Abstract
Pulmonary hypertension (PH) associated with interstitial lung disease (ILD) is an attractive target for clinical trials of PH medications. There are many factors that need to be considered to prime such studies for success. The patient phenotype most likely to respond to the intervention requires weighing the extent of the parenchymal lung disease against the severity of the hemodynamic impairment. The inclusion criteria should not be too restrictive, thus enabling recruitment. The trial should be of sufficient duration to meet the chosen endpoint which should reflect how the patient feels, functions, or survives. This paper summarizes prior studies in PH‐ILD and provides a framework of the type of studies to be considered. Inclusion criteria, clinical trial endpoints, and pharmacovigilance in the context of PH‐ILD trials are also addressed. Through lessons learnt from prior studies, suggestions and guidance for future clinical trials in PH‐ILD are also provided.
Keywords: clinical trial design, interstitial lung disease, pulmonary hypertension
INTRODUCTION
Pulmonary hypertension (PH) complicates the course of many patients suffering from interstitial lung disease (ILD). The initial article of this series from PVRI's Group 3 Pulmonary Hypertension Workstream examined the scope of this issue and its impact on patients as the first step to facilitate and encourage future research in this area. 1 The advent of therapeutics for Group 1 pulmonary arterial hypertension (PAH) has raised interest in studying and using these drugs in Group 3 pulmonary hypertension (PH), especially the ILDs. This paper is designed to provide an overview of prior clinical trials, including lessons from these studies that can help provide a roadmap for future clinical drug studies targeting the pulmonary vasculature or pulmonary hypertension in patients with ILD (PH‐ILD).
PRIOR CLINICAL TRIALS IN PH‐ILD AND ILD WITH A VASCULAR PHENOTYPE
There have only been a few randomized, controlled studies for PH due to ILD. The first of these was the ACTIVE study, which evaluated the use of inhaled iloprost in 51 patients with idiopathic pulmonary fibrosis (IPF). Unfortunately, the drug was not found to be effective based on the primary endpoint of 6‐minute walk distance (6MWD) at 12 weeks and the study was only published in abstract form. 2
The STEP‐IPF study was undertaken by the NIH IPF Network and evaluated the use of sildenafil in patients with advanced IPF. This study did not require a right heart catheterization (RHC) to confirm the presence of PH but was enriched for PH though its main inclusionary criterion of a single breath diffusing capacity for carbon monoxide (DLco) of <35% predicted. 3 This was a negative study based on the primary endpoint of a 20% increase in 6MWD at 12 weeks. However, there were a number of secondary endpoints that were met including quality of life measures, change in DLco, and oxygenation that suggested benefit. Interestingly, a subsequent subgroup analysis of those participants with echocardiographic evidence of right ventricular (RV) dysfunction showed a significant difference in placebo‐corrected change in the 6MWD of 99 m, suggesting perhaps that RV dysfunction could be an enrichment strategy for future clinical trials. 4
The BPHIT study (Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia [IIP]) evaluated the endothelin antagonist, bosentan, in a group of 60 patients with RHC documented PH in the context of fibrotic lung disease. 5 The primary endpoint was change in the pulmonary vascular resistance (PVR) over 16 weeks with multiple secondary endpoints. This was a decidedly negative study based on the primary endpoint, as well as all the secondary endpoints.
The RISE‐IIP study evaluated riociguat in patients with idiopathic interstitial pneumonia (IIP) and associated PH. 6 RISE‐IIP was terminated prematurely, owing to the higher number of deaths and serious adverse events in the active treatment arm. There was no discernible reason for the deleterious outcome, but based on the unfavorable risk/benefit profile, the use of riociguat in patients with PH‐IIP is now contraindicated. A post hoc analysis of those patients with available computed tomographic (CT) scans of the chest suggested that it was the combined pulmonary fibrosis and emphysema (CPFE) patients who were driving the poor outcomes. 7 However, it cannot be ruled out that it was the emphysematous component that drove the poor outcomes, since there were some cases that had predominant emphysema.
The use of ambulatory inhaled nitric oxide has also been studied in patients with ILD on supplemental oxygen. 8 There have been two independent cohorts studied thus far, both suggesting benefit as evaluated through the novel primary endpoint of actigraphy. 8 , 9 This population was enriched for underlying pulmonary hypertension through the need for supplemental oxygen. Since RHC was not required, there is no further insight as to how many of these patients may have had PH. Cohort 3 which is the pivotal Phase 3 study of ambulatory inhaled nitric oxide is currently underway and enrolling (NCT 03267108). 10
The three approved endothelin receptor antagonists have all been studied in IPF for their antifibrotic properties, with inclusion criteria agnostic to the presence of PH. All three randomized controlled trials (RCT) of bosentan (BUILD study), macitentan (MUSIC study), and ambrisentan (ARTEMIS‐IPF) were negative 11 , 12 , 13 In fact, there was the suggestion of harm in the ARTEMIS‐IPF study, which resulted in the study being terminated early at the suggestion of the data safety monitoring board (DSMB). Ambrisentan is therefore now contraindicated in PH due to IPF. In parallel with ARTEMIS‐IPF, there was a “sister” study (ARTEMIS‐PH), which enrolled only patients with IPF who had RHC confirmed PH. This study was very slow to recruit, and the program was halted at the time that the ARTEMIS‐IPF study was terminated. The results from the small number of patients randomized to this trial were never reported. This study's slow recruitment was probably predicated by the serial high hurdles of an accurate IPF diagnosis accompanied by complicating PH. A lesson from the difficulty enrolling this study was to cast a wider net for ILDs, beyond just IPF, for studies targeting associated PH.
Sildenafil has been studied in conjunction with both nintedanib and pirfenidone in patients with advanced IPF as defined by a DLco of ≤35%. The INSTAGE study of nintedanib plus sildenafil was a 24‐week study with the primary endpoint being change in the St. George's respiratory questionnaire (SGRQ) at 12 weeks. 14 There were 274 patients randomized in a one‐to‐one fashion between nintedanib plus placebo versus nintedanib plus sildenafil. The study was negative based on the primary endpoint of change in the SGRQ at 12 weeks. In a longer‐term study, pirfenidone plus sildenafil was compared to pirfenidone alone. 15 The primary endpoint was a composite of disease progression defined as either a relevant decline in the 6‐minute walk distance, respiratory‐related hospital admission, or all‐cause mortality at 52 weeks. There were 177 patients randomized in a one‐to‐one fashion to the two arms, but unfortunately this was also a negative study with no difference in the proportion of patients who had disease progression over the 52 weeks. However, there were no differences in reported serious adverse events or death between both treatment groups. The lack of mandated RHC‐confirmed PH could have played a role in the negative results of some of these clinical trials, especially in the aforementioned studies of sildenafil, although there have been a number of negative studies where RHC was mandatory to confirm the diagnosis of PH. 1 , 16
The largest randomized controlled study targeting PH‐ILD, the INCREASE study, included 346 patients equally randomized between inhaled treprostinil and placebo. 17 The study met its primary endpoint of change in the 6‐minute walk distance at 16 weeks. In addition, multiple secondary endpoints were met, including time to clinical worsening, change in the NT‐proBNP, trough 6‐minute walk test at 15 weeks, and the 6‐minute walk test at 12 weeks. Safety endpoints included the incidence of acute exacerbations, which surprisingly were less in the inhaled treprostinil arm. In addition, spirometry was monitored throughout the study as a safety endpoint; interestingly, there was a suggestion that the group who received inhaled treprostinil had improvements in lung function at 16 weeks compared to the placebo arm. This difference was most evident in the IPF subpopulation. 18 More details will be provided in the upcoming treatment and management article.
