Abstract
Considerable effort has focused on the identification of proteins secreted from Mycobacterium spp. that contribute to the development of protective immunity. Little is known, however, about the release of mycobacterial proteins from the bacterial phagosome and the potential role of these molecules in chronically infected macrophages. In the present study, the release of mycobacterial surface proteins from the bacterial phagosome into subcellular compartments of infected macrophages was analyzed. Mycobacterium bovis BCG was surface labeled with fluorescein-tagged succinimidyl ester, an amine-reactive probe. The fluorescein tag was then used as a marker for the release of bacterial proteins in infected macrophages. Fractionation studies revealed bacterial proteins within subcellular compartments distinct from mycobacteria and mycobacterial phagosomes. To identify these proteins, subcellular fractions free of bacteria were probed with mycobacterium-specific antibodies. The fibronectin attachment protein and proteins of the antigen 85-kDa complex were identified among the mycobacterial proteins released from the bacterial phagosome.
Mycobacterium spp. are the causative agents of a spectrum of diseases. The success of these pathogens lies in their ability to effectively exploit mononuclear phagocytes, where they invade, replicate, and persist within their mammalian hosts. Within these professional antigen-presenting cells, mycobacteria prevent the normal maturation of their phagosome and remain sequestered from the degradative compartments of the endosome/lysosome continuum (2, 4, 11, 28, 35). The mycobacterial phagosome is nonfusigenic with lysosomes (2, 4, 11, 28, 35) and fails to acidify due to lack of accumulation of proton ATPase complexes (30). This is a deviation from the normal maturation process, in which phagosomes of macrophages differentiate into acidic compartments containing proteases, as well as molecules promoting antigen presentation (see reference 21 for a recent review).
Numerous proteins that are secreted from Mycobacterium have been described, and many of these have been postulated to contribute to the development of protective immunity (7, 8, 10, 15, 16, 33, 34). Surface proteins and proteins secreted by mycobacteria are likely preferential targets for the immune system early in infection. However, during chronic infection, in the absence of cell lysis and dispersal of killed bacteria, it is not clear what antigens, if any, are processed and presented for recognition by T cells. Limited acidification of the mycobacterial phagosome and its anomalous distribution of lysosomal markers indicate that it is not an optimal compartment for antigen processing (5, 31). It has previously been shown that mycobacterial lipids are actively released from the mycobacterial phagosome and traffic within the endocytic network of the host macrophage (3, 35). Mycobacterial proteins may have the same fate, providing an alternate mechanism by which bacterial proteins may intersect the antigen-processing pathway of the macrophage. In addition, released bacterial proteins may incorporate into the phagosomal membrane and contribute to modulation of phagosome maturation.
The present study was initiated to identify mycobacterial proteins released from the bacterial phagosome into host subcellular compartments in the context of the infected macrophage. These proteins will be candidate molecules for phagosome biogenesis, antigen presentation, and vaccine development.
MATERIALS AND METHODS
Reagents.
Fluorescein succinimidyl ester and rabbit polyclonal anti-fluorescein were purchased from Molecular Probes (Junction City, Oreg.). Mouse anti-fluorescein antibody was purchased from Boehringer Mannheim (Indianapolis, Ind.). The rabbit polyclonal antibodies to mycobacteria, anti-antigen 85-kDa complex (CS-90) and anti-H37Rv culture filtrate proteins (CFP) (C-193), were obtained through the TB Research Materials and Testing Contract at Colorado State University. Rabbit polyclonal anti-fibronectin attachment protein (FAP) antibody (11516) was kindly provided by Jeffrey Schorey (University of Notre Dame, South Bend, Ind.). The rat hybridoma 9C12.4 that produces monoclonal antibody to FAP was provided by Eric Brown (University of California, San Francisco). 1D4B, a rat monoclonal antibody against Lamp 1, was obtained from the Developmental Hybridoma Bank, National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, Md.). The hybridoma KL295, producing monoclonal antibody recognizing the β subunit of major histocompatibility complex (MHC) class II (I-Ad and I-Ed), was obtained from the American Type Culture Collection (ATCC, Manassas, Va.). Horseradish peroxidase (HRP)- and colloidal gold-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa.). Protein G-coated agarose beads were purchased from Sigma (St. Louis, Mo.).
