Graphical abstract
Keywords: COVID19, COVID19 vaccination, SARS-CoV2 vaccine, AEFI, Vaccine side effect
Abbreviations: ACE-2, angiotensin-converting enzyme 2; ADEM, Acute disseminated encephalomyelitis; AEFI, Adverse events following immunization; AHEM, Acute haemorrhagic encephalomyelitis; BBB, blood–brain barrier; CLOCC, Cytotoxic Lesion of the Corpus Callosum; COVID-19, Coronavirus disease 2019; CSF, cerebrospinal fluid; EEG, electroencephalography; GBS, Guillain-Barré syndrome; IVIg, intravenous immunoglobulin; IQR, Interquartile range; MeSH, Medical Subject Headings; MS, Multiple Sclerosis; MOG, anti-Myelin oligodendrocyte-glycoprotein; MOGAD, MOG associated demyelination; NMDAR, N-methyl-d-aspartate receptor; NMO, neuromyelitis optica; NMOSD, Neuromyelitis optica spectrum disorders; OCB, oligoclonal bands; PLEX, plasma exchange; RTPCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, Standard deviation; VGKC, voltage-gated potassium channel; VVr, viral vector replicating; VVnr, viral vector non-replicating; WHO GACVS, World Health Organization Global Advisory Committee on Vaccine safety
Abstract
Background
Recent studies have shown various neurological adverse events associated with COVID-19 vaccine.
Objective
We aimed to retrospectively review and report the neurological diseases temporally associated with COVID-19 vaccine.
Methods
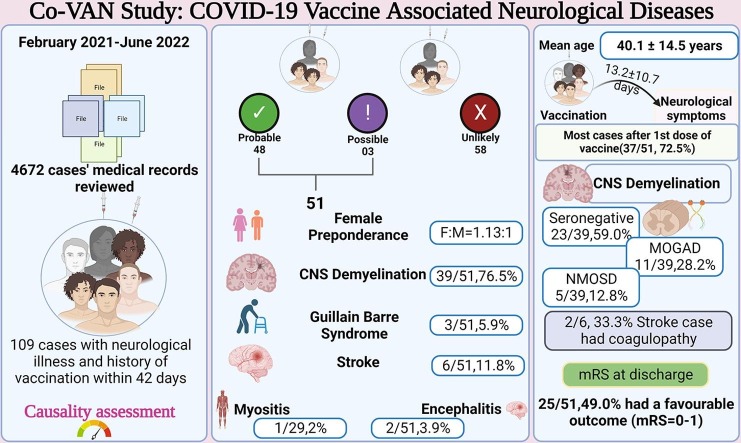
We performed a retrospective chart review of admitted patients from 1st February 2021 to 30th June 2022. A total of 4672 medical records were reviewed of which 51 cases were identified to have neurological illness temporally associated with COVID-19 vaccination.
Results
Out of 51 cases, 48 had probable association with COVID-19 vaccination while three had possible association. Neurological spectrum included CNS demyelination (n = 39, 76.5 %), Guillain-Barré-syndrome (n = 3, 5.9 %), stroke (n = 6, 11.8 %), encephalitis (n = 2, 3.9 %) and myositis (n = 1, 2.0 %). Female gender had a greater predisposition (F:M, 1.13:1). Neurological events were more commonly encountered after the first-dose (n = 37, 72.5%). The mean latency to onset of symptoms was 13.2 ± 10.7 days after the last dose of vaccination. COVIShield (ChAdOx1) was the most commonly administered vaccine (n = 43, 84.3 %). Majority of the cases with demyelination were seronegative (n = 23, 59.0 %) which was followed by anti-Myelin oligodendrocyte-glycoprotein associated demyelination (MOGAD) (n = 11, 28.2 %) and Neuromyelitis optica (NMOSD) (n = 5, 12.8 %). Out of 6 Stroke cases, 2 cases (33.3 %) had thrombocytopenia and coagulopathy. At discharge, 25/51 (49.0 %) of the cases had favourable outcome (mRS 0 to 1). Among six patients of stroke, only one of them had favourable outcome.
Conclusion
In this series, we describe the wide variety of neurological syndromes temporally associated with COVID-19 vaccination. Further studies with larger sample size and longer duration of follow-up are needed to prove or disprove causality association of these syndromes with COVID-19 vaccination.
1. Introduction
In the recent years the world has witnessed an unprecedented challenge of the Coronavirus disease 2019 (COVID19) pandemic caused by a beta coronavirus, the novel severe acute respiratory syndrome coronavirus2 (SARS-CoV2). Vaccination against this virus has emerged as one of the most efficient armours in curbing the pandemic. Several candidate vaccines have been tried and tested in clinical trials. (Refer to Table 1 ). As of 25th March 2022, a total of 153 candidate vaccines are undergoing various phases of clinical trials, whereas 196 candidates are in pre-clinical development. [1] Based on variations in core ingredients and delivery systems, several types of vaccines such as mRNA-1273, viral vector replicating (VVr), viral vector non-replicating(VVnr), inactivated virus, live attenuated, protein subunit, DNA, virus-like particle, Bacterial antigen-spore expression vector, Despite their efficacy, the adverse events following vaccination have also been seen. [2], [3], [4], [5], [6] Many databases including Vaccine Adverse Event Reporting System (VARES), and VigiBase have been dedicated to report these adverse events. A large spectrum indeed has been detected so far. In line with rheumatological, hematological, and cardiac adverse events, neurological complications following COVID19 vaccination have also been witnessed. [7], [8], [9], [10], [11].
Table 1.
Details of vaccines against SARS-CoV2 and its approval and dosing count in India.
| Vaccine generic | Brands | Type of vaccine | Manufacturer | Status in India |
|---|---|---|---|---|
| AZD1222 (ChAdOx1) | COVID-19 Vaccine AstraZeneca, Covishield, Vaxzevria | Adenovirus vaccine | BARDA, OWS, Serum Institute of India | Approved in India, Total vaccine doses administered as on 26/03/22 is 1,50,80,58,152 |
| BBV152 | Covaxin | Inactivated vaccine | Bharat Biotech, ICMR; Ocugen; ViroVax | Approved in India, Total vaccine doses administered as on 26/03/22 is 30,52,68,845 |
| rAd26 and rAd5 | Sputnik V | Recombinant adenovirus vaccine | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Approved in India, Total vaccine doses administered as on 26/03/22 is 12,21,106 |
| Corbevax | Corbevax | Adjuvanted protein subunit vaccine | Biological E, Baylor College of Medicine, Dynavax, CEPI | Approved in India, Total vaccine doses administered as on 26/03/22 is 1,20,88,254 |
| BNT162b2 | COMIRNATY | mRNA-based vaccine | Pfizer, BioNTech, Fosun Pharma | Approved in India |
| ZyCoV-D | ZyCoV-D | DNA vaccine (plasmid) | Zydus Cadila | Approved in India |
| mRNA-1273 | Spikevax | mRNA-based vaccine | Moderna, BARDA, NIAID | Approved in India |
| rAd26 | Sputnik Light | Recombinant adenovirus vaccine | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Approved in India |
| NVX-CoV2373 | Covovax (India), TAK-019(Japan) Nuvaxovid, |
Prefusion protein recombinant nanoparticle vaccine | Novavax; CEPI, Serum Institute of India | Approved in India |
| Sinopharm COVID-19 Vaccine (BBIBP-CorV) | BBIBP-CorV/NVSI-06–07 | Inactivated vaccine | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | |
| EpiVacCorona/ (Aurora-CoV) | EpiVacCorona | Peptide vaccine | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | |
| JNJ-78436735; Ad26.COV2.S | Janssen | Non-replicating viral vector | Janssen Vaccines (Johnson & Johnson) | |
| CoviVac | CoviVac | Inactivated vaccine | Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products | |
| ZIFIVAX | ZF2001 | Recombinant vaccine | Anhui Zhifei Longcom Biopharmaceutical, Institute of Microbiology of the Chinese Academy of Sciences | |
| QazCovid-in | QazVac | Inactivated vaccine | Research Institute for Biological Safety Problems | |
| CoronaVac (formerly PiCoVacc) | CoronaVac | formalin-inactivated and alum-adjuvanted vaccine | Sinovac | |
| Convidicea (Ad5-nCoV) | Ad5-nCoV /PakVac | Recombinant vaccine (adenovirus type 5 vector) | CanSino Biologics | |
| WIBP-CorV | WIBP-CorV | Inactivated vaccine | Wuhan Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | |
| COVIran Barekat | COVIran Barekat | Inactivated vaccine | Shifa Pharmed Industrial Group | |
| CIGB 66 | Abdala | Protein subunit vaccine | Center for Genetic Engineering and Biotechnology | |
| Soberana 02/Soberana Plus | Soberana 02/Soberana Plus | Conjugate vaccine | Finlay Institute of Vaccines; Pasteur Institute | |
| MVC-COV1901 | MVC-COV1901 | Protein subunit vaccine | Medigen Vaccine Biologics Corp.; Dynavax | |
| COVAX-19 | Spikogen | Monovalent recombinant protein vaccine | Vaxine Pty ltd.; CinnaGen | |
| FAKHRAVAC (MIVAC) | FAKHRAVAC (MIVAC) | Inactivated vaccine | The Stem Cell Technology Research Center; Organization of Defensive Innovation and Research | |
| Turkovac (ERUCOV-VAC) | Turkovac (ERUCOV-VAC) | Inactivated vaccine | Health Institutes of Turkey | |
| Covifenz (CoVLP) | Covifenz (CoVLP) | Plant-based adjuvant vaccine | Medicago; GSK; Dynavax | |
| VLA2001 | Valneva;UK National Institute for Health Research; Dynavax | Inactivated vaccine | France, United States | |
| Noora | Noora | Recombinant protein vaccine | Baqiyatallah University of Medical Sciences |
As per government of India database (Co-WIN), till 28th February 2022, a total of 1,48,26,49,754 doses of AstraZeneca, Covishield (ChAdOx-1) and 28, 80, 80,355 doses of COVAXIN (BBV152) was administered.
1.1. Background
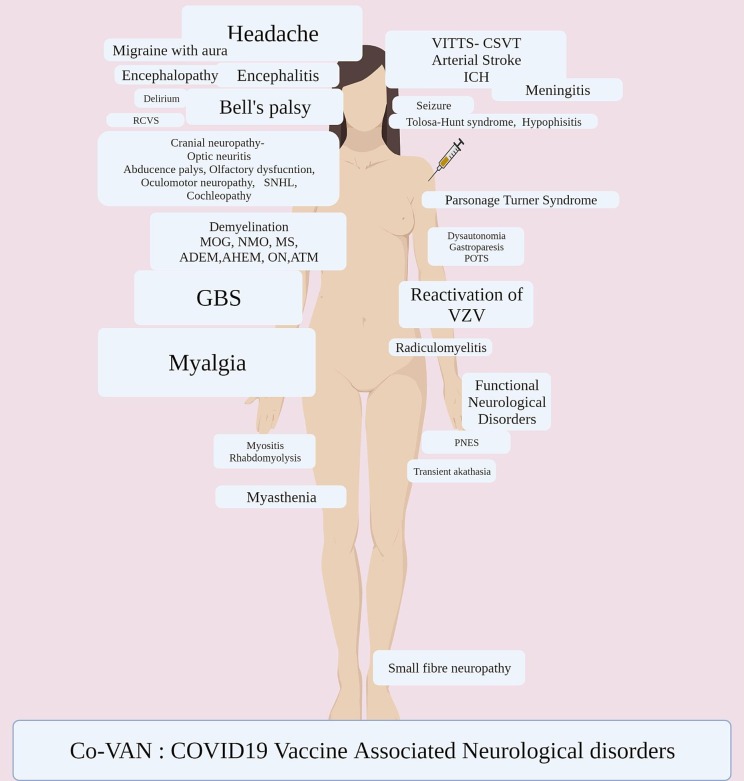
The wide array of neurological adverse events post-COVID-19 vaccination have included vaccine-induced immune thrombotic thrombocytopenia (VITT) and related cerebral thrombosis, [10], [12], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [13], [14], [15], [16], [17], [18], [19], [20] Guillain Barre Syndrome (GBS), [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [55], [56], [57], demyelination spectrum including, neuromyelitis optica spectrum disorders (NMOSD), [58]Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), [59]Multiple sclerosis (MS), [60], [61] Acute disseminated encephalomyelitis (ADEM), [62], [63] acute haemorrhagic encephalomyelitis (AHEM), [64], and optic neuritis. [65].
There has been anecdotal reports describing cases of Bell’s palsy, [66], [67], [68], [69], [70], [71], [72], [73] olfactory dysfunction, hyposmia, phantosmia, [74], [75], [76] oculomotor nerve palsy, [77], [78] abducens nerve palsy, [79], [80] cochleopathy, [81] tinnitus, [82] vertigo, [83] sudden sensorineural hearing loss, [84], [85] encephalitis, [86], [87], [88], [89] autoimmune encephalitis, [90], [91] meningitis, [92], [93] arterial stroke, [94], [95], [96], [97] rhabdomyolysis, [98], [99] myositis, [100], [101] Parsonage-Turner syndrome, [102], [103], [104], [105], [106] small fibre neuropathy, [107] acute on chronic inflammatory polyneuropathy, [108] reversible radiculomyelitis, [109] myasthenia gravis, ocular myasthenia, [110], [111], [112] transient akathisia, [113] dysautonomia, [114], [115] thunderclap headache, [116], [117], [118] reactivation of varicella zoster, [119], [120], [121], [122], [123], [124]functional neurological disorders, [125], [126], [127] reversible cerebral vasoconstriction syndrome (RCVS), [128]Cytotoxic Lesion of the Corpus Callosum (CLOCCs), [129] Gastroparesis, [130] delirium, [131] New-onset refractory status epilepticus (NORSE), [132] non-convulsive status epilepticus, [133] Tolosa-Hunt Syndrome (THS), [134] triggering of moya moya phenomena in existing autoimmune disease, [135] and hypophisitis [136]. While the temporal relation of these adverse events to vaccination were observed, most of the reports couldn’t establish causality.
The type of vaccine and dosing have differed significantly in different parts of the world. The World Health Organization (WHO) has approved nine vaccines so far, while the United States Food and Drug Administration (US-FDA) and European Medicines Agency (EMA) have approved two and five vaccines respectively. The safety and side effect profiles of the individual vaccines are expected to show variation since they are biologically different compounds. [5], [137] Many observations have shown the neurological complications in different populations with different types of COVID19 vaccines. India’s vaccination drive against COVID19 is mostly based on two types of vaccines, i.e. AstraZeneca, Covishield (ChAdOx-1), and COVAXIN (BBV152). As per the government of India database (Co-WIN), till 28th February 2022, a total of 1,482,649,754 doses of AstraZeneca, Covishield (ChAdOx-1), and 28 8,0 80,355 doses of COVAXIN (BBV152) was administered. [138].
Based on this backdrop, we present here a series of 51 cases with various vaccine associated neurological disorders (VAN), temporally associated with vaccination against SARS-CoV2. For delineating the spectrum of the same, we also performed a systematic review of the available medical literature. The proposed hypotheses were reviewed, in accordance of which, the underlying pathophysiological mechanisms were highlighted.
2. Patients and methods
The study was conducted in a tertiary care hospital in India. Retrospective analysis of medical records of all patients who presented to the outpatient, inpatient or emergency services between 1st February 2021 and 30th June 2022 was done for identifying cases with VAN.
Recruitment of patients were conducted in two steps. As a first step, cases with any neurological illness, with a history of a recent vaccination against SARS-CoV2 (i.e. within 6 weeks of onset of the first symptom of neurological disorder), not otherwise explained by any alternate etiology [139] were segregated and then based on the following inclusion and exclusion criteria cases were selected.
Inclusion criteria comprised patients with a new onset neurological syndrome with a) history of first or second or booster dose of COVID-19 vaccination by any route or type, approved in India, b) the last dose of vaccination not beyond 6 weeks (42 days) (as per World Health Organization Global Advisory Committee on Vaccine safety- WHO GACVS) [139], and c) no history of any proven or radiologically suspected COVID-19 infection irrespective of severity, in the past 3 months. Patients with a) history of receipt of any other (non-SARSCoV2) vaccination in the past 6 weeks, b) presence of an alternate diagnosis, c) pre-existing active neurological disease, and d) relapse of a pre-existing neurological syndrome were excluded. Data were extracted with regards to the demographics, clinical examination findings as evaluated by a consultant neurologist, the type, dosing and route of COVID-19 vaccine, investigations, treatment strategies and clinical outcome. The details of investigations including lumbar puncture for cerebrospinal fluid (LP-CSF) analysis, serum with or without CSF anti-Aquaporin 4 antibody i.e. neuromyelitis optica (NMO) antibodies, myelin oligodendrocyte glycoprotein (MOG) antibodies (testing done with IgG1), creatinine phosphokinase (CPK), C- reactive protein (CRP), erythrocyte sedimentation rate (ESR), magnetic resonance imaging (MRI) of the brain and/or spine, muscle MRI, nerve conduction studies, electromyography, evoked potentials including brainstem auditory evoked response (BAER), visual evoked potentials (VEP), somatosensory evoked potential (SSEP), serum and CSF autoimmune antibody profile (NMDA, VGKC, LGI-1, CASPR, GABA-A/B), serum antinuclear antibodies (ANA) profile, antineutrophil cytoplasmic antibodies (ANCA), serum myositis panel, and serum paraneoplastic antibody profile were considered. Other relevant investigations for the exclusion of alternative etiologies were recorded. (Refer to supplementary appendix).
In the second step, the cases were selected for analysis based on the causality label. This was done by two independent authors (SMM, SV) who were blinded to the study design. All selected cases in step 1 were subjected to the proposed criteria for casualty labelling as per the criteria proposed by Butler et al. [140] Accordingly, the cases were categorized to probable, possible and unlikely to be casually related to post-vaccination neurological complication. Only probable and possible cases were included for further analysis, whereas cases with “unlikely” causality association were excluded. Our retrospective recruitment strategy identified some cases of demyelination temporally associated with COVID-19 vaccination which were previously published from the institute (cases 1, 2, 6, 8, 10, 11, 13–15, 16, 17, 20–37). [59] In order to encompass the entire spectrum of COVID-19 vaccine related neurological complications, these cases were included. The cases were reported in accordance with consensus-based clinical case reporting (CARE) guidelines. [141]. Informed consent and ethical committee approval were obtained. A scoping review was done for all published articles pertaining to neurological manifestations following COVID vaccination using PUBMED, SCOPUS, EMBASE, Google Scholar, Ovid and MedRxiv till June 2022.
3. Statistical analysis
In the descriptive statistics, categorical variables were denoted as frequency with percentage while the continuous variables were expressed as median ± IQR and mean ± SD. The categorical variables in multiple groups were analysed with χ2tests to look for any significant difference overall between the groups. If found significant, Fisher exact test was used to compare the two individual subgroups. The quantitative variables, in the three independent demyelination subgroups were tested for significance using one way ANOVA. If found significant, post-hoc analysis was done between the individual groups. A p value of < 0.05 was considered to be statistically significant. Inter-rater reliability was assessed using Cohen’s kappa.IBM-SPSS Version 26 was used for the computation of these statistics.
4. Results
In the given timeframe a total of 4672 medical records were reviewed, out of which 109 cases were identified. Subsequently, 51 cases (probable, n = 48 and possible, n = 3) were included as per casuality assessment based on the criteria by Butler et al by two independent authors SMM and SV Cohen’s kappa was 0.73 and inter-rater agreement was 86.24 %. Amongst these 51 patients, CNS demyelination (n = 39, 76.5 %) was the most common. This was followed by three cases of GBS (5.9 %), six cases of stroke (11.8 %), two cases (3.9 %) of encephalitis and a single case of myositis (Table 2, Table 3 ). Female sex was slightly higher than the male counterpart (F:M, 1.13:1). The mean (±SD) age was 40.1 ± 14.5 years. Majority of the patients belonged to the age group between 25 and 45 years (26, 51.0 %). Majority of the patients received ChAdOx-1 nCoV (COVIShield) vaccine (n = 43, 84.3 %) while the rest of the patients received BBV152 (COVAXIN) (n = 8, 15.7 %). The frequency of neurological complications was higher after the first dose (n = 37, 72.5 %) as compared to the second dose (n = 14, 27.5 %). The latency to the onset of neurological symptoms was 14 (IQR 5.5 to 15) days from the first dose and 12 (IQR 3.3 to 14) days from the second dose. Overall, the latency was 13.2 ± 10.7 days from the last dose of vaccination. Majority of the patients presented in the second week after vaccination (n = 20, 39.2 %).
Table 2.