ROADMAP FOR FUTURE CLINICAL TRIALS
The few successes and many failures in PH‐ILD and ILD clinical trials have afforded a number of lessons but have raised more questions requiring thoughtful deliberation to prime future trials for success.
ILD phenotype
What defines the best patient phenotype to study with pulmonary vasoactive agents? The first issue to address in this regard is which underlying ILDs to include in PH‐ILD studies? Studies of PH complicating specific diseases, such as IPF, are notoriously difficult to recruit as noted with the Artemis‐PH study. It makes sense therefore to cast a wider net for various ILDs as had been successfully employed previously (RISE‐IIP, INCREASE, BPHIT). This approach is supported by the observation that once patients with different ILDs develop PH, their prognosis appears to be equally dismal. In fact, there is greater similarity in the course and prognosis among diverse ILDs with PH than there are among disparate Group 1 PAH diagnoses, whose prognoses differ vastly (e.g., congenital heart disease‐PH vs. connective tissue disease‐PH). Such studies can include all IIPs, with or without chronic hypersensitivity pneumonitis (chronic HP) occupational lung disease patients and select patients with ILD due to connective tissue diseases (CTD‐ILD). For example, in the INCREASE trial of inhaled treprostinil, patients with CTD‐ILD were only eligible for enrollment if their forced vital capacities were <70% of predicted. 17 Sarcoidosis is sufficiently unique that it should ideally be studied separately, although if the cause is clearly parenchymal lung disease, a case can be made for including patients with sarcoid‐associated PH (SAPH). Lymphangioleiomyomatosis and pulmonary Langerhans cell histiocytosis should also probably be studied separately, although these would need to be small studies given their very low prevalence rates. An important lesson from the RISE‐IIP study is that consideration needs to be given to the lung morphology, since the deleterious signal in the study appeared to emanate from the CPFE patients. Specifically, the extent of the underlying parenchymal disease was underestimated by standard pulmonary function testing, due to the counter‐balancing mechanical effects of combined obstructive and restrictive physiology. It might not be necessary to have all HRCTs of the chest centrally adjudicated for an exact characterization or diagnosis; however, it does appear prudent to collect these to validate the presence of ILD, while excluding other disease processes such as excessive emphysema. Another lesson from the RISE‐IIP study was that there were patients included with emphysema that exceeded the extent of the fibrosis, a prespecified exclusionary criteria. This argues for central adjudication in such cases. 7 There is no definition of what constitutes excessive emphysema, but in the context of CPFE, we would posit that this is when the extent of the emphysema exceeds that of the fibrosis. Future studies might also look at objective digital imaging scoring systems for the quantification and distribution of the ILD and any associated emphysema. When considering the appropriate patient phenotype, it is important to rule out other contributory causes to the patients PH, including obstructive sleep apnea, uncorrected hypoxemia, and left‐sided heart failure. In clinical trials without RHC, there needs to be reliance on echocardiographic findings to best rule out significant heart failure, for example, evidence of Grade 3 or 4 diastolic dysfunction, left ventricular basal diameter dilation or hypertrophy, presence of valvular disease or an enlarged left atrium.
Definition and severity of underlying pulmonary hypertension
Should all patients with PH by the prevailing definition be included in any given clinical trial? 16 , 19 , 20 Should clinical trialists look to enrich by only including the most severe patients? The downside of a high hemodynamic bar is that this will impact recruitment and limit the generalizability beyond a highly select group of patients. On the other hand, there are data demonstrating that even slight increases in mPAP together with a PVR ≥ 3 are associated with worse outcomes, supporting broader enrollment criteria. 21 Therefore, trial designs need to have balance between potential enrichment and recruitment. An in‐depth discussion of phenotypes, including the degree of hemodynamic impairment in relation to the extent of the parenchymal lung disease, will be covered in detail in a separate manuscript from our Working Group.
Is a baseline RHC mandatory?
There is precedent through the STEP‐IPF and INSTAGE studies using the inclusionary enrichment strategy of a DLco ≤ 35%. 3 , 14 It has been demonstrated that just over half the IPF patients (56%) with a DLco < 30% of predicted have PH by the old definition. 22 If studies using surrogates to enrich for PH are implemented, then they cannot be categorized as pulmonary hypertension studies, but rather as studies targeting the pulmonary vasculature. For these types of studies, is there a better enrichment strategy than a DLco threshold alone? Various combinations of the DLco, NT‐proBNP, and echocardiographic findings may be considered as noninvasive screening tools and/or inclusion criteria. Enrichment strategies are certainly an area for future research.
Duration of the treatment trial
The optimal duration of a PH‐ILD therapeutic trial depends on the balance between several factors. If the medication being studied is a titratable agent, then sufficient time needs to be allowed for the patient to be escalated to the target dose, while minimizing the risks of dropouts due to intolerable side‐effects. The chosen primary endpoint is important from the standpoint that patients need sufficient time on the target dose to demonstrate a difference; this difference could be constituted by either an improvement in the treatment arm or deterioration in the placebo arm. If more robust endpoints such as hospitalization or mortality are the chosen primary endpoint, then patients with more severe disease followed for a longer period may be required. If functional or surrogate endpoints such as change in the 6‐minute walk distance are chosen, then shorter‐term studies with patients who have less severe disease might suffice. However, for such functional endpoint studies, it should be borne in mind that deconditioning may affect the study result, and longer follow‐up might be needed to allow patients to “recondition” while receiving the new medication. The proposed mechanism of action of the investigational agent also needs to be considered; specifically, when will the effect be seen and through what endpoint measure? While longer‐term studies are more ideal, consideration needs to be given to patient recruitment into longer term studies, as well as patient retention during longer periods of follow‐up. Limiting patient dropouts minimizes the effects of having to impute results for these patients and lessens the controversy as to the best imputation methodology. For the three most robust RCTs in PH‐ILD, the dropout rate for reasons other than death has ranged from 12% (BPHIT trial, 16 weeks, n = 60) to 16% (RISE‐IIP, 26 weeks, n = 147) and 21% (INCREASE study, 16 weeks, n = 326). 5 , 6 , 17 The issue of dropouts is perhaps most germane in patients with severe disease and therefore attention needs to be given to parameters that will preclude significant subject dropouts. For example, consideration should be given to provisions for open label drug or other rescue therapies in the context of disease progression. While there are no set definitions of short, intermediate, or long‐term studies, we suggest the following duration of studies in the context of PH‐ILD. The phase of study, purported mechanism of action, and the study endpoints are some of the factors that play a role in determining the optimal study duration.
