Abstract
CD4+ T-helper type 1 (Th1) responses are essential for the resolution of a primary Chlamydia trachomatis genital tract infection; however, elements of the immune response that function in resistance to reinfection are poorly understood. Defining the mechanisms of immune resistance to reinfection is important because the elements of protective adaptive immunity are distinguished by immunological memory and high-affinity antigen recognition, both of which are crucial to the development of efficacious vaccines. Using in vivo antibody depletion of CD4+ and CD8+ T cells prior to secondary intravaginal challenge, we identified lymphocyte populations that functioned in resistance to secondary chlamydial infection of the genital tract. Depletion of either CD4+ or CD8+ T cells in immune wild-type C57BL/6 mice had a limited effect on resistance to reinfection. However, depletion of CD4+ T cells, but not CD8+ T cells, in immune B-cell-deficient mice profoundly altered the course of secondary infection. CD4-depleted B-cell-deficient mice were unable to resolve a secondary infection, shed high levels of infectious chlamydiae, and did not resolve the infection until 3 to 4 weeks following the discontinuation of anti-CD4 treatment. These findings substantiated a predominant role for CD4+ T cells in host resistance to chlamydial reinfection of the female genital tract and demonstrated that CD8+ T cells are unnecessary for adaptive immune resistance. More importantly, however, this study establishes a previously unrecognized but very significant role for B cells in resistance to chlamydial reinfection and suggests that B cells and CD4+ T cells may function synergistically in providing immunity in this model of chlamydial infection. Whether CD4+ T cells and B cells function independently or dependently is unknown, but definition of those mechanisms is fundamental to understanding optimum protective immunity and to the development of highly efficacious immunotherapies against chlamydial urogenital infections.
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that infects primarily ocular and urogenital mucosal epithelial cells. Over 400 million individuals worldwide are affected by ocular infection, and an estimated 90 million new cases of sexually transmitted chlamydial disease occur yearly. A variety of cellular and humoral immune responses are elicited following human and experimental animal chlamydial infections, but the precise roles of those immune responses in the resolution of chlamydial infection and protection from reinfection remain obscure.
Cell-mediated immune responses play a dominant role in the resolution of chlamydial genital tract infection. The use of adoptive transfer and monoclonal antibody-mediated in vivo depletion have clearly identified CD4+ T cells as a population of cells required for the resolution of genital tract infection in experimental models of chlamydial infection (21, 43). The pattern of cytokines produced by polyclonal populations of protective T cells and protective T-cell clones is consistent with Th1-type cells (15, 25, 29, 43). Lymphocytes isolated from the chlamydia-infected genital tract and homogenates of chlamydia-infected genital tract tissue demonstrate the predominance of Th1-type cytokine and mRNA, respectively (3, 31, 53). Furthermore, anticytokine antibodies that diminish Th2-type responses are beneficial and those that inhibit Th1-type responses are more detrimental (28).
Some of the more definitive studies regarding the contribution of various cell populations and cytokines in resolving a primary chlamydial infection have been those that have used specific gene knockout mice. Those genetic deletions that have a detrimental effect on the ability of the host to resolve a primary infection include strains of mice that lack major histocompatibility complex (MHC) class II molecules, T-cell receptor αβ (TCRαβ), or gamma interferon (IFN-γ) (8, 24, 28). Many other strains of gene knockout mice have been used, including those that affect Th1 and Th2 cytokines and the development of CD8+ cytotoxic T cells (14, 24, 28–30, 32), but none interfere with the development of protective immunity to the level of either MHC class II, TCRαβ, or IFN-γ gene knockout mice.
The importance of MHC class I-restricted cytotoxic CD8+ T cells in protective immunity to chlamydial infection is equivocal because of the discrepancy between the results of in vitro and in vivo studies. For example, in vitro cytotoxicity of chlamydia-infected target cells by CD8+ T cells obtained following both human and experimental animal models of chlamydial infection has been demonstrated (1, 19, 36, 38–40). The importance of those cells in resolving a chlamydial infection or protecting against reinfection, however, is much less certain. Some studies show that immune CD8+ T-cell populations are unable to confer any measurable protection (43), while others demonstrate a very limited protective effect (13, 40). The protection conferred by CD8+ T cells in vivo has been attributed to the production of the chlamydia-inhibitory cytokine IFN-γ rather than cytolysis (13, 20). Differences between the in vitro studies that demonstrated that CD8+ T cells are cytotoxic for chlamydia-infected cells (1, 19, 20, 36, 39, 40) and in vivo studies that failed to demonstrate a convincing role for those cells in protective immunity to chlamydial infection (13, 40, 43) have not been reconciled.
The role of specific antibody in murine and human chlamydial infections has evolved from being the focus of immune protection and vaccine development (2, 11, 26, 44, 47, 57, 58) to being unimportant or having a very subordinate role in protective immunity (16, 17, 45). The discovery of neutralizing monoclonal antibodies to chlamydiae increased interest in the potential role of antibody in protective immunity (57, 58), but interest waned when in vivo studies met with limited success (6, 26, 27, 44). Since then, little attention has been given to a possible role for antibody in protection from chlamydial infection. Arguments against a protective role for antibodies are numerous but generally include the following: (i) the obligate intracellular lifestyle of chlamydiae makes them inaccessible to antibodies, (ii) vaccines or vaccination regimens that elicit high titers of antibody are not protective, and (iii) cell-mediated immunity (CMI) protects. Interest has been recently renewed, however, with the findings that antibody-deficient animals are more susceptible to reinfection than antibody-positive animals (45, 54) and that antibody or B cells may contribute to protective immunity to other intracellular bacterial infections (5, 9, 10, 22, 52, 56). Moreover, it is known that specific antibody plays a substantive role in immunity in the guinea pig model of genital tract infection (33–35).
The use of gene knockout mice, the adoptive transfer of immune lymphocytes, and the manipulation of mice with specific antiantibodies (i.e., anti-CD4, -CD8, -cytokine, etc.) prior to primary infection are approaches currently used to evaluate the in vivo role of lymphocyte subpopulations in host resistance to infectious agents (12). All of those approaches have been used in the study of immunity to primary C. trachomatis infection and have advanced our understanding of the elements of the host's immune response that are needed for the development of adaptive immunity to chlamydial genital tract infection. However, studies that use those in vivo methods to investigate the contribution of lymphocyte populations to resistance to reinfection have not been forthcoming. In this study, we began to decipher the elements of the host immune response that contribute to adaptive immunity to secondary C. trachomatis genital tract infection. Our results suggest that B cells and CD4+ T cells, but not CD8+ T cells, are important elements of resistance to reinfection.
