Abstract
In experimental mycobacterial infection, tumor necrosis factor alpha (TNF-α) is required for control of bacillary growth and the protective granulomatous response, but may cause immunopathology. To directly examine the positive and detrimental effects of this cytokine, a murine model was used in which different amounts of TNF-α were delivered to the site of infection. Mice with a disruption in the TNF-α gene (TNF-KO) or wild-type mice were infected with low or high doses of recombinant Mycobacterium bovis BCG that secreted murine TNF-α (BCG-TNF). Infection of TNF-KO mice with BCG containing the vector (BCG-vector) at a low dose led to increased bacillary load in all organs and an extensive granulomatous response in the lungs and spleen. The mice succumbed to the infection by ∼40 days. However, when TNF-KO mice were infected with low doses of BCG-TNF, bacillary growth was controlled, granulomas were small and well differentiated, the spleen was not enlarged, and the mice survived. Infection with high inocula of BCG-TNF resulted in bacterial clearance, but was accompanied by severe inflammation in the lungs and spleen and earlier death compared to the results from the mice infected with high inocula of BCG-vector. Wild-type mice controlled infection with either recombinant strain, but showed decreased survival following high-dose BCG-TNF infection. The effects of TNF-α required signaling through an intact receptor, since the differential effects were not observed when TNF-α receptor-deficient mice were infected. The results suggest that the relative amount of TNF-α at the site of infection determines whether the cytokine is protective or destructive.
The importance of tumor necrosis factor alpha (TNF-α) in the host defense against mycobacterial infection has been appreciated for some time. In experimental animal models, TNF-α production has been shown to be necessary for the formation and maintenance of the granulomas which seal off foci of infection and thus limit dissemination of the bacteria. When mice infected with Mycobacterium tuberculosis received daily injections of recombinant murine TNF-α, a significant reduction in the number of viable bacteria in the lungs and spleens was observed (8). Similarly, treatment of Mycobacterium bovis bacillus Calmette-Guérin (BCG)-infected mice with TNF and TNF-mimetic peptide (consisting of amino acids 70 to 80 of TNF) resulted in increased organization of the granulomas and a decrease in the bacterial load (25). Conversely, when TNF-α was neutralized by treatment with anti-TNF-α monoclonal antibody, granuloma formation in BCG-infected mice was abrogated, and the bacilli multiplied in an uncontrolled manner, leading to decreased survival of the animals (13). The protective role of TNF-α was further demonstrated in studies with mice in which the genes encoding TNF-α or the TNF-α receptor 1 (TNF-αR1) were disrupted. In TNF-αR1 gene-disrupted (TNFR-KO) mice, infection with M. tuberculosis was not contained and the animals died soon after infection (9). In TNF-α gene-disrupted (TNF-KO) mice, M. tuberculosis infection led to dysregulated granuloma formation, resulting in large accumulations of cells and mycobacteria in the lungs, as well as extensive necrosis and neutrophil infiltration (1).
In addition to its essential protective effects in the generation of immunity against pathogens, TNF-α has been shown in many systems to induce immunopathology in vivo. Tissue necrosis and cachexia or wasting have been associated with elevated TNF-α levels (3, 28). In patients with tuberculosis, increases in this cytokine have been implicated in clinical worsening (2). In a previous study in mice, we showed that a reduction in TNF-α levels in the infected lung was associated with a decrease in granuloma size and less necrosis (22). Although these results are suggestive of a pathogenic role for this cytokine, it has been difficult to directly demonstrate the deleterious effects of TNF-α in murine tuberculosis.
To directly demonstrate the detrimental effects of excess TNF-α in the murine response to mycobacterial infection, we used a strain of recombinant BCG that secretes murine TNF-α to infect TNF-KO mice. Some mice were given an unusually high inoculum of bacilli to increase the amount of TNF-α at the site of infection. Following intravenous infection, we evaluated the growth of the recombinant BCG in the lungs, livers, and spleens; the granulomatous response in the lungs and liver; cytokine mRNA production in the lungs; and survival of the infected animals.
MATERIALS AND METHODS
Mice.
Eight- to 10-week-old C57BL/6 mice (controls), homozygous TNF-α p55 receptor gene-disrupted (TNFR-KO) mice on a C57BL/6 genetic background, and homozygous TNF-α gene-disrupted (TNF-KO) mice on the same genetic background were used (19, 26). The mice were obtained from the University of Cape Town breeding stock and were kept under specific-pathogen-free conditions at the University of Cape Town animal facility until infection.
Recombinant BCG.
Cells of recombinant M. bovis BCG strain Montreal secreting murine TNF-α (BCG-TNF) or containing the vector only (BCG-vector) were a kind gift from Richard Young of the Whitehead Institute, Cambridge, Mass. Murine cDNA for TNF-α was cloned into the plasmids pRBD3 and pRBD4, as described previously (24). The expression vectors contained a kanamycin resistance gene. The BCG-TNF and BCG-vector cells were grown to mid-log phase in Middlebrook 7H9 medium (Difco Laboratories, Detroit, Mich.) containing kanamycin (18 μg/ml) (Sigma, St. Louis, Mo.) with minimal agitation for 7 days and kept frozen in aliquots until use (23).
