Abstract
Background
Chronic pulmonary aspergillosis (CPA) can complicate underlying pulmonary diseases, and clinical management of CPA is challenging. Guidelines support clinicians but due to the complexity of the disease they can be difficult to adhere to.
Objectives
To map current guideline recommendations for the clinical management of CPA into a scoring tool to facilitate and quantify guideline adherence in clinical practice.
Methods
Recommendations for diagnosis, treatment and follow-up of CPA presented in the current ESCMID/ERS/ECMM and CPAnet guidance documents were assembled and weighed on the basis of their strength of recommendation and level of evidence.
Results
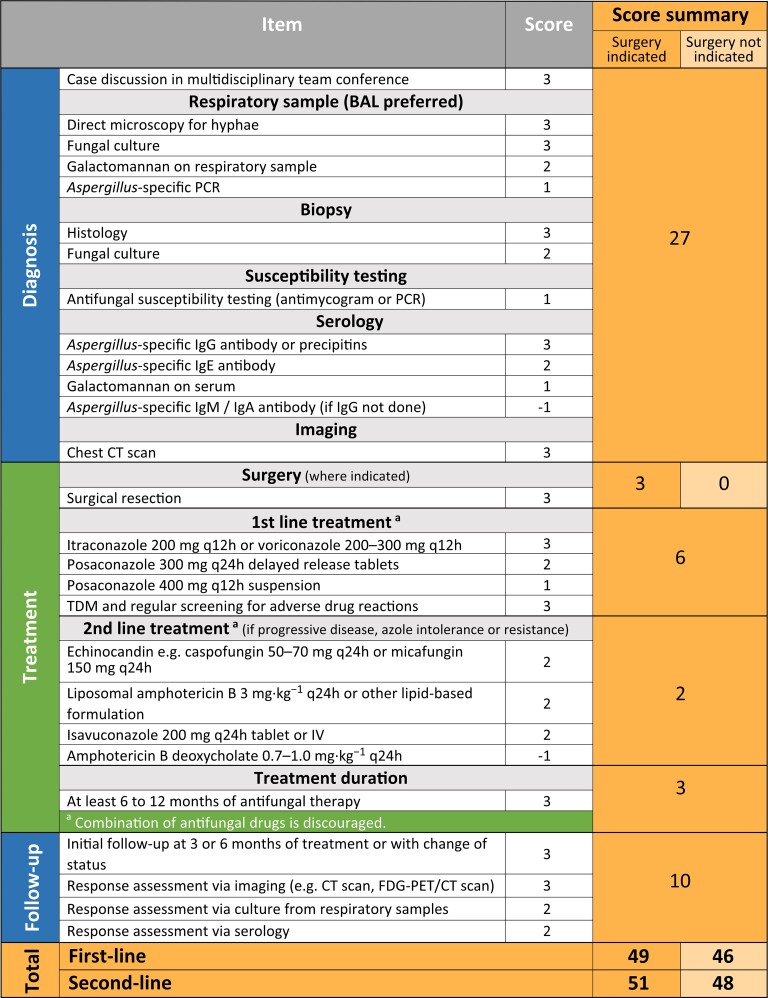
Twenty-seven recommendations were identified, resulting in a total maximum EQUAL CPA Score of 51. For diagnostics (ScoreMax = 27), a strong emphasis on expert consultation, culture, direct microscopy, histopathology, serology and imaging was reflected in respective points, whereas molecular techniques and susceptibility testing count into the diagnostics score to a lesser extent.
Ten treatment recommendations (ScoreMax = 14), including antifungal therapy, therapeutic drug monitoring and treatment duration, were identified. Surgery, where indicated, adds three points. For refractory disease or intolerance of first-line antifungal treatment, optimal second-line treatment added another two points.
During follow-up (ScoreMax = 10), response assessment via imaging gave three points, while culture and serology added two points each to the ScoreMax.
Conclusion
The EQUAL CPA Score intents to be used as a comprehensive tool for measuring guideline adherence. If adherence to current guidelines is associated with clinical outcome, this will be assessed in future studies.
