also caused uniform reduction in the 30 s response (Fig. 5h). While these data show AP5 effects sustained neural activity, the kinetic profile does not strongly affect the late phase over the early phase, suggesting other NMDAR-dependent neurovascular coupling mechanisms downstream of neural activity were affected, such as astrocytes or endothelium58–60.
Epoxyeicosatrienoic acids (EETs) have been demonstrated to contribute to functional hyperemia61–64 and are primarily produced by astrocytes65,66. Whether blocking EETs production has differential effects on brief vs sustained functional hyperemia has not been tested in awake mice. We measured functional hyperemia to 5 and 30 s stimulation before and 1 h after i.p. injection of the epoxygenase inhibitor MSPPOH (30 mg/kg) to c57bl/6 mice (Fig. 6a). Consistent with our proposed model, there was no difference in arteriole dilation to 5 s stimulation in the presence of MSPPOH, compared to before (Fig. 6b–d). However, 30 s stimulation was significantly attenuated by MSPPOH relative to control (Fig. 6b–d). MSPPOH yielded a similar result examining short vs long afferent stimulation-induced arteriole dilation in acute cortical brain slices (Supplementary Fig. 11). These experiments show that EETs, likely downstream of NMDAR activation, are important to maintain sustained functional hyperemia.
Fig. 6. Epoxyeicosatrienoic acids explain amplification of sustained functional hyperemia.
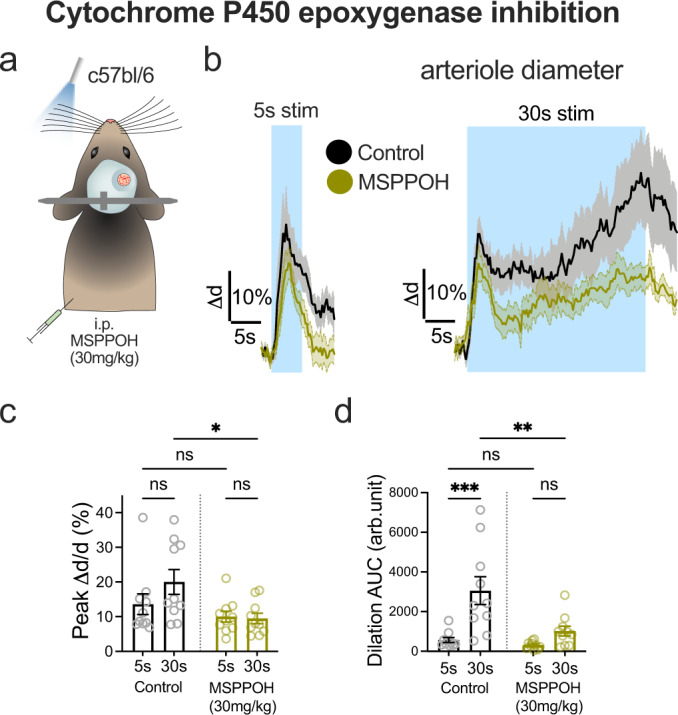
a Cartoon of a c57Bl/6 mouse with a sealed cranial window and a whisker air puffer receiving the epoxygenase inhibitor MSPPOH i.p. b Average traces of penetrating arteriole (PA) diameter in pre drug control (black) or after MSPPOH treatment (green), in response to 5 s (Left) or 30 s (Right) whisker stimulation. N = 10 PA from 4 mice. c Summary data of dilation peak (average of 3–4 trials per vessel). N = 10 PA from 4 mice. d Summary data of dilation AUC. N = 10 PA from 4 mice. All average trace and summary dot plot data show mean ± SEM. All statistical tests were Two-way ANOVA with Tukey’s multiple comparisons (two-sided). For further statistical details see Supplementary Table 6. Source data are provided as a Source Data file.
Discussion
By selectively attenuating or augmenting astrocyte Ca2+ signaling, we provide strong evidence in awake mice that astrocyte Ca2+ does not initiate or mediate functional hyperemia when neuronal activation is brief (<5 s) but is essential to amplify the CBF increase when neuronal activation is prolonged. Our work also reveals the origins of the bimodal neurovascular coupling response in vivo. Our data show that a non-neuronal cell type is late to ‘come online’ and at least partly explain the augmentation of the later phase. Interestingly, earlier work in awake animals showed that surface pial artery dilation to long stimulation was bimodal but the later phase was not amplified15,16. As astrocytes do not directly influence surface pial arterioles, this is congruent with only observing amplification of sustained CBF increases at penetrating arterioles which are wrapped by astrocyte endfeet, and that this astrocyte-mediated response is not conducted upstream to the brain surface.
Previously, controversies about the role of astrocytes in functional hyperemia have arisen from challenges in achieving selective activation67, lack of effect manipulating astrocyte IP3 signaling33–35,53,54, and side effects of anesthesia/sedation13,68. Increased neural activity is the main driver69 and user of fresh O270, yet the linear correlation of BOLD signal to neural activity is lost with sustained brain activation71,72. These results are consistent with a large body of literature showing delayed elevations in astrocyte Ca2+ when neural activity increases, as well as delayed increases in astrocyte metabolism73,74. Our data show that the fully awake animal is necessary to observe sufficiency effects on arteriole diameter driven by astrocyte Gq33, and suggest other IP3R subtypes play a role in CBF regulation. That selectively driving the astrocyte Gq pathway augmented only sustained functional hyperemia, which is impaired in a number of neurological conditions25–28, suggests a potential therapeutic target to improve brain blood flow in these states.
Our ex vivo BAPTA and in vivo astrocyte membrane-tethered GCaMP6 transgenic mouse results, as well as CalEx data indicate that ultrafast astrocyte Ca2+ signals that precede vasodilation19,21 are not involved in the initiation or first dilation peak of functional hyperemia. These same data argue against fast acting astrocyte Ca2+ at the capillary, pre-capillary, or sphincter level4,75,76 driving a rapid conduction-mediated dilation to upstream arterioles77,78. This is because both astrocyte CalEx in vivo and astrocyte BAPTA ex vivo reached these vascular compartments. While indirect effects of astrocyte Ca2+ clamp on neural activity could explain the decrease in sustained functional hyperemia39,79, that 5 s evoked vasodilation was unaffected both in vivo and ex vivo, and that bulk neuronal Ca2+ measures directly around the arteriole in vivo were unchanged by CalEx, argues against this possibility. Furthermore, we found no impact of astrocyte Ca2+ clamp on evoked neural Ca2+ to long stimulation in brain slices, despite reduced vasodilation. Finally, a selective effect of the EETs synthesis blocker MSPPOH on the late phase, a putative NVC astrocyte pathway, further supports a direct role of endfeet to amplify late phase arteriole dilation. However, it was expected that astrocyte CalEx would have some impact on the local neural activity given the literature23,39 and because CalEx targets to both perisynaptic processes as well as endfeet. Indeed, our detailed analysis of neuronal Ca2+ transients in the parenchyma showed some effects, yet without a preference for the late phase of 30 s stimulation. In either scenario (direct effect on the vessel or indirect effect via neural activity), our data points to an important role for astrocyte Ca2+ in this amplification phenomenon.
