Version Changes
Revised. Amendments from Version 1
We modified the original article in response to the reviewers’ comments: 1. We added breast cancer to the keywords. 2. In the sixth paragraph within the introduction section we added: “and treatment improvement”.
Abstract
Background: Traditionally, EB-CPGs have been believed to mainly improve the quality and consistency of health care, but this claim must be conclusively proven. We used the Donabedian three-dimensional model (structure, process, and patient outcomes) to assess improvements in the quality of medical care derived from implementing EB-CPGs. This study corresponds to the second systematic review carried out as a series of studies on different clinical issues that aim to evaluate the effectiveness of the application of the EB-CPG for improving the quality of care.
Methods: We followed the methods described by the Cochrane Handbook and presented a descriptive analysis because of the high heterogeneity found across the included studies. We searched the Cochrane Central Register of Controlled Trials, PubMed, and EBSCO Host databases, as well as the grey literature, between 1990 and April 2021. No language restrictions were applied. Only randomised clinical trials (RCTs) were selected.
Results: Of the total of 364 interventions included in the eleven RCTs evaluated, 11 (3%) were related to healthcare structure, 51 (14%) to the healthcare delivery process and 302 (83%) to patient outcomes. Regarding the impact of using the EB-CPGs, in 303 interventions (83%), there were no significant differences between the control and experimental groups. In 4 interventions (1%), the result favoured the control and intervention groups in 57 of the interventions (16%).
Conclusions: Our study showed that EB-CPGs slightly enhanced the quality of health care in the three dimensions described by Donabedian. Future RCTs should improve their design and methodological rigour by considering the certainty of the evidence supporting the EB-CPGs recommendations. In that context, broader analyses could be performed, having more concise hypotheses for further research.
Registration: PROSPERO CRD42020205594
Keywords: Clinical Practice Guidelines; CPG; effect; health care quality.
Introduction
The emergence of the Evidence-Based Clinical Practice Guidelines (EB-CPGs) in the 1990s has improved decision-making for healthcare personnel and patients with different health conditions ( IOM, 1990; Weisz et al., 2007).
Worldwide, significant efforts have been made to develop and implement EB- CPGs based on scientific evidence. The term “evidence-based” means that the recommendations described in the CPGs derive from the best scientific findings and the highest quality of evidence obtained from applying unbiased, transparent, and rigorous methods to support clinical care ( Watters, 2008; Linskey, 2010).
EB-CPGs are statements that include recommendations intended to improve the quality and consistency of health care ( Woolf et al., 1999; Kwan, 2004) and to assist clinical staff and patients in the decision-making process ( Alonso-Coello et al., 2010; IOM, 2011).
This study corresponds to the second systematic review of a series of studies on different clinical subjects, which aim to assess the success of the use of EB-CPGs ( Ramírez-Morera et al., 2019). Therefore, we evaluated the effectiveness of the application of EB-CPGs for the improvement of the quality of health care in three dimensions: structure, process, and patient outcome in the management of breast cancer (Donabedian Model, Donabedian, 1988).
Studies measuring the effects of EB-CPGs on the quality of health care have mainly focused on the effects on clinical practice ( Lugtenberg et al., 2009), state that some international reviews have demonstrated that most of the studies have resulted in significant improvements to the process of care. However, few studies have focused on the effects of measures on patients’ health outcomes.
We considered for this review breast cancer disease because this is the most common malignancy in women around the world ( Ghoncheh et al., 2016). Although survival has improved in the last 30 years mainly because of the implementation of early detection programs and treatment improvement, it still registers 2.3 million new diagnostics in women during 2020 and 685 000 deaths within the same year ( WHO, 2020).
Strategies to control and prevent this type of cancer must be a high priority for health policymakers ( Ghoncheh et al., 2016). According to the above, hundreds of breast cancer guidelines have been published worldwide to reduce its negative impact on men’s and especially on women’s health.
This review is relevant because there is a growing number of EB-CPGs in different essential areas, and their actual impact on relevant outcomes needs to be assessed. Few systematic reviews evaluate the effect of EBGPC in improving health care; these focus solely on one clinical entity and cover a country or region ( Grimshaw et al., 1993; Worrall et al., 1997; Lugtenberg et al., 2009; Ricci-Cabello et al., 2020). For this reason, we reviewed the evidence on the benefits of implementing EB-CPG to improve the quality of care. This review responds to the need for EB-CPG research synthesis on the overall quality of health care delivery.
This systematic review contributes to meeting the need for research synthesis about EB-CPG by assessing the overall quality of health care delivery. Besides, we visualise the need for a systematic review that conclusively demonstrates the pragmatic impact that evidence-based recommendations have on breast cancer patients.
Methods
We conducted a systematic review to identify and analyse the effect of EB-CPGs on health care quality improvement within the Donabedian Model dimensions: structure, process, and results ( Donabedian,1988). We followed the Cochrane Handbook methodological recommendations described by Higgins et al. (2022a).
This review is registered at PROSPERO (ID: CRD42020205594).
Study search
The research question was translated into the PICO framework for guiding the study search and the criteria selection ( Table 1). We developed a method to incorporate the methodological component of the search strategy combined with selected index and free-text terms.