Introduction
Chronic pulmonary aspergillosis (CPA) is a progressive and destructive pulmonary infection that affects about three million people globally.1 It appears in immunocompetent and mildly immunosuppressed individuals with an underlying pulmonary condition, including chronic obstructive pulmonary disease, lung cancer, tuberculosis or sarcoidosis, and leads to formation of pulmonary nodules, cavities, secondary pleural thickening and fibrosis.1 Diagnosis is challenged by a heterogeneous clinical picture with non-specific symptoms and a variety of radiological findings.2–4 Denning et al. have defined five overlapping forms of CPA that differ in morphological appearance and extent of tissue damage, including simple aspergilloma, chronic cavitary pulmonary aspergillosis, chronic fibrosing pulmonary aspergillosis, Aspergillus nodules and subacute invasive aspergillosis (SAIA).5
Diagnosis is hampered by the absence of a single specific test, instead diagnosis relies on a comprehensive work-up and the exclusion of relevant differential diagnosis.6 Treatment aims are symptom control, preventing haemoptysis, halting disease progression and eventually cure. Triazoles remain the treatment of choice. Itraconazole has been evaluated in randomized controlled trials.7,8 High-quality evidence for other antifungals is still awaited, however, voriconazole is frequently and successfully used for treatment of CPA in clinical practice.9–11 Another challenge of CPA management is the assessment of treatment response in a consistent manner across the phenotypically diverse entities and patient populations.12
The 2016 European guideline on clinical management of CPA jointly published in 2016 by the European Society for Clinical Microbiology and Infectious Diseases (ESCMID), the European Respiratory Society (ERS) and the European Confederation of Medical Mycology (ECMM) provides recommendations that are applicable to a variety of clinical settings.13 Two years after its publication, an international CPA research network (CPAnet) was established. CPAnet recently published consensus definitions for outcome assessment to guide clinical trial design and patient follow-up.12 In routine clinical work, these recommendations can be difficult to comply with.
In 2018, the ECMM introduced the EQUAL scores (ECMM scores to measure quality of disease management) to provide physicians with an easy but comprehensive guidance and to quantify guideline adherence as surrogate for the quality of diagnostic and therapeutic management. Currently, EQUAL scores are available for candidemia, invasive aspergillosis, cryptococcosis, mucormycosis, fusariosis, Scedosporium-, Lomentospora- and Trichosporon-associated infections.14–20 In several subsequent studies, guideline adherence correlated with patient outcome.21–27
Here, we present an EQUAL Score weighing recommendations from current guidelines for the management of CPA. Clinicians may find this tool useful for measuring guideline adherence and adjusting clinical practices as necessary.
Methods
Key recommendations of the current clinical guidance documents were selected.12,13 In the current guidelines of the ESCMID, ERS and ECMM, the provided quality of the evidence (QoE) and strength of recommendations (SoR) is assessed based on the Grading of Recommendations, Assessment, Development and Evaluation method13; whereas in the recent CPAnet consensus document, recommendations on patient follow-up were proposed based on opinions of 29 experts.12
Each recommendation for management of CPA was assigned to one of the three categories: diagnosis, treatment or follow-up. Score points from one to three were assigned to each item according to the guidelines grading and its clinical relevance for patient management. Thereby, three reflects the highest SoR and QoE (e.g. for AI recommendations) and one the lowest (e.g. CIII). Negative score points were assigned for interventions not recommended or discouraged in current guidelines (e.g. DIII recommendations). Furthermore, results from most recent studies that were considered relevant to clinical management by the authors but have not been implemented in the latest guidance documents were also included and weighted as items.