Cells and bacterial cultures.
Bone marrow-derived macrophages (BMMφ) were isolated from the femurs and tibias of BALB/c mice. The cells were cultured on bacterial-grade petri dishes at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 5% horse serum, and 20% L cell-conditioned medium. Macrophages were used between 6 and 10 days in culture.
Mycobacterium bovis BCG (Pasteur), obtained from Barry Bloom (Albert Einstein College of Medicine, New York, N.Y.), was cultured in Middlebrook 7H9 medium (Difco Laboratories, Detroit, Mich.) with OADC supplements (oleic acid, albumin, dextrose, NaCl) (Difco). Macrophages were infected with mid-log-phase mycobacteria at a bacterium-to-macrophage ratio of 10:1. Two hours following the addition of mycobacteria, cells were washed extensively to remove extracellular bacteria and incubated with fresh BMMφ medium for the remaining time indicated in individual experiments.
Succinimidyl ester labeling of mycobacteria.
Surface proteins of Mycobacterium were labeled with fluorescein-tagged succinimidyl ester. Briefly, bacteria were washed twice with phosphate-buffered saline (PBS)–0.5% Tween 80–0.2 M sodium bicarbonate (pH 8.8) and resuspended in the same buffer containing 1 mM fluorescein succinimidyl ester (Molecular Probes). Following a 1-h incubation at 37°C, bacteria were washed three times with PBS-Tween and syringe dispersed prior to infection of macrophages. Bacterial viability following succinimidyl ester labeling was typically >95% as assessed by CFU on Middlebrook 7H10 agar plates (Difco). Macrophages infected with fluorescein succinimidyl ester-labeled mycobacteria were visualized directly using a Zeiss Axioskop 20 fluorescence microscope.
Subcellular fractionation and density gradient electrophoresis (DGE).
Subcellular organelles free of bacteria were isolated as previously described (3). Briefly, macrophages infected with fluorescein succinimidyl ester-labeled mycobacteria were washed and scraped into cold homogenization buffer (0.5 mM EDTA, 0.5 mM EGTA, 20 mM HEPES, and 0.05% gelatin), pH 7, containing 250 mM sucrose and protease inhibitors (50 μg of pepstatin A/ml, 50 μg of leupeptin/ml, 25 μg of E64/ml [Sigma]). Cells were disrupted by multiple passage through a tuberculin syringe with a 25-gauge needle. Following centrifugation at 300 × g for 10 min to remove nuclei, the supernatant was subjected to two postnuclear spins at 100 × g. The resulting supernatant was then carefully layered onto a linear gradient of 30 to 12% sucrose and centrifuged at 800 × g for 1 h at 4°C. Macrophage organelles remained in the upper region of the gradient, distinct from bacteria and bacterial phagosomes, which resolved lower in the gradient. The macrophage subcellular fraction was isolated and ultramicrofuged at 100,000 × g for 45 min.
For analysis of late endosomal/lysosomal compartments, the subcellular pellet was carefully resuspended in DGE buffer (250 mM sucrose, 1 mM EDTA, 0.5 mM EGTA, 10 mM triethanolamine [pH 7.4]) containing 10% Ficoll 70,000 and loaded onto 12% Ficoll in a DGE device resembling that previously described (32). The samples were then separated into a 8 to 0% linear Ficoll gradient at a 10-mA constant current for 2 h. Distinct subcellular fractions were separated according to charge and buoyant density as previously described (3). The late endosomal/lysosomal fraction was isolated and ultramicrofuged at 100,000 × g for 45 min. The resulting subcellular or DGE pellets, free of bacteria, were analyzed directly by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting or subjected to immunoprecipitation as described below.
SDS-PAGE and Western blotting.
For analysis by SDS-PAGE, samples were solubilized by boiling in Laemmli sample buffer and separated by SDS–10% PAGE. Following electrophoretic transfer to nitrocellulose, proteins were probed with the indicated antibodies, followed by the appropriate HRP-conjugated secondary antibody, and detected by enhanced chemiluminescence analysis (Super Signal; Pierce, Rockford, Ill.).
For immunoprecipitation, subcellular organelles were resuspended in PBS–1% Triton X-100 for 1 h at 4°C. Samples were precleared with protein G-coated agarose beads for 30 min, followed by incubation with a mouse anti-fluorescein antibody at 4°C overnight. Protein G beads were added for 2 h at 4°C. The protein G beads were washed extensively and resuspended in 2× Laemmli sample buffer and analyzed by SDS-PAGE.