MATERIALS AND METHODS
Mice.
Female wild-type C57BL/6 (B6) mice were purchased from the National Cancer Institute (Bethesda, Md.) and maintained in the Animal Resources Center at Montana State University. B-cell-deficient (μMT) mice on a B6 genetic background (B6-Igh-6>tm1Cgn) were purchased from Jackson Laboratories (Bar Harbor, Maine) and bred and maintained in the Animal Resources Center at Montana State University. Female mice 8 to 15 weeks old were used throughout this study.
Bacterial growth, purification, and enumeration.
The mouse pneumonitis (MoPn) biovar of C. trachomatis was grown in HeLa 229 cells and purified by discontinuous density gradient centrifugation, and the infectivity titer was determined on HeLa cell monolayers as previously described (4, 24).
In vivo depletion.
Hybridomas secreting monoclonal antibodies used for the in vivo depletion of T-cell subpopulations were clones GK1.5 (anti-CD4) and 2.43 (anti-CD8). Hybridomas were purchased from the American Type Culture Collection and grown in serum-free medium, and antibodies were concentrated and purified by ammonium sulfate precipitation. Purified rat immunoglobulin G (IgG) (Sigma, St. Louis, Mo.) was used as an irrelevant antibody control for in vivo depletion experiments.
In vivo depletion of CD4+ and CD8+ T cells was accomplished by injecting groups of mice intraperitoneally (i.p.) with 0.5 mg of either anti-CD4 or anti-CD8 monoclonal antibody for 3 consecutive days, followed by injections every third day, with the final injection being administered at 23 days. Therefore, mice were injected with antibody on days 50, 51, 52, 55, 58, 61, 64, 67, 70, and 73 post-primary infection. Throughout the course of each experiment, each animal received a total of 5 mg of antibody. Groups of control mice were injected i.p. with either 0.5 mg of purified rat Ig or 0.5 ml of phosphate-buffered saline (PBS; 10 mM phosphate, 0.13 M NaCl, pH 7.4) following the same injection schedule. Depletion of specific lymphocyte subsets was monitored as described below.
Genital tract infection and enumeration of chlamydiae.
To evaluate the effect of the in vivo depletion of T-cell subpopulations on the ability of immune mice to resist a secondary infectious challenge, immune mice were depleted of either CD4+ or CD8+ T cells and rechallenged with infectious chlamydiae and the course of infection was monitored as previously described (24). The experimental design described below was used for both B6 and B-cell-deficient mice. Forty mice (B6 or B-cell-deficient) were injected subcutaneously with 2.5 mg of Depo-Provera (medroxyprogesterone acetate) 5 days prior to intravaginal inoculation of 5 × 104 inclusion forming units (IFU) of C. trachomatis, an inoculum equivalent to 100 50% infectious doses (ID50). The course of infection was monitored in groups of five mice by enumerating the number of IFU recovered from cervicovaginal swabs using indirect immunofluorescence (24). Fifty days following primary infection, a time at which mice had resolved the infection and had acquired a level of resistance to reinfection (45), four groups of immune mice consisting of 10 mice each were treated with either anti-CD4, anti-CD8, rat Ig, or 10 mM PBS (pH 7.2) as described above. Five days prior to a secondary challenge, mice were treated with Depo-Provera as described above. At 56 days following the primary infection, mice were rechallenged vaginally with 100 ID50 of C. trachomatis MoPn and the course of this secondary infection was monitored by enumerating infectious chlamydiae from cervicovaginal swabs (24). Also, at the time of the secondary infectious challenge, a group of 10 naive mice were infected; these served as naive control animals for the secondary challenge. Each group of 10 mice was further divided as follows. Five mice were used to monitor the course of infection (vaginal cultures), and three and two mice were sacrificed on days 3 and 7 following the secondary challenge, respectively. Their genital tracts were removed for immunohistochemical staining (see below), and the spleens were removed for lymphocyte cultures (see cytokines below) and for enumeration of T-cell subpopulations.
Immunohistochemistry.
Immunohistochemistry was used to monitor in vivo depletion of lymphocyte subsets (25). Blood, splenocytes, and genital tract tissue were collected at the indicated times and processed as described below for immunohistological staining. Thin smears of blood and splenocytes were prepared on Superfrost slides (Fisher Scientific, Santa Clara, Calif.), air dried, fixed in acetone for 5 min, and then rehydrated in PBS. Genital tracts were removed, placed in OCT embedding medium (Tissue-Tek, Sakura Finetek, Torrance, Calif.), and snap frozen in dry-ice-cooled 2-methylbutane. Cryostat sections, 5 μm thick, were placed onto Superfrost slides, air dried, fixed in acetone for 5 min at room temperature, and then rehydrated in PBS. Slides containing blood smears, splenocytes, or genital tract tissue were subsequently processed and stained at room temperature as follows. Endogenous peroxidase activity was blocked by incubating the tissues in Peroxo-Block (Zymed Laboratories, San Francisco, Calif.) for 40 s. Slides were washed in PBS for 5 min and blocked with avidin-biotin containing 5% normal goat serum (Vector Laboratories, Burlingame, Calif.) following the manufacturer's protocol. Following a 5-min rinse with PBS, slides were incubated for 1 h with anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), or control (rat IgG2a, clone R35-95) monoclonal antibodies (Pharmingen, San Diego, Calif.) diluted 1/500 in Hank balanced salt solution containing 1% normal goat serum, corresponding to the species from which the secondary antibody was derived. Slides were rinsed in PBS for 5 min and then incubated for 30 min with biotinylated goat anti-rat IgG secondary antibody diluted in Hanks balanced salt solution with 1% normal goat serum. Slides were rinsed with PBS, incubated with Vectastain ABC complex (Vector Laboratories) for 30 min, and washed with PBS, and color was developed by adding 3,3′-diaminobenzidine (Vector Laboratories) substrate. Sections were then counterstained with hematoxylin, rinsed with distilled water, cleared with xylene, and mounted with Permount (Fisher Scientific).