Results
Twenty-seven items that reflect current recommendations for optimal clinical management of patients with CPA were identified, yielding a total sum of 51 in the EQUAL CPA Score (Figure 1). Diagnostics with a ScoreMax of 27 accounted for the greatest proportion of the total score. Case discussion in a multidisciplinary team conference involving pulmonologists, infectious diseases specialists, microbiologists and other specialities to ensure consensus on diagnosis and therapeutic objectives added three points to the score. The combination of diagnostic tools for direct evidence of the organism in clinical respiratory specimens, indirect serological evidence and radiological assessment were equally ranked with three points each. Identification of the fungal pathogen through direct microscopy and culture and performance of galactomannan testing from a respiratory sample preferentially of bronchoalveolar lavage fluid was weighed with two points each. Additional performance of Aspergillus-specific PCR added another point. Histopathology and microbiological evaluation of biopsy or surgical specimen (three and two points, respectively) was considered important to determine invasive growth of Aspergillus and thereby the severity of disease. Antifungal susceptibility testing (one point) by determining in vitro minimum inhibitory concentrations for antifungals or through Aspergillus-specific PCR for sequencing genes associated with drug resistance to inform about the resistance pattern was included.
Figure 1.
EQUAL CPA Score 2022. Items and score points reflecting guideline recommendations and the maximum score (orange, right column) achievable depending on disease complexity. Abbreviations: BAL, bronchoalveolar lavage; h, hour; IV, intravenous; FDG-PET, [18F]-fluorodeoxyglucose positron emission tomography. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Serological evidence through testing of Aspergillus-specific immunoglobulin G (IgG) as the most reliable marker for CPA was considered crucial in the diagnosis of CPA (three points). Alternatively, if IgG cannot be assessed, precipitin should be determined. In regions where non-fumigatus strains account for a high percentage of cases, these assays might have limitations in diagnosing CPA due to potential false negative results.28Aspergillus-specific IgE and the Aspergillus antigen galactomannan from serum should also be assessed for differential diagnosis. Other Aspergillus-specific antibodies such as IgA and IgM have limited diagnostic value and therefore measurement resulted in subtraction of one point if this was done instead of IgG determination.
Computed tomography (CT) is an essential tool in CPA diagnostics and allows assessing treatment response by follow-up comparison (three points).
Ten treatment recommendations were identified, yielding a maximum possible score of 14 if surgical intervention was indicated and 11 points if surgery was not indicated. Surgery might be a curative option for treatment of localized disease such as simple aspergilloma. Oral triazoles are the standard of care for CPA patients and both itraconazole and voriconazole are drugs of first-line choice (three points). Posaconazole was considered an alternative first-line choice despite insufficient evidence for the treatment efficacy for CPA, whereby a tablet formulation (two points) was preferred over a suspension (one point). Monitoring plasma drug concentrations of posaconazole (any formulation) and voriconazole as well as continued screening for untoward effects of treatment have been considered highly relevant (three points).29
For second-line antifungal treatment in case of intolerance to standard of care, treatment failure or disease progression during first-line antifungal treatment, caspofungin and micafungin or lipid-based amphotericin B are recommended with limited evidence for efficacy (two points), as well as isavuconazole, which authors unanimously agreed to add as an alternative second-line treatment option (two points).30 As with first-line therapy, combination therapy is discouraged and does not result in the accumulation of additional points. Antifungal therapy that was continued for at least 6 months scored three points.
Follow-up of CPA patients is essential to determine treatment response and to evaluate disease progression. The first follow-up visit, including assessment of patient reported symptoms with validated scoring systems (e.g. St. George’s Respiratory Questionnaire), thorough clinical examination and pulmonary function tests at 3–6 months, was assigned three points. Additional points were assigned for follow-up radiological evaluation (three points) and microbiological work-up of respiratory samples via culture (two points) and antibody determination (two points).
Overall, a maximum EQUAL CPA Score of 51 points could be achieved. The score was higher if surgical intervention was indicated (ScoreMax = 49 for first-line treatment vs ScoreMax=46 for first-line treatment if surgery was not indicated) or second-line treatment was indicated (ScoreMax =51 if sugery was indicated vs ScoreMax =48 if surgery was not indicated), reflecting more decision points and higher complexity of the disease management.
