Abstract
Since 2013, wild poliovirus (WPV) transmission occurred only for type 1 (WPV1). Following several years of increasing reported incidence (2017-2019) and programmatic disruptions caused by COVID-19 (early 2020), Pakistan and Afghanistan performed a large number of supplementary immunization activities (late 2020-2021). This increased intensity of immunization, following widespread transmission, substantially decreased WPV1 cases and positive environmental samples during 2021. Modeling the potential for undetected circulation of WPV1 after apparent interruption can support regional and global decisions about certification of the eradication of indigenous WPV1 transmission. We apply a stochastic model to estimate the confidence about no circulation (CNC) of WPV1 in Pakistan and Afghanistan as a function of time since the last reported case and/or positive environmental sample. Exploration of different assumptions about surveillance quality suggests a range for CNC for WPV1 as a function of time since the last positive surveillance signal, and supports the potential use of a time with no evidence of transmission of less than 3 years as sufficient to assume die out in the context of good acute flaccid paralysis (AFP) surveillance. We show high expected CNC based on AFP surveillance data alone, even with imperfect surveillance and some use of inactivated poliovirus vaccine masking the ability of AFP surveillance to detect transmission. Ensuring high quality AFP and environmental surveillance may substantially shorten the time required to reach high CNC. The time required for high CNC depends whether immunization activities maintain high population immunity and the quality of surveillance data.
Keywords: Polio, eradication, Pakistan, Afghanistan, infection transmission modeling, vaccination
Social media blurb:
Modeling suggests that the certification of the global of eradication of type 1 wild polioviruses could happen with high confidence sooner than 3 years after the last reported case in Pakistan or Afghanistan
1. Introduction
Review of the prior polio modeling (Thompson & Kalkowska, 2020) published during the period 2000-2019 identified 16 studies related to potential undetected circulation of polioviruses after apparent die out. One additional study since that review (Kalkowska & Thompson, 2021) focused on the last reservoir in Africa (i.e., Nigeria) to support regional certification (World Health Organization, 2020). To date, the Global Commission for Certification of Poliomyelitis Eradication (GCC) applied a criterion of 3 years without observing wild poliovirus (WPV) excretion in individuals with active acute flaccid paralysis (AFP) as an indication of high confidence of elimination of indigenous transmission (World Health Organization, 2019a). Increased use of environmental surveillance (ES), particularly in Pakistan and Afghanistan, also raises some questions about the role of ES information with respect to confidence about no circulation (Kalkowska, Duintjer Tebbens, Pallansch, & Thompson, 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019). Some prior modeling demonstrated the impact of various assumptions that impact population immunity to transmission and showed that high confidence could occur for relatively shorter durations with no observed signals from either AFP or ES for some types of polioviruses and under some conditions (Duintjer Tebbens, Kalkowska, & Thompson, 2019; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019; Kalkowska & Thompson, 2021).
Globally, Pakistan and Afghanistan represent the last remaining epidemiological reservoir for type 1 WPV (WPV1). All other countries remained free of WPV1 for at least 5 years, with the notable exceptions of a reported WPV1 case with onset in November 2021 reported in Malawi and a case with onset in March 2022 reported in Mozambique, which are genetically linked to a WPV1 strain from Pakistan (World Health Organization, 2022). As demonstrated by this recent experience and prior outbreaks, for example Syria 2013 (World Health Organization, 2013), ongoing WPV1 transmission in Pakistan and Afghanistan poses a reintroduction risk for other countries (Thompson, Kalkowska, & Duintjer Tebbens, 2015), even with International Health Regulations (Duintjer Tebbens & Thompson, 2017). However, outbreaks following exportation of WPV do not always occur, for example, a known prior exportation event that did not lead to any cases includes a 2019 detection in Iran (World Health Organization, 2019b).
Numerous modeling studies explored poliovirus transmission dynamics for Pakistan and Afghanistan relevant to WPV1 eradication (Duintjer Tebbens et al., 2018; Duintjer Tebbens & Thompson, 2019; Kalkowska, Badizadegan, & Thompson, 2022; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019; Kalkowska, Pallansch, Cochi, & Thompson, 2021). Most recently, one deterministic dynamic transmission modeling study identified potential immunization strategies that could lead to potential die out of WPV1 in Pakistan and Afghanistan (Kalkowska et al., 2022). Transmission die out occurs stochastically (Eichner & Hadeler, 1995) and depends on the specific population and its epidemiological conditions. Building on prior stochastic studies of the confidence of no circulation (CNC) of WPV1 as a function of time, we consider the current conditions in Pakistan and Afghanistan to update our characterization of CNC for WPV1 as a function of time. This modeling can help to support future deliberations by the Eastern Mediterranean Regional Certification Commission (EMRCC) and GCC as they respectively consider regional and global certification of the eradication of indigenous WPV1 transmission eradication.
2. Methods
Using previously developed methods, most recently applied to Pakistan and Afghanistan in 2019 (pre-COVID-19) (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019), we updated immunization and contact mixing assumptions through the end of 2021 (Kalkowska et al., 2022). With a few of the scenarios in that analysis indicating potential WPV1 die out as early as 2022, we recognized the opportunity to characterize the probability of no circulation of WPV1 as a function of the time since the last detected event specifically for Pakistan and Afghanistan for those scenarios. The transmission model characterizes individuals in the populations in Pakistan and Afghanistan according to 4 preferentially-mixing subpopulations, 8 immunity states subdivided into a 5-stage immunological waning process, 6 stages of infection, and a 20-stage poliovirus reversion process for both fecal-oral and oropharyngeal routes of transmission (Kalkowska et al., 2022). For Pakistan and Afghanistan, we divide the population into 11 age groups and we mimic the tendencies of individuals to mix more with individuals of similar age using 3 preferentially mixing age groups (Kalkowska et al., 2022).