Intravenous infection of mice.
To monitor the course of infection of mice, two different inocula were used: a high dose of about 107 organisms or the lower dose of about 105 organisms. Recombinant bacilli in a volume of 200 μl were injected into the tail vein of mice. The initial infecting load was assayed by plating liver, spleen, and lung homogenates 6 h after infection. Thereafter, groups of infected animals were sacrificed at different time points as indicated. Because mice infected with higher inocula were likely to die quickly, experiments with high inocula were terminated earlier (40 days). Mice were first anesthetized with a solution containing 44 mg of ketamine per kg of body weight (Aveco Co., Inc., Fort Dodge, Iowa) and 5 mg of xylazine per kg of body weight (Rompum; Mobay Corp., Shawnee, Kans.). Blood was collected by cardiac puncture, and serum was prepared and kept frozen at −80°C until assay. Lungs, liver, and spleen were collected aseptically immediately after cardiac puncture, weighed, and then used for evaluation of bacillary load, cytokine mRNA expression levels, and histology. Serum and organs from uninfected mice were used for determination of baseline cytokine and cytokine mRNA levels.
For survival experiments with TNF-KO mice, the mice received either 5 × 106 organisms in 200 μl or a high dose of 2 × 108 organisms in 200 μl. For survival experiments in TNFR-KO mice, the mice were infected with 5 × 106 or 1 × 108 BCG-vector or BCG-TNF cells in 200 μl. The infecting loads in the organs were confirmed by quantitation of the number of viable organisms in the lungs (CFU assay [see below]) 6 h postinfection.
This protocol was approved by the University of Cape Town Animal Ethics Committee.
Histology.
Lungs and livers of mice were fixed in 10% buffered formalin, paraffin embedded, and processed for histology. Sections were stained with hematoxylin and eosin and Ziehl-Neelsen stain for histologic evaluation and photography.
Immunohistology.
Formalin-fixed, paraffin-embedded sections were deparaffinized and rehydrated through graded alcohols. Antigen retrieval was accomplished by boiling the slides in 10 mM citrate buffer (pH 6.0) for 20 min. The staining of the sections was performed in an automated immunostainer (Ventana, Tucson, Ariz.) with a polyclonal rabbit anti-mouse antibody specific for inducible nitric oxide synthase (iNOS) (1:300) (Calbiochem, La Jolla, Calif.) (14).
PCR for cytokine mRNA.
Total cellular RNA was obtained from lungs of mice at 14, 28, and 45 days following intravenous infection with BCG-vector or BCG-TNF. Tissues were homogenized in 3 ml of RNAzol B (Cinna/Biotcex Lab. Inc., Houston, Tex.) and RNA was extracted according to the manufacturer's instructions. The reverse transcription-PCR was carried out as previously described (15). Briefly, 1 μg of RNA was reverse transcribed with a Moloney murine leukemia virus reverse transcriptase and amplified with Taq polymerase according to procedures given in the GeneAmp RNA PCR kit (Perkin-Elmer, Branchburg, N.J.). Primers for TNF-α, interleukin 10 (IL-10), gamma interferon (IFN-γ), IL-12, and β-actin were used as described previously (22). Densitometry of the amplified bands was carried out with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Results were normalized to the density of β-actin.
CFU assay.
Bacterial loads in the lungs, livers, and spleens of infected mice were evaluated by using 10-fold serial dilutions of organ homogenates. Organ homogenates were plated onto Middlebrook 7H10 agar plates (Difco) as well as 7H10 agar supplemented with 18 μg of kanamycin per ml (Sigma). The two sets of plates were incubated at 37°C for 3 weeks. Organisms were enumerated as CFU as described previously (22).
Determination of TNF-α levels in plasma of infected mice.
At the time of sacrifice, mice were bled by cardiac puncture into EDTA-containing tubes. Plasma was stored at −80°C until assay by enzyme-linked immunosorbent assay (ELISA) for TNF-α (Endogen, Inc., Boston, Mass.).
Determination of TNF-α levels in the bacterial culture supernatants.
The ability of the recombinant bacilli to secrete murine TNF-α was verified by measuring by ELISA (Endogen) the concentration of the cytokine in the bacterial culture supernatants. Only mycobacterial suspensions that produced the cytokine were used for infection. To ensure that the recombinant BCG-TNF cells were still secreting the cytokine during the infection, mycobacteria recovered from the lungs of the infected mice (giving rise to colonies in the CFU assay) were grown for 1 week in 7H9 medium supplemented with 18 μg of kanamycin per ml, and the supernatants were tested for TNF-α concentrations by ELISA.
Statistical analysis.
Data were analyzed with an independent t test when indicated. P values of <0.05 were considered statistically significant.
RESULTS
Effect of TNF-α on growth of recombinant BCG in TNF-KO mice.
