Abstract
Introduction
Flibanserin treatment increases sexual desire and satisfying sexual events while decreasing distress in certain women diagnosed with acquired, generalized hypoactive sexual desire disorder (HSDD). Additional aspects of sexual function and the time course of response have not been fully characterized.
Aim
To evaluate changes in sexual function assessed by the subdomains of the Female Sexual Function Index (FSFI) in women with HSDD treated with flibanserin.
Methods
FSFI data pooled from 3 pivotal flibanserin trials in premenopausal women (flibanserin = 1,165; placebo = 1,203) and FSFI data from one complete flibanserin trial in postmenopausal women (flibanserin = 432; placebo = 463) were subjected to post-hoc analyses. For each FSFI subdomain, least squares mean change from baseline was calculated at each assessment visit (treatment weeks 4, 8, 16, 24) and treatment groups were compared using analysis of covariance. Standardized effect size (Cohen's d) was also determined for each FSFI subdomain.
Main Outcome Measure
Changes from baseline in FSFI subdomains.
Results
Compared to placebo, both premenopausal (P < .02) and postmenopausal (P < .045) patients in the flibanserin group reported significantly greater increases over baseline in the FSFI subdomain scores of desire, arousal, lubrication, orgasm, and satisfaction. In premenopausal patients, significant improvements were observed at the first assessment of response (week 4) and were maintained through week 24. In postmenopausal patients, significant improvements were observed at week 4 for desire and arousal, while significant improvements in lubrication, orgasm, and satisfaction were observed at week 8. At week 24, excluding the pain subdomain, standardized effect sizes ranged from 0.18 to 0.28 in the premenopausal cohort and 0.12 to 0.29 in the postmenopausal cohort. In both pre- and postmenopausal patients, improvements in pain were smaller and largely undifferentiated between treatment groups.
Clinical Implications
While variations in time to response should be taken into consideration, on average, the beneficial impact of flibanserin on overall sexual function occurs within the first month of treatment. The data also suggest that the response to flibanserin is sustained for the duration of treatment.
Strengths and Limitations
Sexual function assessments were performed in a large cohort of 2,368 premenopausal women and 895 postmenopausal women. However, the FSFI assesses changes over a 1-month period and time points earlier than 4 weeks could not be assessed.
Conclusion
These analyses suggest that assessment of benefit of flibanserin in HSDD should include improvements across all domains of sexual function, not only desire.
Simon JA, Clayton AH, Goldstein I, et al. Effects of Flibanserin on Subdomain Scores of the Female Sexual Function Index in Women With Hypoactive Sexual Desire Disorder. Sex Med 2022;10:100570.
Key Words: Hypoactive Sexual Desire Disorder, Flibanserin, Female Sexual Function Index Scores, Effect Size
INTRODUCTION
Hypoactive sexual desire disorder (HSDD) is the most commonly reported sexual dysfunction in women and is associated with decreased quality of life, increased health care costs, and increased health burden.1, 2, 3, 4, 5, 6, 7 Flibanserin is the first medication approved for the indication of acquired, generalized HSDD, and is approved in the US for premenopausal women and in Canada for premenopausal women and naturally postmenopausal women, 60 years of age or younger.8,9 These approvals were primarily based upon the risk-benefit assessment from pivotal trials that used changes in satisfying sexual events, sexual desire, and associated distress as co-primary and secondary endpoints.10, 11, 12, 13 The safety profile of flibanserin is well characterized and has been extensively described previously.14
Other aspects of sexual function were evaluated during these trials using the Female Sexual Function Index (FSFI), but these data have only been reported in conference proceedings.15,16 In addition, while the prescribing information for flibanserin states that it may take up to 8 weeks for efficacy to emerge, earliest onset of response regarding overall sexual function has not been fully characterized. Thus, we conducted post-hoc analyses of all FSFI subdomain scores to examine changes in sexual function and the time course of response in patients over the duration of the pivotal trials.
MATERIALS AND METHODS
Study Design
Clinical trials were performed at numerous institutions and clinics in North America between 2006 and 2011. As previously reported,10, 11, 12, 13,17,18 premenopausal or naturally postmenopausal women with a diagnosis of HSDD, as determined by criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR] for ≥24 weeks and in a stable, monogamous, heterosexual relationship that was secure and communicative for ≥1 year were randomly assigned to receive flibanserin (dose groups varied by study) or placebo for up to 24 weeks. The sexual partner had to be sexually functional, both psychologically and physically, and had to be physically present at least 50% of each month. Patients had to indicate a willingness to try to have sexual activity (any act involving direct genital stimulation) at least once each month. Exclusion criteria included sexual dysfunctions other than HSDD, dyspareunia (not caused by inadequate foreplay stimulation or alleviated by lubricants), major depressive disorder within the previous 6 months, substance abuse in the past year, or any ongoing serious clinical disorder. Women with secondary female sexual arousal disorder and/or female orgasmic disorder were allowed in the studies provided that the patient considered HSDD to be of primary importance.