Notably, the profiles of astrocytic Ca2+ responses recorded using Rhod-2/AM and GCaMP6 were different. Rhod-2/AM recorded Ca2+ elevations were largely sustained during 30 s stimulation and returned to baseline only after stimulation. Signals reported by GCaMP6 recovered well before the termination of the stimulus. One possible explanation is that GFP-based GCaMP sensors are sensitive to pH80 and activity-dependent acidification of astrocytes may have led to the quenching of GCaMP fluorescence during the late phase of 30 s stimulation. Alternatively, the difference could be explained by some preferential loading of Rhod-2/AM into mitochondria, an organelle in which GCaMP is excluded. Future experiments should explore whether astrocytic mitochondrial Ca2+ dynamics contribute to sustained functional hyperemia. We also acknowledge that astrocyte endfeet and process Ca2+ signals recorded in a chronic, fully sealed cranial window are different to that recorded in an acutely implanted, sealed or perforated cranial window. For example, the former additionally revealed elevations that were time locked to the onset and offset of whisker stimulation. While this may reflect responses to onset and offset neurons in the barrel cortex46, they could also be explained by astrocyte mechanosensing81 from the blood flow response itself11,82, which would not be observed in an acute window if the integrity of the intracranial pressure is compromised.
Whisker stimulation elevated locomotion and heightened arousal in our experimental paradigm. These behavioral states are often linked48, and activating neuromodulatory pathways can facilitate astrocyte activation in the neocortex42,43. Two of our results suggest that neuromodulatory systems contribute to functional hyperemia directly through astrocytes. First, the level of locomotion impacted only astrocyte endfoot Ca2+ transients and clamping these transients by CalEx significantly reduced late hyperemia83. Second, selective activation of Gq signaling, a common pathway for neuromodulators in astrocytes, amplified late hyperemia without modifying bulk neuronal Ca2+ responses. Identifying the link between behavior-related neuromodulatory inputs and astrocyte-derived vasoactive mediators is warranted.
Surprisingly, we found a temporally distinct contribution of NMDA receptors to eliciting delayed astrocyte Ca2+ transients and mediating the late phase of functional hyperemia. NMDA receptor activation likely controls astrocyte Ca2+ indirectly through diffusible mediators such as nitric oxide released from post-synaptic neurons, because astrocyte-patch based NMDA inhibition with intracellular MK-801 preserves evoked Ca2+ transients 58. Nevertheless, since the block of late phase vasodilation by AP5 is much more pronounced than by astrocyte CalEx, and because AP5 caused a reduction in neuronal activity measured by GCaMP, part of the decrement in the late component of functional hyperemia may be attributed to decreased neuronal activity. It is interesting to speculate that the same synaptic NMDA receptors necessary for long-term potentiation and thus memory are the same for late phase hyperemia, which could have arisen due to the enhanced energy requirements of plasticity84,85. However, late-phase hyperemia could also develop by NMDA receptor activation on endothelial cells. This process requires Ca2+-dependent D-serine release by astrocytes59,86 which would be additionally inhibited by AP5 because this treatment abolished sensory-evoked astrocyte Ca2+ elevations.
In sum, these data (1) help clarify the literature on the role of astrocytes in functional hyperemia—a phenomenon that is fundamental to fueling the brain; (2) provide important insights into the cell-type specific underpinnings of bimodal neurovascular coupling in awake animals and (3) improve our understanding and interpretation of fMRI data which routinely employs stimulations or tasks of long duration.
Methods
Animals
All animal procedures were performed in accordance with guidelines approved by the Animal Care and Use committee of the University of Calgary (protocols AC19-0170 and AC19-0109). Animals were kept on a normal 12–12 h light and dark cycle (7 am: on, 7 pm: off). Room temperature and humidity were set to 21.4 °C and 20%, respectively. Animals had access to food and water ad libitum. Animals were group housed until head-bar installation. Experiments were performed on male P22–90 c57bl/6 mice (N = 41: 5 acute window; 11 CalEx; 11 mutant CalEx control, 4 MSPPOH; 7 AP5; 3 thinned skull), transgenic Aldh1l1-Cre/ERT2 × RCL-GCaMP6s (Ai96)(N = 9), Aldh1l1-Cre/ERT2 × R26-Lck-GCaMP6f (N = 13), PDGFRß-Cre × RCL-GCaMP6s (N = 11), Aldh1l1-Cre/ERT2 × CAG-LSL-Gq-DREADD (N = 11), C57BL/6J-Tg(Thy1-GCaMP6f) (N = 6). For the use of the Ai96 conditional line, three consecutive tamoxifen injections were administered (100 mg/kg, 10 mg/mL corn oil stock, Sigma, St. Louise MO USA) between the age of P19 to P45 and the awake imaging protocol followed at least 2 weeks later. The other ERT2 expressing mice received 4-hydroxy-tamoxifen for 2 consecutive days between P2–4 (16 mg/kg, 4mg/ml corn oil + 12 v/v (%) absolute ethanol) i.p. in a volume of 20 µl.
Two-photon imaging
Two custom-built two-photon laser-scanning microscopes were used, one of which was optimized for acute brain slices and patch-clamp electrophysiology87 and another that was designed for awake in vivo experiments11. Both microscopes were supplied by the same Ti:Sapph laser (Coherent Ultra II, 4 W average power, 670–1080 nm, ~80 MHz) and equipped with the following parts: objectives (Zeiss 40× NA 1.0, Nikon 16× NA 0.8), a green bandpass emission filter (525–40 nm) and an orange/red bandpass emission filter (605–70 nm) coupled to photomultiplier tubes (GaAsP Hamamatsu). The open-source scanning microscope software ScanImage (versions 2.9 and 3.81 HHMI/Janelia Farms) running in Matlab88 was used for image acquisition. During acute brain slice experiments, we acquired time-series images at 0.98 Hz using bidirectional scanning of a 512 × 512 pixel-size area on a single focal plane visualizing fluorescent Ca2+ indicator-labeled cells and the center of a penetrating arteriole lumen in a depth of 40–120 µm. For stimulation experiments, the Ti:Sapph was tuned to 850nm to excite Rhod-2/AM and FITC dextran fluorescent indicators. Filling of the astrocyte network with Alexa-488 was imaged at 780 nm.
In vivo experiments using GCaMP6 signals were imaged at 920–940 nm, mCitrine at 980 nm. Imaging astrocytes and vessels was performed at a standard 3.91 Hz imaging frequency, neuronal Ca2+ in the CalEx and GqDREADD experiments at 7.82 Hz and in the AP5 experiments at 15.64 Hz. Fluorescent signals were recorded at 50–250 µm depth of the sensory cortex.