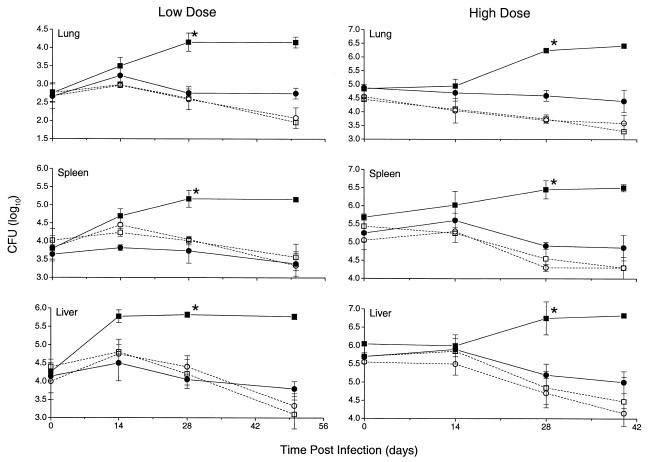
The effect of local production of TNF-α on mycobacterial growth in the organs of mice infected intravenously was evaluated. We compared the bacillary load in the lungs, spleens, and livers of mice following infection with about 105 (low dose) or about 107 (high dose) BCG-TNF cells to the bacillary load in mice infected with the control BCG-vector. When the TNF-KO mice were infected with BCG-vector, the infection was not controlled, and the bacillary load increased in all organs at both doses of infection (Fig. 1). The increase in CFU count from baseline to the 28-day time point of the study was significant at both doses for all organs tested (P < 0.05). On the other hand, when TNF-KO mice were infected with low-dose or high-dose BCG-TNF, bacillary growth was controlled, and CFU decreased slightly from baseline to the final time point, similar to the response seen in wild-type mice (Fig. 1). By 28 days postinfection, there was a significant difference in CFU between the BCG-TNF and BCG-vector in the TNF-KO mice (P < 0.04).
FIG. 1.
Effect of TNF-α on the bacillary load in the tissues of infected mice. TNF-KO mice (solid symbols) or wild-type mice (open symbols) were infected intravenously with a low or high dose of BCG-vector (squares) or BCG-TNF (circles). ∗, statistically significant (P < 0.05) differences between BCG-vector- and BCG-TNF-infected TNF-KO mice. Results are means ± SD of two independent experiments for each dose with 4 mice per group per time point.
No TNF-α was detected in the plasma of any of the mice infected at the low dose. However, this cytokine was detected at days 28 and 40 in the plasma (10 to 13 pg of TNF-α per ml) of TNF-KO mice infected with the high dose of BCG-TNF.
Thus, in the TNF-KO mice, TNF-α produced at the site of infection appeared to reconstitute the host response, resulting in control of bacterial growth even when the initial infecting inoculum was very high.
Effect of TNF-α on lung histopathology following infection of TNF-KO mice with recombinant BCG.
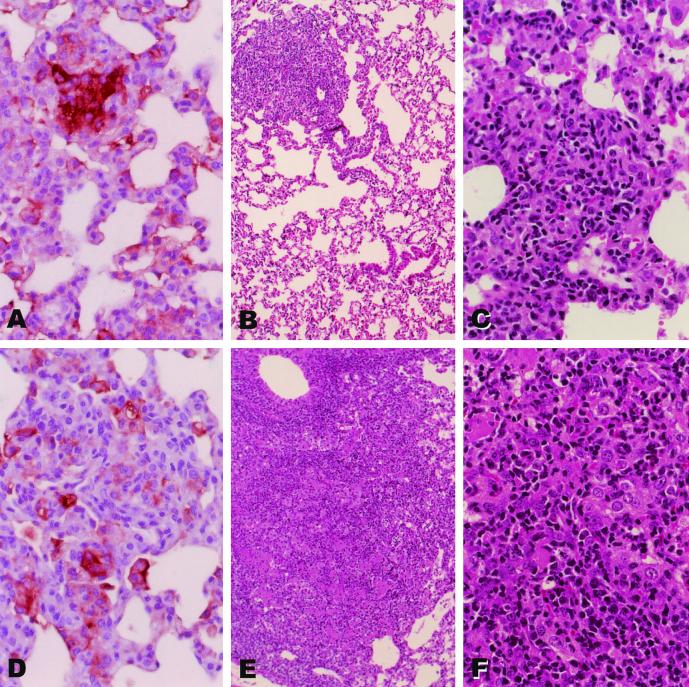
The levels of cellular accumulation and organization (granulomatous response) in the lungs of TNF-KO mice following infection with high or low doses of BCG-TNF or BCG-vector were compared. At 28 days postinfection with about 105 organisms of either recombinant, the lungs of the TNF-KO mice appeared relatively unaffected, with small scattered cellular aggregates in the parenchyma. These aggregates consisted of lymphocytes and macrophages, some of which stained for iNOS expression (Fig. 2A and D). The iNOS-staining macrophages appeared more focused in the BCG-TNF-infected lungs (Fig. 2A) than in BCG-vector infected lungs (Fig. 2D), which showed a scattered pattern. By 45 days, the two infections differed markedly. In mice infected with low-dose BCG-TNF, the granulomas remained small and clearly distinct from the majority of the lung, which appeared normal (Fig. 2B). These granulomas consisted of lymphocytes and macrophages (Fig. 2C), some of which were still iNOS positive (not shown). In contrast, in the TNF-KO mice infected with low-dose BCG-vector, the granulomas had enlarged extensively and occupied most of the lung (Fig. 2E). These contained large undifferentiated macrophages, many lymphocytes, and a few scattered polymorphonuclear leukocytes (Fig. 2F). iNOS staining was still evident (not shown). Infection of wild-type mice with this low dose of BCG-TNF or BCG-vector resulted in little or no granulomatous response (not shown).