We modeled Pakistan and Afghanistan as one epidemiological block, and we divide each country into a general population and an undervaccinated subpopulation, which gives a total of four distinct subpopulations (Duintjer Tebbens et al., 2018; Kalkowska et al., 2022; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). Model inputs included demographics, poliovirus transmissibility and seasonality, time-varying mixing among the four subpopulations (including consideration of the impacts of disruptions caused by COVID-19), and poliovirus vaccination histories for all formulations of oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV) use in routine immunization (RI) and supplemental immunization activities (SIAs). Similar to the 2019 analysis (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019), we considered limited immunization, surveillance, and population mixing assumptions related to insecurity that affected polio program performance in the undervaccinated subpopulations in both countries (Kalkowska et al., 2022).
As with our prior analyses, our transmission modeling approach (using our differential equation based transmission and OPV evolution model (Kalkowska, Wassilak, Cochi, Pallansch, & Thompson, 2021) and a stochastic compartmental model) differed from the use of a theoretical transmission model with far fewer immunity states. Specifically, we made the deterministic model into a stochastic model by first rounding the fractional number of individuals for all compartments to the nearest integer (i.e., up or down depending on the result of a random draw), and then performed stochastic iteration over the prospective model time horizon using a fixed time step of 0.125 day. At each time step, we recalculated the rates that change the current state of the model. We then drew a random number from the Poisson distribution for all of these transition rates with an expected value equal to the transition rate times the fixed time step. Each random Poisson draw returned the expected number of individuals that transition according to the rate using this approach. We then constrained the model to ensure that the sum of all transitions do not exceed the total compartment size. We generated random uniform numbers at each time step to determine which new infection events lead to paralytic cases (if any). The model performed full accounting of the effective proportion of infectious individuals excreting the virus that we used to simulate events that ES could detect. Due to the stochastic nature of the model, we performed 1,000 iterations of different distinct realizations of times at which paralytic cases occur, and we used these modeled times to obtain estimates of confidence about no circulation (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015). Consistent with our prior modeling, reviewed in (Thompson & Kalkowska, 2020) as noted above, we used the same algorithms and specific metrics defined as follows (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015):
POE – “the probability of eradication defined as the fraction of stochastic iterations in which die-out occurs”
DEFP – “the detected-event-free period defined as the time in months since the last detected case (AFP) or positive isolate (environmental surveillance)”
CNC – “confidence about no circulation given the DEFP approximated as (1 - the number of DEFPs equal to t months with ongoing WPV circulation, divided by all DEFPs of t months)”
CNCx% – “the time when the confidence about no circulation exceeds x% (i.e., CNC95%, CNC99%)”
TUC – “the time of undetected circulation after the last detected-event (for those iterations in which extinction occurs)”
TUCx% – “the xth percentile of the TUC (i.e., TUC95%, TUC99%)”
We considered the effect of imperfect information from surveillance using a detection function (DF), which provided a combined indicator of overall surveillance quality. For AFP surveillance alone, we defined the DF as the probability pe of detecting the eth event (polio AFP case) in a cluster of sequentially detected cases within a subpopulation. We define perfect AFP surveillance as a system that detects every polio AFP case that occurs, such that pe = 1 in any population (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). Recognizing that AFP surveillance remains imperfect and the quality varies by subpopulation due to access issues and programmatic performance (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019), we considered different probability values for the general and undervaccinated subpopulations, with a lower and upper bound that reflects a range of possible limited AFP surveillance access levels in the undervaccinated subpopulations (Table 1) (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). For ES alone, we defined the DF as the probability of detecting the event of finding poliovirus in a sewage sample and for which we use the effective (i.e., infectiousness-weighted) number of infected individuals (EI) (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). Prior modeling described substantial challenges related to interpretation of limited ES data as well as its representation in our model as a supplement and/or alternative to AFP surveillance (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). As with the prior analysis, we considered two general approaches for representing ES in the model, which we referred to as either a site-specific (SS) or system-wide (SW) approach (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). For the SS approach, we determined the probability of detecting the event (finding poliovirus in a sewage sample) for each given sampling site using a site-specific detection limit (DL50), which we characterized as the EI per person required in the site catchment area to achieve a 50% probability of detecting poliovirus (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). For the SW approach, we assumed that the DF directly described the probability finding poliovirus in any sampling site given the total catchment area from all ES sites and the prevalence of EI in the country (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). For the SS approach, we consider two ways to allocate EI prevalence to sites: proportional prevalence (PP), which allocated EI proportional to the estimated catchment size of the sites, or isolation-rate based prevalence (IP), which determined whether each