Table 1. Structure of the clinical question.
| Problem | Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|
| Effects of evidence-based clinical practice guidelines for breast cancer in health care quality improvements | Healthcare professionals involved in breast cancer care | EB-CPGs for the management of breast cancer | Standard care for breast cancer | The impact of EB-CPGs for breast cancer on improving the quality of health care (structure, process, patient outcomes) |
We explored the following electronic databases for primary studies: Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Library, including the Cochrane Effective Practice and Organisation of Care (EPOC) group specialised register, Pubmed, Scopus, EBSCO, Academic Search Complete, CINAHL, Biomedical Reference Collection: Comprehensive, APA PsycInfo, Nursing & Allied Health Collection: Comprehensive, Alt HealthWatch, SPORTDiscus with Full Text, Psychology and Behavioral Sciences Collection, Health Source: Nursing/Academic Edition, Biomedical Reference Collection: Basic, AMED - The Allied and Complementary Medicine Database, Consumer Health Complete, Cochrane Database of Systematic Reviews, Cochrane Methodology Register, Rehabilitation & Sports Medicine Source, AgeLine, Global Health, International Pharmaceutical Abstracts, MasterFILE Premier, Rehabilitation & Sports Medicine Source, LILACS, and Health Technology Assessment Database. We also searched the Science Citation Index and Social Sciences Citation Index for papers that refer to studies included in the review.
The PubMed search strategy was executed in the other databases using the appropriate controlled vocabulary. Searching for other resources included grey literature from different sources and hand searching of those high-yield journals and conference proceedings that have not already been hand searched on behalf of the Cochrane Collaboration.
Authors of relevant papers were contacted regarding any further published or unpublished work. Authors of other reviews in the field of effective professional practice were contacted regarding relevant studies of which they may be aware. We searched for studies published between January 1990 and April 2021. The search strategy was not restricted by language. An advanced search strategy and results are available as extended data, Appendix 1 ( Ramírez-Morera et al., 2022).
For the management of bibliographic references of the articles found, the web application “Sciwheel Reference Manager & Generator” was used ( Sciwheel, 2022).
Studies selection
The studies found through the search strategy were screened by two reviewers (AR, JS), and discrepancies about study selection were resolved by a third reviewer (MT). Inclusion criteria were: 1. Randomised Clinical Trial (RCT) or cluster-type RCT measuring the impact of using any implementation model versus passive dissemination or no use of the EB-CPG. 2. The studies evaluated the impact on any of the three domains described in the Donabedian model (structure, process, and patient outcomes) for using EB-GPC in treating breast cancer. 3. No language restriction. 4. Published studies from 1990 to 2021.
Data extraction
Three authors (AR, JS, ALR) independently undertook data extraction. They used a modified version of the Cochrane Collaboration EPOC Group “Data Collection Checklist”, employing an electronic datasheet ( EPOC, 2019).
To assess the bias risk, we used standard Cochrane methods described in chapters 8, 10 and 23 of the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins et al., 2022b; Sterne et al., 2019). In the case of RCTs, bias resulting from several types of systematic errors was assessed according to methods described by Cochrane ( Higgins et al., 2022c) and following the RoB2 instrument ( Sterne et al., 2019).
All studies deemed eligible for the review were assessed independently by the review authors (AR, MT), and discrepancies were resolved by discussion. A summary of the risk of bias assessment is presented as part of the characteristics of included studies table.
We did not find cluster-type RCTs; then, we did not apply what is stated in chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions ( Higgins et al., 2022d) about assessing cluster RCT following the RoB2 instrument ( Sterne et al., 2019).
An analysis of the quality of the evidence related to each of the outcomes was performed using the GRADE approach ( Schünemann et al., 2013). We assessed the certainty of the body of evidence for each key outcome as “high”, “moderate”, “low”, or “very low”, using the GRADEpro GDT platform ( GRADEpro, 2021).
Data were analysed using Review Manager software, version 5.4 ( RevMan, 2020). Revman default templates for data extraction were modified to show the results in a simplified way.
We found very high variability between the measures of effect within the included studies in this review. Then, we decided not to perform a meta-analysis and, therefore, neither to measure statistical heterogeneity.
Results
Study identification and selection
The process describing the analysis of studies retrieved through the systematic search is content in the PRISMA flowchart ( Figure 1, Page et al., 2021). We retrieved 25002 studies from database searching and 20 studies were found from additional sources identified. We excluded 15900 duplicated records, and 6072 studies were excluded after screening by title and abstract. We assessed 83 articles at the full-text level, excluding 72 references which did not meet the selection criteria: 51 (71%) were not randomised controlled trials, and 20 (28%) did not evaluate clinical practice guidelines.
Figure 1. PRISMA flowchart of the studies selection process (PRISMA ; Page et al., 2021).
* The appendix 1 shows the number of records identified from each database or register searched.
** All records were excluded by the authors. Excluded after screening by Title/Abstract.
*** The records were not randomised controlled trials.
**** The records did not evaluate clinical practice guidelines.
***** They published the results included by Irene et al. (2019), whose study described a broader methodology and reported more detailed results.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Stark et al. (2018) was excluded from this systematic review. They published the results included by Irene et al. (2019), whose study described a broader methodology and more detailed reported results. The list of excluded studies and reasons for exclusion are available as extended data, Appendix 2 ( Ramírez-Morera et al., 2022).
Characteristics of the included studies
We included 11 RCTs analysing CPGs for breast cancer ( Boekhout et al., 2015; Greenlee et al., 2016; Grunfeld et al., 2011; Hershman et al., 2013; Irene et al., 2019; Klinkhammer-Schalke et al., 2012; Knobf et al., 2016; Maly et al., 2017; Park et al., 2015; Park et al., 2019; Smith-Turchyn et al., 2020). The list of studies selected after screening and assessing the full text is available as extended data, Appendix 3; Ramírez-Morera et al., 2022).