FIG. 2.
Morphology of the lungs of mice infected with low-dose recombinant BCG. TNF-KO mice were infected intravenously with a low dose of BCG-TNF (A to C) or BCG-vector (D to F). The lungs were examined at 28 days (A and D) or 45 days (B, C, E, and F). Sections were stained with hematoxylin and eosin (B, C, E, and F) or for iNOS protein expression (brown-staining cells) (A and D). Magnifications, panels B and E, ×10; panels A, C, D, and F, ×40.
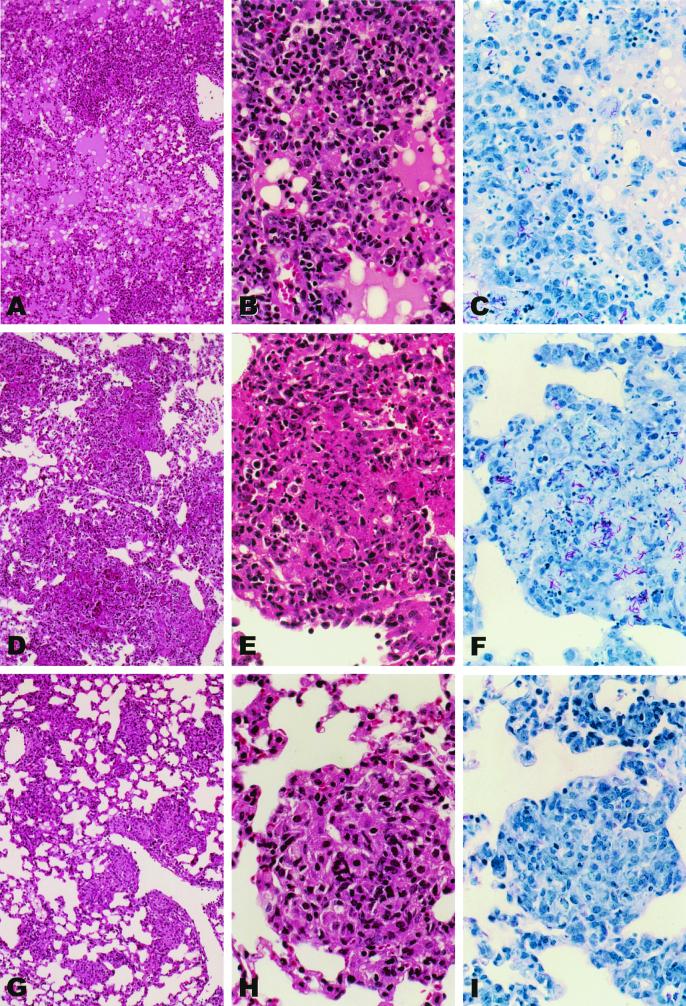
To study the effect of high levels of TNF-α on the cellular inflammatory response in the lungs, TNF-KO mice were infected with high doses (107) of either BCG-TNF or BCG-vector. High doses of infection induced aggressive and rapid responses. Already at 28 days postinfection, extensive cellular recruitment into the lungs was noted in response to either infection (Fig. 3A and D). In mice infected with BCG-TNF, the lung space surrounding the cellular aggregates was almost filled with fluid and infiltrated with predominantly mononuclear cells and scattered polymorphonuclear leukocytes (Fig. 3A to C). In the BCG-vector infected mice, some lung tissue remained uninvolved with intact air spaces (Fig. 3D to F). Staining of the lung sections for acid-fast bacilli revealed fewer mycobacteria in mice infected with BCG-TNF (Fig. 3C) than in mice infected with BCG-vector (Fig. 3F), confirming the CFU data (Fig. 1). When wild-type mice were similarly infected with high doses of BCG-TNF or BCG-vector, multiple small, well-organized granulomas were seen in the lungs at 28 days, with few if any acid-fast bacilli (Fig. 3G to I). In these animals, much more of the lung airspace remained intact.
FIG. 3.
Morphology of the lungs of mice infected with high-dose recombinant BCG. TNF-KO mice were infected with a high dose of BCG-TNF (A to C) or BCG-vector (D to F). Wild-type mice were infected with a high dose of BCG-vector (G to I). The lungs were evaluated at 28 days. Sections were stained with hematoxylin and eosin (A, B, D, E, G, and H) or with Ziehl-Neelsen (C, F, and I). Fluid in the lungs is dark pink in hematoxylin and eosin sections or light pink in Ziehl-Neelsen sections. Magnifications, panels A, D, and G, ×10; panels B, C, E, F, H, and I, ×40.
Thus, the histologic evidence suggests that the absence of TNF-α in the TNF-KO mice infected with BCG-vector led to uncontrolled cellular recruitment into the infected lungs. On the other hand, the TNF-α secreted by the low dose of recombinant mycobacteria appeared to regulate the granulomatous response in the TNF-KO mice, resulting in smaller and better-differentiated granulomas by 45 days of infection. However, high levels of TNF-α (high enough to be detected in the plasma) led to an overwhelming inflammatory response that compromised lung function in the host, despite the successful control of the growth of the infecting mycobacteria (Fig. 1).
