Abstract
Introduction
We studied life satisfaction across Alzheimer's disease (AD) stages and studied mobility and meaningful activities as mediators of the associations between these AD stages and life satisfaction.
Methods
In this cross‐sectional study, we included n = 269 amyloid‐positive patients with subjective cognitive decline (SCD), mild cognitive impairment (MCI), and AD dementia from the Amsterdam Dementia Cohort. Life satisfaction was measured with the satisfaction with life scale. The mediating role of transportation, work, sports, and hobbies on life satisfaction was examined in single and multiple mediator models.
Results
Patients with dementia are less satisfied with life compared to SCD and MCI. These differences in life satisfaction are explained by reduced participation in meaningful activities, which in turn, was largely attributable to decreased transportation use.
Discussion
Our findings suggest that improving access to transportation, therewith allowing participation in meaningful activities help to maintain life satisfaction and may be an important target for intervention.
Keywords: Alhzeimer's disease, dementia, life satisfaction, mild cognitive impairment, mobility, subjective cognitive decline
1. INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disorder, that ultimately leads to dementia. The disease slowly unfolds, and pre‐dementia stages include Mild Cognitive Impairment (MCI) and preclinical AD, which sometimes manifests as Subjective Cognitive Decline (SCD). 1 Most studies on disease progression focus on cognitive deterioration or progression to dementia. 2 , 3 While these studies help to provide an understanding of disease mechanisms, they may not necessarily inform on outcomes that matter most to patients. 4
In an earlier study, we asked patients and their caregivers which outcomes mattered most to them. In particular, participants were asked to answer the questions “What do you want to know about the course of the symptoms?” and “If there was a treatment for AD, on which specific aspect should this have an effect?”. Patients and their caregivers indicated that transportation use (i.e., the ability to use a bike, car or public transportation) and ability to work and to engage hobbies are important outcomes, in both dementia and pre‐dementia stages. 5 Previous studies showed that patients with dementia and MCI reported problems with continuing their hobbies as one of the most important symptoms related to disruptions in everyday life. 6 Physical activity and leisure activities (including volunteering and social activities) are associated with increased life satisfaction in cognitively healthy adults. 7 , 8 , 9 , 10
The Global Action Plan from the World Health Organization calls for action to improve the lives of dementia patients and their caregivers. 11 Life satisfaction indicates how a person assesses his/her quality of life as a whole. 12 A former study showed that dementia and cognitive impairment are associated with a decreased life satisfaction. 13 However, no studies investigated differences in life satisfaction across the AD stages of (amyloid‐positive) SCD, MCI, and dementia. Therefore, we aimed to investigate (1) how life satisfaction changes across the AD stages of amyloid‐positive SCD, MCI, and dementia patients and (2) whether transportation, sports, hobbies, and (voluntary) work mediate the associations between the AD stages and life satisfaction.
2. METHODS
2.1. Amyloid‐positive participants across the AD stages of SCD, MCI, and dementia
In this cross‐sectional study, we included n = 269 amyloid‐positive patients from the Amsterdam Dementia Cohort. 14 All patients visited the memory clinic between 2009 and 2020. Based on information from their last available visit, we included n = 54 patients with SCD, n = 52 patients with MCI and n = 163 with dementia. Inclusion criteria were (1) a diagnosis of AD dementia, MCI, or SCD; (2) amyloid‐positive PET and/or cerebrospinal fluid (CSF) biomarkers; and (3) availability of data on life satisfaction, transportation, sports, hobbies and (voluntary) work. The study was approved by the Medical Ethics Review Committee of the VU University Medical Center. All patients provided written informed consent for the use of their medical data for research propose. We reported this study in accordance with the A Guideline for Reporting Mediation Analyses (AGReMA) statement. The checklist can be found in Appendix A.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using traditional sources (e.g., PubMed). Extending on previous studies on life satisfaction in Alzheimer's disease (AD), we included patients across the biomarker‐confirmed AD stages of SCD, MCI, and dementia and we studied important factors that mediate the associations between AD stage and life satisfaction.
Interpretation: Patients with dementia are less satisfied with life compared to patients with SCD and MCI. These differences in life satisfaction are explained by reduced participation in meaningful activities (i.e., hobbies, sports and [voluntary] work), which in turn, was largely attributable to a decrease in transportation use.
Future directions: Interventions should aim to stimulate transportation and participation in meaningful activities, since this may have a positive effect on life satisfaction in people with AD.
All patients received a standardized dementia diagnostic work‐up, which consisted of medical history, neurological, physical and neuropsychological evaluation, MRI, laboratory tests and lumbar puncture. 14 , 15 In addition, all patients were invited for an annual follow‐up visit at our outpatient clinic to evaluate the AD stage based on clinical assessment and neuropsychological evaluation. 14 , 15 We categorized the patients by the last evaluated AD stage before completing the life satisfaction questionnaire. Clinical diagnosis was determined in a multi‐disciplinary meeting. Patients were diagnosed with AD dementia or MCI according to the National Institute on Aging‐Alzheimer's Association (NIA‐AA) criteria. 16 , 17 Patients were labeled SCD when they presented with cognitive complaints, had normal clinical and cognitive test results and did not meet the criteria for MCI, dementia or other neurologic or psychiatric conditions. 18 We treated the AD stages SCD, MCI, and dementia as determinant in our analyses.