The trials were published between 2011 and 2020, most from 2014 to 2016 (5, 46%). Approximately 55% (6) were carried out in the United States of America. The clinical practice guidelines examined within the selected trials were follow-up (11, 100%) and treatment (4, 36%). When evaluating the outcome categories involving the clinical practice guidelines quoted, most referred to the quality of life (7, 63%). A summary of the characteristics of the included studies (n=11) is available ( Table 2). A broader description of these characteristics is available as extended data ( Appendix 4; Ramírez-Morera et al., 2022) and the list of the CPGs examined in the included studies ( Appendix 5; Ramírez-Morera et al., 2022).
Table 2. Characteristics of all included studies (n=11).
Percentages exceed 100% because the categories are not mutually exclusive (i.e., some studies involved more than one type of guideline scope and more than one outcome category).
Two different studies ( Maly et al., 2017; Irene et al., 2019) evaluated the CPG for Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update ( Loren et al., 2013). Park et al. (2015) and Smith-Turchyn et al. (2020) analysed the American College of Sports Medicine roundtable on exercise guidelines for cancer survivors ( Schmitz et al., 2010).
Additionally, Maly et al. (2017) examined the latest version of the CPG by Khatcheressian et al. (2013), American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting, previously analysed by Hershman et al. (2013) and Greenlee et al. (2016). They assessed Khatcheressian et al. (2006).
Grunfeld et al. (2011) reported the results until 12 months. Extended results (up to 24 months) for the same study were published by Boekhout et al. (2015). Hershman et al. (2013) and Greenlee et al. (2016) published different results of the same study due to the questionaries or scale utilised for measuring outcomes. The first was more interested in the quality of life and treatment satisfaction, and the second in lifestyle behaviours. We found that Knobf et al. (2016) and Park et al. (2019) published the same study. Knobf et al. (2016) focused on describing the effect of exercise on bone density, while Park et al. (2019) described the results concerning the quality of life.
Risk of bias assessment
We assessed the risk of bias with the RoB2 instrument in the eleven included RCTs following the methods described.
We found that a low risk of bias prevailed (6; 55%) in domain 1: randomisation process. Some concerns occurred in domain 2: deviations from the intended interventions (7; 64%). A low risk of bias was found for domain 3: missing outcome data (10; 91%) and domain 4: measurement of the outcome (8; 73%). Finally, we found some concerns in domain 5: selecting the reported result (7; 64%).
Overall, one study (9%) reported low risk ( Irene et al., 2019), some concerns arise from 6 (55%) studies ( Grunfeld et al., 2011; Hershman et al., 2013; Boekhout et al., 2015; Park et al., 2015; Greenlee et al., 2016; Smith-Turchyn et al., 2020) and high risk occurred in 4 (36%) studies ( Knobf et al., 2016; Klinkhammer-Schalke et al., 2012; Maly et al., 2017; Park et al., 2019). A summary of the results is available in Figure 2.
Figure 2. Analysis of the Risk of Bias (RoB2) for all included studies (n=11).
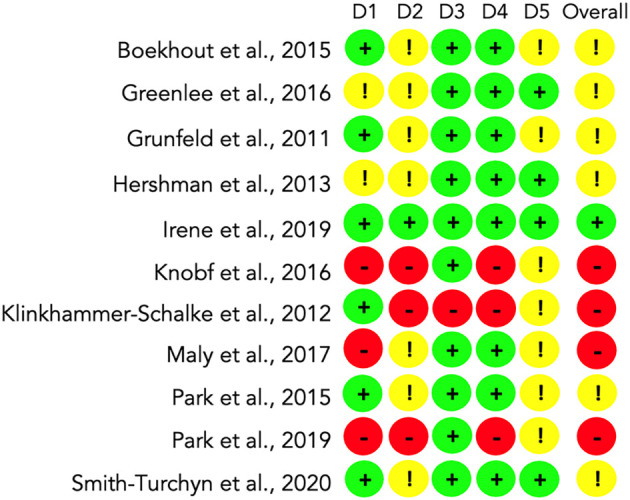
D1: Randomisation process. D2: Deviations from the intended interventions. D3: Missing outcome data. D4: Measurement of the outcome. D5: Selection of the reported result. Overall risk of bias.
Most of the risk of bias found in the included studies occurred due to the lack of existence or some level of blinding. In some cases, outcome data were not available entirely for all randomised participants. Four studies did not analyse the intention to treat, and some outcome measurement methods were not adequately described. A broader description of the risk of bias assessment is available as extended data in Appendix 6 ( Ramírez-Morera et al., 2022).
Quality of evidence assessment
The studies showing the lowest risk of bias ( Irene et al., 2019) and one resulting in the highest risk of bias ( Park et al., 2019) were chosen to be evaluated with the GRADE methodology and to build a summary of the findings. We decided to grade only four outcomes per study described, including two reporting statistically significant results and two not statistically significant results. Performing a GRADE table provided the rank of possible grades for the certainty of the evidence found in the 362 interventions from the 11 studies included.
The results varied from high to very low certainty of the evidence, according to the GRADE classification. We found several types of systematic errors in the studies: random sequence generation (selection bias, in 4 studies), incomplete outcome data (attrition bias, in 3 studies), and imprecision (observed within all the studies, in 303 outcomes evaluated representing 83% of the total).