Acute brain slice preparation
We used Sprague-Dawley rats (p21–33) to prepare acute coronal slices of the sensory-motor cortex. Rats were anesthetized with isoflurane (5% induction, 2% maintenance). Two min after a fluorescent dye injection in the tail vein, animals were decapitated and their brains were quickly extracted and submerged into an ice-cold slicing solution (composition in mM: 119.9 N- methyl D-glucamine, 2.5 KCl, 25 NaHCO3, 1.0 CaCl2-2H2O, 6.9 MgCl2- 6H2O, 1.4 NaH2PO4-H2O, and 20 glucose), continuously bubbled with carbogen (95% O2, 5% CO2) for 2 min. We cut 400 µm thick coronal slices with a vibratome (Leica VT 1200S) and placed them to recover for 45 min on a fine mesh in artificial cerebrospinal fluid continuously bubbled in carbogen at 34 °C. ACSF contained the following (in mM): 126 NaCl, 2.5 KCl, 25 NaHCO3, 1.3 CaCl2, 1.2 MgCl2, 1.25 NaH2PO4, and 10 glucose.
The aCSF was bubbled with a gas mixture containing 30% O2, 5% CO2 and balanced N2 following the recovery for the dye loading period and during imaging experiments in order to enhance vascular dilation responses as described previously89. We performed imaging at room temperature (22 °C) and the imaging bath was perfused with aCSF at 2 ml/min by a sealed, gas pressure driven system. The superfused aCSF was supplemented with the arteriole pre-constrictor U46619 (125 nM) starting at least 30 min prior to each experiment to allow for the vessels to develop a steady tone. Drugs were infused into the main superfusion aCSF line (moving at 2 mL/min) through a side line driven by a syringe pump at a rate of 0.2 mL/min at ten-fold of the desired concentration to achieve the intended final concentration in the bath.
Dye loading
We labeled penetrating arterioles with Fluorescein Isothiocyanate (FITC) dextran (2000 KDa, Sigma Aldrich) via a tail vein injection (15 mg dissolved in 300 µl lactated Ringer’s solution (5%)) under anesthesia immediately before slicing. Following slice recovery, slices were bulk loaded in Rhodamine-2 Acetoxymethyl Ester (Rhod-2/AM)(15 µM, dissolved in 0.2% DMSO; 0.006% Pluronic Acid; 0.0002% Cremophore EL) in 3 mL of aCSF while bubbled with a 30% O2-containing gas mixture (see above) in a small incubation well for 45 min at 33 °C. In acute slice experiments astrocytes were identified by a brighter baseline dye fluorescence than neurons due to their preferential Rhod-2/AM uptake and by the presence of endfeet on vasculature.
Materials
FITC-dextran (2000KDa), rhodamine B isothiocyanate-dextran (Rhodamine-B-dextran, 70KDa), dexamethasone 21-phopshate disodium, Chremophore® EL, DMSO were obtained from Sigma Aldrich. D-(-)-2-Amino-5-phosphonopentanoic acid (AP5) and Compound 21 dihydrochloride was purchased from Hello Bio Inc. (Princeton, NJ). U46619, N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MSPPOH), medotomidine hydrochloride, and atipamezole hydrochloride were ordered from Cayman Chemicals. BAPTA-tetrapotassium and Alexa 488 hydrazide were obtained from Invitrogen, isoflurane from Fresenius Kabi Canada Ltd., Pluronic F127 and Rhod-2/AM from Biotium, buprenorphine from Champion Alstoe Animal Health, and enrofloxacin from Bayer, meloxicam from Boehringer Ingelheim, ketamine from Vétoquinol N.-A. Inc. AAV2/5.GfaABC1D.GCamP6f and AAV9.hSyn.GCaMP6f were ordered from Penn Vector Core, PA, USA, pZac2.1-GfaABC1D-mCherry-hPMCA2w/b plasmid was ordered from Addgene.
Electrical stimulation
The activation of afferent fibers was elicited by electric stimulation using an assembly of a Grass S88X stimulator, a voltage isolation unit and a concentric bipolar electrode (FHC). We softly positioned the electrode onto the surface of the slice by a micro-manipulator ~250 µm lateral to the penetrating arteriole. The tissue was stimulated for 5 s or 30 s with a theta burst pattern (trains of 1 ms mono-polar pulses at 100 Hz for 50 ms, repeated at 4 Hz) using a 0.1 V higher intensity than the threshold of astrocyte endfoot activation (1.1–1.3 V). This threshold was determined by gradually ramping the voltage (+0.1 V) of a low frequency (20 Hz) 5 s stimulation until an endfoot Ca2+ transient was observed. For some experiments (see Supplementary Fig. 11), we employed a 20 Hz stimulation for 5 s with low intensity that did not activate astrocyte endfeet and subsequent 30 s stimulations used the same voltage.
Astrocyte patch clamping experiments
Patching experiments were conducted similar to our previous publication32. Before the patching process, slices were electrically stimulated for 5 or 30 s with a theta burst pattern. Next, astrocytes were selected in the 30–70 µm proximity of an arteriole at a 30–60 µm depth, typically above the imaging plane of the arteriole (Supplementary Fig. 4a). A 3–5 MΩ resistance glass electrode formed a GΩ seal on the cell body membrane and when whole cell configuration was achieved astrocytes were filled with an internal solution containing the fluorescent dye Alexa-488 (100–200 µM) to visualize diffusion, and the following substances (in mM): 8 KCl, 8 Na- gluconate, 2 MgCl2, 10 HEPES, 4.38 CaCl2, 4 K-ATP, and 0.3 Na-GTP, 10 K-BAPTA (1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid) with 96 K-gluconate. Osmolality was adjusted to ~285 mOsm, pH was corrected with KOH to 7.2. We voltage clamped astrocytes at −80 mV and confirmed the cell type by a low input resistance (10–20 MΩ) and dye filling of the endfeet circumventing a large section of the arteriole (Supplementary Fig. 4a, b). Importantly, dye filling of the endfeet with a clamping solution of 100 nM Ca2+ did not change arteriole tone per se (see Supplementary Fig. 5). We allowed 15–30 min for any internal solution in the extracellular space from the patching process to washout from the tissue and for the arteriole tone to return to baseline. Then, while in stable whole-cell configuration, we repeated the electric stimulation to compare to the first response.