2-D gel electrophoresis.
For 2-dimensional (2-D) analysis, proteins of subcellular fractions isolated from infected BMMφ were separated according to their isoelectric point (pI) by isoelectric focusing as described previously by Sturgill-Koszycki et al. (29). Ampholytes of pH 5 to 8 were used in combination with broad-range ampholytes of pH 3 to 10. Samples were separated in the 1st-dimension cylindrical gels consisting of 4% acrylamide for 18 h at 400 V and an additional 2 h at 800 V. Gels were extruded from glass tubes and layered on SDS slab gels for electrophoresis in the 2nd dimension (as described above). Following electrophoretic transfer to nitrocellulose and polyvinylidene difluoride (PVDF) membranes, the proteins recognized by the anti-fluorescein were excised from a Coomassie-stained PVDF membrane, and N-terminal amino acid sequences were determined by Midwest Analytical (St. Louis, Mo.).
Immunoelectron microscopy.
Infected macrophages were processed for immunoelectron microscopy as previously described with slight modifications (26). Cells were fixed with 4% paraformaldehyde in 200 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)–0.5 mM MgCl2 (pH 7.1) for 30 min on ice and then scraped and pelleted. Pellets were washed once with PIPES-MgCl2, embedded in gelatin, and infiltrated with 2.3 M sucrose–20% polyvinyl pyrrolidone in PIPES-MgCl2. The samples were trimmed, frozen, and sectioned with an RMC MT7/CR21 cryoultramicrotome. Cryosections of infected macrophages and subcellular compartments were probed with the indicated antibodies followed by the appropriate gold-conjugated secondary antibody.
RESULTS AND DISCUSSION
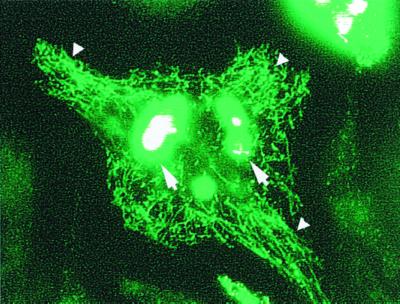
To facilitate the analysis of bacterial proteins released from the mycobacterium-containing phagosome, surface proteins of Mycobacterium were labeled with fluorescein-tagged succinimidyl ester prior to infection of macrophages. Succinimidyl ester is an amine-reactive probe which will conjugate with aliphatic nonprotonated amines (lysine residues and free amines at the N terminus) and has low reactivity with aromatic amines (tyrosine and histidine) of proteins and other molecules. The fluorescent tag was used for direct analysis of the fate of labeled bacterial amine-reactive surface molecules by fluorescence microscopy and indirect biochemical analysis using antibodies specific to fluorescein. Visualization of live infected macrophages by fluorescence microscopy revealed a striking release of fluorescent label from the bacterial phagosome which penetrated host subcellular compartments (Fig. 1). The release of labeled bacterial constituents was evident as early as 1 h postinfection and continued for several days in culture. Fluorescent labeling was not present in control macrophages or macrophages infected with unlabeled bacteria (data not shown). Visualization of bacteria labeled with fluorescein succinimidyl ester by electron microscopy, using anti-fluorescein and colloidal gold-conjugated secondary antibodies, revealed that the succinimidyl ester label was restricted to the surfaces of mycobacteria (data not shown).
FIG. 1.
Release of labeled mycobacterial proteins from the phagosome in infected macrophages. Live BMMφ infected for 24 h with fluorescein succinimidyl ester-labeled BCG were analyzed by fluorescence microscopy. Labeled bacterial proteins were released from the mycobacterial phagosome into subcellular compartments of the infected macrophage (small arrowheads). The labeled bacteria are intensely fluorescent and are indicated by the large arrows.