infection happens in the catchment area of any ES site based on the total estimated catchment size and assigned the infection to a specific site proportionally to the isolation rates of the sites (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). Our model approach did not link or attempt to match modeled subpopulations with specific geographical locations in either country, but instead used more abstract characterization of the different subpopulations (Kalkowska et al., 2022). As such we cannot explicitly match actual ES sampling sites to the modeled subpopulations. Therefore, as with prior modeling, we considered three approaches to distribute ES sites to the four subpopulations: national sites (NS), undervaccinated subpopulation sites (US), or general population sites (GS) distribution. These options covered the bounds of possible distributions of ES sites to the subpopulations (see appendix for the updated coefficients based on updated information on ES sites during 2009-2021 and perspective on how ES surveillance increased over time in both countries) (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019)). Consistent with our prior modeling we considered following nine options to characterize ES (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019):
Table 1:
List of model inputs for the transmission model and the surveillance component
| Model input | Symbol | Value |
|---|---|---|
| Deterministic and stochastic transmission model inputs | ||
| Stochastic model start date | January 1, 2021 | |
| Paralysis-to-infection ratio for WPV1 | 1/200 | |
| Average R0 (WPV1) considering the seasonal changes over the year | 11 | |
| Annual SIA frequencies from 2021 (cumulative fraction targeted) | 4.5 | |
| True SIA coverages from 2021 (general populations) | 0.80 | |
| Relative SIA coverage from 2021 (undervaccinated subpopulations) | 0.45 | |
| National RI coverage from 2021 | ||
| Pakistan | 0.75 | |
| Afghanistan | 0.57 | |
| Relative RI coverage from 2021 (undervaccinated subpopulation) | ||
| Pakistan | 0.40 | |
| Afghanistan | 0.60 | |
| Surveillance model inputs | ||
| Cluster length (days) | cl | 90 |
| Probability of detecting a case by perfect AFP surveillance in either population | p = (p1, p2, …, pi) | (1, 1, 1, 1, …, 1) |
| Probability of detecting a case by imperfect AFP surveillance in general population | p = (p1, p2, …, pi) | (0.75, 0.80, 0.85, 0.90, …, 0.90) |
| Probability of detecting a case by imperfect AFP surveillance in undervaccinated subpopulation, lower bound | p = (p1, p2, …, pi) | (0.10, 0.10, …, 0.10) |
| Probability of detecting a case by imperfect AFP surveillance in undervaccinated subpopulation, upper bound | p = (p1, p2, …, pi) | (0.50, 0.53, 0.57, 0.60, …, 0.60) |
| Effective (i.e., infectiousness-weighted) number of infectious individuals (infections) | EI | Obtained from transmission model |
| Number of people in the population (people) | N | Obtained from transmission model |
| Catchment area population of the ith sampling site (people) | Ni | Varies per site |
| Detection limit of ith ES sampling site (infections/person) | Varies per site |
Abbreviations: AFP, acute flaccid paralysis; ES, environmental surveillance; PV, poliovirus; R0, basic reproductive number; RI, routine immunization; SIA, supplementary immunization activity; WPV, wild poliovirus
SS-PP-NS – “site-specific approach with proportional prevalence allocation and national sites distribution”,
SS-PP-US – “site-specific approach with proportional prevalence allocation and undervaccinated sites distribution”,
SS-PP-GS – “site-specific approach with proportional prevalence allocation and general sites distribution”,
SS-IP-NS – “site-specific approach with isolation-rate based prevalence allocation and national sites distribution”,
SS-IP-US – “site-specific approach with isolation-rate based prevalence allocation and undervaccinated sites distribution”,
SS-IP-GS – “site-specific approach with isolation-rate based prevalence allocation and general sites distribution”,
SW-NS – “system-wide approach with national sites distribution”,
SW-US – “system-wide allocation with undervaccinated sites distribution”,
SW-GS – “system-wide allocation with general sites distribution”.
Overall, the different approaches and assumptions used to model the information from ES provide a means to characterize its uncertain potential role as a tool to increase sensitivity of the overall poliovirus surveillance system and to increase confidence about no circulation.
For the this analysis, we started with the WPV1 transmission results from a companion study (Kalkowska et al., 2022) and transformed the deterministic model into a discrete, stochastic model (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015)). We defined Scenario 1 as the increased reach and coverage scenario that assumes 36% true coverage in the undervaccinated subpopulations for both Pakistan and Afghanistan from October 2020 (i.e., IRC 36) (Kalkowska et al., 2022). We selected this option as Scenario 1 because as of November 2021, this scenario led to small numbers of estimated WPV1-associated cases in 2021 and led to die out in the deterministic model in 2022, which appeared consistent with epidemiology in late 2021. For this scenario, we focused on characterization of the modeled confidence about no circulation (CNC) as a function of the DEFP in the context of both perfect and imperfect AFP, ES, and combined surveillance for 95% and 99% confidence. As of May 2022, recently reported AFP cases and active ES in both countries indicate ongoing transmission of WPV1 (Kalkowska et al., 2022; World Health Organization, 2022).
Recognizing uncertainty about the epidemiological situations in both countries, in late 2021 we also performed the analysis for a second scenario (Scenario 2) that assumed the same level of programmatic improvement as in Scenario 1 (i.e., 36% true coverage in undervaccinated subpopulations of both Pakistan and Afghanistan), but shifts this improvement such that it began from January 1, 2021 (Kalkowska et al., 2022). This scenario also led to die out of WPV1 transmission in early 2022 (Kalkowska et al., 2022). Both of these scenarios assumed sustained improvements once they began (Kalkowska et al., 2022), and we did not model any scenarios with wavering levels of commitment to improvement (Duintjer Tebbens & Thompson, 2009; Thompson & Duintjer Tebbens, 2007).