Participants were categorized as amyloid‐positive, based on a positive amyloid‐PET scan (n = 59) or abnormal CSF amyloid‐ß1‐42 (Aβ42) values (n = 210). If both amyloid‐PET and CSF values were available, we used the result of the amyloid‐PET scan (n = 59). CSF was obtained by lumbar puncture, collected in polypropylene tubes (Sarstedt Nurnberg, Germany), and processed according to international guidelines. 19 Before 2018, amyloid beta (Aβ42), total tau (t‐tau) and phosphorylated threonine 181 (p‐tau) were measured using sandwich enzyme‐linked immunosorbent assays (ELISAs; Innotest, Fujirebio, Gent, Belgium). 20 Amyloid‐beta values were drift‐corrected. 21 After 2018, CSF was analyzed using Elecsys (Roche, Rotkreuz, Swiss). CSF concentrations were considered amyloid‐positive if CSF Aβ42 drift‐corrected ELISA <813 or CSF Aβ42 Elecsys <1000 pg/ml. Amyloid‐PET scans made using 3‐Tesla Ingenuity TF PET/MRI, Ingenuity TF PET/CT and Gemini TF PET/CT scanners (Philips healthcare, the Netherlands) were visually rated by an experienced nuclear medicine physician. The amyloid‐PET procedure using 18F‐florbetaben, 18F‐Florbetapir, 18F‐flutemetamol or 11C‐Pittsburgh compound B (PiB) have been described in detail elsewhere. 22 , 23
2.2. Life satisfaction and mediator variables
In 2020, we started onlineADC; an online data collection of questionnaires related to Patient Relevant Outcomes (PROs), including questions about life satisfaction, hobbies, sports, (voluntary) work, and mobility. 24 We invited patients who had ever visited the memory clinic and their caregivers by email to complete the questionnaires in our online platform. In total, we invited n = 356 patients diagnosed with amyloid‐positive SCD, MCI, and dementia during the first visit at the memory clinic. Of these invited patients, n = 279 patients completed the questionnaires (n = 10 patients were excluded due to diagnosis other than SCD, MCI, or dementia diagnosed at the follow‐up visits) and n = 87 patients did not complete the questionnaire and therefore had no data on life satisfaction of the mediators. The mean (SD) time between the last available AD stage diagnosis at the memory clinic and completing the onlineADC questionnaires was 2.4 (2.3) years.
Life satisfaction was measured using the satisfaction with life scale (SWLS). 24 The SWLS included five items, each rated on a 7‐point Likert‐scale, scored from 1 (totally disagree) to 7 (totally agree). The total score ranges from 5 (extremely dissatisfied) to 35 (extremely satisfied). We treated life satisfaction as the outcome in our analyses.
Transportation was measured using three questions from the comprehensive geriatric assessment (CGA): ‘Do you cycle?’, ‘Do you drive a car?’ and ‘Are you able to use public transport completely independently?’. 25 The questions regarding cycling and driving had three possible responses: I never cycled/driven a car, I still cycle/drive and I stopped cycling/driving. The public transport question had two possible responses: yes and no. Based on the responses on these three questions we created a new dichotomous variable that indicated (1) whether patients were able to use at least one of the aforementioned forms of transportation or never have cycled/driven a car and (2) whether patients stopped using at least one of the aforementioned forms of transportation. Hobbies were measured with two questions: ‘What is or was your hobby?’ and ‘Have you stopped doing this hobby?’. Playing sports was measured with two questions: ‘Do or did you do sports?’ and ‘Have you stopped playing sports?’. Patients could also fill in that he/she had no hobby and did not participate in sports (this was one item). We created two (hobbies and sports) dichotomous variables that indicated (1) whether patients were able to do a hobby/sport or never had a hobby/sport and (2) whether patients stopped doing a hobby/sports.
Continuing sports was defined as playing sports at the time of completing the questionnaire. (Voluntary) work was measured using two questions from the iMTA productivity cost questionnaire: ‘Do you currently work for pay?’ and ‘Do you currently do unpaid volunteer work?’. 26 Maintaining (voluntary) work was defined as doing (voluntary) work at the time of completing the questionnaire. We treated hobbies, sports, (voluntary) work and transportation as dichotomous mediator variables.
2.3. Confounders
We adjusted the models for age, sex, education, and comorbidity. All confounders were measured at the time of last available AD stage diagnosis. Education was measured using the Dutch Verhage Scale. 27 Comorbidity was defined using the Charlson Comorbidity Index (CCI), which was calculated based on medical history and medication use (CCI score ranges from 0 [no comorbidity] to 37 [high comorbidity]). 28
2.4. Statistical analysis
Descriptive analyses were performed using STATA SE version 14.0. To evaluate differences between AD stages we used ANOVAs for normally distributed continuous variables, Kruskal‐Wallis tests for non‐normally distributed continuous variables, and chi‐square tests for categorical variables.
Regression and mediation analyses were performed using Rstudio version 4.0.3. We used multiple linear and logistic regression to estimate the associations between (1) AD stages and life satisfaction; (2) AD stages and the mediators (i.e., transportation, sports, hobbies and [voluntary] work); (3) AD stages, transportation, and meaningful activities (i.e., sports, hobbies and [voluntary] work); and (4) AD stages, mediators, and life satisfaction. We varied the reference group of AD stages to compare life satisfaction, transportation, sports, hobbies, and (voluntary) work across the three AD stages (i.e., dementia vs. SCD, dementia vs. MCI, and MCI vs. SCD). All analyses were adjusted for age, sex, education, and comorbidity (CCI).
