Abstract
Recruitment of neutrophils to lung tissue and airspaces is a hallmark of inflammatory events following inhalation of endotoxins. We studied the role of different lymphocyte subsets in this inflammation, which is assumed to primarily involve the innate immune system. Inhalation of aerosolized Escherichia coli lipopolysaccharide (LPS) in mice induced a dose-dependent increase in neutrophils in bronchoalveolar lavage fluid, reaching a maximum after 12 h at a low dose and after 24 h at a high dose. Profiles of gene expression in lung tissue indicated an early (2 h) and transient onset of proinflammatory cytokines and chemokines by a low dose of LPS, while a high dose caused more delayed and sustained (6 to 12 h) activation. Gamma interferon, interleukin-2 (IL-2), RANTES, and the α chain of the IL-2 receptor were not expressed at a low dose, whereas a high dose of LPS induced a strong expression of these genes, indicating a dose-dependent activation of T cells. A similar pattern was observed for IL-17, supporting a contribution of T cells to the neutrophilic inflammation only at high-dose exposure to LPS. The involvement of lymphocytes in the inflammatory response was further studied using mice with functional deficiencies in defined lymphocyte subsets. Both γδ T-cell- and B-cell-deficient mice displayed a response similar to that of the corresponding wild-type strains. Selective depletion of NK cells by in vivo administration of the pk136 antibody did not significantly affect the recruitment of neutrophils into airspaces. Thus, neither NK cells, B cells, nor γδ T cells appeared to participate in the host response, suggesting that among the lymphocyte subsets, αβ T cells are exclusively involved in endotoxin-induced airway inflammation.
Bacterial endotoxins (lipopolysaccharides [LPS]) are components of the outer membrane which play an important role in the pathogenesis of infections with gram-negative bacteria. It has long been recognized that alveolar macrophages (AM) have a most important function in mediating the effects of LPS entering airways and lungs. Most research in this area has thus focused on the role of macrophages and mediators, e.g., cytokines, released from those cells during the host response. Studies in vivo and in vitro have demonstrated that LPS exerts adjuvant effects on macrophages, resulting in an inflammatory cascade defined by early production of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), followed by subsequent induction of interleukin-1 (IL-1) and IL-6 (7, 33). TNF-α and IL-1 play key roles in inflammatory processes, as indicated by significantly diminished inflammatory responses and lethality by use of anticytokine antibodies and soluble receptors (8, 13, 36) or by using gene knockout (KO) technology (14, 24). However, both synthesis of TNF-α in macrophages and recruitment of inflammatory cells are controlled, at least in part, by T cells, as demonstrated by depletion of CD4+ cells before LPS challenge (10). The decreased TNF-α secretion and inflammatory cell recruitment could be fully restored by pretreatment with the potent macrophage-activating cytokine gamma interferon (IFN-γ), thus indicating the need for IFN-γ-producing cells in the inflammatory response. T-cell release of IFN-γ is reported to occur in the septic state of endotoxemia, and anti-IFN-γ antibody administration enhanced survival through macrophage modulation, although endotoxemia was already established (32). Mattern et al. demonstrated that monocyte-dependent stimulation of human T cells by LPS is not major histocompatibility complex restricted but involves CD80-CD28 interactions and IL-12 secretion (26). This group recently reported a new pathway for LPS-induced human T-cell stimulation, identifying the rare CD34+ stem cell population as a requisite for CD80 expression on monocytes and thus facilitating the CD80-CD28 interaction required for T-cell activation (25). Together these findings indicate a lymphocyte-driven modulation of macrophage effector mechanisms, suggesting a contribution to classical innate immune activation, which may thus proceed independently of specific antigen recognition.
To more thoroughly dissect the influence of different lymphocyte populations on the endotoxin-induced host response, we established a mouse model for acute airway inflammation induced by inhalation of LPS. After defining the dose dependency and time kinetics for cytokine and chemokine expression in lung tissue and the subsequent migration of neutrophils into airspaces, the model was applied with mice deficient in subpopulations of T cells and B cells. The role of NK cells was investigated by in vivo depletion before LPS challenge. The results indicate that a high dose of LPS induces a concordant activation of both T cells and macrophages, while a lower dose of LPS seems to induce a response exclusively dependent on macrophages. B cells, γδ T cells, or NK cells did not contribute to the inflammatory response after LPS exposure.
MATERIALS AND METHODS
Mice.
In dose-response and kinetic studies, C57BL/6JBom mice (M&B A/S, Ry, Denmark) were used. Animals were fed with standard chow and water ad libitium and allowed to acclimatize for at least 7 days. For studies on T- and B-lymphocyte involvement, KO and corresponding wild-type mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and bred in our animal facility. T-cell-receptor-deficient mice lacking either αβ T cells (TCR-β−/−), γδ T cells (TCR-δ−/−), or both T cell types (TCR-β−/−δ−/−) were of C57BL/6 origin, while the B-cell-deficient mice (Igh-6tm1Cgn) were of strain C57BL/10. In all experiments mice of ages between 10 and 14 weeks were used. The study was approved by the Regional Animal Research Ethics Committee according to national laws.
Inhalation of LPS.