Discussion
The EQUAL CPA Score 2022 is a 27-item assessment tool that summarized the diagnostic, treatment and follow-up recommendations from the current guidance documents of ESCMID, ERS and ECMM and CPAnet. Equivalent to the other EQUAL scores, this tool was designed as a bedside instrument for self-assessment of management quality and to support antifungal stewardship.14–20
One of the main objectives of CPA management is to reliably diagnose or rule out CPA when clinically suspected. A multidisciplinary team conference between healthcare professionals with different medical expertise is crucial for obtaining consensus on the appropriate diagnostic and therapeutic approaches in difficult to manage fungal infections. For pulmonary diseases as for CPA, a team conference should involve experts in the fields of pulmonology, infectious diseases and microbiology, as well as other specialities (e.g. immunology, radiology, thoracic surgery) as appropriate. A CPA diagnosis relies on a combination of radiological and microbiological, histopathological and serological methods. For microbiological disease evaluation, bronchoalveolar lavage is preferred over sputum samples, however, bronchoscopy might be contraindicated in some cases leaving sputum as the alternative type of sample. Comprehensive microbiological work-up including direct microscopy, culture, nucleic acid amplification testing and galactomannan assay is recommended. The usefulness of sputum galactomannan analysis remains controversial.31 Histopathology and culture results from biopsy or surgical resection is recommended to confirm disease stage and to exclude the numerous differential diagnoses, including other infectious diseases and malignancies. Repeated antifungal susceptibility testing informs about resistance development, especially in patients undergoing long-term antifungal treatment. In vitro susceptibility data guide treatment decisions and are useful for in-house disease surveillance and epidemiological investigations. In regions with high incidence of resistance reported for Aspergillus, in vitro susceptibility testing or alternative methods such as resistance gene sequencing especially in culture-negative cases are recommended.32
Serum marker detection is considered the most effective method for the diagnosis of CPA; however, many different methods exist, and data on cut-off values, sensitivity and reproducibility are limited. Elevated Aspergillus-specific IgG is found in most cases of CPA but is also common in allergic bronchopulmonary aspergillosis and invasive aspergillosis, as well as in patients with lung diseases that may mimic CPA.33 Other serological markers i.e. Aspergillus-specific IgE and galactomannan also present a considerable overlap to other forms of aspergillosis.34 This underlines the necessity for a combined approach to diagnose CPA. Diagnosis of CPA is particularly complex and crucial for subsequent decisions on disease management. The complexity of the diagnostic work-up is reflected by the highest score possible to achieve in the EQUAL Score in comparison to treatment and follow-up.
Imaging is an important component for the diagnosis and response assessment for CPA. There is continuous evidence building that [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT can be used to estimate disease activity in infectious diseases including CPA.6,35 However, conventional diagnostics such as CT is often preferred over PET/CT as patient preparation and scanning is less time consuming and radiation dose is lower. The author group considers [18F]-FDG-PET/CT a useful alternative to CT for response assessment in CPA if appropriate baseline imaging is available. Regardless of imaging modality, histology is indispensable if radiologic features are suggestive of malignancy.
Recent advances in the understanding of antifungal pharmacokinetics are not represented in the most recent CPA guideline.13 For posaconazole, delayed release tablets with a more predictable pharmacokinetic profile than the oral suspension became available. The second-generation triazole isavuconazole with efficacy against a broad spectrum of fungi has been approved for treatment of invasive aspergillosis and mucormycosis. Its favourable safety profile is beneficial for the long treatment durations required in CPA patients but costs are high compared to the older triazoles. Posaconazole and voriconazole serum concentrations have non-linear pharmacokinetics with interpatient variability influenced by various factors, thus, therapeutic drug monitoring is required during treatment.
All echinocandins are considered to demonstrate equal clinical efficacy in fungal infections.36 In CPA, the highest quality of evidence has been obtained with the use of micafungin.37 In the light of data showing an association of micafungin with the development of hepatocellular carcinoma in rats, caspofungin may be the drug of choice in the absence of contraindications.38 Caspofungin and micafungin demonstrate similar efficacy to voriconazole in the treatment of CPA with a favourable safety profile, which makes them preferred second-line choices. An alternative with a less favourable safety profile is lipid-based amphotericin B, which should be preferred over amphotericin B deoxycholate. The added value of inhaled amphotericin B for treatment of CPA has not been comprehensively evaluated for all subtypes but first studies suggested a beneficial effect.39 Association of in vivo efficacy of antifungal drugs and clinical outcome must be considered as an interplay with patients underlying condition and its control during clinical course, as prognosis may vary widely between patient populations at risk.