Awake in vivo two-photon imaging of acute cranial window
Male mice were used according to the protocol previously developed by our laboratory11. Briefly, on day 1, under isoflurane anesthesia (induction 4%, maintenance 1.5–2%), pain control (buprenorphine 0.05 mg/kg) and antibiotic premedication (enrofloxacin 2.5 mg/kg), a light (0.5 g) titanium headbar was glued on the occipital bone under aseptic conditions with a three-component dental glue (C&B Metabond, Parkell Inc, NY, USA) and dental cement (OrthoJet Acrylic Resin, Land Dental MFG. CO., Inc., IL, USA). After a recovery period of 24 h, the mice were trained on days 2 and 3 to habituate to imaging and air puff stimulation to the whiskers while head fixed but free to move on a passive, air supported spherical Styrofoam ball in a light tight environment (Fig. 1a). After 15 min of rest, 5 s continuous air puffs (30 psi, Picospritzer III, General Valve) were delivered to the contralateral whiskers 15 times every 10 s, 15 s, 30 s, and 60 s, respectively on day 2 and 3. On day 4, a craniotomy was performed over the barrel cortex. Bone and dura were gently removed. For Aldh1l1-Cre/ERT2 × CAG-LSL-Gq-DREADD mice, Rhod-2/AM (15 µM) in aCSF was incubated on the brain surface for 45 min. Next, the window was fully sealed with a coverslip. In experiments where astrocyte endfeet were labeled with a red fluorescent dye, 0.2 mL 5% FITC dextran solution was injected into the tail vein immediately before imaging, whereas Rhodamine-B dextran (0.2 mL, 2.3%) was injected when astrocytes or neurons expressed GCaMP6. The animals were transferred to the imaging rig, where imaging started ~30 min after the animals awoke. Behavior was continuously monitored by a camera detecting the light of an infrared LED and was recorded during imaging. A 16× or a 40× water-immersion objective was positioned square to the surface of the window. Imaging was performed at 3.91 or at 0.49 Hz when drug application was recorded. Rhod-2/AM only loaded astrocytes in mice in vivo (Fig. 4a) as neuronal compartments were neither labeled at rest nor appeared in response to stimulation11.
Awake in vivo two-photon imaging of chronic cranial window
Male, 3–4 months old c57bl/6 and Aldh1l1-Cre/ERT2 × R26-Lck-GCaMP6f mice were used. The surgical procedure was based on Tournissac et al. with modifications90. For head bar installation mice were premedicated with dexamethasone (2 h prior, 6 mg/kg s.c.). Under isoflurane anesthesia and aseptic conditions, the parietal, occipital and temporal bones were carefully separated from the surrounding soft tissue and a U-shaped metal headbar with 2 lateral arms was cemented to the occipital and temporal bones by a UV glue (Ivoclar vivident Tetric Evoflow). A conical cement wall was built around the right parietal cortex. The surgery was supported with antibiotic (enrofloxacin), analgesic (buprenorphen 0.1 mg/kg s.c.) and anti-inflammatory (meloxicam 5 mg/kg s.c.) medication. After a minimum of 5-day recovery, mice were premedicated with dexamethasone (2–4 h prior), enrofloxacin, meloxicam, buprenorphine and anesthetized with a mixture of ketamine (100 mg/kg) and medotomidine (0.5 mg/kg) i.p. The mice were temperature controlled and inhaled 100% O2 during cranial window implantation. A 3 mm circular area was removed from the parietal bone over the whisker barrel area, leaving the dura intact. To prevent bone regrowth, a custom-cut T-shaped coverslip (inner diameter: 3 mm; outer diameter: 3.5 mm, central thickness: 250 µm)(Laser Micromachining Ltd.) was sealed into the hole with UV cement. Anesthesia was reversed with atipamezole (0.5 mg/kg s.c.). Post-operative supportive therapy included a heated cage with O2-enriched air (until the mouse turned active) and recovery jello for diet. For CalEx, and mutant CalEx control mice, a tiny slit was cut in the dura, through which the viral construct was injected into the barrel cortex before sealing the craniotomy. Six weeks later, mice were trained the same as in the acute cranial window experiments applying 5 s continuous air puffs to the whiskers. For ball movement and pupil diameter recording 2 near-infrared LEDs (780 nm) and 2 infrared-sensitive cameras (Basler) were pointed at a small area of the Styrofoam ball and the right eye of the mouse, respectively. We recorded pupil + ball movement at 40 Hz, while ball movement for 2P imaging was recorded at 62.5 Hz with the software Pylon Viewer (version 6.2.4.9387) by Basler. An open-source pulse train generator (Sanworks, Pulse Pal v2) was configured to synchronize trigger signals between the cameras, LEDs, the Picospritzer III and the microscope.
CalEx Plasmid construction
To generate the AAV astrocyte-targeted expression construct of HA-tagged CalEx, the DNA sequence encoding hPMCA2w/b was PCR amplified from pZac2.1-GfaABC1D-mCherry-hPMCA2w/b (a gift from Baljit Khakh (Addgene plasmid # 111568 ; http://n2t.net/addgene:111568; RRID:Addgene_111568) with primers designed to incorporate a N-terminal HA tag encoding the amino acids YPYDVPDYA and used to replace mCherry-PMCA2w/b in the same backbone at the 5’ NheI and 3’ XbaI restriction sites using the NEBuilder Hifi DNA assembly kit, generating pZac2.1-GfaABC1D-HA-hPMCA2w/b23. For the control construct, the critical Ca2+ binding site residue glutamic acid 457 was converted to Alanine using PCR site-directed mutagenesis, generating a mutant control CalEx (hPMCA2w/b (E457A)) incapable of binding or membrane transport of Ca2+36,37. The DNA sequences of all constructs were verified by Sanger DNA sequencing.
AAV production
AAV viral vectors were generated using the methods of Challis et al. 91. Briefly, 293FT cells (ThermoFisher Scientific) were grown to ~90% confluency in Corning hyperflasks (Corning) and co-transfected with 129 µg pHELPER (Agilent), 238 µg rep-cap plasmid encoding AAV5 capsid proteins (pAAV2/5 was a gift from Melina Fan (Addgene plasmid # 104964 ; http://n2t.net/addgene:104964; RRID:Addgene_104964) and 64.6 µg of either the CalEx plasmid (pZac2.1-GfaABC1D-HA-hPMCA2w/b) or control (pZac2.1-GfaABC1D-HA-hPMCA2w/b (E457A)) using the PEIpro transfection reagent (Polyplus). The AAV viral vector purification, concentration and titer determination was performed as previously described91–93.
AAV CalEx injection
A viral cocktail containing AAV2/5.GfaABC1D GCaMP6f (3 × 1012 gc/ml, Addgene) for astrocyte Ca2+ detection or AAV9.hSyn.GCaMP6f (3 × 1012 gc/ml, Addgene) for neuronal Ca2+ detection was mixed with the astrocyte-targeted CalEx encoding construct AAV2/5.GfaABC1D-HA-hPMCA2w/b (1.18 × 1013 gc/ml) in a 1:3 ratio and delivered by a Nanoject III (Drummond Scientific, Broomall, PA, USA) in a volume of 400 nL. For the best possible control, we designed a mutated version of the CalEx virus in which changing the critical glutamic acid residue in TM4 (E457) to Alanine to render the pump incapable of transporting Ca2+. Training and imaging took place 6 weeks later, because this is sufficient time to achieve functionally effective CalEx expression23.