Animals were exposed for 15 min to an aerosol of LPS (Escherichia coli O128:B12; Sigma, St. Louis, Mo.) using a nose-only Battelle exposure chamber. Aerosols were generated by a compressed-air Collison six-jet nebulizer at an airflow of 7 liters/min, yielding a particle size of 0.1 to 0.3 μm and a lung deposition of approximately 20% (20). After establishing the dose-dependent accumulation of granulocytes in bronchoalveolar lavage fluid (BALF) (range of 1 to 1,000 μg of LPS per ml), further experiments were confined to two levels (low or high) by using two different nebulizer concentrations (100 or 1,000 μg/ml).
Analysis of leukocytes in bronchoalveolar fluid.
At specified times after exposure animals were killed by cervical dislocation, and their tracheae were cannulated with polyethylene tubing. Bronchoalveolar lavage was conducted with 1-ml aliquots of ice-cold Hanks balanced salt solution in a total volume of 4 ml. The fluid was centrifuged at 4°C for 5 min at 250 × g, and the total leukocyte number in BALF was determined using a hemacytometer and Türchs reagents. The number of granulocytes in BALF was determined by flow cytometry analysis using a granulocyte-specific monoclonal antibody (MAb) and by differential counting of cytospin-centrifuged cells stained with May-Grünewald Giemsa stain. The specific staining of granulocytes was performed by incubating 2 × 105 cells (2 × 106 cells/ml) with 2 μl of rat serum and 2 μl (1:5) of Fc block (Pharmingen, San Diego, Calif.) for 5 min and thereafter incubating the cells with 2 μl (1:10) of a fluorescein isothiocyanate-conjugated GR-1 MAb (Ly-6G; Pharmingen) for 30 min at 4°C in the dark. At least 10,000 cells were analyzed with a FACSort (Becton Dickinson, San Jose, Calif.). The number of granulocytes in BALF was determined by analyzing the percentage of positive cells in fluorescence channel 1 (fl.1). A fluorescein isothiocyanate-conjugated isotype-matched control antibody (IgG2b; Pharmingen) typically stained less than 1% of collected cells.
Analysis of cytokine and chemokine and IL-2 receptor mRNA in lung tissue.
Lung tissue was removed 2 h after LPS exposure and immediately frozen in liquid nitrogen. The frozen lung tissue (100 to 200 mg) was homogenized in 2 ml of TRIzol (GIBCO BRL, Gaithersburg, Md.). Total cytoplasmic RNA was prepared according to the manufacturer's instructions. The purified RNA was quantified spectrophotometrically by measuring the absorbance at 260 nm. First-strand cDNA synthesis of mRNA was performed by using oligo(dT) primers. In brief, 3 μg of heat-denatured RNA (70°C for 10 min) was subjected to cDNA synthesis in a standardized buffer system (reverse transcriptase [RT] buffer; GIBCO BRL) including deoxynucleotides (dATP, dCTP, dGTP, and dTTP), dithiothreitol, the cDNA primer, and RT (Superscript; GIBCO BRL). The reaction was conducted at 42°C for 60 min. PCR amplification of cDNA was performed using oligonucleotide primers specific for the genes for TNF-α (Clontech, Palo Alto, Calif.), IL-1α (2), IL-1β (12), IL-2 (12), IL-4 (12), IL-5 (5′GGCTTCCTGTCCCTACTCATAA3′ and 5′CTTCCATTGCCCACTCTGTA3′), IL-6 (12), IL-10 (12), IL-12 p40 chain (41), IL-17 (5′GGTCAACCTCAAAGTCTTTAACTC3′ and 5′TTAAAAATGCAAGTAAGTTTGCTG3′), IL-18 (29), IFN-γ (Clontech), RANTES (37), macrophage inflammatory protein 1α (MIP-1α) (37), MIP-2 (37), monocyte chemoattractant protein 1 (MCP-1) (37), MCP-3 (6), eotaxin (22), the α chain of the IL-2 receptor (IL-2Rα) (12), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (9). Two microliters of cDNA was added to a 23-μl PCR mixture consisting of 1× GenAmp buffer (Perkin-Elmer, Norwalk, Conn.), 1.5 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate, 0.4 μM sense and antisense primers, and 0.025 U of Taq polymerase (AmpliTaq Gold; Perkin-Elmer) per μl. The PCR profile used was the following: denaturation at 94°C for 30 s, annealing at 60°C for 30 s (annealing for IL-17 was at 50°C), and extension at 72°C for 60 s. The optimal number of amplification cycles for each primer pair was carefully monitored to ensure that the reaction was not run to completion (TNF-α, 28 cycles; IL-1α, 28 cycles; IL-1β, 26 to 28 cycles; IL-2, 35 cycles; IL-5, 32 cycles; IL-6, 30 cycles; IL-10, 35 cycles; IL-12, 30 cycles; IL-17, 35 cycles; IL-18, 24 cycles; IFN-γ, 30 cycles; RANTES, 22 cycles; MIP-1α, 26 cycles; MIP-2, 28 cycles; MCP-1, 28 cycles; MCP-3, 28 cycles; eotaxin, 26 cycles; IL-2Rα, 30 cycles; and GAPDH, 20 to 21 cycles). Negative controls were included in each experiment to ensure that the reagents were free of contamination. PCR products were electrophoresed in 1.5% agarose gels (180 V for 45 min) and stained with ethidium bromide. Each PCR product yielded a single band corresponding to the expected fragment size (ranging between 148 and 620 bp). The signal intensity of each band was scanned using an image analyzing system. A ratio was calculated by dividing the cytokine and chemokine signals by the signal from the amplified housekeeping gene that encodes GAPDH.