Patients in the study group were diagnosed with acquired, generalized HSDD and randomized to receive either flibanserin 100 mg qhs or placebo for 24-week duration.10, 11, 12, 13,17,18 Some premenopausal patients in the flibanserin group received a dose of 50 mg qhs for the first 2 weeks and were uptitrated to 100 mg qhs for the remainder of the trial.11
Assessment of sexual function using the FSFI was conducted at study entry (baseline) and at weeks 4, 8, 16, and 24. The FSFI is a validated instrument consisting of 19 items across 6 subdomains (desire, arousal, lubrication, orgasm, satisfaction, pain).19 For each FSFI subdomain, the maximum score is 6.0. Total scores can range from 2 to 36 with higher scores indicating better sexual function. FSFI subdomain data were pooled from 3 pivotal multicenter, randomized, placebo-controlled, double-blind trials for premenopausal women treated with flibanserin 100 mg qhs (n = 1,165) or placebo (n = 1,203) and one similarly designed trial in naturally postmenopausal women (Snowdrop trial; flibanserin, n = 432; placebo, n = 463).10, 11, 12, 13,17
Data from the Plumeria trial in postmenopausal women18 (flibanserin, n = 376; placebo, n = 369) with identical design and endpoints is also included for comparative purposes but was not pooled with the Snowdrop postmenopausal study because the Plumeria trial was terminated early by the sponsor due to commercial reasons. At trial termination, 32.5% of patients completed the 24-week study while 50.1% of patients were unable to complete the study due to early discontinuation. The remaining patients (n = 130; 17.4% of treated set) discontinued before the trial was closed, consistent with the dropout rate of the Snowdrop trial in postmenopausal women (see Data Analysis section). As previously reported, the primary analysis timepoint of the Plumeria trial in postmenopausal women was at 16 weeks when 45.3% of patients had completed treatment and assessments.18
Studies were conducted in accordance with the principles of the Declaration of Helsinki (1996) and the International Conference on Harmonisation Good Clinical Practice Guidelines. All studies were approved by an institutional review board or independent ethics committee at each investigative site, and all participants provided written informed consent before the initiation of study procedures. Clinical trials were funded by Boehringer Ingelheim while post-hoc analyses were funded by Sprout Pharmaceuticals.
Data Analysis
The full dataset of efficacy assessments from the clinical trials was made available to the authors and data were analyzed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). For each FSFI subdomain and FSFI total scores, least squares mean change from baseline was calculated at each assessment visit. Post-hoc exploratory analyses were conducted to compare flibanserin 100 mg qhs vs placebo treatment groups using an analysis of covariance with treatment and study center as the fixed effects and the baseline score as the covariate. Mean differences between flibanserin and placebo treatment groups with 95% confidence intervals were also calculated to determine raw effect size. Cohen's d (standardized effect size), odds ratios, and number needed to treat (NNT) were also determined to provide additional estimates of effect size.
In the 3 pivotal trials with premenopausal women, dropout rates ranged between 18.6% and 27.6% for the placebo group (combined mean rate = 24.7%) and 25.7–32.2% for the flibanserin group (combined mean rate = 30.3%). In the Snowdrop trial with postmenopausal women, the dropout rate was 17.3% for the placebo group and 21.8% for the flibanserin group. Missing data were handled by using the method of last observation carried forward, as prespecified by the statistical analysis plan. Sensitivity analyses using mixed model repeated measures and missing at random were also conducted, as prespecified by the statistical analysis plan. Additional more conservative post-hoc sensitivity analyses using methods which impute missing data assuming no treatment benefit (ie, baseline observation carried forward, control-based imputation, and jump to reference) were also conducted. While each domain of the FSFI was not tested, these missing data imputation methods were consistent with last observation carried forward when comparing differences between flibanserin and placebo treatment for the main trial endpoints of FSFI desire domain, distress associated with decreased sexual desire, and satisfying sexual events.
RESULTS
Patient Demographics
In the premenopausal group, patients were 36 ± 7 years old (mean ± SD), mean duration of HSDD was 4.5–4.8 years, and mean duration of relationship was approximately 11 years (Table 1). In the Snowdrop postmenopausal trial, patients were 55 ± 5 years old (mean ± SD), mean duration of HSDD was 4.9–5.1 years, and mean duration of relationship was approximately 21 years. Mean age (56 ± 5 years), duration of HSDD (4.7–5.2 years), and duration of relationship (22 years) was not significantly different for postmenopausal patients in the Plumeria trial. Demographic characteristics were similar between the flibanserin and placebo treatment groups within each analysis cohort.
Table 1.