Enumerates the clinical details of the cases.
| Demyelination | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serial No | Age(years) | Gender | Presenting Complaints | Total Duration (days) of Illness | Type of Vaccine/dose | Interval from last vaccination to the onset of first neurological symptoms | Examination finding | Investigations | Diagnosis | Treatment | Prognosis | Causality label$ |
| 1 | 35 | F | Body ache, headache, vomiting followed by altered sensorium and, inability to walk, excessive sleepiness and bladder retention. Known case of well controlled T2DM | 10 | ChAdOx-1/1st dose | 9 days | Hypotonia in both lower limbs and lower limb power 2/5 with biceps, supinator and triceps hyperreflexia and knee and ankle hyporeflexia and left extensor plantar. | CRP, RA factor, ANA profile and ANCA- negative. LP-CSF: Cells- 58/hpf cells (50 L),protein- 47 mg/dl. VEP b/l and BAER, SSEPs – Normal. MRI of Brain and spine T2/FLAIR hyperintensities in mid brain, pons, left MCP, bilateral posterior internalcapsule, thalamus, bilateral centrum semiovale and longitudinally extensive transverse myelitis involving cervical cord and conus. Serum MOG was positive | MOGAD | IV MP (1gm) * 7daysFollowed Mycophenolate mofetil maintenance | Improved (mRS = 2) | Probable |
| 2 | 34 | M | Headache, right eye visual diminution | 14 | ChAdOx-1/1st dose | 1 days | Rt eye- Visual acuity-perceptionof light present, Lteye 6 /18 | CRP, RA factor, ANA profile and ANCA- negative. LP-CSF: Cells- 4/hpf cells (2 L),protein- 26.6 mg/dl. VEP- right eye prolonged P100 and BAER, SSEPs – Normal. MRI of Brain suggestive of right optic neuritis. Serum and CSF aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative Optic neuritis | IV MP (1gm) * 5 days followed by oral prednisolone gradual tapering | Improved (mRS = 0) | Probable |
| 3 | 27 | F | Hiccups and vomiting, tingling numbness in all four limbs and decreased sensation over trunk and lower limbs, weakness in left upper and lower limbs, weakness in right upper limb and lower limb, spasms and pain in right upper limb and lower limb and neck | 80 | BBV152/1st dose | 17 days | Right hemiparesis, Tone:- Tone increased in right upper and lower limbsRight upper and lower limb flexor spam present every 30 min. Right Biceps, triceps,knee,ankle jerks brisk, plantar no response b/l. Sensory- Touch, vibration, JPS impaired b/l Ul and LL. | ESR, and CRP – Elevated. LP-CSF: cells-2(lymphocytes-100 %) protein-23.8 mg/dlSSEP showed absence of wave forms. MRI of Brain and spine – s/o cervical myelitis and medullary involvementSerum aNTI-AQ-4 ANTIBODY – Strongly positive. | NMOSD | LVPP*5 cylcles f/bRituximab | Improved (mRS = 1) | Probable |
| 4 | 38 | M | Urinary incontinence, and weakness in all 4 limbs. Known case of well controlled T2DM | 4 | ChAdOx-1/1st dose | 14 days | Quadriparesis with brisk DTRs andsensory loss over V3 division of trigeminal nerve bilaterally, trunk (till C4 level) and all 4 limbs. | LP-CSF- 370 cells (80 percent neutrophils and 20 percent lymphocytes), protein 174 mg/dl. CSF OCB is positive, serum OCB is negative. ACE, RA factor, ANA profile and ANCA- negative. MRI of Brain and spine – longitudinally extensive transverse myelitis from cervico-medullary junction upto D1 and hyper intensity in left middle cerebellar peduncle and pons. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | IV MP (1gm) * 5 days followed by PLEX * 7 cycles followed by Rituximab | Mild Improvement (mRS = 2) | Probable |
| 5 | 54 | M | Tingling paresthesia of right Lower limb and associated with transient tonic posturing of right upper limb lasting for seconds. | 6 | ChAdOx-1/1st dose | 14 days | Tone and power normal, brisk DTRs and flexor plantar response. Sensory examination normal. | MRI of Brain and spine – symmetrical T2/FLAIR hyperintensities in b/l corticospinal tract and, cerebral peduncles and middle cerebellar peduncle. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | Symptomatic management of paresthesia and antiepileptic | Improved (mRS = 0) | Probable |
| 6 | 36 | F | Tingling parasthesia in both lower limbs,weakness of both lower limb and urinary symptoms | 20 | ChAdOx-1/2nd dose | 32 days | Hypotonia with sluggish DTRs in lower limb and lower limb power 0–1/5, sensory loss till D4. | CRP, RF, ANA, ANCA and Paraneoplastic profile -negative. LP-CSF: 720 cells (lymphocytes-580, polymorphs-20, degenerated cells-120), elevated protein (144 mg/dl), elevated lactate (32 mg/dl) and normal glucose. VEPwas absent in right eye and prolonged in left eye. SSEP- absent wave forms LL and prolonged in UL. MRI of Brain and spine – longitudinally extensive transverse myelitis predominantly involving central and posterior cord sparing anterior part extending from obex till conus with cord swelling with left optic neuritis. Serum MOG – Positive | MOGAD | IV MP (1gm) * 5 days followed by PLEX * 7 cycles | Improved (mRS = 1) | Probable |
| 7 | 30 | M | Pain in the right eye and diminution of vision,and pain in left eye and diminution of vision. | 13 | ChAdOx-1/1st dose | 14 days | Right RAPD was present. Right eye perception on light was absent. Left eye 6/60.Fundus showed bilateral papilledema grade 3 (right more than left) | ANA profile and ANCA were negative. Serum NMO MOG panel was negative. Viral markers were negative. CSF analysis showed 1 cell with normal protein. Evoked potentials showed bilateral absence of P100 and BAER and SSEP were normal. MRI brain showed optic nerves hyperintensities bilaterally with volume loss more on left side. MRI spine screening was normal. | Bilateral Optic neuritis | LVPP*5 cylcles f/b1gm IVMP*2 days f/b oral steroid and Rituximab | No improvement (mRS = 5) | Probable |
| 8 | 50 | F | Tingling paresthesia and both upper and right lower limbs weakness. Known case of hypothyroidism on treatment. | 10 | ChAdOx-1/1st dose | 28 days | Right lower limb power 3–4/5, spastic and DTRs in right side, Knee and ankle jerks are brisk with right extensor plantar | ANA profile – PCNA 1 +.LP-CSF: Cells- 2/hpf cells (2 L),protein- 28.3 mg/dl. MRI of spine C7 level short segment T2/FLAIR hyperintensities. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Short segment transverse myelitis | Oral prednisolone and mycophenolate mofetil | Improved (mRS = 1) | Probable |
| 9 | 44 | M | Imbalance while walking and vomiting, acute urinary retention, band like sensation and double vision | 12 | ChAdOx-1/1st dose | 13 days | Quadriparesis with brisk DTRs andsensory loss over V3 division of trigeminal nerve bilaterally, trunk (till C4 level) and all 4 limbs. | LP-CSF: Lymphocytic pleocytosis with elevated proteinMRI of Brain and spine – T2/FLAIR long segment non expansile hyperintensities in the cervical and dorsal cord and conus medullaris with involvement of 2/3rd cross sectional area of cord. Serum SARS-CoV2 S1,S2 (IgG&IgM)- PositiveSerum MOG – Positive | MOGAD | IV MP (1gm) * 5 days followed by Mycophenolate mofetil | Improved (mRS = 0) | Probable |
| 10 | 38 | M | Vertigo, double vision on looking left, Imbalance while walking and blurring of vision in Right eye with Headache | 26 | ChAdOx-1/1st dose | 6 days | Pupils:3 mm equal and reactiveV/A- 6/9 in RE, 6/6 in LEFundus – NormalEOM: fullGaze evoked horizontal and torsional nystagmus. | CRP,RF, ANA profile and ANCA- Negative. LP-CSF- Traumatic tap. MRI of Brain and spine – patchy areas of demyelination in left MCP, right corona radiata with T2/FLAIR hyperintensity in right vestibular apparatus. VEP- Prolonged P100 latency and low amplitude BAER waveforms. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | CNS demyelination with Vestibulopathy | IVMP 1gm *5 days f/b oral steroid | Mild Improvement(mRS = 2) | Probable |
| 11 | 53 | F | Paresthesia of both lower limb, urinary hesitancy, paresthesia and tightness of both upper limbs over trunk,and band like sensation over chest. Known case of medically controlled hypertension since 1 year. | 12 | ChAdOx-1/2nd dose | 1 day | Fine touch reduced bilaterally from toes to epigastrium and in bilateral medial part of forearm and middle and little fingersPain: decreased bilaterally from toes to epigastriumVibration: Absent on both sides till knee. Joint position sense: Absent in great toes, thumbs on both sides. Plantar: Bilateral extensor. Rhomberg s: Positive | ACE levels, ANA Profile, ANCA, CRP, RA Factor- Negative. LP-CSF showed 6 cells, 57 mg/dl protein. Serum anti-recoverin- Positive. MRI of Brain and spine – T2/FLAIR hyper-intensities in the bilateral periventricular white matter, bilateral insula and bilateral cerebellar hemispheres. Few short segment expansive T2 hyperintensities are noted in the cervical cordat C5,6,7 levels and dorsal cord at D6-7 level with involvement of central cord. SARS-CoV2 S1,S2 (IgG&IgM)- PositiveSerum and CSF aNTI-AQ-4 ANTIBODY and MOG – Negative | CNS demyelination | IVMP 1gm *5 days f/b oral steroid | Mild Improvement (mRS = 1) | Probable |
| 12 | 35 | F | Blurring of vision of both eyes, walking difficulty, mild pain thorax and breathing problem in supine position. | 20 | ChAdOx-1/2nd dose | 14 days | Visual acuity-bilateral 6/9. E.O.M.-full. Pupils-bilateral 3 mm,pupils equally reactive to light. Lower limb power 3–4/5, Sensory-90 percent loss of pain,touch,temperature in bilateral lower limbs,bilateral upper limbs. 100 percent pain,touch,temperature sensation present in right side of face. Joint,position sensation, and vibration impaired in bilateral lower limbs. | ESR-raised, CRP,ANA-Negative. LP-CSF: cells-17(all lymphocytes), protein-64 mg/dlV.E.P.-left(P100-115.8), right(P100-125.7),prolonged S.S.E.P inlower limb(P37-43),normal S.S.E.P. in upper limb(N20-19.3)and normal value of ABR. MRI of Brain and spine – few short segment T2 hyperintensities in thecervical (C2-3 level) and dorsal cord (D1 to D3) with patchy heterogeneous enhancement. Posterior intra-orbital segment of bilateral optic nerves, optic chiasm and the bilateral proximal optic tracts also showed T2/ FLAIR hyperintensity with patchy contrast enhancement along with signal change in the hypothalamus, left trigeminal nerve (root entry zone and cisternal segment), right lateral medulla extending to the cervicomedullary junction. Serum aNTI-AQ-4 ANTIBODY and MOG – NegativeCSF OCB- Pattern 4. | Bilateral Optic Neuritis and Brainstem demyelination | LVPP*5 cylcles f/b1gm IVMP*5 days f/b oral steroid and Rituximab | Improved (mRS = 0) | Probable |
| 13 | 30 | F | Shock like sensation on flexing the neck and tingling paraesthesia of B/l hand | 3 months | ChAdOx-1/2nd dose | 15 days | Tone- Normal. Power-normal in U/L and L/L including intrinsic muscles of handReflexes −2Plantar bilateral- flexorSensory system −40 percent reduction in sensation to touch over both palms. | ESR-68 mm, ACE,RA, ANA profile-negativeMRI of Brain and spine – T2 hyperintensities short segment at C3 level. Evoked potentials are normal. Serum SARS-CoV2 S1,S2 (IgG&IgM)- Positive. CSF OCB- Positive. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | LVPP*5 cylcles f/b1gm IVMP*5 days f/b oral steroid | Improved (mRS = 0) | Probable |
| 14 | 26 | F | Weakness of bilateral lower limbs,sensory loss below the chest, urinary retention, weakness and paresthesias of both upper limbs | 4 | BBV152/1st dose | 5 days | Quadriparesis Sensory examination – absent sensation to touch and pin prick below T4 Level. JPS and vibrationimpaired in lower limbs. DTRs – upper limb 2, lower limbs absent | ANCA, RA factor, and CRP – negative. ANA profile – anti PCNA strongly positive. LP-CSF: cells-207(lymphocytes-40 %, PMN-60 %), protein-95.8 mg/dlSSEP showed absence of wave forms. MRI of Brain and spine – long egment transverse myelitis from cervical region to lower lumbar region. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | LVPP*5 cylcles f/b1gm IVMP*5 days f/b oral steroid | Improved (mRS = 2) | Probable |
| 15 | 27 | F | Pain in left upper and lower limb and right lower limb, headache, weakness of left upper and lower limb and right lower limb | 30 | ChAdOx-1/ 1st dose | 5 days | MotorGrade 1 spasticity in left upper limbPower- 5/5Tendon reflexes- 3Plantars- Bilaterally flexorSensory- Touch, pain, joint position sense- Normal | ANA profile, ANCA, ACE – negative. LP-CSF: cells-0, protein-27.7 mg/dlMRI Brain – multifocal mildly expansile discrete T2 heterogeneously hyperintense lesions without FLAIR suppression in periventricular white matter along lateral ventricles, subcortical -deep white matter of bilateral frontal -parietal – temporal lobes, right caudate nucleus body, right PLIC -adjacent thalamus. Larger lesion in bilateral corona radiata show peripheral diffusion restriction and peripheral thin rim of blooming on SWI. Post contrast enhancementin few lesions in bilateral periventricular -deep white matter. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Acute disseminated encephalomyelitis(ADEM) | IVMP 1gm*5 days f/b oral steroid | Improved (mRS = 2) | Probable |
| 16 | 45 | F | Bilateral visual loss | 4 | ChAdOx-1/ 1st dose | 5 days | VA- Bilateral lowMotor, sensory, cerebellar- normal | RA factor, and ANA profile – negativeLP-CSF: cells-2(lymphocytes-100 %), protein-52.3 mg/dlVEP- b/l prolonged P100. CSF OCB- Negative. MRI of Brain and spine – No significant signal changes. Serum MOG – Positive | MOGAD | LVPP*5 cylcles f/b1gm IVMP*5 days f/b mycophenolate mofetil | Improved (mRS = 1) | Probable |
| 17 | 20 | F | Double vision | 5 | ChAdOx-1/1st dose | 3 days | Brisk DTRs and mild spatic lower limbs. | CRP, RA factor, ANA profile and ANCA- negative. MRI of Brain multple discrete T2/FLAIR hyperintensities in pericallosal, callosal and frontal regions. Serum aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | IV MP (1gm) * 5 days followed by oral prednisolone gradual tapering | Improved (mRS = 0) | Probable |
| 18 | 55 | F | Right lower limb pain and weakness and then after 2 month paresthesia left lower limbKnown case of medically controlled T2DM | 60 | ChAdOx-1/ 1st dose | 2 days | Pupil, EOM- fullRight hemiparesisRight UL and LL DTRs brisk | ESR (57 mm) and CRP(11 mg/L) – elevated. ANA profile – NegativeParaneoplastic profile: anti-Tr and anti-GAD65, LP-CSF: cells-2(lymphocytes-100 %), protein-28.3 mg/dlSSEP showed absence of wave forms. MRI of Brain and spine – multiple T2 hyper intensities in the cervico-dorsal spine. CT abdomen, pelvis, thorax- negative for malignancy. Serum and CSF aNTI-AQ-4 ANTIBODY and MOG – Negative | Seronegative CNS demyelination | 1gm IVMP*5 days f/b oral steroid | Improved (mRS = 1) | Probable |
| 19 | 16 | F | Recurrent vomiting, burning sensation of both upper limbs, tremuloousness of b/l upper limbs, imbalance while walking, double vision and swallowing difficulty | 90 | BBV152/2nd dose | 14 | EOM: Bilaterally abduction, Upbeat nystagmus in all directions ofgaze. Bilateral LMN facial palsy. Trismus, jaw opening restricted. Power 4/5Cerebellar signs present b/l, DTRs brisk, plantar b/l extensorSevere gait ataxia | Serum ANA, ANCA negative. MRI brain-T2/Flair diffuse white matter hyper-intensities involving lower mid brain to C4 level of spinal cord. LP-CSF: nil cells-2, protein-28.0 mg/dl. Serum and CSF NMO was strongly positive. | NMOSD | LVPP*5 cylcles f/b1gm IVMP*5 days f/b oral steroid and Rituximab | Mild Improved (mRS = 3) | Probable |
| 20 | 54 | M | Imbalance, Dysarthria, weakness of both lower limbs, dysphagia | 10 | ChAdOx-/2nd dose | 14 | Dysarthria-scanningVA-Right eye-6/36,Left eye-6/36Tone- Hypotonia b/l LLPower- LL 4/5DTRs- BriskPlantar- Extensor b/lJPS- impairedCerebellar signs- present | ANA profile: AntiRNP,Anti JO 2 + ANCA,Serum.NMOMOG:negative. ESR was 90 mm/hr. MRI Brain:T2 /FLAIR patchy hyper intense lesion in pontine region | Seronegative CNS demyelination | 1gm IVMP*5 days f/b oral steroid and Rituximab | Improved (mRS = 1) | Probable |
| 21 | 29 | F | Headache, Rt eyeblurring of vision | 15 | ChAdOx1nCoV- 19 /1st dose | 11 | Rt: eye RAPD, VA –Rt: hand movementclose to face; Lt − 6/6 | CSF: 0 cells, P:18 mg/dl, G: 61 mg/dl Serumand CSF OCB absentANA, ANCA, RAfactor, CRP -negativeSerum MOG- positiveVEP: Rt – absentwaveform, Lt – normalMRI brain: T2 /FLAIRhyperintensity of longintraorbital segment ofRt optic nerve withcontrast enhancement | MOGAD | Inj. MP 1 gm × 5 days 1 cycle ofLVPP T. Prednisolone 40 mg OD followedby taperingdoses | Improved (mRS = 1) | Probable |
| 22 | 54 | F | Progressivequadriparesisfollowed byaltered sensorium | 42 | ChAdOx1nCoV- 19 /1st dose | 14 | Drowsy, not openingeyes, bl UL flexionposturing, quadriparesis with2/5 power in UL and0/5 power in LL. | CSF: 8 cellslymphocyticpredominant, P:77 mg/dl, G:98 mg/dlANA, ANCA, CRP-negative Serum NMOMOG-negative MRIbrain: T2/FLAIRhyperintensities in thecorpus callosum, blperiventricular andsubcortical whitematter, infratentorialregion with patchycontrast enhancement | ADEM | Inj. MP 1 gm × 5 days 5 cycles ofLVPP Inj. Iv Ig100 g T. Prednisolone 40 mg OD followedby taperingdoses | Mild Improved (mRS = 2) | Probable |
| 23 | 44 | M | Hiccups, vomiting, urinaryretention, doublevision, Imbalance onwalking | 12 | ChAdOx1nCoV- 19 /1st dose | 7 | Lt VA: 6/9, Rt – 6/6. spasticquadriparesis, bilateral cerebellarsigns in UL | CSF: Lymphocytic pleocytosis with elevated protein. ANA, ANCA -negativeSerum and CSF MOGStronglypositive, MRI: T2 hyperintensities inthe cervico-dorsalcord and conus | MOGAD | Inj. MP 1 gm × 5 days 5 cycles ofLVPP T. Prednisolone 40 mg OD | Mild Improved (mRS = 2) | Probable |
| 24 | 39 | M | Rt eye painfollowed byblurring of vision | 20 | ChAdOx1nCoV- 19 /1st dose | 14 | RT eye-RAPD, Rt VA: Finger counting at 2 m Visual field- rightinferonasal quadrantinvolvement | ANA, ANCA, APLA-negative, Serum MOG- positive, VEP- bl prolonged (Right-132 ms, left-115 ms) MRI: T2 /FLAIRhyperintensity of longintraorbital segment ofRt optic nerve withcontrast enhancement | MOGAD | Inj. MP 1 gm × 5daysT. Prednisolone 40 mg OD | Improved (mRS = 0) | Probable |
| 25 | 54 | M | Left eye blurringof vision | 21 | ChAdOx1nCoV- 19 /1st dose | 14 | VA: Bl 6/12, Lt eyeRAPD present, Rteye-normal pupillaryreaction. | ANA profile anti Jo1 1 + positive, ANCA, VDRL-negative, VEP: Rt- 127 ms, Lt-absentwaveform Serum MOG–Strongly positive MRIbrain and spine: T2/FLAIR hyperintensityin Rt pons | MOGAD | Inj. MP 1 gm × 5 days T. Prednisolone 40 mg OD | Mild Improved (mRS = 1) | Probable |
| 26 | 31 | M | Bladderdisturbancesfollowed byprogressivenumbness ofwhole body andLL weakness | 5 | ChAdOx1nCoV- 19 /1st dose | 14 | Lower limbspasticity, paraparesis withpower 1/5, decreased sensationsby 70 % below L1, plantars extensor, ULDTRs-3 + and LL 2+ | CSF: 370 cells -polymorphicpredominant, P: 174 mg/dl, G: 168 mg/dlANA profile, ANCA, VDRL, RA factor, CRPnegativeSerum andCSF NMO-MOG –negative VEP andBERA- normal, SSEP ofLt. LL prolonged (55.9 ms) MRI: long segmentcervico-dorsal T2/ FLAIR hyperintensitywith subtleenhancement | Seronegative CNS demyelination | Inj. MP 1 gm × 5 days T. Prednisolone 40 mg OD 7 cyclesof LVPP Inj. Rituximab 1 gm | Mild Improved (mRS = 2) | Probable |
| 27 | 20 | F | Rt ULparaesthesiasfollowed byparaparesis &altered sensorium | 2 | BBV152 /1st dose | 1 | VA: Bl 6/6. LLproximal weakness (3/5), distal 4/5, DTRs- 3+, Rt LL50% decreasedsensation, PlantarsEquivocal | CSF: 8 cells -lymphocyticpredominant,P:24.9 mg/dl, G:61 mg/dlANA profile, ANCA, VDRL, RA factor, CRP-negative Serum andCSF NMO-MOGnegative, CSF OCB –Positive VEP, BERA, SSEP- normal MRI: few juxtacortical andshort segment cervicalT2/FLAIRhyperintensity at C5level with subtleenhancement | Seronegative CNS demyelination | Inj. MP 1 gm × 5 days T. Prednisolone 40 mg OD 5 cyclesof LVPP | Mild Improved (mRS = 2) | Probable |
