Abstract
Glycosylation, which consists of the enzymatic addition of sugars to proteins and lipids, is one of the most important post-co-synthetic modifications of these molecules, profoundly affecting their activity. Although the presence of carbohydrate chains is crucial for fine-tuning the interactions between cells and molecules, glycosylation is an intrinsically stochastic process regulated by the relative abundance of biosynthetic (glycosyltransferases) and catabolic (glycosidases) enzymes, as well as sugar carriers and other molecules. Non-coding RNAs, which include microRNAs, long non-coding RNAs and circRNAs, establish a complex network of reciprocally interacting molecules whose final goal is the regulation of mRNA expression. Likewise, these interactions are stochastically regulated by ncRNA abundance. Thus, while protein sequence is deterministically dictated by the DNA/RNA/protein axis, protein abundance and activity are regulated by two stochastic processes acting, respectively, before and after the biosynthesis of the protein axis. Consequently, the worlds of glycosylation and ncRNA are closely interconnected and mutually interacting. In this paper, we will extensively review the many faces of the ncRNA–glycosylation interplay in cancer and other physio-pathological conditions.
Keywords: glycosylation, glycosyltransferases, non-coding RNAs, miRNA, sugar antigens
1. Introduction
Glycosylation consists of the enzymatic addition of sugars or sugar chains to proteins or lipids, giving rise to glycoproteins and glycolipids, respectively. Glycans are attached to proteins usually through an amidic linkage to asparagine (N-linked) or to the hydroxyl group of serine or threonine (O-linked). The presence of these sugar chains exerts a subtle but crucial functional effect, modulating the interactions between molecules and cells. Glycans are deeply altered in pathological conditions, including cancer [1], inflammation [2] and aging [3,4]. Glycan structure is not under direct genetic control, but results from the cooperative and competitive interaction between glycosyltransferases. These enzymes transfer a monosaccharide from an activated sugar donor (frequently a nucleotide-sugar) to an acceptor, which can be an amino acid, a lipid or another sugar. Consequently, glycosylation can be considered a stochastic rather than a deterministic process. The biological role of glycans is frequently mediated by sugar-binding molecules (lectins) which, upon recognition of specific carbohydrate structures, trigger a broad range of cellular effects, including proliferation, apoptosis and cell migration. Galectins and siglecs are among these sugar-binding molecules. The extraordinary importance of non-coding RNAs (ncRNAs)—which include micro RNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs)—in gene expression regulation, both at the transcriptional and the post-transcriptional level, is increasingly being recognized. Together, ncRNAs form an extremely complex non-deterministic network of gene expression regulation. Many glycosyltransferases and sugar-binding molecules have been shown to be regulated by ncRNAs. On the other hand, glycosylation has been demonstrated to modulate ncRNA expression in some instances. Finally, small ncRNAs have recently been shown to undergo canonical N-glycosylation [5]. These glyco-RNAs bear terminal sialic acid (Sia) and fucose (Fuc) residues and can interact with sugar-binding molecules, such as siglecs [5]. Thus, ncRNA network and glycosylation can be considered as two stochastic mechanisms affecting the role of protein: the first acting before, and the second after, protein axis biosynthesis. The purpose of this review is to provide an overview of the emerging picture of the glycans–ncRNA bi-directional relationship.
2. The Essentials of Non-Coding RNAs
RNA-seq technologies revealed that while the human genome is widely transcribed, only a small percentage of RNA (~2%) is protein-coding, broadening the spectrum of RNAs involved in gene expression regulation. According to their length, ncRNAs can be grouped in two main classes: miRNAs and lncRNAs [6].
2.1. miRNA
Mature miRNAs are 22-nucleotide (nt)-long RNAs. They are transcribed by RNA polymerase II into a primary form called (pri)-miRNA and undergo a maturation process, first in the nucleus (cropping), then in the cytosol (dicing), by the ribonucleases Drosha and Dicer, respectively. In the nucleus, (pri)-miRNA are converted by Drosha into miRNA precursors, then translocated in the cytosol where they are cleaved by Dicer into double-stranded mature miRNA. Only the guide strand from the miRNA duplex is incorporated in RNA-induced silencing complex (RISC), and it directs the RISC to degrade the complementary target-mRNA. Although post-transcriptional gene regulation represents the main function of miRNAs, they also exert control over other ncRNAs, interacting with lncRNAs and circRNAs [7]. Typically, both the gene locus and precursor miRNA (pre-miRNA) of a miRNA is referred as “mir”, while the mature miRNA product is designated “miR”. miRNA are defined by the prefix miR, followed by a number (e.g., miR-515). A three-letter code prefix defines the species (e.g., hsa-mir-515 indicates Homo sapiens). This can be followed by a letter (e. g. miR-515a and miR-515b) if the miRNA with the same number diverge for one or two nucleotides. These miRs belong to the same family. If two diverse loci produce identical mature products, an additional number is given after the full name. For instance, mir-515-1 and mir-515-2 produce the same final miRNA product: miR-515. To indicate whether the mature sequence comes from the 5’ arm or the 3’ arm of the precursor, the -5p or the -3p suffix are added (e.g., miR-515-5p or miR-515-3p).
2.2. lncRNAs
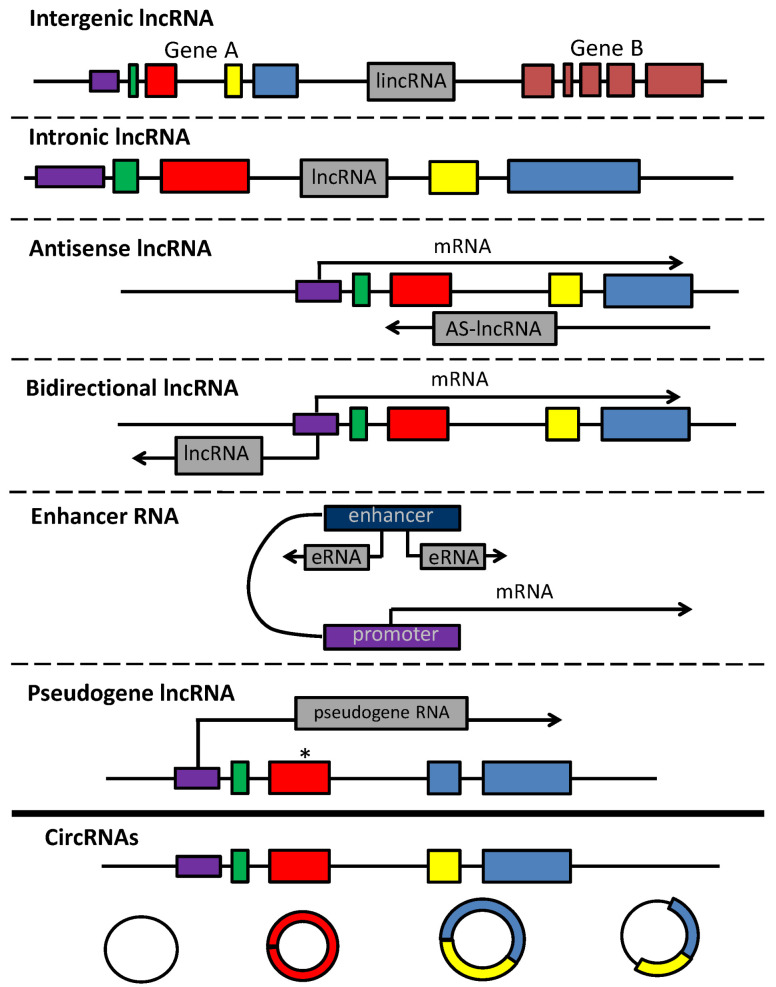
LncRNAs are >200-nt-long transcripts that do not encode for proteins. However, lncRNAs share common traits with mRNAs, since most of them are transcribed by RNA polymerase II and undergo specific post-transcriptional modifications, such as capping, polyadenylation and alternative splicing [8]. Depending on their position in the genome, lncRNAs are referred to as “intergenic” (lincRNAs) when localized between two protein-coding genes (Figure 1); “intronic” (lncRNA) when situated within the intronic portion of a protein-coding gene; “antisense” (AS-lncRNAs) when derived from the antisense RNA strand of protein-coding gene; “bidirectional” when transcribed from the same promoter of protein-coding gene, but in the opposite direction; and enhancer RNAs (eRNAs) when derived from enhancer regions, aiding the transcription factor placement in the proximity of promoters. LncRNAs are also generated by transcription of pseudogenes (genes carrying one or more mutations affecting their RNA translation). Other than linear lncRNAs, circRNAs represent the most abundant isoform, resulting from both canonical and back-splicing [6]. Due to their strong tissue-specific expression, lncRNAs exert a key role in several physiological processes such as cell cycle, differentiation and metabolism, and their dysregulation may lead to disease, such as cancer and infections. LncRNA display different mechanisms of action depending on their localization in the nucleus or cytoplasm [8]. In the nucleus, they can modulate transcription through the recruitment of TF to the promoter (eRNAs) or through the direct interaction with the RNA pol II (circRNAs). LncRNAs can also induce epigenetic modifications via histone remodeling. In the cytoplasm, lncRNAs act post-transcriptionally by interacting with RNAs and/or proteins. For instance, lncRNAs regulate alternative splicing, mRNA stability and RNA availability. Both linear and circular lncRNAs can act as molecular sponges by harboring a genomic region complementary to miRNA that competes with miRNA for the target site of mRNA (competitive endogenous RNAs, ceRNA). lncRNAs sequester miRNA, thus impairing the interaction with miRNA-target RNA and preventing targeted mRNA degradation [6].
Figure 1.
LncRNAs and circRNAs. Gene A comprises 4 exons (in green, red, yellow and blue, respectively). Its promoter is depicted in violet. Intergenic lncRNAs are generated by transcription of sequences between genes; intronic lncRNAs are generated by transcription of intronic regions between exons of a coding gene; antisense lncRNAs are produced by transcription of the antisense DNA strand; bidirectional lncRNAs are produced from the antisense transcription starting from the promoter of the coding gene; enhancer RNAs are derived from enhancer sequences; pseudogene lncRNAs are produced by transcription of genes carrying inactivating mutations (pseudogenes). Mutation is marked by an asterisk. circRNAs are produced from (left to right) intronic sequences, single exons, multiple exons and intronic and exonic sequences.