In the 2016 guideline of the ESCMID, ERS and ECMM, a treatment duration of 4–6 months for initial therapy is recommended.13 A significant relapse rate in cases with discontinued antifungal treatment after 6 months has been described.7 A recent study suggests that a longer treatment duration of 12 months prevents relapses more effectively compared to 6 months of treatment.40 Therefore, we consider a treatment duration of up to 12 months appropriate.
The EQUAL CPA Score should be used considering its limitations. With the paucity of comprehensive studies on CPA, strong guideline recommendations are still awaited. Given the low level of scientific evidences available to support recommendations for clinical management of CPA, the correlation between the score and patient outcome remains unknown and will be assessed in future studies. In addition, the diagnosis of CPA requires a high level of clinical suspicion, and when CPA is considered as a differential diagnosis, this is usually because of a high level of expertise in the respective centre. Therefore, the differences in clinical management are unlikely to be as pronounced as in more common infections such as candidemia. Furthermore, we did not differentiate the management of the proposed CPA subtypes in detail, as subtypes overlap and evolve over time, making it difficult to build on high-quality evidence.
Our scoring tool includes recommendation that cannot be found in currently used guidance documents. Despite the overall aim to provide a tool to quantify guideline adherence, our scoring system is intended to be used as a bedside tool for self-assessment that may affect the clinical practice. For educational purposes and to ensure optimal patient management, aspects considered relevant by experts in the field that did not find their way into guidelines yet should be considered.
An update of the EQUAL CPA Score is necessary if new evidence becomes available; this may include data for new antifungal agents that are currently in late-stage clinical development, including drugs with enhanced pharmacokinetic properties such as the second-generation echinocandin rezafungin or novel antifungals with oral route of administration such as fosmanogepix or ibrexafungerp.41
Conclusion
The EQUAL CPA Score provides a tool for clinicians to easily follow current guideline recommendations for the clinical management of patients with CPA. Adherence to guidelines is particularly challenging for complex diseases such as CPA that are therefore often left undiagnosed and untreated if expertise in a health care facility is limited. The EQUAL CPA Score may be used as an easy self-assessment tool in clinical practice.
Contributor Information
Rosanne Sprute, Faculty of Medicine and University Hospital Cologne, University of Cologne, Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, University of Cologne, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf (CIO ABCD) and Excellence Center for Medical Mycology (ECMM), Cologne, Germany; German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, Cologne, Germany.
Eva Van Braeckel, Department of Internal Medicine and Paediatrics, Ghent University, Ghent, Belgium; Department of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium.
Holger Flick, Division of Pulmonology, Department of Internal Medicine, Medical University of Graz, Graz, Austria.
Martin Hoenigl, Division of Infectious Diseases, Medical University of Graz, Graz, Austria; Biotech Med, Graz, Austria.
Chris Kosmidis, Division of Evolution, Infection and Genomics, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, University of Manchester, Manchester, UK.
Ritesh Agarwal, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Jesper R Davidsen, Department of Respiratory Medicine, Pulmonary Aspergillosis Center Denmark (PACD), Odense University Hospital, Odense, Denmark; Department of Clinical Research, Odense Respiratory Research Unit (ODIN), University of Southern Denmark, Odense, Denmark.
Christian B Laursen, Department of Respiratory Medicine, Pulmonary Aspergillosis Center Denmark (PACD), Odense University Hospital, Odense, Denmark; Department of Clinical Research, Odense Respiratory Research Unit (ODIN), University of Southern Denmark, Odense, Denmark.
Oliver A Cornely, Faculty of Medicine and University Hospital Cologne, University of Cologne, Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, University of Cologne, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf (CIO ABCD) and Excellence Center for Medical Mycology (ECMM), Cologne, Germany; German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, Cologne, Germany; Faculty of Medicine and University Hospital Cologne, University of Cologne, Clinical Trials Centre Cologne (ZKS Köln), Cologne, Germany.