We used causal mediation analysis based on the potential outcomes framework to investigate to what extent transportation, sports, hobbies, and work explained differences in life satisfaction across AD stages. We used the imputation‐based approach in the "medflex" package in Rstudio version 4.0.3 to estimate natural direct and indirect effects. 29 , 30 All mediation analyses were based on linear regression models for the continuous life satisfaction variable. We tested for determinant‐mediator interaction, a situation in which the mediator is an effect modifier of the direct effect and the determinant is an effect modifier of the indirect effects. Determinant‐mediator interaction was tested by adding a determinant‐mediator interaction term to the mediation models. Determinant‐mediator interaction was considered present if the mediated interaction was significant (p < 0.05). We found that none of the mediated interactions were significant, so the reported results are based on models without determinant‐mediator interactions. All direct and indirect effect estimates are accompanied by 95% percentile bootstrap confidence intervals based on 1000 bootstrap resamples. 31
We hypothesized transportation to be the first mediator in the causal chain, we used a single mediator model to estimate the indirect effect of AD stage on life satisfaction through transportation (Figure 1). Subsequently, we estimated three multiple mediator models which each contained two mediators: (1) transportation and sports, (2) transportation and hobbies, and (3) transportation and (voluntary) work. The multiple mediator models resulted in indirect effect estimates of AD stages on life satisfaction through the two mediators jointly. The differences between the indirect effect estimates from the single and multiple mediator models indicate to what extent the total effect of AD stages on life satisfaction is additionally explained by sports, hobbies, and (voluntary) work.
FIGURE 1.
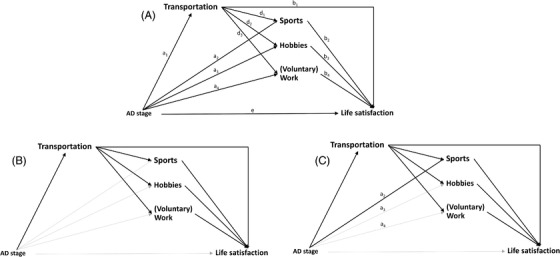
(A) Directed acyclic graph of the hypothesized mediation model. (B) The bold arrows highlight the indirect effect through transportation only from the single mediator model. (C) The bold arrows highlight the total indirect effect through transportation and sports from a multiple mediator model. Each multiple mediator model contained two mediators. For the total indirect effects for transportation and hobbies, we included path a3 instead of a2, and for the total indirect effects for transportation and (voluntary) work, we included path a4 instead of a2
2.5. Sensitivity analyses
In a sensitivity analysis, we repeated the analyses based on the dichotomized life satisfaction variable to assess whether the effect estimates are generally in line with the results based on the continuous life satisfaction variable. We dichotomized the continuous life satisfaction score into satisfied with life (score 21–35) and dissatisfied with life (score 5–20) as described in previous literature. 24
In another sensitivity analysis, we performed the analyses without patients who have never cycled, driven a car, had a hobby, or played sports. For this analyses, we included n = 52 patients with SCD, n = 50 patients with MCI, and n = 143 with dementia.
3. RESULTS
3.1. Patient characteristics
Patient characteristics are summarized in Table 1 and in Appendix B. Compared to SCD, dementia patients had a lower educational level. Dementia patients had a lower MMSE and more comorbidity (CCI) compared to SCD or MCI individuals. There were no differences in age and sex between dementia and SCD or MCI. Compared to SCD, MCI patients had a lower MMSE.
TABLE 1.
Patient characteristics
| SCD (n = 54) | MCI (n = 52) | Dementia (n = 163) | p‐value * | |
|---|---|---|---|---|
| Characteristics at time of AD stage diagnosis | ||||
| Age, mean years (SD) | 64.1 (6.9) | 67.3 (6.7) | 64.8 (7.0) | 0.03 |
| Female, n(%) | 27 (50) | 25 (48) | 82 (50) | 0.96 |
| Education Verhage, mean (SD) | 5.7 (1.1) | 5.6 (1.0) | 5.2 (1.0) | <0.001 |
| MMSE, median (IQR) | 29 (28–30) | 27 (24–28) | 23 (20–26) | <0.001 |
| CCI, mean (SD) | 2.4 (1.2) | 2.9 (1.3) | 3.4 (1.1) | <0.001 |
| online ADC: Life satisfaction and mediator variables | ||||
| Satisfaction with life scale, median (IQR) | 28 (23–30) | 28 (25–30) | 23 (16–29) | <0.001 |
| Satisfied with life (score >20), n(%) | 45 (83) | 44 (85) | 99 (61) | <0.001 |
| Using transportation: ability to drive, cycle or use public transport, n(%) | 44 (81) | 28 (54) | 25 (15) | <0.001 |
| Maintaining hobbies, n(%) | 42 (78) | 43 (83) | 112 (69) | 0.10 |
| Maintaining (voluntary) work, n(%) | 26 (48) | 13 (25) | 19 (12) | <0.001 |
| Playing sports, n(%) | 23 (43) | 13 (25) | 35 (21) | 0.009 |
Note: To evaluate differences between AD stages we used ANOVAs for normally distributed continuous variables, Kruskal‐Wallis tests for non‐normally distributed continuous variables, and chi‐square tests for categorical variables.
Abbreviations: CCI, charlson comorbidity index; MCI, mild cognitive impairment; MMSE, mini‐mental state examination; SCD, subjective cognitive decline.