Short‐term studies (4–12 weeks). This appears to be a reasonable timeframe for Phase 2 studies.
Intermediate‐term studies (12–26 weeks). This appears to be a reasonable timeframe for either Phase 2 or Phase 3 studies with functional endpoints.
Long‐term studies >26 weeks. This appears to be a reasonable minimal period required to demonstrate a difference in more robust endpoints, such as time to clinical worsening/exacerbation, mortality, or hospitalization.
Types of trials
-
(a)
Event‐driven trials do not have a fixed duration but rather rely on the number of accrued events to demonstrate a difference in the treatment arms. The rate of study recruitment also factors into the total time to complete the trial; the more robust the initial recruitment, the greater the number of patients with accrued time to experience an event. Enrichment strategies, both in patient and endpoint selection, are also very important. If hospitalization and death are components of the composite, then patients with more severe diseases are more likely to meet one of these. For endpoint selection, casting a wider net through composite endpoints also enables more patients to reach the endpoint as well as capturing multiple domains of disease progression.
-
(b)
Hybrid trials. This term has been used to describe a mix of site‐based patient visits as well as remote visits conducted via telemedicine. 23 The latter provision is an increasingly attractive option with the COVID‐19 pandemic, given the unpredictable nature of surges or other unanticipated events (e.g., wars) in different geographic areas that may preclude study participants from returning for scheduled research visits. This is especially important for end of study visits to capture the primary endpoint parameter, for example, the 6‐minute walk test. Having a backup primary endpoint such as home‐based actigraphy is a reasonable contingency strategy in the event a predetermined threshold of subjects with evaluable end of study primary endpoint data is not met. The ability to participate in clinical trials through remote visits might also increase access to research trials for patients living in rural areas. For such an approach to be successful, a priori regulatory approval and buy‐in is essential. This safeguard might be complicated in multinational studies, where the same logistical support for home‐based monitoring might not exist on a country‐by‐country basis.
-
(c)
Seamless trials. These are a type of hybrid trial that can be used to describe a study designed to incorporate both a fixed short to intermediate primary endpoint (such as change in the 6MWD) together with a longer‐term supportive endpoint. This can be achieved by allowing all patients to continue beyond the primary endpoint in their blinded arms until the last patient crosses the primary endpoint time threshold. This would enable a longer‐term time to clinical worsening composite as a key secondary endpoint to support the primary analysis.
-
(d)
Adaptive trial designs. The term “adaptive design” refers to a clinical trial in which data collected during the course of the trial are used to change aspects of the trial design while not compromising the validity and integrity of the trial. 24 There are many elements to clinical trial design that could be regarded as adaptive. For example, an event driven study is “adapted” to how quickly the events occur. Another version of an adaptive trial, which especially pertains to PH‐ILD, is to have all patients on the study drug for a short period of time to ensure tolerability and no deleterious effects. Patients would then enter and complete a washout period of the drug for a few weeks (dependent on the half‐life of the agent), before randomization into the respective arms of the study. This strategy serves to limit dropouts during the randomized controlled phase. The run‐in period could also be regarded as an enrichment strategy, in that patient who experience any untoward effects from the drug would be screened out before randomization. Another form of clinical trial adaptation is to seamlessly transition from a Phase 2a (safety tolerability) into a Phase 2b efficacy study, and from 2b into Phase 3 studies. These types of strategies appear especially attractive for rare disorders, where recruitment is difficult and maximizing each subject's study participation can improve the trials efficiency. In addition, such an approach may save development time and be more resource effective. In these situations, the study protocol should detail how sufficient evidence might be expected from the trial, with regulatory feedback on the study design. 24 Changes in the study drug dosing can also be adapted based on interim analyses of either efficacy and/or safety.
-
(e)
Master Protocols. This concept has been proposed by the FDA to orchestrate efficient clinical trial strategies in the development of novel therapeutic agents and biologics for cancer. 25 These protocols, which are adaptive in nature, are designed to facilitate late‐stage oncologic drug development with one protocol that tests multiple drugs and or multiple cancer subpopulations. Whether a similar type of approach can or should be undertaken in diseases such as PH‐ILD, with a prognosis similar to or worse than many cancers, remains to be explored.
-
(f)
Basket trial designs. This describes the use of an agent in multiple disease subpopulations. This might be an attractive option for PH‐ILD clinical trials in that different phenotypes could be studied together with adaptation based on interim analyses.
-
(g)
Umbrella trial design. This is a master protocol designed to evaluate a number of investigational drugs alone or in combination versus placebo in a single disease population.
-
(h)
Platform trials are also an extension of adaptive trials. 26 This type of trial design enables the evaluation of multiple agents and are similar to umbrella trials but allow for adaptation using interim evaluations and addition of new potential therapeutics during the course of the trial. These studies are regarded as more “disease‐focused” (what works best?) rather than “intervention” focused (does this therapy work?). These types of studies might not be feasible for PH‐ILD given the different stakeholders with drugs at various stages of development for this indication. These might also only be feasible if undertaken by a large funding or governmental agency.
-
(i)
Crossover studies. These make sense from the standpoint that each patient receives active therapy and placebo in a blinded, sequential fashion and thereby serve as their own controls. This is especially attractive for rare disorders since subjects can contribute to both arms of the study. The optimal time for study drug washout is uncertain and should not only be dependent on the half‐life of the drug, but also on how long it takes for the benefit to dissipate. The optimal duration of each phase of the study is also uncertain but would likely be best suited to more short‐term intervals (4–12 weeks). While “hard” endpoints such as mortality and hospitalization would need to be accounted for, cross‐over studies are more suited for “softer” reversible endpoints as the primary outcome measure; these could include quality of life measures, or functional measures such as actigraphy or the 6MWD. For those patients who receive active drug during the first phase, it might be difficult to account for any “reconditioning” that might persist beyond the half‐life of the drug, and thereby impact the subsequent placebo phase of the study. One area of additional concern in such a study design is withdrawal of the proposed PH therapeutic and the risk of rebound pulmonary hypertension. This does raise the notion of whether there is a threshold of PH severity that should preclude enrollment in this type of clinical trial design. Open label therapy for all patients in the context of a crossover study might provide further validation and safety information while optimizing each patient's participation in the clinical trial.