In vivo depletion of NK1.1+ cells.
Depletion of NK1.1+ cells in wild-type and TCR-αβ/γδ double KO mice was performed by intraperitoneal (i.p.) injection of 200 μg (400 μl) of anti-NK1.1 MAb (pk136) 2 days before LPS challenge. The purified pk136 MAb was kindly provided by Hans-Gustaf Ljunggren, Karolinska Institute, Stockholm, Sweden. The efficacy of depletion was confirmed by fluorescence-activated cell sorter analysis of NK1.1+ cells in BALF and spleen from both strains of animals. A control group of mice received 400 μl of solvent alone (phosphate-buffered saline [PBS]).
Statistical analysis.
All statistical comparisons were performed by analysis of variances. If differences were significant (P < 0.05, two-tailed), this was followed by Dunnett's multiple-comparison test. The data presented in Results are means ± standard errors of the means (SEM) for all results.
RESULTS
Dose response of inhaled LPS.
Inhalation of LPS induced a dose-dependent accumulation of leukocytes in BALF (Fig. 1). At nebulizer concentrations of 100 and 1,000 μg/ml the number of total leukocytes was significantly increased, and flow cytometric analysis revealed a major contribution of GR-1-positive cells among the accumulated cells. Since the GR-1 MAb binds to both neutrophils and eosinophils, we performed a standard staining of cytospin preparations which confirmed a predominant neutrophil influx into airways during the inflammatory response. In subsequent experiments, the 100- and 1,000-μg/ml concentrations of LPS were used, referred to as low and high dose, respectively.
FIG. 1.
Dose-dependent induction of airway inflammation by aerosolized LPS. Withdrawal and analysis of BALF was performed 16 h after LPS exposure. The total number of leukocytes was determined by cell counting, and the proportion of granulocytes was analyzed by flow cytometric straining using the GR-1 MAb. Control mice (0 μg of LPS per ml) were exposed to an aerosol of solvent alone (endotoxin-free distilled water). The leukocytes recovered from these mice were predominantly alveolar macrophages (>95%), with a proportion of granulocytes generally less than 5%. At nebulizer concentrations of 100 and 1,000 μg/ml, the numbers of granulocytes and total leukocytes were significantly increased in BALF compared to that of animals not exposed to LPS. ∗∗, P < 0.01; ∗∗∗, P < 0.001. Mean values and SEM are shown (three to five animals in each group).
Kinetics of the inflammatory response.
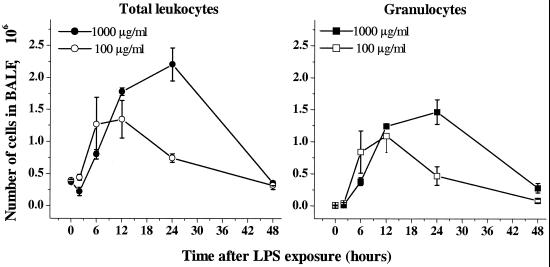
High-dose LPS provoked a more severe and sustained inflammation than low-dose LPS (Fig. 2). The two doses of LPS did not differ significantly in granulocyte accumulation during the first 12 h. However, at the high dose, granulocytes continued to accumulate for a further 12 h, while low-dosed animals started resolving inflammation 12 h after LPS exposure. The total number of leukocytes was significantly decreased 2 h after high-dose exposure (P < 0.05 versus untreated animals), followed by a somewhat delayed increase in cell number compared to low-dose LPS. The BALF leukocyte numbers were nearly normalized to preexposure levels within 48 h for both doses.
FIG. 2.
Kinetics for the inflammatory response after inhalation of low-dose (100 μg/ml) and high-dose (1,000 μg/ml) LPS. The total number of leukocytes in BALF was determined by cell counting, and the number of granulocytes was determined by flow cytometry staining using the GR-1 MAb. Mean values and SEM are shown (four animals in each group).
Profiles of cytokine and chemokine and IL-2Rα mRNA in lung tissue.
By using a panel of sequence-specific primers in a semiquantitative RT-PCR assay, we determined the kinetics of gene expression for defined chemokines, cytokines, and IL-2Rα in lung tissue after inhalation of LPS.
Low-dose LPS induced an early (2 h after LPS exposure) and transient onset of genes for all chemokines analyzed (MIP-1α, MIP-1β, MIP-2, MCP-1, and MCP-3), except RANTES and eotaxin (Fig. 3a). A similar pattern was observed for the proinflammatory cytokines generally associated with early macrophage activation, TNF-α, IL-1α, IL-1β, and IL-6 (Fig. 3c), but not for the p40 chain of the T- and NK-cell-activating cytokine IL-12 (IL-12 p40) or the lymphocyte-derived cytokines (IFN-γ, IL-2, and IL-5) (Fig. 3e). The IL-2Rα mRNA was not induced at low-dose exposure to LPS (Fig. 3e).