Danila Seidel, Faculty of Medicine and University Hospital Cologne, University of Cologne, Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, University of Cologne, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf (CIO ABCD) and Excellence Center for Medical Mycology (ECMM), Cologne, Germany; German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, Cologne, Germany.
Funding
This study was carried out as part of our routine work.
Transparency declarations
RS reports lecture honoraria from Pfizer, outside the submitted work. EB received travel support and speaker fees, all paid to her institution, from Pfizer, Gilead and MSD. HF received travel support and speaker fees from Pfizer, Gilead, Astellas, Roche, Boehringer Ingelheim, Chiesi, Actelion, Merck/MSD, Actavis, AstraZeneca, BMS, GSK, INSMED, Novartis and Sandoz. MH reports grants and personal fees from Astellas, Pfizter, Gilead, MSD, Pulmocide, F2G, Mundipharma, Scynexis and Euroimmune. JRD reports personal fees from Roche, Boehringer Ingelheim and Chiesi for lectures, and has received support for congresses, attending meetings and travel, all outside the submitted work. RA reports receiving grants from Cipla Pharmaceuticals, India, outside of the submitted work. JRD reports personal fees from Roche, Boehringer Ingelheim and Chiesi for lectures, and has received support for congresses, attending meetings and travel, all outside the submitted work. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Abbvie, Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, IQVIA, Janssen, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pardes, Pfizer, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Abbvie, Al-Jazeera Pharmaceuticals, Astellas, Gilead, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Noscendo, Pfizer, Shionogi; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, Pulmocide, Shionogi, The Prime Meridian Group; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Stocks from CoRe Consulting; Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley. DS reports grants and lecture honoraria from Pfizer outside the submitted work. CK and CBL have nothing to disclose.
References
- 1. Brown GD, Denning DW, Gow NAet al. Hidden killers: human fungal infections. Sci Transl Med 2012; 4: 165rv13. 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 2. Sprute R, Salzer HJF, Seidel D. CPAnet: the challenges of gaining evidence-based knowledge in chronic pulmonary aspergillosis. Eur Respir J 2022; 59: 2102879. 10.1183/13993003.02879-2021 [DOI] [PubMed] [Google Scholar]
- 3. Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141: 121–31. 10.1016/j.rmed.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 4. Laursen CB, Davidsen JR, Van Acker Let al. CPAnet registry—an international chronic pulmonary aspergillosis registry. J Fungi (Basel) 2020; 6: 96. 10.3390/jof6030096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denning DW, Riniotis K, Dobrashian Ret al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003; 37Suppl 3: S265–80. 10.1086/376526 [DOI] [PubMed] [Google Scholar]
- 6. Jenks JD, Salzer HJF, Hoenigl M. Improving the rates of Aspergillus detection: an update on current diagnostic strategies. Expert Rev Anti Infect Ther 2019; 17: 39–50. 10.1080/14787210.2018.1558054 [DOI] [PubMed] [Google Scholar]
- 7. Agarwal R, Vishwanath G, Aggarwal ANet al. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses 2013; 56: 559–70. 10.1111/myc.12075 [DOI] [PubMed] [Google Scholar]
- 8. Sehgal IS, Dhooria S, Prasad KTet al. Anti-fungal agents in the treatment of chronic pulmonary aspergillosis: systematic review and a network meta-analysis. Mycoses 2021; 64: 1053–61. 10.1111/myc.13324 [DOI] [PubMed] [Google Scholar]
- 9. Cucchetto G, Cazzadori A, Conti Met al. Treatment of chronic pulmonary aspergillosis with voriconazole: review of a case series. Infection 2015; 43: 277–86. 10.1007/s15010-014-0711-4 [DOI] [PubMed] [Google Scholar]