| 28 | 33 | F | Fever, vomitingfollowed byaltered sensoriumand persistentparaesthesiasbelow midthoracic level | 28 | ChAdOx1nCoV- 19 /1st dose | 14 | VA: Rt 6/12, Lt 6/9, Bl normal pupillaryreaction, no otherfocal deficits | CSF: 105 cells -lymphocyticpredominant, P: 28.12 mg/dl, G: 70.4 mg/dlSerum MOG –Stronglypositive MRI brain: T2/FLAIRhyperintensity in Blfronto parietal region, no enhancement | MOGAD | Inj. MP 1 gm × 5 days T. Prednisolone 40 mg OD | Minimal improvement (mRS = 3) | Probable |
| 29 | 60 | M | Acute onsettinglingparaesthesias andmotor weaknessin left upper andlower limb, followed bybehavioural andmemorydisturbances | 34 | ChAdOx1nCoV- 19 /2nd dose | 14 | MMSE-27/30 Cranialnerves-VA:R-6/6, L-6/9, nystagmuspresent Motorsystem-Power: normal,DTRs-brisk | CSF: 9 cells – 90 %lymphocytes, P:68.3 mg/dl, G:132 mg/dl, OCBs-negative ANA, ANCA,B12, Homocysteine,VDRLnegative, ACE-normalSerum NMO and MOG-negative, VEP-normalMRI brain: multiplefocal lesions in rightpons, midbrain, medial temporal lobes, splenium of corpuscallosum, high parietallobe with tumefactionand peripheralenhancement | ADEM | Inj MP 1 gm × 5 days T. Prednisolone 40 mg OD T. MMF (1gm) | Mild Improved (mRS = 2) | Probable |
| 30 | 23 | F | Burningparaesthesias inright palmassociated withnumbness andmotor weaknessfollowed byburning sensationin right foot overnext 7 days | 41 | ChAdOx1nCoV- 19 /2nd dose | 7 | VA-6/6 Bl Cranialnerves-normal Motorsystem-normalSensory systemdecreasedvibrationalong distal rightupper and lower limbjoints | CRP- 23 mg/dl ANAnegativeSerum NMOand MOG-negativeCSF-OCB negative MRIbrain-T2/flairhyperintensitiesadjacent to rightfrontal horn, ependymal margins ofbilateral lateralventricles MRI spineshortsegmenthyperintensities at C2-C3,C5,D4 | SeronegativeCNS demyelination | Inj MP 1 gm × 5 days T. Prednisolone 40 mg OD | Minimal Improved (mRS = 3) | Probable |
| 31 | 40 | M | Blurring of visionfrom left eyefollowed by acuteurinary retentionnd right eyevision loss | 77 | ChAdOx1nCoV- 19 /1st dose | 10 | VA- 6/18 Bl Cranial, motor and sensoryexamination-normal | CSF: 8 cells – 100 %lymphocytes, P:32 mg/dl, G:68 mg/dl, OCB-positive ANA, ANCA,VDRL-negative, Serum MOG-positive MRI brain: T2 Hyperintensities inpons, bilateralthalami, right frontalcortex MRI spinelongitudinallyextensive myelitisfrom C4-D3 | MOGAD | Inj MP 1 gm × 5 days T. Prednisolone 60 mg OD T. MMF (2gm) | Mild Improved (mRS = 2) | Probable |
| 32 | 45 | M | H/o feveraccompanied byurinary retentionand difficulty inwalkingprogressing toaltered sensorium | 5 | ChAdOx1nCoV- 19 /1st dose | 10 | VA-6/6 BL Cranialnerves-normal Motorsystem-Tone andpower normal inupper limbs LLhypotonia, grade-0 power withhyporeflexia, plantars mute | CSF: 44 cells – 44 %lymphocytes, P:90.9 mg/dl, G:68 mg/dl, rabies CSF PCRNegativeVEP-L-141,R-129,BERA-normal, N20-normal, P37–40 (mildly prolonged), ANA-U1RNP-1+,CANCA-,Serum MOG –strongly positive S. NMO–Negative MRI of brain and spinehyperintensitiesinbrainstem, cervicodorsal cord andsupratentorial regionswith central cordswelling | MOGAD | INJ MP-5 days, LVPP 3 CYCLESTABWYSOLONE 40MG TAB MMF1.5 GM | Mild Improved (mRS = 1) | Probable |
| 33 | 34 | F | H/o recurrentvomiting andhiccupsprogressing toimbalance whilewalking | 60 | ChAdOx1nCoV- 19 /2nd dose | 36 | Cranial nerves: Rightgaze evokednystagmus, restnormal Motorexamination::Toneand power normal, DTRs brisk BLSensoryexamination: pseudoathetosisLeft > Right,, Romberg’s positive, Tandem gaitimpaired | CSF-1 cell,P-15,3 mg/dl, 63 mg/dl,OCBNegative ESR-46 mm/hr Serum NMO-weaklypositive Serum MOGnegativeANA:Ro-521+,ANCA-negativeMRI brain:T2hyperintensity indorsal aspect ofmedulla | NMOSD | I/V MP-5 daysLVPP-3 cyclesTab Wysolone40 mg InjRituximab | Mild Improved (mRS = 2) | Probable |
| 34 | 31 | M | H/o progressiveupper and lowerlimb tingling f/bdifficulty inwalking, urinaryurgency, andconstipation | 17 | ChAdOx1nCoV- 19 /1st dose | 42 | Cranial nervesnormalUL motorexamination-normal, LL power-4/5,briskDTRs, extensorplantars Sensorylevel at T4 | CSF: 32 cells – 100 %lymphocytes, P:49.2 mg/dl, G:74 mg/dlANA,ANCA,VDRL-negative, Serum NMOand MOG -negativeMRI brain: T2Hyperintensities incervicomedullaryjunction, right frontalsubcortical region MRIspine-cervical cord HIC2-C5,also in dorsalcord | SeronegativeCNS demyelination | I/V MP-5 daysLVPP-4 cyclesTab Wysolone40 mg Tab MMF1.5 gm | Mild Improved (mRS = 1) | Probable |
| 35 | 52 | F | H/o progressiveslurring of speechwith right upperlimb and lowerlimb weakness, followed byappearance ofswallowingdifficulty | 51 | ChAdOx1nCoV- 19 /1st dose | 35 | Spastic anarthria + Gaze restrictedleft > right Rightfacial weaknessMotor examinationhypotonicrightupper and lower limbwith 0/5 power, leftsided power-5/5,BLDTRs brisk andplantars extensor | CSF-2 CELLS,P-40.5 mg/dl,G-56 mg/dlESR-18,CRP-POSITIVEANA,ANCA-Negative, VDRL-Negative S. NMO and MOGNegativeMRI brain: tumefactivedemyelination in leftfrontal hemispherewith insularinvolvement alongwith left more thanright midbraininvolvement | ADEM | I/V MP-5 daysLVPP-4 cyclesTab Wysolone40 mg InjRituximab | Minimal Improved (mRS = 3) | Probable |
| 36 | 65 | F | H/o urinaryretentionfollowed bynumbness andweakness of bothhands andblurring of visionof right eye | 30 | ChAdOx1nCoV- 19 /1st dose | 42 | V/A-R- handmovements close toface,L-6/18 UL: motor examinationnormal LL: Power-0/5 DTRs absent in LLSensory level:T6 | CSF-17 CELLS,P-49 mg/dl,G-59 mg/dlESR-97 ANA,ANCANegative, VDRLNegativeS.NMOStronglypositive S. MOG-Negative VEP-RNotrecordable, LNormalSSEP-LLabsent MRI brain: fewhyperintensities infrontal subcorticalwhite matter MRISpine: D2-D11hyperintensity withpatchy contrastenhancement andbright spotty areas | NMOSD | LVPP – 3 cyclesI/V MP-5 daysTab Wysolone40 mg Tab MMF1.5 gm | Mild Improved (mRS = 2) | Probable |
| 37 | 20 | F | H/o tingling intips of right handfollowed byprogressive imbalance whilewalking | 24 | ChAdOx1nCoV- 19 /2nd dose | 39 | V/A-6/6 BL Motorexamination: Toneincreased in rightupper limb and lower limb Power − 5/5 inall 4 limbs DTRs: normal Plantar rightextensor and leftflexor Sensorysystem- Pain andtouch decreased by10 percent in rightupper and lower limbJPS normal Vibrationnormal Rombergpositive Gait ataxic | CSF- 4 CELLS,P-23 mg/dl,G-111 mg/dl, CSF- OCB + ANA-,ANCA-,CRP-13 mg/dl,,EBV-IGG + S.NMO and MOG-NegativeMRI brain: hyperintensities in BLjuxtacortical, subcortical, periventricular whitematter, anteriortemporal lobes as wellas infratentorialregions includingpons, MCP andmedulla MRI Spine: short segment lesionsin cervical and dorsalspine | CNS Demyelination- MS | I/V MP-5 daysTab Wysolone40 mg InjRituximab | Mild Improved (mRS = 1) | Probable |
| 38 | 23 | F | Heaviness in the legs followed by weakness of both legs over 7 days | 13 | ChAdOx1nCoV- 19 /2nd dose | 1 | VA-Right- 6/24, Left- 6/9Power- UL 5/5, LL-0–1/5, DTRs- BriskPlantars- B/l extensorPain touch decreased below T4, JPS- impaired in LL | ANA screening positive (1:80 titres), and anti sm-RNP 2 positive. CSF −9 cells (all lymphocytes) with normal protein and glucose. Serum and CSF NMO- MOG strongly positive for NMO. MRI spine – long segment transverse myelitis in thoracic spinal cord. | NMOSD | LVPP*5 cylcles f/b1gm IVMP*2 days f/b oral steroid and Rituximab | Mild Improved (mRS = 1) | Probable |
| 39 | 28 | M | Right eye visual loss | 12 | ChAdOx1nCoV- 19 /1st dose | 11 | RAPD right eyeVA- right 6/36, left-6/6 | LP-CSF- Normal cell and proteinMRI Brain-Intraneural T2WI-FLAIR hyperintensity noted involving right optic nerve intraconal & intracanalicular segments. | SeronegativeCNS demyelination | IVMP*5 days f/b oral steroid | Mild Improved (mRS = 1) | Probable |
| Guillain Barre Syndrome | ||||||||||||
| 40 | 34 | F | Numbness in both upper and lower limbs, weakness in all limbs, speech disturbances and swallowing difficulty. Is a known patient of Rheumatoid arthritis since 2014. Currently asymptomatic since 2 years, not on any medication. | 10 | ChAdOx-1/ 2nd dose | 14 days | Bifacial weakness present. tongue movements reduced. Tone: hypotonia in all 4 limbs. Quadriparesis, global areflexia | NCS- Motor axonopathyLP-CSF: Albuminocytological dissociation (cells-Nil, protein-147.0 mg/dl) LFT, RFT, Serum electrolytes, CBC, homocysteine,folate, Vit B12, thyroid function test were within normal limits. Antiganglioside antibody IgM,IgG negative. Serum Rheumatoid factor elevated (33 Iu/ml) | Guillain Barre Syndrome | LVPP * 7 cycles | Improved (mRS = 2) | Probable |
| 41 | 34 | F | Weakness of both lower limbs, weakness of both upper limbs and paresthesias of all 4 limbs | 20 | ChAdOx-1/ 2nd dose | 3 days | Tone: hypotonia in all 4 limbs. Quadriparesis, global areflexia | NCS- Axonal and demyelinating neuropathyLP-CSF: Albuminocytological dissociation (cells-Nil, protein-123.6 mg/dl) ANA profile, ANCA, ACE levels and anti-ganglioside antibodies werenegative. Urine for Bence jones proteins was negative. Serum Rheumatoid factor elevated (33 Iu/ml) | Guillain Barre Syndrome | LVPP * 7 cycles f/b IVMP 1gm * 5 days | Improved (mRS = 2) | Probable |
| 42 | 44 | M | eakness of both upper and lower limbs, and paresthesias of all 4 limbs | 10 | ChAdOx-1/ 1st dose | 16 days | Tone: hypotonia in all 4 limbs. Quadriparesis, global areflexia | NCS- Axonal and demyelinating neuropathyLP-CSF: Albuminocytological dissociation (cells-Nil, protein-75.7 mg/dl) ANA profile, ANCA, ACE levels and anti-ganglioside antibodies werenegative. Urine for Bence jones proteins was negative. Serum Rheumatoid factor elevated (33 Iu/ml) | Guillain Barre Syndrome | IvIg 0.4 g/kg/day * 5 days | Improved (mRS = 1) | Probable |
| Stroe | ||||||||||||
| 43 | 16 | F | Headache followed by right upper and lower limb weakness with slurred speech | 3 | BBV152/1st dose | 5 days | right upper and lower limbs spastic hemiparesis | MRI- acute infarcts in left MCA territory with left M1 MCA occlusionESR-51mmPlatelet-57Lakh/cmmPT,INR,aPTT-NormalANA Profile, ANCA- NegativeFasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalSickling test- NegativeCardiac evaluation-Normal | Acute ischemic stroke | Statin, antiplatelet and antioedema measures | Status quo (mRS = 3) | Probable |
| 44 | 35 | Headache and left upper limb and face paresthesia and weaknsess | 2 | ChAdOx-1/ 2nd dose | 10 days | left upper and lower limbs spastic hemiparesis | MRI- venous sinus filling defect involving the anterior 2/3rd of the superior sagittal sinus and bilateral frontal and parietal infarctESR-12 mm, CRP- NegativePlatelet-376Lakh/cmmPT,INR,aPTT-NormalPCV-NormalHomocysteine, Vitamin B12-Folate- Normal. Fasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalCardiac evaluation-Normal | Cerebral Sinus Venous Thrombosis | Anticoagulation | Status quo (mRS = 3) | Probable | |
| 45 | 80 | M | Sudden onset right upper and lower limbs weakness. | 1 | ChAdOx-1/ 1st dose | 15 days | Right hemiparesis | MRI-left basal ganglia infarctPlatelet-96Lakh/cmmaPTT-79secCRP-Negative) d-dimer-1381 ng/mlFibronogen- 443 mg/dlFasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalCardiac evaluation-Normal | Acute ischemic stroke with coagulopathy | Statin, antiplatelet | Status quo (mRS = 4) | Probable |
| 46 | 56 | M | Sudden onset left upper and lower limbs weakness | 2 | BBV152/1st dose | 14 days | left upper and lower limbs spastic hemiparesis | MRI- right MCA-PCA territory watershed infarctPlatelet-254Lakh/cmmPT,INR,aPTT-NormalFasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalCardiac evaluation-Normal | Acute ischemic stroke | Statin, antiplatelet | Status quo(mRS = 3) | Probable |
| 47 | 65 | M | Tingling paresthesia of left half of the body. Known case of medically well controlled dyslipidemia and T2DM | 4 | BBV152/1st dose | 3 days | Tone, power-normal | MRI- right thalamic infarctPlatelet-293Lakh/cmmPT,INR,aPTT-NormalFasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalCardiac evaluation-Normal | Acute ischemic stroke | Statin, antiplatelet | Status quo (mRS = 1) (mRS = 1 | Possible |
| 48 | 55 | M | Headache, and right upper and lower limbs weakness. Known case of medically controlled hypertension | 1 | ChAdOx-1/ 2nd dose | 2 days | Right spastic hemiparesis | MRI-Acute infarct noted involving left corona radiata, posterior putamen and posterior limb of internal capsule. And Eccentric vessel wall enhancement noted involving left MCA distal M1 and M2 segment (inferior division). Platelet-275Lakh/cmmPT,INR,aPTT-NormalCRP-6 mg/dl(Positive) Fasting lipid profile-Normal panelHbA1C,FBS,PPBS-NormalCardiac evaluation-Normal | Acute ischemic stroke | Statin, antiplatelet | Status quo (mRS = 4) | Possible |
| Encephalitis | ||||||||||||
| 49 | 23 | F | Irrelevant talkConfusion and disorientation | 2 | ChAdOx-1/ 1st dose | 2 days | Alopecia, knuckle hyperpigmentationMMSE:9/30Speech- suggestive of transcortical sensory aphasiaNo meningeal signsEOM- fullPupils- Equal, reactive to lightOther cranial nerves- normalSensory, motor, cerebellar signs- negativeGait- normalPlantars- flexors | CRP-24 mg/L. Serum homocysteine- 132 umol/LVitamin B12- 50 pg/ml (low) LP-CSF: cells-14(PMN-10), protein-27.5 mg/dl. Normal sugar. HSV and other viral agents including chikunguniya, AFB staining, culture sensitivity. ANA profile, ANCA, serum and CSF autoimmune encephalitis panel, RF, creatine kinase, TFT, lipid profile, viral markers including HIV, HbSAg, HCV, VDRL were all normal or negative. Serum dengue and chikunguniya was negative. EEG showed bilateral intermittent slowing (Left more than right). MRI of Brain and spine – left temporal lobe FLAIR hyperintensity suggestive of cerebritis. Serum lactate was persistently elevated (70 mg/dl). | Possible Postvaccinal encephalitis with pre-existing possible mitochondrial cytopathy with primary hyper homocysteinemia | Acyclovir 500 mg iv TID × 7 daysCeftriaxone 1gm iv BD × 7 daysAnd Inj Methyl prednisolone 1gm iv OD × 5 daysFollowed by mitochondrial supplements and oral steroid. | Improved (mRS = 1) | Possible |
| 50 | 52 | F | Pain in the both lower limbs and Stiffness of both lower limbs | 360 | ChAdOx-1/ 22nd dose | 7 | Severe spasticity (grade 4) in both lower limbs(left > right) Plantar- b/l extensor | LP-CSF: nil cell, protein-26.7 mg/dl. ANA profile- anti- SS-A and AntiRo-52 positive. Serum and CSF NMO-MOG were negative. Paraneoplastic antibody- Anti GAD65 ab strongly positive. MRI Brain and Spine: Unremarkable. Whole bodyPET MRI: Normal tracer uptake | Stiff Person Syndrome | Oral steroidDiazepamBaclofen | Mild Improved (mRS = 2) | Probable |
| Myositis | ||||||||||||
| 51 | 58 | M | Pains of both lower limbs, weakness of both lower limbs, weakness of both upper limbs. | 60 | BBV152/1st dose | 15 days | Wasting of bilateral supraspinatus, ifraspinatus, deltoid, biceps, and triceps was noted. Tone- Hypotonia in all 4 limbs. Quadriparesis, proximal and flexor group predominant weakness in UL and LL. DTRs- Hyporeflexic | ESR was 22 mm/hr and CRP was positiveSerum Creatine kinase (CPK) was elevated (13,786 U/L at presentation). Urine routine showed 2 plus blood and myoglobin was positive. ANA profile showed anti-RO52 1plus positive. Myositis profile showed anti-SRP 3 plus positive. Muscle biopsy: polygonal to rounded, myofibers with moderate variation in fiber size and prominent features of active myopathy in the form of myonecrosis. ACR/EULAR2017: Definite myositis | Inflammatory Myositis | IVMP 1gm *5days f/b Rituximab | Improved (mRS = 1) | Probable |
Table 3.
Spectrum of COVID19 vaccine associated neurological disorders (Co-VAN).
| Overall |
CNS Demyelination |
GBS |
Stroke |
Encephalitis |
Myositis |
|
|---|---|---|---|---|---|---|
| Number of cases (%) | 51 | 39 (76.5) | 3 (5.9) | 6 (11.8) | 2 (3.9) | 1 (2.0) |
| Demographics | ||||||
| Mean Age(±SD) | 40.1 (14.5) | 37.8 (12.6) | 44.3(10.5) | 51.1(22.6) | 37.5(20.5) | |
| Age group < 25 years | 8 (15.7) | 6 (15.4) | – | 1(16.7) | 1 [50] | – |
| Age group 25–45 years | 26 (51.0) | 23 (59.0) | 2(66.7) | 1(16.7) | – | – |
| Age group 46–60 years | 14 (27.5) | 9 (23.1) | 1(33.3) | 2(33.3) | 1 [50] | 1 |
| Age group > 60 years | 3 (5.9) | 1 (2.3) | – | (33.3) | – | – |
| Female/Male | 27/24 | 22/17 | 2/1 | 1/5 | Both females | Male |
| Female: Male | 1.13:1 | 1.29:1 | 2:1 | 0.2:1 | ||
| Vaccine details | ||||||
| COVIShield (ChAdOx1)(%) | 43 (84.3) | 35 (89.7) | 3 [100] | 3 (50.0) | 2 [100] | 0 |
| COVAXIN (BBV152) (%) | 8 (15.7) | 4 (10.3) | 0 | 3 (50.0) | 0 | 1 [100] |
| First dose (%) | 37 (72.5) | 29(74.4) | 2(66.7) | 4(66.7) | 1(50.0) | 1 [100] |
| Second dose (%) | 14 (27.5) | 10(25.6) | 1(33.3) | 2(33.3) | 1(50.0) | – |
| Timelines | ||||||
| Mean interval from last dose (in days ± SD) | 13.2 (10.7) | 14.6 (11.6) | 13.(5.8) | 8.2 (5.6) | 5.5(2.1) | – |
| Median interval (days) from first dose (IQR) | 14 (5.5–15) | 14 | 9.5 | 9.5 | – | – |
| Median interval (days) from second dose (IQR) | 12 (3.3–14) | 14 | 14 | 6.0 | – | – |
| 1st week | 14 (27.5) | 9 (23.1) | 1(33.3) | 2(33.3) | 1 | – |
| 2nd week | 20 (39.2) | 17 (43.6) | 1(33.3) | 2(33.3) | 1 | – |
| 3rd week | 6 (11.8) | 3 (7.7) | 1(33.3) | 1(16.7) | – | – |
| 4th week | 1 (2.0) | 1 (2.6) | – | 0 | – | – |
| >4 week | 10 (19.6) | 9 (23.1) | 1(33.3) | 1(16.7) | – | – |
| Mean duration of disease (in days ± SD) | 29.5(52.9) | 26.4(24.8) | 13.3(5.8) | 2.2(1.2) | – | – |
| Causality label | ||||||
| Probable (%) | 48(94.1) | 39 [100] | 3 [100] | 4(66.7) | 1 [50] | 1 |
| Possible (%) | 3(5.9) | – | – | 2(33.3) | 1 [50] | – |
| Clinical outcomes | ||||||
| Favourable (mRS 0–1) (%) | 25 (49.0) | 21 (53.8) | 1 (33.3) | 1 (16.7) | 1 [50] | – |
4.1. Demyelination (patient 1–18)
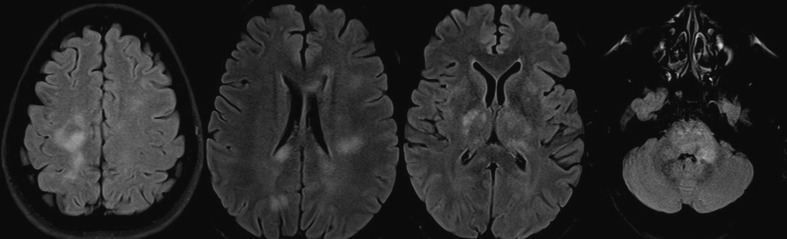
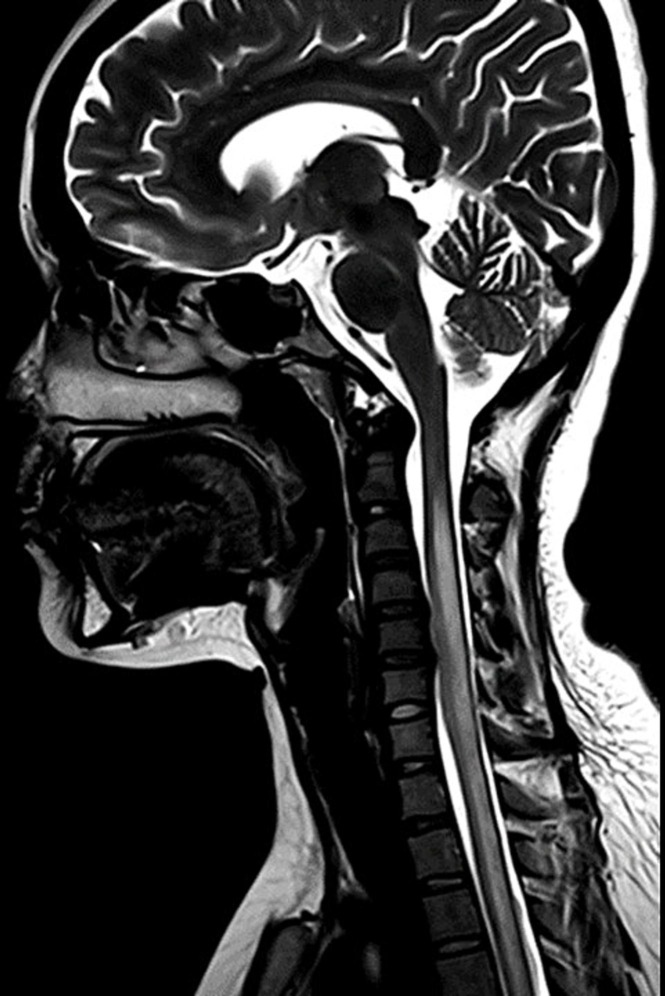
Out of 39 cases with CNS demyelination majority had received ChAdOX-1 vaccine (n = 39, 76.5 %). Majority of the patients were of female sex (F:M, 1.3:1). The mean age of presentation was lower compared to that of overall age in this series (37.8 ± 12.6 years vs 40.1 ± 14.5 years). Majority of the patients belonged to the group of 25 to 45 years. (Table 2, Table 4 )The median interval from the last dose to the onset of the neurological symptoms was 13 (10to14) days. Majority of the cases were vaccinated with COVIShield (ChAdOx1) vaccine (n = 35, 89.7 %). The clinical manifestations occurred after first dose in 29/39 (74.4 %) cases. Majority of the cases were seronegative (n = 23, 59.0 %) which was followed by MOGAD (n = 11, 28.2 %) and NMOSD (n = 5, 12.8 %). LETM was the most common mode of presentation (n = 19, 48.7 %). ON was the presentation in 9/39 cases (23.1 %) cases. Interestingly, none of the cases of NMOSD presented with ON. Neuroimaging showed supratentorial lesions in 16/39 (41.0 %) cases while infratentorial lesions were present in 15/39 (38.5 %) cases. (Fig. 1, Fig. 2 ) As per casuality labelling, all cases were found to be probable temporal association. CSF analysis revealed pleocytosis in 19/37 (77.8 %) and elevated CSF protein in 14/37 (37.8 %), respectively. Favorable mRS scores (0to1) were attained by 21/39 (81.9 %) patients at discharge. There was no significant difference with regards to the latency to presentation, investigational profile or clinical outcomes among the various demyelination subgroups. (Refer to Table 4)
Table 4.