FIG. 3.
Expression profiles for chemokines (a and b), proinflammatory cytokines (c and d), and immunoregulatory cytokines and IL-2Rα (e and f) after exposure to low-dose (a, c, and e) and high-dose (b, d, and f) LPS. Expression of mRNA was semiquantitatively analyzed in lung tissue at different time points after LPS exposure. The relative amount of each transcript is indicated in relation to the corresponding amount of the housekeeping gene that encodes GAPDH.
At high-dose exposure we recorded a strong induction of all chemokines, including RANTES and eotaxin (Fig. 3b), all proinflammatory cytokines, including IL-12 p40 (Fig. 3d), and the immunosuppressive cytokine IL-10 (Fig. 3f). The mRNA expression was somewhat delayed and more sustained compared to that at the low-dose exposure. The depressed induction of cytokine and chemokine mRNA 2 h after high-dose exposure was confirmed in a repeated experiment (data not included).
Inhalation of high-dose LPS induced mRNA expression of the lymphocyte-derived cytokines IFN-γ, IL-2, and IL-17, as well as IL-2Rα, a marker for activated T cells (Fig. 3f). LPS-induced expression of IL-5, IL-18 (Fig. 3f), or IL-4 (data not included) was not observed, at either high- or low-dose exposure. In a separate dose-response experiment, we confirmed that proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-12 p40) and the markers for T-cell activation (IFN-γ, IL-2, and IL-2Rα) were strongly expressed in the late phase (20 h) of the inflammatory process only after high-dose exposure (data not included). The results from the cytokine analyses are summarized in Table 1.
TABLE 1.
Grouping of chemokines, cytokines, and IL-2Rα based on the expression profiles of mRNA in lung tissue following low- and high-dose exposure to aerosolized LPS
| Expression pattern | Chemokines | Cytokines |
|---|---|---|
| Early and transient expression at low dose, delayed and sustained expression at high dose | MIP-1α, MIP-1β, MIP-2, MCP-1, MCP-3 | IL-1α, IL-1β, IL-6, TNF-α |
| No detectable expression at low dose, strong expression at high dose | RANTES, eotaxin | IL-2, IL-10, IL-12 (p40), IL-17, IFN-γa |
| No detectable expression at either high or low dose | IL-4, IL-5, IL-18 |
IL-2Rα was also present with this expression pattern.
The inflammatory response in γδ T-cell- and B-cell-deficient mice.
The observation that high-dose LPS induced expression of genes for lymphocyte-derived cytokines prompted studies aimed at defining the roles of lymphocyte subsets during acute inflammation. Mice deficient in γδ T cells (TCR-δ−/−) were exposed to low and high doses of aerosolized LPS. The response in γδ T-cell-deficient mice was similar to that of wild-type controls at both doses (Fig. 4).
FIG. 4.
The inflammatory response in KO mice lacking γδ T cells. The total number of granulocytes in BALF was analyzed 16 h after LPS exposure. The response did not differ significantly between the TCR-δ−/− and the corresponding wild-type strain, at either low- or high-dose exposure. Mean values and SEM are shown (three to five animals in each group).
The role of B cells in LPS-induced airway inflammation was studied by comparing the response in Igh-6tm1Cgn mutant mice lacking functional B cells with that in wild-type mice with an identical genetic background (C57BL/10). Inhalation of LPS evoked a dose-dependent accumulation of granulocytes in BALF which was similar in the two strains. Although the inflammatory response tended to be weaker in the B-cell KO strain (Fig. 5), this difference was not statistically significant and could not be reproduced in a second experiment.
FIG. 5.
The inflammatory response in B-cell-deficient mice. The total number of granulocytes in BALF was analyzed 16 h after LPS exposure in B-cell KO mice (the Igh-6tm1Cgn strain) and in the corresponding wild-type strain (C57BL/10). The response did not differ significantly between these two strains, at either low- or high-dose exposure. Unexposed animals from the B-cell KO strain did not display detectable airway inflammation (not shown). Mean values and SEM are shown (three to five animals in each group).
Depletion of NK cells in T-cell-deficient and wild-type mice.
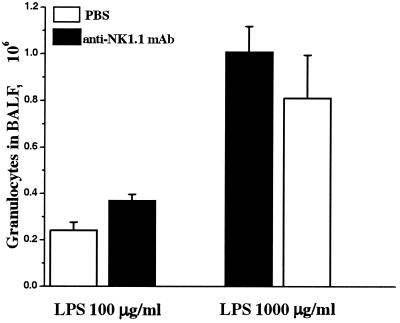
In order to investigate the role of NK cells in acute airway inflammation, we performed a depletion of this lymphocyte subset by injection of the pk136 MAb before exposure to LPS. The pk136 MAb binds to the NK1.1 receptor expressed on NK cells but may also bind to and activate a subset of T cells (NK T cells) in vivo (3). To exclude a hypothetical interference of activated NK1.1+ T cells, the experiment was performed with wild-type C57BL/6 and T-cell-deficient (TCR-β−/−δ−/−) mice. Injection of 200 μg of pk136 i.p. 2 days before LPS exposure completely eliminated the NK1.1−/− CD3− population in BALF (Fig. 6) and spleen (data not shown), confirming the ability of the MAb to deplete NK cells. However, depletion of NK cells did not affect the accumulation of granulocytes in BALF after inhalation of LPS in either wild-type (Fig. 7) or T-cell-deficient (data not included) mice.