Demographic and baseline clinical characteristics
| Premenopausal |
Postmenopausal |
|||
|---|---|---|---|---|
| Characteristic | Placebo (n = 1,238) | Flibanserin (n = 1,227) | Placebo (n = 480) | Flibanserin (n = 467) |
| Age, mean (SD), y | 36.2 (7.3) | 35.9 (7.5) | 55.5 (5.3) | 55.4 (5.4) |
| Race, n (%) | ||||
| White* | 1,089 (88.0) | 1,073 (87.4) | 444 (92.5) | 425 (91.0) |
| Black | 119 (9.6) | 131 (10.7) | 27 (5.6) | 35 (7.5) |
| Asian | 21 (1.7) | 20 (1.6) | 4 (0.8) | 4 (0.9) |
| Other | 9 (0.7) | 3 (0.2) | 5 (1.0) | 3 (0.6) |
| Duration of present relationship, mean (SD), y | 11.0 (6.7) | 10.7 (7.0) | 20.6 (12.6) | 21.6 (12.3) |
| Duration of HSDD, mean (SD), y | 4.8 (3.9) | 4.5 (3.6) | 5.1 (4.3) | 4.9 (3.8) |
| Female Sexual Function Index baseline scores | Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean (SD), n |
| Desire | 1.85 (0.7), 1,203 | 1.85 (0.7), 1,165 | 1.84 (0.71), 463 | 1.78 (0.68), 432 |
| Arousal | 2.69 (1.4), 1,203 | 2.63 (1.3), 1,165 | 2.18 (1.17), 463 | 2.19 (1.20), 432 |
| Lubrication | 3.80 (1.8), 1,202 | 3.72 (1.7), 1,165 | 2.64 (1.60), 463 | 2.63 (1.62), 432 |
| Orgasm | 3.16 (1.8), 1,202 | 3.06 (1.8), 1,164 | 2.44 (1.67), 463 | 2.46 (1.65), 432 |
| Satisfaction | 2.78 (1.1), 1201 | 2.82 (1.1), 1,165 | 2.78 (1.22), 463 | 2.72 (1.19), 432 |
| Pain | 5.07 (1.7), 1,204 | 5.06 (1.7), 1,164 | 4.03 (2.12), 463 | 4.16 (2.13), 432 |
| Total | 19.37 (6.4), 1,200 | 19.14 (6.2), 1,163 | 15.88 (6.40), 463 | 15.94 (6.52), 432 |
Changes in Sexual Function Assessed by the Female Sexual Function Index
At study entry (baseline), premenopausal patients had mean total FSFI scores of 19.37 (placebo) and 19.14 (flibanserin) and postmenopausal patients had mean total FSFI scores of 15.88 (placebo) and 15.94 (flibanserin). Mean baseline FSFI subdomain scores ranged from 1.84 to 5.07, as shown in Table 1.
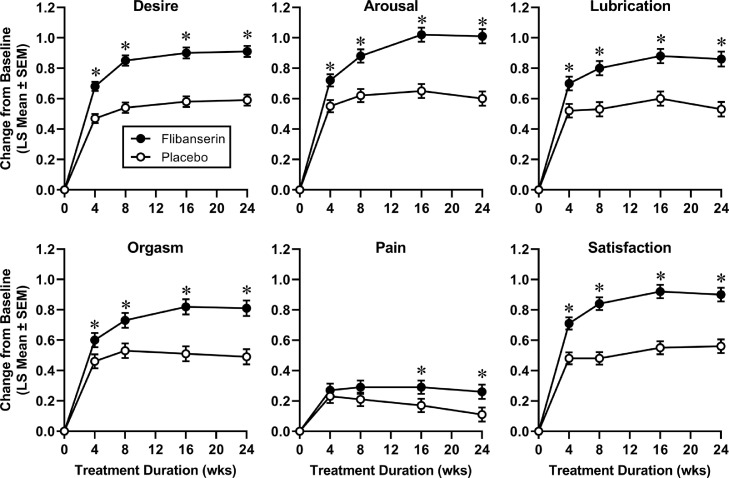
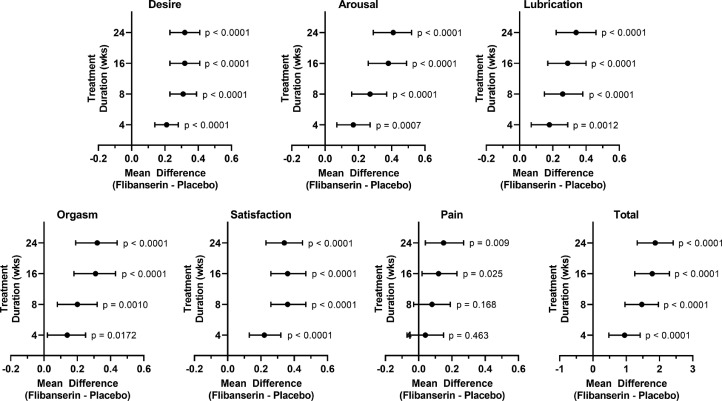
Premenopausal patients reported increases over baseline in all FSFI subdomain scores. Compared to the placebo treatment group, these improvements were significantly greater for those in the flibanserin treatment group for desire, arousal, lubrication, orgasm, and satisfaction subdomains (Figures 1 and 2, Table 2). Excluding the pain subdomain, mean differences between flibanserin and placebo groups (raw effect size) ranged from 0.32 to 0.41 and standardized effects sizes (Cohen's d) ranged from 0.18 to 0.28 (Table 2). In addition, the odds ratio for self-reported perceived clinical benefit was 2.4 and the NNT was 4.7. Improvements in the flibanserin group were evident at the earliest on-treatment assessment that took place at Week 4, continued to increase through Week 16, and was maintained through Week 24 (end of trial). Improvement in the pain subdomain was significantly greater in the flibanserin group compared to placebo at Weeks 16 and 24. However, mean baseline scores for the pain subdomain indicated that, on average, premenopausal women experienced discomfort or pain with vaginal penetration “less than half the time” or “almost never or never” at a level or degree that was “low” or “very low or none at all.”
Figure 1.
Change from baseline in Female Sexual Function Index subdomain scores in premenopausal women during treatment with flibanserin 100 mg qhs (n = 1165) or placebo (n = 1203). Sample sizes vary slightly by domain based on available data for each treatment group (see Table 2). *P < .025.
Figure 2.
Mean difference in Female Sexual Function Index subdomain scores between flibanserin and placebo treatment groups in premenopausal women (see Figure 1). For each mean difference, 95% confidence intervals and p values are shown.
Table 2.