Characteristics of cases with CNS demyelination.
| MOGAD |
NMOSD |
Seronegative Demyelination |
p value |
|
|---|---|---|---|---|
| Number of cases (%) | 11 (28.2) | 5 (12.8) | 23 (59.0) | -- |
| Demography | ||||
| Mean Age (±SD) | 41.5 (7.0) | 37.25 (19.0) | 23.1 (21.7) | 0.566 |
| Age < 25 years (%) | 0 | 2 [40] | 4(17.4) | 0.111 |
| Age 25–45 years (%) | 10(90.9) | 2 [40] | 11(47.8) | 0.038*# |
| Age 46–60 years (%) | 1(9.1) | 0 | 8(34.8) | 0.106 |
| Age > 60 years (%) | 0 | 1 [20] | 0 | – |
| Gender (Female:Male) | 4:7 | All females | 13:10 | – |
| Vaccine details | ||||
| COVIShield (ChAdOx1) (%) | 11 [100] | 3 (60.0) | 21 (91.3) | – |
| COVAXIN (BBV152) (%) | 0 | 2 (40.0) | 2 (8.7) | |
| First dose (%) | 10 (90.9) | 2 (40.0) | 17 (73.9) | 0.096 |
| Second dose (%) | 1 (9.1) | 3 (60.0) | 6 (26.1) | |
| Timelines | ||||
| Median latency from last vaccination (IQR) (days) | 13 [10–14] | 17 [14–36] | 14 [4–14] | 0.309 |
| Median interval (days) from 1st dose (IQR) | 12 [10–14] | 29.5(23.3–35.8) | 14 [5–14] | 0.097 |
| Median interval (days) from 2n dose (IQR) | 32 | 14(7.5–25) | 10.5(2.5–14) | 0.528 |
| 1st week (%) | 1(9.1) | 1 [20] | 7(30.4) | 0.379 |
| 2nd week (%) | 7(63.6) | 1 [20] | 9(39.1) | 0.211 |
| 3rd week (%) | 2(18.2) | 1 [20] | 0 | 0.096 |
| 4th week (%) | 0 | 0 | 1(4.3) | 0.700 |
| >4 week (%) | 1(9.1) | 2 [40] | 6(26.1) | 0.344 |
| Mean duration of disease (in days ± SD) | 20.5 (20.0) | 54.6 (32.6) | 23.1 (21.7) | 0.019*$ |
| Causality label | All probable | All probable | All probable | |
| Investigations | ||||
| CSF | ||||
| Pleocytosis (%) | 7/9 (77.8) | 2/5 (40.0) | 10/22 (45.5) | 0.217 |
| Protein elevation (%) | 4/9 (44.4) | 1/5 (20.0) | 9/22 (40.9) | 0.636 |
| MRI | ||||
| LETM | 6/11 (54.5) | 4/5 [80] | 9/23 (39.1) | 0.228 |
| ON | 5/11 (45.5) | – | 4/23 (17.4) | 0.081 |
| Supratentorial lesion | 4/11 (36.4) | 2/5 (40.0) | 10/23 (43.5) | 0.924 |
| Infratentorial lesion | 3/11 (27.3) | 3/5 (60.0) | 9/23 (39.1) | 0.457 |
| Outcome | ||||
| Favourable (mRS 0–1) (%) | 7/11 (63.3) | 2/5 (40.0) | 12/23 (52.2) | 0.658 |
* Denotes p value < 0.05.
$ p value of 0.023 between MOGAD and other demyelination; p value of 0.023 between NMOSD and other demyelination.
# p value of 0.014 between MOGAD and rest of the demyelination group; p value of 0.631 between NMOSD and rest of the demyelination group and 0.111 between other demyelination group and combined NMOSD and MOGAD.
Fig. 1.
MRI brain T2/FLAIR shows hyperintensities in mid brain, pons, left MCP, bilateral posterior internal capsule, thalamus, bilateral centrum semiovale in a case of MOGAD. (Case 01).
Fig. 2.
MRI spine T2 weighted image shows longitudinally extensive cervico-dorsal cord hyperintensities in a case of probable post vaccination myelitis. (Case 14).
4.2. Guillain-Barré syndrome (patient 40–42)
Patients with a diagnosis of GBS constitutes 10.3 % (3/29) of the total post COVID19 vaccination related neurological diseases. All of them had received ChAdOx-1 vaccine. The mean age of presentation was higher (44.3 ± 10.5 years) than the overall mean age (40.1 ± 14.5 years). Out of three cases, two were female and first clinical symptom started after a mean of 11.0 ± 7.0 days from last vaccination. All three of them had albumin-cytological dissociation with a mean CSF cell of 0 and protein of 115.2 ± 36.2 mg/dl. Nerve conduction studies of sampled nerves were suggestive of motor axonopathy in one case (case 40) and mixed axonal and demyelinating neuropathy (case 41 and 42) in two cases. All patients were treated with large volume plasma exchange for five cycles. One of the patients had favorable mRS at discharge. (Refer to Table 2, Table 3).
4.3. Stroke (patient 43–47)
Out of six cases of stroke, three (50 %) had received ChAdOx-1 and 3 (50 %) were vaccinated with BBV152 vaccine. Based on the [125] criteria for causality labeling four patients were considered as probable vaccine related event. The mean age of presentation (51.1 ± 22.6 years) was higher than the overall mean. Majority of the patients were of male sex (F:M 1:5). They experienced first symptoms after a mean interval of 8.2 ± 5.6 days post vaccination. The spectrum comprised three cases of anterior circulation arterial stroke, and single case each of posterior circulation, watershed infarct and venous stroke. Two cases (Case 47 & 48) were considered to have possible associations since they had vascular risk factors which were well controlled at the time of onset of symptoms. Two cases (33.3 %) had thrombocytopenia and coagulopathy. None of the cases had any definitive evidence of Vaccine induced immune thrombotic thrombocytopenia (VITT) based on American Haematology Society guidelines. Patients were treated as per standard treatment protocols. At discharge, one of the patients (16.7 %) had favorable mRS (0to1). (Refer to Table 3).
4.4. Encephalitis (n = 2)
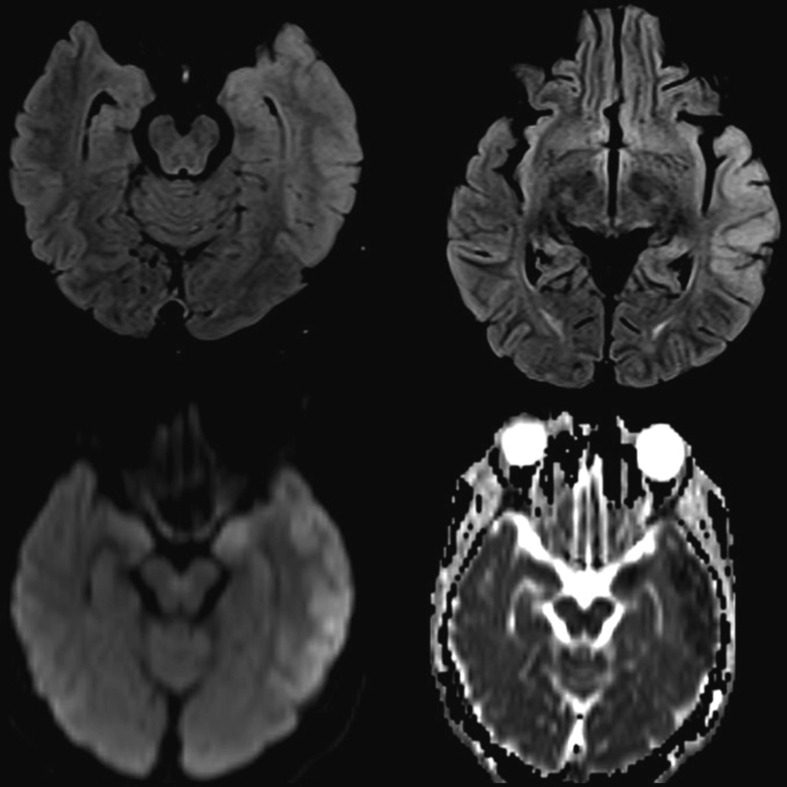
Patient 49: A 23-year-old lady developed encephalopathy-two days after first dose of ChAdOx 1 vaccination. Brain MRI revealed T2/FLAIR hyperintensities with areas of diffusion restriction predominantly involving cortical grey matter of left parahippocampal gyrus, amygdala, lateral temporal lobe, parieto-temporal junction in a gyriform pattern on left side and deep grey matter of left pulvinar nucleus. (Fig. 3 ). LP-CSF analysis showed polymorphonuclear cells with predominant pleocytosis with normal protein and sugars. Extensive evaluation for CSF and serum viral markers were unremarkable. Electroencephalogram showed bilateral intermittent slowing (left more than right). Serum and CSF autoimmune mosaic panel were negative. She was empirically treated with antivirals and as there was no response, steroids were started following which she improved completely. Hence a diagnosis of possible post-COVID19 vaccination autoimmune encephalitis was considered.
Fig. 3.
MRI brain T2/FLAIR hyperintensities with restricted diffusion predominantly involving cortical grey matter of left parahippocampal gyrus, amygdala, lateral temporal lobe, parieto-temporal junction in a gyriform pattern on left side and deep grey matter of left pulvinar nucleus. (Case 28).
Patient 50: A 52-year-old lady presented with pain in the bilateral lower limbs and stiffness, 7 days post vaccination with ChAdOx-1 (second dose). Examination revealed severe spasticity in both the lower limbs and extensor plantar response. Secondary work-ups revealed strong positivity for anti-GAD-65 antibody. Neuroimaging including brain and spine MRI, CSF analysis, serum and CSF NMO/MOG antibody titres were negative. PET-MR brain was normal. She was diagnosed as Stiff person syndrome. She was treated with oral steroids and symptomatic measures. At discharge, she made a mild recovery to mRS of 2.
4.5. Myositis (n = 1)
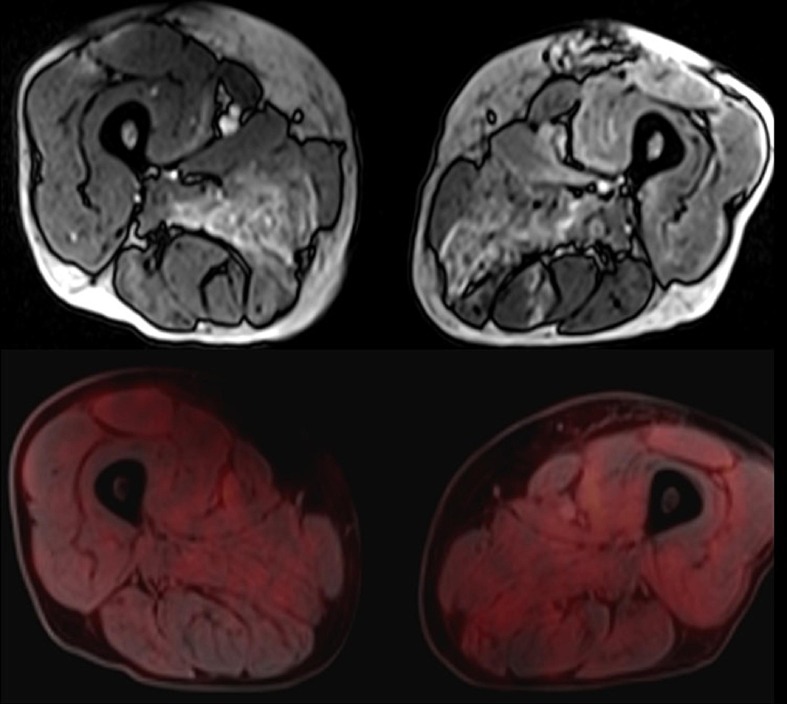
Patient 51: A 58-year aged male, developed myalgia and progressive weakness of limbs, 15 days post-BBV152 vaccination. He presented to us 2 months after symptom onset and was wheel chair bound at the time of admission. He had Creatine Kinase value of 13786U/L with anti-SRP-antibody positivity, hence diagnosed as definite inflammatory myopathy (ACR/EULAR 2017) [142]. Muscle MRI was suggestive of myositis. PET MRI showed increased tracer uptake in the muscles without any sign of malignancy. (Fig. 4 ) He was treated with intravenous methylprednisolone pulse therapy followed by rituximab 6 monthly regime. At 6 months follow-up, patient was ambulant with mild support. (Refer to Table 3).
Fig. 4.
Muscle MRI shows T2 hyperintensities in the muscles of the anterior, posterior & adductor compartment of thigh bilaterally. 18FDG-PET shows increased tracer uptake in the muscles of the anterior, posterior & adductor compartment of thigh bilaterally.
5. Discussion
In this series of 51 cases, we present multiple neurological diseases which were found to be temporally associated with COVID19 vaccination. Vaccination-associated neurological diseases are well known in the medical literature. Several vaccines, such as influenza, rabies, mumps-measles-rubella (MMR), yellow fever have reported neurological adverse events. [143] However, presence of coexisting confounding factors enhances the risk of false association of any adverse event to a particular vaccine. For instance, several series of post-vaccination GBS were reported following mass vaccination against novel A/NJ/76 (Hsw1N1) influenza, the association which was later refuted in a few observations. [144], [145] Similarly, measles vaccines were claimed to be associated with the development of autism, [146] the same was clearly rejected in subsequent studies. [147], [148].
In the current scenario, when the mass vaccination campaign is underway with the majority of the world population are in the process of vaccination [149], the coincidental occurrence of a disease, can lead to false labelling of a condition as a vaccine related adverse outcome. Multiple types of vaccines from different manufacturers, different routes of administration, and administration of vaccine candidates in different phases of clinical trials (i.e., phase III or IV) have added to the existing dilemma of causality labelling of AEFIs. (Refer to supplementary appendix). In due course of time, with evolving evidence from larger studies, some of the reports of vaccine-related adverse events get refuted as was seen with sudden sensorineural hearing loss post COVID-19 vaccination. [150], [151], [152] A higher incidence, well and above the background incidence of a given clinical entity can serve as an important surrogate marker of a probable vaccine induced association. Post-vaccination GBS had an approximately-four times the higher incidence among Ad26.COV2.S recipients, with an estimated rate of 9.8 cases per million doses. [43], [143] Association of ChadOx1 nCoV-19/AZD1222 and Ad26.COV2.S vaccines to a small risk of thrombotic thrombocytopenia, [153], [154] and myocarditis with mRNA vaccines, BNT162b2, [155] are pointed out in many observations. In India, the adenoviral vector vaccine was mostly used. We found three cases of vaccination associated with GBS over 1 year, when a total of 1,48,26,49,754 doses of AstraZeneca, COVIShield (ChAdOx-1), and 28, 80, 80,355 doses of COVAXIN (BBV152) are already administered. This implies the incidence of the event lies within the usual incidence of GBS. [156].
In contrast to the higher association of the mRNA-based vaccine with demyelination as shown in the systematic review of 32 cases of post-COVID19 vaccination-associated demyelination, we found a majority (16/18, 69.6 %) to be associated with adenoviral vector vaccine (ChAdOx-1). The similar female predominance, the median age of presentation, median interval from the last dose, and clinical presentation as pointed out in the review are also observed in our series. Similar to previous studies, the most common antibody associated with post-vaccination demyelination in our study was MOG. [59], [157], [158]. MOG associated demyelination has been reported to occur following vaccinations with Japanese encephalitis, tetanus, measles, rubella etc. Various mechanisms proposed are autoantibody production due to molecular mimicry, induction of autoreactive T cells via bystander activation due to ongoing response against vaccine antigen or adjuvant. Vaccines may also cause unmasking of a preexisting autoimmune disorder [59]. Our series on post-vaccination stroke revealed coagulopathy in two cases, wherein vaccine induced thrombocytopenia, could be a potential consideration. The more frequent occurrence of the neurological events among the ChAdOx-1 recipients could probably be the reflection of the more widespread administration of the ChAdOx-1 vaccine in India. [138].
5.1. Spectrum of COVID vaccine associated neurological symptoms (Co-VAN)
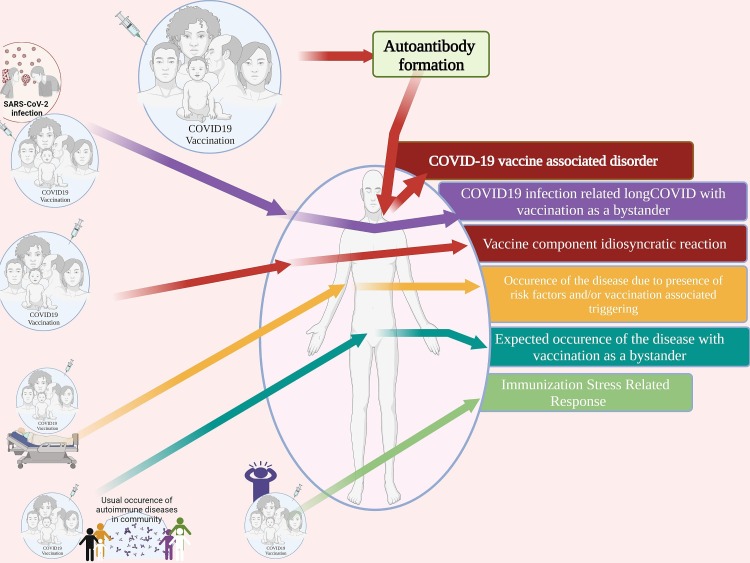
The spectrum of the neurological diseases associated with COVID19 vaccination is yet to be completely explored. Reports of COVID19 vaccine-related adverse events have been tabulated for providing an updated list of neurological diseases attributed to the receipt of COVID-19 vaccine. (Refer to Table 5 Refer to Fig. 5, Fig. 6 ) (Refer to supplementary appendix for detailed search terms) Although the causality label wasn’t justified in many of these reports, awareness of the smallest possibility of any adverse event could enable prompt recognition in subsequent cases. Presence of clustering or detection of signals of AEFI would prompt further investigations. In the current context, an individual developing any neurological illness after the COVID19 vaccination could potentially satisfy-one or more of the following: a) COVID19 vaccine-associated disorder, b) remote COVID19 infection-related, or “long COVID” with vaccination as a bystander, c) vaccine component induced idiosyncratic reaction, d) occurrence of the disease due to the presence of risk factors and/ or vaccination associated triggering, e) expected occurrence of the disease with vaccination as a bystander, or f) immunization stress-related response. (Refer to Fig. 3 for details) (Refer to Supplementary appendix for vaccination related terms).