3. Results
Figures 1-4 present the model results for Scenario 1 of the CNC as a function of DEFP with black horizontal lines at the top shown for reference to indicate the 99% (small dots) and 95% (larger dots) confidence levels. For consistent context in all figures, we show the curve that would result from assuming perfect AFP surveillance only as a point of reference. Estimating the CNC as a function of time since the last detected event simply requires quantifying the time period since the last case or positive ES isolate, going across the x-axis to that time and then up to the appropriate curve, and then comparing that point to the location on the y-axis. With the 95% and 99% confidence levels provided for reference, the curves also allow simple identification of the time required to reach those levels of confidence. Prior assessments of CNC focused on AFP cases, largely because these analyses preceded the development of ES systems. In the absence of ES, the time since the last reported case (or case-free period) represents the key observation, but ES adds the potential to instead consider the time since the last event detected by either AFP (i.e., a reported case) or ES (i.e., a positive isolate).
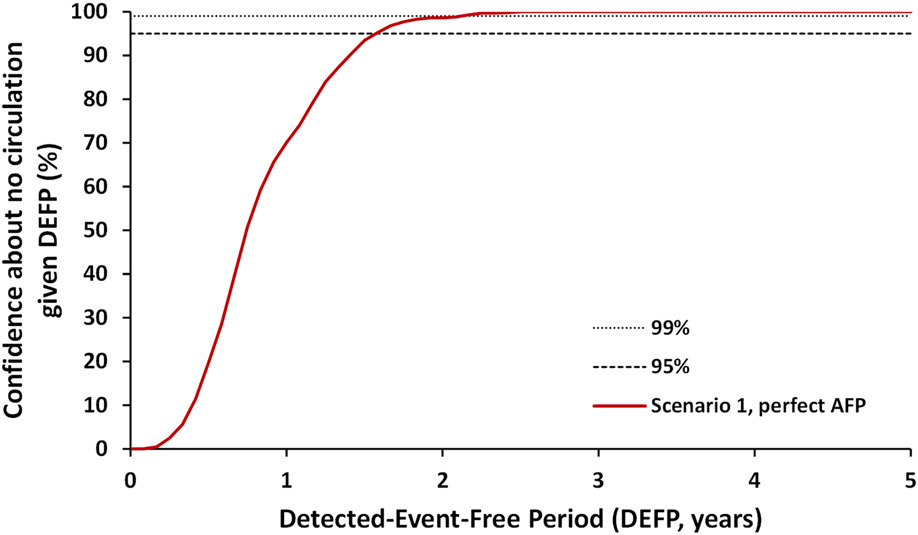
Figure 1:
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event-free period (DEFP) assuming perfect AFP surveillance, and reference lines provided to indicate 95% and 99% confidence for WPV1 for Scenario 1
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; WPV1, serotype 1 wild poliovirus
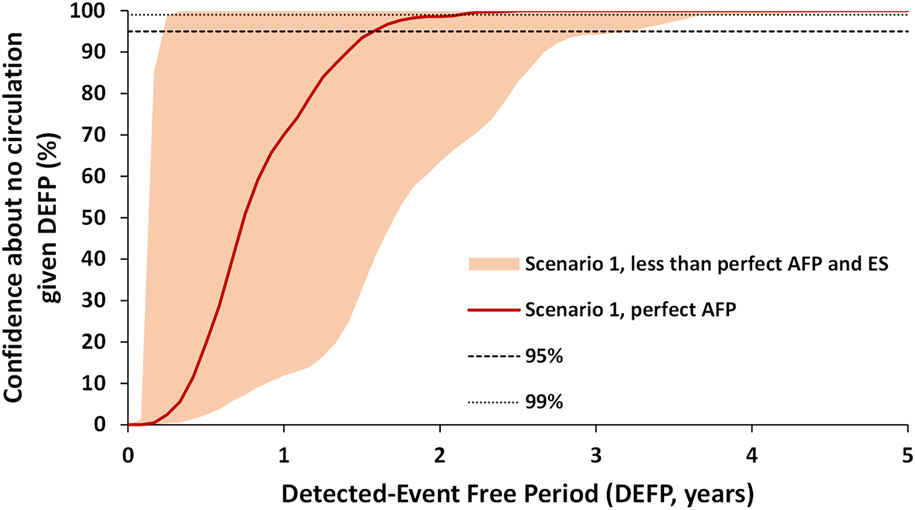
Figure 4:
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event-free period (DEFP) assuming a range of less than perfect AFP surveillance and a range of ES, with perfect AFP surveillance without ES, and reference lines provided to indicate 95% and 99% confidence for WPV1 for Scenario 1
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; ES, environmental surveillance, WPV1, serotype 1 wild poliovirus
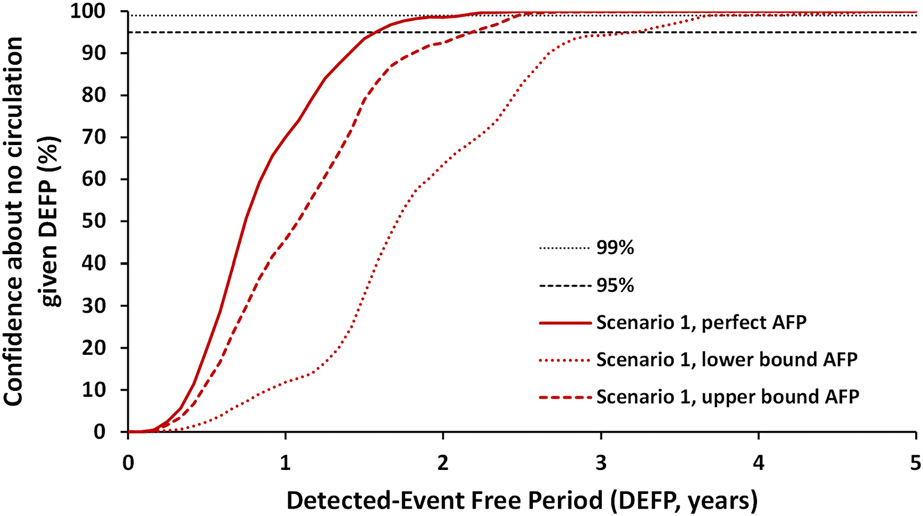
Figure 1 shows the CNC curve for WPV1 given currently assumed levels of population immunity necessary to interrupt WPV1 transmission and assuming perfect AFP surveillance, which is an updated result similar to the “increased relative SIA coverage 0.15” scenario in a 2019 study (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019). Figure 2 explores the uncertainty in CNC as a function of AFP quality only, and shows the extent to which imperfect AFP surveillance pulls the CNC curve to the right implying longer times to reach high confidence. Figure 2 shows the CNC curves for imperfect AFP surveillance for the lower and the upper bound assumptions for the undervaccinated subpopulations (Table 1, in both bounds, the assumptions of imperfect AFP surveillance in the general population remain the same). Figure 2 shows that poor AFP surveillance quality in inaccessible areas may result in more than 1.5 years longer required DEFPs to obtain equal levels of CNC compared to perfect AFP. As shown in Figure 2, however, even with the worst performing AFP surveillance sensitivity modeled (and no ES), the model anticipates high confidence (CNC95%) of WPV1 eradication with 3 years of no reported AFP cases. The relevant bounding curve depends on the “weakest link,” such that whichever country performs worse drives the rightmost and relevant location of the CNC curve.