We found for the study with the lowest risk of bias ( Irene et al., 2019) a range for the certainty of the evidence between high (for fertility-related concerns scale scores ≤3 and improvement with no or low fertility or pregnancy concerns) to moderate certainty of the evidence (for improvement in at least one women’s health issue and 50% decrease in the hot flash score), as described in Table 3a.
Table 3a. Summary of findings: Low risk of bias ( Irene et al., 2019).
| Patient or population: young breast cancer survivors
Setting: web-based, women’s health survivorship care plan (SCP) Intervention: implementation of the recommendations of the CPG Comparison: usual care Outcomes: improve hot flashes, fertility-related concerns, contraception, and vaginal symptoms | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Effects | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Importance | |
| With usual care | With web-based women’s health survivorship care plan (SCP) | |||||
| Fertility-related concerns scale scores ≤3; assessed with: Reproductive Concerns After Cancer scale (RCAC) follow-up: mean 24 weeks | 146 per 1,000 | 279 per 1,000 (158 to 452) | OR 2.27 (1.10 to 4.84) | 182 (1 RCT) | ⨁⨁⨁⨁ High a , b , h | Important |
| Improvement with no or low fertility or pregnancy concerns; assessed with: Reproductive Concerns After Cancer scale (RCAC), follow-up: mean 24 weeks | 304 per 1,000 | 533 per 1,000 (329 to 733) | OR 2.61 (1.12 to 6.29) | 91 (1 RCT) | ⨁⨁⨁⨁ High a , c , h | Important |
| Improvement in at least one women’s health issue, follow-up: mean 24 weeks | 573 per 1,000 | 709 per 1,000 (570 to 820) | OR 1.82 (0.99 to 3.40) | 182 (1 RCT) | ⨁⨁⨁◯ Moderate a , e | Important |
| 50% decrease in hot flash score; assessed with: Hot Flashes Score, follow-up: mean 24 weeks | 552 per 1,000 | 578 per 1,000 (413 to 729) | OR 1.11 (0.57 to 2.18) | 182 (1 RCT) | ⨁⨁⨁◯ Moderate a , f | Important |
CI: confidence interval; OR: odds ratio.
GRADE Working Group grades of evidence: High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Risk of bias. Lack of blinding in clinical staff.
Imprecision. 95% CI: 1.10 to 4.84. p: 0.03. Statistically significant.
Imprecision. 95% CI: 1.12 to 6.29. p: 0.03. Statistically significant.
Imprecision. 95% CI: 0.99 to 3.40. p: 0.057. Not statistically significant. Downgraded -1 for imprecision.
Imprecision. 95% CI: 0.57 to 2.18. p: 0.75. Not statistically significant. Downgraded -1 for imprecision.
Strong association. OR > 2. Large effect. Upgraded +1.
In the case of Park et al. (2019) study reporting a high risk of bias, we found a range for the certainty of the evidence between low (moderate MET-min/wk and walk MET-min/wk at six months) and very low certainty of the evidence (Moderate MET-min/wk and Walk MET-min/wk at 12 months), as described in Table 3b. Our results after grading the certainty of the evidence for both studies were consistent with the findings from the RoB2 instrument.
Table 3b. Summary of findings: High risk of bias ( Park et al., 2019).
| Patient or population: peri-menopausal and early postmenopausal female cancer survival
Setting: Yale Fitness Intervention Trial Intervention: adherence to the ACS guidelines Comparison: usual care Outcome: improvement of the quality of life (QoL) | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Mean effects min/wk ± SD | Absolute effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Importance | |
| With usual care | With adherence to the ACS guidelines | |||||
| Moderate MET-min/wk; assessed with: International Physical Activity Questionnaire (IPAQ), follow-up: mean 6 months | 33.15 ± 123.87 | 681.23 ± 127.38 | 648.08 min/wk (606.091 to 690.069) | 138 (1 RCT) | ⨁⨁◯◯ Low a , b | Important |
| Moderate MET-min/wk; assessed with: International Physical Activity Questionnaire (IPAQ), follow-up: mean 12 months | 38.19 ± 128.25 | 115.69 ± 133.59 | 77.5 min/wk (32.289 to 122.711) | 130 (1 RCT) | ⨁◯◯◯ Very low a , c | Important |
| Walk MET-min/wk; assessed with: International Physical Activity Questionnaire (IPAQ), follow-up: mean 6 months | -178.80 ± 124.52 | 236.63 ± 127.78 | 415.43 min/wk (373.268 to 457.592) | 138 (1 RCT) | ⨁⨁◯◯ Low a , d | Important |
| Walk MET-min/wk; assessed with: International Physical Activity Questionnaire (IPAQ), follow-up: mean 12 months | -126.27 ± 127.75 | 157.37 ± 132.40 | 283.64 min/wk (238.718 to 328.562) | 130 (1 RCT) | ⨁◯◯◯ Very low a , e | Important |
CI: confidence interval.
GRADE Working Group grades of evidence: High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Risk of bias. Lack of allocation concealment. Selection bias. Lack of blinding. Downgraded -2 for risk of bias.
Imprecision. 95% CI: 606.091 to 690.069. p: 0.0004. Statistically significant.
Imprecision. 95% CI: 32.289 to 122.711. p: 0.67. Not statistically significant. Downgraded -1 for imprecision.
Imprecision. 95% CI: 373.268 to 457.592. p: 0.02. Statistically significant.