3. The Essentials of Glycosylation
3.1. N-Glycosylation
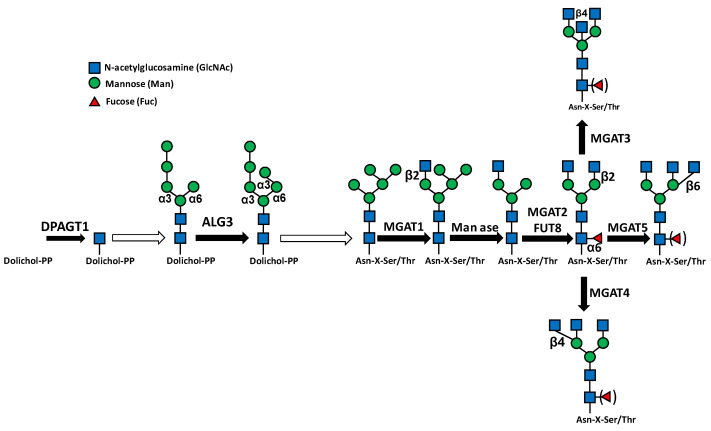
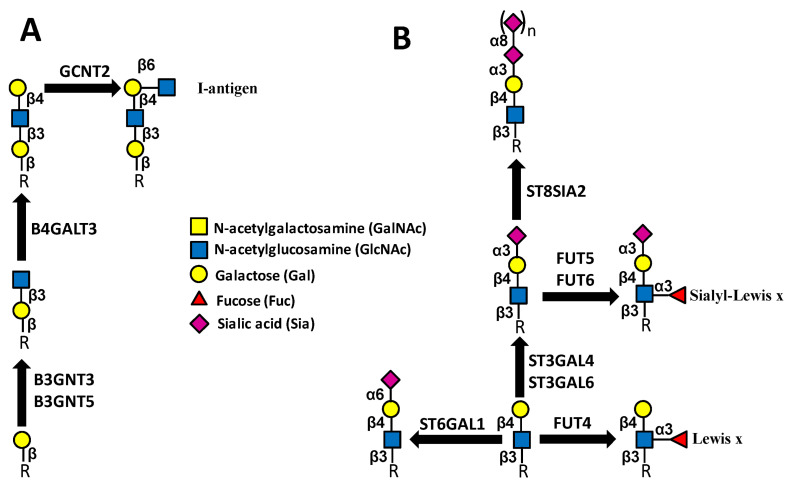
N-glycosylation starts in the rough endoplasmic reticulum (RER) with the building of an oligosaccharide comprising three N-acetylglucosamine (GlcNAc), nine mannose (Man) and three glucose (Glc) residues on the dolichol phosphate lipid-carrier [9,10] (Figure 2). This “high mannose” molecule is subsequently transferred “en bloc” to an Asn-X-Ser/Thr consensus motif of a nascent polypeptide chain. After transfer to protein, the high-mannose oligosaccharide undergoes sequential trimming of the three Glc residues and of six Man residues, followed by the building and elongation of the outer branches by the addition of GlcNAc and Gal residues. At these stages, the addition of a “core fucose” to the innermost GlcNAc residue can also occur. Finally, these branches can be elongated and capped, usually by Sia and/or Fuc. Sialic acid is always in the terminal position of the branches and can be followed only by successive Sia residues.
Figure 2.
N-glycosylation steps regulated by ncRNAs. Black arrows indicate single-step reactions. White arrows indicate that the indicated transition is the product of multiple steps.
3.2. O-Glycosylation
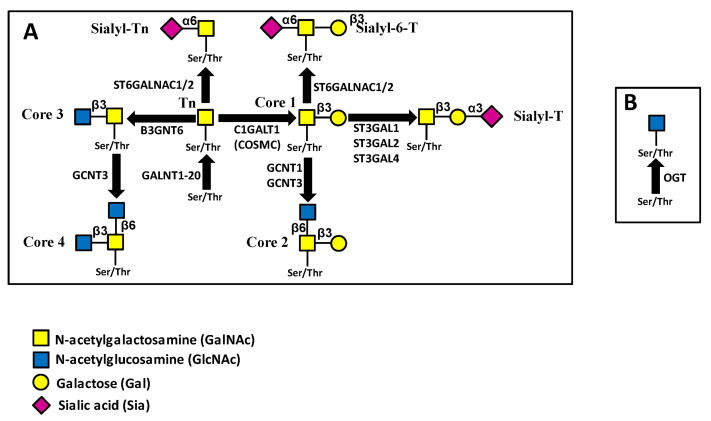
O-glycosylation consists of the stepwise addition of single sugars to the oligosaccharide chains of glycoproteins during their transit along the exocytic pathway [11]. In the canonical “mucin-type” O-glycosylation, linkage with peptide involves the N-acetylgalactosamine (GalNAc) terminal residue (Figure 3A). This step can be catalyzed by 20 different GalNAc transferases, with subtle differences in substrate specificities [12]. Subsequently, a defined number of basic “core” structures are synthesized, elongated and finally “capped”, usually by Sia and Fuc (Figure 3A). A peculiar type of glycosylation, represented by the addition of a single GlcNAc residue to Ser/Thr (O-GlcNAcylation), is mediated by the O-GlcNAc transferase product of the OGT gene [13] (Figure 3B). Unlike conventional glycosylation, O-GlcNAcylation regards cytoplasmic and nuclear proteins and competes with phosphorylation for the post-translational modification of Ser/Thr residues. OGT plays a particularly relevant role in regulating gene expression because GlcNAcylation of Polycomb group proteins is necessary for their transcriptional repression activity [14,15].
Figure 3.
Pathways of O-glycosylation. (A) mucin-type; (B) O-GlcNAc. Enzymes catalyzing the same reactions but not mentioned in the text are not shown.
3.3. Glycolipids
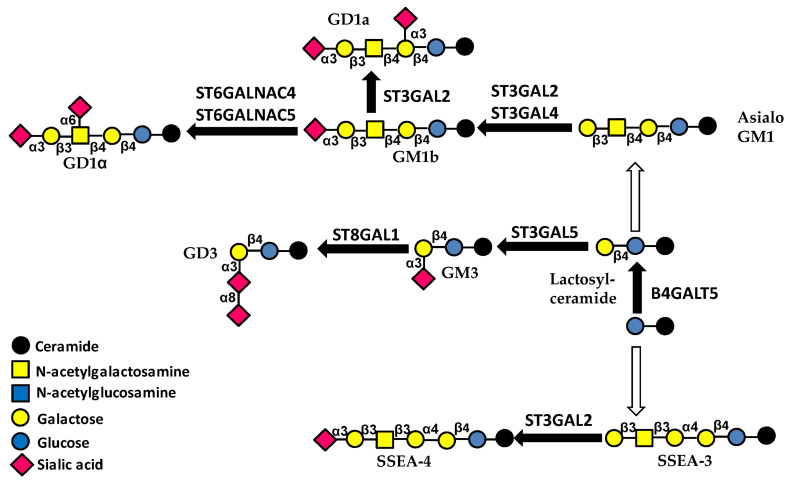
Glycolipid biosynthesis starts with the addition of glucose (Glc) to the lipid ceramide, followed by the addition of galactose by B4GALT5 (Figure 4). To this core structure, a Sia residue can be added by sialyltransferase ST3GAL5 forming GM3 ganglioside. Gangliosides are sialylated glycolipids, and GM3 is the simplest member of this category.
Figure 4.
Pathways of glycolipid biosynthesis. Black arrows indicate single-step reactions. White arrows indicate that the indicated transition is the product of multiple steps. Enzymes catalyzing the same reactions but not mentioned in the text are not shown.
4. Regulation of Glycosylation by ncRNAs
Non-coding RNAs regulate multiple steps of the biosynthesis of N- and O-glycans. In a pioneering work, Kurcon et al. [16] used miR of the 200 family (miR-200f), known to regulate the epithelial to mesenchymal transition (EMT), as a proxy to identify glycosyltransferases involved in EMT. ST3GAL5 and ST6GALNAC5 were among the identified enzymes. The proxy approach to the study of glycosylation control by miRNAs has been recently reviewed in detail [17]. In this section, we will discuss how glycosyltransferase genes are regulated by ncRNAs. We have classified glycosyltransferases in the following groups: (i) initiating glycosyltransferases, which catalyze the initial steps of the biosynthesis of N- and O-glycans and glycolipids; (ii) core-extending glycosyltransferases, which elaborate core structures of N- and O-glycans and glycolipids; (iii) elongating glycosyltransferases, which elongate carbohydrate chains shared by different glycoconjugate classes; and (iv) capping glycosyltransferases, which cap carbohydrate chains shared by different glycoconjugate classes. In addition, we have considered two main classes of sugar-binding molecules, namely galectins and siglecs. A large part of the literature deals with cancer, but a relevant number of studies deal with the ncRNA regulation of glycosylation in other pathological contexts. The numerous examples of glycogene regulation by ncRNAs are summarized in Table 1.
Table 1.
Glycogenes modulated by ncRNAs.