Study drug attribution
How long after the study drug is withheld do the outcomes or adverse events still get attributed to the agent being studied? Not only is this important in the interpretation of crossover studies, but it is perhaps more germane to randomized controlled trials where study drug is withdrawn from both arms at the end of the study, or the placebo arm gets rolled over to open label drug. Two examples of this in the PH‐ILD literature are illustrative; first, the STEP IPF study of sildenafil where the strict intent to treat analysis resulted in a numeric difference in the number of deaths in the placebo arm (N = 11) versus the treatment arm (N = 4). 3 However, seven of the deaths in the placebo arm occurred after 12 weeks during the open label period when the patients were receiving sildenafil. If mortality was attributed to being on active drug versus placebo, then these numbers would have “flipped” and there would have been 4 deaths on placebo (over 12 weeks) versus 11 deaths on sildenafil (over 24 weeks on sildenafil for the active arm and 12 weeks on sildenafil for the former placebo arm). The opposite approach was adopted for the RISE‐IIP study where the 8 deaths in the former placebo arm (out of 38 patients) who were rolled over to open label drug were attributed to riociguat rather than placebo. This mortality during the open‐label phase, together with the mortality in the blinded phase (8/73 in the active treatment arm) weighed heavily in the decision of the data safety monitoring board in recommending early termination of the study. Whatever approach is adopted, it is important to have input from all stakeholders including the regulatory authorities, and the prespecified approach should be clearly outlined in the protocol.
Pandemic and other unanticipated global events
The impact of the COVID‐19 pandemic on patient care has been profound, as it has been on clinical trial implementation and maintaining the integrity of existing studies. While it is hoped that the impact of the virus on future clinical trials will diminish, this remains largely unpredictable, especially in the context of multinational studies. Lessons from the COVID‐19 pandemic extend to other unanticipated global events, such as wars and cyberattacks. Therefore, it is strongly recommended that appropriate contingencies be built into all future trial designs. Considerations in this regard include flexibility around data collection, incorporating innovative endpoints, harnessing digital technology, and embracing off‐site data collection as well as remote monitoring. 27
ILD‐PH CLINICAL TRIAL ENDPOINTS
A robust clinical endpoint should measure a relevant improvement or deterioration of the clinical condition. For Phase 2 and short to intermediate‐term Phase 3 studies, less robust surrogate outcomes or composites thereof may be employed. There are many potential endpoints from which to choose in the context of PH‐ILD clinical trials, some of which might be more suited to Phase 2 and others to Phase 3 studies. Figure 1 depicts the various endpoints that may have a role in PH‐ILD clinical trials.
-
(a)
Hemodynamic endpoints. Change in the PVR is the most commonly evaluated hemodynamic endpoint for PAH clinical trials. This has mostly been employed in the context of Phase 1 and Phase 2a studies. However, change in PVR might not be necessary for PH‐ILD clinical trials, since most agents being studied in this disease entity have previously been studied for Group 1 PAH, where hemodynamic target engagement has already been demonstrated. Hemodynamic endpoints are best suited to Phase 1 and 2 clinical trials only. 28
-
(b)
Functional endpoints and exercise capacity. The most commonly employed in this regard has been the 6‐minute walk test, which has resulted in many of the PAH agents being approved, as well as the one agent approved for PH‐ILD in the USA. Notably, the FDA would be comfortable with other functional endpoints, but clinical trialists have continued to employ this functional measure since the first oral drug approval for PAH. 29 Although it remains a somewhat controversial primary endpoint for PAH clinical trials, it is still deeply embedded as a component of time to clinical worsening which is the preferred endpoint in the modern era of PAH clinical trials. The pros and cons of the 6‐minute walk are beyond the scope of this current manuscript. However, in the context of PH‐ ILD clinical trials, there are parameters that are easily obtained during the 6MWD test, aside from just the distance, that might be worth further consideration with regard to supporting the primary endpoint. Specifically, the amount of supplemental oxygen used, oxygen desaturation, heart rate recovery and the Borg dyspnea score all provide more insight into the patient's condition, as well as change in the patient's condition. If the 6‐minute walk distance is a standalone endpoint, then it should be analyzed as a continuous variable. However, within the context of a composite endpoint that defines clinical worsening or improvement, a categorical change is acceptable. The best threshold to define a significant change is open to debate. Should this be a set distance that approximates the minimally important difference or a percentage of the patient's baseline; for example, a 15% increase or decrease. The minimally important difference in IPF has previously been estimated to be in the range of 22–45 m, while in PAH it has a similar estimated range of 25–39 m. 30 , 31 , 32 The drawbacks to using categorical changes are that these are statistically inefficient, and it can be difficult to ensure that patients who satisfy these changes are consistent responders versus day‐to‐day variability. If categorical changes are used, then other parameters within the 6‐minute walk may also be used to validate smaller changes as being clinically meaningful. Indeed, both desaturation and a low pulse rate recovery have been noted previously to be associated with worse outcomes. 33 , 34 Therefore, as an example, a smaller decrement in the walk distance might be significant if accompanied by worse desaturation, greater oxygen requirements, a lower pulse rate recovery or an increase in the Borg dyspnea score. 35 , 36 , 37 , 38
-
(c)
Accelerometry/actigraphy. Patients with PH‐ILD may have severe limitations on physical activity which impacts their quality of lives to varying extents. They may struggle to perform basic activities of daily living (ADLs), such as walking, climbing stairs, or showering. The ability to monitor changes in the level of physical activity accurately, specifically moderate physical activity, which correlates to household tasks and ADLs, has the potential to inform directly on the patient's overall health, well‐being, and quality of life. With currently available accelerometry technology, these changes can be assessed as direct quantitative measures in activity counts or energy expenditure, as well as categorized into activity intensity levels including time spent in sedentary, light, or moderate activities. Moderate activity includes ADLs, such as walking, climbing stairs, or washing dishes. Generally, a change in moderate activity of 10%–20% has been considered clinically relevant in cardiopulmonary diseases. 39 , 40 , 41 This nascent emerging technology appears to be an attractive endpoint that requires further refinement and ongoing validation. Whereas the 6‐minute walk test informs (at set intermittent intervals) on what patients are capable of doing, actigraphy informs on what patients actually do, through continuous monitoring of their daily activity. This also has the advantage of reflecting patients' reality as it is outside the confines of the clinic and research visit. This emerging technology will be further addressed by the PVRI workgroup dealing with new Modalities and Technologies.