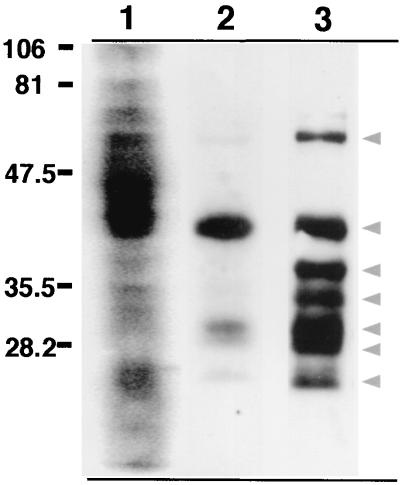
To clearly distinguish bacterial proteins released from the phagosome, subcellular organelles distinct from bacterial phagosomes were isolated and analyzed by SDS-PAGE. BMMφ infected with fluorescein succinimidyl ester-labeled BCG for 24 h were homogenized and subjected to centrifugation in a continuous sucrose gradient to separate bacteria and bacterial phagosomes from macrophage subcellular organelles as previously described (3). The resulting crude organellar fraction, free of bacteria, was isolated. Western blot analysis of fluorescein succinimidyl ester-labeled BCG probed with anti-fluorescein antibodies revealed a profile of fluorochrome-tagged bacterial proteins (Fig. 2, lane 1). Comparison of this profile to that of the bacterium-free macrophage organelle fraction revealed that a subset of fluorescein-labeled bacterial surface proteins were released from the mycobacterial phagosome, the most prominent being approximately 45 kDa (Fig. 2, lane 2). When a mouse anti-fluorescein antibody was used to immunoprecipitate fluorescein-tagged bacterial proteins, a profile of approximately seven proteins was identified in macrophage subcellular compartments (Fig. 2, lane 3). Immunoprecipitation of uninfected control macrophages confirmed that the anti-fluorescein antibodies did not recognize host cellular proteins (data not shown). Mouse anti-fluorescein immunoglobulin G (IgG) used for immunoprecipitation was not recognized by the anti-rabbit secondary antibody (data not shown). This confirmed that the 1-dimensional profile of immunoprecipitated proteins (Fig. 2, lane 3) did not include mouse IgG heavy and light chains.
FIG. 2.
Labeled mycobacterial proteins are present in subcellular compartments of infected macrophages. BMMφ were infected with fluorescein succinimidyl ester-labeled M. bovis BCG for 24 h. The infected cell lysates were then subjected to fractionation on sucrose gradients to isolate a macrophage subcellular membrane fraction free of bacteria. The bacterium-free subcellular compartments were then analyzed for the presence of released fluorescein-labeled bacterial proteins by probing with HRP-conjugated rabbit anti-fluorescein antibody. Lane 1, fluorescein succinimidyl ester-labeled BCG; lane 2, a bacterium-free subcellular fraction isolated from fluorescein succinimidyl ester-labeled mycobacterium-infected macrophages; lane 3, mouse anti-fluorescein immunoprecipitation of a bacterium-free macrophage subcellular fraction. Arrowheads indicate bacterial proteins immunoprecipitated from macrophage subcellular organelles.
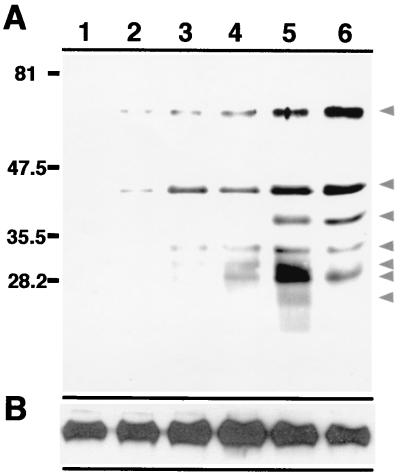
To elucidate the time frame involved in the release of bacterial proteins from the mycobacterial phagosome, subcellular fractions free of bacteria were isolated from BMMφ infected with fluorescein succinimidyl ester-labeled BCG. As shown in Fig. 3, the release of bacterial constituents into host cell organelles was a time-dependent process. At 30 min postinfection, released bacterial constituents could be detected in subcellular organelles immunoprecipitated with anti-fluorescein antibody. The levels and number of bacterial proteins increased with time, revealing differential release of bacterial proteins.
FIG. 3.