Effect sizes (from baseline to end-of-trial) for flibanserin therapy in premenopausal and postmenopausal women with HSDD
| Assessment | Difference between flibanserin and placebo treatment groups |
Cohen's d |
Odds ratio (95% CI) | NNT§ (95% CI) | ||
|---|---|---|---|---|---|---|
| Mean (95% CI) | P Value | Unadjusted (95% CI) | Adjusted† (95% CI) | |||
| Premenopausal (Pooled) | 2.4 (2.0, 2.9)‡ | 4.7 (3.9, 6.0) | ||||
| Desire | 0.32 (0.23, 0.41) | <.0001 | 0.28 (0.20–0.37) | 0.28 (0.21–0.36) | ||
| Arousal | 0.41 (0.29, 0.52) | <.0001 | 0.28 (0.20–0.36) | 0.26 (0.19–0.34) | ||
| Lubrication | 0.34 (0.22, 0.46) | <.0001 | 0.22 (0.14–0.30) | 0.20 (0.13–0.26) | ||
| Orgasm | 0.32 (0.19, 0.44) | <.0001 | 0.21 (0.13–0.29) | 0.18 (0.12–0.25) | ||
| Pain | 0.15 (0.04, 0.27) | .009 | 0.09 (0.01–0.17) | 0.09 (0.03–0.15) | ||
| Satisfaction | 0.34 (0.23, 0.45) | <.0001 | 0.22 (0.14–0.30) | 0.22 (0.15–0.29) | ||
| Total | 1.87 (1.33, 2.41) | <.0001 | 0.27 (0.19–0.36) | 0.26 (0.19–0.33) | ||
| Postmenopausal (Snowdrop) | 1.6 (1.2, 2.2)‡ | 9.4 (6.1, 21.2) | ||||
| Desire | 0.29 (0.15, 0.44) | <.0001 | 0.29 (0.16–0.42) | 0.26 (0.14–0.39) | ||
| Arousal | 0.29 (0.10, 0.48) | .003 | 0.19 (0.06–0.32) | 0.19 (0.07–0.31) | ||
| Lubrication | 0.22 (0.01, 0.44) | .044 | 0.13 (-0.00 to 0.26) | 0.12 (0.01–0.24) | ||
| Orgasm | 0.23 (0.01, 0.45) | .044 | 0.12 (-0.01 to 0.25) | 0.12 (0.01–0.24) | ||
| Pain | 0.20 (-0.04, 0.43) | .099 | 0.06 (-0.07 to 0.19) | 0.09 (-0.01 to 0.20) | ||
| Satisfaction | 0.27 (0.09 - 0.46) | .003 | 0.20 (0.07–0.33) | 0.18 (0.06–0.29) | ||
| Total | 1.49 (0.52, 2.47) | .003 | 0.19 (0.05–0.32) | 0.19 (0.07–0.30) | ||
| Postmenopausal (Plumeria)* | 1.4 (1.2–1.7) | 12.3 (8.2, 25.1) | ||||
| Desire | 0.20 (0.05, 0.35) | .011 | 0.18 (0.03–0.33) | 0.19 (0.05–0.32) | ||
| Arousal | 0.27 (0.07, 0.48) | .009 | 0.20 (0.05–0.35) | 0.19 (0.06–0.32) | ||
| Lubrication | 0.24 (0.01, 0.48) | .042 | 0.15 (0.00–0.30) | 0.14 (0.02–0.27) | ||
| Orgasm | 0.20 (-0.04, 0.43) | .109 | 0.14 (-0.01 to 0.29) | 0.11 (-0.01 to 0.24) | ||
| Pain | 0.20 (-0.05, 0.45) | .122 | 0.15 (0.00–0.30) | 0.11 (-0.02 to 0.23) | ||
| Satisfaction | 0.10 (-0.10, 0.30) | .344 | 0.06 (-0.09to 0.20) | 0.07 (-0.06 to 0.20) | ||
| Total | 1.21 (0.17, 2.26) | .023 | 0.18 (0.03–0.33) | 0.17 (0.03–0.30) | ||
Study was terminated early and data are from week 16.
Adjusted for covariates using ANCOVA.
Simon et al. Sex Med 2022;10:100476.
NNT = number needed to treat; calculations were based on changes in sexual desire for patients who completed the study (instead of the full analysis set) and "effective intervention" was defined by the responder analyses described in Simon et al. Sex Med 2022;10:100476.
Postmenopausal patients in the Snowdrop trial also reported increases over baseline in all FSFI subdomain scores. Compared to placebo, these improvements were significantly greater for those in the flibanserin treatment group as early as Week 4 for desire and arousal subdomains, and as early as Week 8 for lubrication, orgasm, and satisfaction subdomains, as indicated in Figures 3 and 4. Excluding the pain subdomain, raw effect sizes ranged from 0.22 to 0.29 and standardized effect sizes ranged from 0.12 to 0.29 (Table 2). The odds ratio for self-reported perceived clinical benefit was 1.6 and the NNT was 9.4. Improvement in the pain subdomain was significantly greater for the flibanserin treatment group only at Week 8, compared to the placebo group. Mean baseline scores for the pain subdomain indicated that postmenopausal women experienced more discomfort or pain with vaginal penetration than premenopausal women with a frequency ranging from “less than half the time” to “about half the time” at a level or degree that was “low” to “moderate.” For the Plumeria study, raw effect sizes (range = 0.10–0.27) and standardized effect sizes (range = 0.06–0.20) for FSFI subdomains were generally smaller at 16 weeks of treatment. When the study was terminated, less than half the patients (45.3%) had completed 16 weeks. Nevertheless, in our post-hoc exploratory analyses, compared to placebo, increases in desire, arousal, lubrication, and total scores were significantly greater in the flibanserin group (Table 2). The odds ratio for self-reported perceived clinical benefit was 1.4 and the NNT was 12.3.