Immunohistochemistry
Slices were collected from control and CalEx mice at the end of the in vivo experiments and post-fixed in 4% paraformaldehyde overnight and then cryoprotected in 30% sucrose for another 24 h. Sections were cut on a cryostat to 30 µm and free-floated in PBS. The sections were first washed in PBS (phosphate buffered saline) for 20 min, followed by 3 × 5 min washes in PBS; agitated at room temperature. Sections were permeabilized in 0.1% Triton X in PBS for 10 min. Anti-HA (rabbit) primary antibody (1:100: Abcam) was diluted in 2% BSA in PBS and incubated for 1 h and agitated at room temperature. After primary antibody incubation, sections were washed three times with PBS and then incubated for 1 h with Alexa Fluor 555 (1:1000: Invitrogen) diluted in 2% BSA in PBS and agitated at room temperature. Sections were then washed again three times with PBS before mounting coverslips with ImmunoMount (Epredia) and read on a confocal fluorescence microscope (Leica TCS SP8, Leica Microsystems).
Functional hyperemia
A two-photon imaging session started by confirming Rhod-2/AM, GCaMP6 or Gq DREADD-linked mCitrine expression and identifying the vascular network by z-stack imaging to a depth of ~300 µm with a 16× objective. Pial arterioles were distinguished from veins by spontaneous vasomotion and by positive responses to contralateral whisker air puff. Penetrating arteriole branches of the pial arteries were tracked and imaged at a depth of 50–250 µm with a 40× or a 16× objective. Air puff was delivered through 2 glass capillary tubes positioned to deflect as many whiskers as possible without blowing air on the face (Fig. 1a). A 10 s baseline period was recorded after which a continuous or an intermittent (4 Hz, 125 ms pulses) 5 s or 30 s air puff (30psi) was employed. Stimulation was repeated 3–6 times and averaged for each arteriole. In case of chronic cranial window experiments, on the day before two-photon imaging we mapped the cortical center of activation to whisker stimulation by collecting intrinsic optical signals (IOS) of hemoglobin under the cranial window. We flashed green light with a stable light-emitting diode (M530L3, Thorlabs Inc.) equipped with filtering (Semrock) and collimation optics (Thorlabs) at 10 Hz and collected reflected light with a Basler Ace U acA1920–40 μm camera with Fujinon HF50XA-5M lens (pixel size = 5.86 μm), during 30 s whisker stimulations. IOS was recorded with Pylon Viewer (version 5.0.11.10913) by Basler. Penetrating arterioles for two-photon imaging were selected from a region where green light reflectance showed a major decrease during the period of whisker stimulation overlapping with the expression of the viral construct (good neuronal or astrocytic GCaMP6 expression). We measured pupil diameter changes in a separate set of trials with no stimulation, 30 s ipsilateral air puff to the body, or 30 s whisker stimulation while the pupils were constricted to mid-size with a low intensity continuous green light in the background.
Data processing and statistical analysis
Ca2+ signals were analyzed in ImageJ 2.9.0 (NIH) and calculated in Prism software 9.4.0 (GraphPad Software LLC., La Jolla, CA) as follows: ∆F/F = ((F1 − F0)/F0) × 100, where F is fluorescence, 1 is at any given time point, and 0 is an average baseline value of 60 s ex vivo or 2 s in vivo pre stimulus. Ca2+ responses in brain slices were obtained by selecting a region of interest (ROI) and running the ‘intensity vs. time monitor’ plugin over the following compartments: (1) neuropil was outlined as an acellular region next to the region of vasodilation on the side closer to the stimulating electrode, (2) neuron soma responses were the average of 5 neuronal cell bodies visible in the imaging plane closest to the arteriole, (3) astrocyte soma signal was the average of 1–3 cells closest to the arteriole, (4) astrocyte endfoot was chosen as a clearly visible continuous structure adjacent to the area of vasodilation. The same ROIs were used after patching in vitro or after drug administration in vivo. Astrocyte GCaMP6 signals of endfeet and processes in all experiments were outlined by including regions around the arteriole where an increase in the fluorescent signal was visible after stimulation onset. On neuronal GCaMP6 recordings ROI was determined as a 5000 µm2 polygonal perivascular area avoiding the shadow of the proximal section of the vessel.
In the absence of stimulus-evoked fluorescence changes, areas of basal GCaMP6 fluorescence were selected. Ca2+ signals are labeled as ∆F/Fc in control conditions, ΔF/FBAPTA after BAPTA loading. Pre-processing of intraluminal dye recordings in ex vivo slices included xy-motion correction, median filter (0.5–2 pixel radius), and Gaussian filter (1–4 pixel radius) performed by ImageJ plugins. Intraluminal area was selected by intensity thresholding and quantified using the ‘Analyze particles’ function of ImageJ.
To determine the cross-sectional area of intraluminal dye-loaded arterioles in vivo, we used three independent methods to outline the edges of the vascular lumen. For Gq-DREADD (Fig. 4e, f) and MSPPOH experiments (Fig. 6i) we used manual outlining of individual images via Amazon Mechanical Turk (see section below), for thinned skull, and acute window experiments (Fig. 1a–h, Fig. 5a–g, and Supplementary Figs. 1a, 3), we used ImageJ analysis (see above). For CalEx experiments and Aldh1l1-Cre/ERT2 × R26-Lck-GCaMP6f chronic window experiments (Supplementary Fig 2d–f and Fig. 2), for each recording, we quantified cross-sectional area of the imaged penetrating arteriole using the ImageJ analysis approach outlined above and again by using a semi-automated tracking approach detailed in Haidey et al.82 (see below), and then averaged the two. Briefly, the latter approach involved: (1) defining regions of interest from blood-vessel cross-sections at different imaging angles to create tracking templates; (2) selecting a bounding box constraining the tracking algorithm to the maximum observed dilation in the recording; (3) applying MATLAB’s implementation of the Thirion’s DEMONS algorithm (with default options) for non-rigid image matching to estimate ROI deformation within the bounding box relative to the tracking template, which provides the cross-sectional area estimate. A color-coded image of the input template with the detected edges is shown in Supplementary Fig. 12i. Arteriole diameter changes were quantified as the relative change in the area of intraluminal dye along the longitudinal section (in slice) or calculated from the cross-sectional area (in vivo) of the vascular lumen, assuming a circular shape, as follows: diameter = 2× √(area/π). Stimulation-evoked vasodilation was first calculated as Δ diameter (Δd) = (diameter1 − diameter0)/diameter0) × 100 (%), where diameter1 is the diameter of the vascular lumen at any time point and diameter0 is the average baseline vascular lumen diameter 2 s prior to stimulation onset. Response latency was determined as the time from stimulation onset until 2 consecutive values exceeded 3 standard deviations of the 2 s baseline. All peak responses during topical C21 application (first 10 min from Ca2+ increase onset) were compared to the peak value of the 500 s baseline period (see Fig. 4c). AUC values were calculated from Δd traces as the summary of all positive peaks over the baseline value for 35 min from stimulation onset. For data presentation and statistical analysis averaged arteriole diameter traces of whisker stimulation trials served as single data points as we expected a uniform and reproducible response across trials. For astrocyte and neuronal Ca2+ response analysis we used data points of individual trails because we considered the response of an individual cell less stereotypical and predictable (based on citation11).