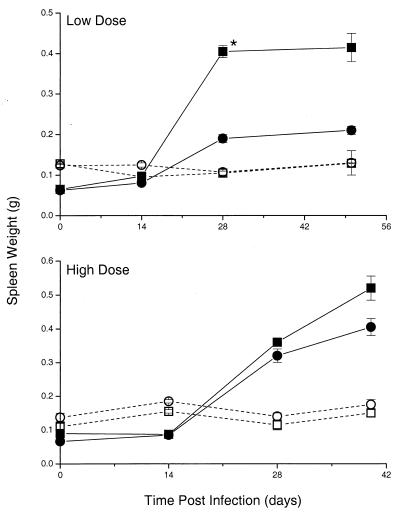
Effect of TNF-α on spleen weight in TNF-KO mice infected with recombinant BCG.
Spleen weight, which may be used as an indicator of the systemic immune response to infection, was monitored in the gene-disrupted mice infected with either recombinant. At each time point, the spleens recovered from mice were weighed. When TNF-KO mice were infected with about 105 BCG-vector cells, there was a dramatic increase in mean spleen weight from 0.065 mg to 0.395 mg (P < 0.01) (Fig. 4). In contrast, when TNF-KO mice were infected with 105 BCG-TNF cells, there was less increase in the mean spleen weight, from 0.062 mg to 0.215 mg (P < 0.03). From 28 days postinfection, a statistically significant difference in spleen weight between infection with BCG-TNF and infection with BCG-vector was noted (P < 0.01). At this time, the spleens from the TNF-KO mice infected with BCG-TNF were not much larger than those from wild-type mice infected with BCG-vector or BCG-TNF, which did not change in weight (Fig. 4).
FIG. 4.
Effect of TNF-α on the weight of spleens of TNF-KO mice (solid symbols) or wild-type mice (open symbols) infected with BCG-vector (squares) or BCG-TNF (circles). ∗, statistically significant (P < 0.05) difference between mice infected with BCG-vector and those infected with BCG-TNF. Results are means ± standard deviations of two independent experiments for each dose with 4 mice per group per time point.
Following infection with the high doses of BCG-vector, the spleens recovered from TNF-KO mice were enlarged (from 0.09 mg to 0.565 mg; P = 0.006) (Fig. 4). Interestingly, infection with the high dose of BCG-TNF also resulted in enlarged spleens at day 28, although the size did not increase as much by day 40 (from 0.055 mg to 0.425 mg; P = 0.002). The wild-type mouse spleens showed no increase in size in response to high doses of either infection (Fig. 4). Thus, the TNF-α produced by the recombinant BCG reduced the degree of spleen enlargement seen in the absence of TNF-α in the TNF-α gene-disrupted mice. However, the presence of a larger amount of TNF-α appeared to exacerbate the inflammatory process, leading to an increase in spleen weight despite the control of bacillary growth (Fig. 1).
Effect of TNF-α on survival of mice infected with recombinant BCG.
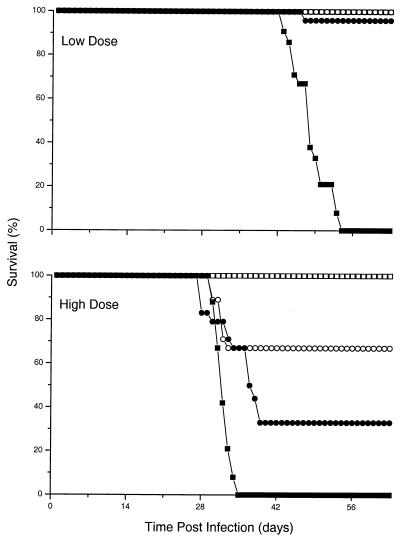
The infection of TNF-KO mice with 5 × 106 BCG-vector cells resulted in early death of the mice (Fig. 5). By day 56, all animals had succumbed to the infection. Following infection with 5 × 106 BCG-TNF cells, however, the TNF-KO mice survived (P < 0.001 for BCG-TNF versus BCG-vector). The wild-type mice infected at this dose with BCG-vector or BCG-TNF had no deaths over the period of the experiment. Following infection of TNF-KO mice with high-dose (2 × 108) BCG-vector, 100% mortality had been observed already by day 35 (P < 0.001 for TNF-KO versus wild-type mice infected with BCG-vector) (Fig. 5). Infection with high-dose (2 × 108) BCG-TNF resulted in similar mortality (P = 0.4 for BCG-TNF versus BCG-vector in TNF-KO mice), with about 70% of the TNF-KO mice succumbing by day 39 (P = 0.006 for TNF-KO mice infected with BCG-TNF versus wild-type mice infected with BCG-vector) (Fig. 5). When wild-type mice were infected with this dose of BCG-vector, 100% survived. Interestingly, wild-type mice infected with this dose of BCG-TNF showed some early mortality. By day 32, 33% of the mice had succumbed (P = 0.06 for wild-type mice infected with BCG-TNF versus those infected with BCG-vector). Therefore, it appears that the TNF-α from the recombinant mycobacteria restored the ability of the TNF-KO mice to control the infection and survive. However, excess levels of TNF-α due to the presence of unusually large numbers of recombinant BCG-TNF cells compromised the survival of the mice, whether or not the growth of the bacilli was controlled (Fig. 1).