Imprecision. 95% CI: 238.718 to 328.562. p: 0.12. Not statistically significant. Downgraded -1 for imprecision.
Assessment of the studies outcomes
The outcomes were grouped in simple relative and absolute numbers. A global estimate of the measurements of the effects included in the studies is lacking because of the significant variability of measuring units combined with the clinical heterogeneity found among the studies included.
There was significant variability in the measurement of the outcomes reported in the studies. Most were continuous, e.g., to assess the quality of life, which corresponds to the dimension of patient outcome. A total of 362 were included in the 11 RCTs evaluated; 11 (3%) corresponded to the health care structure dimension, 51 (14%) interventions to the dimension process and 302 (83%) interventions to the dimension of patient outcomes. A broader description of the main findings by the dimensions evaluated in the included studies is available as extended data, Appendix 7 ( Ramírez-Morera et al., 2022).
Regarding the impact of using EB-CPG, we found 303 (83%) interventions with no significant difference between the control and experimental groups. The outcome favoured the control group in 4 (1%). Three outcomes interfered with the patient’s adherence (predictable variables: age, marital status, and hot flashes), as reported by Maly et al. (2017). Also, the fourth outcome informed by Klinkhammer-Schalke et al. (2012) reported rates of therapeutic options for Physiotherapy 16 (experimental group) vs 30 (control group), p: <0.02, at six months. The result favoured the intervention group for 57 interventions (16%) ( Table 4).
Table 4. Summary of results for all included studies by dimensions and effect (n=11).
| Citation | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| Dimension | In favour CPG n (%) | Equal n (%) | In favour control n (%) | Total n (%) | ||||
| Structure | Process | Patient outcome | ||||||
| 1 | Boekhout et al., 2015 | 7 (17%) | 20 (49%) | 14 (34%) | 8 (17%) | 33 (83%) | 0 | 41 (100%) |
| 2 | Greenlee et al., 2016 | 0 | 0 | 16 (100%) | 4 (25%) | 12 (75%) | 0 | 16 (100%) |
| 3 | Grunfeld et al., 2011 | 0 | 6 (25%) | 18 (75%) | 3 (13%) | 21 (87%) | 0 | 24 (100%) |
| 4 | Hershman et al., 2013 | 0 | 0 | 27 (100%) | 2 (8%) | 25 (92%) | 0 | 27 (100%) |
| 5 | Irene et al., 2019 | 0 | 5 (42%) | 7 (58%) | 3 (25%) | 9 (75%) | 0 | 12 (100%) |
| 6 | Klinkhammer-Schalke et al., 2012 | 0 | 8 (12%) | 60 (88%) | 10 (15%) | 57 (84%) | 1 (1%) | 68 (100%) |
| 7 | Knobf et al., 2016 | 0 | 0 | 24 (100%) | 7 (29%) | 17 (71%) | 0 | 24 (100%) |
| 8 | Maly et al., 2017 | 2 (5%) | 10 (23%) | 32 (72%) | 3 (7%) | 38 (86%) | 3 (7%) | 44 (100%) |
| 9 | Park et al., 2015 | 2 (5%) | 0 | 38 (95%) | 6 (15%) | 34 (85%) | 0 | 40 (100%) |
| 10 | Park et al., 2019 | 0 | 0 | 53 (100%) | 6 (11%) | 47 (89%) | 0 | 53 (100%) |
| 11 | Smith-Turchyn et al., 2020 | 0 | 0 | 13 (100%) | 3 (23%) | 10 (77%) | 0 | 13 (100%) |
| TOTAL | 11 (3%) | 51 (14%) | 302 (83%) | 57 (16%) | 303 (83%) | 4 (1%) | 364 (100%) | |
Discussion
For more than two decades, governmental and non-governmental institutions have been making economic and methodological efforts to develop more and better quality EB-CPGs, seeking to deal with different issues most healthcare systems face. Such as the ageing population, rising costs motivated by increased demand for quality care, increasingly expensive emerging health technologies, variability in the provision of health by presuming that part of this disparity could cause inadequate care (either overuse or underuse of supplies), and the desire for clinicians and patients to provide and to receive, respectively, the best possible care with measurable clinical effect. However, it still appears that some of these EB-CPGs are far from contributing to an effective, standardised clinical practice based on the best available evidence ( Woolf et al., 1999; IOM, 2011; Alonso-Coello et al., 2010). We agree with Woolf et al. (1999) that EB-CPGs that promote proven benefits and discourage ineffective interventions could reduce morbidity and mortality, and improve quality of life, at least for some conditions. EB-CPGs can also improve the consistency of care.
The effects of the recommendations in the interventions included in the 11 RCTs considered in this project were in the structure of medical care (3%) and the care provided (14%); both were the least explored. Surprisingly, patient outcomes were the most evaluated domain (83%), with significant results in 43 of 302 (14%), representing 75% of all significant results.
This fact could lead us to suppose that researchers finally focused on the importance of evaluating the patient health, solely prioritising evaluating the adherence to the CPG (assess the process dimension). They are trying to glimpse more clearly what the use of the CPG represents for patients and not only for clinical staff. As this review considered breast cancer, we could not ignore that there is more social and economic pressure to know the patient outcome.