Table 5.
CO-VAN study: scoping review of literature. Spectrum of COVID19 vaccine associated neurological disorders (Co-VAN) – a review of the literature.
| Spectrum of COVID19 vaccine associated neurological disorders (Co-VAN) | ||||||
|---|---|---|---|---|---|---|
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose & Symptoms | Description and observation |
| Guillain Barre Syndrome | ||||||
| Fernandez et al. 2021[31] | Pfizer-BioNTech (BNT162b2) = 22Moderna (mRNA1273) = 9AstraZeneca (ChAdOx1) = 3Janssen = 3 andJohnson & Johnson = 1 | GBS | 24 cases | 1st | 7 days (average) | 7 patients had CSF albuminocytological dissociation, andAll had a predominant demyelinating pattern |
| Maramattom et al. 2021[32] | AstraZeneca (ChAdOx1) = 7 | GBS | Seven cases of GBS | 1st | 2 weeks | All patients developed severe GBS. The frequency of GBS was 1.4- to 10-fold higher than that expected. |
| Woo et al. 2021[43] | Jannsen (Ad26.COV2.S) = 130 | GBS | Median age = 56 years; (IQR, 45–62 years) | Median time to onset of GBS following vaccination = 13 days (IQR, 10–18 days) | Estimated absolute rate increase of 6.36 per 100 000 person-years | |
| Dang et al. 2021[52] | Miller-Fisher Syndrome and Guillain-Barre Syndrome overlap syndrome | 63/M | 1st | 9 days laterExperienced new-onset lower back pain and 5 days after developed bilateral oculomotor nerve palsy, ataxia, facial diplegia and lower limb weakness. Later developed diplopia on lateral gaze bilaterally. Examination revealed impaired adduction, restricted upward gaze and intorsion with down gaze bilaterally, consistent with partial cranial nerve III palsies. | LP-CSF: Protein- 2.99 g/L Cells- 5/hpf: Albuminocytological dissociation. NCS- long-standing axonal neuropathy with reduced motor and sensory amplitudes. EMG- and length-dependent chronic neurogenic changes. MRI Brain- enhancement of the facial and oculomotor nerves bilaterally. Serum anti-GQ1b antibody- negative. Showed partial improvement with IvIg 2 g/kg over 5 days. | |
| Kim et al. 2022[53] | Pfizer-BioNTech (BNT162b2) | Pediatric Case of Sensory PredominantGuillain-Barré Syndrome | 16/F | 2nd | 2 days afterAscending numbness and paresthesia of her bilateral lower and upper extremities | MRI – mild thickening and enhancement of the anterior and posterior spinal nerve roots of the cauda equine. LP-CSF: 1cell/cmm, Protein- 112 mg/dlNCS- prolonged latency and slowed conduction velocity in multiple sensory and motor nerves |
| David et al. 2021[54] | Pfizer-BioNTech (BNT162b2) | Recurrence of GBS | Out of 702 patients of previous GBS, 1 had recurrence. | NCS s/o sensorimotor demyelinating polyneuropathy. Was treated with PLEX and improved. | ||
| Demyelination | ||||||
| Ismail et al. 2022[60] | Pfizer-BioNTech (BNT162b2) = 11AstraZeneca (ChAdOx1) = 8Moderna (mRNA-1273) = 6Sinovac/ Sinopharm = 5 Sputnik = 1Johnson&Johnson = 1 | Transverse myelitisADEMMS-like illnessNMOSD | 32 cases of with demyelination. Female predominance (68.8 %) and median age of 44 years. | 71.8 % occurred after the first dose of the vaccine, with a median of 9 days. | Types: Transverse myelitis = 12/32MS-like pictures (first diagnosis or a relapse) = 12/32ADEM- like 5/32NMOSD- like = 3/32. | Most MS-like episodes (9/12) were triggered by mRNA-based vaccines, TM occurred following both viral vector and mRNA-based vaccines. |
| Netravathi et al. 2022[59] | AstraZeneca (ChAdOx1) = 27COVAXIN (BBV152) = 2 | MOGAD& other demyelinations | Myelitis = 11, Optic neuritis = 6, Acute demyelinating encephalomyelitis = 5, Brainstem demyelination = 3, andMultiaxial involvement = 4 | MOG positive = 10Postvaccinial cases were found to have a significantly higher-Mean age, Presence of encephalopathy (p value:0.0007), CSF pleocytosis (p value: 0.0094) andRaised CSF protein (p value: 0.0062). | ||
| Chen et al. 2021[58] | Inactivated virus vaccine | NMOSD | A middle aged female | 1st | After 3 days of vaccine developed mild fever, vomiting, diarrhoea, cough and unsteadiness and dizziness. | MIR Brain- area postrema and bilateral hypothalamus lesions without Gd enhancement. Investigations: leucopenia of 2.36 × 109/Land positive antibodies for AQP4, ANA, SSA, SSB, Ro-52, and p-ANCA. CSF- Mononuclear pleocytosis with normal protein and negative OCB. Treated with intravenous steroid pulse and patient responded well. |
| Khayat-Khoei et al. 2021[61] | Pfizer-BioNTech (BNT162b2) = 4Moderna (mRNA-1273) = 3 | Exacerbation of known stable MS = 4, New onset MS = 2, New onset NMO = 1 | 24 to 64 (mean 39.1) years. Male = 2, Female = 5 | First (n = 2), Second(n = 5) | 1–21 daysSymptoms: visual loss, dysmetria, gait instability, paresthesias, sphincter disturbance, and limb weakness. | All responded to corticosteroid (n = 7) or plasma exchange (n = 1) therapy. |
| Arnao et al. 2022[65] | AstraZeneca (ChAdOx1) | Bilateral optic neuritis | A middle aged female, | After 2 weeks | First dose of vaccine. Developed headache and painful blurred vision worsened by movement in both eyes, decreased bilateral vision acuity. | MRI of the brain in FLAIR axial showed increased signal of the left optic nerve. LP-CSF analysis normal cells and protein. Aquaporin 4 (AQP4)-IgG and MOG-IgG negative. Treated with intravenous steroid pulse and patient responded well. |
| Ancau1 et al. 2022[64] | AstraZeneca (ChAdOx1) | Acute HemorrhagicEncephalomyelitis (AHEM) | 61Y/M | 1st | 2daysp/w- fever, headache and apathy followed by seizure and coma. | MRI Brain- bilateral confluent cortical and subcortical FLAIR hyperintense lesions with haemorrhagic involvement of the basal ganglia. CSF- revealed normal cell counts (1 leukocyte per μl) and moderate disturbance of the blood–brain-barrier. Treated with PLEX and IVMP, poorly responded. |
| 25Y/F | 1st | 9 days. P/w severe cephalgia, thoracic back pain, mild weakness and ascending numbness in her legs. | MRI- longitudinal edema throughout the thoracic spinal cord exhibiting mild contrast enhancement as well as focal central haemorrhages and brain showed bi-hemispheric white matter lesions with focal contrast enhancement. CSF- granulocytic pleocytosis | |||
| VITTS and associated strokes: CSVT | ||||||
| Sangli et al. 2021[26] | Moderna (mRNA-1273) | VITTS with CSVT | 65/F | 2nd | 10 days after. With symptoms of headache, lower limb discomfort and breathing difficulties. | She was found to have catastrophic thrombosis including deep venous and cerebral sinus venous thrombosis. |
| See et al. 2022[27] | AstraZeneca (ChAdOx1) Janssen (Ad26.COV2.S) | VITTS and venous and/or arterial ischemic strokes/ intracerebral haemorrhage | Younger age (median age 46), female preponderance and 12 days as median time after vaccination are reported. | Vaccine-induced immune thrombotic thrombocytopenia (VITT) is mainly reported in adenovirus vector based vaccines, ChAdOx1 CoV-19 vaccine and Ad26.COV2.S. According to VARES data the incidence of VITT is approximately 1 in 263,000 recipients of Ad26.COV2.S. (PMID 35,038,274) | ||
| Krzywicka et al. 2021[30] | AstraZeneca (ChAdOx1) Jannsen (Ad26.COV2.S) Pfizer-BioNTech (BNT162b2) Moderna (mRNA-1273) | CVST | Vaccine types | Absolute risk of CVST within 28 days of per million of first-dose vaccination | The absolute risk of CVST with thrombocytopenia within 28 days of per million of first-dose vaccination | Age group between 18 and 24 years had the highest absolute risk of CSVT, with thrombocytopenia (7.3 per million, 95 % CI 2.8–18.8) or without thrombocytopenia (3.7 per million, 95 % CI 1.0–13.3). |
| ChAdOx1 nCov-19 | 7.5 (95 % confidence interval [CI] 6.9–8.3) | 4.4 (95 % CI 3.9–4.9) | ||||
| Ad26.COV2.S | 0.7 (95 % CI 0.2–2.4) | 0.7 (95 % CI 0.2–2.4) | ||||
| BNT162b2 | 0.6 (95 % CI 0.5–0.7) | 0.0 (95 % CI 0.0–0.1) | ||||
| mRNA-1273 | 0.6 (95 % CI 0.3–1.1) | 0.0 (95 % CI 0.0–0.2) | ||||
| Stroke-Arterial | ||||||
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose | Description and observation |
| Walter et al. 2021[94] | AstraZeneca (ChAdOx1) | Thrombosis of Carotid Artery | 31/M | 1st | 8 days. with acute headache, aphasia, and hemiparesis. | MRI brain showed main stem occlusion of middle cerebral artery. Had elevated d-dimer, normal platelet and fibrinogen level. Positive IgG PF4 antibody. |
| Tiede et al. 2021[95] | AstraZeneca (ChAdOx1) | Ischemic stroke-arterial | Ischemic stroke in ICA and MCA territory with haemorrhagic transformation in one patient and another had cortical infarctions and aortic arch thrombi. Both had thrombocytopenia, increased d-dimer level, and positive anti-PF4 antibody. | |||
| Al-Mayhani et al. 2021[96] | AstraZeneca (ChAdOx1) | Strokes | 3 patients with MCA infarct, ICA infarct and CVST, and MCA infarct respectively. All had thrombocytopenia, positive anti-PF4 antibody, and increased d-dimer level. | |||
| Cari et al. 2021[97] | AstraZeneca (ChAdOx1), Jannsen (Ad26.COV2.S) | Post vaccinal thrombosis | Most of the ChAdOx-1 and Ad26.COV2.S vaccine associated venous thrombotic serious adverse events were not associated with thrombocytopenia. | |||
| Bell’s Palsy | ||||||
| Burrows et al. 2021[66] | Pfizer-BioNTech (BNT162b2) | Sequential contralateral facial nerve palsies | 61/M | 1st | 5 h. Developed unilateral LMN facial palsy. | 2 days after the 2nd dose – contralateral LMN facial palsy. Significant improvement with oral steroid course in either occasions. |
| Wan et al. 2022[67] | Number of cases | Age-standardised incidence (cases per 100 000 person-years) | Age-standardiseddifference for the incidence compared with the background population | Equivalent to additional cases per 100 000 people | Odds ratio | |
| CoronaVac | 28 | 66·9 | 41·5 | 4.8 cases | 2·385 (95 % CI 1·415 to 4·022) | |
| Pfizer-BioNTech (BNT162b2) | 16 | 42·8 | 17·0 | 2·0 cases | 1·755 (0·886 to 3·477) | |
| Olfactory dysfunction | ||||||
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose | Description and observation |
| Konstantinidis et al. 2021[74] | Pfizer-BioNTech (BNT162b2) | Hyposmia | 42y/F | 2nd dose | 3 days after presented with decreased olfactory ability. | Showed partial improvement on olfactory testing after olfactory training with four odors (lemon, rose, eucalyptus, and cloves). |
| 39y/F | 2nd dose | 5 days of 2nd dose of vaccine presented with hyposmia. | Improved within a week after the initial assessment. | |||
| Keir et al. 2021[75] | Pfizer-BioNTech (BNT162b2) | Phantosmia | 57y/F | 2nd dose | Complaining of constantly ‘‘smelling smoke’’ and headaches. Associated with hyposmia to additional odorants and was affecting her quality of life. | CTA postcontrast showed a faint enhancement of left olfactory tract. MRI brain – Asymmetric enlargement and increased T2 hyperintensity in the left olfactory bulb and tract extending posteriorly and thickened, clumped olfactory nerve filia. |
| Vestibulo-cochlear nerve dysfunction | ||||||
| Jeong et al. 2021[84] | AstraZeneca (ChAdOx1) | Sudden sensorineural hearing loss | 64/F | 1st | 1 day afterSudden hearing loss in the right ear. | Initially treated with oral steroid and followed by intratympanic steroid following which had complete recovery |
| Pfizer-BioNTech (BNT162b2) | 42/M | 1st | Same daysudden hearing loss in the left ear | Responded oral steroid and followed by intratympanic steroid injection. | ||
| Pfizer-BioNTech (BNT162b2) | 18/M | 2nd | 2 days aftersudden hearing loss in the right ear | Temporal magnetic resonance imaging showed normal findings. Detriment on steroid therapy. | ||
| Parrino et al. 2021[82] | Pfizer-BioNTech (BNT162b2) = 3 | Tinnitus | 37y/F | 1st dose | 7 h after had right ear tinnitus | |
| 63/M | 1st dose | 20 h after had left tinnitus associated to hyperacusis and dysacusis, | ||||
| 30y/M | 2nd dose | 1 week after vaccine presented with left tinnitus, hyperacusis and dysacusis. | ||||
| P -T Tseng et al. 2021[81] | AstraZeneca (ChAdOx1) | Cochleopathy | 37Y/M | 1st dose | 5 h. Iintermittent, right ear, high-pitch tinnitus which progressed into continuous high-pitch tinnitus and disturbed the normal hearing along with fever and myalgia. | Audiological evaluation s/o cochleopathy. Responded to short course of steroid. |
| Zhao et al. 2021[85] | Sinovac Coronavirus vaccine = 2 | SNHL | 30Y/M, and 64Y/F | 1st dose | 4 daysDeveloped hearing loss in the right ear with tinnitus and dizziness. | CT temporal bone and MRI brain were normal. Blood investigations were not remarkable. Poorly responded to vitamin B12 and steroid. |
| Mauro et al. 2022[83] | Pfizer-BioNTech (BNT162b2) = 23 | Objective vertigo = 16Subjective vertigo = 14Dizziness = 3 | Associated ENT symptoms: Hearing loss = 4 Tinnitus = 6Ear fullness = 2Hypersensitivity to noise = 1 | No presence of nystagmus = 7Presence of horizontal or rotatory nystagmus = 9Presence of positive HST/ “central HINTS” or vertical or oblique nystagmus/ “central HINTS”= 17 | Probable clinical diagnosis: No presence of vestibular impairment or central etiology of vertigo/dizziness = 7Benign paroxysmal positional vertigo = 9Probable central etiology = 17 | |
| AstraZeneca (ChAdOx1) = 5 | ||||||
| Moderna (mRNA-1273) = 4 | ||||||
| Jannsen (Ad26.COV2.S) = 1 | ||||||
| Abducens nerve palsy | ||||||
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose | Description and observation |
| Reyes-Capo et al. 2021[79] | Pfizer-BioNTech (BNT162b2) | Abducens nerve palsy | 59Y/F | 2 days, after vaccineAcute binocular and painless, horizontal diplopia. And had h/o fever for 1 day. | Mild elevation in ESR and CRP. MRI and other blood investigations were unremarkable. Had persistent deficit on follow up. | |
| Pawar et al. 2021[80] | AstraZeneca (ChAdOx1) | Recurrent Abducens nerve palsy | 23Y/M | 1st | 1 weekWith sudden-onset diplopia along with severe headache of 1 week’s duration. On examination had left esotropia with limited abduction of the left eye (LE 6th cranial nerve palsy) | MRI and blood investigations were unremarkable. Improved in follow up. H/o 2 episodes of similar 6th nerve palsy, one 5 years back following a febrile illness and another 2 years back following chicken pox. |
| Oculomotor nerve palsy | ||||||
| Cicalese et al 2021[77] | Moderna (mRNA-1273) | Third cranial nerve palsy | 88/M | 1st | 3 daysWith objective dizziness, diplopia andgait instability. k/c/o IHD, HTN, Paroxysmal AF. Non diabetic. | Brain CT scan, CT angiographyand MRI ruled out a vascular accident. Treated with oral steroid, made complete recovery. Later vaccinated again with different vaccine at different injection site. |
| Kerbage et al. 2021[78] | Pfizer-BioNTech (BNT162b2) | Oculomotor nerve palsy | 84/F | 1st | 1 day, Presented with mydriasis, ptosis, and a “down and out” gaze. | MRI Brain (plain) normal. Serum anti AChR Ab, ANA screening and EMG were unremarkable. Treated with prednisone 40 mg daily for 5 days, followed by valacyclovir 500 mg twice daily for 7 days. On 2 months follow up patient improved completely. |
| Encephalitis | ||||||
| Zuhorn et al. 2021[86] | AstraZeneca (ChAdOx1) | Postvaccinal Encephalitis (Possible Autoimmune Encephalitis) | 21/F | 1st | 1 day afterdeveloped headache and progressive neurological symptoms includingattention and concentration difficulties starting on day 5 after vaccination, resulting in admission to hospital11 days after vaccination. Subsequently had seizure. | MRI Brain- NormalCSF- 46 leukocytes/cmm(lymphocytic). EEG- diffuse abnormally slow thetarhythms without epileptiform activity. Responded to steroid therapy. |
| 63/F | 1st | 2 days later diagnosed to have DVT in left left- started on anticoagulation. 6 days post vaccination – gait deteriorated, she developed a vigilance disorder and a twitching all over her body. Later developed severe immobilizing opsoclonusmyoclonus syndrome. | MRI Brain- NormalCSF- 115 leukocytes/cmm(lymphocytic). EEG- diffuse abnormally slow theta and delta rhythms without epileptiform activity. No response to initial antibiotic therapy. Later, Responded to steroid therapy. | |||
| 63/M | 8 days afterisolated aphasia and fever. | MRI Brain- NormalCSF- 7 leukocytes/cmm(lymphocytic). Testing for neurotropic viruses in serum and CSF- Negative. EEG- NormalResponded to steroid therapy. | ||||
| Baldelli et al. 2021[87] | AstraZeneca (ChAdOx1) | Hyperacute reversible encephalopathy | 77/M | 1st | 1 day afterConfusion and agitation consistent with delirium with extreme agitation. k/c/osarcoidosis and polymyalgiarheumatica in clinical remission with Methylprednisolone 4 mg/day. Mild COVID-19 five months prior to vaccination. | CRP- elevatedEEG – moderate diffuse slowingCT (contrast)- unremarkableCSF: cell-3, protein-119 mg/dl, glucose-52 mg/dl, IL6-194(high), IL8-162(high) Microbiological testing on CSF-negativeCSFoligoclonal bands, CSF and serum autoimmune encephalitis antibodies, serum onconeural, antinuclear and antineutrophil cytoplasmic antibodies: Negative. Responded to intravenous methylprednisolone pulse therapy. |
| Moslemi et al. 2022[88] | AstraZeneca (ChAdOx1) | Herpes simplex encephalitis | 27/M | 1st | 3 days aftersevere headache and altered mental status began to appear, including slowed psychomotor activity and loss of alertness. Subsequently severeheadache, agitation, delirium, and disorientation | LP-CSF: protein levels (3.05 mg/dl), WBC count of 600 per mm3 (predominance of lymphocyte) CSF HSV PCR- +veMRI brain and EEG- Unremarkable. Treated with antiviral, and improved over 21 days. |
| Al-Mashdali et al. 2021[89] | Moderna (mRNA-1273) | Acute hyperactive encephalopathy | 32/M | 1st | 2 days afterdeveloped acuteconfusion, memory disturbances, and auditory hallucination | EEG showed features of encephalopathy, CSF: elevated protein levels (0.76 gm/L, reference range = 0.15–0.45) with average cell counts (white blood cells of 3 u/L) and glucose levels., MRI brain- UnremarkableCSF autoimmune encephalitis (including anti-aquaporin-4, anti-myelin basic protein, anti-myelin oligodendrocyte glycoprotein, anti-glial fibrillary acidic protein, anti-NMDAR, anti-GAD, andother autoimmune encephalitis antibodies) was negative. Responded to intravenous steroid pulse therapy. |
| Autoimmune Encephalitis | ||||||
| Zlotnik et al. 2022[91] | Pfizer-BioNTech (BNT162b2) | LGI-1 associated autoimmune encephalitis | 48/M | 2nd | 2.5 weeks later, Started to have memory deficits and anterograde amnesia. O/E- Montreal Cognitive Assessment (MoCA) score of 18/30 | Serum sodiumlevel of 132 mEq/ L (normal range 135–145), Tumor markers (CEA, AFP, CA125, CA19–9, CA15–3) andParaneoplastic neuronal antibodiesincluding anti-Hu, Ri, Yo, Ma/Ta, Amphiphysin, CV2, SOX1, Tr (Euroimmun) were negative. EEG- UnremarkableMRI Brain – intense signal on both medial temporal lobes (more on the left) including theparahyppocampal gyrus on T2/FLAIR and DWI. Whole body CT- liver cyst and adrenal adenoma. CSF- Cell, protein and sugar normal. CSF- LGI-Ab + Treated with methylprednisolone (1gramdailyfor5consecutivedays) with a good response. |
| Shi et al. 2022[90] | AstraZeneca (ChAdOx1) | Autoimmune encephalitis | 35/F | 1st | 5 days afterDeveloped dysarthria, abnormalMovements, extreme anxiety, and reduced voluntary movements | MRI brain- mild swelling of the right hippocampus without abnormal enhancementin contrast-enhanced fluid-attenuated inversion recovery (FLAIR) and T1-weightedimages. CSF- WBC-37/cmm (poly 67.6 %, mono 32.4 %)CSF- RBC-14800/cmmCSF-Protein- 50.7 mg/dl, Glucose-68 mg/dlSerum paraneoplastic antibodies, anti-myelin oligodendrocyte (MOG) antibody, serum and CSF synaptic antibodies, serum antiganglioside anti-bodies, and CSF oligoclonal band: Negative. Treated with weekly rituximab. |
| Meningitis | ||||||
| Fernandes et al. 2021[92] | AstraZeneca (ChAdOx1) | Ascetic Meningitis retention syndrome | 61/F | 1st | 18 daysWith headache, fever, paresthesias of the calves and thighs bilaterally and an unsteady gait, diplopia, and urinary retention. O/E: Neck stiffness + | MRI brain- non-enhancing, nonspecific deep white matter lesions. CSF − 200 WBCcells per mm3 with lymphocytic predominance, Mildly elevated protein (65 mg/dl, reference 12–60 mg/dl) and glucose CSF to serum ratio of 0.5. Infection work up and paraneoplastic panel were negative. Treated with IV steroid, responded partially. |
| Kang et al. 2022[93] | Pfizer-BioNTech (BNT162b2) | Ascetic Meningitis | 32/M | 2nd | 2 week afterHeadache for 1 week, O/E: Neck stiffness + | LP-CSF: Cells-480/cmm(90 %Lymphocyte) Protein- 118 mg/dlSugar- 56 mg/dl (RBS-91 mg/dl) No response to intravenpus acyclovir. Responded to methylprednisolone. |
| Myositis | ||||||
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose | Description and observation |
| Faissner et al. 2021[100] | Moderna (mRNA-1273) = 12 | Myositis | 28Y/F, | 1st dose | 5 days after the first dose of vaccine presented with muscle pain of herthigh muscles, radiating to the lower legs, accompanied by an asymmetrical weakness of the lower limbs. Creatine kinase (CPK) was 17,959 U/l (normal range 26–140 U/l). | Myositis profile was negative. MRI muscles- left-dominant edematous signal alterations with contrast enhancement of the quadriceps muscles sparing the M. rectus femoris, and diffuse subcutaneous fluid retention with contrast enhancement, suggestive of fasciitisTreated with steroid, patient improved. |