FIG. 6.
Depletion of NK cells in lungs by treatment with the pk136 MAb. Mice were injected i.p. either with 200 μg of pk136 or with solvent alone (PBS) 2 days before LPS exposure. Withdrawal of cells in airspaces was performed 16 h after inhalation of LPS. Flow cytometric staining of NK cells in BALF from untreated wild-type C57BL/6 (a) and TCR-αβ/γδ double KO mice (c) is illustrated. Data for mice treated with the pk136 MAb are shown in panels b (wild type) and d (TCR-αβ/γδ double KO). Gates were set for lymphocytes in forward scatter and side scatter, and within the lymphocyte population CD3-negative cells expressing NK1.1 were identified as NK cells (upper left quadrant). In BALF of T-cell-deficient mice treated with pk136, only scarce lymphocytes were found, in contrast to the large number of lymphocytes, predominantly NK cells, found in untreated mice. The proportion of NK cells in BALF was smaller in wild-type C57BL/6 mice than in T-cell KO mice (<1% of total BALF leukocytes, compared to about 5% in TCR-β−/−δ−/− mice). FITC, fluorescein isothiocyanate. PE, phycoerythrin.
FIG. 7.
Unaffected airway inflammation in mice depleted of NK cells. C57BL/6 mice were injected i.p. with 200 μg of anti-NK1.1 MAb (pk136) and 2 days thereafter were exposed to aerosolized LPS at a high or low dose. Withdrawal of BALF cells was performed 16 h after inhalation of LPS. The number of granulocytes in BALF did not differ significantly between the groups of NK-cell-depleted mice and the corresponding groups of control mice injected with solvent alone (PBS). Mean values and SEM are shown (four to five animals in each group).
DISCUSSION
In this study we utilized a mouse model for airway inflammation induced by inhalation of an aerosol of E. coli LPS in order to define early events in the inflammatory cascade, including kinetics and dose dependency of cytokine and chemokine expression in target tissue. We also investigated the roles of different lymphocyte subsets in the endotoxin-induced inflammation, which is assumed to primarily involve the innate immune system. The two concentrations of LPS, 100 and 1,000 μg/ml, caused a significant dose-dependent inflammatory response characterized by a massive recruitment of neutrophils into the airway lumen, reaching a maximum 12 and 24 h, respectively, after exposure. These two nebulizer concentrations, referred to as the low and high doses, respectively, could be calculated to give a lung burden of approximately 0.2 and 2 μg/mouse, assuming 20% lung deposition of aerosol particles. The terms low and high dose were defined by the host response, i.e., the lower dose induced a fast and transient neutrophilic airway inflammation, while the higher dose provoked an exaggerated and more sustained response. The mRNA levels in lung tissue of the proinflammatory cytokines TNF-α, IL-1α, IL-1β, and IL-6 reached a maximum 2 h after administration of low-dose LPS, while at the high dose, quite the opposite to what was expected, the onsets of gene activation were delayed and did not reach a maximum until 12 h after exposure. Concurrent with depressed cytokine induction 2 h after administration of high-dose LPS, the recovered numbers of BALF cells were significantly decreased compared to those of untreated animals. At the same time point, as evidenced by cytospin preparations, more than 95% of the BALF cells had macrophage morphology, indicating that the reduced BALF cell recovery could be accounted for either by an actual reduction of macrophages in the alveolar region or by increased adhesion of existing cells to the alveolar epithelium, as has been reported to occur after LPS stimulation (11, 18). However, since the analysis of cytokine mRNA was performed in tissue samples containing both adherent and nonadherent alveolar cells, the delayed gene expression at high-dose exposure cannot solely be explained by an increased adherence of AM. Instead, the reduced BALF cell numbers and depressed induction of cytokine mRNA in lung tissue after 2 h suggest that the LPS-induced adhesion of AM to alveolar structures is accompanied by apoptosis or necrosis. The time needed for recruitment of new cells to restore and fulfill the inflammatory process could thus explain the delayed inflammatory response after administration of high-dose LPS. This scenario is supported by previous studies demonstrating reduction of mononuclear BALF cells between 1 and 6 h after intratracheal administration of LPS to rats (27) and mice (10). In addition, in vitro studies have demonstrated apoptosis of rodent peritoneal macrophages (1, 38) and human AM (5) after high-dose treatment with LPS.