Temporal release of bacterial proteins from the mycobacterial phagosome. Bacterium-free subcellular organelles isolated from uninfected macrophages (lane 1) and from macrophages infected with fluorescein succinimidyl ester-labeled BCG for 30 min (lane 2), 1 h (lane 3), 4) 2 h (lane 4), 4 h (lane 5), and 12 h (lane 6) were immunoprecipitated with a mouse anti-fluorescein antibody. The resulting material was analyzed by probing a Western blot with a rabbit anti-fluorescein antibody (A) or anti-Lamp 1 (B). Lamp 1 served as an internal control for the total amount of protein present in each sample. Arrowheads indicate bacterial proteins immunoprecipitated from macrophage subcellular organelles.
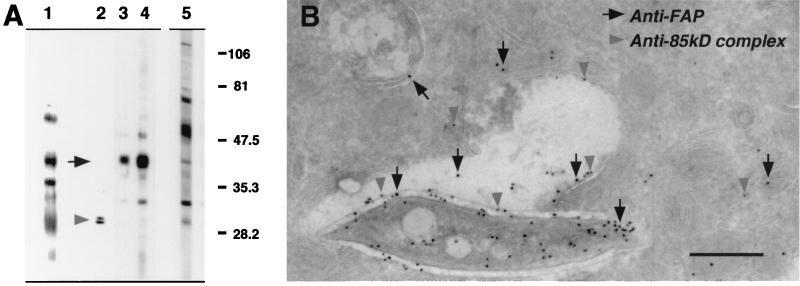
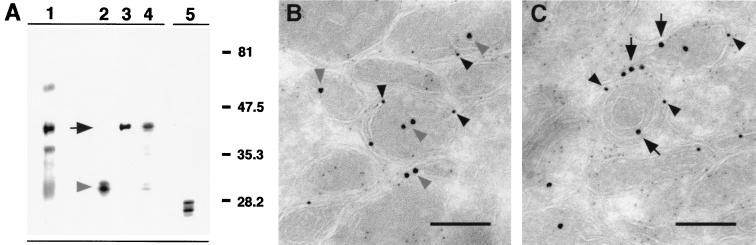
To identify the released bacterial proteins, anti-fluorescein-precipitated subcellular fractions were probed with mycobacterium-specific antibodies. Of those obtained from colleagues and through the TB Research Materials and Testing Contract at Colorado State University, two antibodies with specificity to the released mycobacterial proteins were identified. The rabbit polyclonal anti-antigen 85-kDa complex identified a doublet of 32 kDa (Fig. 4A, lane 2). Rabbit polyclonal anti-FAP antibody identified a band at approximately 45 kDa (Fig. 4A, lane 3). Anti-H37Rv CFP were used to confirm that the bacterial proteins identified in the subcellular organelles were not a result of contamination of this fraction with whole bacteria. Anti-H37Rv CFP recognized only a subset of the bacterial proteins in the subcellular fraction (Fig. 4A, lane 4) compared to the protein profile when probed against BCG itself (Fig. 4A, lane 5).
FIG. 4.
Identification of mycobacterial proteins released from the phagosome. (A) Bacterium-free subcellular organelles of macrophages infected for 24 h with fluorescein succinimidyl ester-labeled BCG were immunoprecipitated with a mouse anti-fluorescein antibody. The resulting material was analyzed by Western blot probing with rabbit antibodies specific to fluorescein (lane 1), the antigen 85-kDa complex of mycobacteria (lane 2), mycobacterial FAP (lane 3), and M. tuberculosis H37Rv CFP (lane 4). (Lane 5) BCG was probed with anti-H37Rv CFP to illustrate the mycobacterial protein profile recognized by this antibody. (B) Cryosections of BMMφ infected for 24 h with BCG and probed with rat anti-FAP (anti-rat 18-nm colloidal gold) (solid arrows) and rabbit anti-antigen 85-kDa complex (anti-rabbit 12-nm colloidal gold) (shaded arrowheads) revealed the release of these proteins into the bacterial phagosome and compartments of the host macrophage. Bar, 0.5 μm.
To further confirm the identities of these released fluorescein-labeled bacterial proteins, anti-fluorescein immunoprecipitations of subcellular fractions isolated from infected BMMφ membrane preparations were subjected to 2-D gel electrophoresis. Samples were electrophoretically transferred to nitrocellulose and probed with a rabbit anti-fluorescein antibody. Proteins recognized by this antibody were excised from a Coomassie-stained PVDF membrane, and N-terminal amino acid sequences were determined. Due to the small quantity of protein, an N-terminal sequence was obtained for only one protein of 45 kDa. The sequence TPNAQAGDPN matched that of M. bovis FAP (GenBank accession number AAB71842) with 90% identity.