| Target Glycogene | Upstream ncRNA | Downstream ncRNA | Downstream Target |
Tissue and Reference |
|---|---|---|---|---|
| A/B glycosyltransferases | miR-331-3p miR-1908-5p | Biosynthesis of AB0 antigens [18] | ||
| ALG3 | miR-98-5p | Lung cancer [19] | ||
| B3GNT3 | miR-149-5p | Lung cancer [20] | ||
| B3GNT5 | miR1365-5p | MIR44352HG | Liver cancer [21] | |
| B4GALT3 | miR-27 | β1 integrins | Cervical cancer [22] | |
| miR-338-3p | DANCR | Neuroblastoma [23] | ||
| B4GALT5 | miR-491-5p | circ_0009910 | miR-491-5p | Acute myeloid leukemia [24] |
| C1GALT1 | miR-181d-5p | RAC1 | Lung cancer [25] | |
| miR-1-3p | circ HP1BP3 | Bladder cancer [26] | ||
| miR-124-3p | Aging colon [27] | |||
| COSMC | miR-374b | IgA nephropathy [28] | ||
| DPAGT1 | miR-485-5p | LINC00467 | Esophageal cancer [29] | |
| FUT4 | miR-200c | Colorectal cancer [30] | ||
| miR-26a/b | MALAT1 | PI3/AKT | Colorectal cancer [31,32] | |
| miR-224-3p | Breast cancer [33] | |||
| miR-493 | GAS6-AS2 | Breast cancer [34,35] | ||
| miR-200c | Breast cancer [36] | |||
| miR-493-5p | Breast cancer [34] | |||
| miR-200b | Breast cancer [37] | |||
| miR-371b-5p | AC114812.8 | Bladder cancer [38] | ||
| miR-29b | Sp1 | CD44 | Leukemia stem cells [39] | |
| HOXB-AS1 | Multiple myeloma [40] | |||
| miR-199b-5p | Medulloblastoma [41] | |||
| miR26a/b | NFkB | Osteoarthritis [42] | ||
| miR200b | Arthritis [43] | |||
| miR200c | Uterine receptivity [44] | |||
| FUT5 | miR-125a-3p | PI3K/AKT | Colorectal cancer [45] | |
| FUT6 | miR-125a-3p | PI3K/AKT | Colorectal cancer [45] | |
| miR-326 | HOTAIR | CD44/PI3K/AKT | Colorectal cancer [46] | |
| miR-106b | Breast cancer [47] | |||
| FUT8 | miR-34a, miR-122 | Liver cancer [48] | ||
| HOTAIR | JAK/STAT3 | Liver cancer [49] | ||
| miR-198-5p | Lung cancer [50] | |||
| miR-186 | SNHG1 | MMP2/MMP9 | Oral cancer [51] | |
| miR-34c-5p | Renal interstitial fibrosis [52] | |||
| GALNT1 | miR-129 | Bladder cancer [53] | ||
| let-7i-5p | Kidney fibrosis [54] | |||
| GALNT10 | mir-505 | DLGAP1-AS2 | Cholangiocarcinoma [55] | |
| miR-122 | Liver cancer [56] | |||
| GALNT14 | miR-125a | MMP2 and MMP9 | Ovarian cancer [57] | |
| GALNT3 | miR-378a-3p | PSMA3-AS1 | PI3K/Akt | Ovarian cancer [58] |
| GALNT4 | miR-506-3p | Lung cancer [59] | ||
| miR-365b | Lung cancer [60] | |||
| miR-9 | Liver cancer [61] | |||
| GALNT7 | miR-30b/30d | Melanoma [62] | ||
| miR-34a | SNHG7 | PI3K/Akt/mTOR | Colorectal cancer [63] | |
| miR-30e | Cervical cancer [64] | |||
| miR-214 | Cervical cancer [65] | |||
| miR-34a/c | Laryngeal cancer [66] | |||
| miR-214 | Esophageal cancer [67] | |||
| miR-17-5p/miR-17-3p | Liver cancer [68] | |||
| miR-30c | PI3K/AKT | Natural killer activity [69] in lung cancer | ||
| miR-378 | Osteoblast differentiation [70] | |||
| GCNT2 | miR-199a/b-5p | Colorectal cancer [71] | ||
| GCNT3 | miR-15b | Pancreatic and colorectal cancer [72] | ||
| miR-BART1-5p | EBV-induced gastric cancer [73] | |||
| miR-195-5p | LINC00511 | Lung cancer [74] | ||
| LGALS3 | miR-424-3p | Ovarian cancer [75] | ||
| miR-128 | Colorectal cancer [76] | |||
| miR-128-3p | Pancreatic cancer [77] | |||
| miR-299-5p | circRERE | Apoptosis of nucleus polposum cells [78] | ||
| LGALS9 | miR-455-5p | Colorectal cancer [79] | ||
| miR-22 | Liver cancer [80] | |||
| MGAT1 | LINC00173 | mucin 3A | Wilms’ tumor [81] | |
| MGAT3 | miR-23b | Tau protein | Alzheimer’s disease [82] | |
| MGAT4A | miR-424 | cyclin D1 | Breast cancer [83] | |
| MGAT5 | miR-124 | Breast cancer [84] | ||
| OGT | miR-485-5p | Esophageal cancer [85] | ||
| miR-15a/miR-26a | Kidney cancer [86] | |||
| miR-424-5p | XIST | Raf1 | Liver cancer [87] | |
| miR-122 | RYR1 | Breast cancer [88] | ||
| miR-15b | RORγt | Th17 differentiation [89] | ||
| miR-423-5p | Apoptosis of cardiomyocytes [90] | |||
| SIGLEC15 | miR-7109-3p | LINC00973 | Kidney cancer [91] | |
| miR-582-5p | TUG1 | Liver cancer [92] | ||
| ST3GAL1 | MEG3 | EGFR/PI3K/AKT | Kidney cancer [93] | |
| ST3GAL2 | Gut infection [94] | |||
| ST3GAL4 | miR-193a-3p miR-224 | PI3K/AKT | Kidney cancer [95] | |
| miR-224, let-7i | Chronic myeloid leukemia [96] | |||
| miR-370 | Colorectal cancer [97] | |||
| miR193-b | CD44/NF-kB | Arthritis [98] | ||
| ST3GAL5 | miR-26a, miR-548l, miR-34a | Liver cancer [99] | ||
| ST3GAL6 | miR-26a | AKT/mTOR | Liver cancer [100] | |
| ST3GAL6-AS1 | EGFR | Lung cancer [101] | ||
| ST3GAL6-AS1 | MLL1 | ST3GAL6 | Colorectal cancer [102] | |
| ST3GAL6-AS1 | HNRNPA2B1 | ST3GAL6 | Multiple myeloma [103] | |
| ST6GAL1 | miR-9 | β1-integrins/FAK | Liver cancer [104] | |
| miR-195-3p | TINCR | NFkB | Liver cancer [105] | |
| miR-214-3p | Breast cancer [106] | |||
| miR-150 | ZF-AS1 | EGFR/PI3K/Akt | Acute lymphoblastic leukemia [107] | |
| miR-199a | ErbB2/ErbB3 | Various cancers [108] | ||
| ST6GALNAC2 | miR-182/miR-135b | PI3K/AKT | Colorectal cancer [109,110] | |
| ST6GALNAC4 | miR-4299 | Thyroid cancer [111] | ||
| ST6GALNAC5 | miR-182 | Prostate cancer [112] | ||
| ST8SIA1 | miR-33a/let-7e | Colorectal cancer [113] | ||
| MIR44352HG | FAK/AKT/β-catenin | Prostate cancer [114] | ||
| ST8SIA2 | miR-3072-3p | TUG1 | Brain ischemia [115] | |
| ST8SIA4 | miR-26a/b | MALAT1 | Breast cancer [116,117] | |
| miR-146a/b | PI3K-AKT-mTOR | Thyroid cancer [118] | ||
| miR-144-5p/miR-451a | Cholangiocarcinoma [119] |
4.1. Initiating Glycosyltransferases
4.1.1. N-Linked Chains
The initial step of N-glycans biosynthesis consists of the addition of GlcNAc to dolichol phosphate and is mediated by DPAGT1 (Figure 2). In esophageal squamous cell carcinoma, DPAGT1 promotes growth and is down-regulated by miR-485-5p which, in turn, is sponged by lncRNA LINC00467. Consequently, the latter behaves as an oncogene [29]. A second example of regulation of an initiating N-glycosyltransferase is provided by the α3 mannosyltransferase ALG3, which is involved in a biosynthetic step of the GlcNAc2, Man9, Glc3 precursor (Figure 2). ALG3 contributes to the malignancy of non-small-cell lung cancer (NSCLC) and is negatively regulated by miR-98-5p [19].
4.1.2. Mucin-Type O-Glycosylation
In bladder cancer, miR129 exerts growth inhibition by repressing GALNT1 [53] (Figure 3A). In ovarian cancer, lncRNA PSMA3-AS1 promotes cell proliferation, migration and invasion by sponging miR-378a-3p, which targets GALNT3. This leads to GALNT3 overexpression and PI3K/Akt pathway activation [58]. GALNT4 is one of the few GALNTs with preference for partially GalNAc-glycosylated substrates, modifying the available sites not employed by other GALNTs. In prostate cancer [59] and NSCLC [60], GALNT4 behaves as a tumor-promoting gene. In the former it is targeted by miR-506-3p, whereas in the latter it is targeted by miR-365b. By contrast, in hepatocellular carcinoma, GALNT4 behaves as a tumor-restraining enzyme, inhibited by miR-9 [61]. GALNT7 also prefers partially GalNAc-glycosylated substrates. Reports indicate opposite roles in different contexts. In fact, GALNT7 is tumor-promoting in cervical carcinoma (targeted by miR-30e [64] and miR-214 [65]), esophageal squamous cell cancer (targeted by miR-214 [67]), laryngeal carcinoma [66] and colorectal cancer [63], both targeted by miR-34a. On the contrary, in melanoma [62] and hepatocellular carcinoma [68] GALNT7 plays a tumor-restraining role. GALNT7 inhibits cytolytic activity of natural killer cells associated with lung cancer. Consistently, miR-30c which targets GALNT7 enhances NK cytotoxicity [69]. GALNT10 exerts a tumor-promoting activity in cholangiocarcinoma [55] and in hepatitis B virus-associated hepatocellular carcinoma [56], regulated by DLGAP1-AS2/miR -505 in the former and by miR-122 in the latter. miR-125a inhibits ovarian cancer proliferation and invasion by repressing GALNT14 expression [57].
The following are examples of mucin-type O-glycosylation regulation by ncRNA in non-neoplastic conditions. miRNA let-7i-5p exacerbates kidney fibrosis by targeting GALNT1 [54]. MiR-378 binds competitively to both the 3’ UTR of the nephronectin (an extracellular glycoprotein increasing osteoblast differentiation) mRNA and the GALNT7 transcript. Nephronectin glycosylation by GALNT7 creates a complex balance modulating osteoblast differentiation [70].
4.1.3. O-Linked GlcNAc
In esophageal cancer, malignancy is increased by OGT over-expression due to down-regulation of miRNA-485-5p [85]. A similar condition was observed by miR-15a and miR-26a in clear-cell renal cell carcinoma [86]. In hepatocarcinoma, the regulation of RAF1 oncogene, which is involved in progression, offers a good example of the interplay between a glycosyltransferase, such as OGT, miRNAs and lncRNAs. In fact, OGT mediates RAF1 O-GlcNAcylation, promoting its stability. MiR-424-5p targets OGT but it is sponged by the lncRNA XIST (which plays a major role in the mechanisms of chromosome X inactivation in females) [87]. O-GlcNAcylation is involved in muscular homeostasis. In cancer, a decline in skeletal muscle mass is often observed. It has been shown that miR-122, encapsulated in extracellular vesicles and released by breast cancer cells, suppresses OGT, reducing O-GlcNAcylation of ryanodine receptor RYR1, resulting in skeletal muscle proteolysis [88]. Besides the many examples of glycosylation regulation by ncRNA, opposite cases also exist. In particular, the miR-483-3p production in liver cancer cells is made possible by the O-GlcNAcylation of the transcriptional complex at the miR-483 promoter [120]. Other examples of miRNA-mediated OGT regulation in non-neoplastic conditions are presented as follows. OGT targeting by miR-501-3p and miR-619-3p is a key factors in the regulation of hepatitis C virus assembly and infectivity [121]. Multiple sclerosis is a de-myelinating autoimmune disease in which the helper T cell subpopulation Th17 plays a major role. The transcription factor RORγt, which is the key determinant for Th17 differentiation, requires O-GlcNAcylation. OGT targeting by miRNA-15b suppresses Th17 differentiation, ameliorating demyelination in animal models of multiple sclerosis [89]. Apoptosis of cardiomyocytes, an event closely associated with congestive heart failure, is prevented by O-GlcNAcylation. Targeting of OGT by miR-423-5p promotes apoptosis in cardiomyocytes [90]. As shown in a recent review [17], OGT is a glycosyltransferase tightly regulated by miRNAs.
4.1.4. Glycolipids
The number of studies reporting regulation by the ncRNA network of the first steps of glycolipid biosynthesis is surprisingly small. B4GALT5 synthesizes lactosylceramide, the core portion of glycolipids (Figure 4). Acute myeloid leukemia progression is promoted by B4GALT5 and circ0009910 which sponges miR-491-5p, activating the PI3K/AKT signaling pathway [24].
4.2. Core-Extending Glycosyltransferases
In this class are included the glycosyltransferases acting directly on the core structures of N- and O-linked chains of glycoproteins and of glycolipids.
4.2.1. N-Linked Chains
Core-extending glycosyltransferases of N-linked chains include fucosyltransferase 8 (FUT8), mediating the addition of α6-linked fucose to the innermost GlcNAc of the core, and GlcNAc transferases 1-5, product of genes MGAT1-MGAT5, which add GlcNAc to the Man residues of the trimannosyl core (Figure 2).