Induction of chemokine genes followed to a large extent that of proinflammatory cytokines, i.e., a fast and transient increase in mRNA levels at the low dose, with a peak expression 2 h after LPS exposure and a delayed peak response at the high dose. This pattern of expression was observed for the C-X-C chemokine MIP-2 and for the C-C chemokines MIP-1α, MIP-1β, MCP-1, and MCP-3. MIP-2 is an important chemoattractant for granulocytes, previously reported to be required for the full recruitment of neutrophils into the rat lung following LPS challenge (35), while the C-C group of chemokines are chemoattractants for a broader range of leukocytes, including monocytes, T and NK cells, eosinophils, and, to some extent, neutrophils (31). In contrast, the C-C chemokine RANTES, which is largely produced by activated T cells (34), was not detected after low-dose exposure to LPS, but a 10-fold increase of the LPS dose induced a strong expression with a peak level of mRNA 12 h after exposure. Taken together, the pattern of chemokine expression at low-dose exposure to LPS is compatible with a fast and transient activation of primarily AM, causing a short-lived neutrophilic inflammation in airspaces. The dose-dependent induction of RANTES indicates a contributory activity of T cells only at high-dose exposure to LPS. This hypothesis was supported by our data for the expression of immunoregulatory cytokines predominantly produced by T cells (IL-2 and IFN-γ) and IL-2Rα, which is a marker for activated T cells. mRNA of neither the lymphocyte-derived cytokines nor IL-2Rα was induced at significant levels after low-dose exposure, while the high dose induced a strong expression with peak levels after 12 h. The strong expression of the TH1-associated cytokine IFN-γ but without induction of the TH2 cytokines IL-4 (data not included) and IL-5 imply a primary contribution of T cells to the inflammatory process by promotion of a type 1 response. Further support of the hypothesis of T-cell activation only after administration of high-dose LPS is our finding of the dose-dependent expression of the inducible p40 chain of IL-12. This cytokine is highly associated with adjuvant-induced activation of the innate immune system, leading to cytotoxic activity and production of IFN-γ by T cells and NK cells (39). IL-18, another cytokine with the capacity to induce IFN-γ, was not detected in lung tissue, however, at either a low or high dose of LPS.
The central role of AM in LPS-induced accumulation of neutrophils into airspaces has previously been demonstrated by transfer of AM from LPS-exposed mice to naive recipients (17) and by depletion of these cells, which markedly reduced both neutrophil recruitment and release of TNF-α in the alveolar space (4). Our results for cytokine expression after high-dose exposure to LPS suggest a key role for macrophage-derived IL-12 in the activation of T cells and also, eventually, of NK cells. That AM are a cellular source of IL-12 was supported by in vitro studies performed in our laboratory, demonstrating a high capacity of AM to express IL-12 p40 mRNA after stimulation with LPS compared to peritoneal macrophages (data not included). The enhanced and prolonged inflammatory response observed in airways after inhalation of high-dose LPS may thus be explained by a significant contribution of activated lymphocytes to the recruitment of neutrophils. In this context, our finding of a strong expression of IL-17 mRNA after high-dose exposure is intriguing. This recently identified T-cell-derived cytokine dose-dependently induces production of IL-8, a major neutrophil chemoattractant in humans, in vitro (40). In addition, it is reported that instillation of human IL-17 in rats significantly and selectively increases the number of neutrophils in the airways (19, 23).
In order to determine the influence of cells belonging to the adaptive immune system in adjuvant-induced airway inflammation, we performed experiments using genetically modified mice with target deletions in the TCR (TCR-β−/−, TCR-δ−/−, and TCR-β−/−δ−/−) or immunoglobulin M (Igh-6tm1Cgn). These mice were deficient in functional αβ T cells, γδ T cells, both T-cell subsets (double KO mice), or B cells, respectively. Since animals lacking αβ or both αβ and γδ T cells are severely immunocompromised, these mice often suffer from infections with opportunistic organisms that may cause pneumonia, e.g., Pneumocystis carinii (15, 16). A health test on the T-cell-deficient strains revealed occurrence of P. carinii in the TCR-β−/− and TCR-β−/−δ−/− strains. Due to the hypothetical risk for a major influence of this infection on the responsiveness to endotoxin, data from the TCR-αβ and TCR-αβ/γδ double-KO mice were excluded from the study. The γδ T-cell- and B-cell-deficient strains were, however, free from P. carinii infection.
Previous studies have demonstrated a contribution of γδ T cells to airway inflammation, but interestingly the influence seems to be limited to the recruitment of eosinophils into airspaces. γδ T cells are reported to be required for LPS-induced eosinophil migration into the mouse pleural cavity after intrathoracic injection of LPS (30). In a model of airway inflammation provoked by sensitization to ovalbumin, we observed a significant diminished eosinophilia in TCR-δ−/− mice compared to the wild-type strain (data not included), an observation also reported by others (42). The mechanism for the γδ T-cell-dependent migration of eosinophils into the lungs remains to be defined, but it is proposed that γδ T cells are directly involved in IL-4 production, thereby contributing to TH2-mediated eosinophilic inflammation (42). Our finding of γδ T-cell-independent inflammation after inhalation of LPS is fully compatible with such a TH2 bias of γδ T cells, since our model appears to be dependent on a type 1, rather than a type 2, response and is consequently dominated by accumulation of neutrophils in airspaces, with only occasional appearance of eosinophils.