FIG. 5.
Effect of TNF-α on survival of mice infected with recombinant BCG. TNF-KO mice (solid symbols) or wild-type mice (open symbols) were infected with BCG-vector (squares) or BCG-TNF (circles). Results expressed as percent survival represent the means of two independent experiments for each dose with 12 mice per group. A significant (P < 0.001) difference in survival of TNF-KO mice infected with low doses of BCG-vector versus those infected with BCG-TNF was noted. (For P values for the high doses of infection, see text.)
Survival of TNF-αR1-deficient mice infected with recombinant BCG.
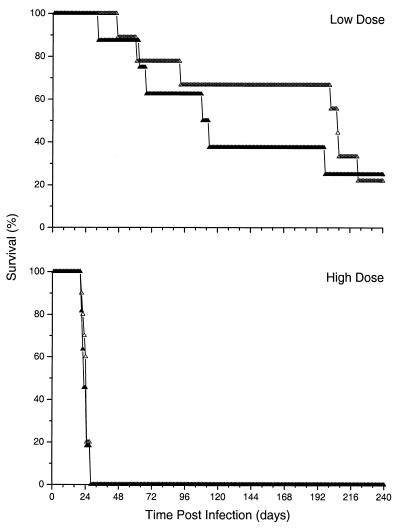
To determine whether the differences in survival of mice required signaling by TNF-αR1, TNFR-KO mice were infected intravenously with either BCG-vector or BCG-TNF at a low (5 × 106) or a high (1 × 108) dose. There was no difference in survival between the TNFR-KO mice infected with BCG-vector and those infected with BCG-TNF at either dose (P > 0.1) (Fig. 6). This result indicated that the absence of a functional TNF-α signaling pathway due to the lack of TNF-α was the cause of the reduced survival observed in the TNF-KO mice infected with BCG-vector (Fig. 5).
FIG. 6.
Effect of TNF-α on survival of TNFR-KO mice infected with recombinant BCG. TNFR-KO mice were infected with BCG-vector (open triangles) or BCG-TNF (solid triangles). Results expressed as percent survival are from a single experiment for each dose with 12 mice per group. No significant difference in survival of mice infected with BCG-TNF versus those infected with BCG-vector was noted (P > 0.1).
Cytokine expression in tissues of TNF-KO mice infected with recombinant BCG.
The early (14 day) expression of IFN-γ, IL-12, and IL-10 mRNA in the lungs of TNF-KO-infected mice was studied. In response to infection of TNF-KO mice with BCG-vector, relatively low levels of IFN-γ mRNA were induced compared to the amount of IFN-γ mRNA induced by infection with BCG-TNF (Table 1). The levels of IL-12 and IL-10 mRNA expressed in the lungs were also lower in response to infection with BCG-vector than those in response to BCG-TNF. By later time points, as differences in the bacillary load became apparent, cytokine mRNA levels increased in the lungs of mice infected with BCG-vector relative to the lungs of mice infected with BCG-TNF (not shown). The results taken together suggested that TNF-α is required for the efficient generation of the Th1-type (IFN-γ and IL-12) cellular immune response to mycobacterial infection in mice.
TABLE 1.
Cytokine mRNA levels in infected lungs (14 days).
| Cytokine | Infecting strain | Activity level (SD)a |
|---|---|---|
| IFN-γ | BCG-vector | 2,239 (200) |
| BCG-TNF | 16,014 (6,000) | |
| IL-12 | BCG-vector | 11,401 (3,120) |
| BCG-TNF | 21,045 (124) | |
| IL-10 | BCG-vector | 19,937 (4,397) |
| BCG-TNF | 57,142 (9,250) |
Results are expressed as mean density units normalized to β-actin from 4 animals per group (standard deviation in parentheses).
DISCUSSION
Here we directly demonstrate with the murine model of mycobacterial infection that TNF-α is not only essential for protection, but at high levels is also seriously detrimental. We show that when excess levels of the cytokine are present at the site of infection, as in the TNF-KO or wild-type mice infected with a high inoculum of BCG-TNF, the toxic sequelae of excess TNF-α override its demonstrated protective effects, and the animals die in spite of control of bacterial growth.