Cytokine production by immune splenocytes.
Chlamydia-specific cytokine responses of splenocyte cultures were assayed as previously described (46). Briefly, 107 splenocytes, pooled from two or three mice, were mixed with 4 × 107 heat-killed (56°C, 30 min) MoPn elementary bodies (EBs) at 37°C for 90 min and then added to 24-well tissue culture plates and incubated for 72 h at 37°C. Supernatants were then collected and analyzed by enzyme-linked immunosorbent assay (OptEIA; Pharmingen) for interleukin-4 (IL-4), IL-10, IL-12, and IFN-γ following the manufacturer's instructions.
Statistical analysis.
Student's t test of log-transformed data was used to analyze differences between IFU counts of control and experimental groups and to determine differences between cytokine responses.
RESULTS
Duration of in vivo depletion.
To evaluate the importance of immune CD4+ and CD8+ T cells in resistance to secondary chlamydial infection, it would be necessary to maintain the depletion of T-cell subsets for the duration of a typical infection (3 to 4 weeks). To determine the extent and duration of depletion following our treatment schedule, four groups of three mice each were treated with either anti-CD4, anti-CD8, rat Ig, or PBS as described in Materials and Methods. At weekly intervals, mice were bled and the percentages of CD4+ and CD8+ T cells were determined by immunoperoxidase staining (Table 1). Mice treated with anti-CD4 antibody were depleted of peripheral blood CD4+ T cells throughout the duration of the antibody treatment (23 days), and the level of CD4+ T cells in peripheral blood did not return to normal until ∼2 to 3 weeks following the final injection. Similarly, anti-CD8 treatment effectively eliminated CD8+ T cells from the peripheral blood throughout the duration of antibody treatment but CD8+ T-cell levels remained diminished even at 36 days following the last injection. Mice treated with either rat Ig or PBS consistently had normal levels of CD4+ and CD8+ T cells (19 and 12%, respectively) in the peripheral blood. Thus, using the schedule of antibody injections described, we could effectively maintain depletion of CD4+ and CD8+ T-cell subsets for at least 4 to 5 weeks.
TABLE 1.
Maintenance of lymphocyte subpopulation depletion in noninfected B6 micea
| In vivo treatmentc | % Positive staining cells at indicated day following initiation of antibody treatmentb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
7
|
14
|
21
|
28
|
35
|
42
|
59
|
|||||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| Anti-CD4 | 16 | 11 | 2 | 18 | 1 | 18 | 0 | 16 | 0 | 16 | 5 | 19 | 10 | 21 | 15 | 16 |
| Anti-CD8 | 20 | 12 | 20 | 1 | 22 | 1 | 25 | 0 | 22 | 1 | 22 | 1 | 30 | 4 | 25 | 9 |
Groups of mice were treated i.p. with either anti-CD4 or anti-CD8 on days 0, 1, 2, 5, 8, 11, 14, 17, 20, and 23. At the indicated days, blood smears were prepared and lymphocyte subpopulations were enumerated by immunoperoxidase staining. Details of the experimental protocols are provided in Materials and Methods.
Data are presented as the mean percentage of either CD4+ or CD8+ T cells from three mice per treatment group.
Groups of mice were also treated with either buffer or rat Ig. The proportions of CD4+ and CD8+ T cells in those mice did not change appreciably and remained at approximately 19 and 12%, respectively.
Secondary chlamydial genital tract infection in CD4-depleted or CD8-depleted wild-type B6 mice.
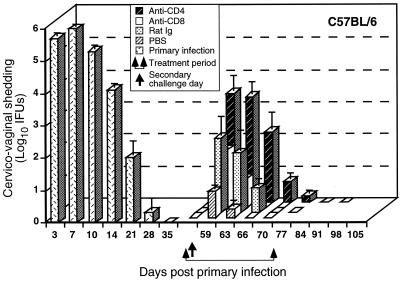
Previous studies have shown that adaptive immunity to chlamydial genital tract infection develops in B6 mice following the resolution of primary infection and that CD4+ T cells and Th1-type cytokines are important in that response (28, 46). The protective response is characterized by a >4- to 5-log10 reduction in chlamydial shedding and an infection of much shortened duration (24, 45). To begin to identify the cell populations involved in host immunity to reinfection, we analyzed the effect of eliminating subpopulations of immune CD4+ and CD8+ T cells on the outcome of a secondary chlamydial infection (Fig. 1). Genital tract inoculation of naive B6 mice with C. trachomatis MoPn resulted in a self-limiting infection that resolved by approximately 4 weeks. Following resolution of the primary infection, a significant level of protective immunity to a secondary infection developed, as shown by decreased shedding of chlamydiae (>5 log10) and an infection of much shorter duration (Fig. 1, PBS treated). Treatment of immune mice with either rat Ig or anti-CD8 antibody had a minimal effect on the resolution of a secondary infection. Secondary infections of both rat Ig- and anti-CD8-treated groups of immune mice resolved by 10 to 14 days postchallenge and were characterized by the shedding of 3.5 to 4 log10 fewer chlamydiae at all of the times analyzed. The depletion of CD4+ T cells had a limited effect on protective immunity. A secondary challenge of CD4-depleted immune mice produced an infection that took longer to resolve than a secondary infection in other treatment groups. However, most of the anti-CD4-treated mice resolved the secondary infection by 2 weeks postchallenge (only one of five mice remained culture positive for >2 weeks). CD4-depleted infected mice shed higher numbers of chlamydiae than other treatment groups but significantly fewer organisms than noted during primary infection (>2 log10 fewer). Both CD4-depleted and CD8-depleted groups of mice resolved the infection despite continued depletion of the CD4+ and CD8+ T-cell subpopulations, respectively (Table 1). Thus, depletion of CD4+, but not CD8+, immune T cells affects the course of a secondary infection but does not compromise the ability of animals to resolve a secondary infection.
FIG. 1.