The release of FAP and the antigen 85-kDa complex from the bacterial phagosome in infected macrophages was confirmed by immunoelectron microscopy. Cryosections of infected macrophages were probed with rat anti-FAP and rabbit polyclonal anti-antigen 85-kDa complex followed by the appropriate gold-conjugated secondary antibody. This approach confirmed that mycobacterial proteins of the antigen 85-kDa complex, as well as FAP, were released into the mycobacterial phagosome and could be identified associated with the phagosome membrane and within subcellular compartments distinct from mycobacteria (Fig. 4B).
Previous studies have shown that mycobacterial lipids are released from the bacterial phagosome and accumulate in late endosomal/lysosomal compartments (3). Analysis of these compartments by electron microscopy revealed an abundance of multilamellar vesicles morphologically reminiscent of MHC class II-enriched compartments (MIIC) (3). Although MHC class II is not exclusive to the late endosomal/lysosomal fraction, these compartments are enriched for class II molecules (9). To identify an association between released mycobacterial proteins and class II molecules, bacterium-free subcellular organelles isolated from macrophages infected with fluorescein succinimidyl ester-labeled BCG were subject to DGE for isolation of late endocytic compartments. Anti-fluorescein immunoprecipitation revealed that fluorescein-labeled bacterial proteins traffic to the late endocytic/lysosomal compartments (Fig. 5A, lane 1). When these fractions were probed with mycobacterium-specific antibodies, the antigen 85-kDa complex and FAP were identified in this fraction (Fig. 5A, lanes 2 and 3). Western blot analysis also revealed the presence of MHC class II in this fraction (Fig. 5A, lane 5). To determine if class II molecules and bacterial proteins were present in the same compartments, the late endosomal/lysosomal fraction was analyzed by electron microscopy. Both antigens of the 85-kDa complex and FAP localized to vesicles containing MHC class II (Fig. 5B and C). This fraction included multilamellar MIIC-like compartments containing both mycobacterial proteins and MHC class II molecules.
FIG. 5.
Mycobacterial proteins traffic to late endocytic compartments. (A) Subcellular organelles of macrophages infected for 24 h with fluorescein succinimidyl ester-labeled BCG were further fractionated by DGE to isolate late endosomal/lysosomal compartments free of bacteria. Following immunoprecipitation with mouse anti-fluorescein antibody, the resulting material was analyzed by Western blot probing with rabbit antibodies specific to fluorescein (lane 1), the antigen 85-kDa complex of mycobacteria (lane 2), mycobacterial FAP (lane 3), and M. tuberculosis H37Rv CFP (lane 4). (Lane 5) The late endosomal/lysosomal fraction was probed with an antibody recognizing the β subunit of MHC class II (KL295). (B and C) Cryosections of DGE-isolated late endosome/lysosome compartments from BMMφ infected for 24 h with BCG were probed with rabbit anti-antigen 85-kDa complex (anti-rabbit 18-nm colloidal gold) (shaded arrowheads) (B) and rabbit anti-FAP (anti-rabbit 18-nm colloidal gold) (solid arrows) (C). These compartments were also probed with mouse anti-MHC class II (KL295) (anti-mouse 12-nm colloidal gold) (solid arrowheads) and an antibody to the lysosomal marker Lamp 1 (rat anti-Lamp 1; anti-rat 6-nm colloidal gold) as shown in both panels B and C. Bar, 0.1 μm.
FAP belongs to a family of highly homologous proteins of mycobacteria (27). The attachment and internalization of several mycobacterial species to their host cell is dependent on bacterial attachment to fibronectin (24), and FAP has been proposed as the bacterial mediator of this process (23). Interestingly, an immune response to this protein, originally designated the 45/47-kDa complex of M. bovis BCG, was observed in guinea pigs inoculated with live BCG but not heat-killed BCG (25). This protein is a prime candidate for release within the context of the infected macrophage because it induces a strong T-cell response in mice (14) and is secreted in culture fluids (23).