FUT8: In cancer, FUT8 increase is unambiguously associated with malignancy. FUT8 is directly targeted by miR-122 and miR-34a in hepatocarcinoma [48], as well as by miRNA-198-5p in NSCLC [50], leading to reduced malignancy in both cases. In oral squamous cell carcinoma, FUT8 inhibitor miR-186 is sponged by lncRNA SNHG1 [51]. In breast cancer, miR-10b enhances FUT8 expression through noteworthy mechanisms, highlighting a more complex glycosyltransferase/miRNA relationship [122]. In fact, FUT8 transcription requires phosphorylated STAT3. In turn, the transcription factor activator protein 2γ (AP-2γ), which is targeted by miR-10b, binds to STAT3, preventing its phosphorylation. Thus, inhibition of AP-2γ by miR-10b results in FUT8 activation [122]. FUT8 transcription in liver cancer involves the indirect (through Hsp90 and MUC1) activation of the STAT3/JAK1 cascade, which is potentiated by antisense RNA HOTAIR [49]. Another interesting mechanism is the basis of FUT8 regulation by lncRNA LEF1-AS1 in colorectal cancer. This lncRNA recruits the histone methyltransferase MLL1 (product of the KMT2A gene) to the LEF1 promoter site, resulting in increased LEF1 expression and FUT8 transcription via the Wnt/β-catenin pathway [123]. FUT8-mediated core fucosylation of various profibrotic signals is a crucial event in the pathogenesis of renal interstitial fibrosis, a pathology secondary to chronic kidney diseases. FUT8 targeting by miR-34c-5p delivered by mesenchymal stem cells ameliorates the disease [52].
MGATs: MGAT5 is a well-known GlcNAc transferase involved in malignancy, particularly in metastasis formation in various systems [124]. In breast cancer, decreased miR-124-3p, which targets MAGAT5, promotes proliferation and metastasis [84]. On the other hand, in mammary cells, miR-424 has been shown to down-regulate the expression of MGAT4A, the GlcNAc transferase which adds GlcNAc in β4 linkage to the trimannosyl core (Figure 2). The presence of this modification promotes malignancy through cyclin D1 activation [83]. A complex mechanism of glycosylation regulation by ncRNAs is provided by LINC00173, which promotes Wilms’ tumor progression. LINC00173 stabilizes MGAT1 mRNA by recruiting the HNRPA2B1 ribonucleoprotein, resulting in mucin MUC3A N-glycosylation and tumor progression [81]. One of the hallmarks of Alzheimer’s disease is the presence of a hyperphosphorylated form of tau protein, which is the basis of neurofibrillary tangle formation. Tau pathology is attenuated by miRNA-23b through MGAT3 targeting [82].
4.2.2. O-Linked Chains
Core 1 structures: The biosynthesis of core 1 O-linked structures starts with the addition of Gal to GalNAc by galactosyltransferase C1GALT1 (Figure 3), which requires the presence of the molecular chaperone COSMC. C1GALT1 expression promotes lung cancer progression by oncogene RAC1 up-regulation. This activity is negatively regulated by miR-181d-5p, which targets C1GALT1 [25]. C1GALT1 promotes malignancy even in bladder cancer, but it is inhibited by miR-1-3p, which is sponged by circHP1BP3 [26]. In aging colons, C1GALT1 expression is decreased, making mucus glycosylation defective and increasing susceptibility to colitis. This is partially due to overexpression of miR-124-3p, which targets C1GALT1 [27]. In IgA nephropathy, IgGA1 antibodies are aberrantly O-glycosylated because of the increased expression of miR-374b, which targets COSMC [28].
Core 2 and 4 structures: The β6 GlcNAc transferase GCNT3 is responsible for core 2 and core 4 O-glycan biosynthesis (Figure 3). Down-regulation by miR15b inhibits colon and pancreatic cancer growth [72]. MiR-BART1-5p is an Epstein–Barr virus (EBV)-encoded miRNA expressed in all stages of the viral infection. In EBV-associated gastric cancer, miR-BART1-5p targets GCNT3 to repress cell proliferation and migration [73]. In lung cancer, GCNT3 is up-regulated by LINC00511, which sponges its inhibitor miR-195-5p [74]. Noteworthy, GCNT3 appears to exert a tumor-restraining activity in colon and pancreatic cancer [72] and a tumor-promoting activity in gastric and lung cancers [73,74].
Core 3 structures: Core 3 glycans provide another example of miRNA regulation by glycosylation rather than the opposite. MUC1 is a heavily O-glycosylated membrane glycoprotein comprising an extracellular amino-terminal domain involved in cell adhesion and an intracellular C-terminal domain involved in cell signaling. In colon cancer, core 3 glycans synthesized by B3GNT6 decorate the N-terminal portion of MUC1, hindering the nuclear migration of the C-terminal portion of the protein. The absence of the C-terminal portion of MUC1 in the nucleus triggers the transcription of p53 and miR-200c, enhancing the mesenchymal to epithelial transition (which is the opposite of the more popular epithelial to mesenchymal transition) [125].
α2,6-Sialylation: ST6GALNAC transferases 1 and 2 mediate the α2,6-sialylation of the innermost GalNAc of O-linked chains. MiR-30d-5p is involved in NSCLC progression and is proposed to act by regulating several genes and their downstream pathways. ST6GALNAC1 is among these genes, indicating a possible role of mucin-type O-glycans [126]. MiR-182 and miR-135b increase malignancy of colorectal cancer cells by targeting ST6GALNAC2 which behaves as a tumor-restraining enzyme in this system [109,110].
4.2.3. Glycolipids
The core-extending glycolipid sialyltransferase ST3GAL5 is targeted by miR-26a, miR-548l and miR-34a, resulting in inhibition of hepatocarcinoma progression [99].
4.3. Elongating Glycosyltransferases
Elongating glycosyltransferases add sugars, such as GlcNAc and Gal, to core structures of N- and O-linked chains and glycolipids, forming linear or branched polylactosaminic structures (Figure 5A).
Figure 5.
Elongating (A) and capping (B) glycosyltransferases. Enzymes catalyzing the same reactions but not mentioned in the text are not shown.
4.3.1. GlcNAc Transferases
The enzyme B3GNT3, involved in polylactosamine biosynthesis, is targeted by miR-149-5p. In lung cancer, it promotes progression and is associated with poor prognosis [20]. B3GNT5 participates in elongation of glycolipids. LncRNA MIR44352HG is a well-recognized oncogene, regulating various signaling pathways. In liver cancer, it promotes progression by sponging miR1365p, leading to B3GNT5 upregulation [21]. GCNT2, a β6 GlcNAc transferase crucial for the biosynthesis of I antigen (Figure 5) whose expression is positively associated with malignancy in colon cancer cell lines, is targeted by miR-199a/b-5p [71].
4.3.2. Gal Transferases
B4GALT3 adds Gal to GlcNAc, forming type 2 lactosaminic chains. In human cervical cancer cells, B4GALT3 is unconventionally up-regulated by miR-27, contributing to oncogenic activity through stabilization of β1 integrins [22]. LncRNA DANCR inhibits cell differentiation often associated with cancer. In neuroblastoma, DANCR promotes B4GALT3 expression and malignancy by sponging miR-338-3p [23].
4.4. Capping Glycosyltransferases
Capping glycosyltransferases add terminal sugars to extended carbohydrate chains. This group includes mainly, but not exclusively, fucosyltransferases and sialyltransferases. Among the most relevant terminal-fucosylated structures are the Lewisx (Lex) and its sialylated counterpart sialyl Lewisx (sLex) (Figure 5). The overexpression of these structures in several cancers correlates with malignancy through different mechanisms [127], including binding to cells’ adhesion molecules of the selectin family [128,129]. While the biosynthesis of Lex occurs through the simple addition of a α1,3-linked Fuc on a type 2 chain, the biosynthesis of sLex requires the preliminary addition to the type 2 chains of a α2,3-linked Sia (Figure 5)
4.4.1. Fucosyltransferases
FUT4. FUT4 is mainly responsible for the biosynthesis of Lex, while its contribution to the biosynthesis of sLex is considered marginal [130]. Numerous studies report FUT4 regulation by ncRNAs in various cancers and its contribution to malignancy. In colorectal cancer, FUT4 is down-regulated by miR-200c [30] and by miR-26a/26b [31]. The latter is sponged by lncRNA MALAT1, delivered through exosomes [32]. Additionally, in breast cancer, FUT4 and its associated glycans exert a cancer-promoting activity, which is limited by miR-224-3p [33], miR-200b/c [36,37] and miR-493-5p [34], which is sponged by lncRNA GAS6-AS2 [35]. In bladder cancer, FUT4 is controlled by miR-371b-5p, which is sponged by lncRNA AC114812.8 [38]. Among hematological cancers, FUT4 has been shown to increase malignancy of leukemia stem cells due to miR-29b, which inhibits the transcription factor Sp1 binding to FUT4 promoter [39]. In multiple myeloma, lncRNA HOXB-AS1 promotes growth. ELAVL1 is a member of the ELAVL family of RNA-binding proteins whose role is to stabilize mRNAs by binding to their 3′UTR. The tumor-promoting activity of HOXB-AS1 in myeloma is partly due to its ability to promote ELAVL1 binding to FUT4 mRNA, resulting in its stabilization [40]. Medulloblastoma cancer stem cells are positive for CD133, as well as for sLex (CD15). MiR199b-5p has been reported to target FUT4 in these cells [41] even if FUT4 is a poor sLex synthase. In a non-neoplastic context, FUT4 targeting by miR-26a/b [42] and miR-200b [43] reduces articular inflammation and uterine receptivity [44].
FUT5 and FUT6: MiR-125a-3p can reduce malignancy of colorectal cancer cells by targeting FUT5 and FUT6 and the downstream PI3K-AKT pathway [45]. Additionally, lncRNA HOTAIR supports colorectal cancer progression by interacting with miR-326 and FUT6, increasing CD44 fucosylation and stimulating the PI3K/AKT/mTOR pathway [46]. On the other hand, in breast cancer, FUT6 appears to play a tumor-restraining role. In fact, FUT6 inhibition by miRNA-106b promotes cell migration, invasion and proliferation [47].
4.4.2. Sialyltransferases
We will distinguish between ST3GAL—which catalyzes the addition of Sia in α2,3 linkage to Gal—ST6GAL and ST6GALNAC—which mediate the addition of Sia in α2,6-linkage to Gal or GalNAc, respectively—and polysialyltransferases, which add Sia in α2,8 linkage to an underlying Sia residue.