Event-based analyses of Ca2+ signals in neuronal compartments were performed by an automated analysis toolkit (https://github.com/yu-lab-vt/AQuA)39 on 5000 µm2 peri-arteriole ROIs of image stacks recorded at 7.82 Hz. A Gaussian spatial filter was applied blindly to each trial (1–4 standard deviation of the mean) to optimize the fidelity of event detection. Movement-related event detection artefacts were excluded. Absolute event frequency (calculated as the average number of events for each sec), peak fluorescence value (dF/F Max), integrated Ca2+ event value (AUC dF/F), event area size (µm2) and the duration (time of rise and fall between the 10% of peak) were summarized from the analysis output.
Locomotion analysis: Recordings of a small area of the Styrofoam ball were analyzed by a custom MATLAB script to quantify ball movement on a relative locomotion scale where ‘0’ is assigned to the least movement (stationary ball) and 1 is the highest speed an animal reached during the experiment.
To quantify locomotion activity from video recordings of a small region of the Styrofoam ball, we implemented a simple algorithm in MATLAB94: briefly, the image stack was read in and duplicated; the first frame from ‘Stack1’ and the last frame from ‘Stack2’ were deleted to yield an image stack that reported pixel motion within the recorded video (which directly related to the animal’s location); a pixel-by-pixel subtraction was performed for every image in the stack (i.e., Stack1–Stack2); mean gray value was then plotted against time for the resulting stack, providing an estimate of relative locomotion within the imaging trial.
Pupil diameter analysis: Pupil diameter changes were recorded at 40 Hz using an infrared camera mounted close to the animal’s eye. To quantify pupil diameter from the videos, we used single-animal DeepLabCut to train a model to track four points on the circumference of the pupil (two along the vertical axis and two along the horizontal axis)95. The following parameters were used: TrainingFraction = 0.95; net_type = resnet_50; augmenter_type = imgaug; max_iters = 500,000. Tracking performance was assessed by visual examination of the processed videos and the filtered position traces; frames where the animal blinked (<0.5%) were discarded. The average of the horizontal and vertical distance was used as pupil diameter. A custom MATLAB script (version 2021b) was used to determine pairwise distances and perform median filtering of the diameter traces (https://github.com/tpgovind/Locomotion-Pupil-Tracking, 10.5281/zenodo.7246747).
Refer to Supplementary Tables 1–6 for the statistical details that pertain to the six main text figures. Statistical analyses were performed on averaged trials of PA responses and on Ca2+ responses of individual stimulation trials in vivo. Normality was tested with Shapiro–Wilk test. Normally distributed data were evaluated with a paired or unpaired Student’s t-test, and non-normally distributed with Mann–Whitney (Fig. 3a, b) or Wilcoxon test (astrocyte Ca2+ signals in Supplementary Fig. 5). The non-parametric Friedman test and Dunn’s multiple comparison test was applied to compare in vivo peak diameter changes on stimulation length (1–5–30 s), astrocyte process Ca2+ peak at various time periods of stimulation (Supplementary Fig. 2f) and to compare methods of arteriole cross sectional area analysis (Supplementary Fig. 12b). Mixed effects model with Holm–Sidak’s multiple comparison test was used for response latency comparison (Fig. 1e). Response latency of arteriole, endfoot and process Ca2+ was analyzed with Kruskal-Wallis test and Dunn’s multiple comparison test (Supplementary Fig. 2d, e). One-way ANOVA with Tukey’s post hoc test was applied on pupil response comparisons (Fig. 3g) Two-way ANOVA was run to compare the effects of stimulation length (5 s vs 30 s), external manipulations (CalEx, or drug treatments) and their interaction, followed by Tukey’s post hoc test for pairwise comparison. Baseline arteriole diameter (intravascular area) changes were analyzed with the two-tailed non-parametric Wilcoxon test. Data are expressed as mean ± standard error of the mean (SEM). We analyzed the effect of locomotion as a co-variate for each trial on group differences in arteriole diameter, astrocyte and neuronal Ca2+ signals of the CalEx experiment using generalized linear models in SPSS (IBM SPSS Statistics, Version 25). Every other statistical analysis was performed in Prism 9.4.0 (GraphPad Software, Inc., La Jolla, CA, USA). Levels of significance used were: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Amazon mechanical Turk analysis
Arteriole diameter changes of the CalEx experiments were analyzed by manual outlining of the FITC dextran loaded vascular lumen frame by frame in an outsourced and randomized fashion, using Amazon Mechanical Turk (Supplementary Fig. 12)96. It is an online crowdsourcing website, through which remotely located “crowd-workers” are hired to perform a discrete on-demand task. A custom-made GUI (https://github.com/leomol/vessel-annotation, 10.5281/zenodo.7231728) facilitated the manual outlining of the vascular cross-section over the course of the recording (https://codepen.io/leonardomt/full/jOEvPvY). An annotation task consisted of adjusting a polygonal shape with a fixed number of vertices on the top of the vessel. To keep annotations consistent, crowd-workers could inspect the time evolution of the vessel and their annotations by freely scrolling through the time-lapse images. Annotation tasks were randomly assigned to 10 different crowd-workers, blind to the experiment. Each stack of images was then visually examined and accepted or rejected based on whether the instructions were followed or not, i.e., if outlines were not consistently labeled over time or if there was a clear mismatch in the outlines with respect to the brain vessel. The area of each polygonal shape was then calculated and normalized (Supplementary Fig. 12).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Our work is supported by grants to G.R.G from the Canadian Institutes of Health Research (PJT-173468 and FDN-148471) and by the Hotchkiss Brain Institute and the University of Calgary. We appreciate Dr. Marine Tournissac’s assistance in establishing the chronic cranial window model. We thank Dr. David Rosenegger for preliminary patch experiments. We thank Drs. Ciaran Murphy-Royal, Brian MacVicar and Rebecca Williams and Darren Clark for their valuable scientific comments. We thank Dr. Vincent Ebacher and the HBI advanced microscopy platform for the confocal microscope images. We thank the CSM Optogenetics Facility for hosting the web services for crowdsourced annotations. We are grateful for Dr. Frank Visser for transgenic mouse sample genotyping. We thank the developers and distributors of ScanImage open-source control and acquisition software for two-photon laser-scanning microscopy.