The Igh-6tm1Cgn strain, lacking functional B cells, displayed a significantly reduced eosinophila when sensitized to aerosolized ovalbumin compared to the wild-type C57BL/10 mice (our unpublished observation), supporting an important role in airway inflammations that are dependent on a specific immune response against immunized antigens. In contrast, the B-cell-deficient strain did not differ significantly from the corresponding wild-type strain in the number of neutrophils in BALF following exposure to LPS, arguing against a major contribution of β cells to the acute inflammatory response evoked by endotoxin stimulating the innate immune system.
In addition to lymphocytes involved in the antigen-specific immune response (i.e., γδ T cells and B cells), we also investigated the role of NK cells in LPS-induced airway inflammation. Depletion of NK cells was performed in vivo by i.p. injection of the pk136 MAb, recognizing the NK1.1 receptor, before exposure to aerosolized LPS. Administration of this antibody selectively eliminated the NK cells, as demonstrated by the disappearance of NK1.1+ cells in BALF and spleen. Since elimination of NK cells did not influence the inflammatory response, we conclude that the recruitment of neutrophils into airspaces proceeds independently of NK cells. In contrast to our results, Korsgren et al. reported that depletion of NK cells by the pk136 MAb inhibits allergic eosinophilic airway inflammation (21). Interestingly, these authors demonstrated that the effect of NK cells is exerted during the primary phase of immunization with allergen and not during the subsequent aerosol challenge. Thus, it appears that NK cells play a major role in the initiation of the (TH2-dependent) immune response and do not directly participate in the recruitment of inflammatory cells into the airways.
In summary, we demonstrated a dose-dependent involvement of lymphocytes in endotoxin-induced airway inflammation. At low-dose exposure to LPS, the inflammatory response was predominantly driven by macrophages, while at the high dose, activation of both macrophages and lymphocytes was evident. Surprisingly, lymphocyte subsets assumed to be involved in such innate immune activation, i.e., NK cells and γδ T cells, appeared not to participate in the inflammatory process. These data, together with a previous study suggesting a role for CD4+ cells in LPS-induced inflammation (10), indicate an exclusive contribution of CD4+ αβ T cells to the host response.
ACKNOWLEDGMENTS
We thank Thorsten Johansson for technical assistance, Hans-Gustaf Ljunggren for providing us with purified pk136 MAb, and Robert A. Harris for linguistic advice.
REFERENCES
- 1.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 2.Allen R D, Staley T A, Sidman C L. Differential cytokine expression in acute and chronic graft-versus-host-disease. Eur J Immunol. 1993;23:333–337. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- 3.Asea A, Stein-Streilein J. Signalling through NK1.1 triggers NK cells to die but induces NK T cells to produce interleukin-4. Immunology. 1998;93:296–305. doi: 10.1046/j.1365-2567.1998.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg J T, Lee S T, Thepen T, Lee C Y, Tsan M F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 5.Bingisser R, Stey C, Weller M, Groscurth P, Russi E, Frei K. Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am J Respir Cell Mol Biol. 1996;15:64–70. doi: 10.1165/ajrcmb.15.1.8679223. [DOI] [PubMed] [Google Scholar]
- 6.Chensue S W, Warmington K, Ruth J H, Lukacs N, Kunkel S L. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-γ and IL-4 knockout mice. Analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- 7.de Rochemonteix-Galve B, Marchat-Amoruso B, Dayer J-M, Rylander R. Tumor necrosis factor and interleukin-1 activities in free lung cells after single and repeated inhalation of bacterial endotoxin. Infect Immun. 1991;59:3646–3650. doi: 10.1128/iai.59.10.3646-3650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello C A. Interleukin-1 and interleukin-1 receptor antagonist. Nutrition. 1995;11:492–494. [PubMed] [Google Scholar]
- 9.Driscoll K E, Howard B W, Carter J M, Asquith T, Johnston C, Detilleux P, Kunkel S L, Isfort R J. Alpha-quartz-induced chemokine expression by rat lung epithelial cells. Am J Pathol. 1996;149:1627–1637. [PMC free article] [PubMed] [Google Scholar]
- 10.Dsouza N B, Mandujano F J, Nelson S, Summer W R, Shellito J E. CD4(+) T lymphocyte depletion attenuates lipopolysaccharide-induced tumor necrosis factor secretion by alveolar macrophages in the mouse. Lymphokine Cytokine Res. 1994;13:359–366. [PubMed] [Google Scholar]
- 11.Edelman J, Cardozo C, Lesser M. Lipopolysaccharide stimulates alveolar macrophage adherence in vivo and in vitro. Agents Actions. 1989;26:287–291. doi: 10.1007/BF01967292. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers S, Mielke M E A, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 13.Gatti S, Faggioni R, Echtenacher B, Ghezzi P. Role of tumour necrosis factor and reactive oxygen intermediates in lipopolysaccharide-induced pulmonary oedema and lethality. Clin Exp Immunol. 1993;3:456–461. doi: 10.1111/j.1365-2249.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez-Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 15.Hanano R, Kaufmann S H. Immune responses to naturally acquired Pneumocystis carinii in gene disruption mutant mice. Res Immunol. 1998;149:429–435. doi: 10.1016/s0923-2494(98)80766-9. [DOI] [PubMed] [Google Scholar]