Effect of anti-CD4 or anti-CD8 treatment on the resolution of secondary C. trachomatis genital tract infection of wild-type B6 mice. Mice were infected vaginally with 100 ID50 of C. trachomatis, and the course of the primary infection was monitored. Following resolution of the primary infection, immune mice were divided into four groups of five mice each and treated with either anti-CD4, anti-CD8, rat Ig, or PBS as described in Materials and Methods. Treated mice were challenged vaginally with 100 ID50 of C. trachomatis on day 56 following primary infection, and the infection was monitored by swabbing the vaginal vault and enumerating IFU on HeLa cell monolayers. Data are presented as log10 IFU and represent the mean ± the standard error of the mean of triplicate determinations of five mice. P < 0.05 for anti-CD4-, anti-CD8-, rat Ig-, and PBS-treated mice compared to primary infection of mice at days 3, 7, 10, and 14 post infectious challenge.
Secondary chlamydial genital tract infection in CD4-depleted or CD8-depleted B-cell-deficient mice.
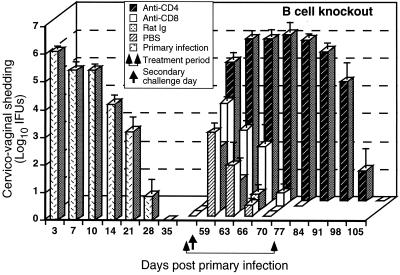
B6 mice resolved their infections despite depletion of CD4+ or CD8+ T cells. We reasoned that immune B cells may have contributed to the protection of T-cell-depleted animals. To assess the contribution of B cells to immunity to reinfection, the course of a secondary genital tract infection was evaluated in CD4+ T-cell-depleted or CD8+ T-cell-depleted B-cell-deficient mice. Primary chlamydial genital tract infection of B-cell-deficient mice resulted in a self-limiting infection that resolved within 5 weeks (Fig. 2), and the level of chlamydial shedding was not significantly different from that of wild-type B6 mice (Fig. 1). As noted previously (45), B-cell-deficient mice were more susceptible to reinfection (Fig. 2, PBS treated) than wild-type B6 mice (Fig. 1, PBS treated). However, B-cell-deficient mice developed a level of protective immunity following primary infection, as indicated by the shortened duration of the secondary infection. Depletion of CD8+ T cells in immune B-cell-deficient mice had no significant effect on the course of a secondary infection (Fig. 2), whereas depletion of CD4+ T cells resulted in a much prolonged course of a secondary infection that did not resolve until 4 weeks following the discontinuation of anti-CD4 treatment (Fig. 2, anti-CD4 treated). CD4-depleted immune B-cell-deficient mice continued to shed high numbers of chlamydiae (∼106 IFU) throughout anti-CD4 treatment and for 3 weeks after discontinuation of antibody treatment. Resolution of the infection in those mice coincided with the return of CD4+ T cells (Table 1). The shedding of such high numbers of chlamydiae during the duration of anti-CD4 treatment was very reminiscent of the type of infection that is seen in MHC class II and TCRαβ strains of gene knockout mice (24, 28). Our results argue that immune B cells and CD4+ T cells, functioning dependently or independently, play important roles in adaptive immunity to chlamydial genital tract infection and that immune CD8+ T cells are inconsequential.
FIG. 2.
Effect of anti-CD4 or anti-CD8 treatment on the resolution of a secondary C. trachomatis genital tract infection of B-cell-deficient mice. Mice were infected vaginally with 100 ID50 of C. trachomatis, and the course of the primary infection was monitored. Following resolution of the primary infection, immune mice were divided into four groups of five mice each and treated with either anti-CD4, anti-CD8, rat Ig, or PBS as described in Materials and Methods. Treated mice were challenged vaginally with 100 ID50 of C. trachomatis on day 56, and the infection was monitored by swabbing the vaginal vault and enumerating IFU on HeLa cell monolayers. Data are presented as log10 IFU and represent the mean ± the standard error of the mean of triplicate determinations of five mice. P < 0.05 for anti-CD8-, rat Ig-, and PBS-treated mice compared to primary infection of mice at days 3, 7, 10, 14, and 21 days post infectious challenge. P < 0.05 for anti-CD4-treated mice compared to primary infection of mice at days 3, 14, 21, 28, 35, and 42 post infectious challenge.
In vivo depletion of T-cell subpopulations in chlamydia-infected wild-type B6 mice and B-cell-deficient mice.
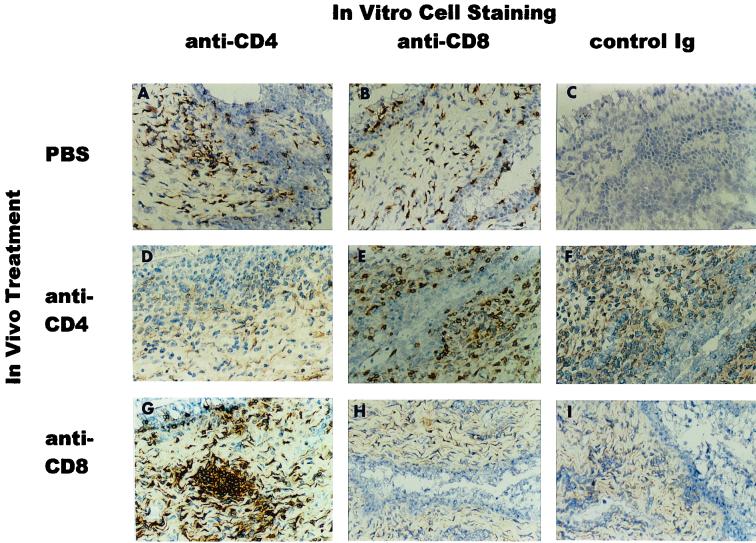
We demonstrated that peripheral blood CD4+ and CD8+ T-cell populations could be depleted for at least 4 weeks in naive, noninfected mice (Table 1). However, because CD4-depleted and CD8-depleted B6 mice and CD8-depleted B-cell-deficient mice resolved a secondary chlamydial genital tract infection, it was necessary to confirm that those groups of mice resolved the infection in the absence of the indicated cell population. To confirm the depletion of CD4+ and CD8+ T cells, peripheral blood, splenocytes, and genital tract tissue taken from groups of mice at various times following a secondary infectious challenge were analyzed. Analysis of peripheral blood and splenocyte cell populations from B6 and B-cell-deficient mice following a secondary infection demonstrated effective depletion of CD4+ and CD8+ T cells (Table 2). In addition, during the course of a secondary infection, genital tract tissue from groups of anti-CD4-, anti-CD8-, or PBS-treated mice were analyzed by immunohistochemistry for CD4+ and CD8+ T cells. Anti-CD4 and anti-CD8 treatment of B6 and B-cell-deficient mice during a secondary infection effectively depleted genital tract tissue of CD4+ and CD8+ T cells, respectively (Fig. 3 and 4). CD4+ T cells and CD8+ T cells were found scattered throughout the mucosal epithelium and submucosa of PBS-treated mice, and CD4+ T cells tended to form perivascular clusters. CD4+ T cells were not observed in genital tract tissue of anti-CD4-treated mice, and only a rare CD8+ T cell was observed in genital tract tissue of anti-CD8-treated B6 mice (Fig. 3). A similar pattern of localization and depletion of CD4+ and CD8+ T cells was found in B-cell-deficient mice (Fig. 4). Therefore, the resolution of a secondary infection in either anti-CD4-treated or anti-CD8-treated B6 mice or in anti-CD8-treated B-cell-deficient mice was not attributable to ineffective depletion of specific cell populations.