*See appendix B for post hoc analysis.
Dementia patients were less satisfied with life compared to SCD and MCI. The distribution of satisfaction with life for all stage are shown in Appendix C. In addition, dementia patients use transport less often compared to SCD or MCI. Compared to SCD, dementia patients less often maintained (voluntary) work and less frequently played sports. MCI individuals less often use transportation and less often maintained (voluntary) work compared to SCD.
3.2. Mediators of the relationship between AD stage and life satisfaction
Linear regression analysis showed that life satisfaction was lower in dementia compared to SCD and MCI, while there was hardly any difference between the latter two (Table 3). To further investigate the associations between AD stages, life satisfaction, transportation, sports, hobbies, and work, we estimated the pathways depicted in Figure 1. These effect estimates are provided in Table 2. Subsequently, we performed mediation analyses, to investigate to what extent the differences in life satisfaction are attributable to decreased use of transportation and meaningful activities (indirect effect) and to what extent factors other than transportation and meaningful activities are responsible (direct effect). Table 3 shows the results from these mediation analyses. The results from the unadjusted models (i.e., no covariates) are provided in Appendix D and E. The results generally followed the same pattern as the main results.
TABLE 3.
Total, indirect, and direct effects in terms of mean differences with 95% percentile bootstrap confidence intervals for mediation by transportation, sports, hobbies, and work in the association between AD stages and life satisfaction
| Comparison | Effect of stage on life satisfaction ‡ (Total effect) | Mediator(s) | Effect explained by mediator (Indirect effect ‡ ) | Effect not explained by mediator (Direct effect ‡ ) |
|---|---|---|---|---|
| Dementia vs. SCD | −3.24*, † (−5.53; −0.96) | Transportation | −1.81 (−3.22; −0.49)*, † | −1.44 (−4.09; 1.04) |
| Transportation and sports | −1.97 (−3.43; −0.64)*, † | −1.27 (−3.78; 1.16) | ||
| Transportation and hobbies | −1.78 (−3.23; −0.48)*, † | −1.46 (−3.93; 1.14) | ||
| Transportation and (voluntary) work | −2.52 (−4.20; −1.06)*, † | −0.72 (−3.46; 1.76) | ||
| Dementia vs. MCI | −3.42*, † (−5.67; −1.17) | Transportation | −1.09 (−2.00; −0.29)*, † | −2.33 (−4.49; −0.13)* |
| Transportation and sports | −1.02 (−2.03; −0.14)*, † | −2.39 (−4.56; −0.13)* | ||
| Transportation and hobbies | −1.36 (−2.32; −0.41)*, † | −2.06 (−4.02; 0.05) | ||
| Transportation and (voluntary) work | −1.29 (−2.30; −0.32)*, † | −2.13 (−4.34; 0.15) | ||
| MCI vs. SCD | 0.17 (−2.42; 2.78) | Transportation | −0.72 (−1.63; −0.13)*, † | 0.89 (−0.60; 3.22) |
| Transportation and sports | −0.95 (−1.94; −0.18)*, † | 1.12 (−1.38; 3.48) | ||
| Transportation and hobbies | −0.42 (−1.41; 0.39) | 0.59 (−1.91; 2.94) | ||
| Transportation and (voluntary) work | −1.23 (−2.38; −0.45)*, † | 1.41 (−1.25; 3.87) |
Abbreviations: MCI, mild cognitive impairment; SCD, subjective cognitive decline.
Significant based on p < 0.05.
Significant based on Bonferroni‐corrected p‐value of <0.017 (98.3% percentile bootstrap confidence intervals not shown).
All effect estimates are adjusted for age, sex, education and comorbidities.
An indirect effect is the part of the total effect of AD stage on life satisfaction that is explained by the mediator.
TABLE 2.
Associations among AD stage, transportation, meaningful activities, and life satisfaction
| Stage → mediator † (a paths in Figure 1A) | Transportation → mediator † , ‡ (d paths in Figure 1A) | Mediator → life satisfaction † , ‡ (b paths in Figure 1A) | ||
|---|---|---|---|---|
| Comparison stages | Mediator | OR (95% CI) | OR (95% CI) | Β (95% CI) |
| Dementia vs. SCD | Transportation | 0.03 (0.01; 0.08)* | NA | 2.74 (0.68; 4.82)* |
| Sports | 0.45 (0.21; 0.97)* | 2.04 (1.01; 4.14)* | 2.54 (0.64; 4.44)* | |
| Hobbies | 0.64 (0.28; 1.38) | 2.29 (1.09; 5.02)* | 3.60 (1.82; 5.39)* | |
| (voluntary) work | 0.16 (0.07; 0.37)* | 2.48 (1.17; 5.34)* | 3.45 (1.34; 5.56)* | |
| Dementia vs. MCI | Transportation | 0.12 (0.05; 0.27)* | NA | 2.74 (0.68; 4.82)* |
| Sports | 0.84 (0.38; 1.92) | 2.04 (1.01; 4.14)* | 2.54 (0.64; 4.44)* | |
| Hobbies | 0.46 (0.19; 1.03) | 2.29 (1.09; 5.02)* | 3.60 (1.82; 5.39)* | |
| (voluntary) work | 0.39 (0.16; 0.94)* | 2.48 (1.17; 5.34)* | 3.45 (1.34; 5.56)* | |
| MCI vs. SCD | Transportation | 0.25 (0.10; 0.62)* | NA | 2.74 (0.68; 4.82)* |
| Sports | 0.54 (0.22; 1.28) | 2.04 (1.01; 4.14)* | 2.54 (0.64; 4.44)* | |
| Hobbies | 1.38 (0.52; 3.75) | 2.29 (1.09; 5.02)* | 3.60 (1.82; 5.39)* | |
| (voluntary) work | 0.42 (0.17;0.98)* | 2.48 (1.17; 5.34)* | 3.45 (1.34; 5.56)* |
Note: Odds ratios or beta coefficients and 95% confidence intervals for the direct associations between AD stages, transportation, sports, hobbies, work and life satisfaction. Life satisfaction was used as a continuous variable.