ST3GAL: ST3GAL1 is the major sialyltransferase catalyzing the sialylation of core 1 O-linked chains, leading to sialyl-T formation (Figure 3). The lncRNA MEG3, expressed only by the maternally inherited chromosome, shows tumor-suppressor activity. In renal cell carcinoma, it regulates binding of the transcription factor JUN to the ST3GAL1 promoter, reducing its transcription and leading to reduced EGFR sialylation, increased phosphorylation and activation of the PI3/AKT pathway [93]. ST3GAL2 is a crucial sialyltransferase acting on O-linked chains and glycolipids (Figure 3 and Figure 4). The intestinal bacterial pathogen Campylobacter jejuni empowers its infectivity, inducing glycosylation changes. One mechanism involves the inhibition of ST3GAL2 by miR-615-3p [94]. Sialylation of a Galβ1,4GlcNAc unit, operated by ST3GAL4 or ST3GAL6, is a crucial step in the biosynthesis of sLex antigen, which is followed by subsequent α1,3-fucosylation (Figure 5). In kidney cancer, ST3GAL4 is targeted by miR-193a-3p and miR-224 and seems to play a tumor-restraining role through the PI3K/AKT pathway [95]. By contrast, in chronic myeloid leukemia cells, ST3GAL4 up-regulation resulting from the downregulation of their inhibitors miR-224 and let-7i contributes to cell survival and chemoresistance [96]. In colon carcinoma cells, ST3GAL4 targeting by miR-370 inhibits P-selectin-induced cell adhesion by targeting ST3GAL4 [97]. Modulation of ST3GAL4 by miR-193b also plays a role in inflammatory disease, such as osteoarthritis, by regulating CD44 sialylation through the NF-kB pathway [98]. In hepatocarcinoma, ST3GAL6 promotes malignancy and is targeted by miR-26a [100], while in lung cancer it reduces malignancy, acting on EGFR signaling [101]. Besides coding transcript(s), the ST3GAL6 gene also generates antisense transcript ST3GAL6-AS1, derived from the promoter region and circRNA. In lung cancer, ST3GAL6-AS1 expression parallels that of ST3GAL6, restraining malignancy [101]. In colorectal cancer, ST3GAL6-AS1 exerts a tumor-restraining activity by recruiting histone methyltransferase MLL1 to the ST3GAL6 promoter, resulting in increased ST3GAL6 transcription and α2,3-sialylation and PI3K/AKT inhibition [102]. On the other hand, in multiple myeloma, ST3GAL6-AS1 promotes invasion [131] by increasing ST3GAL6 expression. This was obtained through ST3GAL6-AS1-mediated inhibition of the heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1 gene), a protein which stabilizes the ST3GAL6 transcript [103].
ST6GAL and ST6GALNAC: α2,6sialyltransferases include ST6GAL1 and 2, which mediate the addition of α2,6-linked Sia to Gal and ST6GALNAC transferases which add sialic acid to GalNAc. ST6GAL1 is by far the major ST6GAL. In liver cancer, ST6GAL1 stimulates progression, and its expression is regulated by miR-9 [104] and by miR-195-3pc/lncRNA TINCR [105]. ST6GAL1 also affects the exosomal release of a broad range of miRNA. In fact, the activity of neutral sphingomyelinase-2, a key enzyme in exosomal sorting of miRNA, is regulated by α2,6-sialylation. Consequently, differential expression of ST6GAL1 underlies differential miRNA sorting [132]. In T-cell acute lymphoblastic leukemia, high ST6GAL1 is associated with drug resistance. It is regulated by miR-150, which is sponged by ZF-AS1 and modulates sialylation of EGFR via the PI3K/Akt pathway [107]. On the other hand, in triple-negative breast cancer, ST6GAL1 exerts a tumor-restraining activity. In fact, up-regulation of miR-214-3p, which targets ST6GAL1, is associated with malignancy [106]. ST6GAL1 has been shown to be regulated by miR199a, affecting sialylation of nectin-like molecule 2 and increasing ErbB2/ErbB3 signaling [108]. An in silico study in alcohol-related esophageal cancer has identified some lncRNA/miRNA interactions potentially regulating ST6GAL1 [133]. Both ST6GALNAC4 and ST6GALNAC5 are mainly involved in the biosynthesis of the GD1α ganglioside. In human follicular thyroid carcinoma, ST6GALNAC4, which is inhibited by miR-4299, promotes malignancy [111]. On the other hand, in prostate cancer, ST6GALNAC5, which is targeted by miR182, exerts a tumor-restraining role [112].
Polysialyltransferases. Several ST8SIA, including ST8SIA1 (GD2 synthase), mount a single Sia unit on Sia, generating the Siaα2,8Sia disaccharide. However, only two members of the ST8SIA family, namely ST8SIA2 and ST8SIA4, can synthesize long linear chains of polysialic acid, such as those present on the neural cell adhesion molecule (NCAM) and a few other glycoproteins. ST8SIA1 is generally associated with malignancy [134]. In colorectal cancer progression, it is inhibited by miRNA-33a and let-7e ST8SIA1 [113], while in prostate cancer progression, it is stimulated by the lncRNA MIR44352HG, resulting in FAK/AKT/β-catenin signaling pathway activation [114]. In ischemia/reperfusion brain models, ST8SIA2 is increased. This change is mediated by increased expression of the lncRNA TUG1, which sponges miR-3072-3p targeting ST8SIA2 [115]. The miR-26a-b/MALAT1 axis already described for FUT4 regulation in colorectal cancer also modulates ST8SIA4 in breast cancer cell lines [116,117]. ST8SIA4, targeted by miR-144-5p and miR-451a, also promotes growth in cholangiocarcinoma cells [119]. On the other hand, in follicular thyroid carcinoma, ST8SIA4, targeted by miR-146a and miR-146b, inhibits proliferation, migration and invasion [118].
4.4.3. AB0 Glycosyltransferases
Three allelic forms of a single genetic locus regulate the biosynthesis of the AB0 antigens. The allele responsible for the “A” blood group encodes a α1,3GalNAc transferase; the one responsible for the “B” group encodes a highly homologous α1,3 Gal transferase; the “0” antigen results from a null allele. In rare cases, the weak expression of the A/B antigens cannot be explained by genetic variations in the glycosyltransferase coding region. Even the disappearance of AB0 antigens during carcinogenesis is not fully explained. A possible explanation is provided by the observation that miR-331-3p and miR-1908-5p directly target the mRNA of glycosyltransferases A and B [18].
5. Regulation of Sugar-Binding Molecules by ncRNAs
5.1. Galectins
Galectins are epigenetically regulated [135] soluble galactose-binding molecules, which exert an extremely wide array of biological functions [136]. In cancer, some galectins exert tumor-promoting activity, while others play the opposite role. Galectin-3, a product of the LGALS3 gene, is frequently associated with malignancy. In ovarian [75] and colorectal [76] cancer, it is targeted by miR-424-3p and miR-128, respectively. Growth of pancreatic cancer cells is inhibited by miRNA-128-3p, delivered by exosomes released from human umbilical cord mesenchymal stem cells. In addition, the apoptosis of the nucleus pulposus cells (cells of the intervertebral disc) induced by galectin-3 is inhibited by miR-299-5p, which is in turn sponged by circRNA RERE [78]. Galectin-9, a product of the LGALS9 gene, exerts a tumor-promoting activity in liver cancer, targeted by miR-22 [80], and a tumor-restraining activity in colon cancer [79] in which it is targeted by miR-455-5p.
5.2. Siglecs
Siglecs are sialic acid receptors of the immunoglobulin family expressed mainly by cells of the immune system, playing a fundamentally inhibitory role and aiding tumors in escaping immune recognition [137]. SIGLEC15 behaves as a tumor immune suppressor. In clear-cell renal cell carcinoma, LINC00973 sponges miR-7109-3p, resulting in increased SIGLEC15 expression [91]. Analogously, in hepatocellular carcinoma, SIGLEC15 is targeted by miR-582-5p, which is sponged by lncRNA TUG1 [92].
6. Non-Coding RNAs Derived from Glycosyltransferase Genes but Not Involved in Glycogene Regulation
A number of ncRNAs derived from glycosyltransferase genes do not modulate glycogenes but exert a function on other basic cellular mechanisms. MGAT3-AS1 is an lncRNA derived from the antisense transcription of an intronic sequence of the MGAT3 gene. Low levels of this transcript correlate with delayed rejection [138] but an increased risk for viremia of polyomavirus and cytomegalovirus after kidney transplantation [139].
Several antisense glycosyltransferase lncRNAs affect cancer cell malignancy. B3GALT5-AS1 contributes to the progression of gastric cancer by up-regulating the expression of the α1 subunit of casein kinase Ii (product of the CSNK2A1 gene), which is involved in a variety of signaling pathways [140]. ST8SIA6-AS1 lncRNA (also known as APAL) is overexpressed and associated with poor prognosis in a variety of cancers [141]. Its silencing causes mitotic catastrophe and massive apoptosis in human cancer cells [141]. ST8SIA6-AS1 increases malignancy by regulating miR-142-3p [142], miR-338-3p [143] and miR-651-5p [144] in hepatocellular carcinoma cells, while in triple-negative breast cancer it drives cell proliferation and metastasis by targeting miR-145-5p, resulting in p53 pathway inactivation [145].
Even circRNAs contribute to regulate cancer cell growth. CircRNA ST3GAL6 displays a tumor-restraining activity in gastric cancer through autophagy set by the FOXP2/MET/mTOR axis [146]. In bladder cancer cells, circRNA ST6GALNAC6 behaves as a tumor-suppressor by increasing the sensitivity to ferroptosis, a type of programmed cell death induced by iron accumulation [147]. Finally, circRNA FUT10 sponges miR-365a-3p, inhibiting its binding with homeobox A9. These mechanisms regulate the regenerative potential of aged skeletal muscle stem cells [148].
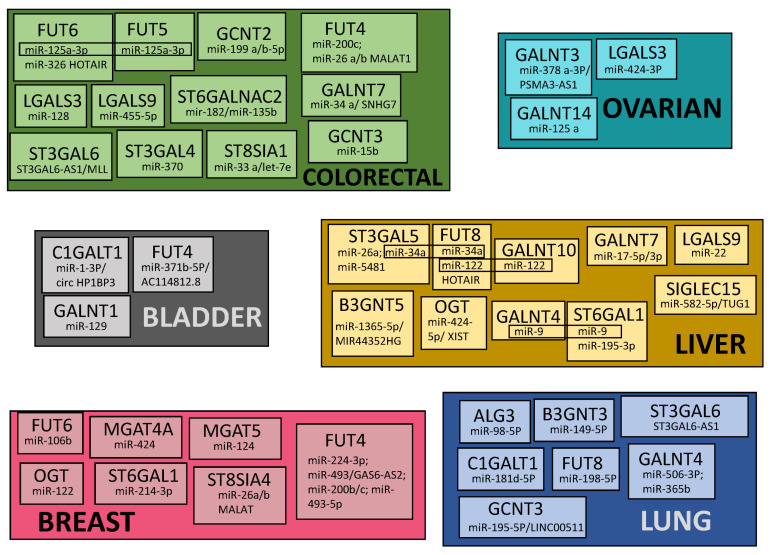
7. Common Patterns of Glycogene Modulation by ncRNA in Cancers
In Figure 6 are shown the glycogenes modulated by ncRNAs in different cancer types (only cancers with at least three modulated glycogenes are reported). These data, together with those reported in Table 1, show that only a few molecules undergo common modulation by ncRNAs in different cancers. In particular, FUT4 displays common modulation in breast and colon cancers by miR-200c. On the other hand, some miRNAs modulate different enzymes of the same malignancy. This is the case of liver cancer, in which miR-9 modulates GALNT4 and ST6GAL1, miR-122 modulates FUT8 and GALNT10 and miR-34 modulates ST3GAL5 and FUT8. In addition, miR-125a-3p modulates FUT5 and FUT6 in colorectal cancer. Together, these data are consistent with the existence of a very intricate and fragmented network of glycosylation regulation by ncRNAs.