Figure 2:
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event-free period (DEFP) assuming different estimates of less than perfect AFP surveillance, with perfect AFP surveillance without ES, and reference lines provided to indicate 95% and 99% confidence for WPV1 for Scenario 1
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; ES, environmental surveillance, WPV1, serotype 1 wild poliovirus
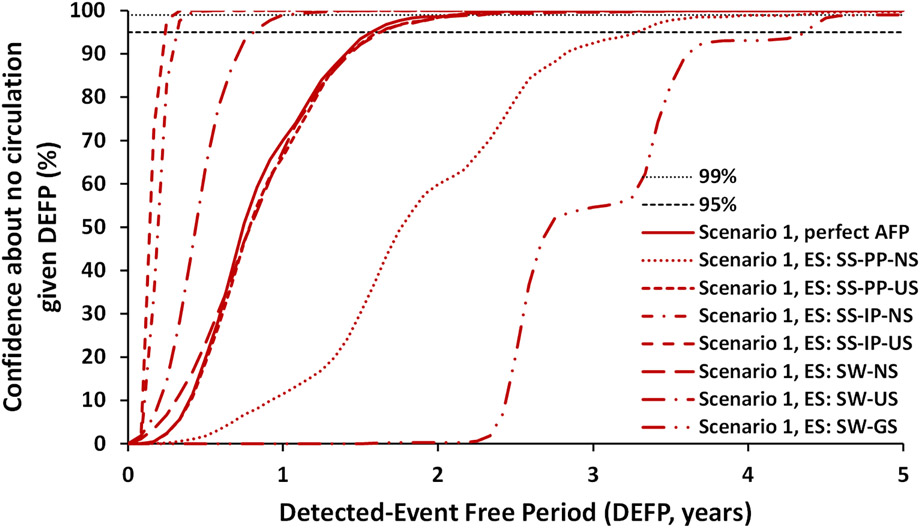
Figure 3 compares the CNC curves for ES alone using the different approaches, but includes the perfect AFP only surveillance curve for reference. Figure 3 shows only one of the GS methods (i.e., SW-GS, no observable difference compared to SS-PP-GS or SS-IP-US approach, since most of the transmission takes place in the undervaccinated subpopulations). Figure 3 demonstrates a range of possible CNC estimates based on our understanding of the ES system in Pakistan and Afghanistan and corresponding different approaches to model the system and to allocate observed prevalence to the sampling sites (Kalkowska, Duintjer Tebbens, & Thompson, 2019). Figure 3 suggests that the SS approach continues to perform better than the SW approach (since in the SS approach detection by the system on a given scheduled sampling day requires detection by just one of the active sampling sites), while both approaches continue to perform best with sites located in the undervaccinated subpopulations, in which poliovirus circulates the most at the time of sampling, see similar results shown in an earlier study (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019).
Figure 3:
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event-free period (DEFP) assuming different ES approaches compared to perfect AFP surveillance without ES, and reference lines provided to indicate 95% and 99% confidence for WPV1 for Scenario 1
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; ES, environmental surveillance; GS, general population sites distribution; IP, isolation-rate based prevalence; NS, national sites distribution; PP, proportional prevalence; SS, site-specific; SW, system-wide; US, undervaccinated subpopulation sites distribution, WPV1, serotype 1 wild poliovirus
Figure 4 shows the space of possible outcomes following the joint effect of the range of imperfect AFP surveillance and the spectrum of the investigated ES approaches. The lower bound of the space in Figure 4 corresponds to the worst performing AFP surveillance sensitivity, which reflects the assumption of not doing worse than the worst AFP surveillance sensitivity independent of the quality of ES. The upper bound curve represents the upper bounds of AFP and the best performing ES.