TABLE 2.
In vivo depletion of T-cell subpopulations in C. trachomatis-infected B6 and B-cell deficient micea
| In vivo treatmentb | B6 mice
|
B-cell-deficient mice
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3
|
Day 7
|
Day 3
|
Day 7
|
|||||||||||||
| Blood
|
Spleen
|
Blood
|
Spleen
|
Blood
|
Spleen
|
Blood
|
Spleen
|
|||||||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| Anti-CD4 | 0 | 15 | 0 | 17 | 0 | 19 | 0 | 15 | 1 | 32 | 0 | 30 | 2 | 22 | 0 | 41 |
| Anti-CD8 | 30 | 1 | 27 | 0 | 27 | 2 | 25 | 0 | 60 | 0 | 50 | 0 | 55 | 0 | 48 | 1 |
Groups of B6 and B-cell-deficient mice that had resolved a primary C. trachomatis genital tract infection were injected i.p. with either anti-CD4 or anti-CD8 antibody as described in Materials and Methods. Mice were administered a secondary infectious challenge on day 56 post primary infection. Anti-CD4 or anti-CD8 antibody was administered on days 50, 51, 52, 55, 58, 61, 64, 67, 70, and 73. Mean percentages of cells that stained positively for CD4 or CD8 in the peripheral blood and in the splenocyte population of antibody-treated mice at 3 and 7 days post secondary infectious challenge are shown (three mice per determination).
Treatment of B6 or B-cell-deficient mice with buffer or rat Ig did not affect the distribution of CD4+ or CD8+ T cells in the peripheral blood or splenocyte population. The proportions of CD4+ and CD8+ T cells in the peripheral blood and spleens of infected B6 mice treated with either buffer or rat Ig were approximately 30 and 18%, respectively. For infected B-cell-deficient mice, the percentages of CD4+ and CD8+ T cells were approximately 42 and 35%, respectively.
FIG. 3.
Tissue depletion of CD4+ and CD8+ T cells following secondary infectious challenge of antibody-treated wild-type B6 mice. Seven days following a secondary C. trachomatis infection, mice were sacrificed and their genital tracts were removed and processed for immunohistochemical analysis. In vivo treatment: PBS (A to C), anti-CD4 (D to F), and anti-CD8 (G to I). In vitro immunohistochemical staining: anti-CD4 (A, D, and G), anti-CD8 (B, E, and H), and control rat Ig (C, F, and I). Representative tissues from two or three mice are shown. Tissues harvested at 3 days post secondary infectious challenge showed a similar staining pattern. Original magnification, ×300.
FIG. 4.
Tissue depletion of CD4+ and CD8+ T cells following secondary infectious challenge of antibody-treated, B-cell-deficient mice. Seven days following secondary C. trachomatis infection, mice were sacrificed and their genital tracts were removed and processed for immunohistochemical analysis. In vivo treatment: PBS (A to C), anti-CD4 (D to F), and anti-CD8 (G to I). In vitro immunohistochemical staining: anti-CD4 (A, D, and G), anti-CD8 (B, E, and H), and control rat Ig (C, F, and I). Representative tissues from two or three mice are shown. Tissues harvested at 3 days post secondary infectious challenge showed a similar staining pattern. Original magnification, ×300.
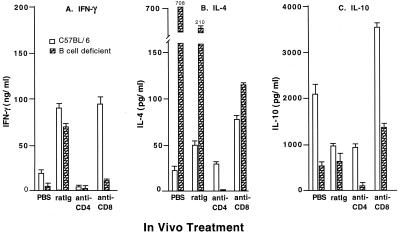
Perturbations in the splenic cytokine response of CD4- or CD8-depleted B6 and B-cell-deficient mice.
Cytokines have been shown to play an important role in the development of adaptive immunity to chlamydial genital tract infection (8, 28). To identify changes in the chlamydia-specific cytokine response of immune mice depleted of either CD4+ or CD8+ T cells, splenic lymphocytes were cultured in the presence of heat-killed chlamydiae and specific cytokine production was evaluated by analyzing culture supernatants by enzyme-linked immunosorbent assay (Fig. 5). Compared to splenocytes from PBS-treated B6 mice, splenocytes from rat Ig-treated mice produced increased amounts of IFN-γ and IL-4 and decreased amounts of IL-10. Anti-CD4 treatment diminished the IFN-γ and IL-10 responses but not the IL-4 response. Anti-CD8 treatment resulted in increased production of IFN-γ, IL-4, and IL-10. Splenocytes from PBS-treated, immune, B-cell-deficient mice produced very high levels of IL-4, and treatment with anti-CD4 nearly abolished the IL-4 response and anti-CD8 treatment reduced the response. Anti-CD4 treatment of B-cell-deficient mice diminished the IL-10 response, whereas anti-CD8 was minimally enhancing. Splenocytes from PBS-treated B-cell-deficient mice produced less IFN-γ than did those of B6 mice, and IFN-γ production was only minimally affected by anti-CD4 or anti-CD8 treatment. IL-12 was not detected in any of the lymphocyte cultures (data not shown). This analysis demonstrates alterations in the in vitro cytokine response of treated animals, but it does not specifically address the importance of those alterations in adaptive immunity. Additional studies are necessary to determine if the alterations in the cytokine responses merely reflect the in vivo depletion of specific cell populations or whether the alterations contribute directly to the observed changes in adaptive immunity.