-
(d)
Functional class. The New York Heart Association functional class or World Health Organization modification thereof has been used in many prior PAH clinical trials. However, this is a rather blunt instrument and is subject to much interobserver variability. Functional class has been used as a component of risk scores 20 , 42 as well as some composite endpoints, 43 or as a further validation of meaningful change in other endpoints. 44
-
(e)
Supplemental oxygen needs. This has not previously been used as an endpoint in either PAH or ILD clinical trials but appears to be an attractive patient‐centric endpoint that warrants further consideration. It is perhaps best used in the context of a composite endpoint. This might be best suited to studies of patients with early disease who are oxygen‐independent, since the initiation of supplemental oxygen is more meaningful than increasing oxygen needs in patients who already are on supplemental oxygen. The criteria for initiating supplemental oxygen will need to be standardized in the clinical trial, and, if based on the 6MWD results, there needs to be recognition of factors that might impact the measurement of desaturation via pulse oximetry, including the speed of the walk and skin pigmentation. 45 , 46
-
(f)
Patient reported outcomes (PRO). Accounting for the patients’ perspective when designing a clinical study is needed to provide direct feedback on whether the magnitude of the outcomes seen with the selected primary endpoint translate into benefits that the patient experiences and perceives. The best PRO to use in patients with PH‐ILD remains uncertain, as both PAH‐specific instruments as well as ILD instruments may have a role. Currently there is no standardized approach to PROs, and instruments in use have not been adequately validated with regard to content validity for PH‐ILD. Therefore, which PROs are best suited to capture meaningful change in PH‐ILD patients requires further investigation. Since both conditions manifest primarily with shortness of breath, a generic dyspnea PRO might well suffice in conjunction with a more general health related quality of life instrument. Whether or not a PH‐ILD specific questionnaire needs to be developed remains to be answered. Currently several QOL measures validated in other cardiopulmonary diseases are being applied in PH‐ILD studies, including the SGRQ, the SF 36, which is a 36 item Short Form Health Survey, and the University of California, San Diego Shortness of Breath Questionnaire (UCSD‐SOBQ). In a recent prospective multicentre study in IPF the King's Brief Interstitial Lung Disease Questionnaire (K‐BILD) describing psychometric properties had the strongest relationship with 1‐year mortality in comparison to the modified Medical Research Council (mMRC) dyspnea scale, the SGRQ and the UCSD‐SOBQ. 47 Another rapid, specific tool that could be used for patients with IPF during routine clinic visits is the R‐scale which has a five‐item numerical rating scale. This has been shown to correlate well with the K‐BILD and EQ‐5D‐5L but should be subjected to further validation. 48 There are shorter questionnaires that focus on key elements of concern to IPF patients which include the PROMIS® Short Form v1.0 for Fatigue and the Short Form v2.0 for Physical Function. The Patient Global Impression of Severity (PGIS) and Change (PGIC) are also very short questionnaires that are employed to capture the patient's perception of the severity and change in their disease symptoms. These can also serve to validate and anchor the magnitude of the primary endpoint outcome.
-
(g)
Biomarkers. There is increasing evidence that NT‐pro BNP can be an important biomarker in PH‐ILD clinical trials. 17 , 49 While this can be used as a supportive secondary endpoint, it would not suffice as the primary endpoint in a Phase 3 clinical trial but could provide proof of concept in a Phase 2 program. This or other biomarkers may have important roles in supporting the validity of the primary endpoint, as was seen in the INCREASE study. 17
-
(h)
Hospitalization. Hospitalization is a meaningful endpoint in and of itself, since it has implications not only for patients and their prognosis, but also for healthcare resource utilization. It does have well‐established prognostic implications in patients with ILD. 50 , 51 Whether it should be all‐cause hospitalization or cardiopulmonary hospitalization is open to some debate. The latter appears more attractive as it is more specific for the direct consequences of PH‐ILD. What is important to bear in mind is that the threshold for hospitalization might differ among centers, regions, and in different countries. Whether it should be hospitalization or “need for hospitalization” is also a subject for debate. Need for hospitalization would require central adjudication, but would homogenize this endpoint among different hospitals, regions, and countries. Another yet unanswered question, is the prognostic implications of emergency room (ER) visits, which do not result in hospitalization, as well as unscheduled doctor visits. These arguably are also important endpoints, as they also have healthcare resource utilization implications. Whereas hospitalization has been incorporated in many composite endpoints, details pertaining to each hospitalization are generally not reported. Such details might also be important and provide meaningful insight; for example, need for ICU care, need for mechanical ventilation and duration of hospitalization are rarely, if ever reported.
-
(i)
Lung transplantation. How best to deal with transplant? This can be dealt with in two ways; in an outcomes analysis, it is frequently incorporated with mortality as time to death or transplant. However, transplant is by no means equivalent to death and is somewhat arbitrary and variable based on different regions and countries. It is dependent on the availability of transplant, as well as donor availability. Subjects ultimately attain this endpoint through the donor's demise and sometimes in the absence of meaningful change in the patient's clinical status. It is also an endpoint that might only be available to those within the study who are medically qualified to be transplant candidates. An alternative method for dealing with transplant is to censor those patients as alive on the date of the transplant. Thus far in all PH and ILD clinical trials, the number of transplant events has been very small and therefore has not impacted the analyses meaningfully.
-
(j)
Mortality. Mortality is the most meaningful endpoint, and certainly this is the case for PH‐ILD clinical trials. Whatever endpoint is chosen, be it a functional endpoint or a time to clinical worsening endpoint, mortality needs to be accounted for. The issue of whether it should be all‐cause mortality or cardiopulmonary mortality is another area for debate. All‐cause mortality is preferred since this is definitive and does not require any adjudication. Indeed, when these patients succumb, it is often very difficult to pinpoint the exact cause of their demise. Mortality as an endpoint typically necessitates longer follow‐up and more patients, exacerbating issues of patient retention and increased funding requirements.
-
(k)
Composite endpoints. Composite endpoints with two or more distinct efficacy outcomes with recognized clinical meaningful implications have numerous advantages including increased number of events with a smaller sample size. Composite endpoints have gained increasing use in the context of PAH, ILD and PH‐ILD clinical trials. The use of composites is attractive as they incorporate multiple domains of disease progression, thus enabling more events to be captured in a shorter time period, as well as reflecting the global benefits of the study drug in question. They are typically constituted by some of the endpoints discussed above. Which of these to incorporate is also open to some debate. Composites should not include components that the intervention is not expected to impact. Composite endpoints are driven by the most common events, which are not necessarily the most severe or clinically relevant. For example, death and hospitalization are more important events than a categorical change in the 6‐minute walk distance, actigraphy or change in a PRO. This raises the concept of weighted composite endpoints to reflect the relative importance of these various composite components. Most composites consist of elements that herald worsening of the disease and are “packaged” together as time to clinical worsening. However, in the context of PH‐ILD studies, it is not unreasonable to constitute a composite of events that herald improvement in the patient's clinical status. Examples of composite endpoints that might be considered in PH‐ILD trials are shown in Figure 2. The feasibility of demonstrating a difference in clinical improvement in PH‐ILD studies was recently demonstrated in a post hoc analysis of the INCREASE trial; specifically, more patients receiving inhaled treprostinil improved their 6MWD by 15% in association with a 30% reduction in the NT‐pro‐BNP in comparison to the placebo arm. 52
Figure 1.