Figure 3.
Change from baseline in Female Sexual Function Index subdomain scores in naturally postmenopausal women (Snowdrop trial) during treatment with flibanserin 100 mg qhs (n = 432) or placebo (n = 463). *P < .045.
Figure 4.
Mean difference in Female Sexual Function Index subdomain scores between flibanserin and placebo treatment groups in naturally postmenopausal women (Snowdrop trial; see Figure 3). For each mean difference, 95% confidence intervals and p values are shown.
Despite the differences in baseline total FSFI scores between premenopausal and postmenopausal women, the overall magnitude of change during treatment with flibanserin or placebo was similar for both groups (Figures 2, 4, and 5). Arguably, premenopausal women sustained a more robust improvement in sexual function compared to postmenopausal women, mostly due to better desire, arousal, and satisfaction subdomain scores (Figure 5).
Figure 5.
Change from baseline in Female Sexual Function Index total scores in premenopausal and postmenopausal women during treatment with flibanserin 100 mg qhs or placebo (see Figures 1 & 3). The contributions of each subdomain to the total score at Week 4 and Week 24 are shown in the middle and lower panels. *P < .0035.
DISCUSSION
Data from these post-hoc analyses demonstrated that treatment with flibanserin 100 mg qhs significantly improved not only sexual desire, but other relevant aspects of sexual function that included arousal, lubrication, orgasm, and satisfaction, relative to placebo. These benefits were consistently reported by both premenopausal and naturally postmenopausal women with acquired, generalized HSDD.
In premenopausal women taking flibanserin, improved sexual function in all FSFI subdomains except pain was significantly greater than those taking placebo at the first on-treatment assessment at Week 4. Since the FSFI has been validated as an instrument that retrospectively assesses 4 weeks of sexual activity,19 earlier assessment time points were not conducted. Postmenopausal women taking flibanserin also reported enhanced sexual desire and arousal that was significantly greater than those taking placebo at Week 4. Improvements in other FSFI subdomains that were significantly greater for flibanserin-treated postmenopausal women than those treated with placebo were detected at the second on-treatment assessment at Week 8.
Thus, our data suggest that both premenopausal and postmenopausal women experienced a beneficial impact of flibanserin that was greater than placebo on improved sexual desire and arousal within the first month. It is interesting to note that women may poorly differentiate between sexual desire and sexual arousal, often conflating these responses.20 This may, in part, explain the parallel changes in sexual desire and arousal. However, the eleventh revision of the International Classification of Diseases (ICD-11) defines disorders of sexual arousal and desire as separate entities.21 This is in accordance with the recommendations of the Fourth International Consultation on Sexual Medicine22 and the expert consensus nomenclature of the International Society for the Study of Women's Sexual Health.23 Irrespective of how these sexual disorders may be defined and classified, sexual desire is primarily a cognitive process while sexual arousal has both cognitive and peripheral physiological (eg, increases in genital blood flow, heart rate, respiration) components.24,25 The FSFI does not distinguish between the various components of arousal but merely provides the terms “turned on” or “excitement” parenthetically next to “sexual arousal” or “arousal” in the questions of the arousal subdomain.19
Lubrication, also termed “wet” or “wetness” in the FSFI,19 may be more closely associated with sexual arousal than sexual desire, while orgasm (also termed “climax”) and satisfaction may be understood as being more “downstream” aspects of sexual function, relative to sexual desire. Nevertheless, each of these subdomains are on a continuum of overall sexual function and influence each other. It should also be noted that postmenopausal women reported lower baseline lubrication and orgasm subdomain scores than premenopausal women. This is not surprising, since decreased levels of sex steroid hormones in postmenopausal women lead to significant changes in genital tissue structure and function.26, 27, 28
Pain associated with sexual penetration may be the most distinct aspect of sexual function. While pain is mitigated by higher levels of arousal and lubrication, pain subdomain scores did not change consistently in either premenopausal or postmenopausal women. As noted in the results section, postmenopausal women did have a lower mean baseline score for the pain subdomain compared to premenopausal women, although neither group experienced significant pain, since dyspareunia was a specific exclusion criteria for the trials. Flibanserin has not been studied in women with significant pain associated with sexual penetration. However, multiple factors and conditions can contribute to pain during sexual activity, including pelvic floor dysfunction, vestibulodynia, and genitourinary syndrome of menopause,29, 30, 31 none of which are indications for flibanserin therapy.
It is important to note that the FSFI was always administered as a whole and was not modified in any way. The FSFI instrument was used in the same way that it was validated and analysis of subdomain scores between different groups of patients does not impact the validity. This view is supported by a preliminary cross-validation study by Wiegel et al that used discriminant validity testing to confirm the ability of both total and subdomain scores of the FSFI to differentiate between various sexual dysfunctions and those without sexual dysfunctions in a mixed population of pre- and postmenopausal women.32 While the FSFI is not used as a diagnostic instrument, these results suggest that subdomain scores may be used to assess changes in sexual function. Beyond assessing changes in domains specific to sexual function, assessing aspects of health-related quality of life are also very important, as emphasized in a study of quality-of-life measures in women with sexual dysfunction.33 However, the FSFI was not designed to make these assessments and broader quality of life topics such as physical functioning, overall mental well-being, partner relationship, and life satisfaction were not evaluated in the flibanserin trials.