Grimshaw and Russell (1993) findings described in their systematic review, “Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations”, reported more than 80% significant improvements among the included studies. Contrasting their results, in this second systematic review, we found 57 interventions on breast cancer in favour of the use of EB-CPGs distributed in all the studies included (16% of all the interventions evaluated). Then, compared to our first systematic review, only half of the measures with statistically significant results favour using EB-CPGs ( Ramírez-Morera et al., 2019). However, as 75% of these results were found for the dimension of results in patients, we keep optimistic about the finding on incoming reviews an increasingly more significant impact.
Four studies (36%) reported a high risk of bias ( Knobf et al., 2016; Klinkhammer-Schalke et al., 2012; Maly et al., 2017; Park et al., 2019), and some concerns arise from 6 (55%) studies ( Grunfeld et al., 2011; Hershman et al., 2013; Boekhout et al., 2015; Park et al., 2015; Greenlee et al., 2016; Smith-Turchyn et al., 2020). Because of that, we agree with Ivers et al. (2012) that EB-CPGs must be evaluated, including more remarkable methodological quality studies to provide feedback and corrective measures for clinical practice through audits, promoting improvements in the quality of care.
We also concur with Lugtenberg et al. (2009) about the need to focus on the strength of the recommendations for determining what factors influence the use of the guidelines and the improvement of the results of the patients.
Then we identified the need to perform recommendations distinguishing between the level of certainty of evidence (stratified analysis) as this could have a more significant impact on the results when the best available certainty of evidence recommendation is implemented. We keep in mind that explicit EB-CPG improves clinical practice when introduced within a context of rigorous evaluations ( Grimshaw & Russell, 1993; Ricci-Cabello et al., 2020).
Some studies included in the review five reported outcomes in the process area ( Boekhout et al., 2015; Grunfeld et al., 2011; Irene et al., 2019; Klinkhammer-Schalke et al., 2012; Maly et al., 2017). All of them described favourable results for the intervention in 14 of 51 measurements (27%). This fact reflects that researchers have continued to endeavour to measure when EB-CPGs should be used or not, but this time to a much lesser extent (14% vs 64%) when compared to the first review.
Lugtenberg et al. (2009) reported that the size of the effects observed in their systematic review varied considerably between the recommendations within the guidelines. We repeated this finding in our study and the previous one ( Ramírez-Morera et al., 2019). We found that in many evaluated interventions (303, 83%), the use of EB-CPG did not reflect any impact in any dimension. The approach followed to report the current effectiveness of EB-CPGs remains incomplete ( Woolf et al., 1999), and a strategy that captures better results has not yet been found.
EB-CPGs may contribute to improving the quality of healthcare. However, it is still necessary to integrate them with strategies that enhance their use and effect, such as academic and educational visits as part of ongoing training programs ( O’Brien et al., 2007). Repeatedly, advocates for EB-CPGs consider their only existence as a magic solution to solve health care problems; however, they ignore other practical actions that should be implemented along with the guidelines ( Woolf et al., 1999).
EB-CPG has an essential role when clinicians do not clearly know the appropriate practice and which scientific evidence should support their decisions ( Woolf et al., 1999). Then, EB-CPG developers should be vigilant in identifying these needs to help close this information gap and increase the enthusiasm for employing them.
Conclusions
Developing strategies for a more standardised implementation of the EB-CPGs through structured programs within health systems is essential. Improving the awareness of clinical staff about the possibility of enhancing clinical practice and patient outcomes by using evidence-based recommendations with an expected effect is a need.
There is an imbalance between the number of EB-CPGs developed for breast cancer and the number of high-quality studies evaluating their effectiveness. Due to the limited results found on the benefit of using EB-CPG, we must continue investigating the subject. We could gradually structure a more robust hypothesis about the variables influencing this issue.
The variation in the effects found for the recommendations included in the EB-CPGs suggests that it would be helpful to change strategies and focus on the analysis of the limitations of adherence and on designing implementation approaches adapting each recommendation.
In addition, future RCTs should distinguish the levels of certainty of the evidence supporting each recommendation in their evaluations. Researchers should focus on evaluating recommendations expected to have the most significant impact (superior levels of certainty of the evidence: High or Moderate).
More research is necessary to define which factors related to the implementation of EB-CPG and its specific recommendations are essential to predict the application of EB-CPG and, therefore, achieve better patient results.
Implications for the practice
This systematic review aimed to support the development of programs evaluating the effects of EB-CPG on the quality of health care. Also, to provide reliable evidence sustaining the decision-making process related to the production of EB-CPGs.
Even when some of the results of this systematic review were statistically significant, supporting the use of EB-CPG as a tool to improve clinical practice and quality of care, the results of this review need to be interpreted with caution.
The implementation of EB-CPGs must consider the differences in the measures of effect to define customised approaches and specific recommendations within the guideline to enhance health care.
For the adequate implementation of EB-CPGs, it is necessary to consider the possible costs, risks, and benefits and the expected effects derived from EB-CPGs recommendations. The efforts to build EB-CPGs must be complemented with psychosocial strategies promoting health personnel to follow the recommendations of the EB-CPG and evaluate their impact.
Greater methodological rigour in the development of CPGs is needed. It is also required to carry out this process within a standardised formal program in the health systems. Greater credibility could be achieved if recommendations are based on the best available evidence, improving the credibility of their positive effect among health personnel. It could also lead to a more willingness to implement the CPGs and to participate in evaluating their impact.
Implications for the research
This study corresponds to the second systematic review of a series of studies aiming to assess the effect of Evidence-Based Clinical Practice Guidelines (EB-CPGs) on improving health care quality. Our next and last project will investigate Covid-19 disease.