Figure 6.
Glycogenes modulated by ncRNAs in different cancer types. Only cancers with at least 3 modulated glycogenes are reported. MiRNAs modulating different enzymes in the same cancer type are boxed.
8. Conclusions
Although many examples of glycosylation control by the ncRNA network have been published in recent years, they probably represent just the tip of the iceberg. It is reasonable to hypothesize that nearly all the components of the glycosylation machinery undergo regulation by ncRNAs because both kinds of molecules concur to define precisely the amount of protein molecules and their biological function. These mechanisms are crucial to the health of highly complex multicellular organisms, such as mammals. However, some glycogenes appear to be more frequently regulated by ncRNAs than others, suggesting that they require a particularly precise regulation. According to a recent hypothesis, deregulation of these genes is associated with complex diseases, such as such cancer and inflammatory conditions [17,149]. The recently discovered glyco-RNAs [5] are sialylated and located on the plasma membrane and found to be able to interact with siglecs. The intrinsic nature of the technique used for glyco-RNA isolation, which is based on a sialic acid analogue, limits—for the moment—the study to sialylated glyco-RNAs, but it is likely that neutral glyco-RNAs will be discovered in the future. The existence of this new kind of glycoconjugate establishes a new paradigm in glycobiology. Its impact on human health is, at the moment, unpredictable. Unlike other small RNAs, which are intracellular, glyco-RNA are exposed on the cell membrane in close contact with the immune system. On this basis, their possible involvement in auto-immune diseases has been proposed [5]. The emerging picture of the mutual relationship between ncRNA and glycosylation paves the way for conceptually new therapies.
Abbreviations
| APAL | aurora A/polo kinase 1 |
| AP-2γ | activator protein 2γ |
| circRNA | circular RNA |
| DANCR | differentiation antagonizing non-protein-coding RNA |
| DLGAP1 | discs large-homolog-associated protein 1 |
| DPAGT1 | dolichyl-phosphate N-acetylglucosaminephosphotransferase 1 |
| EMT | epithelial to mesenchymal transition |
| EBV | Epstein–Barr virus |
| HNRPA2B1 | Heterogeneous Nuclear Ribonucleoprotein A2/B1 |
| HOTAIR | HOX transcript antisense RNA |
| LEF1 | Lymphoid enhancer binding factor 1 |
| Lex | Lewisx |
| lncRNA | long non-coding RNA |
| MALAT1 | metastasis-associated lung adenocarcinoma 1 |
| miRNA | micro RNA |
| MUC1 | mucin 1 |
| NCAM | neural cell adhesion molecule |
| ncRNA | non-coding RNA |
| NSCLC | non-small-cell lung cancer |
| OGT | O-GlcNActransferase |
| PSMA3 | proteasome 20S subunit alpha 3 |
| RER | rough endoplasmic reticulum |
| RISC | RNA-induced silencing complex |
| RYR1 | ryanodine receptor 1 |
| sLex | sialyl Lewisx |
| SNHG1 | small nuclear RNA host gene 1 |
| TINCR | TINCR ubiquitin domain containing |
| TUG1 | Taurine up-regulated 1 |
| XIST | X-inactivated specific transcript |
Author Contributions
Conceptualization, F.D.; writing—original draft preparation, F.D.; writing—review and editing, N.M., M.D. and F.D.; supervision, F.D.; funding acquisition, F.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the University of Bologna and by the “Pallotti” Legacy for Cancer Research to FDO.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012;17:670–699. doi: 10.2741/3951. [DOI] [PubMed] [Google Scholar]
- 2.Dall’Olio F., Malagolini N. Immunoglobulin G Glycosylation Changes in Aging and Other Inflammatory Conditions. Exp. Suppl. 2021;112:303–340. doi: 10.1007/978-3-030-76912-3_10. [DOI] [PubMed] [Google Scholar]
- 3.Dall’Olio F., Vanhooren V., Chen C.C., Slagboom P.E., Wuhrer M., Franceschi C. N-glycomic biomarkers of biological aging and longevity: A link with inflammaging. Ageing Res. Rev. 2013;12:685–698. doi: 10.1016/j.arr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Dall’Olio F. Glycobiology of Aging. Subcell. Biochem. 2018;90:505–526. doi: 10.1007/978-981-13-2835-0_17. [DOI] [PubMed] [Google Scholar]
- 5.Flynn R.A., Pedram K., Malaker S.A., Batista P.J., Smith B.A.H., Johnson A.G., George B.M., Majzoub K., Villalta P.W., Carette J.E., et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021;184:3109–3124. doi: 10.1016/j.cell.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes J.C.R., Acuna S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non-Coding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan H., Bu P. Non-coding RNA in cancer. Essays Biochem. 2021;65:625–639. doi: 10.1042/EBC20200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges M.C., Daulagala A.C., Kourtidis A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021;220:e202009045. doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin A., Imperiali B. The expanding horizons of asparagine-linked glycosylation. Biochemistry. 2011;50:4411–4426. doi: 10.1021/bi200346n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz F., Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R., Leon F., Rauth S., Batra S.K., Ponnusamy M.P. A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis. Cells. 2020;9:446. doi: 10.3390/cells9020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K., Hansen L., Clausen H. Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals. Molecules. 2021;26:5504. doi: 10.3390/molecules26185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.B., Pyo K.H., Kim H.R. Role and Function of O-GlcNAcylation in Cancer. Cancers. 2021;13:5365. doi: 10.3390/cancers13215365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambetta M.C., Oktaba K., Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–96. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- 15.Sinclair D.A., Syrzycka M., Macauley M.S., Rastgardani T., Komljenovic I., Vocadlo D.J., Brock H.W., Honda B.M. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc. Nat. Acad. Sci. USA. 2009;106:13427–13432. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurcon T., Liu Z., Paradkar A.V., Vaiana C.A., Koppolu S., Agrawal P., Mahal L.K. miRNA proxy approach reveals hidden functions of glycosylation. Proc. Nat. Acad. Sci. USA. 2015;112:7327–7332. doi: 10.1073/pnas.1502076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thu C.T., Mahal L.K. Sweet Control: MicroRNA Regulation of the Glycome. Biochemistry. 2020;59:3098–3110. doi: 10.1021/acs.biochem.9b00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronstein-Wiedemann R., Nowakowska P., Milanov P., Gubbe K., Seifried E., Bugert P., Chavakis T., Tonn T. Regulation of ABO blood group antigen expression by miR-331-3p and miR-1908-5p during hematopoietic stem cell differentiation. Stem Cells. 2020;38:1348–1362. doi: 10.1002/stem.3251. [DOI] [PubMed] [Google Scholar]
- 19.Ke S.B., Qiu H., Chen J.M., Shi W., Han C., Gong Y., Chen Y.S. ALG3 contributes to the malignancy of non-small cell lung cancer and is negatively regulated by MiR-98-5p. Pathol. Res. Pract. 2020;216:152761. doi: 10.1016/j.prp.2019.152761. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y., Liu T., Xian L., Liu W., Liu J., Zhou H. B3GNT3, a Direct Target of miR-149-5p, Promotes Lung Cancer Development and Indicates Poor Prognosis of Lung Cancer. Cancer Manag. Res. 2020;12:2381–2391. doi: 10.2147/CMAR.S236565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y., Li B., Xu G., Han C., Xing G. lncRNA MIR44352HG promotes the progression of liver cancer by upregulating B3GNT5 expression. Mol. Med. Rep. 2022;25:38. doi: 10.3892/mmr.2021.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y., Yang X., Liu M., Tang H. B4GALT3 up-regulation by miR-27a contributes to the oncogenic activity in human cervical cancer cells. Cancer Lett. 2016;375:284–292. doi: 10.1016/j.canlet.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Bi C., Shan J., Li M., Zhang Q., Li C., Tong J., Huang Q. Long noncoding RNA differentiation antagonizing nonprotein coding RNA promotes the proliferation, invasion and migration of neuroblastoma cells via targeting β-1, 4-galactosyltransferase III by sponging miR-338-3p. Neuroreport. 2021;32:965–974. doi: 10.1097/WNR.0000000000001664. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y., Zhao B., Chen X., Geng X., Zhang Z. Circ_0009910 sponges miR-491-5p to promote acute myeloid leukemia progression through modulating B4GALT5 expression and PI3K/AKT signaling pathway. Int. J. Lab. Hematol. 2022;44:320–332. doi: 10.1111/ijlh.13742. [DOI] [PubMed] [Google Scholar]
- 25.Dong X., Liu Y., Deng X., Shao J., Tian S., Chen S., Huang R., Lin Z., Chen C., Shen L. C1GALT1, Negatively Regulated by miR-181d-5p, Promotes Tumor Progression via Upregulating RAC1 in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2021;9:707970. doi: 10.3389/fcell.2021.707970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Z., Jiang Y., Liang L., Wu J., Cao L., Zhou X., Song Z., Ye Z., Zhao Z., Feng H., et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/ miR-1-3p axis in bladder cancer. J. Exp. Clin. Cancer Res. 2022;41:228. doi: 10.1186/s13046-022-02438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang L., Sun T.Y., Hu L.J., Hu S.L., Sun H.M., Zhao F.Q., Wu B., Yang S., Ji F.Q., Zhou D.S. Elevated miR-124-3p in the aging colon disrupts mucus barrier and increases susceptibility to colitis by targeting T-synthase. Aging Cell. 2020;19:e13252. doi: 10.1111/acel.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu S., Bao H., Xu X., Zhou X., Qin W., Zeng C., Liu Z. Increased miR-374b promotes cell proliferation and the production of aberrant glycosylated IgA1 in B cells of IgA nephropathy. FEBS Lett. 2015;589:4019–4025. doi: 10.1016/j.febslet.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z., Yang S., Chen X., Dong S., Zhou S., Xu S. LncRNA LINC00467 acted as an oncogene in esophageal squamous cell carcinoma by accelerating cell proliferation and preventing cell apoptosis via the miR-485-5p/DPAGT1 axis. J. Gastroenterol. Hepatol. 2021;36:721–730. doi: 10.1111/jgh.15201. [DOI] [PubMed] [Google Scholar]