| Maramattom et al. 2021[101] | AstraZeneca (ChAdOx1) = 3 | Inflammatory myositis | 74/M | 1st | 48hoursPresented with a 3-week history of intermittent low-grade fever and polyarthralgia. ESR-123 mm/hr (<15 mm/ hr) CRP-269 (<5mg/L) CPK-24 (25 – 170 U/L) ANCA- negativeANA- negativeMyositis profile- negative | 18FDG-PET-CT: a tree-root-like uptake pattern in the lower limbs suggestive of small–medium vessel vasculitis. Whole-body short tau inversion recovery (STIR)-MRI showed diffuse ill-defined muscle hyperintensities suggestive of inflammatory myositis. EMG- fibrillations, positive sharp waves, and complex repetitive discharges in the distal leg muscles. Skin and muscle biopsy showed features of small–medium vessel vasculitis. Remission achieved with oral steroid therapy. |
| 75/F | 1st. | 2 days afterFever, arthralgia, myalgia, tachycardia. ESR-120 (<15 mm/ hr) CRP-271 (<5mg/L) CPK-30 ((25 – 170 U/L) ANCA- negativeANA- negativeMyositis profile- negative | 18 FDG-PET CT- Day 25; Diffuse patchy minimallyincreased FDG avidity in skeletal muscles moreevident in lower limb. Arteries show ‘tree root’patterns. MRI – Day 27-Multiple patchy areas of STIRhyperintensity involving the muscles of both thighsincluding all compartments, posterior compartment ofboth legs and pelvic girdle. Treated with Oral Prednisolone 1 mg/ kg + Mycophenolate mofetil. Achieved remission. | |||
| 80/F | 2nd | 2 days. Fever, fatigue, tachycardia. CPK-40 ((25 – 170 U/L) ESR-59 (<15 mm/ hr) CRP-102 (<5mg/L) Myositis profile/ ANCA- negative. | MRI- Hyperintense signal in STIR MRI in mostmuscles of both upper and lower limbs. Treated with oral steroid. Achieved remission. | |||
| Rhabdomyolysis | ||||||
| Gelbenegger et al. 2021[98] | Janssen (Ad26.COV2.S) | Rhabdomyolysis | 18/M | 1st | 2 days aftermyalgia, muscle weakness, and darkened urine. Creatine kinase (CK) level of 15,638 U/L, serum creatinine of 1.06 mg/dL, a lactate dehydrogenase (LDH) level of 428 U/L and elevated liver enzymes (aspartate transaminase (AST) 340 U/L, alanine transaminase (ALT) 70 U/L), C-reactive protein 1.61 mg/ dL | ANA profile and myositis panel was negative. With symptomatic management (fluid therapy) CPK increased in first week and normalized by 15 days. |
| Nassar et al. 2021[99] | Pfizer-BioNTech (BNT162b2) | Rhabdomyolysis | 21/M | 1st | 1 day afterprogressively worsening pain and swelling in the lower back. O/E- tenderness to the paraspinal lumbar area upon palpation. | CPK- 22000U/LAldolase- 97.8U/LAST-675U/LALT-165U/LCRP-6.4 mg/LLDH-1525U/LUrine blood + Myositis profile- NegativeHydrated with high volume IV normalSaline and pain controlled with morphine. Improved. |
| Parsonage Turner Syndrome | ||||||
| Mahajan et al. 2021[102] | Pfizer-BioNTech (BNT162b2) | Parsonage Turner syndrome | 50/M | 2nd | 1weekPain and left hand grip and left wrist extension weakness with no sensory disturbances or other symptoms. Examination – weaknessof left finger extension and left hand grip. Weak(MRC 3/5) – left dorsal interossei, extensor digitorum, extensorindicis, and flexor carpi ulnaris. DTR- mildly brisk b/l and symmetrical. | MR brachial plexography and NCS- was normal (done early in the disease course). Treated with oral steroid and patient responded significantly. |
| Shields et al. 2021[103] | Pfizer-BioNTech = 4, Moderna = 2 | Parsonage Turner Syndrome | 36/F, 74/M, 50/M53/M, 84/F, 46/F | 2 patient after 1st dose4 after 2nd dose | Mean duration of 17 days (5 days–8 weeks). Initial symptom was pain in the shoulder girdle/upper limb, followed within days by muscle weakness. | Examination and investigation s/o- upper trunk brachial plexopathy in 2 patients, lower trunk plexopathy in 1 patient, posterior cord brachial plexopathy in 1 patient, andanterior/posterior interosseous nerve involvement in 2 patients. All patients either improved or attained complete resolution of the arm pain at follow-up. |
| Queler et al. 2021[106] | Pfizer-BioNTech (BNT162b2) = 1, Moderna (mRNA-1273) = 1 | Parsonage Turner Syndrome | 49/M | 1st | 13 hoursPain followed by weakness of left upper limb. | MR Neurography- Within the arm, four severe hourglass- like constrictions and T2-weighted signal hyperintensity of the anteromedially positioned fascicular bundle of the median nerve were detected; this bundle represents the PT/FCR bundle based on the known topographic fascicular arrangement of the median nerve. EDx- severe denervation and no motor unit recruitment within thePT or FCR muscles. 3 month follow up- pain decreased but weakness increased. |
| 44/M | 2nd | 18 days after developed sudden-onset, Intense, cramping pain in the left lateral deltoid region. Examination- severe weakness in left shoulder abduction (2/5) and external rotation (3/5) Reported hyperesthesias in the left lateral shoulderAnd had diminished sensation to pinprick in the radial nerve distribution. | NCS- mild slowing of the left median and radial sensory responses. EMG- denervation and poor motor unit recruitment in the infraspinatus muscle. MRI- left brachial plexus MR neurography demonstrated enlargement, T2-weighted signal hyperintensity and multiple focal hourglass-like constrictions of the suprascapular nerve with accompanying denervation edemapattern of the supraspinatus and infraspinatus muscles. | |||
| Other Neuropathies | ||||||
| Waheed et al. 2021[107] | Pfizer-BioNTech (BNT162b2) | Small fiber neuropathy | 57/F | 2nd | 1weekWith subacute onset of intense burning dysesthesias in the feet, gradually spreading to the calves and minimally into the hands, unaccompanied by other neurological or constitutional symptoms. Nerve conduction study was unremarkable. | Skin biopsies showed multifocal involvement. Relevent workups for neuropathy were negative. Treated with gabapentin and improved in 2 weeks. |
| Souza et al. 2022[108] | AstraZeneca (ChAdOx1) = 4 | Acute onset- Chronic inflammatory demyelinating polyneuropathy (aCIDP) | Between 51 and 72 years. All male | 1st | 2–3 weeks | In aCIDP a/w COVID vaccination: the acute illness may be severe and associated with cranial nerve dysfunction, particularly bifacial weakness. |
| Spataro et al. 2022[109] | AstraZeneca (ChAdOx1) | Reversible radiculomyelitis | Woman in her 20 s | 1st | 3–4 days after, subacute onset of legs’ weakness, cramping pain and fever (38 °C − 39 °C). O/E: Power LL- 2/5 (b/l) Spastic LLPlantar- equivocalVery brisk patellar, abductor and Achilles tendon reflexes with horizontal and vertical extension, and legs paraesthesia. Tactile and pinpricksensation was decreased from T4 dermatomedownward. Passive and active leg movementselicited rigidity and tenderness. | CSF- Albuminocytological dissociationOCB (CSF and Serum): Pattern IVMRI Brain & Spine- NormalElectromyography and electroneurography – NegativeNear complete recovery in 2 months of steroid therapy. |
| Myasthenia gravis | ||||||
| Chavez et al. 2021[110] | Pfizer-BioNTech (BNT162b2) | Myasthenia | 82/M | 2nd | 2 days afterWith intermittent bulbar symptoms, present in the evenings. history of laryngeal cancer status post hemi-laryngectomy 40 years previously, Barrett’s esophagus, and stage 3a chronic kidney disease | Ach receptor binding Ab 11.4 (normal < 0.02) RNST- Decrement patternSecondary evaluation for thymoma was negative. Treated with pyridostigmine and IVIG. Had improving course. |
| Galassi et al. 2022[111] | AstraZeneca (ChAdOx1) | Ocular Myasthenia | 73/M | 1st | 8 days laterPainless left-sided ptosis without diplopia. K/c/o Psoriasis and hypertension, IHD | MRI Brain- NormalPositive rheumatoid factor (240 IU/ml, normal < 20 IU/ml). Low-frequency repetitive nerve stimulation − 14.7 % decrement in amplitude of nasalis muscle of the compound muscle action potential. Serum titer of anti- AChR antibodies (Day 20 after vaccine) = 1.9 nmol/l (normal < 0.25 nmol/L). Positive pyridostigmine test. |
| Lee et al. 2022[112] | Triggering of Early-Onset Myasthenia Gravis | 33/F | 2nd | On the same day: bilateral ptosis and binocular diplopia. On 3rd day: Developed bilateral ptosis. On 4th day: difficulty in raising her arms and moving her neck with a diurnal fluctuation. | RNST- Significant decremental response. CT- Mild thymic hyperplasia. Anti AchR Ab and anti MUSK Ab – NegativeNeostigmine test- Positive. Responded to pyridostigmine. | |
| Movement disorders | ||||||
| Salinas et al 2021[113] | Pfizer-BioNTech (BNT162b2) | Transient akathesia | 36/F | 2nd | 12 hoursStarted to experience an urge to move which she described as “restless body syndrome.”. k/c/o atopic dermatitis, allergic rhinitis and anxiety (on sertraline 50 mg/day) | She derived temporary relief of symptoms from volitional movement but the internal discomfort and urge to move would soon return. Her movements were alleviated by flexing/extending her trunk and legs as well as getting up and constantly moving. This was followed by fever and myalgia. Her symptoms improved after 24 h. |
| Dysautonomia | ||||||
| Galougahi et al. 2021[114] | AstraZeneca (ChAdOx1) | Autonomic dysfunction | 29/M | 1st | 4 days afterWith intermittent paraesthesia in extremities, which gradually became persistent. Initially was treated with vitamin b12 injection and amitriptyline. 2 months after had increased heart rate, with a significant change when standing (80– 120b.p.m.) vs lying (50–60b.p.m.) and skin colour changes (dark-blue/white/ dark-red) in acral areas (hands/ feet/penis) which is intermittent. | Antinuclear antibody (ANA) was positive at low titre (speckled pattern, 1:40) with elevated IgA level [5.06 g/L (0.60–3.96)]. MRI brain and nerve conduction study was unremarkable. Treated with short course of oral steroid. His postural tachycardia improved, but paraesthesia and skin colour changes persisted at 6-months. |
| Reddy et al. 2021[115] | Pfizer-BioNTech (BNT162b2) | Postural orthostatic tachycardia syndrome (POTS) | 42/M | 1st | 1 week after vaccination presented with sinus tachycardia, dizziness, headaches, and fatigue that are often triggered after a large meal or standing for a longer duration. | Investigations were not remarkable. Treated with life style modification. |
| Hadache | ||||||
| Oonk et al. 2022[116] | Pfizer-BioNTech (BNT162b2) | Thunderclap headache | 62/M | Recurrent episodic thunderclap headache. k/c/o- ocular melanoma. | Laboratory analysis, brain CT and MRI, EEG and CSF analysis including blood pigment and cytologyanalysis were all unremarkable. | |
| AstraZeneca (ChAdOx1) | 21/F | 1st | 2 h afterDeveloped general malaise with subfebrile temperature6hours later experienceda thunderclap headache, with nausea and vomiting | Neurological examination, blood analysis, and brain CT including CTangiography and venography were all normal. Symptoms improved over 1 day with paracetamol, NSAIDs, intravenous morphine, and oxygen therapy | ||
| Mattiuzzi et al. 2021[117] | Rate of headache/migraine episodes (per 100,000) voluntarily reported by recipients of COVID-19 vaccines up to May 9, 2021: | Risk of developing headache/migraine episodes(Odds) | ||||
| AstraZeneca | 129 | AstraZeneca | 3.50; 95 % CI, 3.12–3.93; P < 0.001 | |||
| Pfizer | 103 | Pfizer | 2.78; 95 % CI, 2.47–3.13; P < 0.001 | |||
| Moderna | 21 | Moderna | 0.58; 95 % CI, 0.49–0.68; P < 0.001 | |||
| The cumulative rate of headache/migraine episodes after receiving all COVID-19 vaccines was 2.25-fold higher than the daily frequency of headache disorders (odds ratio, 2.25; 95 % CI, 0.83–6.11). | ||||||
| Suwanwela et al. 2022[118] | Corona Vac | Prolonged migraine aura resemblingischemic stroke | Age between 24 and 48 years and 75 % female. | Interval from vaccination: within the first 24 h: 75 %between 1 and 7d:25 %. | All presented with lateralizedsensory deficits, motor deficits, or both, of 2–14 day duration. Migraine headache occurred in half of the patients. | MRI brain during and after the attacks did not demonstrate any abnormalities suggesting ischemic stroke. All patients showed moderately large regions of hypoperfusion and concurrent smaller regions of hyperperfusion on SPECT imaging while symptomatic. None developed permanent deficits or structural brain injury. |
| Reactivation of Varicella Zoster | ||||||
| Desai et al. 2021[119] | mRNA vaccine = 45/54 (86.27 %)Inactivated COVID-19 vaccine = 5/54 (5.88 %)Non-replicatingviral vector = 4/51 (7.84 %) | Reactivation of Varicella Zoster cutaneous infection | 27 male and 27 female | 2nd dose = 36 | Mean interval = 7.64 (6.92) days | Based on the criteria of temporal connection with vaccination anda plausible biological link, HZ appears to be a “possible”. |
| Maldonado et al. 2021[120] | Pfizer-BioNTech (BNT162b2) | 79/M | 1st | 4 days afterelevated erythematous lesions with vesicles on his right-handsidelumbar area that quickly spread to his lower back, hip, groin, and right-hand-side front and inner thigh, corresponding to L1, L2 and L3 dermatomes. K/c/o Hypercholesterolemia, hyperuricemia and hypertension | Responded to: 800 mg/day of acliclovir for one week; 50 mg of acyclovir applied topically onthe vesicles. | |
| Pfizer-BioNTech (BNT162b2) | 56/F | 2nd | 16 days afterFever, with haemorrhagic vesicles upon an erythematous base spreading on her arm, hand, and left side ofher chest, with chest pain, and pain in her arm on the same side | Treated with 400 mg/8h of gabapentin and 25 mg/12 h of a vitamin B complex. | ||
| Functional Neurological Disorders (FND) | ||||||
| Butler et al. 2021[125] | Pfizer-BioNTech (BNT162b2) | Functional Neurological disorder | 38/F | 1st | After twenty minutes of receiving the vaccine, developed an odd sensation that she described as “weakness” around her left ear. During the rest of the day, this weakness spread to her mouth, left arm, and leg. | The next morning, she had difficulty moving the left side of her face and experienced heaviness in her left leg. Her hoover's sign, hip abduction test results, were positive and symptoms were variabile. Investigations including neuroimaging was unremarkable. |
| Moderna (mRNA-1273) | Functional Neurological disorder | 36/F | 1st | Few minutes after experienced weakness in her right hand and new right-leg limping, which lasted about 2 h. On the second day after vaccination, she experienced severe bilateral leg heaviness and difficulties in fine movements of the right hand. In addition, she had exertional fatigue after walking short distances. | These symptoms persisted for several days. Examination and neuroimaging, routine investigations were unremarkable. | |
| Ercoli et al. 2021[126] | Functional Neurological disorder | 41/M | 1st followed by second dose | After a few minutes fromthe injection, reported bilateral facial paralysis withdifficulty to blink and move the facial muscles properly. All the symptoms resolved spontaneously within 40 min. Three weeks later, a few minutes after the second dose, he complained of swollen tongue and respiratory impairment, which was quickly resolved by corticosteroid therapy. Later he developed right-sided weakness, at the same side of the injection, lasting for about 40 min. | Few weeks later, he suddenly manifested left-sided facial hypoesthesia. Examination- midline splitting of sensory deficit in the face with tacto-dolorific hypoesthesia. Brain MRI, CT, & carotid artery Doppler ultrasonography: Normal. Sensory disturbance resolved, and the neurological examination become normal in next 2 weeks. | |
| Fasano et al. 2022[127] | Pfizer-BioNTech (BNT162b2) | PNES | 2nd dose | 20 min aftershort episode of generalised tonic–clonic psychogenic non-epileptic seizures (PNES) which was followed byanother episode of-inability to move the whole body with preserved level of consciousness). No post-ictal period followed these episodes. | VEEG during few events- normal. | |
| AstraZeneca (ChAdOx1) | Subjective sensory symptoms- FND | 2 weeks afterpersistent dizziness and a subjective loss of tactile sensitivity in the right arm and leg. | Brain CT- Normal | |||
| Others | ||||||
| Author | Vaccine type | Neurological diseases | Age/Sex | Dose of vaccine | Interval from last dose | Description and observation |
| Finsterer et al. 2021[128] | Moderna (mRNA-1273) | Reversible cerebral vasoconstriction syndrome (RCVS) | 38/F | 2nd | 18daysdeveloped visual impairment due to scotomas and thunderclapheadache. | Multimodal cerebral MRI: Acute cortical ischemic lesion in the territory of the right PCA on T2- weighted images, DWI,ADC maps and absence of the PCA on MRA. Partially responded to Nimodipine(90 mg/d) and Levetiracetam (1 g/d). |
| Youn et al. 2021[129] | Pfizer-BioNTech (BNT162b2) | Cytotoxic Lesion of the Corpus Callosum (CLOCCs) | 22/M | 1st dose | 3 daysWith febrile sensation and headache around the eyes and forehead. CSF- Normal cells and protein. | MRI brain- oval shaped restricted diffusion in the corpus callosum with low apparent diffusion coefficient (ADC) values and lack of contrast mediated enhancement |
| Scott et al. 2021[130] | Pfizer-BioNTech (BNT162b2) | Gastroparesis | 57/M | 1st | 5daysStarted to have nausea, intractable vomiting and hiccups. Treated with metoclopramide, and erythromycin. Recurred again after receiving the second dose. | Investigation showed significant delay in gastric emptying. No response to H2 receptor blocker, but responded to oral steroid. |
| Zavala-Jonguitud et al. 2021[131] | Pfizer-BioNTech (BNT162b2) | Delirium | 89/M | 1st | 2 dayswith a 24-h history of confusion, fluctuating attention, anxiety and inversion of the sleep–wake cycle. | K/c/o type 2 diabetes mellitus, hypertension, stage III-b chronic kidney disease, prostatic hyperplasia, mild hearing impairment and depressive disorder. Managed with antipsychotic, improved in 1 week. |
| Aladdin et al. 2021[132] | AstraZeneca (ChAdOx1) | New-onset refractory status epilepticus (NORSE) | 42/F | 1st | 10 days of vaccination presented with f headache and subjective fever that started one day prior and a rising epigastric, jamais vu and followed by new onset generalized tonic-clonic seizure. Brain MRI showed a subtle increase in the signal on FLAIRimages at bilateral hippocampi and insula that was correlating with Postictal changes. | Cerebrospinal fluid analysis showed normal cell count, normal protein at 0.31 g/L, elevated glucose at 4 mmol/L, and negative microbial cultures and serological tests. EEG showed moderate slowing. Treated with 3 AEDs levetiracetam, phenytoin and lacosamide. Responded to pulse intravenous steroid followed by two sessions of plasma exchange on alternate days. |
| Liu et al. 2021[133] | Moderna (mRNA-1273) = 2 | Encephalopathy Associated With Nonconvulsive Status Epilepticus | 86/F | 1st | 7dayswith acute confusion with visual hallucinations and left frontal headache. k/c/o: diastolic dysfunction, chronic kidney disease stage 3, glaucoma, cataracts, and Type 2 diabetes mellitus. | CSF studies, including meningitis/encephalitis panel NAAT, oligoclonal bands, and Lyme antibody, were negative except for West Nile virus IgG but no IgM antibodies with minimal protein elevation. CT head without contrast and MRI brain with and without contrast showed no acute findings. Continuous EEG –non-convulsive focal status epilepticus treated with lorazepam and fosphenytoin. |
| 73/M | 1st | 21 dayswith staring episodes, restlessness, and cognitive deficits. K/c/o Crohn’s, hereditary hemochromatosis, hypertension, and hyperlipidemia | CSF studies, including meningitis/encephalitis panel Nucleic Acid Amplification Tests (NAAT), autoimmune encephalitis, and toxoplasma, were negative except for mildly elevated protein and glucose. CT head and MRI brain showed no acute findings. EEG- non-convulsive status epilepticus, which was treated with lorazepam and levetiracetam loading and maintenance. | |||
| Chuang et al. 2021[134] | Moderna (mRNA-1273) | Tolosa-Hunt Syndrome (THS) | 45/M | 7 days after severe left-sided headache, pain with progressive ptosis in left eye, decreased vision, and binocular diplopia. | Had left RAPD and left eye complete ophthalmoplegia. MRI brain s/o THS. | |
| Lin et al. 2021[135] | Moderna (mRNA-1273) | Triggered Moyamoya disease with Sjogren disease andautoimmune thyroiditis | 40/F | 2nd | 3 days aftersevere headaches with a decreased level ofconsciousness and a tonic-clonic seizure. k/c/o- Sjogren disease andautoimmune thyroiditisO/E- Febrile with high Blood pressure and PR. | Elevated CRP, anti-PF4 Ab, SSA, fibrinogen, CT Brain – left caudate nucleus, temporal lobe IVH and ICH with hydrocephalusDSA- bilateral distal ICAsteno-occlusion with the constricted flow in middle cerebral arteries and anterior cerebral arteries with cortical collateralization pattern from the external carotid artery system that was consistent with typical moyamoya angiopathy (MMA)-Willis and the Suzuki staging system was stage V. |
| Murvelashvili et al. 2021[137] | Moderna (mRNA-1273) | Hypophisitis | 51/M | 2nd | 3 days after vaccination with headache, nausea, vomiting, malaise, and diffuse arthralgias | MRI brain suggestive of diffusely enlarged pituitary gland consistent with acute hypophysitis |
Abbreviations: GBS- Guillain-Barré syndrome; NMOSD- Neuromyelitis optica spectrum disorders; MOGAD- Myelin oligodendrocyte glycoprotein antibody-associatd disease; MS- Multiple sclerosis; CSVT- Cerebral Venous Sinus Thrombosis; RCVS- Reversible cerebral vasoconstriction syndrome; PNES- Psychogenic Nonepileptic Seizures; POST- Postural orthostatic tachycardia syndrome; MRI- Magnetic resonance imaging; O/E- On examination; k/c/o- Known case of; LP-CSF- Lumbar puncture cerebrospinal fluid; CSF- cerebrospinal fluid; EEG- Electroencephalogram; CT- computerized tomography; ADC- Apparent diffusion coefficient; FLAIR- fluid attenuation inversion recovery DWI- Diffusion weighted imagine.