Excluding pain, Wiegel and colleagues found that mean differences in FSFI subdomain scores between women with HSDD and those without any sexual dysfunctions ranged between 0.84 and 1.43 with mean change in FSFI total score of 6.86.32 In our analysis, we noted that mean change from baseline in FSFI domain scores (excluding pain) in the flibanserin group ranged from 0.81 to 1.01 in premenopausal women with mean change in FSFI total score of 4.75. In postmenopausal women (Snowdrop trial), mean change from baseline in FSFI domain scores (excluding pain) in the flibanserin group ranged from 0.66 to 0.85 with mean change in FSFI total score of 4.22. Since the analyses by Wiegel et al used a mixed population of women and did not examine effects of treatments, it is difficult to directly compare our data.
However, other analyses have shown self-reported clinically meaningful benefit for both pre- and post-menopausal women treated with flibanserin.34 Using the Patient Global Impression of Improvement,35 a higher percentage of premenopausal patients reported clinically meaningful benefit with flibanserin (49.8%) than with placebo (33.6%).34 Likewise, more postmenopausal patients reported clinically meaningful benefit with flibanserin (40.5%) than with placebo (28.7%). Further, odds ratios for assessments of sexual desire, distress, and satisfying sexual events indicated that premenopausal women were 2.0–2.4 times as likely to be responders (those self-reporting benefit) with flibanserin as with placebo.34 Postmenopausal women were 1.6 times as likely to be responders with regard to sexual desire, as assessed by the desire subdomain of the FSFI.34 In the context of these findings, we believe that the changes in FSFI subdomain scores from our post-hoc analyses reflect impactful changes in sexual function.
Additional perspective on the effect of flibanserin may be gleaned from examining standardized effect sizes (Cohen's d) and the NNT. In general, both adjusted and unadjusted Cohen's d values were similar for any given FSFI subdomain within each pre- or postmenopausal cohort. Although the findings of the Plumeria trial were likely influenced by the lack of completers, leading to “artificial dropouts,” all effect size estimates tended to be higher in premenopausal women. It should be emphasized that Cohen's benchmarks for small (0.2), medium (0.5) and large (0.8) effect sizes are arbitrary and should only be used if no other indices of standardized effect size are available.36 Effect size estimates are influenced by the magnitude of the response to placebo, standard deviations, and sample size.36,37 Evaluation of subjective outcomes such as sexual desire can also be associated with lower effect size estimates compared to objective indices like blood pressure, serum cholesterol, or glucose levels.37
For context, in a review of various meta-analyses for psychiatric disorders, antidepressants were reported to have a standardized effect size of 0.32 for major depressive disorder, SSRIs had a standardized effect size of 0.44 for obsessive-compulsive disorder, and tricyclic antidepressants, SSRIs and benzodiazepines had standardized effect sizes of 0.40–0.41 for anxiety symptoms.37 Further, in a systematic review of antidepressant therapies, tricyclic antidepressants had a median NNT of 9 (range = 7–16) and SSRIs had a median NNT of 7 (range = 7–8).38 Similar large data sets for either standardized effect size or NNTs are not currently available for HSDD therapies. One previous publication estimated the standardized effect sizes of the 3 pivotal flibanserin trials in premenopausal women (Cohen's d = 0.43–0.50) and the Snowdrop flibanserin trial in postmenopausal women (Cohen's d = 0.43).39 However, these previously published estimates used the baseline SD for the calculations while we used the pooled SD from baseline to the end of trial for our calculations. In our analyses, standardized effect sizes for the FSFI sexual desire subdomain in both pre- and postmenopausal women taking flibanserin (0.26–0.29) approached that of treatments for major depressive disorder and NNT values for flibanserin therapy were similar (NNT for postmenopausal women = 9.4) or lower (NNT for premenopausal women = 4.7) than those for antidepressant therapies (median NNT = 7–9).37,38
In general, while the change from baseline in FSFI scores was smaller for the placebo group than the flibanserin group, both treatment groups followed a similar trajectory over time and the response to placebo was also sustained through the end of the trial at 24 weeks. There is a growing body of literature that supports the perspective that placebo is a form of active therapy that is associated with changes in brain activity.40, 41, 42 Previous publications have also discussed significant placebo effects in clinical trials of treatments for female sexual dysfunctions.43, 44, 45 There is also some evidence to suggest that establishing a therapeutic alliance between patient and healthcare practitioner through repeated communications is a predictor of therapeutic success.39 The flibanserin clinical trials required multiple office visits and assessments over a 24-week period and there was likely benefit from external validation of the patient's condition, education about HSDD, and development of a therapeutic alliance in addition to any central effects of undergoing placebo therapy.
CONCLUSIONS
The efficacy of therapeutic interventions should be viewed in the context of outcomes assessed, the natural course of the condition, and associated suffering/distress. Patients in the flibanserin trials experienced statistically significant improvements in sexual desire, arousal, lubrication, orgasm, and satisfaction over a substantial placebo response with effect sizes that approximate those of other centrally acting medications that treat multifactorial conditions with subjective assessments. Improvements in sexual function were observed within the first 4 weeks of treatment for premenopausal women. Improvement in sexual desire and sexual arousal also occurred within the first 4 weeks for postmenopausal women and within the first 8 weeks for other subdomains. Thus, early assessment of benefit of flibanserin in treating HSDD should include improvements across all domains of sexual function, not only desire.