The protective role of TNF-α in mycobacterial infections has been well established in experimental models (1, 8–10, 13, 25). The cytokine can exert a number of effects. TNF-α has been shown to modify the endothelium and induce chemokine expression, thereby facilitating extravasation of monocytes and T cells from the blood and directing this migration of cells to the infected site (13, 20, 32). In our previous studies, we showed that the local immune response to mycobacterial antigens induces the expression of the CXC chemokine IP10 (12), which is recognized by the T-cell and NK-cell chemokine receptor CXCR3 (16, 17). The expression of IP10 and other TNF-α-induced molecules by macrophages, fibroblasts, and endothelial cells served to retain the activated T cells at the site of infection (27). Indeed, in this study, we show that TNF-α is necessary for the accumulation and organization of monocytes, macrophages, and lymphocytes into well-differentiated granulomas (Fig. 2). In addition, TNF-α activates T cells or T-cell subsets, thereby facilitating the generation of cytokines and/or cytotoxic effector molecules and ensuring their sustained expression by the cells (11, 30). TNF-α also activates and fosters differentiation of dendritic cells for enhanced IL-12 production and antigen presentation (11). This effect is confirmed by our results, which show levels of IFN-γ and IL-12 mRNA induced in the BCG-TNF-infected lungs in the presence of TNF-α higher than those induced by the BCG-vector infection in the absence of this cytokine.
TNF-α may also directly activate macrophages to control the growth of and/or kill the intracellular mycobacteria (7). TNF-α-activated murine macrophages can kill mycobacteria via the generation of reactive nitrogen intermediates. It has previously been shown that iNOS induction is associated with killing of mycobacteria in vivo and in vitro (4, 18). However, our study reported here shows that the presence of the iNOS protein in the macrophages of the infected lungs is not sufficient to control the growth of the bacilli. Tissue sections of the lungs and livers of the TNF-KO mice infected with BCG-vector stained for iNOS (Fig. 2D), yet the infection was not controlled (Fig. 1). Similar observations were recently made by Bean et al. (1). Thus, it appears that in the absence of TNF-α, iNOS expression alone is not sufficient for the killing of intracellular mycobacteria. Further aspects of TNF-α-mediated macrophage activation and protection against mycobacteria are currently being explored.
In addition to these protective effects, TNF-α has detrimental effects. When TNF-α is present in large amounts systemically, the resulting inflammatory cascade causes leaky capillaries, leukocyte infiltration, neutrophil-mediated endothelial damage, and inhibition of pulmonary surfactant (28, 31). This damaging inflammation is analogous to that seen in the lungs of adults with respiratory distress syndrome (6, 14). TNF-α-mediated inflammatory damage is also easily demonstrated in the central nervous system. For example, in rabbits, intrathecal infection with virulent M. bovis strain Ravenel results in local production of relatively high levels of TNF. Excessive vasculitis, more extensive leukocyte migration into the cerebrospinal fluid, and more severe pathology in the brain are observed compared to inoculation with M. bovis strains that induced less TNF (29). This pathologic inflammation in the central nervous system is similar to that observed in patients with tuberculous meningitis.
Our studies suggest that the extent of inflammation and cellular recruitment to a site of mycobacterial infection are determined by a number of independent factors. One of these is the load of infecting bacilli. This is clearly demonstrated by the observation that following infection of TNF-KO or TNFR-KO mice with the high dose of BCG-vector, when no TNF-α or TNF-α activity is present, the inflammatory cellular response was augmented and the mice died sooner than following infection with the lower dose of BCG-vector. The other determinant of inflammation is the level of TNF-α. When TNF-KO mice were infected with a high dose of BCG-TNF, the inflammatory response was more severe than following infection with the same dose of BCG-vector, and the mice died. Therefore, it appears that excessive inflammation, whether due to an unusually high level of bacteria or of TNF-α, leads to occupation of the air space by cells and fluid, compromised lung function, and death of the animals.
Cytokines secreted by recombinant mycobacteria within the host cell vacuoles have been shown to be biologically active. In a recent study, infection of IFN-γ gene-disrupted mice with recombinant BCG secreting murine IFN-γ (BCG-IFN-γ) resulted in reconstitution of the protective immune response against BCG (21). In the same study, infection in vitro of peritoneal macrophages obtained from IFN-γ gene-disrupted mice with BCG-IFN-γ induced specific activation of the nuclear factor STAT-1 and IFN regulatory factor 1 as well as iNOS. Similarly, in the present study, the antimycobacterial immune response of TNF-KO mice was restored by infection with recombinant BCG secreting murine TNF-α, and iNOS was induced in the infected tissues. In vitro, we observed that macrophages obtained from TNF-KO mice infected with BCG-TNF are activated, as demonstrated by induction of iNOS (G. Kaplan et al., unpublished data). These results, taken together, demonstrate that the cytokines secreted by recombinant bacteria can be biologically active. However, the mechanisms by which the cytokines exert their effects, whether intracellular or extracellular, are not clear. The cytokines can be measured in the culture supernatants of infected macrophages, as well as in the plasma of mice infected at very high doses. This suggests that, at least in some cases, active cytokine is secreted into the extracellular milieu.
The protective and detrimental effects of TNF-α in an infection have also been similarly observed in a rodent model of malaria. In rats, Plasmodium chaboudi infection cures spontaneously and TNF-α is not detectable in the blood (5). Plasmodium vinckei infection on the other hand, leads to death of infected rats after several weeks. In the latter animals, high parasitemia leading to high TNF-α levels results in a shock-like condition and focal liver necrosis. Although ultimately the parasitemia in this infection is cleared, death of the animal from TNF-α-mediated shock still ensues.