Significant based on p < 0.05.
All odds ratios and beta coefficients are adjusted for age, sex, education and comorbidities.
Odds ratios and beta coefficients are additionally adjusted for AD stages.
3.2.1. Dementia versus SCD
Dementia patients were less satisfied with life compared to SCD patients (total effect Β [95% confidence interval {CI}] = ‐3.24[‐5.53; ‐0.96]) (Table 3). The significant indirect effect for transportation indicates that a decreased use of transportation (indirect effect Β [95% CI] = ‐1.81 [‐3.22; ‐0.49]) accounted for a substantial portion of this difference.
The indirect effect increased in magnitude after adding sports and (voluntary) work to the mediation model (indirect effect for transportation and sports B [95% CI] = ‐1.97 [‐3.43; ‐0.64]; indirect effect for transportation and [voluntary] work B [95% CI] = ‐2.52 [‐4.20; ‐1.06]). This indicates that there were additional differences in the ability to maintain sports and to do (voluntary) work between patients with SCD and dementia that were not explained by transportation, which also affected life satisfaction.
3.2.2. Dementia versus MCI
Dementia patients were less satisfied with life compared to MCI patients, (total effect Β [95% CI] = ‐3.42 [‐5.67; ‐1.17]) (Table 3). The significant indirect effect for transportation indicates that dementia patients were less satisfied with life compared to MCI individuals, due to a decreased use of transportation (indirect effect Β [95% CI] = ‐1.09 [‐2.00; ‐0.29]).
The indirect effect increased in magnitude after adding hobbies and (voluntary) work to the mediation model (indirect effect for transportation and hobbies B [95% CI] = ‐1.36 [‐2.32; ‐0.41]; indirect effect for transportation and [voluntary] work B [95% CI] = ‐1.29 [‐2.30; ‐0.32]). This indicates that there were additional differences in the ability to maintain hobbies and to do (voluntary) work between patients with MCI and dementia that were not explained by transportation, which also affected life satisfaction.
3.2.3. MCI versus SCD
There was no difference in life satisfaction between MCI and SCD individuals (total effect B [95% CI] = 0.17 [‐2.42; 2.78]). Nonetheless, the indirect effect for transportation indicates that a decreased use of transportation in MCI patients may have led to somewhat reduced satisfaction compared to SCD patients (indirect effect B [95%CI] = ‐0.72 [‐1.63; ‐0.13]).
The indirect effect increased in magnitude after adding sports and (voluntary) work to the mediation model (indirect effect of transportation and sports together B [95% CI] = ‐0.95 [‐1.94; ‐0.18]; indirect effect for transportation and (voluntary) work B [95% CI] = ‐1.23 [‐2.38; ‐0.45]). This indicates that there were differences in the ability to do sports and to do (voluntary) work between patients with MCI and SCD that were not explained by transportation, and that these differences in sports and (voluntary) work also affected life satisfaction.
3.3. Sensitivity analyses
The results of two sensitivity analyses based on (1) the dichotomous life satisfaction variable and (2) without patients who have never done the activity generally followed the same pattern as the main results. Dementia patients were less often satisfied with life compared to SCD or MCI, which could for a substantial part be explained by the decreased use of transportation. In addition, (voluntary) work (across all stages) and hobbies (dementia vs. MCI) also affected life satisfaction. The results of these sensitivity analyses can be found in Appendix F and G.
4. DISCUSSION
The main findings of our study are (1) satisfaction with life along the AD continuum particularly drops in the dementia stage, and (2) that this drop can be explained by reduced participation in meaningful activities (i.e., hobbies, sports, and [voluntary] work), which in turn was attributable to decreased use of transportation. These results provide important targets for both pharmacological and non‐pharmacological interventions, as our results suggest that improving access to transportation could lead to improved participation in meaningful activities, which could positively impact life satisfaction.
Our results indicate that the ability to use transportation is key for AD patients to participate in meaningful activities, which in turn influences life satisfaction. Our findings are in line with recommendations of the World Health Organization to achieve physical, mental and social wellbeing in dementia patients. 11 They recommend to include dementia patients in activities of the wider community and foster cultural, social, and civic participation to enhance their autonomy. We show that ability to use transportation plays a pivotal role in reaching this goal. Improving access to transportation may help provide patients with AD with more options to participate in meaningful activities, such as hobbies and voluntary work.