ACKNOWLEDGMENTS
Boehringer Ingelheim was involved in design, data collection, and data analysis of the clinical trials. Boehringer Ingelheim had no involvement in the interpretation of the data, writing of the report, or the decision to submit the report for publication. Funding for the post-hoc analyses and the preparation of this manuscript was provided by Sprout Pharmaceuticals, Inc, Raleigh, NC.
Statement of Authorship
Conceptualization, JAS, NNK, SP; Writing – Original Draft, JAS, NNK; Writing – Review & Editing, JAS, NNK, AHC, IG, SAK, MS, SP; Visualization, NNK; Supervision, NNK, SP; Funding Acquisition, SP.
Footnotes
Conflict of interest and funding: Dr Simon has grant/research support from: AbbVie, Bayer Healthcare, Endoceutics, Ipsen, Myovant Sciences, ObsEva, Therapeutics MD, Viveve Medical; has been a consultant or on advisory boards of Allergan, AbbVie, AMAG Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Camargo Pharmaceutical Services, CEEK Enterprises, Covance, Dare´ Bioscience, Duchesnay USA, Hologic, KaNDy/NeRRe Therapeutics, Madorra, Mitsubishi Tanabe Pharma Development America, Sebela Pharmaceuticals, Shionogi, Sprout2, Therapeutics MD; has served on the Speaker's bureaus of: AMAG Pharmaceuticals, Duchesnay USA, Therapeutics MD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. Dr Clayton reports receiving research and grant support from Daré Bioscience, Janssen Pharmaceuticals, Praxis Precision Medicines, Relmada Therapeutics, and Sage Therapeutics; serving as a consultant to or on the advisory board for Fabre-Kramer, Janssen Research & Development, MindCure, Ovoca Bio, Praxis Precision Medicines, PureTech Health, S1 Biopharma, Sage Therapeutics, Takeda/Lundbeck, Vella Bioscience, and WCG MedAvante-ProPhase; being a stock shareholder of Euthymics Bioscience, Mediflix, and S1 Biopharma; and having royalties and copyrights at Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, and Guilford Publications. Dr Goldstein is a consultant to Sprout Pharmaceuticals, Inc. Dr Kingsberg is consultant or advisory board member for Alloy, Astellas Pharma, Bayer, Daré Bioscience, Lupin Pharmaceuticals, Madorra, Materna, Ovoca, Palatin Technologies, Pfizer, Sprout Pharmaceuticals, Strategic Science & Technologies, TherapeuticsMD, Ms. Medicine, and Mithra; and is a stock shareholder of Viveve Medical, Field Trip Health, and Materna. Dr Shaprio is a consultant or serves on the advisory boards of Amgen, Aspen, Astellas, BioSyent, Bayer, Duchesnay, GSK, Merck, Mithra, Pfizer, Searchlight, Sprout, Sunovion, and TherapeuticsMD. Dr Patel is an employee of Sprout Pharmaceuticals. Dr Kim is a consultant to Strategic Science & Technologies and Sprout Pharmaceuticals.
Funding: Sprout Pharmaceuticals, Inc.
REFERENCES
- 1.Graziottin A. Prevalence and evaluation of sexual health problems–HSDD in Europe. J Sex Med. 2007;4(Suppl 3):211–219. doi: 10.1111/j.1743-6109.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayes RD, Dennerstein L, Bennett CM, et al. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007;87:107–112. doi: 10.1016/j.fertnstert.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 3.Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health (Larchmt) 2014;23:817–823. doi: 10.1089/jwh.2014.4743. [DOI] [PubMed] [Google Scholar]
- 4.Foley K, Foley D, Johnson BH. Healthcare resource utilization and expenditures of women diagnosed with hypoactive sexual desire disorder. J Med Econ. 2010;13:583–590. doi: 10.3111/13696998.2010.518114. [DOI] [PubMed] [Google Scholar]
- 5.Biddle AK, West SL, D'Aloisio AA, et al. Hypoactive sexual desire disorder in postmenopausal women: quality of life and health burden. Value Health. 2009;12:763–772. doi: 10.1111/j.1524-4733.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 6.Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 7.West SL, D'Aloisio AA, Agans RP, et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Intern Med. 2008;168:1441–1449. doi: 10.1001/archinte.168.13.1441. [DOI] [PubMed] [Google Scholar]
- 8.Addyi® . Sprout Pharmaceuticals; Raleigh, NC: 2021. (flibanserin) tablets, for oral use. [Google Scholar]
- 9.Addyi® . Searchlight Pharma, Inc.; Montreal, QC, CANADA: 2021. (flibanserin) tablets, for oral use. [Google Scholar]
- 10.DeRogatis LR, Komer L, Katz M, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET Study. J Sex Med. 2012;9:1074–1085. doi: 10.1111/j.1743-6109.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 11.Thorp J, Simon J, Dattani D, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(4):793–804. doi: 10.1111/j.1743-6109.2011.02595.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz M, DeRogatis LR, Ackerman R, et al. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10:1807–1815. doi: 10.1111/jsm.12189. [DOI] [PubMed] [Google Scholar]
- 13.Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: Results of the SNOWDROP trial. Menopause. 2014;21:633–640. doi: 10.1097/GME.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 14.Clayton AH, Brown L, Kim NN. Evaluation of safety for flibanserin. Expert Opin Drug Saf. 2020;19:1–8. doi: 10.1080/14740338.2020.1707804. [DOI] [PubMed] [Google Scholar]
- 15.Simon J, Millheiser L, Clayton A, et al. Improvements in Female Sexual Function Index (FSFI) domains over time after flibanserin treatment in premenopausal women with hypoactive sexual desire disorder (HSDD) J Sex Med. 2020;17:S260. [Google Scholar]