The antigen 85-kDa complex consists of three highly related proteins of approximately 30 kDa, often referred to as the 30/31-kDa doublet (34). These proteins are associated with the bacterial surface (22) and are major secretory products in Mycobacterium tuberculosis and M. bovis BCG culture fluids (1, 6, 12). In addition, the 30-kDa protein of this complex, referred to as antigen 85B, has been found to be among the most abundant proteins produced intracellularly in human mononuclear phagocytes (18) and has been demonstrated to be immunoprotective in a guinea pig model of pulmonary tuberculosis (15). Immunocytochemical analysis by Harth et al. localized the antigen 85-kDa complex to the bacterial cell wall and the phagosomal space in infected macrophages (13). In addition, the proteins were found in cytoplasmic vacuoles distinct from the mycobacterium-containing phagosome. This complements the present study, further confirming the identification of the antigen 85-kDa complex among mycobacterial proteins that are released from the phagosome and traffic within the host macrophage.
Immunoregulation of mycobacterial infections is mediated primarily by CD4+ T cells in response to antigen in association with MHC class II molecules (17). The release of bacterial moieties from the phagosome and subsequent trafficking within macrophages provides a mechanism by which mycobacterial proteins may intersect antigen-processing compartments. Our previous studies demonstrated the presence of bacterial lipid-containing surface components in multilamellar compartments morphologically reminiscent of MIIC, suggesting the intersection of bacterial constituents with the MHC class II transport pathway (3). The intersection of specific mycobacterial peptide antigens with MHC class II molecules in the context of the infected macrophage has not been analyzed. The present study confirms that a subset of mycobacterial proteins are released differentially from the mycobacterial phagosome and intersect compartments containing class II molecules, making these proteins prime candidates for processing and presentation by MHC class II molecules.
Among the highest priorities of tuberculosis research is the identification of immunoprotective determinants for use in the production of more-effective vaccines. Numerous studies have described Mycobacterium proteins found predominantly in culture supernatants that induce strong humoral and cellular immune responses (7, 8, 16, 33, 34). However, evidence suggests that immunizations with live bacteria evoke much better protection than immunizations with dead bacteria or bacterial extracts (19, 20, 25). The chronic nature of mycobacterial infections emphasizes the importance of the identification of mycobacterial antigens released from the phagosome that are relevant to the chronic stage of the host-pathogen interaction. These would include both secreted proteins and proteins released from the bacterial surface upon contact with the intracellular environment of the macrophage. Further elucidation of released bacterial proteins in the context of an intracellular infection, and their association with specific subcellular compartments, is currently under investigation. These studies will contribute to the understanding of fundamental interactions between mycobacteria and their host cells, as well as providing relevant antigens for inclusion in a subunit vaccine.
ACKNOWLEDGMENT
This work was supported by USPHS grants HL55936 and AI33348 to D. G. Russell.
REFERENCES
- 1.Abou-Zeid C, Smith I, Grange J M, Ratliff T L, Steele J, Rook G A. The secreted antigens of Mycobacterium tuberculosisand their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988;134:531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J A, Hart P D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Rhoades E R, Ullrich H-J, Chatterjee D, Heuser J E, Russell D G. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 4.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosisphagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowle A J, Dahl R, Ross E, May M H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium aviumin cultured human macrophages are not acidic. Infect Immun. 1991;59:1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bruyn J, Bosmans R, Nyabenda J, Van Vooren J P. Effect of zinc deficiency on the appearance of two immunodominant protein antigens (32 kDa and 65 kDa) in culture filtrates of mycobacteria. J Gen Microbiol. 1989;135:79–84. doi: 10.1099/00221287-135-1-79. [DOI] [PubMed] [Google Scholar]
- 7.Espitia C, Cervera I, Gonzalez R, Mancilla R. A 38-kD Mycobacterium tuberculosisantigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol. 1989;77:373–377. [PMC free article] [PubMed] [Google Scholar]