- 16.Hanano R, Reifenberg K, Kaufmann S H E. Naturally acquired Pneumocystis carinii pneumonia in gene disruption mutant mice: roles of distinct T-cell populations in infection. Infect Immun. 1996;64:3201–3209. doi: 10.1128/iai.64.8.3201-3209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmsen A G. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano S. Interaction of rat alveolar macrophages with pulmonary epithelial cells following exposure to lipopolysaccharide. Arch Toxicol. 1996;70:230–236. doi: 10.1007/s002040050265. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino H, Lotvall J, Skoogh B E, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- 20.Koch B L, Edvinsson Å A, Koskinen L-O D. Inhalation of substance P and thiorphan: acute toxicity and effects on respiration in conscious guinea pig. J Appl Toxicol. 1999;19:19–23. doi: 10.1002/(sici)1099-1263(199901/02)19:1<19::aid-jat533>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Korsgren M, Persson C G, Sundler F, Bjerke T, Hansson T, Chambers B J, Hong S, Van Kaer L, Ljunggren H G, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;189:553–562. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krzesicki R F, Winterrowd G E, Brashler J R, Hatfield C A, Griffin R L, Fidler S F, Kolbasa K P, Shull K L, Richards I M, Chin J E. Identification of cytokine and adhesion molecule mRNA in murine lung tissue and isolated T cells and eosinophils by semi-quantitative reverse transcriptase-polymerase chain reaction. Am J Respir Cell Mol Biol. 1997;16:693–701. doi: 10.1165/ajrcmb.16.6.9191471. [DOI] [PubMed] [Google Scholar]
- 23.Laan M, Cui Z H, Hoshino H, Lotvall J, Sjostrand M, Gruenert D C, Skoogh B E, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 24.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattern T, Girroleit G, Flad H D, Rietschel E T, Ulmer A J. CD34(+) hematopoietic stem cells exert accessory function in lipopolysaccharide-induced T cell stimulation and CD80 expression on monocytes. J Exp Med. 1999;189:693–700. doi: 10.1084/jem.189.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattern T, Flad H-D, Brade L, Rietschel E T, Ulmer A J. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–3418. [PubMed] [Google Scholar]
- 27.Miller-Larsson A, Runstrom A, Brattsand R. Adrenalectomy permits a late, local TNF-α release in LPS-challenged rat airways. Eur Respir J. 1999;13:1310–1317. [PubMed] [Google Scholar]
- 28.Mombaerts P, Mizoguchi E, Grusby M J, Glimcher L H, Bhan A K, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 29.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T-cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 30.Penido C, Castro-Faria-Neto H C, Larangeira A P, Rosas E C, Ribeiro-dos-Santos R, Bozza P T, Henriques M G. The role of γδ T lymphocytes in lipopolysaccharide-induced eosinophil accumulation into the mouse pleural cavity. J Immunol. 1997;159:853–860. [PubMed] [Google Scholar]
- 31.Proost P, Wuyts A, van Damme J. The role of chemokines in inflammation. Int J Clin Lab Res. 1996;26:211–223. doi: 10.1007/BF02602952. [DOI] [PubMed] [Google Scholar]
- 32.Redmond H P, Chavin K D, Bromberg J S, Daly J M. Inhibition of macrophage-activating cytokines is beneficial in the acute septic response. Ann Surg. 1991;214:502–508. doi: 10.1097/00000658-199110000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruggiero V, Chiapparino C, Manganello S, Pacello L, Foresta P, Martelli E A. Beneficial effects of a novel platelet-activating factor receptor antagonist, ST 899, on endotoxin-induced shock in mice. Shock. 1994;2:275–280. doi: 10.1097/00024382-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Schall T J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- 35.Schmal H, Shanley T P, Jones M L, Friedl H P, Ward P A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- 36.Shvedova A A, Kramarik J A, Keohavong P, Chumakov K M, Karol M H. Use of anti-TNF-α antiserum to investigate toxic alveolitis arising from cotton dust exposure. Exp Lung Res. 1994;20:297–315. doi: 10.3109/01902149409064389. [DOI] [PubMed] [Google Scholar]
- 37.Su Y-H, Yan X-T, Oakes J E, Lausch R N. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terenzi F, Diaz-Guerra M J, Casado M, Hortelano S, Leoni S, Bosca L. Bacterial lipopeptides induce nitric oxide synthase and promote apoptosis through nitric oxide-independent pathways in rat macrophages. J Biol Chem. 1995;270:6017–6021. doi: 10.1074/jbc.270.11.6017. [DOI] [PubMed] [Google Scholar]
- 39.Wolf S F, Sieburth D, Sypek J. Interleukin-12—a key modulator of immune function. Stem Cells. 1994;12:154–168. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- 40.Yao Z, Painter S L, Fanslow W C, Ulrich D, Macduff B M, Spriggs M K, Armitage R J. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 41.Yoshida A, Koide Y, Uchijima M, Yoshida T O. IFN-γ induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–861. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- 42.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig B B, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]