Table 2 reports the POE, CNC95%, CNC99%, TUC95% and TUC99% estimates assuming perfect AFP surveillance only and with either the worst or best modeled estimates of actual, imperfect surveillance quality. The model suggests that with perfect AFP only (no ES), achieving 95% CNC about the interruption of WPV1 transmission requires 1.6 years without any WPV1 cases (Table 2). Depending on the amount of poliovirus excreted into the sewage system and the quality of sampling sites at detecting those viruses, good quality ES used in addition to a good quality AFP surveillance could reduce the CNC95%. For example, the best performing ES can reduce the DEFP required by 16 months compared to perfect AFP surveillance alone, and by up to 36 months compared to the worst performing modeled AFP surveillance alone (Table 2). This interval changes depending on the quality of the AFP surveillance and sensitivity of ES.
Table 2:
Expected detected-event-free period (DEFP) required for 95% and 99% confidence about no circulation (CNCx%) and time of undetected circulation between the last paralytic case and die-out (TUCx%) in Pakistan and Afghanistan assuming perfect and imperfect surveillance (based on 1,000 iterations)
| Scenario 1 | Scenario 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| POE | 100.0% | 99.9% | ||||||
| Metric | CNCx% | TUCx% | CNCx% | TUCx% | ||||
| x% | 95% | 99% | 95% | 99% | 95% | 99% | 95% | 99% |
| DEFP values assuming perfect AFP surveillance without ES | 1.58 | 2.17 | 1.49 | 1.98 | 1.50 | 2.08 | 1.45 | 2.08 |
| DEFP values assuming worst performing surveillance (worst AFP surveillance sensitivity and no ES) | 3.25 | 3.75 | 3.19 | 3.74 | 2.67 | 3.25 | 2.58 | 3.24 |
| DEFP values assuming best performing surveillance (AFP with best ES sensitivity) | 0.25 | 0.33 | 0.20 | 0.25 | 0.25 | 0.25 | 0.18 | 0.23 |
Abbreviations: AFP, acute flaccid paralysis; CNCx%, DEFP at which the confidence about no circulation exceeds x%; cVDPV, circulating vaccine-derived poliovirus; DEFP, detected-event-free period; ES, environmental surveillance; PIR, paralysis-to-infection ratio; POE, probability of eradication; TUCx%, time at which the probability of undetected WPV circulation after the true last case becomes exceeds x%; WPV1, serotype 1 wild poliovirus.
For Scenario 2, Figure 5 shows the comparable results to those shown in Figure 4 for Scenario 1. The 3-month later shift in coverage improvement of Scenario 2 (relative to Scenario 1) leads to lower population immunity to WPV1 transmission and more WPV1 cases with shorter times between cases, which implies shorter times required to reach confidence about no undetected circulation. The reduction in DEFP for the lower bound curve for Scenario 2 compared to Scenario 1 translates to reaching a CNC95% approximately 7 months earlier than in the lower bound of Scenario 1 (Table 2). This occurs, because increased occurrence of cases implies more chances to detect cases even with the worst performing AFP surveillance sensitivity.
Figure 5:
Confidence about no circulation in Pakistan and Afghanistan as a function of the detected-event-free period (DEFP) assuming a range of less than perfect AFP surveillance and a range of ES, with perfect AFP surveillance without ES, and reference lines provided to indicate 95% and 99% confidence for WPV1 for Scenario 2
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; ES, environmental surveillance, WPV1, serotype 1 wild poliovirus
4. Discussion
As the EMRCC and GCC consider the evidence relevant to certifying the region and world, respectively, as free of indigenous WPV1 transmission, these results show that a 3-year period with no cases in the context of continued AFP surveillance alone supports certification with high confidence. If the EMRCC and GCC assess the quality of the AFP surveillance system as high in all areas or very high overall, then this could reduce the time with no observed cases AFP cases to 2 years to reach high confidence considering only AFP. Our analysis suggests that if assessments support assumptions of good AFP surveillance sensitivity, then the DEFP could shift closer to 2-2.5 years for both countries based on AFP alone (see the upper bound curve in Figure 2).
We also provide a bounding analysis that can support GCC deliberations as it considers both AFP and ES information for Pakistan and Afghanistan, which will raise questions about how to consider the information from both parts of the polio surveillance systems in these countries. Given the existence of ES in Pakistan and Afghanistan, the EMRCC and GCC may benefit from ES information, and this may support some reduction in the DEFP required to achieve high CNC. The GCC may find it useful to separately consider the value of the different types of surveillance information, and this analysis supports that by providing separate discussions of AFP and ES (Figures 2 and 3). For example, characterization of the quality of AFP surveillance may prove useful with respect to considering the likely trajectory for CNC as a function of DEFP based on the quality of AFP only. Then, consideration of the information from ES may help to inform the extent to which the combined CNC as a function of DEFP could shift the curve to the left (toward shorter times). The information provided by ES provides an opportunity to potentially shift the curve over toward shorter DEFPs, but counting the DEFP would need to begin at the time of the last positive isolate, which would likely occur after the last reported case.