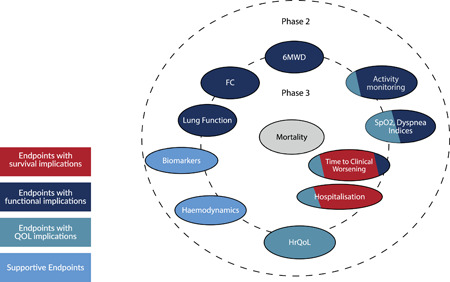
Endpoints that may have a role in PH‐ILD clinical trials. The closer to the center, the greater the correlation with mortality. Those crossing into both the Phase 2 and Phase 3 circles might be suitable for both types of study phases. 6MWD, 6‐minute walking distance; FC, functional class; HrQOL, health‐related quality of life; SpO2, oxygen saturation as measured by pulse oximetry; QoL, quality of life.
Figure 2.
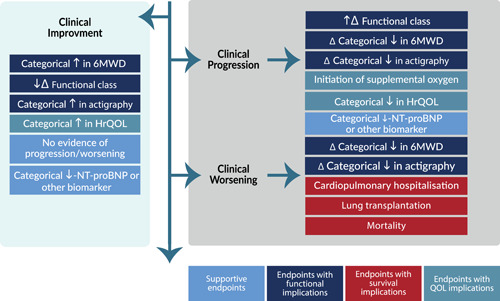
Clinical trial outcome measures that may be considered for inclusion as components of composite endpoints in PH‐ILD trials. Large Δ infers larger changes, while smaller Δ infers lesser changes. Clinical progression composites are heralded by lesser change, while clinical worsening infers greater change or more meaningful events. The NT‐proBNP can be used either with a progression or worsening categorical change event to provide biomarker validation. 6MWD six‐minute walk distance; HrQOL, health‐related quality of life; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; QoL, quality of life.
PHARMACOVIGILANCE
Pharmacovigilance (PV) can be described as the process of collecting, evaluating, and reporting on the safety of medicines and in doing so, taking timely and appropriate action to minimize the risks and increase the benefits of these medicines. The decision to approve a drug is based on it having a satisfactory balance of benefits and risks within the conditions specified in the product labeling. It is based on the information available at the time of approval that is also subject to change over time through expanded use. Global PV systems that have been implemented today are continually being optimized with evolving awareness of its critical need, supported by advancing technology. PV allows the continual rebalance of the risk/benefit ratio of drugs through the various stages of pre‐ and postmarketing safety monitoring.
Safety evaluation and monitoring with regard to PH‐ILD patients is in its infancy when compared to other disease areas. First, there is only one approved treatment (in the USA only) in this patient population and, therefore, post market surveillance is very limited compared to other disease states. Second, there are limited clinical trials data from this population and arguably, the most appropriate ILD phenotype awaits further refinement. In this regard, PV in PH‐ILD plays an even more critical role in supporting the continued discovery of new therapies from initial prehuman trials, through clinical development, regulatory approval and in post marketing surveillance. As an example, one potential safety issue pertains to worsening of ventilation/perfusion matching. In addition, pulmonary veno‐occlusive like lesions have been described in fibrotic lung disease which raises the notion that there is perhaps a specific phenotype that does not do well with vasoactive therapy. 53
There have been a number of trials studying the treatment of PH‐ILD. These have demonstrated a wide range of results that have spanned the spectrum from the positive INCREASE study of inhaled treprostinil to the harmful RISE‐IIP study of oral riociguat. Specifically, the RISE‐IIP study, a multicenter double‐blind RCT evaluating Riociguat in PH‐IIP showed increased rates of serious adverse events and mortality in the treatment arm leading to early termination of the study. 6 On the other hand, the INCREASE study, a 16‐week double‐blind RCT and the largest study to date in PH‐ILD, demonstrated the safety and efficacy of inhaled treprostinil in patients with PH‐ILD. 17 Although these two studies were of different drugs with different routes of administration, the negative signal from the RISE‐IIP study does underscore the importance of ongoing PV in studies of PH‐ILD, since early positive outcomes may not necessarily translate to improved longer‐term outcomes.
CONCLUSION
There are many elements to a successful trial design. These include a drug with biologic activity against an aberrant pathway with target engagement through a route of administration that enables this. The study drug needs to have an acceptable safety profile and tolerable side‐effects. The clinical trial should target a patient phenotype enriched to demonstrate treatment responsiveness, but who are also readily recruitable (and retainable) with inclusion criteria that are not onerously restrictive. The study needs to be adequately powered with sufficient patients followed for an appropriate length of time to demonstrate efficacy through a carefully constructed and meaningful endpoint. To align all these elements requires multidisciplinary expertise, great diligence, methodical planning, and strategic alignment from all key stakeholders. It is hoped that this manuscript helps to lay the foundation for the future success of clinical trials in PH‐ILD, which are sorely needed for this population in dire need of more treatment options.
AUTHOR CONTRIBUTIONS
Steven D. Nathan was involved in the conception and design of the manuscript, conducted the searches and data extraction as well as wrote the first draft of the manuscript. All authors analyzed and interpreted the data, revised the manuscript critically for important intellectual content, approved the final manuscript, and agreed to be accountable for its overall content.
CONFLICTS OF INTEREST
Steven D. Nathan is a consultant for United Therapeutics, Bellerophon, Third Pole, Roche, Boehringer‐Ingelheim, Merck, and Daewoong. Peter Fernandes is an employee of Bellerophon Pharma; Lucilla Piccari has received research funding from and served as a speaker for Janssen as well as received support for attending congresses from Janssen, MSD and Ferrer, not related to this manuscript; and Sylvia M. Nikkho is an employee of Bayer AG. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
The engaged PVRI's IDDI—Group 3 PH members: Steven H. Abman, Jonathan Chung, Paul Hassoun, Howard M. Lazarus, Horst Olschewski, Manuel J. Richter, Rajan Saggar, Eric Shen, Oksana Shlobin, Carmine Dario Vizza, and S. John Wort. IDDI Leads: Paul Corris, Raymond Benza, and Mark Toshner, supported by the PVRI. This work did not receive any funding.