In summary, the results presented here show that TNF-α is a double-edged sword. While it is an essential component of the host protective response against mycobacterial infection, high levels of the cytokine at the site of infection induce an excessive inflammatory response that overwhelms the beneficial effects of the cytokine.
ACKNOWLEDGMENTS
We thank Victoria Freedman for help with preparation of the manuscript, Marguerite Nulty for secretarial assistance, and Judy Adams for preparation of the figures.
These studies were supported in part by Direct Effect (New York, N.Y.) and by AI42056 (G.K.). L.G.B. was a Fogarty International Fellow (AITRP TW00231 to Columbia University, New York, N.Y.).
REFERENCES
- 1.Bean A G, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 2.Bekker L G, Maartens G, Steyn L, Kaplan G. Selective increase in plasma TNF-α and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–584. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Britton W J, Meadows N, Rathjen D A, Roach D R, Briscoe H. A tumor necrosis factor mimetic peptide activates a murine macrophage cell line to inhibit mycobacterial growth in a nitric oxide-dependent fashion. Infect Immun. 1998;66:2122–2127. doi: 10.1128/iai.66.5.2122-2127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark I A, Cowden W B, Butcher G A, Hunt N H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987;129:192–199. [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras M, Hariharan N, Lewandoski J R, Ciesielski W, Koscik R, Zimmerman J J. Bronchoalveolar oxyradical inflammatory elements herald bronchopulmonary dysplasia. Crit Care Med. 1996;24:29–37. doi: 10.1097/00003246-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Denis M, Gregg E O, Ghandirian E. Cytokine modulation of Mycobacterium tuberculosis growth in human macrophages. Int J Immunopharmacol. 1990;12:721–727. doi: 10.1016/0192-0561(90)90034-k. [DOI] [PubMed] [Google Scholar]
- 8.Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leukoc Biol. 1991;50:495–501. doi: 10.1002/jlb.50.5.495. [DOI] [PubMed] [Google Scholar]
- 9.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-γ in the differentiation of protective granulomas. Eur J Immunol. 1997;27:3182–3190. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 11.Josien R, Wong B R, Li H L, Steinman R M, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562–2568. [PubMed] [Google Scholar]
- 12.Kaplan G, Luster A D, Hancock G, Cohn Z A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kindler V, Sappino A-P, Grau G E, Pignet P F, Vassalli P. The inducing role of tumour necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 14.Kristof A S, Goldberg P, Laubach V, Hussain S N. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 15.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loetscher M, Gerber B, Loetscher P, Jones S A, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luster A D, Greenberg S M, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ming W J, Bersani L, Mantovani A. Tumor necrosis factor is chemotactic for monocyte and polymorphonuclear leukocytes. J Immunol. 1987;138:1469–1474. [PubMed] [Google Scholar]
- 21.Moreira A L, Tsenova L, Murray P J, Freeman S, Bergtold A, Chiriboga L, Kaplan G. Aerosol infection of mice with recombinant BCG secreting murine IFN-γ reconstitutes local protective immunity. Microb Pathog. 2000;29:175–185. doi: 10.1006/mpat.2000.0382. [DOI] [PubMed] [Google Scholar]
- 22.Moreira A L, Tsenova-Berkova L, Wang J, Laochumroonvorapong P, Freeman S, Freedman V H, Kaplan G. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tuber Lung Dis. 1997;78:47–55. doi: 10.1016/s0962-8479(97)90015-0. [DOI] [PubMed] [Google Scholar]
- 23.Murray P J, Aldovini A, Young R A. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell M A, Aldovini A, Duda R B, Yang H, Szilvasi A, Young R A, DeWolf W C. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–2514. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach D R, Briscoe H, Baumgart K, Rathjen D A, Britton W J. Tumor necrosis factor (TNF) and a TNF-mimetic peptide modulate the granulomatous response to Mycobacterium bovis BCG infection in vivo. Infect Immun. 1999;67:5473–5476. doi: 10.1128/iai.67.10.5473-5476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 27.Taub D D, Longo D L, Murphy W J. Human interferon-inducible protein-120 induces mononuclear cell infiltration in mice and promotes the migration of human T lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood. 1996;87:1423–1431. [PubMed] [Google Scholar]
- 28.Tracey K J, Cerami A. Tumor necrosis factor and regulation of metabolism in infection: role of systemic versus tissue levels. Proc Soc Exp Biol Med. 1992;200:233–239. doi: 10.3181/00379727-200-43426. [DOI] [PubMed] [Google Scholar]
- 29.Tsenova L, Bergtold A, Freedman V H, Young R A, Kaplan G. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci USA. 1999;96:5657–5662. doi: 10.1073/pnas.96.10.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueta C, Kawasumi H, Fujiwara H, Miyagawa T, Kida H, Ohmoto Y, Kishimoto S, Tsuyuguchi I. Interleukin-12 activates human gamma delta T cells: synergistic effect of tumor necrosis factor-alpha. Eur J Immunol. 1996;26:3066–3073. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]
- 31.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 32.Wuyts A, Proost P, VanDamme J. Interleukin-8 and other CXC-chemokines. In: Thomson A, editor. The cytokine handbook. New York, N.Y: Academic Press; 1998. pp. 271–311. [Google Scholar]