- 8.Espitia C, Sciutto E, Bottasso O, Gonzalez-Amaro R, Hernandez-Pando R, Mancilla R. High antibody levels to the mycobacterial fibronectin-binding antigen of 30–31 kD in tuberculosis and lepromatous leprosy. Clin Exp Immunol. 1992;87:362–367. doi: 10.1111/j.1365-2249.1992.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Borja M, Verwoerd D, Sanderson F, Aerts H, Trowsdale J, Tulp A, Neefjes J. HLA-DM and MHC class II molecules co-distribute with peptidase-containing lysosomal subcompartments. Int Immunol. 1996;8:625–640. doi: 10.1093/intimm/8.5.625. [DOI] [PubMed] [Google Scholar]
- 10.Fifis T, Costopoulos C, Radford A J, Bacic A, Wood P R. Purification and characterization of major antigens from a Mycobacterium bovisculture filtrate. Infect Immun. 1991;59:800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–562. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui Y, Hirai T, Uchida T, Yoneda M. Extracellular proteins of tubercle bacilli. IV. Alpha and beta antigens as major extracellular protein products and as cellular components of a strain (H37Rv) of Mycobacterium tuberculosis. Biken J. 1965;8:189–199. [PubMed] [Google Scholar]
- 13.Harth G, Lee B Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holsti M A, Schorey J S, Brown E J, Allen P M. Identification of epitopes of fibronectin attachment protein (FAP-A) of Mycobacterium aviumwhich stimulate strong T-cell responses in mice. Infect Immun. 1998;66:1261–1264. doi: 10.1128/iai.66.3.1261-1264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackett P S, Bothamley G H, Batra H V, Mistry A, Young D B, Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 18.Lee B Y, Horwitz M A. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J Clin Investig. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovik M, Closs O. Induction of immunity against live Mycobacterium lepraemurium: a requirement for viable bacilli? Immunology. 1984;53:165–173. [PMC free article] [PubMed] [Google Scholar]
- 20.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandra L, Noss E, Boom W H, Harding C V. Phagocytic processing of antigens for presentation by class II major histocompatibility complex molecules. Cell Microbiol. 1999;1:205–214. doi: 10.1046/j.1462-5822.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 22.Rambukkana A, Das P K, Chaud A, Baas J G, Groothuis D G, Kolk A H J. Subcellular distribution of monoclonal antibody defined epitopes on immunodominant 33-kilodalton protein(s) of Mycobacterium tuberculosis: identification and localization of 29/33-kilodalton doublet proteins on mycobacterial cell wall. Scand J Immunol. 1991;33:763–775. doi: 10.1111/j.1365-3083.1991.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 23.Ratliff T L, McCarthy R, Telle W B, Brown E J. Purification of a mycobacterial adhesin for fibronectin. Infect Immun. 1993;61:1889–1894. doi: 10.1128/iai.61.5.1889-1894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratliff T L, McGarr J A, Abou-Zeid C, Rook G A, Stanford J L, Aslanzadeh J, Brown E J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988;134:1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- 25.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. Identification of a Mycobacterium bovisBCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993;61:742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell D G. Immunoelectron microscopy of endosomal trafficking in macrophages infected with microbial pathogens. Methods Cell Biol. 1994;45:277–288. doi: 10.1016/s0091-679x(08)61857-9. [DOI] [PubMed] [Google Scholar]
- 27.Schorey J S, Holsti M A, Ratliff T L, Allen P M, Brown E J. Characterization of the fibronectin-attachment protein of Mycobacterium aviumreveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol. 1996;21:321–329. doi: 10.1046/j.1365-2958.1996.6381353.x. [DOI] [PubMed] [Google Scholar]
- 28.Sibley L D, Franzblau S G, Krahenbuhl J L. Intracellular fate of Mycobacterium lepraein normal and activated mouse macrophages. Infect Immun. 1987;55:680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturgill-Koszycki S, Haddix P L, Russell D G. The interaction between Mycobacteriumand the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1997;18:2558–2565. doi: 10.1002/elps.1150181411. [DOI] [PubMed] [Google Scholar]
- 30.Sturgill-Koszycki S, Schaible U E, Russell D G. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 31.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacteriumphagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 32.Tulp A, Verwoerd D, Fernandez-Borja M, Neefjes J, Hart A A. High resolution density gradient electrophoresis of cellular organelles. Electrophoresis. 1996;17:173–178. doi: 10.1002/elps.1150170128. [DOI] [PubMed] [Google Scholar]
- 33.Vordermeier H M, Harris D P, Friscia G, Roman E, Sourcel H M, Moreno C, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to immunodominant epitope of 38-kDa antigen. Eur J Immunol. 1991;22:2631–2337. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 34.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis- and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]