Future interventions should stimulate independence of patients to such an extent that they can continue to use transportation and participate in activities that are meaningful to them. This holds for non‐pharmacological interventions, which could also foster access to transportation. Accessible transportation can be improved by the availability of door‐to‐door (regional) transportation services. Furthermore, dementia‐friendly travelling with public transport or taxi can stimulate mobility, including clear signage and education for transport employees (i.e., taxi drivers, bus drivers, staff at the train station) to help and support individuals with dementia. 32 In addition, people can be advised to travel with a companion (i.e., family member or volunteer). Finally, tools can be offered to stimulate leisure activities at home, including (hybrid/online) physical activities. 33 Nonetheless, these results also have relevance for future pharmacological treatment. There is currently much debate about the clinical relevance of these types of treatment: What is the minimum important change (MIC) that is necessary and how does a difference of x points on a memory test translate to daily living? 34 In addition to the outcome cognition, there are other outcomes relevant to patients and their caregivers that can be used in future research of pharmacological treatments. In our previous study, we showed that transportation is an important outcome for patients and their caregivers. 5 Our results confirm that treatment should ultimately contribute to keeping patients mobile and able to participate in meaningful activities, as these are a major contributor to life satisfaction.
A previous study showed that approximately 83% of the elderly with dementia were satisfied with their life. 7 Our results show a lower percentage of life satisfaction in the AD dementia group. A possible explanation for this might be the method of measuring life satisfaction. In the previous study, life satisfaction was measured using a single question. Among the strengths of our study is that we used the validated multi‐item Satisfaction with Life scale (SWLS). 24 Furthermore, Statistics Netherlands also investigated life satisfaction using the SWLS and showed an average (SD) SWLS score of 26 (6) in the Dutch population aged 65 and older. 35 This score is consistent with the SWLS scores we found in SCD and MCI individuals, and suggests that life satisfaction is not yet much impacted in the pre‐dementia stages of AD.
In this study, we investigated work as a mediator of the associations between AD stages and life satisfaction. Although in general AD patients are typically of retirement age, our study is based on a relatively young population for whom work is an important factor. The Amsterdam Dementia Cohort includes relatively young AD patients, as the memory clinic is neurology based and the memory clinic has specific expertise on dementia at younger age. A strength of this study is that we were able to show that maintaining work is indeed an important factor that contributes to life satisfaction in this relatively young cohort of AD patients. Another strength of our study are that we included a large sample of patients across the biomarker‐confirmed AD stages of SCD, MCI, and dementia.
A limitation of this study is the possibility of bias due to unmeasured confounders. We adjusted our analyses for sex, age, education, and comorbidity, but there are likely more confounders that affect two or more variables in our mediation models. Changes in the social environment (e.g., the passing of family members or friends), social support (i.e., people who have a buddy, may have more access to transportation), home environment (e.g., nursing home placement), and functional ability may have affected the mediators and life satisfaction. 7 , 36 , 37 We were not able to adjust for these confounders, as we either did not have data on these confounders or the available data were sparse. Future studies should investigate the impact of these factors on the indirect effects through transportation and meaningful activities.
Another limitation of this study is that the length of time between AD stage diagnosis at the memory clinic and completing the onlineADC questionnaires differs between the patients. This could have resulted in misclassification and selective dropout. However, we classified patients based on the last available diagnosis in order to minimize these effects. Selective drop‐out may have led to an underestimation of the effect estimates, because patients diagnosed in a more advanced disease stage may have died before the onlineADC questionnaires were administered and patients in a more advanced disease stage may not have been able to fill out the online questionnaires. Nonetheless, the online questionnaire allows us to include a large group of patients, including a large group of amyloid‐positive dementia patients.
Finally, the last limitation of this study is the use of cross‐sectional data, which has implications for the interpretation of the results. 38 , 39 First, the results can only be interpreted as between‐person effects. This means that the effect estimates are based on differences between people, while ultimately we are interested in the effects observed within people. Second, because transportation, meaningful activities and life satisfaction were measured at the same time point, there is a possibility of reverse causality. However, transportation, meaningful activities, and life satisfaction are measured in such a way that the time lag between these variables is not a strict time‐lag of zero. 40 Specifically, the questions used to measure transportation and meaningful activities are phrased in such a way that they cover the time till filling out the questionnaire (e.g., Have you stopped doing a hobby?). The life satisfaction questionnaire measures the patients’ life satisfaction at the time of filling out the questionnaire. There is a possibility that meaningful activities affect transportation instead of vice versa or that the effects are reciprocal. However, the total indirect effects estimates in Table 3 do not assume a specific causal direction of the association between transportation and meaningful activities. 41 If transportation does not causally affect meaningful activities, then the total indirect effects can still be interpreted as the portion of the total effect of stage on life satisfaction that is explained by transportation and hobbies, sports, or work. Nonetheless, future studies are needed that measure transportation, meaningful activities and life satisfaction at multiple time points, so that it is possible to estimate within‐person effects and to determine whether the discontinuation of transportation and meaningful activities indeed precede a decrease in life satisfaction.
This study showed that along the continuum of AD, satisfaction with life drops in the dementia stages. The decreased use transportation by dementia patients, which leads to reduced participation in meaningful activities (i.e., hobbies, sports, and [voluntary] work) is a major explaining factor of the observed drop in life satisfaction. Our focus on outcomes that matter to patients is of great relevance, as it easily translates to daily practice. Future treatment should ultimately contribute to patients maintaining their independence, to such an extent that they can use transportation, to enable them to participate in meaningful activities. This also holds for non‐pharmacological interventions, both at the level of the patient and their family and at the level of society (dementia friendly society), which should aim to stimulate transportation and participation in meaningful activities, since this may have a positive effect on life satisfaction in people with AD.