- 30.Cong J., Gong J., Yang C., Xia Z., Zhang H. MiR-200c/FUT4 axis prevents the proliferation of colon cancer cells by downregulating the Wnt/beta-catenin pathway. BMC Cancer. 2021;21:2. doi: 10.1186/s12885-020-07670-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Li Y., Sun Z., Liu B., Shan Y., Zhao L., Jia L. Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death. Dis. 2017;8:e2892. doi: 10.1038/cddis.2017.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J., Xiao Y., Liu B., Pan S., Liu Q., Shan Y., Li S., Qi Y., Huang Y., Jia L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2020;39:54. doi: 10.1186/s13046-020-01562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng X., Zhao L., Gao S., Song X., Dong W., Zhao Y., Zhou H., Cheng L., Miao X., Jia L. Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4. Gene. 2016;578:232–241. doi: 10.1016/j.gene.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L., Feng X., Song X., Zhou H., Zhao Y., Cheng L., Jia L. miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4. Oncol. Rep. 2016;36:1007–1015. doi: 10.3892/or.2016.4882. [DOI] [PubMed] [Google Scholar]
- 35.Li W., Jin X., Zhao Y., Dai J., Cai Y. Long noncoding RNA GAS6-AS2 sponges microRNA-493, thereby enhancing the malignant characteristics of breast cancer cells via upregulation of FUT4. Pathol. Res. Pract. 2020;216:152772. doi: 10.1016/j.prp.2019.152772. [DOI] [PubMed] [Google Scholar]
- 36.Yuan X., Liu J., Ye X. Effect of miR-200c on the proliferation, migration and invasion of breast cancer cells and relevant mechanisms. J. Buon. 2019;24:61–67. [PubMed] [Google Scholar]
- 37.Zheng Q., Cui X., Zhang D., Yang Y., Yan X., Liu M., Niang B., Aziz F., Liu S., Yan Q., et al. miR-200b inhibits proliferation and metastasis of breast cancer by targeting fucosyltransferase IV and alpha1,3-fucosylated glycans. Oncogenesis. 2017;6:e358. doi: 10.1038/oncsis.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W., Li Y., Ma W., Zhou J., Sun Z., Yan X. Long noncoding RNA AC114812.8 promotes the progression of bladder cancer through miR-371b-5p/FUT4 axis. Biomed. Pharmacother. 2020;121:109605. doi: 10.1016/j.biopha.2019.109605. [DOI] [PubMed] [Google Scholar]
- 39.Liu B., Ma H., Liu Q., Xiao Y., Pan S., Zhou H., Jia L. MiR-29b/Sp1/FUT4 axis modulates the malignancy of leukemia stem cells by regulating fucosylation via Wnt/β-catenin pathway in acute myeloid leukemia. J. Exp. Clin. Cancer Res. 2019;38:200. doi: 10.1186/s13046-019-1179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen R., Zhang X., Wang C. LncRNA HOXB-AS1 promotes cell growth in multiple myeloma via FUT4 mRNA stability by ELAVL1. J. Cell Biochem. 2020;121:4043–4051. doi: 10.1002/jcb.29573. [DOI] [PubMed] [Google Scholar]
- 41.Andolfo I., Liguori L., De Antonellis P., Cusanelli E., Marinaro F., Pistollato F., Garzia L., De Vita G., Petrosino G., Accordi B., et al. The micro-RNA 199b-5p regulatory circuit involves Hes1, CD15, and epigenetic modifications in medulloblastoma. Neuro. Oncol. 2012;14:596–612. doi: 10.1093/neuonc/nos002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J., Wang Z., Pan Y., Ma J., Miao X., Qi X., Zhou H., Jia L. MiR-26a and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-kB signaling pathway. Int. J. Biochem. Cell Biol. 2018;94:79–88. doi: 10.1016/j.biocel.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Wei S., Zhang Z. MicroRNA-200b relieves LPS-induced inflammatory injury by targeting FUT4 in knee articular chondrocytes in vitro. Exp. Ther. Med. 2021;21:407. doi: 10.3892/etm.2021.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Q., Zhang D., Yang Y.U., Cui X., Sun J., Liang C., Qin H., Yang X., Liu S., Yan Q. MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and alpha1,3-fucosylation. Cell Death. Differ. 2017;24:2161–2172. doi: 10.1038/cdd.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang L., Gao C., Li Y., Sun M., Xu J., Li H., Jia L., Zhao Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death. Dis. 2017;8:e2968. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan S., Liu Y., Liu Q., Xiao Y., Liu B., Ren X., Qi X., Zhou H., Zeng C., Jia L. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:750–760. doi: 10.1016/j.bbamcr.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Li N., Liu Y., Miao Y., Zhao L., Zhou H., Jia L. MicroRNA-106b targets FUT6 to promote cell migration, invasion, and proliferation in human breast cancer. IUBMB Life. 2016;68:764–775. doi: 10.1002/iub.1541. [DOI] [PubMed] [Google Scholar]
- 48.Bernardi C., Soffientini U., Piacente F., Tonetti M.G. Effects of microRNAs on fucosyltransferase 8 (FUT8) expression in hepatocarcinoma cells. PLoS ONE. 2013;8:e76540. doi: 10.1371/journal.pone.0076540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y., Liu B., Huang T., Qi X., Li S. HOTAIR modulates hepatocellular carcinoma progression by activating FUT8/core-fucosylated Hsp90/MUC1/STAT3 feedback loop via JAK1/STAT3 cascade. Dig. Liver Dis. 2022. Online ahead of print . [DOI] [PubMed]
- 50.Wang S., Zhang X., Yang C., Xu S. MicroRNA-198-5p inhibits the migration and invasion of non-small lung cancer cells by targeting fucosyltransferase 8. Clin. Exp. Pharmacol. Physiol. 2019;46:955–967. doi: 10.1111/1440-1681.13154. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y., Shi J., Zhao Y., Lu Z. SNHG1/miR-186/FUT8 regulates cell migration and invasion in oral squamous cell carcinoma. Oral Dis. 2021. Online ahead of print . [DOI] [PubMed]
- 52.Hu X., Shen N., Liu A., Wang W., Zhang L., Sui Z., Tang Q., Du X., Yang N., Ying W., et al. Bone marrow mesenchymal stem cell-derived exosomal miR-34c-5p ameliorates RIF by inhibiting the core fucosylation of multiple proteins. Mol. Ther. 2022;30:763–781. doi: 10.1016/j.ymthe.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyrskjot L., Ostenfeld M.S., Bramsen J.B., Silahtaroglu A.N., Lamy P., Ramanathan R., Fristrup N., Jensen J.L., Andersen C.L., Zieger K., et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 54.Sun C.M., Zhang W.Y., Wang S.Y., Qian G., Pei D.L., Zhang G.M. microRNA let-7i-5p aggravates kidney fibrosis via targeting GALNT1. Gen. Physiol. Biophys. 2021;40:147–154. doi: 10.4149/gpb_20210031. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z., Pan L., Yan X., Duan X. The long noncoding RNA DLGAP1-AS2 facilitates cholangiocarcinoma progression via miR-505 and GALNT10. FEBS Open Bio. 2021;11:413–422. doi: 10.1002/2211-5463.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Q., Liu H.O., Liu Y.D., Liu W.S., Pan D., Zhang W.J., Yang L., Fu Q., Xu J.J., Gu J.X. Decreased Expression of Hepatocyte Nuclear Factor 4α (Hnf4α)/MicroRNA-122 (miR-122) Axis in Hepatitis B Virus-associated Hepatocellular Carcinoma Enhances Potential Oncogenic GALNT10 Protein Activity. J. Biol. Chem. 2015;290:1170–1185. doi: 10.1074/jbc.M114.601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J., Li G., Zhang K. MiR-125a regulates ovarian cancer proliferation and invasion by repressing GALNT14 expression. Biomed. Pharmacother. 2016;80:381–387. doi: 10.1016/j.biopha.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 58.Xu Z., Jin H., Duan X., Liu H., Zhao X., Fan S., Wang Y., Yao T. LncRNA PSMA3-AS1 promotes cell proliferation, migration, and invasion in ovarian cancer by activating the PI3K/Akt pathway via the miR-378a-3p/GALNT3 axis. Environ. Toxicol. 2021;36:2562–2577. doi: 10.1002/tox.23370. [DOI] [PubMed] [Google Scholar]
- 59.Hu C.Y., You P., Zhang J., Zhang H., Jiang N. MiR-506-3p acts as a novel tumor suppressor in prostate cancer through targeting GALNT4. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5133–5138. doi: 10.26355/eurrev_201906_18177. [DOI] [PubMed] [Google Scholar]
- 60.Xing L., Hong X., Chang L., Ren P., Zhang H. miR-365b regulates the development of non-small cell lung cancer via GALNT4. Exp. Ther. Med. 2020;20:1637–1643. doi: 10.3892/etm.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Liu H., Yang L., Wu Q., Liu W., Fu Q., Zhang W., Zhang H., Xu J., Gu J. Loss of N-acetylgalactosaminyltransferase-4 orchestrate oncogenic microRNA-9 in hepatocellular carcinoma. J. Biol. Chem. 2017;292:3186–3200. doi: 10.1074/jbc.M116.751685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaziel-Sovran A., Segura M.F., Di Micco R., Collins M.K., Hanniford D., Vega-Saenz D.M., Rakus J.F., Dankert J.F., Shang S., Kerbel R.S., et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y., Zeng C., Hu J., Pan Y., Shan Y., Liu B., Jia L. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J. Hematol. Oncol. 2018;11:89. doi: 10.1186/s13045-018-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu H., Chen J., Li D., Liu X., Li L., Wang K. MicroRNA-30e Functions as a Tumor Suppressor in Cervical Carcinoma Cells through Targeting GALNT7. Transl. Oncol. 2017;10:876–885. doi: 10.1016/j.tranon.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pedersen M.E., Snieckute G., Kagias K., Nehammer C., Multhaupt H.A., Couchman J.R., Pocock R. An epidermal microRNA regulates neuronal migration through control of the cellular glycosylation state. Science. 2013;341:1404–1408. doi: 10.1126/science.1242528. [DOI] [PubMed] [Google Scholar]
- 66.Li W., Ma H., Sun J. MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol. Med. Rep. 2014;9:1293–1298. doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- 67.Lu Q., Xu L., Li C., Yuan Y., Huang S., Chen H. miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer. Tumour. Biol. 2016;37:14605–14614. doi: 10.1007/s13277-016-5320-7. [DOI] [PubMed] [Google Scholar]
- 68.Shan S.W., Fang L., Shatseva T., Rutnam Z.J., Yang X., Du W., Lu W.Y., Xuan J.W., Deng Z., Yang B.B. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 2013;126:1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 69.Gao F., Han J., Jia L., He J., Wang Y., Chen M., Liu X., He X. MiR-30c facilitates natural killer cell cytotoxicity to lung cancer through targeting GALNT7. Genes Genom. 2022. Online ahead of print . [DOI] [PubMed]