Nathan SD, Fernandes P, Psotka M, Vitulo P, Piccari L, Antoniou K, Nikkho SM, Stockbridge N. Pulmonary hypertension in interstitial lung disease: clinical trial design and endpoints: a consensus statement from the Pulmonary Vascular Research Institute's Innovative Drug Development Initiative—Group 3 Pulmonary Hypertension. Pulm Circ. 2022;12:e12178. 10.1002/pul2.12178
REFERENCES
- 1. Nikkho SM, Richter MJ, Shen E, Abman SH, Antoniou K, Chung J, Fernandes P, Hassoun P, Lazarus HM, Olschewski H, Piccari L, Psotka M, Saggar R, Shlobin OA, Stockbridge N, Vitulo P, Vizza CD, Wort SJ, Nathan SD. Clinical significance of pulmonary hypertension in interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute's innovative drug development initiative—Group 3 pulmonary hypertension. Pulm Circ. 2022;12:e12127. 10.1002/pul2.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krowka MJ, Ahmad S, de Andrade JA, Frost A, Glassberg MK, Lancaster LH, Lasky J, Mathier MA, Stocks J. A randomized, double‐blind, placebo‐controlled study to evaluate the safety and efficacy of iloprost inhalation in adults with abnormal pulmonary arterial pressure and exercise limitation associated with idiopathic pulmonary fibrosis. Chest. 2007;132:633A. 10.1378/chest.132.4_MeetingAbstracts.633a [DOI] [Google Scholar]
- 3. Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–8. 10.1056/NEJMoa1002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, Anstrom KJ, Martinez FJ. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right‐sided ventricular dysfunction. Chest. 2013;143:1699–1708. 10.1378/chest.12-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corte TJ, Keir GJ, Dimopoulos K, Howard L, Corris PA, Parfitt L, Foley C, Yanez‐Lopez M, Babalis D, Marino P, Maher TM, Renzoni EA, Spencer L, Elliot CA, Birring SS, O'Reilly K, Gatzoulis MA, Wells AU, Wort SJ. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190:208–17. 10.1164/rccm.201403-0446OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nathan SD, Behr J, Collard HR, Cottin V, Hoeper MM, Martinez FJ, Corte TJ, Keogh AM, Leuchte H, Mogulkoc N, Ulrich S, Wuyts WA, Yao Z, Boateng F, Wells AU. Riociguat for idiopathic interstitial pneumonia‐associated pulmonary hypertension (RISE‐IIP): a randomised, placebo‐controlled phase 2b study. Lancet Respir Med. 2019;7:780–90. 10.1016/s2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 7. Nathan SD, Cottin V, Behr J, Hoeper MM, Martinez FJ, Corte TJ, Keogh AM, Leuchte H, Mogulkoc N, Ulrich S, Wuyts WA, Yao Z, Ley‐Zaporozhan J, Müller‐Lisse UG, Scholle FD, Brüggenwerth G, Busse D, Nikkho S, Wells AU. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia:a post hoc subgroup analysis of the RISE‐IIP study. J Heart Lung Transplant. 2021;40:494–503. 10.1016/j.healun.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 8. Nathan SD, Flaherty KR, Glassberg MK, Raghu G, Swigris J, Alvarez R, Ettinger N, Loyd J, Fernandes P, Gillies H, Kim B, Shah P, Lancaster L. A randomized, double‐blind, placebo‐controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest. 2020;158:637–45. 10.1016/j.chest.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 9. King CS, Flaherty KR, Glassberg MK, Lancaster L, Raghu G, Swigris JJ, Argula RG, Dudenhofer RA, Ettinger NA, Feldman J, Johri S, Fernandes P, Parsley E, Shah PS, Nathan SD. A phase‐2 exploratory randomized controlled trial of INOpulse in patients with fibrotic interstitial lung disease requiring oxygen. Ann Am Thorac Soc. 2022;19:594–602. 10.1513/AnnalsATS.202107-864OC [DOI] [PubMed] [Google Scholar]
- 10. Bellerophon . A study to assess pulsed inhaled nitric oxide in subjects with pulmonary fibrosis at risk for pulmonary hypertension (REBUILD). ClinicalTrialsorg: NCT03267108; 2022.
- 11. King TE Jr, Brown KK, Raghu G. BUILD‐3: a randomized, controlled trial of Bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–9. 10.1164/rccm.201011-1874OC [DOI] [PubMed] [Google Scholar]
- 12. Raghu G, Million‐Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42:1622–32. 10.1183/09031936.00104612 [DOI] [PubMed] [Google Scholar]
- 13. Raghu G, Behr J, Brown KK, Egan, JJ, Kawut SM, Flaherty KR, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, de Andrade J, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O'Riordan TG. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:634–649. 10.7326/0003-4819-158-9-201305070-00003 [DOI] [PubMed] [Google Scholar]
- 14. Kolb M, Raghu G, Wells AU, Behr J, Richeldi L, Schinzel B, Quaresma M, Stowasser S, Martinez FJ. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018;379:1722–31. 10.1056/NEJMoa1811737 [DOI] [PubMed] [Google Scholar]
- 15. Behr J, Nathan SD, Wuyts WA, Mogulkoc Bishop N, Bouros DE, Antoniou K, Guiot J, Kramer MR, Kirchgaessler KU, Bengus M, Gilberg F, Perjesi A, Harari S, Wells AU. Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: a double‐blind, randomised, placebo‐controlled, phase 2b trial. Lancet Respir Med. 2021;9:85–95. 10.1016/s2213-2600(20)30356-8 [DOI] [PubMed] [Google Scholar]
- 16. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke‐Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waxman A, Restrepo‐Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng C, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–34. 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 18. Nathan SD, Waxman A, Rajagopal S, Case A, Johri S, DuBrock H, De La Zerda DJ, Sahay S, King C, Melendres‐Groves L, Smith P, Shen E, Edwards LD, Nelsen A, Tapson VF. Inhaled treprostinil and forced vital capacity in patients with interstitial lung disease and associated pulmonary hypertension: a post‐hoc analysis of the INCREASE study. Lancet Respir Med. 2021;9:1266–74. 10.1016/s2213-2600(21)00165-x [DOI] [PubMed] [Google Scholar]
- 19. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 21. Piccari L, Wort SJ, Meloni F, Rizzo M, Price LC, Martino L, Salvaterra E, Scelsi L, López Meseguer M, Blanco I, Callari A, Pérez González V, Tuzzolino F, McCabe C, Rodríguez Chiaradía DA, Vitulo P. The effect of borderline pulmonary hypertension on survival in chronic lung disease. Respiration. 2022;101:717–27. 10.1159/000524263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nathan SD, Shlobin OA, Ahmad S, Urbanek S, Barnett SD. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest. 2007;131:657–63. 10.1378/chest.06-2485 [DOI] [PubMed] [Google Scholar]
- 23. ICON . Practical considerations for decentralised and hybrid clinical trials. ICON 2021, White Paper. ICONplc.com/DCT.
- 24. Nikkho S, Fernandes P, White RJ, Deng C, Farber HW, Corris PA. Clinical trial design in phase 2 and 3 trials for pulmonary hypertension. Pulm Circ. 2020;10:1–10. 10.1177/2045894020941491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Administration FaD . Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry—guidance for industry. US Department of Health and Human Services; March 2022.