CONFLICTS OF INTEREST
Arenda Mank reports no financial disclosures or conflicts of interest. Ingrid S. van Maurik is consultant to Roche. All funding is paid to her institution. Argonde van Harten was supported by funding from Alzheimer Netherlands, The Alzheimer Drug Discovery Foundation and the VUmc fund. Argonde van Harten has a collaboration contract with Quanterix corp. Hanneke F.M. Rhodius‐Meester is recipient of the Memorabel Dementia Fellowship 2021 (ZonMw projectnumber 10510022110004). Hanneke F.M. Rhodius‐Meester performs contract research for Combinostics; all funding is paid to her institution. Research of Charlotte E. Teunissen is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), and JPND), Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association. CT is recipient of ABOARD, which is a public‐private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). More than 30 partners participate in ABOARD. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes‐Strijbisfonds. CET has a collaboration contract with ADx Neurosciences, Quanterix and Eli Lilly, performed contract research or received grants from AC‐Immune, Axon Neurosciences, Biogen, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, PeopleBio, Roche, Toyama, Vivoryon. CET serves on editorial boards of Medidact Neurologie/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is editor of a Neuromethods book Springer. Bart van Berckel has received research support from EU‐FP7, CTMM, ZonMw, NWO and Alzheimer Nederland. BvB has performed contract research for Rodin, IONIS, AVID, Eli Lilly, UCB, DIAN‐TUI and Janssen. BvB was a speaker at a symposium organized by Springer Healthcare. BvB has a consultancy agreement with IXICO for the reading of PET scans. BvB is a trainer for GE. Johannes Berkhof reports no financial disclosures or conflicts of interest. Wiesje M. van der Flier: Research programs of Wiesje M. van der Flier have been funded by ZonMW, NWO, EU‐FP7, EU‐JPND, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis‐NL, Life‐MI, AVID, Roche BV, Fujifilm, Combinostics. Wiesje M. van der Flier holds the Pasman chair. Wiesje M. van der Flier is recipient of ABOARD, which is a public‐private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). Wiesje M. van der Flier has performed contract research for Biogen MA Inc, and Boehringer Ingelheim. Wiesje M. van der Flier has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), Springer Healthcare. Wiesje M. van der Flier is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc. Wiesje M. van der Flier participated in advisory boards of Biogen MA Inc and Roche. All funding is paid to her institution. Wiesje M. van der Flier was associate editor of Alzheimer, Research & Therapy in 2020/2021. Wiesje M. van der Flier is associate editor at Brain. Judith J. M. Rijnhart received a grant from the Amsterdam Public Health Research Institute, which was paid to the Amsterdam UMC. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
Research of Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The chair of Wiesje M. van der Flier is supported by the Pasman stichting. Arenda Mank is appointed at the EU Joint Programme‐ Neurodegenerative Disease Research (JPND) ADDITION project (ZonMW no. 733051083). Wiesje M. van der Flier and Ingrid S. van Maurik are recipients of the collaboration project ABIDE‐clinical utility, which is co‐funded by the PPP Allowance made available by health‐Holland, Top Sector Life Sciences & Health, to stimulate public‐private partnerships and Life Molecular Imaging GmbH (grant no.: LSHM18075). Wiesje M. van der Flier, Johannes Berkhof and Charlotte E. Teunissen are recipients of ABOARD, which is a public‐private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). Wiesje M. van der Flier and Hanneke F. M. Rhodius‐Meester are recipients of the collaboration project Dementia diagnostics using Artificial Intelligence (DAILY), which is co‐funded by the PPP Allowance made available by Health‐Holland, Top Sector Life Sciences and Health, to stimulate public–private partnerships and Combinostics (grant no: LSHM19123‐HSGF). Argonde C. van Harten is a recipient of an Alzheimer Netherlands impulse grant.
Mank A, van Maurik IS, van Harten AC, et al. Life satisfaction across the entire trajectory of Alzheimer's disease: A mediation analysis. Alzheimer's Dement. 2022;14:e12389. 10.1002/dad2.12389
REFERENCES
- 1. Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet. 2016;388(10043):505‐517. [DOI] [PubMed] [Google Scholar]
- 2. van Maurik IS, Slot RER, Verfaillie SCJ, et al. Personalized risk for clinical progression in cognitively normal subjects‐the ABIDE project. Alzheimers Res Ther. 2019;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Maurik IS, Vos SJ, Bos I, et al. Biomarker‐based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol. 2019;18(11):1034‐1044. [DOI] [PubMed] [Google Scholar]
- 4. Patrick DL, Burke LB, Powers JH, et al. Patient‐reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125‐S137. [DOI] [PubMed] [Google Scholar]
- 5. Mank A, van Maurik IS, Bakker ED, et al. Identifying relevant outcomes in the progression of Alzheimer's disease; what do patients and care partners want to know about prognosis? Alzheimer's & dementia (N Y). 2021;7(1):e12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunn T, Howlett SE, Stanojevic S, Shehzad A, Stanley J, Rockwood K. Patterns of symptom tracking by caregivers and patients with dementia and mild cognitive impairment: cross‐sectional study. J Med Internet Res. 2022;24(1):e29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ataollahi Eshkoor S, Hamid TA, Nudin SSH. Mun CY. The effects of social support, substance abuse and health care supports on life satisfaction in dementia. Soc Indic Res.116:535‐544. [Google Scholar]