- 10. Felton TW, Baxter C, Moore CBet al. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin Infect Dis 2010; 51: 1383–91. 10.1086/657306 [DOI] [PubMed] [Google Scholar]
- 11. Tashiro M, Takazono T, Saijo Tet al. Selection of oral antifungals for initial maintenance therapy in chronic pulmonary aspergillosis: a longitudinal analysis. Clin Infect Dis 2020; 70: 835–42. 10.1093/cid/ciz287 [DOI] [PubMed] [Google Scholar]
- 12. Van Braeckel E, Page I, Davidsen JRet al. Treatment outcome definitions in chronic pulmonary aspergillosis: a CPAnet consensus statement. Eur Respir J 2022; 59: 2102950. 10.1183/13993003.02950-2021 [DOI] [PubMed] [Google Scholar]
- 13. Denning DW, Cadranel J, Beigelman-Aubry Cet al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016; 47: 45–68. 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 14. Cornely OA, Koehler P, Arenz Det al. EQUAL aspergillosis score 2018: an ECMM score derived from current guidelines to measure quality of the clinical management of invasive pulmonary aspergillosis. Mycoses 2018; 61: 833–6. 10.1111/myc.12820 [DOI] [PubMed] [Google Scholar]
- 15. Koehler P, Mellinghoff SC, Lagrou Ket al. Development and validation of the European QUALity (EQUAL) score for mucormycosis management in haematology. J Antimicrob Chemother 2019; 74: 1704–12. 10.1093/jac/dkz051 [DOI] [PubMed] [Google Scholar]
- 16. Spec A, Mejia-Chew C, Powderly WGet al. EQUAL cryptococcus score 2018: a European Confederation of Medical Mycology Score derived from current guidelines to measure quality of clinical cryptococcosis management. Open Forum Infect Dis 2018; 5: ofy299. 10.1093/ofid/ofy299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sprute R, Bethe U, Chen SCet al. EQUAL trichosporon score 2022: an ECMM score to measure quality of the clinical management of invasive trichosporon infections. J Antimicrob Chemother 2022; 77: 1779–84. 10.1093/jac/dkac085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mellinghoff SC, Hoenigl M, Koehler Pet al. EQUAL candida score: an ECMM score derived from current guidelines to measure quality of clinical candidaemia management. Mycoses 2018; 61: 326–30. 10.1111/myc.12746 [DOI] [PubMed] [Google Scholar]
- 19. Guarana M, Nouér SA, Nucci M. EQUAL fusariosis score 2021: a European Confederation of Medical Mycology Score derived from current guidelines to measure QUALity of the clinical management of invasive fusariosis. Mycoses 2021; 64: 1542–5. 10.1111/myc.13321 [DOI] [PubMed] [Google Scholar]
- 20. Stemler J, Lackner M, Chen SCet al. EQUAL score scedosporiosis/lomentosporiosis 2021: a European confederation of medical mycology (ECMM) tool to quantify guideline adherence. J Antimicrob Chemother 2021; 77: 253–8. 10.1093/jac/dkab355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bal AM. European Confederation of medical mycology quality of clinical candidaemia management score: a review of the points based best practice recommendations. Mycoses 2021; 64: 123–31. 10.1111/myc.13196 [DOI] [PubMed] [Google Scholar]
- 22. Bal AM, Palchaudhuri M. Candidaemia in the elderly: epidemiology, management and adherence to the European Confederation of Medical Mycology quality indicators. Mycoses 2020; 63: 892–9. 10.1111/myc.13134 [DOI] [PubMed] [Google Scholar]
- 23. Budin S, Salmanton-García J, Koehler Pet al. Validation of the EQUAL aspergillosis score by analysing guideline-adherent management of invasive pulmonary aspergillosis. J Antimicrob Chemother 2021; 76: 1070–7. 10.1093/jac/dkaa518 [DOI] [PubMed] [Google Scholar]
- 24. Huang HY, Lu PL, Wang YLet al. Usefulness of EQUAL candida score for predicting outcomes in patients with candidaemia: a retrospective cohort study. Clin Microbiol Infect 2020; 26: 1501–6. 10.1016/j.cmi.2020.01.029 [DOI] [PubMed] [Google Scholar]