Source data
Author contributions
A.I. designed and performed experiments, analyzed results, and wrote the paper. M.V. and G.P. performed experiments and analyzed results, C.C. analyzed results, C.H.T. performed experiments, X.Y., and B.S.K., designed CalEx virus construct, F.V. designed the mutant CalEx virus construct, C.B. did immunohistochemistry, L.M. designed platform for crowdsourcing annotations with Amazon Mechanical Turk and supervised analysis. M.N. provided trainee supervision, experiment design, and edited the manuscript. R.J.T. provided trainee supervision, experiment design, and edited the manuscript. G.R.G. conceived the project, designed experiments, and wrote the paper.
Peer review
Peer review information
Nature Communications thanks Alexander Gourine, Barbara Lind and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Source data are provided with this paper. Raw image files are stored on servers at Hotchkiss Brain Institute owing to their large size. These raw images can be provided from the corresponding author upon request.
Code availability
The code for the custom-made automated luminal area tracking algorithm is available at: https://senselab.med.yale.edu/modeldb/enterCode?model=266929. The code for the custom-made GUI for the Amazon Mechanical Turk analysis has been deposited to Zenodo: https://zenodo.org/record/7231728. The code for the pupil tracking and locomotion analysis have been posted to Github and Zenodo: https://github.com/tpgovind/Locomotion-Pupil-Tracking. 10.5281/zenodo.7279708, 10.5281/zenodo.7246747. The code for event-based analyses of Ca2+ signals in neuronal compartments: https://github.com/yu-lab-vt/AQuA.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35383-2.
References
- 1.He Y, et al. Ultra-slow single-vessel BOLD and CBV-based fMRI spatiotemporal dynamics and their correlation with neuronal intracellular calcium signals. Neuron. 2018;97:925–939.e5. doi: 10.1016/j.neuron.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent ‘resting-state’ connectivity. Neuron. 2017;96:936–948.e3. doi: 10.1016/j.neuron.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow BW, et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579:106–110. doi: 10.1038/s41586-020-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb S, et al. Precapillary sphincters maintain perfusion in the cerebral cortex. Nat. Commun. 2020;11:395. doi: 10.1038/s41467-020-14330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill RA, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rungta RL, Chaigneau E, Osmanski B-F, Charpak S. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron. 2018;99:362–375.e4. doi: 10.1016/j.neuron.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front. Neurosci. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra A. Binaural blood flow control by astrocytes: listening to synapses and the vasculature. J. Physiol. 2017;595:1885–1902. doi: 10.1113/JP270979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masamoto, K. et al. Unveiling astrocytic control of cerebral blood flow with optogenetics. Sci. Rep.5, 11455 (2015). [DOI] [PMC free article] [PubMed]
- 10.Takata N, et al. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia. 2018;66:2013–2023. doi: 10.1002/glia.23454. [DOI] [PubMed] [Google Scholar]
- 11.Tran CHT, Peringod G, Gordon GR. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron. 2018;100:1133–1148.e3. doi: 10.1016/j.neuron.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 12.Schulz K, et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods. 2012;9:597–602. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- 13.Thrane AS, et al. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl Acad. Sci. USA. 2012;109:18974–18979. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EMC. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc. Natl Acad. Sci. USA. 2011;108:8473–8478. doi: 10.1073/pnas.1100428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takuwa H, et al. Long-term adaptation of cerebral hemodynamic response to somatosensory stimulation during chronic hypoxia in awake mice. J. Cereb. Blood Flow. Metab. 2013 doi: 10.1038/jcbfm.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyre B, et al. The effects of locomotion on sensory-evoked haemodynamic responses in the cortex of awake mice. Sci. Rep. 2022;12:6236. doi: 10.1038/s41598-022-10195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, et al. Synchronized astrocytic Ca2+ responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell Rep. 2018;23:3878–3890. doi: 10.1016/j.celrep.2018.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind BL, et al. Fast Ca2+ responses in astrocyte end-feet and neurovascular coupling in mice. Glia. 2018;66:348–358. doi: 10.1002/glia.23246. [DOI] [PubMed] [Google Scholar]
- 20.Otsu Y, et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 2015;18:210–218. doi: 10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stobart JL, et al. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron. 2018;98:726–735.e4. doi: 10.1016/j.neuron.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, et al. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron. 2018;99:1170–1187.e9. doi: 10.1016/j.neuron.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosford PS, et al. CO2 signaling mediates neurovascular coupling in the cerebral cortex. Nat. Commun. 2022;13:2125. doi: 10.1038/s41467-022-29622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber P, Lütschg J, Fahnenstich H. Cerebral hemodynamic changes in response to an executive function task in children with attention-deficit hyperactivity disorder measured by near-infrared spectroscopy. J. Dev. Behav. Pediatr. 2005;26:105–111. doi: 10.1097/00004703-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts SARB, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage. 2005;26:1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Rombouts SARB, Scheltens P, Kuijer JPA, Barkhof F. Whole brain analysis of T2* weighted baseline FMRI signal in dementia. Hum. Brain Mapp. 2007;28:1313–1317. doi: 10.1002/hbm.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- 29.Mishra A, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016;19:1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng K, et al. Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca(2+) in neurons and astroglia. Neuron. 2015;88:277–288. doi: 10.1016/j.neuron.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenegger DG, Tran CHT, Wamsteeker Cusulin JI, Gordon GR. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J. Neurosci. 2015;35:13463–13474. doi: 10.1523/JNEUROSCI.1780-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J. Neurosci. 2014;34:13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nizar K, et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takata N, et al. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS ONE. 2013;8:e66525. doi: 10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerini D, Foletti D, Vellani F, Carafoli E. Mutation of conserved residues in transmembrane domains 4,6, and 8 causes loss of Ca2+ transport by the plasma membrane Ca2+ pump. Biochemistry. 1996;35:3290–3296. doi: 10.1021/bi952572f. [DOI] [PubMed] [Google Scholar]