- 8. Maher JP, Pincus AL, Ram N, Conroy DE. Daily physical activity and life satisfaction across adulthood. Dev Psychol. 2015;51(10):1407‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An HY, Chen W, Wang CW, Yang HF, Huang WT, SY Fan. The Relationships between physical activity and life satisfaction and happiness among young, middle‐aged, and older adults. Int J Environ Res Public Health. 2020;17(13):4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoon H, Lee WS, Kim KB, Moon J. Effects of leisure participation on life satisfaction in older Korean adults: a panel analysis. Int J Environ Res Public Health. 2020;17(12):4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Global Action Plan On The Public Health Response To Dementia 2017‐2025. World Health Organization (WHO). https://www.who.int/publications/i/item/global‐action‐plan‐on‐the‐public‐health‐response‐to‐dementia‐2017—2025 [Google Scholar]
- 12. Shin DC, Johnson DM. Avowed happiness as an overall assessment of the quality of life. Soc Indic Res. 1978;5:475‐492. [Google Scholar]
- 13. St John PD, Montgomery PR. Cognitive impairment and life satisfaction in older adults. Int J Geriatr Psychiatry. 2010;25(8):814‐821. [DOI] [PubMed] [Google Scholar]
- 14. van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Flier WM, Pijnenburg YA, Prins N, et al. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41(1):313‐327. [DOI] [PubMed] [Google Scholar]
- 16. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch neurol. 1999;56(3):303‐308. [DOI] [PubMed] [Google Scholar]
- 18. Jessen F. Subjective and objective cognitive decline at the pre‐dementia stage of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 2014;264(Suppl 1):S3‐S7. [DOI] [PubMed] [Google Scholar]
- 19. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duits FH, Prins ND, Lemstra AW, et al. Diagnostic impact of CSF biomarkers for Alzheimer's disease in a tertiary memory clinic. Alzheimer's dement. 2015;11(5):523‐532. [DOI] [PubMed] [Google Scholar]
- 21. Tijms BM, Willemse EAJ, Zwan MD, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid Amyloid‐β 1‐42 analysis results. Clin Chem. 2018;64(3):576‐585. [DOI] [PubMed] [Google Scholar]
- 22. de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE Project. JAMA Neurol. 2018;75(9):1062‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Konijnenberg E, Carter SF, Ten Kate M, et al. The EMIF‐AD PreclinAD study: study design and baseline cohort overview. Alzheimers Res Ther. 2018;10(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71‐75. [DOI] [PubMed] [Google Scholar]
- 25. Federatie Medisch Specialisten . Comprehensive Geriatric Assessment (CGA). Federatie Medisch Specialisten; 2021. https://richtlijnendatabase.nl/richtlijn/comprehensive_geriatric_assessment_cga/startpagina_‐_comprehensive_geriatric_assessment_cga.html [Google Scholar]
- 26. Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart‐van Roijen L. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health‐related productivity losses. Value Health. 2015;18(6):753‐758. [DOI] [PubMed] [Google Scholar]
- 27. Verhage F. Intelligence and age; research among the Dutch aged 12 to 77 [in Dutch]. Assen: van Gorcum; 1964. [Google Scholar]
- 28. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 29. Team R. RStudio . Integrated Development for R. RStudio. PBC. Team R. RStudio; 2020. http://www.rstudio.com/ [Google Scholar]
- 30. Steen J, Loeys T, Moerkerke B, Vansteelandt S. Medflex: an R package for flexible mediation analysis using natural effect models. J Stat Softw. 2017;76:1‐46. [Google Scholar]
- 31. Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alzheimer's society . Dementia‐Friendly Transport. Alzheimer's society. https://www.alzheimers.org.uk/get‐involved/dementia‐friendly‐communities/organisations/transport [Google Scholar]
- 33. Alzheimer Nederland . Activiteiten voor thuis. https://www.dementie.nl/omgaan‐met‐dementie/corona‐en‐dementie/omgaan‐met‐corona/activiteiten‐voor‐thuis
- 34. Dubbelman MA, Verrijp M, Terwee CB, et al. Pursuing clinical meaningfulness: determining the minimal important change of everyday functioning in dementia. Neurology. 2022;99(9):e954‐e964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jv Beuningen. The Satisfaction with Life Scale Examining Construct Validity. Statistics Netherlands; 2012. [Google Scholar]
- 36. Wu YT, Nelis SM, Quinn C, et al. Factors associated with self‐ and informant ratings of quality of life, well‐being and life satisfaction in people with mild‐to‐moderate dementia: results from the improving the experience of dementia and enhancing active life programme. Age Ageing. 2020;49(3):446‐452. [DOI] [PubMed] [Google Scholar]
- 37. Martyr A, Nelis SM, Quinn C, et al. Living well with dementia: a systematic review and correlational meta‐analysis of factors associated with quality of life, well‐being and life satisfaction in people with dementia. Psychol Med. 2018;48(13):2130‐2139. [DOI] [PubMed] [Google Scholar]
- 38. O'Laughlin KD, Martin MJ, Ferrer E. Cross‐sectional analysis of longitudinal mediation processes. Multivariate Behav Res. 2018;53(3):375‐402. [DOI] [PubMed] [Google Scholar]
- 39. Maxwell SE, Cole DA. Bias in cross‐sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23‐44. [DOI] [PubMed] [Google Scholar]
- 40. Reichardt CS. Commentary: are three waves of data sufficient for assessing mediation? Multivariate Behav Res. 2011;46(5):842‐851. [DOI] [PubMed] [Google Scholar]
- 41. VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Methods. 2014;2(1):95‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