Due to recent research in this field, and the results of this study were not conclusive, more research is necessary to evaluate how EB-CPGs could impact the quality of health care, especially emphasising fewer investigated areas, such as the structure of healthcare services and patient outcomes.
Additionally, the design and methodological rigour applied to future RCTs should improve by considering the certainty of the evidence supporting the EB-CPGs recommendations. Besides, focusing on those with a greater level of evidence (high or moderate) which could lead to determining more clearly the effect that these recommendations have on the quality of health care.
Data (and software) availability
Data availability
Underlying data
All data underlying the results are available as part of the article, and no additional source data are required.
Extended data
Open Science Framework: Extended data for the second SR CPG Breast Cancer. DOI: https://osf.io/6h9pm/?view_only= ( Ramírez-Morera et al., 2022).
This project contains the following extended data:
Appendix 1. Advanced search strategy and results.pdf
Appendix 2. List of excluded studies and reasons for exclusion.pdf
Appendix 3. List of selected studies after screening and assessing the full text.pdf
Appendix 4. Characteristics of the included studies.pdf
Appendix 5. List of the CPGs examined in the included studies.pdf
Appendix 6. Risk of Bias 2 assessment.pdf
Appendix 7. Main findings by dimensions of the included studies.pdf
Reporting guidelines
We followed the PRISMA 2020 statement for reporting systematic reviews ( Page et al., 2021). We did the PRISMA 2020 checklist and the flow diagram for new systematic reviews, which included searches of databases, registers, and other sources.
Open Science Framework: PRISMA checklist and flow chart for Effects of evidence-based clinical practice guidelines for breast cancer in health care quality improvements. A second systematic review. DOI: https://osf.io/k7f5x/?view_only=7d6b4a63853c43e4b233424ea226e66a.
Author contributions
Anggie Ramírez-Morera: Conceptualisation, Data Curation, Formal Analysis, Funding Acquisition, Methodology, Project Administration, Writing – Original Draft Preparation, Writing – Review & Editing.
Mario Tristán: Conceptualisation, Data Curation, Formal Analysis, Funding Acquisition, Methodology, Resources, Software, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing.
Jordan Salazar-Vargas: Data Curation, Formal Analysis, Funding Acquisition, Resources, Writing – Original Draft Preparation, Writing – Review & Editing.
Ana Leonor Rivera-Chavarría: Data Curation, Writing – Original Draft Preparation, Writing – Review & Editing.
Leading author information
Anggie Ramírez-Morera is PhD candidate Program in Biomedical Research Methodology and Public Health, Universitat Autònoma de Barcelona.
Funding Statement
IHCAI Foundation, the sponsor of the Cochrane Center for Central America and the Spanish-speaking Caribbean, provided the necessary to carry out this review, such as access to databases, full-text articles, and other resources. The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- Alonso-Coello P, et al. : The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual. Saf. Health Care. 2010;19(6):e58. 10.1136/qshc.2010.042077 [DOI] [PubMed] [Google Scholar]
- Boekhout AH, et al. : A survivorship care plan for breast cancer survivors: extended results of a randomised clinical trial. J. Cancer Surviv. 2015;9(4):683–691. 10.1007/s11764-015-0443-1 [DOI] [PubMed] [Google Scholar]
- Donabedian A: The quality of care. How can it be assessed? J. Am. Med. Assoc. 1988;260(12):1743–1748. 10.1001/jama.260.12.1743 [DOI] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care (EPOC): Data Collection Checklist. EPOC Resources for review authors, 2017. (updated March 2019). Cochrane. Reference Source
- Greenlee H, et al. : Survivorship care plans and adherence to lifestyle recommendations among breast cancer survivors. J. Cancer Surviv. 2016;10(6):956–963. 10.1007/s11764-016-0541-8 [DOI] [PubMed] [Google Scholar]
- Ghoncheh M, Pournamdar Z, Salehiniya H: Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016;17(S3):43–46. 10.7314/apjcp.2016.17.s3.43 [DOI] [PubMed] [Google Scholar]
- GRADEpro GDT: GRADEpro Guideline Development Tool: [Computer program]. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.). 2021. Reference Source
- Grimshaw JM, Russell IT: Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883):1317–1322. 10.1016/0140-6736(93)92244-n [DOI] [PubMed] [Google Scholar]
- Grunfeld E, et al. : Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update). CMAJ. 2005;172(10):1319–1320. 10.1503/cmaj.045062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld E, et al. : Evaluating survivorship care plans: results of a randomised, clinical trial of patients with breast cancer. J. Clin. Oncol. 2011;29(36):4755–4762. 10.1200/JCO.2011.36.8373 [DOI] [PubMed] [Google Scholar]
- Hershman DL, et al. : Randomised controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res. Treat. 2013;138(3):795–806. 10.1007/s10549-013-2486-1 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, et al. : Cochrane Handbook for Systematic Reviews of Interventions version 6.3. (updated February 2022). Cochrane. 2022a. Reference Source
- Higgins JPT, et al. : Chapter 6: Choosing effect measures and computing estimates of effect. Higgins JPT, Thomas J, Chandler J, et al.(editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane;2022b. Reference Source [Google Scholar]
- Higgins JPT, et al. : Chapter 8: Assessing risk of bias in a randomised trial. Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane;2022c. Reference Source [Google Scholar]
- Higgins JPT, et al. : Chapter 23: Including variants on randomised trials. Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane;2022d. Reference Source [Google Scholar]
- Ivers N, et al. : Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012;6:CD000259. 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines: Clinical practice guidelines: directions for a new program. Field MJ, Lohr KN, editors. Washington (DC): National Academies Press (US);1990. 10.17226/1626 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines: Clinical practice guidelines we can trust. Graham R, et al., editors. Washington (DC): National Academies Press (US);2011. 10.17226/13058 [DOI] [PubMed] [Google Scholar]
- Irene S, H., et al. : Efficacy of a web-based women’s health survivorship care plan for young breast cancer survivors: a randomised controlled trial. Breast Cancer Res. Treat. 2019;176(3):579–589. 10.1007/s10549-019-05260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatcheressian JL, et al. : American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J. Clin. Oncol. 2006;24(31):5091–5097. 10.1200/JCO.2006.08.8575 [DOI] [PubMed] [Google Scholar]
- Khatcheressian JL, et al. : Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013;31(7):961–965. 10.1200/JCO.2012.45.9859 [DOI] [PubMed] [Google Scholar]
- Klinkhammer-Schalke M, et al. : Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. Br. J. Cancer. 2012;106(5):826–838. 10.1038/bjc.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobf MT, et al. : Effect of a randomised controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res. Treat. 2016;155(3):491–500. 10.1007/s10549-016-3693-3 [DOI] [PubMed] [Google Scholar]
- Kwan TJ: Do evidence-based guidelines bridge the gaps in research?. Bridging the gaps. Abstracts of the 12th Cochrane Colloquium. 2004 2-6 Oct; Ottawa, Canada. 2004.