FIG. 5.
In vitro production of IFN-γ (A), IL-4 (B), and IL-10 (C) by spleen cells from wild-type B6 and B-cell-deficient mice following in vivo treatment with either PBS, rat Ig, anti-CD4, or anti-CD8. Spleens were harvested from two or three mice per group 7 days after secondary infection with C. trachomatis and restimulated in vitro with heat-killed chlamydiae. The values shown are the concentrations of cytokines (mean ± standard deviation of triplicate determinations) present in the supernatants after 72 h of culture. At 24 and 72 h, supernatants were also analyzed for IL-12 but IL-12 was never detectable.
DISCUSSION
Gene knockout mice have proven valuable in the identification of immunological mediators and cellular responses necessary for the development of protective immunity to chlamydial infection in naive mice (8, 14, 16, 24, 28–30, 32). An equally important but as yet unanswered question regarding protective immunity to chlamydial infection is the contribution of specific cell populations and immunological mediators in adaptive immune resistance to a secondary infectious challenge. Animal models clearly establish that marked resistance to reinfection develops following the resolution of a primary infection (24, 45). Since vaccination is a reasonable approach for the prevention of chlamydial urogenital infections, it is of fundamental importance that the cellular and humoral mediators of acquired resistance to reinfection be identified. In this study, we addressed the role of T cells and B cells in resistance to a secondary infection by manipulating the immune response of wild-type and B-cell gene knockout mice prior to a secondary infectious challenge. Our data further substantiate the prominent role of CD4+ T cells in protective immunity to chlamydial genital tract infection by extending their importance to include acquired immune resistance to reinfection. Furthermore, we document a previously unappreciated role for B cells in immunity to reinfection and convincingly demonstrate that immune CD8+ T cells are inconsequential for protective immunity to secondary infection.
Previous studies of chlamydial infections in humans and mice provide evidence that specific antibody or B cells confer a level of protective immunity to chlamydial infection, but that general conclusion is arguable because the data are indirect or only demonstrate a subordinate role for antibody (2, 6, 17, 27, 45, 57). Those studies invariably documented only a limited protective effect of antibody in vivo; therefore, we reasoned that the dominant protective CD4+ T-cell response possibly masked the detection of a protective humoral response. To address that premise, we eliminated CD4+ T cells in immune wild-type B6 and B-cell-deficient mice and assessed immunity to reinfection. We clearly demonstrate that B cells and/or antibodies are important constituents of the adaptive immune response to chlamydial urogenital infection (Fig. 1 and 2).
Arguments against a protective role for antibodies are numerous but generally include the following: (i) the obligate intracellular lifestyle of chlamydiae renders them inaccessible to antibodies, (ii) vaccination regimens eliciting high titers of antibody are not protective, and (iii) CMI confers protection. Several recent studies have renewed interest in evaluating the contribution of antibodies and B cells to immunity to intracellular microbial pathogens. Antibody imparts a level of protective immunity to intracellular cryptococcal infection (5) and to infection by the intracellular bacterial pathogen Mycobacterium tuberculosis (52). Although the mechanism by which antibody augmented immunity to M. tuberculosis was not defined, increased protection was thought to result from antibody enhancement of CMI. B cells have also been implicated in immunity to the intracellular bacterial pathogens Francisella tularensis (10) and Salmonella typhimurium (23). Other studies did not directly implicated B cells in the effector immune response to intracellular bacterial pathogens but instead suggested that B cells are important for priming protective T-cell responses (22, 56).
We previously assessed the role of Th cells in the immune response to chlamydial genital tract infection (24). We noted that after primary chlamydial genital tract infection, mice deficient in the CD4 cell surface molecule (CD4 gene knockout mice) resolved the infection more slowly than wild-type B6 mice. The production of both serum and mucosal antichlamydial IgA antibodies, but not IgG antibodies, was delayed in CD4-deficient mice. The delay in resolution of the primary infection coincided with delayed production of mucosal antichlamydial IgA antibodies. We concluded that a correlation exists between mucosal antichlamydial IgA antibodies and resolution of infection. In our current study, we demonstrated that B cells or antibody played a critical role in adaptive immunity to chlamydial genital tract infection. The mechanism(s) by which antibody or B cells contributed to adaptive immunity in murine genital tract infection is not understood, but our past and current experimental results have renewed interest in and lend support to continued investigations of the interaction of B-cell immunity and CMI.
A reasonable inference from our data is that B cells and CD4+ T cells function synergistically in the protective immune response to chlamydial infection. Although our data do not address a specific mechanism, (i) recognition of chlamydial antigen on the infected-cell surface by a specific antibody and subsequent lysis of the infected cell by an antibody-dependent cellular cytotoxicity (ADCC) mechanism and (ii) arming of an immune cell population with a specific antibody which subsequently inactivates chlamydial EBs are two possible mechanisms. There exists no precedent that those mechanisms function in immunity to chlamydial infection. However, several laboratories have demonstrated cell surface localization of chlamydial antigens (18, 37, 42, 55), thus establishing the precedent that chlamydial antigen or a form of antigen may be expressed on the surface of infected cells and accessible to immune mechanisms. ADCC-type immune mechanisms have been shown to function in host immunity to other intracellular bacterial pathogens. For example, in a series of studies, Tagliabue et al. addressed the role of secretory IgA-dependent cellular cytotoxicity as an immune mechanism that killed the facultative intracellular pathogens Shigella sp. and Salmonella sp. (48–51). Both Salmonella and Shigella spp. were inhibited by an ADCC-type mechanism, which was dependent on CD4+ T cells and specific IgA antibodies. Because chlamydial infection is mucosal and generates both antichlamydial IgA and immunoprotective CD4+ T cells (24), it may be that protective immunity to chlamydial infection is mediated by a similar ADCC mechanism.