As shown in several prior analyses (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019), population immunity to transmission, which depends on type, quality, and scope of activities, and the quality of surveillance both substantially impact confidence. Given that IPV protects individuals from paralysis but does not prevent infection, immunization with IPV can reduce the ability to detect transmission of polioviruses with AFP surveillance alone without stopping transmission (see (Thompson & Kalkowska, 2020, Table 4) for modeling studies that mentioned “silent transmission on an IPV background and/or delayed detection of transmission due to IPV use” and the “role of IPV after OPV cessation”). Notably, the extensive ES system in Israel detected WPV1 importations and transmission that did not result in any AFP cases due to the high immunization IPV coverage and intensive response to the detection (Anis et al., 2013; Kalkowska, Duintjer Tebbens, Grotto, et al., 2015). The relatively low coverage with IPV in Pakistan and Afghanistan limits the extent to which IPV delays the detection of cases by AFP surveillance, but the use of IPV in both countries may increasingly mask some transmission. Our modeling includes immunization with IPV in both countries at current levels. Immunization coverage remains uneven in both countries, with some areas not accessible for polio immunization or surveillance activities, leading to uncertainty about immunity and surveillance levels in some areas. The extent of interactions with hard-to-reach populations impacts their role in certification (Duintjer Tebbens et al., 2019). The importance of continuing to maintain both intensive OPV1-containing SIAs and AFP surveillance and ES despite apparent or potential die out may present challenges in both countries (potentially for different reasons), but should remain a priority with respect to supporting the certification of WPV1 eradication. Counterintuitively, IPV immunization may extend the time required to observe AFP cases, and increase dependence on ES.
Although not generally appreciated, some individuals infected with WPV1 may continue to excrete the virus for an extended period of time (i.e., more than 60 days) prior to clearing their infections. The time delay between the last reported case and the disappearance of signals from ES depends on many factors, including prolonged excretion and continued silent transmission. The relatively recent expansion of ES limited its prior use in national, regional, and global certification decisions. However, sensitive ES may detect transmission after the last case, because of the time taken to fully clear the virus. Prior experience with detection of polioviruses by ES after die out or cessation of use should prove helpful with respect to communicating this point (Blomqvist et al., 2012; Huang et al., 2005; World Health Organization, 2004), but the relevant time for Pakistan and Afghanistan will depend on the specific conditions in that reservoir and the locations of ES sites (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019).
ES coverage remains limited to relatively small geographies (Duintjer Tebbens, Zimmermann, Pallansch, & Thompson, 2017). For Pakistan and Afghanistan, we estimate that ES may cover a small fraction of the total population with periodic sampling (e.g., on the order of 3-4%, see Table A1(c)), although we remain uncertain due to limitations of the available information about catchment areas. The distribution of sampling sites relative to where transmission occurs remains a substantial source of uncertainty (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019). If the ES provides good information about transmission (or lack thereof) in undervaccinated populations, then this may increase confidence and support a lower DEFP required to achieve high CNC. However, if the ES mainly provides no evidence of transmission in areas unlikely to experience transmission, then placing too much weight on ES could lead to a false sense of security or greater confidence than warranted.
Our results come with the same limitations as prior similar modeling (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019). In particular, the ability to estimate confidence about no undetected circulation depends on the occurrence of the transmission die-out, for which we use a transmission threshold criterion rather than absolute 0 total infected individuals. Thus, our model represents a simplified construct of the complex dynamics of transmission die-out. As shown in Figure 3, the results of the model remain sensitive to the chosen ES approach and the assumptions about the site distribution, but also depend on the information quality about existing ES sampling sites themselves (see Appendix A1). Since the available data on catchment population of ES sampling sites still remain incomplete (see appendix and compare it with similar earlier analyses (Kalkowska, Duintjer Tebbens, Pallansch, et al., 2019; Kalkowska, Duintjer Tebbens, & Thompson, 2019)), we continue to assume country average catchment population in place of missing catchment estimates. Therefore, we may misrepresent the true catchment population for some sites, and our model remains limited with respect to its representation of the ES data from Pakistan and Afghanistan.
In depth reviews of surveillance quality (for both AFP and ES) and intensified efforts to look for any indications of children missed by surveillance in the recent past would help to support potential consideration of a shorter DEFP to certify WPV1 eradication. The challenges and situations in both countries differ, and perceptions of WPV1 dying out as the time since the last reported AFP case increases may make investment in further intensive efforts difficult. However, if Pakistan and Afghanistan can both contemporaneously achieve and maintain sufficiently high population immunity for WPV1 and achieve good quality surveillance, then WPV1 certification appears possible within the next couple of years based on this modeling.
5. Conclusion
The time required to achieve high confidence of no circulation of WPV1 will depend on the degree to which polio immunization activities increase and maintain high population immunity to transmission, and on the quality of the surveillance data in Pakistan and Afghanistan. However, modeling supports the potential use of a criterion of less than 3 years since the last reported AFP case for certification of global WPV1 eradication, and may support accelerated timelines for EMRCC and GCC deliberations.
Supplementary Material
Acknowledgments
We acknowledge support for this publication under Cooperative Agreement Number 5NU2RGH001915-01-00 funded by the Centers for Disease Control and Prevention. The views expressed are solely those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or Department of Health and Human Services. We thank Richard Franka, Mark Pallansch, and Steven Wassilak for helpful information and discussions.