- Loren AW, et al. : Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013;31(19):2500–2510. 10.1200/JCO.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskey ME: Defining excellence in evidence-based medicine clinical practice guidelines. Clin. Neurosurg. 2010;57:28–37. [PubMed] [Google Scholar]
- Lugtenberg M, Burgers JS, Westert GP: Effects of evidence-based clinical practice guidelines on quality of care: a systematic review. Qual. Saf. Health Care. 2009;18(5):385–392. 10.1136/qshc.2008.028043 [DOI] [PubMed] [Google Scholar]
- Maly RC, et al. : Randomized Controlled Trial of Survivorship Care Plans Among Low-Income, Predominantly Latina Breast Cancer Survivors. J. Clin. Oncol. 2017;35(16):1814–1821. 10.1200/JCO.2016.68.9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MA, et al. : Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2007;4:CD000409. 10.1002/14651858.CD000409.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, et al. : The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.). 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, et al. : The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomised controlled trial. Cancer. 2015;121(16):2740–2748. 10.1002/cncr.29400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-H, et al. : Adherence to American cancer society guidelines on nutrition and physical activity in female cancer survivors: results from a randomised controlled trial (yale fitness intervention trial). Cancer Nurs. 2019;42(3):242–250. 10.1097/NCC.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Morera A, Tristan M, Vazquez JC: Effects of evidence-based clinical practice guidelines in cardiovascular health care quality improvements: A systematic review [version 3; peer review: 2 approved]. F1000Res. 2019;8:1041. 10.12688/f1000research.18865.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Morera A, et al. : Extended data for the second SR CPG Breast Cancer. osf.io/6h9pm. 2022.
- Review Manager (RevMan): [Computer program]. Version 5.4.1 for Mac. The Cochrane Collaboration;2020. [Google Scholar]
- Ricci-Cabello I, et al. : Adherence to breast cancer guidelines is associated with better survival outcomes: a systematic review and meta-analysis of observational studies in EU countries. BMC Health Serv. Res. 2020;20(1):920. 10.1186/s12913-020-05753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann H, et al. : GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. 2013. 2013. Reference Source
- Schmitz KH, et al. : American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010;42(7):1409–1426. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- Sciwheel Reference Manager & Generator: Computer program. MacOS Monterey. London: Sciwheel Ltd.;2022. [Google Scholar]
- Smith-Turchyn J, et al. : Bridging the gap: incorporating exercise evidence into clinical practice in breast cancer care. Support Care Cancer. 2020;28(2):897–905. 10.1007/s00520-019-04897-9 [DOI] [PubMed] [Google Scholar]
- Sterne JAC, et al. : RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.). 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Stark SS, et al. : Randomised controlled trial of the effect of a reproductive health survivorship care plan on fertility and pregnancy concerns, vasomotor symptoms, sexual health, and contraception in young breast cancer survivors. Fertil. Steril. 2018;110(4):e48. 10.1016/j.fertnstert.2018.07.149 [DOI] [Google Scholar]
- Watters W: Defining evidence-based clinical practice guidelines. “AOOS Now”. 2008. American Academy of Orthopaedic Surgeons. RESEARCH2. Reference Source Reference Source
- Weisz G, et al. : The Emergence of Clinical Practice Guidelines. Milbank Q. 2007;85(4):691–727. 10.1111/j.1468-0009.2007.00505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO: Breast Cancer. Cancer prevention. Early Diagnosis and Screening. Geneva: World Health Organization;2020. Reference Source [Google Scholar]
- Woolf SH, et al. : Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ (Clinical Research Ed.). 1999;318(7182):527–530. 10.1136/bmj.318.7182.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall G, Chaulk P, Freake D: The effects of clinical practice guidelines on patient outcomes in primary care: a systematic review. Can. Med. Assoc. J. 1997;156(12):1705–1712. [PMC free article] [PubMed] [Google Scholar]