The function of CD8+ cytotoxic T lymphocytes (CTLs) in protective immunity to chlamydial infection is controversial. That controversy arises from in vivo data that fail to show a convincing protective role for CTLs (13, 24, 40, 43) and in vitro data which consistently document CTL cytotoxicity against chlamydia-infected targets (1, 19, 20, 36, 39, 40). The in vivo studies that fail to show a protective role for CD8+ T cells are not based solely on a single experimental approach, yet the data are surprisingly consistent. (i) The adoptive transfer of immune CD4+ T cells, but not immune CD8+ T cells, to naive mice confers a level of protective immunity upon infectious challenge (43). (ii) CD4+ T-cell clones and lines confer a greater level of protective immunity on naive recipients than do CD8+ T-cell clones and lines (13, 15, 40). (iii) The resolution of a chlamydial genital tract infection in β2-microglobulin gene knockout mice is indistinguishable from that in wild-type mice (24). (iv) Mice genetically deficient in perforin, Fas, Fas ligand, or both perforin and Fas ligand resolve the infection similarly to wild-type mice (30). In this latter study, the fact that mice deficient in both Fas ligand and perforin did not display altered kinetics of bacterial clearance is of particular importance because it argues against both CD8+ CTL cytotoxicity and CD4+ T-cell-mediated apoptosis as protective mechanisms in immunity to chlamydial genital tract infection. Herein we report that the presence or absence of immune CD8+ T cells was inconsequential with regard to protective immunity. Thus, our data add another level of confidence for the protective role of CD4+ T cells in adaptive immunity to chlamydial genital tract infection and convincingly demonstrate that CD8+ T cells are neither required nor necessary for protective immunity.
In striking contrast to the convincing in vivo data that argue against protective CD8+ T cells, numerous in vitro studies have consistently demonstrated that immune CD8+ T cells are cytolytic for chlamydia-infected target cells (1, 19, 20, 36, 39, 40). Those data are derived from a number of different laboratories using different CD8+ T-cell and target cell populations. For example, immune murine splenic CD8+ T cells, murine CD8+ T-cell lines and clones, and human CD8+ T cells have been utilized to demonstrate antichlamydial CTL activity and some studies have shown that the responses are restricted by MHC class I molecules. Although differences exist among the details of the various studies, a common theme that has emerged is that cytolysis occurs rather late in the chlamydial growth cycle. Intracellular chlamydial growth and development are unique among bacterial pathogens. Early during intracellular growth (<24 h), chlamydiae exist as metabolically active, noninfectious replicative forms termed reticulate bodies. As intracellular infection progresses (24 to 72 h), reticulate bodies differentiate into metabolically inactive infectious forms termed EBs. Thus, for CTLs to be protective, an early lytic event which would eliminate infected cells and disperse only noninfectious chlamydiae would be much more beneficial than a late lytic event which would release infectious chlamydiae and potentially facilitate the infectious process. Nevertheless, because of the frequency with which antichlamydial CD8+ CTLs have been documented, it is difficult to argue that CD8+ CTLs are not generated following chlamydial infection.
An apparent discrepancy appears to exist between the in vivo data that argue against a protective role for CTLs and the in vitro data that support a protective function. The answer to that apparent contradiction may be provided by recent findings by Zhong et al., who demonstrated that chlamydiae secrete a proteosome-like activity into the host cell cytosol that suppresses IFN-γ-inducible MHC class I and II expression (59, 60). Therefore, despite the possible secretion of chlamydial molecules into the cytosol via a type III secretion system (41), the suppression of MHC class I synthesis would circumvent the processing and presentation of chlamydial epitopes on the infected-cell surface and preclude recognition and subsequent lysis by CTLs. This novel parasite strategy was proposed by Zhong et al. to be an important mechanism which allows chlamydiae to evade host immune recognition and facilitates the establishment of persistent infections (60). In the murine model of chlamydial genital tract infection, however, the infection resolves in ∼4 weeks and long-term persistent infections rarely occur in animals having a full complement of immune responses (7, 24). Alternatively, though, the data from the study by Zhong et al. (60) provide a plausible explanation by which CTLs do not function as a protective T-cell phenotype in vivo, as reported in this paper and by others (43). Despite this novel mechanism of immune evasion, the host has evolved alternative immune strategies that function very effectively to eliminate infection in the absence of CTLs.
Our data confirm the important role of CD4+ T cells in protective immunity to chlamydial genital tract infection and describe a previously unappreciated protective role for B cells and/or antibody. Although we do not understand the mechanism(s), our data raise a number of questions regarding the role of B cells, antibody, and CD4+ T cells in immunity to chlamydial infection that require further investigation. Furthermore, we show that CD8+ T cells are not necessary for adaptive immunity to chlamydial genital tract infection, suggesting that the host has devised effector strategies other than CD8+ CTLs for eliminating an intracellular infection and for providing immunity against reinfection. Definition of B-cell and CD4+ T-cell interactions resulting in antichlamydial responses and how those responses contribute to immune protection will be fundamental for the development of an efficacious vaccine against chlamydial urogenital infections.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant AI 38991 (R.P.M.).