Fig. 5.
Depicts the spectrum of possible COVID19 vaccine associated neurological diseases.
Fig. 6.
Illustrates the various possibilities of neurological illness among the recipients of vaccines against SARS-CoV2.
5.2. Pathogenesis
AEFI may occur due to vaccine product-related reaction, vaccine quality defect-related reaction, immunization error-related reaction, immunization stress-related reaction, or an unrelated incidental event. Although the underlying pathomechanisms are yet to be completely elucidated, based on the available limited observations and hypotheses the following possible mechanisms are proposed. (Refer to Fig. 7 ).
Fig. 7.
Section A- Enumerates various types of vaccine candidates and their principle components. Se6tion B- Illustrates the post vaccination mechanisms of immunogenicity Section C- Demonstrates the anti-idiotype antibody hypothesis Section D- Explains the role of adjuvants and mast cell activation and mechanism of anaphylaxis. Section E- Depicts the autoantibodies formation and ACE2 down regulation leading to various neurological diseases.
5.2.1. Autoimmunity
Similarity of vaccine component with human protein can lead to the production of antibodies which are directed against host’s own protein. This mechanism is known as molecular mimicry. [159] Genetic predisposition and pre-existing antibodies may recognize the vaccine components and adjuvants which can activate the mast cells leading to degranulation, and hypersensitivity reactions including anaphylaxis. Vaccine adjuvants may also activate the inflammasome pathway leading to interleukin productions and subsequent activation of nuclear factor kB, Th17, and Th1 cells. [160], [161] Antibody dependent COVID-19 enhancement has also been attributed to be one of the pathophysiology of the post-vaccinal complications. [162], [163].
5.2.2. Theory of anti-idiotype antibodies
SARS-CoV2 virus uses its spike protein (S) to bind to the angiotensin-converting–enzyme 2 (ACE2) receptors on the target cell. Viral infection and its vaccines mount antibodies to the S protein which is called as Ab1. A distinctive sequence in the complementarity-determining region 3 (CDR3), of the idiotype portions of the Ab1 binds and neutralizes the S protein. Subsequently, these antibody-binding regions get down-regulated through generation of antibody responses against themselves which is called anti-idiotype (Ab2) antibodies.
Ab2 antibodies bind to the earlier formed protective neutralizing Ab1 antibody, which results in immune-complex formation and clearance. This impairs the Ab1 efficacy. As the Ab1 is directed against the S protein and the Ab2 is directed against the Ab1, a few binding regions, or paratopes of Ab2 antibodies mirror the S protein. Hence, the Ab2 binds to the same target as the S protein would bind, i.e. the ACE2 receptor. This Ab2-ACE2 interaction blocks the ACE2 function by competitive inhibition of the normal ligand interactions. As Ab2 is an immune response, it may persist even after the original antibody gets cleared off and may lead to the long term adverse events. [164], [165].
5.2.3. Immunization stress related response (ISRR)
In a prospective study consisting eight patients who experienced post vaccination neurological adverse events, 18F-FDGPET/MRI, and 15O-water PET scans were performed at the baseline (immediately following neurological adverse event after the vaccination) and after 7 days of vaccination. All had hypometabolism in the bilateral parietal lobes on both the first and follow-up scans. Metabolic changes in the bilateral cuneus including hypometabolism in six and hypermetabolism in two patients were observed. One showed mildly significant decreases in perfusion in the bilateral thalamus and bilateral cerebellum, whereas another patient was found to have a diffuse increase in the cerebral white matter perfusion. The areas of metabolic abnormalities indicates towards the involvement of the fear network model which has been implicated in anxiety. [166].
5.3. Limitations
Retrospective study design and small size are important limitations in this study. Further studies with larger sample size are needed to establish the causal association with these disorders.
6. Conclusion
The advent of newer vaccines raises the possibility of emergence of novel AEFI. While causality may not always be proven, the replication of similar events over a period of time, serve to generate speculations over a new AEFI. Though subject to further investigations, this study will sensitize the neurologists and vaccine stakeholders regarding the spectrum of neurological diseases of probable or possible temporal association with COVID-19 vaccination. It will also enlighten the practitioner regarding the possible underlying pathophysiology of this evolving entity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jocn.2022.12.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Organization WH. Worldwide, COVID-19 - Landscape of novel coronavirus candidate vaccine development [Internet]. 2022. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 2.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines [Internet]. 2021;6(1). Available from: http://dx.doi.org/10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed]
- 4.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet [Internet] 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., et al. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open [Internet] 2021;4(12):e2140364–e. doi: 10.1001/jamanetworkopen.2021.40364. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M., Yuan Y., Zhou Y., Deng Z., Zhao J., Feng F., et al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty [Internet] 2021;10(1):94 doi: 10.1186/s40249-021-00878-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho J.S., Sia C.-H., Ngiam J.N., Loh P.H., Chew N.W., Kong W.K., et al. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med J. 2021 doi: 10.11622/smedj.2021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination [Internet]. Vol. 43, Neurological Sciences. Springer International Publishing; 2022. 3–40 p. Available from: https://doi.org/10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed]
- 9.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA [Internet] 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ [Internet] 2021:374. doi: 10.1136/bmj.n1931. https://www.bmj.com/content/374/bmj.n1931 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Ali D., Elshafeey A., Mushannen M., Kawas H., Shafiq A., Mhaimeed N., et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J Cell Mol Med. 2022;26(3):636–653. doi: 10.1111/jcmm.17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Munckhof A., Krzywicka K., Aguiar de Sousa D., Sánchez van Kammen M., Heldner M.R., Jood K., et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS-CoV-2 vaccination. Eur J Neurol. 2022;29(1):339–344. doi: 10.1111/ene.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhashim A., Hadhiah K., Al Khalifah Z., Alhaddad F.M., Al Arhain S.A., Bin Saif F.H., et al. Extensive Cerebral Venous Sinus Thrombosis (CVST) After the First Dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine without Thrombotic Thrombocytopenia Syndrome (TTS) in a Healthy Woman. Am J Case Rep. 2022;23(1):1–7. doi: 10.12659/AJCR.934744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanni D., Saba L., Demontis R., Gerosa C., Chighine A., Nioi M., et al. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: A report of an autoptic case and review of the literature. Eur Rev Med Pharmacol Sci. 2021;25(15):5063–5069. doi: 10.26355/eurrev_202108_26464. [DOI] [PubMed] [Google Scholar]
- 15.Siegler J.E., Klein P., Yaghi S., Vigilante N., Abdalkader M., Coutinho J.M., et al. Cerebral Vein Thrombosis with Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke. 2021:3045–3053. doi: 10.1161/STROKEAHA.121.035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pietro M., Dono F., Consoli S., Evangelista G., Pozzilli V., Calisi D., et al. Cerebral venous thrombosis without thrombocytopenia after a single dose of COVID-19 (Ad26.COV2.S) vaccine injection: a case report. Neurol Sci [Internet] 2022;19(0123456789) doi: 10.1007/s10072-022-05965-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiteley W.N., Ip S., Cooper J.A., Bolton T., Keene S., Walker V., et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLOS Med [Internet] 2022;19(2):e1003926. doi: 10.1371/journal.pmed.1003926. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez EVC, Bouazza FZ, Dauby N, Mullier F, d’Otreppe S, Jissendi Tchofo P, et al. Fatal vaccine-induced immune thrombotic thrombocytopenia (VITT) post Ad26.COV2.S: first documented case outside US. Infection [Internet]. 2021;(0123456789). Available from: https://doi.org/10.1007/s15010-021-01712-8. [DOI] [PMC free article] [PubMed]
- 19.Mirandola L., Arena G., Pagliaro M., Boghi A., Naldi A., Castellano D., et al. Massive cerebral venous sinus thrombosis in vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 serum: case report of a successful multidisciplinary approach. Neurol Sci [Internet] 2022;43(3):1499–1502. doi: 10.1007/s10072-021-05805-y. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotal R., Jacob I., Rangappa P., Rao K., Hosurkar G., Anumula S.K., et al. A rare case of vaccine-induced immune thrombosis and thrombocytopenia and approach to management. Surg Neurol Int. 2021;12(June):1–5. doi: 10.25259/SNI_689_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maramattom B.V., Moidu F.M., Varikkottil S., Syed A.A. Cerebral venous sinus thrombosis after ChAdOx1 vaccination: The first case of definite thrombosis with thrombocytopenia syndrome from India. BMJ Case Rep. 2021;14(10):1–4. doi: 10.1136/bcr-2021-246455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J.K., Kim S., Kim S.R., Jin J.Y., Choi S.W., Kim H., et al. Intracerebral Hemorrhage due to Thrombosis with Thrombocytopenia Syndrome after Vaccination against COVID-19: the First Fatal Case in Korea. J Korean Med Sci. 2021;36(31):1–6. doi: 10.3346/jkms.2021.36.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedmann M., Skattør T., Stray-Pedersen A., Romundstad L., Antal E.A., Marthinsen P.B., et al. Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series. Front Neurol. 2021;12(July):1–7. doi: 10.3389/fneur.2021.721146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso M., Lauretti D.L., Cecconi N., Santini M., Lami V., Orlandi G., et al. Arterial intracranial thrombosis as the first manifestation of vaccine-induced immune thrombotic thrombocytopenia (VITT): a case report. Neurol Sci [Internet] 2022;43(3):2085–2089. doi: 10.1007/s10072-021-05800-3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H.P., Selvaratnam V., Rajasuriar J.S. Thrombotic thrombocytopenic purpura after ChAdOx1 nCoV-19 vaccine. BMJ Case Rep. 2021;14(10):4–6. doi: 10.1136/bcr-2021-246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangli S., Virani A., Cheronis N., Vannatter B., Minich C., Noronha S., et al. Thrombosis With Thrombocytopenia After the Messenger RNA-1273. Vaccine. 2021;Vol. 174:1480–1482. doi: 10.7326/L21-0244. Annals of internal medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See I., Lale A., Marquez P., Streiff M.B., Wheeler A.P., Tepper N.K., et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022 doi: 10.7326/M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Günther A., Brämer D., Pletz M.W., Kamradt T., Baumgart S., Mayer T.E., et al. Complicated long term vaccine induced thrombotic immune thrombocytopenia—a case report. Vaccines. 2021;9(11):1–10. doi: 10.3390/vaccines9111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.J. Cleaver R. Ibitoye H. Morrison R. Flood K. Crewdson A. Marsh et al. Endovascular treatment for vaccine-induced cerebral venous sinus thrombosis and thrombocytopenia following ChAdOx1 nCoV-19 vaccination: a report of three cases J Neurointerv Surg. 2021 neurintsurg-2021-018238. [DOI] [PubMed]
- 30.Krzywicka K., Heldner M.R., Sánchez van Kammen M., van Haaps T., Hiltunen S., Silvis S.M., et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;28(11):3656–3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahoz Fernandez P.E., Miranda Pereira J., Fonseca Risso I., Baleeiro Rodrigues Silva P., Freitas Barboza I.C., Vieira Silveira C.G., et al. Guillain-Barre syndrome following COVID-19 vaccines: A scoping review. Acta Neurol Scand. 2022;145(4):393–398. doi: 10.1111/ane.13575. [DOI] [PubMed] [Google Scholar]
- 32.Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., et al. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 33.Biswas A, Pandey SK, Kumar D, Vardhan H. Post Coronavirus Disease ‑ 2019 Vaccination Guillain ‑ Barré Syndrome. 2021. 2019–22. [DOI] [PubMed]
- 34.Scendoni R., Petrelli C., Scaloni G., Logullo F.O. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vaccines Immunother [Internet] 2021;17(11):4093–4096. doi: 10.1080/21645515.2021.1954826. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva GF, da Silva CF, Oliveira REN da N, Romancini F, Mendes RM, Locks A, et al. Guillain–Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin Exp Neuroimmunol. 2021;(September):1–3. [DOI] [PMC free article] [PubMed]
- 36.Bonifacio G.B., Patel D., Cook S., Purcaru E., Couzins M., Domjan J., et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2022;93(3):341–342. doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 37.Thant H.L., Morgan R., Paese M.M., Persaud T., Diaz J., Hurtado L. Guillain-Barré Syndrome After Ad26.COV2.S Vaccination. Am J Case Rep. 2022;23(1):1–5. doi: 10.12659/AJCR.935275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao S.J., Khurana S., Murthy G., Dawson E.T., Jazebi N., Haas C.J. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J Community Hosp Intern Med Perspect [Internet] 2021;11(5):597–600. doi: 10.1080/20009666.2021.1954284. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossetti A., Gheihman G., O’Hare M., Kosowsky J.M. Guillain-Barré Syndrome Presenting as Facial Diplegia after COVID-19 Vaccination: A Case Report. J Emerg Med [Internet] 2021;61(6):e141–e145. doi: 10.1016/j.jemermed.2021.07.062. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen C.M., Ramsamy S., Tarr A.W., Tighe P.J., Irving W.L., Tanasescu R., et al. Guillain-Barré Syndrome Variant Occurring after SARS-CoV-2 Vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 41.James J., Jose J., Gafoor V.A., Smita B., Balaram N. Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: A case series. Neurol Clin Neurosci. 2021;9(5):402–405. doi: 10.1111/ncn3.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Čenščák D., Ungermann L., Štětkářová I., Ehler E. Guillan-Barré Syndrome after First Vaccination Dose against COVID-19: Case Report. Acta medica (Hradec Kral [Internet] 2021;64(3):183–186. doi: 10.14712/10.14712/18059694.2021.30. Available from: [DOI] [PubMed] [Google Scholar]
- 43.Woo E.J., Mba-Jonas A., Dimova R.B., Alimchandani M., Zinderman C.E., Nair N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. JAMA. 2021;326(16):1606–1613. doi: 10.1001/jama.2021.16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanabar G., Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021;14(10):1–3. doi: 10.1136/bcr-2021-244527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim N., Kim J.H., Park J.S. Guillain-Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea. Neurol Sci [Internet] 2022;43(3):1491–1493. doi: 10.1007/s10072-021-05849-0. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aldeeb M., Okar L., Mahmud S.S., Adeli G.A. Could Guillain-Barré syndrome be triggered by COVID-19 vaccination? Clin Case Reports. 2022;10(1):17–20. doi: 10.1002/ccr3.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kripalani Y., Lakkappan V., Parulekar L., Shaikh A., Singh R., Vyas P. A Rare Case of Guillain-Barré Syndrome following COVID-19 Vaccination. Eur J Case Reports. Intern Med. 2021:3–6. doi: 10.12890/2021_002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J.W., Kim Y.G., Park Y.C., Choi S., Lee S., Min H.J., et al. Guillain-Barre Syndrome After Two COVID-19 Vaccinations: Two Case Reports With Follow-up Electrodiagnostic Study. J Korean Med Sci. 2022;37(7):1–9. doi: 10.3346/jkms.2022.37.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagalli S, Shankar Kikkeri N. Sub-acute Onset of Guillain-Barré Syndrome Post-mRNA-1273 Vaccination: a Case Report. SN Compr Clin Med [Internet]. 2022;4(1):1–5. Available from: https://doi.org/10.1007/s42399-022-01124-1. [DOI] [PMC free article] [PubMed]
- 50.Chang Y.L., Chang S.T. The effects of intravascular photobiomodulation on sleep disturbance caused by Guillain-Barré syndrome after Astrazeneca vaccine inoculation: Case report and literature review. 2022;101(6) doi: 10.1097/MD.0000000000028758. Med (United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Cherukudal Vishnu Nampoothiri S., Syed A.A., et al. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 52.Dang Y.L., Bryson A. Miller-Fisher Syndrome and Guillain-Barre Syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021;14(11):1–3. doi: 10.1136/bcr-2021-246701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Y. Kim Z. Zhu P. Kochar P. Gavigan D. Kaur A. Kumar A Pediatric Case of Sensory Predominant Guillain-Barré Syndrome Following COVID-19 Vaccination Child Neurol Open. 2022;9:2329048X2210745. [DOI] [PMC free article] [PubMed]
- 54.Shapiro Ben David S., Potasman I., Rahamim-Cohen D. Rate of Recurrent Guillain-Barré Syndrome After mRNA COVID-19 Vaccine BNT162b2. JAMA Neurol. 2021;78:1409–1411. doi: 10.1001/jamaneurol.2021.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouattour N, Hdiji O, Sakka S, Fakhfakh E, Moalla K, Daoud S, et al. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci [Internet]. 2022;43(2):755–61. Available from: https://doi.org/10.1007/s10072-021-05733-x. [DOI] [PMC free article] [PubMed]
- 56.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14(7):1–4. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finsterer J. Guillain-Barre syndrome 15 days after COVID-19 despite SARS-CoV-2 vaccination. IDCases [Internet]. 2021;25:e01226. Available from: https://doi.org/10.1016/j.idcr.2021.e01226. [DOI] [PMC free article] [PubMed]
- 58.Chen S, Fan X-R, He S, Zhang J-W, Li S-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci [Internet]. 2021;42(9):3537–9. Available from: https://doi.org/10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed]
- 59.Netravathi M., Dhamija K., Gupta M., Tamborska A., Nalini A., Faheem A., et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. 2022;60(March) doi: 10.1016/j.msard.2022.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ismail I.I., Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362(January) doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khayat-Khoei M., Bhattacharyya S., Katz J., Harrison D., Tauhid S., Bruso P., et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2022;269(3):1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frontera J.A., Tamborska A.A., Doheim M.F., Garcia-Azorin D., Gezegen H., Guekht A., et al. Neurological Events Reported after COVID -19 Vaccines: An Analysis of VAERS. Ann Neurol. 2022 doi: 10.1002/ana.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Permezel F, Borojevic B, Lau S, de Boer HH. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol [Internet]. 2022;18(1):74–9. Available from: https://doi.org/10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed]
- 64.Ancau M., Liesche-Starnecker F., Niederschweiberer J., Krieg S.M., Zimmer C., Lingg C., et al. Case Series: Acute Hemorrhagic Encephalomyelitis After SARS-CoV-2 Vaccination. Front Neurol. 2022;12(February):16–21. doi: 10.3389/fneur.2021.820049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnao V, Maimone MB, Perini V, Giudice G Lo, Cottone S. Bilateral optic neuritis after COVID vaccination. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022. p. 1–2. [DOI] [PMC free article] [PubMed]
- 66.Burrows A., Bartholomew T., Rudd J., Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan E.Y.F., Chui C.S.L., Lai F.T.T., Chan E.W.Y., Li X., Yan V.K.C., et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cellina M, D’Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell’s palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imaging [Internet]. 2022;82(November 2021):1–4. Available from: https://doi.org/10.1016/j.clinimag.2021.10.010. [DOI] [PMC free article] [PubMed]
- 69.Mussatto CC, Sokol J, Alapati N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Reports [Internet]. 2022;25(November 2021):101259. Available from: https://doi.org/10.1016/j.ajoc.2022.101259. [DOI] [PMC free article] [PubMed]