References
- Anis E, Kopel E, Singer S, Kaliner E, Moerman L, Moran-Gilad J, … Grotto I (2013). Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveillance, 18(38), 20586. [DOI] [PubMed] [Google Scholar]
- Blomqvist S, El Bassioni L, El Maamoon Nasr EM, Paananen A, Kaijalainen S, Asghar H, … Roivainen M (2012). Detection of imported wild polioviruses and of vaccine-derived polioviruses by environmental surveillance in Egypt. Appl Environ Microbiol, 78(15), 5406–5409. doi: 10.1128/aem.00491-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, & Thompson KM (2019). Global certification of wild poliovirus eradication: insights from modelling hard-to-reach subpopulations and confidence about the absence of transmission. BMJ Open, 9(1), e023938. doi: 10.1136/bmjopen-2018-023938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Ehrhardt DT, Farag NH, Hadler SC, … Thompson KM (2018). Modeling poliovirus transmission in Pakistan and Afghanistan to inform vaccination strategies in undervaccinated subpopulations. Risk Anal, 38(8), 1701–1717. doi: 10.1111/risa.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2009). Priority shifting and the dynamics of managing eradicable infectious disease. Management Science, 55(4), 650–663. [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2017). Modeling the costs and benefits of temporary recommendations for poliovirus exporting countries to vaccinate international travelers. Vaccine, 35(31), 3823–3833. doi: 10.1016/j.vaccine.2017.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2019). Evaluation of proactive and reactive proactive strategies for polio eradication activities in Pakistan and Afghanistan. Risk Anal, 39(2), 389–401. doi: 10.1111/risa.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Zimmermann M, Pallansch MA, & Thompson KM (2017). Insights from a systematic search for information on designs, costs, and effectiveness of poliovirus environmental surveillance systems. Food Environ Virol, 9(4), 361–382. doi: 10.1007/s12560-017-9314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, & Hadeler KP (1995). Deterministic models for the eradication of poliomyelitis: vaccination with the inactivated (IPV) and attenuated (OPV) polio virus vaccine. Math Biosci, 127(2), 149–166. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Greening G, Baker M, Grimwood K, Hewitt J, Hulston D, … Pallansch MA (2005). Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet, 366(9483), 394–396. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Badizadegan K, & Thompson KM (2022). Modeling scenarios for ending poliovirus transmission in Pakistan and Afghanistan. Risk Anal, Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Grotto I, Shulman LM, Anis E, Wassilak SGF, … Thompson KM (2015). Modeling options to manage type 1 wild poliovirus imported into Israel in 2013. J Infect Dis, 211(11), 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, & Thompson KM (2015). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Implications for surveillance and vaccination. BMC Infect Dis, 15, 66. doi: 10.1186/s12879-015-0791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, & Thompson KM (2019). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Anal, 39(2), 402–413. doi: 10.1111/risa.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2019). Environmental surveillance system characteristics and impacts on confidence about no undetected serotype 1 wild poliovirus circulation. Risk Anal, 39(2), 414–425. doi: 10.1111/risa.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Cochi SL, & Thompson KM (2021). Updated characterization of poliovirus transmission in Pakistan and Afghanistan and the impacts of different outbreak response options. J Infect Dis, 224(9), 1529–1538. doi: 10.1093/infdis/jiab160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, & Thompson KM (2021). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Borno and Yobe in northeast Nigeria. Risk Anal, 41(2), 303–311. doi: 10.1111/risa.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Wassilak SGF, Cochi SL, Pallansch MA, & Thompson KM (2021). Global transmission of live polioviruses: Updated integrated dynamic modeling of the polio endgame. Risk Anal, 41(2), 248–265. doi: 10.1111/risa.13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2007). Eradication versus control for poliomyelitis: an economic analysis. Lancet, 369(9570), 1363–1371. doi: 10.1016/s0140-6736(07)60532-7 [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2020). Review of poliovirus modeling performed from 2000-2019 to support global polio eradication. Expert Rev Vaccines, 19(7), 661–686. doi: 10.1080/14760584.2020.1791093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Kalkowska DA, & Duintjer Tebbens RJ (2015). Managing population immunity to reduce or eliminate the risks of circulation following the importation of live polioviruses Vaccine, 33(3), 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2004). Report on the Second Meeting of the Regional Technical Advisory Group for Poliomyelitis Eradication. Retrieved from https://apps.who.int/iris/bitstream/handle/10665/255054/who_em_pol_251_e_en.pdf
- World Health Organization. (2013, 11 Nov 2013). Polio in the Syrian Arab Republic. Retrieved from http://polioeradication.org/news-post/polio-in-the-syrian-arab-republic/
- World Health Organization. (2019a). Report from the Twentieth Meeting of the Global Commission for Certification of Poliomyelitis Eradication, Geneva, Switzerland, 17-18 October 2019. Retrieved from http://polioeradication.org/wp-content/uploads/2016/07/20th-meeting-of-the-Global-Commission-for-the-Certification-of-Eradication-of-Poliomyelitis-17-18-October-2019.pdf
- World Health Organization. (2019b). Wild poliovirus type 1 - Iran (Islamic Republic of) Retrieved from https://www.who.int/emergencies/disease-outbreak-news/item/24-may-2019-wild-polio-virus-islamic-republic-of-iran-en
- World Health Organization. (2020). Africa kicks out wild polio. Retrieved from https://polioeradication.org/news-post/africa-kicks-out-wild-polio/
- World Health Organization. (2022). Wild poliovirus list: List of wild poliovirus by country and year. Retrieved from https://polioeradication.org/wp-content/uploads/2022/05/weekly-polio-analyses-WPV-20220517.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.