- 25. Koehler P, Mellinghoff SC, Stemler Jet al. Quantifying guideline adherence in mucormycosis management using the EQUAL score. Mycoses 2020; 63: 343–51. 10.1111/myc.13047 [DOI] [PubMed] [Google Scholar]
- 26. Mellinghoff SC, Hartmann P, Cornely FBet al. Analyzing candidemia guideline adherence identifies opportunities for antifungal stewardship. Eur J Clin Microbiol Infect Dis 2018; 37: 1563–71. 10.1007/s10096-018-3285-8 [DOI] [PubMed] [Google Scholar]
- 27. Nemer S, Imtiaz T, Varikkara Met al. Management of candidaemia with reference to the European Confederation of Medical Mycology quality indicators. Infect Dis (Lond) 2019; 51: 527–33. 10.1080/23744235.2019.1606436 [DOI] [PubMed] [Google Scholar]
- 28. Takeda K, Suzuki J, Watanabe Aet al. Non-fumigatus aspergillus infection associated with a negative aspergillus precipitin test in patients with chronic pulmonary aspergillosis. J Clin Microbiol 2022; 60: e0201821. 10.1128/JCM.02018-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussaini T, Rüping MJ, Farowski Fet al. Therapeutic drug monitoring of voriconazole and posaconazole. Pharmacotherapy 2011; 31: 214–25. 10.1592/phco.31.2.214 [DOI] [PubMed] [Google Scholar]
- 30. Nwankwo L, Gilmartin D, Matharu Set al. Experience of isavuconazole as a salvage therapy in chronic pulmonary fungal disease. J Fungi (Basel) 2022; 8: 362. 10.3390/jof8040362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fayemiwo S, Moore CB, Foden Pet al. Comparative performance of aspergillus galactomannan ELISA and PCR in sputum from patients with ABPA and CPA. J Microbiol Methods 2017; 140: 32–9. 10.1016/j.mimet.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 32. Singh A, Sharma B, Mahto KKet al. High-frequency direct detection of triazole resistance in Aspergillus fumigatus from patients with chronic pulmonary fungal diseases in India. J Fungi (Basel) 2020; 6: 67. 10.3390/jof6020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rønberg R, Davidsen JR, Salzer HJFet al. Prevalence of chronic pulmonary aspergillosis in patients suspected of chest malignancy. J Fungi (Basel) 2022; 8: 297. 10.3390/jof8030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis–quo vadis? Med Mycol 2015; 53: 417–39. 10.1093/mmy/myv020 [DOI] [PubMed] [Google Scholar]
- 35. Schiraldi G, Popescu C, Chiericozzi Met al. Role and sensitivity of positron emission tomography with [18 F] fluorodeoxyglucose in diagnosis and follow-up of patients affected by chronic pulmonary aspergillosis (CPA). J Health Soc Sci 2018; 3: 49–58. [Google Scholar]
- 36. Chen SC, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 2011; 71: 11–41. 10.2165/11585270-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 37. Alastruey-Izquierdo A, Cadranel J, Flick Het al. Treatment of chronic pulmonary aspergillosis: current standards and future perspectives. Respiration 2018; 96: 159–70. 10.1159/000489474 [DOI] [PubMed] [Google Scholar]
- 38. EMA . Summary of the risk management plan. https://www.ema.europa.eu/en/documents/rmp-summary/mycamine-epar-risk-management-plan-summary_en.pdf. Accessed 3 August 2022.
- 39. Ray A, Manikanta J, Singh Ket al. An open-label non-inferiority randomised control trial comparing nebulised amphotericin B with oral itraconazole in patients with pulmonary aspergilloma. Mycoses 2021; 64: 1038–44. 10.1111/myc.13329 [DOI] [PubMed] [Google Scholar]
- 40. Sehgal IS, Dhooria S, Muthu Vet al. Efficacy of 12-months oral itraconazole versus 6-months oral itraconazole to prevent relapses of chronic pulmonary aspergillosis: an open-label, randomised controlled trial in India. Lancet Infect Dis 2022; 22: 1052–61. 10.1016/S1473-3099(22)00057-3 [DOI] [PubMed] [Google Scholar]
- 41. Hoenigl M, Sprute R, Egger Met al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021; 81: 1703–29. 10.1007/s40265-021-01611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]