- 70.Ozonoff A., Nanishi E., Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahsanuddin S., Nasser W., Roy S.C., Povolotskiy R., Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. 2022;39(1):80–84. doi: 10.1093/fampra/cmab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ish S., Ish P. Isolated peripheral facial nerve palsy post COVID-19 vaccination with complete clinical recovery. Indian J Ophthalmol. 2022;70(1):347. doi: 10.4103/ijo.IJO_2425_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato K., Mano T., Niimi Y., Toda T., Iwata A., Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. 2021;111(January):310–312. doi: 10.1016/j.ijid.2021.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konstantinidis I., Tsakiropoulou E., Hähner A., de With K., Poulas K., Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399–1401. doi: 10.1002/alr.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keir G., Maria N.I., Kirsch C.F.E. Unique Imaging Findings of Neurologic Phantosmia Following Pfizer-BioNtech COVID-19 Vaccination: A Case Report. Top Magn Reson Imaging. 2021;30(3):133–137. doi: 10.1097/RMR.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 76.Vaira L.A., De Vito A., Lechien J.R., Chiesa-Estomba C.M., Mayo-Yàñez M., Calvo-Henrìquez C., et al. New Onset of Smell and Taste Loss Are Common Findings Also in Patients With Symptomatic COVID-19 After Complete Vaccination. Laryngoscope. 2022;132(2):419–421. doi: 10.1002/lary.29964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cicalese M.P., Ferrua F., Barzaghi F., Cerri F., Moro M., Aiuti A., et al. Third cranial nerve palsy in an 88-year-old man after SARS-CoV-2 mRNA vaccination: Change of injection site and type of vaccine resulted in an uneventful second dose with humoral immune response. BMJ Case Rep. 2022;15(2):2021–2023. doi: 10.1136/bcr-2021-246485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.A. Kerbage S.F. Haddad F. Haddad Presumed oculomotor nerve palsy following COVID-19 vaccination SAGE Open Med Case Reports. 2022;10:2050313X2210744. [DOI] [PMC free article] [PubMed]
- 79.Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J Am Assoc Pediatr Ophthalmol Strabismus {JAAPOS} [Internet]. 2021 Oct 1;25(5):302–3. Available from: https://doi.org/10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed]
- 80.Pawar N., Ravindran M., Padmavathy S., Chakrabarty S. Acute abducens nerve palsy after COVID-19 vaccination in a young adult. Indian J Ophthalmol. 2021;69:3764–3766. doi: 10.4103/ijo.IJO_1968_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng P.T., Chen T.Y., Sun Y.S., Chen Y.W., Chen J.J. The reversible tinnitus and cochleopathy followed first-dose AstraZeneca COVID-19 vaccination. QJM. 2021;114(9):663–664. doi: 10.1093/qjmed/hcab210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol [Internet]. 2021;0(0):1–4. Available from: https://doi.org/10.1080/14992027.2021.1931969. [DOI] [PubMed]
- 83.Di Mauro P., La Mantia I., Cocuzza S., Sciancalepore P.I., Rasà D., Maniaci A., et al. Acute Vertigo After COVID-19 Vaccination: Case Series and Literature Review. Front Med. 2022;8(January):1–9. doi: 10.3389/fmed.2021.790931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeong J, Choi HS. Sudden sensorineural hearing loss after COVID-19 vaccination. Vol. 113, International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021. p. 341–3. [DOI] [PMC free article] [PubMed]
- 85.Zhao H., Li Y., Wang Z. Adverse event of Sinovac Coronavirus vaccine: Deafness. Vaccine [Internet] 2022;40(3):521–523. doi: 10.1016/j.vaccine.2021.11.091. https://www.sciencedirect.com/science/article/pii/S0264410X21015760 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuhorn F., Graf T., Klingebiel R., Schäbitz W.R., Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baldelli L., Amore G., Montini A., Panzera I., Rossi S., Cortelli P., et al. Hyperacute reversible encephalopathy related to cytokine storm following COVID-19 vaccine. J Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moslemi M, Ardalan M, Haramshahi M, Mirzaei H, Sani SK, Dastgir R, et al. Herpes simplex encephalitis following ChAdOx1 nCoV-19 vaccination: a case report and review of the literature. BMC Infect Dis [Internet]. 2022;22(1):22–5. Available from: https://doi.org/10.1186/s12879-022-07186-9. [DOI] [PMC free article] [PubMed]
- 89.Al-Mashdali AF, Ata YM, Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann Med Surg [Internet]. 2021;69(August):102803. Available from: https://doi.org/10.1016/j.amsu.2021.102803. [DOI] [PMC free article] [PubMed]
- 90.Shin H.-R., Kim B.-K., Lee S.-T., Kim A. Autoimmune Encephalitis as an Adverse Event of COVID-19 Vaccination. J Clin Neurol. 2022;18(1):114–116. doi: 10.3988/jcn.2022.18.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zlotnik Y, Gadoth A, Abu-Salameh I, Horev A, Novoa R, Ifergane G. Case Report: Anti-LGI1 Encephalitis Following COVID-19 Vaccination. Vol. 12, Frontiers in immunology. 2021. p. 813487. [DOI] [PMC free article] [PubMed]
- 92.Fernandes J., Jaggernauth S., Ramnarine V., Mohammed S.R., Khan C., Panday A. Neurological Conditions Following COVID-19 Vaccinations: Chance or Association? Cureus. 2022;14(2):1–8. doi: 10.7759/cureus.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang HS, Kim JE, Yoo JR, Oh H, Kim M, Kim YR, et al. Aseptic Meningitis Following Second Dose of an mRNA Coronavirus Disease 2019 Vaccine in a Healthy Male: Case Report and Literature Review. Infect Chemother [Internet]. 2022;54. Available from: https://doi.org/10.3947/ic.2021.0131. [DOI] [PMC free article] [PubMed]
- 94.Walter U., Fuchs M., Grossmann A., Walter M., Thiele T., Storch A., et al. Adenovirus-Vectored COVID-19 Vaccine-Induced Immune Thrombosis of Carotid Artery. Neurology. 2021;97(15):716–719. doi: 10.1212/WNL.0000000000012576. [DOI] [PubMed] [Google Scholar]
- 95.Tiede A., Sachs U.J., Czwalinna A., Werwitzke S., Bikker R., Krauss J.K., et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138(4):350–353. doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Mayhani T., Saber S., Stubbs M.J., Losseff N.A., Perry R.J., Simister R.J., et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Journal of neurology, neurosurgery, and psychiatry England. 2021;92:1247–1248. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 97.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: An analysis of European data. J Autoimmun [Internet] 2021;122 doi: 10.1016/j.jaut.2021.102685. https://www.sciencedirect.com/science/article/pii/S0896841121000937 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gelbenegger G., Cacioppo F., Firbas C., Jilma B. Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination. Vaccines. 2021;9(9) doi: 10.3390/vaccines9090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nassar M., Chung H., Dhayaparan Y., Nyein A., Acevedo B.J., Chicos C., et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faissner S, Richter D, Ceylan U, Schneider-Gold C, Gold R. COVID-19 mRNA vaccine induced rhabdomyolysis and fasciitis. J Neurol [Internet]. 2021;(0123456789):10–1. Available from: https://doi.org/10.1007/s00415-021-10768-3. [DOI] [PMC free article] [PubMed]
- 101.Maramattom B.V., Philips G., Thomas J., Santhamma S.G.N. Inflammatory myositis after ChAdOx1 vaccination. Lancet Rheumatol. 2021;3(11):e747–e749. doi: 10.1016/S2665-9913(21)00312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahajan S., Zhang F., Mahajan A., Zimnowodzki S. Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve. 2021;64(1):E3–E4. doi: 10.1002/mus.27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shields L.B.E., Iyer V.G., Zhang Y.P., Burger J.T., Shields C.B. Parsonage-Turner Syndrome Following COVID-19 Vaccination: Clinical and Electromyographic Findings in 6 Patients. Case Rep Neurol. 2022:58–67. doi: 10.1159/000521462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vitturi BK, Grandis M, Beltramini S, Orsi A, Schenone A, Icardi G, et al. Parsonage–Turner syndrome following coronavirus disease 2019 immunization with ChAdOx1-S vaccine: a case report and review of the literature. J Med Case Rep [Internet]. 2021;15(1):1–4. Available from: https://doi.org/10.1186/s13256-021-03176-8. [DOI] [PMC free article] [PubMed]
- 105.Nawaz SB, Raigam WA. Parsonage Turner Syndrome Following COVID-19 Vaccine. 2022;4(1):13–4.
- 106.Queler SC, Towbin AJ, Milani C, Whang J, Sneag DB. Parsonage-Turner Syndrome Following COVID-19 Vaccination: MR Neurography. Radiology [Internet]. 2022;302(1):84–7. Available from: https://doi.org/10.1148/radiol.2021211374. [DOI] [PMC free article] [PubMed]
- 107.Waheed W., Carey M.E., Tandan S.R., Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Souza A., Oo W.M., Giri P. Inflammatory demyelinating polyneuropathy after the ChAdOx1 nCoV-19 vaccine may follow a chronic course. J Neurol Sci. 2022;436 doi: 10.1016/j.jns.2022.120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spataro R., Fisco G., La Bella V. Reversible radiculomyelitis after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2022;15(2):20–23. doi: 10.1136/bcr-2021-247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chavez A, Pougnier C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J Prim Care \& Community Heal [Internet]. 2021;12:21501327211051932. Available from: https://doi.org/10.1177/21501327211051933. [DOI] [PMC free article] [PubMed]
- 111.Galassi G., Rispoli V., Iori E., Ariatti A., Marchioni A. Coincidental Onset of Ocular Myasthenia Gravis Following ChAdOx1 n-CoV-19 Vaccine against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Isr Med Assoc J [Internet] 2022;24(1):9–10. http://europepmc.org/abstract/MED/35077038 Available from. [PubMed] [Google Scholar]
- 112.Lee MA, Lee C, Park JH, Lee JH. Early-Onset Myasthenia Gravis Following COVID-19 Vaccination. J Korean Med Sci [Internet]. 2022 Mar;37(10). Available from: https://doi.org/10.3346/jkms.2022.37.e50. [DOI] [PMC free article] [PubMed]
- 113.Salinas MR, Dieppa M. Transient akathisia after the SARS-Cov-2 vaccine. Clin Park Relat Disord [Internet]. 2021;4(May):100098. Available from: https://doi.org/10.1016/j.prdoa.2021.100098. [DOI] [PMC free article] [PubMed]
- 114.Karimi G.K. Autonomic dysfunction post-inoculation with ChAdOx1 nCoV-19 vaccine. Eur Hear J - Case Reports. 2021;5(12):1–2. doi: 10.1093/ehjcr/ytab472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reddy S., Reddy S., Arora M. A Case of Postural Orthostatic Tachycardia Syndrome Secondary to the Messenger RNA COVID-19 Vaccine. Cureus. 2021;13(5):13–16. doi: 10.7759/cureus.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oonk NGM, Ettema AR, van Berghem H, de Klerk JJ, van der Vegt JPM, van der Meulen M. SARS-CoV-2 vaccine-related neurological complications. Neurol Sci [Internet]. 2022;43(4):2295–7. Available from: https://doi.org/10.1007/s10072-022-05898-z. [DOI] [PMC free article] [PubMed]
- 117.Mattiuzzi C, Lippi G. Headache after COVID-19 vaccination: updated report from the Italian Medicines Agency database. Neurol Sci [Internet]. 2021;42(9):3531–2. Available from: https://doi.org/10.1007/s10072-021-05354-4. [DOI] [PMC free article] [PubMed]
- 118.Suwanwela N.C., Kijpaisalratana N., Tepmongkol S., Rattanawong W., Vorasayan P., Charnnarong C., et al. Prolonged migraine aura resembling ischemic stroke following CoronaVac vaccination: an extended case series. J Headache Pain. 2022;23(1):1–7. doi: 10.1186/s10194-022-01385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Desai H.D., Sharma K., Shah A., Patoliya J., Patil A., Hooshanginezhad Z., et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of Varicella zoster? A systematic review J Cosmet Dermatol. 2021;20(11):3350–3361. doi: 10.1111/jocd.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maldonado MD, Romero-Aibar J. The Pfizer-BNT162b2 mRNA-based vaccine against SARS-CoV-2 may be responsible for awakening the latency of herpes varicella-zoster virus. Brain, Behav Immun - Heal [Internet]. 2021;18(July):100381. Available from: https://doi.org/10.1016/j.bbih.2021.100381. [DOI] [PMC free article] [PubMed]
- 121.Santovito LS, Pinna G. A case of reactivation of varicella–zoster virus after BNT162b2 vaccine second dose? Inflamm Res [Internet]. 2021;70(9):935–7. Available from: https://doi.org/10.1007/s00011-021-01491-w. [DOI] [PMC free article] [PubMed]
- 122.Atiyat R., Elias S., Kiwan C., Shaaban H.S., Slim J. Varicella-Zoster Virus Reactivation in AIDS Patient After Pfizer-BioNTech COVID-19 Vaccine. Cureus. 2021;13(12):10–13. doi: 10.7759/cureus.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abu-Rumeileh S, Mayer B, Still V, Tumani H, Otto M, Senel M. Varicella zoster virus-induced neurological disease after COVID-19 vaccination: a retrospective monocentric study. J Neurol [Internet]. 2021;(0123456789). Available from: https://doi.org/10.1007/s00415-021-10849-3. [DOI] [PMC free article] [PubMed]
- 124.Triantafyllidis K.K., Giannos P., Mian I.T., Kyrtsonis G., Kechagias K.S. Varicella zoster virus reactivation following COVID-19 vaccination: A systematic review of case reports. Vaccines. 2021;9(9):1–4. doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Butler M., Coebergh J., Safavi F., Carson A., Hallett M., Michael B., et al. Functional Neurological Disorder After SARS-CoV-2 Vaccines: Two Case Reports and Discussion of Potential Public Health Implications. J Neuropsychiatry Clin Neurosci. 2021;33(4):345–348. doi: 10.1176/appi.neuropsych.21050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ercoli T, Lutzoni L, Orofino G, Muroni A, Defazio G. Functional neurological disorder after COVID-19 vaccination. Neurol Sci [Internet]. 2021;42(10):3989–90. Available from: https://doi.org/10.1007/s10072-021-05504-8. [DOI] [PMC free article] [PubMed]
- 127.Fasano A., Daniele A. Functional disorders after COVID-19 vaccine fuel vaccination hesitancy. Journal of neurology, neurosurgery, and psychiatry England. 2022;93:339–340. doi: 10.1136/jnnp-2021-327000. [DOI] [PubMed] [Google Scholar]
- 128.Finsterer J. First Reported Case of Reversible Cerebral Vasoconstriction Syndrome After a SARS-CoV-2 Vaccine. Vol. 13, Cureus. 2021. p. e19987. [DOI] [PMC free article] [PubMed]
- 129.Youn T., Yang H. Cytotoxic Lesion of the Corpus Callosum (CLOCCs) after SARS-CoV-2 mRNA Vaccination. J Korean Med Sci. 2021;36(31):1–2. doi: 10.3346/jkms.2021.36.e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scott J, Anderson J, Mallak N, Beitinjaneh B, Wei K, Otaki F. Gastroparesis After Pfizer-BioNTech COVID-19 Vaccination. Vol. 116, The American journal of gastroenterology. United States; 2021. p. 2300. [DOI] [PubMed]
- 131.Zavala-Jonguitud L.F., Pérez-García C.C. Delirium triggered by COVID-19 vaccine in an elderly patient. Geriatr Gerontol Int. 2021;21(6):540. doi: 10.1111/ggi.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aladdin Y., Shirah B. New-onset refractory status epilepticus following the ChAdOx1 nCoV-19 vaccine. J Neuroimmunol. 2021;357 doi: 10.1016/j.jneuroim.2021.577629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu BD, Ugolini C, Jha P. Two Cases of Post-Moderna COVID-19 Vaccine Encephalopathy Associated With Nonconvulsive Status Epilepticus. Vol. 13, Cureus. 2021. p. e16172. [DOI] [PMC free article] [PubMed]
- 134.Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-Hunt Syndrome Presenting After COVID-19 Vaccination. Vol. 13, Cureus. 2021. p. e16791. [DOI] [PMC free article] [PubMed]
- 135.Lin Y.-H., Huang H., Hwang W.-Z. Moyamoya disease with Sjogren disease and autoimmune thyroiditis presenting with left intracranial hemorrhage after messenger RNA-1273 vaccination: A case report. Medicine (Baltimore) [Internet] 2022;101(6) doi: 10.1097/MD.0000000000028756. https://journals.lww.com/md-journal/Fulltext/2022/02110/Moyamoya_disease_with_Sjogren_disease_and.16.aspx Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.N. Murvelashvili A. Tessnow A Case of Hypophysitis Following Immunization With the mRNA-1273 SARS-CoV-2 Vaccine J Investig Med high impact case reports. 9 2021 23247096211043384. [DOI] [PMC free article] [PubMed]
- 137.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 138.https://www.mohfw.gov.in/. Vaccination By Type [Internet]. Available from: https://dashboard.cowin.gov.in/.
- 139.Organization WH. Extract from report of GACVS meeting of 30 November-1 December 2016, published in the WHO Weekly Epidemiological Record on 13 January 2017 [Internet]. Available from: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/yellow-fever-vaccines/campaigns.
- 140.Butler M., Tamborska A., Wood G.K., Ellul M., Thomas R.H., Galea I., et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92(11):1144–1151. doi: 10.1136/jnnp-2021-326924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Heal Med. 2013;2(5):38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lundberg I.E., Tjärnlund A., Bottai M., Werth V.P., Pilkington C., de Visser M., et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thakur K.T., Epstein S., Bilski A., Balbi A., Boehme A.K., Brannagan T.H., et al. Neurologic Safety Monitoring of COVID-19 Vaccines: Lessons from the Past to Inform the Present. Neurology. 2021;97(16):767–775. doi: 10.1212/WNL.0000000000012703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soni R., Heindl S.E., Wiltshire D.A., Vahora I.S., Khan S. Antigenic Variability a Potential Factor in Assessing Relationship Between Guillain Barré Syndrome and Influenza Vaccine - Up to Date Literature Review. Cureus. 2020;12(9):e10208. doi: 10.7759/cureus.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fiore A.E., Bridges C.B., Cox N.J. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 146.Wakefield A.J., Murch S.H., Anthony A., Linnell J., Casson D.M., Malik M., et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet (London, England) 1998;351(9103):637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 147.Wilson K, Mills E, Ross C, McGowan J, Jadad A. Association of Autistic Spectrum Disorder and the Measles, Mumps, and Rubella Vaccine: A Systematic Review of Current Epidemiological Evidence. Arch Pediatr Adolesc Med [Internet]. 2003;157(7):628–34. Available from: https://doi.org/10.1001/archpedi.157.7.628. [DOI] [PubMed]
- 148.Eggertson L. Lancet retracts 12-year-old article linking autism to MMR vaccines. Vol. 182, CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2010. p. E199-200. [DOI] [PMC free article] [PubMed]
- 149.Https://www.nytimes.com/. Tracking Coronavirus Vaccinations Around the World [Internet]. Available from: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html.
- 150.Amanzio M, Mitsikostas DD, Giovannelli F, Bartoli M, Cipriani GE, Brown WA. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: A systematic review. Lancet Reg Heal - Eur [Internet]. 2022;12:100253. Available from: https://doi.org/10.1016/j.lanepe.2021.100253. [DOI] [PMC free article] [PubMed]
- 151.Formeister EJ, Wu MJ, Chari DA, Meek R 3rd, Rauch SD, Remenschneider AK, et al. Assessment of Sudden Sensorineural Hearing Loss After COVID-19 Vaccination. JAMA Otolaryngol Head Neck Surg. 2022 Feb. [DOI] [PMC free article] [PubMed]
- 152.Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McGrogan A., Madle G.C., Seaman H.E., de Vries C.S. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review Neuroepidemiology. 2009;32(2):150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 157.Kumar N, Graven K, Joseph NI, Johnson J, Fulton S, Hostoffer R, et al. Case Report: Postvaccination Anti-Myelin Oligodendrocyte Glycoprotein Neuromyelitis Optica Spectrum Disorder: A Case Report and Literature Review of Postvaccination Demyelination. Vol. 22, International journal of MS care. 2020. p. 85–90. [DOI] [PMC free article] [PubMed]
- 158.Azumagawa K., Nomura S., Shigeri Y., Jones L.S., Sato D.K., Nakashima I., et al. Post-vaccination MDEM associated with MOG antibody in a subclinical Chlamydia infected boy. Brain and Development. 2016;38(7):690–693. doi: 10.1016/j.braindev.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 159.Kowarz E, Krutzke L, Külp M, Streb P, Larghero P, Reis J, et al. Vaccine-induced COVID-19 mimicry syndrome. Middeldorp S, Barton M, ten Cate H, editors. Elife [Internet]. 2022 Jan;11:e74974. Available from: https://doi.org/10.7554/eLife.74974. [DOI] [PMC free article] [PubMed]
- 160.Chen Y., Xu Z., Wang P., Li X.M., Shuai Z.W., Ye D.Q., et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2021 doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 161.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int [Internet]. 2021;41(3):509–18. Available from: https://doi.org/10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed]
- 162.Kelleni M.T. SARS CoV-2 Vaccination Autoimmunity, Antibody Dependent Covid-19 Enhancement and Other Potential Risks: Beneath the Tip of the Iceberg. Int J Pulm Respir Sci. 2021;5(2):1–10. [Google Scholar]
- 163.Xu L., Ma Z., Li Y., Pang Z., Xiao S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv Immunol. 2021;151:99–133. doi: 10.1016/bs.ai.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murphy W.J., Ph D., Longo D.L. Cl inic a l I m pl ic a t ions of B a sic R e se a rch A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and. Vaccination. 2022:394–396. doi: 10.1056/NEJMcibr2113694. [DOI] [PubMed] [Google Scholar]
- 165.Mastellos D.C., Skendros P., Lambris J.D. Is complement the culprit behind COVID-19 vaccine-related adverse reactions? J Clin Invest. 2021;131(11) doi: 10.1172/JCI151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siripongsatian D., Kunawudhi A., Promteangtrong C., Kiatkittikul P., Jantarato A., Choolam A., et al. Alterations in 18F-FDG PET/MRI and 15O-Water PET Brain Findings in Patients with Neurological Symptoms after COVID-19 Vaccination: A Pilot Study. Clin Nucl Med. 2022;47(3):E230–E239. doi: 10.1097/RLU.0000000000004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.