- 16.Simon JA, Kingsberg SA, Shapiro M, et al. North American Menopause Society Annual Meeting; Washington, DC: 2001. Onset of flibanserin treatment effect in postmenopausal women assessed by subdomain scores of the female sexual function index (FSFI) September 22-25. [Google Scholar]
- 17.Simon JA, Thorp J, Millheiser L. Flibanserin for premenopausal hypoactive sexual desire disorder: pooled analysis of clinical trials. J Womens Health. 2019;28:769–777. doi: 10.1089/jwh.2018.7516. [DOI] [PubMed] [Google Scholar]
- 18.Portman DJ, Brown L, Yuan J, et al. Flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the PLUMERIA study. J Sex Med. 2017;14:834–842. doi: 10.1016/j.jsxm.2017.03.258. [DOI] [PubMed] [Google Scholar]
- 19.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 20.Brotto LA. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav. 2010;39:221–239. doi: 10.1007/s10508-009-9543-1. [DOI] [PubMed] [Google Scholar]
- 21.Reed GM, Drescher J, Krueger RB, et al. Disorders related to sexuality and gender identity in the ICD-11: revising the ICD-10 classification based on current scientific evidence, best clinical practices, and human rights considerations. World Psychiatr. 2016;15:205–221. doi: 10.1002/wps.20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016;13:135–143. doi: 10.1016/j.jsxm.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions—part II. J Sex Med. 2016;13:1888–1906. doi: 10.1016/j.jsxm.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Pfaus JG, Ismail N, Coria-Avila GA. Sexual motivation. In: Koob G.F., Le Moal M. and Thompson R.F. (eds.) Encyclopedia of behavioral neuroscience, volume 3, pp. 201–9 Oxford, UK: Academic Press, 2010.
- 25.Parish SJ, Meston CM, Althof SE, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions-part III. J Sex Med. 2019;16:452–462. doi: 10.1016/j.jsxm.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Portman DJ, Gass ML, Vulvovaginal Atrophy Terminology Consensus Conference Panel Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063–1068. doi: 10.1097/GME.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 27.Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Menopause. 2018;25:837–847. doi: 10.1097/GME.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 28.Traish AM, Vignozzi L, Simon JA, et al. Role of androgens in female genitourinary tissue structure and function: Implications in the genitourinary syndrome of menopause. Sex Med Rev. 2018;6:558–571. doi: 10.1016/j.sxmr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Al-Abbadey M, Liossi C, Curran N, et al. Treatment of female sexual pain disorders: a systematic review. J Sex Marital Ther. 2016;42:99–142. doi: 10.1080/0092623X.2015.1053023. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572–590. doi: 10.1016/j.jsxm.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Phillips NA, Bachmann GA. The genitourinary syndrome of menopause. Menopause. 2021;28:579–588. doi: 10.1097/GME.0000000000001728. [DOI] [PubMed] [Google Scholar]
- 32.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 33.Lim-Watson MZ, Hays RD, Kingsberg S, et al. A systematic literature review of health-related quality of life measures for women with hypoactive sexual desire disorder and female sexual interest/arousal disorder. Sex Med Rev. 2022;10:23–41. doi: 10.1016/j.sxmr.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Simon JA, Clayton AH, Kim NN, et al. Clinically meaningful benefit in women with hypoactive sexual desire disorder treated with flibanserin. Sex Med. 2022;10 doi: 10.1016/j.esxm.2021.100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute of Mental Health. Patient global impressions scale - change, Improvement, Severity (PGI-C, PGI-I, PGI-S). Mapi Research Trust. Available at:https://eprovide.mapi-trust.org/instruments/patient-global-impressions-scale-change-improvement-severity. Accessed December 18, 2021.
- 36.Bakker A, Cai J, English L, et al. Beyond small, medium, or large: points of consideration when interpreting effect sizes. Educ Stud Math. 2019;102:1–8. [Google Scholar]
- 37.Leucht S, Hierl S, Kissling W, et al. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psych. 2012;200:97–106. doi: 10.1192/bjp.bp.111.096594. [DOI] [PubMed] [Google Scholar]
- 38.Arroll B, Elley CR, Fishman T, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Syst Rev. 2009;3 doi: 10.1002/14651858.CD007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyke RE, Clayton AH. Effect size in efficacy trials of women with decreased sexual desire. Sex Med Rev. 2018;6:358–366. doi: 10.1016/j.sxmr.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Wager RD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tetreault P, Mansour A, Vachon-Presseau E, et al. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedetti F, Piedmonte A, Frisaldi E. How do placebos work? Eur J Psychotraumatol. 2018 doi: 10.1080/20008198.2018.1533370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradford A, Bradford A. Listening to placebo in clinical trials for female sexual dysfunction. J Sex Med. 2013;10:451–459. doi: 10.1111/j.1743-6109.2012.02941.x. [DOI] [PubMed] [Google Scholar]
- 44.Bradford A, Meston CM. Placebo response in the treatment of women's sexual dysfunctions: a review and commentary. J Sex Marital Ther. 2009;35:164–181. doi: 10.1080/00926230802716302. [DOI] [PubMed] [Google Scholar]
- 45.Bradford A, Meston CM. Behavior and symptom change among women treated with placebo for sexual dysfunction. J Sex Med. 2011;8:191–201. doi: 10.1111/j.1743-6109.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]