- 37.Gong D, et al. Structure of the human plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018;9:3623. doi: 10.1038/s41467-018-06075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu Y, et al. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell. 2019;178:27–43.e19. doi: 10.1016/j.cell.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc. Natl Acad. Sci. USA. 2016;113:E2675–E2684. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, et al. Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat. Neurosci. 2019;22:1936–1944. doi: 10.1038/s41593-019-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slezak M, et al. Distinct mechanisms for visual and motor-related astrocyte responses in mouse visual cortex. Curr. Biol. 2019;29:3120–3127.e5. doi: 10.1016/j.cub.2019.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Georgiou L, Echeverría A, Georgiou A, Kuhn B. Ca+ activity maps of astrocytes tagged by axoastrocytic AAV transfer. Sci. Adv. 2022;8:eabe5371. doi: 10.1126/sciadv.abe5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paukert M, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Echagarruga CT, Gheres KW, Norwood JN, Drew PJ. nNOS-expressing interneurons control basal and behaviorally evoked arterial dilation in somatosensory cortex of mice. Elife. 2020;9:e60533. doi: 10.7554/eLife.60533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayaz A, et al. Layer-specific integration of locomotion and sensory information in mouse barrel cortex. Nat. Commun. 2019;10:2585. doi: 10.1038/s41467-019-10564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGinley MJ, et al. Waking state: rapid variations modulate neural and behavioral responses. Neuron. 2015;87:1143–1161. doi: 10.1016/j.neuron.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reimer J, et al. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron. 2014;84:355–362. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kristjansson SD, Stern JA, Brown TB, Rohrbaugh JW. Detecting phasic lapses in alertness using pupillometric measures. Appl. Erg. 2009;40:978–986. doi: 10.1016/j.apergo.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Shimaoka D, Harris KD, Carandini M. Effects of arousal on mouse sensory cortex depend on modality. Cell Rep. 2018;22:3160–3167. doi: 10.1016/j.celrep.2018.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 53.Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J. Cereb. Blood Flow. Metab. 2014 doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Franco AP, Chiang P-P, Newman EA. Dilation of cortical capillaries is not related to astrocyte calcium signaling. Glia. 2022;70:508–521. doi: 10.1002/glia.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busija DW, Bari F, Domoki F, Louis T. Mechanisms Involved in the cerebrovascular dilator effects of N-methyl-D-aspartate in cerebral cortex. Brain Res. Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front. Neuroenerget. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosford PS, Gourine AV. What is the key mediator of the neurovascular coupling response? Neurosci. Biobehav. Rev. 2019;96:174–181. doi: 10.1016/j.neubiorev.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehina EMF, Murphy-Royal C, Gordon GR. Steady-state free Ca2+ in astrocytes is decreased by experience and impacts arteriole tone. J. Neurosci. 2017;37:8150–8165. doi: 10.1523/JNEUROSCI.0239-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L, et al. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J. Cereb. Blood Flow. Metab. 2019;39:481–496. doi: 10.1177/0271678X17734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogan-Cann AD, Lu P, Anderson CM. Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc. Natl Acad. Sci. USA. 2019;116:10229–10231. doi: 10.1073/pnas.1902647116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am. J. Physiol. Cell Physiol. 2010;299:C1068–C1078. doi: 10.1152/ajpcell.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lecrux C, et al. Pyramidal neurons are ‘neurogenic hubs’ in the neurovascular coupling response to whisker stimulation. J. Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, et al. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng X, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am. J. Physiol.-Heart Circulatory Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 65.Alkayed NJ, et al. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.STR.27.5.971. [DOI] [PubMed] [Google Scholar]
- 66.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J. Cereb. Blood Flow. Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 68.Tran CHT, Gordon GR. Astrocyte and microvascular imaging in awake animals using two-photon microscopy. Microcirculation. 2015;22:219–227. doi: 10.1111/micc.12188. [DOI] [PubMed] [Google Scholar]
- 69.Lee JH, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow. Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kahn I, et al. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J. Neurosci. 2011;31:15086–15091. doi: 10.1523/JNEUROSCI.0007-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 73.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 74.Zuend M, et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020;2:179–191. doi: 10.1038/s42255-020-0170-4. [DOI] [PubMed] [Google Scholar]
- 75.Hall CN, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khennouf L, et al. Active role of capillary pericytes during stimulation-induced activity and spreading depolarization. Brain. 2018;141:2032–2046. doi: 10.1093/brain/awy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longden TA, et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian P, et al. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc. Natl Acad. Sci. USA. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adamsky A, et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018;174:59–71.e14. doi: 10.1016/j.cell.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Kneen M, Farinas J, Li Y, Verkman AS. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys. J. 1998;74:1591–1599. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marina N, et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat. Commun. 2020;11:131. doi: 10.1038/s41467-019-13956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haidey JN, et al. Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback. Cell Rep. 2021;36:109405. doi: 10.1016/j.celrep.2021.109405. [DOI] [PubMed] [Google Scholar]
- 83.Peringod, G., Yu, L., Murari, K. & Gordon, G. R. Spatiotemporal components of sustained functional hyperemia are differentially modulated by locomotion and silenced with vascular chemogenetics. Preprint at bioRxiv10.1101/2021.09.15.460557 (2021).
- 84.Mery F, Kawecki TJ. A cost of long-term memory in drosophila. Science. 2005;308:1148–1148. doi: 10.1126/science.1111331. [DOI] [PubMed] [Google Scholar]
- 85.Plaçais P-Y, Preat T. To favor survival under food shortage, the brain disables costly memory. Science. 2013;339:440–442. doi: 10.1126/science.1226018. [DOI] [PubMed] [Google Scholar]
- 86.Stobart JLL, Lu L, Anderson HDI, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenegger DG, Tran CHT, LeDue J, Zhou N, Gordon GR. A high performance, cost-effective, open-source microscope for scanning two-photon microscopy that is modular and readily adaptable. PLoS One. 2014;9:e110475. doi: 10.1371/journal.pone.0110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Institoris Á, Rosenegger DG, Gordon GR. Arteriole dilation to synaptic activation that is sub-threshold to astrocyte endfoot Ca2+ transients. J. Cereb. Blood Flow. Metab. 2015;35:1411–1415. doi: 10.1038/jcbfm.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tournissac M, et al. Cranial window for longitudinal and multimodal imaging of the whole mouse cortex. Neurophoton. 2022;9:031921. doi: 10.1117/1.NPh.9.3.031921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Challis RC, et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019;14:379–414. doi: 10.1038/s41596-018-0097-3. [DOI] [PubMed] [Google Scholar]
- 92.Batool S, et al. Neuronal Menin overexpression rescues learning and memory phenotype in CA1-specific α7 nAChRs KD mice. Cells. 2021;10:3286. doi: 10.3390/cells10123286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong Y, et al. Single-cell and spatial RNA sequencing identify perturbators of microglial functions with aging. Nat. Aging. 2022;2:508–525. doi: 10.1038/s43587-022-00205-z. [DOI] [PubMed] [Google Scholar]
- 94.Peringod, G. MATLAB code estimating motion spherical treadmill images, 10.5281/zenodo.7279708 (2022).
- 95.Peringod, G. Pupil diameter Track. tool., 10.5281/zenodo.7246747 (2022).
- 96.Molina, L. Blood vessel annotation tool. Zenodo, 10.5281/zenodo.7226673 (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided with this paper. Raw image files are stored on servers at Hotchkiss Brain Institute owing to their large size. These raw images can be provided from the corresponding author upon request.
The code for the custom-made automated luminal area tracking algorithm is available at: https://senselab.med.yale.edu/modeldb/enterCode?model=266929. The code for the custom-made GUI for the Amazon Mechanical Turk analysis has been deposited to Zenodo: https://zenodo.org/record/7231728. The code for the pupil tracking and locomotion analysis have been posted to Github and Zenodo: https://github.com/tpgovind/Locomotion-Pupil-Tracking. 10.5281/zenodo.7279708, 10.5281/zenodo.7246747. The code for event-based analyses of Ca2+ signals in neuronal compartments: https://github.com/yu-lab-vt/AQuA.