- 26. Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. J Clin Epidemiol. 2020;125:1–8. 10.1016/j.jclinepi.2020.04.025 [DOI] [PubMed] [Google Scholar]
- 27. Psotka MA, Abraham WT, Fiuzat M, Filippatos G, Lindenfeld J, Ahmad T, Bhatt AS, Carson PE, Cleland JGF, Felker GM, Januzzi JL, Kitzman DW, Leifer ES, Lewis EF, McMurray JJV, Mentz RJ, Solomon SD, Stockbridge N, Teerlink JR, Vaduganathan M, Vardeny O, Whellan DJ, Wittes J, Anker SD, O'Connor CM. Conduct of clinical trials in the era of COVID‐19. J Am Coll Cardiol. 2020;76:2368–78. 10.1016/j.jacc.2020.09.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ventetuolo CE, Gabler NB, Fritz JS, Smith KA, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Are hemodynamics surrogate end points in pulmonary arterial hypertension. Circulation. 2014;130:768–75. 10.1161/circulationaha.114.009690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubin LJ, Badesch DB, Barst RJ, Galiè N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. 10.1056/NEJMoa012212 [DOI] [PubMed] [Google Scholar]
- 30. Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, King TE. Six‐minute‐walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–37. 10.1164/rccm.201007-1179OC [DOI] [PubMed] [Google Scholar]
- 31. Nathan SD, du Bois RM, Albera C, Bradford WZ, Costabel U, Kartashov A, Noble PW, Sahn SA, Valeyre D, Weycker D, King TE. Validation of test performance characteristics and minimal clinically important difference of the 6‐minute walk test in patients with idiopathic pulmonary fibrosis. Respir Med. 2015;109:914–22. 10.1016/j.rmed.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 32. Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6‐minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:428–33. 10.1164/rccm.201203-0480OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP, Martinez FJ. Prognostic value of desaturation during a 6‐minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–90. 10.1164/rccm.200302-219OC [DOI] [PubMed] [Google Scholar]
- 34. Swigris JJ, Swick J, Wamboldt FS, Sprunger D, du Bois R, Fischer A, Cosgrove GP, Frankel SK, Fernandez‐Perez ER, Kervitsky D, Brown KK. Heart rate recovery after 6‐min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest. 2009;136:841–848. 10.1378/chest.09-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, Pott GB, Vnencak‐Jones CL, Arneson C, Wade M, White RJ. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo‐controlled trial. J Heart Lung Transplant. 2010;29:137–49. 10.1016/j.healun.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 36. Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6‐minute walk distance. J Heart Lung Transplant. 2015;34:362–368. 10.1016/j.healun.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 37. Pesonen I, Gao J, Kalafatis D, Carlson L, Sköld M, Ferrara G. Six‐minute walking test outweighs other predictors of mortality in idiopathic pulmonary fibrosis. A real‐life study from the Swedish IPF registry. Respir Med X. 2020;2:100017. 10.1016/j.yrmex.2020.100017 [DOI] [Google Scholar]
- 38. Jing ZC, Parikh K, Pulido T, Jerjes‐Sanchez C, White RJ, Allen R, Torbicki A, Xu KF, Yehle D, Laliberte K, Arneson C, Rubin LJ. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624–33. 10.1161/circulationaha.112.124388 [DOI] [PubMed] [Google Scholar]
- 39. Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, Janssens W, Troosters T. The minimal important difference in physical activity in patients with COPD. PLoS One. 2016;11:e0154587. 10.1371/journal.pone.0154587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG, Paul R. Analysis of daily activity data from implanted cardiac defibrillators: the minimum clinically important difference and relationship to mortality/life expectancy. World Journal of Cardiovascular Diseases. 2012;2:129–35. 10.4236/wjcd.2012.23021 [DOI] [Google Scholar]
- 41. Teylan M, Kantorowski A, Homsy D, Kadri R, Richardson C, Moy M. Physical activity in COPD: minimal clinically important difference for medical events. Chron Respir Dis. 2019;16:147997311881642. 10.1177/1479973118816424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sitbon O, Nikkho S, Benza R, Deng C, Farber HW, Gomberg‐Maitland M, Hassoun P, Meier C, Pepke‐Zaba J, Prasad K, Seeger W, Corris PA. Novel composite clinical endpoints and risk scores used in clinical trials in pulmonary arterial hypertension. Pulm Circ. 2020;10:1–11. 10.1177/2045894020962960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez‐Sanchez M, Siedentop H, Seeger W. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. [DOI] [PubMed] [Google Scholar]
- 45. Bangash MN, Hodson J, Evison F, Patel JM, Johnston AM, Gallier S, Sapey E, Parekh D. Impact of ethnicity on the accuracy of measurements of oxygen saturations: a retrospective observational cohort study. EClinicalMedicine. 2022;48:101428. 10.1016/j.eclinm.2022.101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383:2477–78. 10.1056/NEJMc2029240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JW, Clark A, Birring SS, Atkins C, Whyte M, Wilson AM. Psychometric properties of patient reported outcome measures in idiopathic pulmonary fibrosis. Chron Respir Dis. 2021;18:147997312110339. 10.1177/14799731211033925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scallan C, Strand L, Hayes J, Kadura S, Collins B, Ho L, Spada C, Canestaro W, Kolb M, Raghu G. R‐scale for pulmonary fibrosis: a simple, visual tool for the assessment of health‐related quality of life. Eur Respir J. 2022;59:2100917. 10.1183/13993003.00917-2021 [DOI] [PubMed] [Google Scholar]
- 49. Andersen C, Mellemkjær S, Hilberg O, Bendstrup E. NT‐proBNP <95 ng/l can exclude pulmonary hypertension on echocardiography at diagnostic workup in patients with interstitial lung disease. Eur Clin Respir J. 2016;3:32027. 10.3402/ecrj.v3.32027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Durheim MT, Collard HR, Roberts RS, Brown KK, Flaherty KR, King TE, Palmer SM, Raghu G, Snyder LD, Anstrom KJ, Martinez FJ. Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials. Lancet Respir Med. 2015;3:388–96. 10.1016/s2213-2600(15)00093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown AW, Fischer CP, Shlobin OA, Buhr RG, Ahmad S, Weir NA, Nathan SD. Outcomes after hospitalization in idiopathic pulmonary fibrosis. Chest. 2015;147:173–179. 10.1378/chest.13-2424 [DOI] [PubMed] [Google Scholar]
- 52. Nathan SD, Deng C, King CS, DuBrock HM, Elwing J, Rajagopal S, Rischard F, Sahay S, Broderick M, Shen E, Smith P, Tapson VF, Waxman AB. Inhaled treprostinil dosage in pulmonary hypertension associated with interstitial lung disease and its effects on clinical outcomes. Chest. 2022;S0012–3692(22):03725–4. 10.1016/j.chest.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Colombat M, Mal H, Groussard O, Capron F, Thabut G, Jebrak G, Brugière O, Dauriat G, Castier Y, Lesèche G, Fournier M. Pulmonary vascular lesions in end‐stage idiopathic pulmonary fibrosis: histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol. 2007;38:60–5. 10.1016/j.humpath.2006.06.007 [DOI] [PubMed] [Google Scholar]