REFERENCES
- 1.Beatty P R, Stephens R S. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–4595. [PubMed] [Google Scholar]
- 2.Brunham R C, Kuo C-C, Cles L, Holmes K K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983;39:1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 6.Cotter T W, Meng Q, Shen Z-L, Zhang Y-X, Su H, Caldwell H D. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter T W, Miranpuri G S, Ramsey K H, Poulsen C E, Byrne G I. Reactivation of chlamydial genital tract infection in mice. Infect Immun. 1997;65:2067–2073. doi: 10.1128/iai.65.6.2067-2073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelson B T, Cossart P, Unanue E R. Paradigm revisited: antibody provides resistance to Listeria infection. J Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 10.Elkins K L, Bosio C M, Rhinehart-Jones T R. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Fancisella tularensis live vaccine strain. Infect Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaem-Maghami S, Hay P E, Lewis D J M. Antigen and isotype specificity of human B cell responses in immunopathogenesis of genital Chlamydia trachomatis infection. Infect Dis Obstet Gynecol. 1996;4:179–180. [Google Scholar]
- 12.Harty J T, Tvinnereim A R, White D W. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 13.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme J U, Perry L L, Ananaba G A, Uriri I M, Ojior O O, Kumar S H, Caldwell H D. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect Immun. 1998;66:1282–1286. doi: 10.1128/iai.66.4.1282-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Regional Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 16.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson M, Ward M, Lycke N. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology. 1997;92:422–428. doi: 10.1046/j.1365-2567.1997.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimi S T, Schloemer R H, Wilde C E., III Accumulation of chlamydial lipopolysaccharide antigen in the plasma membranes of infected cells. Infect Immun. 1989;57:1780–1785. doi: 10.1128/iai.57.6.1780-1785.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S-K, Angevine K, Demick K, Ortiz L, Rudersdorf R, Watkins D, DeMars R. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J Immunol. 1999;162:6855–6866. [PubMed] [Google Scholar]
- 20.Lampe M F, Wilson C B, Bevan M J, Starnbach M N. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun. 1998;66:5457–5461. doi: 10.1128/iai.66.11.5457-5461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landers D V, Erlich K, Sung M, Schachter J. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect Immun. 1991;59:3774–3777. doi: 10.1128/iai.59.10.3774-3777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki G, Vordermeier H M, Hashimoto A, Nomoto K, Ivanyi J. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell Immunol. 1999;194:178–785. doi: 10.1006/cimm.1999.1503. [DOI] [PubMed] [Google Scholar]
- 23.Mittrucker H-W, Raupach B, Kohler A, Kaufmann S H E. Role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 24.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison S G, Morrison R P. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect Immun. 2000;68:2870–2879. doi: 10.1128/iai.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murdin A D, Su H, Klein M H, Caldwell H D. Poliovirus hybrids expressing neutralization epitopes from variable domains I and IV of the major outer membrane protein of Chlamydia trachomatis elicit broadly cross-reactive C. trachomatis-neutralizing antibodies. Infect Immun. 1995;63:1116–1121. doi: 10.1128/iai.63.3.1116-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal S, Theodor I, Peterson E M, de la Maza L. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 28.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 29.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry L L, Feilzer K, Hughes S, Caldwell H D. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infect Immun. 1999;67:1379–1385. doi: 10.1128/iai.67.3.1379-1385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-γ-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 32.Ramsey K H, Miranpuri G S, Poulsen C E, Marthakis N B, Braune L M, Byrne G I. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect Immun. 1998;66:835–838. doi: 10.1128/iai.66.2.835-838.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rank R G, Barron A L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983;39:463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rank R G, Batteiger B E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989;57:299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rank R G, White H J, Barron A L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979;26:573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen S J, Timms P, Beatty P R, Stephens R S. Cytotoxic-T-lymphocyte-mediated cytolysis of L cells persistently infected with Chlamydia spp. Infect Immun. 1996;64:1944–1949. doi: 10.1128/iai.64.6.1944-1949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richmond S J, Stirling P. Localization of chlamydial group antigen in McCoy cell monolayers infected with Chlamydia trachomatis or Chlamydia psittaci. Infect Immun. 1981;34:561–570. doi: 10.1128/iai.34.2.561-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieper J, Kingsley G, Palacios-Boix A, Pitzalis C, Treharne J, Hughes R, Keat A, Panayi G S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991;34:588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- 39.Starnbach M N, Bevan M J, Lampe M F. Murine cytotoxic T lymphocytes induced following Chlamydia trachomatis intraperitoneal or genital tract infection respond to cells infected with multiple serovars. Infect Immun. 1995;63:3527–3530. doi: 10.1128/iai.63.9.3527-3530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T-lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 41.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 42.Stuart E S, Wyrick P B, Choong J, Stoler S B, MacDonald A B. Examination of chlamydial glycolipid with monoclonal antibodies: cellular distribution and epitope binding. Immunology. 1991;74:740–747. [PMC free article] [PubMed] [Google Scholar]
- 43.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su H, Caldwell H D. Immunogenicity of a synthetic oligopeptide corresponding to antigenically common T-helper and B-cell neutralizing epitopes of the major outer membrane protein of Chlamydia trachomatis. Vaccine. 1993;11:1159–1166. doi: 10.1016/0264-410x(93)90080-h. [DOI] [PubMed] [Google Scholar]
- 45.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su H, Morrison R P, Messer R, Whitmire W, Hughes S, Caldwell H D. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999;180:1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 47.Su H, Morrison R P, Watkins N G, Caldwell H D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tagliabue A, Boraschi D, Villa L, Keren D F, Lowell G H, Rappuoli R, Nencioni L. IgA-dependent cell-mediated activity against enteropathogenic bacteria: distribution, specificity, and characterization of the effector cells. J Immunol. 1984;133:988–992. [PubMed] [Google Scholar]
- 49.Tagliabue A, Nencioni L, Villa L, Keren D F, Lowell G H, Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature. 1983;306:184–186. doi: 10.1038/306184a0. [DOI] [PubMed] [Google Scholar]
- 50.Tagliabue A, Villa L, Boraschi D, Peri G, De Gori V, Nencioni L. Natural anti-bacterial activity against Salmonella typhi by human T4+ lymphocytes armed with IgA antibodies. J Immunol. 1985;135:4178–4182. [PubMed] [Google Scholar]
- 51.Tagliabue A, Villa L, De Magistris M T, Romano M, Silvestri S, Boraschi D, Nencioni L. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty21a vaccine. J Immunol. 1986;137:1504–1510. [PubMed] [Google Scholar]
- 52.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins J B, Unanue A, Casadevall A, Bloom B R. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1999;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng C-T K, Rank R G. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams D M, Grubbs B, Kelly K A, Rank R G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wyrick P B, Choong J, Knight S T, Goyeau D, Stuart E S, MacDonald A B. Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immunol Infect Dis. 1994;4:131–141. [Google Scholar]
- 56.Yang X, Brunham R C. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 57.Zhang Y-X, Stewart S, Joseph T, Taylor H R, Caldwell H D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 58.Zhang Y-X, Stewart S J, Caldwell H D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong G, Fan T, Liu L. Chlamydia inhibits interferon γ-inducible major histocompatibility complex II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999;189:1931–1937. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong G, Liu L, Fan T, Fan P, Ji H. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon-γ-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J Exp Med. 2000;191:1525–1534. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]