- 70.Kahai S., Lee S.C., Lee D.Y., Yang J., Li M., Wang C.H., Jiang Z., Zhang Y., Peng C., Yang B.B. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS ONE. 2009;4:e7535. doi: 10.1371/journal.pone.0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chao C.C., Wu P.H., Huang H.C., Chung H.Y., Chou Y.C., Cai B.H., Kannagi R. Downregulation of miR-199a/b-5p is associated with GCNT2 induction upon epithelial-mesenchymal transition in colon cancer. FEBS Lett. 2017;591:1902–1917. doi: 10.1002/1873-3468.12685. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez-Vallinas M., Molina S., Vicente G., Zarza V., Martin-Hernandez R., Garcia-Risco M.R., Fornari T., Reglero G., Ramirez de Molina A. Expression of MicroRNA-15b and the Glycosyltransferase GCNT3 Correlates with Antitumor Efficacy of Rosemary Diterpenes in Colon and Pancreatic Cancer. PLoS ONE. 2014;9:e98556. doi: 10.1371/journal.pone.0098556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J., Zhang Y., Liu W., Zhang Q., Xiao H., Song H., Luo B. MiR-BART1-5p targets core 2 β-1,6-acetylglucosaminyltransferase GCNT3 to inhibit cell proliferation and migration in EBV-associated gastric cancer. Virology. 2020;541:63–74. doi: 10.1016/j.virol.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Xiao P., Hu X. LINC00511 enhances LUAD malignancy by upregulating GCNT3 via miR-195-5p. BMC Cancer. 2022;22:389. doi: 10.1186/s12885-022-09459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bieg D., Sypniewski D., Nowak E., Bednarek I. MiR-424-3p suppresses galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch. Gynecol. Obstet. 2019;299:1077–1087. doi: 10.1007/s00404-018-4999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu W., Wang J., Yang G., Yu N., Huang Z., Xu H., Li J., Qiu J., Zeng X., Chen S., et al. Posttranscriptional regulation of Galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget. 2017;8:15242–15251. doi: 10.18632/oncotarget.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie X., Ji J., Chen X., Xu W., Chen H., Zhu S., Wu J., Wu Y., Sun Y., Sai W., et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin. Transl. Oncol. 2021;24:517–531. doi: 10.1007/s12094-021-02705-7. [DOI] [PubMed] [Google Scholar]
- 78.Wang R., Zhou X., Luo G., Zhang J., Yang M., Song C. CircRNA RERE Promotes the Oxidative Stress-Induced Apoptosis and Autophagy of Nucleus Pulposus Cells through the miR-299-5p/Galectin-3 Axis. J. Healthc. Eng. 2021;2021:2771712. doi: 10.1155/2021/2771712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Yang Q., Hou C., Huang D., Zhuang C., Jiang W., Geng Z., Wang X., Hu L. miR-455-5p functions as a potential oncogene by targeting galectin-9 in colon cancer. Oncol. Lett. 2017;13:1958–1964. doi: 10.3892/ol.2017.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Q., Jiang W., Zhuang C., Geng Z., Hou C., Huang D., Hu L., Wang X. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol. Rep. 2015;34:1771–1778. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 81.Zhu Q., Zhan D., Yang Y., Chong Y., Xue H., Zhu P. LINC00173 Promotes Wilms’ Tumor Progression Through MGAT1-mediated MUC3A N-glycosylation. Cell Cycle. 2022;21:1795–1810. doi: 10.1080/15384101.2022.2070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan K., Chen S., Wang Y., Yao W., Gao X. MicroRNA-23b attenuates tau pathology and inhibits oxidative stress by targeting GnT-III in Alzheimer’s disease. Neuropharmacology. 2021;196:108671. doi: 10.1016/j.neuropharm.2021.108671. [DOI] [PubMed] [Google Scholar]
- 83.Vaiana C.A., Kurcon T., Mahal L.K. MicroRNA-424 Predicts a Role for β1,4 Branched Glycosylation in Cell Cycle Progression. J. Biol. Chem. 2016;291:1529–1537. doi: 10.1074/jbc.M115.672220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan G., Li Y., Zhan L., Sun S., Yuan J., Wang T., Yin Y., Dai Z., Zhu Y., Jiang Z., et al. Decreased miR-124-3p promoted breast cancer proliferation and metastasis by targeting MGAT5. Am. J. Cancer Res. 2019;9:585–596. [PMC free article] [PubMed] [Google Scholar]
- 85.Han D.L., Wang L.L., Zhang G.F., Yang W.F., Chai J., Lin H.M., Fu Z., Yu J.M. MiRNA-485-5p, inhibits esophageal cancer cells proliferation and invasion by down-regulating O-linked N-acetylglucosamine transferase. Eur. Rev. Med. Pharmacol. Sci. 2019;23:2809–2816. doi: 10.26355/eurrev_201904_17556. [DOI] [PubMed] [Google Scholar]
- 86.Kalantzakos T.J., Sullivan T.B., Sebel L.E., Canes D., Burks E.J., Moinzadeh A., Rieger-Christ K.M. MicroRNAs MiR-15a and MiR-26a cooperatively regulate O-GlcNAc-transferase to control proliferation in clear cell renal cell carcinoma. Cancer Biomark. 2021;30:343–351. doi: 10.3233/CBM-200553. [DOI] [PubMed] [Google Scholar]
- 87.Ning D., Chen J., Du P., Liu Q., Cheng Q., Li X., Zhang B., Chen X., Jiang L. The crosstalk network of XIST/miR-424-5p/OGT mediates RAF1 glycosylation and participates in the progression of liver cancer. Liver Int. 2021;41:1933–1944. doi: 10.1111/liv.14904. [DOI] [PubMed] [Google Scholar]
- 88.Yan W., Cao M., Ruan X., Jiang L., Lee S., Lemanek A., Ghassemian M., Pizzo D.P., Wan Y., Qiao Y., et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat. Cell Biol. 2022;24:793–804. doi: 10.1038/s41556-022-00893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu R., Ma X., Chen L., Yang Y., Zeng Y., Gao J., Jiang W., Zhang F., Li D., Han B., et al. MicroRNA-15b Suppresses Th17 Differentiation and Is Associated with Pathogenesis of Multiple Sclerosis by Targeting O-GlcNAc Transferase. J. Immunol. 2017;198:2626–2639. doi: 10.4049/jimmunol.1601727. [DOI] [PubMed] [Google Scholar]
- 90.Luo P., He T., Jiang R., Li G. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol. Med. Rep. 2015;12:1163–1168. doi: 10.3892/mmr.2015.3491. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y., Li X., Zhang C., Zhang H., Huang Y. LINC00973 is involved in cancer immune suppression through positive regulation of Siglec-15 in clear-cell renal cell carcinoma. Cancer Sci. 2020;111:3693–3704. doi: 10.1111/cas.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren Y., Lyu J., Guo Y., Yao Y., Hu L. Long Noncoding RNA TUG1 Inhibits Tumor Progression through Regulating Siglec-15-Related Anti-Immune Activity in Hepatocellular Carcinoma. J. Immunol. Res. 2022;2022:9557859. doi: 10.1155/2022/9557859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong A., Zhao X., Pan Y., Qi Y., Li S., Huang Y., Guo Y., Qi X., Zheng W., Jia L. The lncRNA MEG3 mediates renal cell cancer progression by regulating ST3Gal1 transcription and EGFR sialylation. J. Cell Sci. 2020;133:jcs244020. doi: 10.1242/jcs.244020. [DOI] [PubMed] [Google Scholar]
- 94.Xi D., Hofmann L., Alter T., Einspanier R., Bereswill S., Heimesaat M.M., Golz G., Sharbati S. The glycosyltransferase ST3GAL2 is regulated by miR-615-3p in the intestinal tract of Campylobacter jejuni infected mice. Gut Pathog. 2021;13:42. doi: 10.1186/s13099-021-00437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan Y., Hu J., Ma J., Qi X., Zhou H., Miao X., Zheng W., Jia L. MiR-193a-3p and miR-224 mediate renal cell carcinoma progression by targeting α2,3-sialyltransferase IV and the phosphatidylinositol 3 kinase/Akt pathway. Mol. Carcinog. 2018;57:1067–1077. doi: 10.1002/mc.22826. [DOI] [PubMed] [Google Scholar]
- 96.Zhou H., Li Y., Liu B., Shan Y., Li Y., Zhao L., Su Z., Jia L. Downregulation of miR-224 and let-7i contribute to cell survival and chemoresistance in chronic myeloid leukemia cells by regulating ST3GAL IV expression. Gene. 2017;626:106–118. doi: 10.1016/j.gene.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 97.Wei Y., Shao J., Wang Y., Shen H., Yu S., Zhang J., Yin L. Hsa-miR-370 inhibited P-selectin-induced cell adhesion in human colon adenocarcinoma cells. Mol. Cell Biochem. 2019;450:159–166. doi: 10.1007/s11010-018-3382-0. [DOI] [PubMed] [Google Scholar]
- 98.Wang T., Hao Z., Liu C., Yuan L., Li L., Yin M., Li Q., Qi Z., Wang Z. MiR-193b modulates osteoarthritis progression through targeting ST3GAL4 via sialylation of CD44 and NF-kB pathway. Cell Signal. 2020;76:109814. doi: 10.1016/j.cellsig.2020.109814. [DOI] [PubMed] [Google Scholar]
- 99.Cai H., Zhou H., Miao Y., Li N., Zhao L., Jia L. MiRNA expression profiles reveal the involvement of miR-26a, miR-548l and miR-34a in hepatocellular carcinoma progression through regulation of ST3GAL5. Lab. Investig. 2017;97:530–542. doi: 10.1038/labinvest.2017.12. [DOI] [PubMed] [Google Scholar]
- 100.Sun M., Zhao X., Liang L., Pan X., Lv H., Zhao Y. Sialyltransferase ST3GAL6 mediates the effect of microRNA-26a on cell growth, migration, and invasion in hepatocellular carcinoma through the protein kinase B/mammalian target of rapamycin pathway. Cancer Sci. 2017;108:267–276. doi: 10.1111/cas.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J., Long Y., Sun J., Wu J., He X., Wang S., Wang X., Miao X., Huang R., Yan J. Comprehensive landscape of the ST3GAL family reveals the significance of ST3GAL6-AS1/ST3GAL6 axis on EGFR signaling in lung adenocarcinoma cell invasion. Front. Cell Dev. Biol. 2022;10:931132. doi: 10.3389/fcell.2022.931132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu J., Shan Y., Ma J., Pan Y., Zhou H., Jiang L., Jia L. LncRNA ST3Gal6-AS1/ST3Gal6 axis mediates colorectal cancer progression by regulating α2,3 sialylation via PI3K/Akt signaling. Int. J. Cancer. 2019;145:450–460. doi: 10.1002/ijc.32103. [DOI] [PubMed] [Google Scholar]
- 103.Shen Y., Feng Y., Li F., Jia Y., Peng Y., Zhao W., Hu J., He A. lncRNA ST3GAL6AS1 promotes invasion by inhibiting hnRNPA2B1mediated ST3GAL6 expression in multiple myeloma. Int. J. Oncol. 2021;58:5. doi: 10.3892/ijo.2021.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han Y., Liu Y., Fu X., Zhang Q., Huang H., Zhang C., Li W., Zhang J. miR-9 inhibits the metastatic ability of hepatocellular carcinoma via targeting β-galactoside α-2,6-sialyltransferase 1. J. Physiol. Biochem. 2018;74:491–501. doi: 10.1007/s13105-018-0642-0. [DOI] [PubMed] [Google Scholar]
- 105.Mei J., Lin W., Li S., Tang Y., Ye Z., Lu L., Wen Y., Kan A., Zou J., Yu C., et al. Long noncoding RNA TINCR facilitates hepatocellular carcinoma progression and dampens chemosensitivity to oxaliplatin by regulating the miR-195-3p/ST6GAL1/NF-kB pathway. J. Exp. Clin. Cancer Res. 2022;41:5. doi: 10.1186/s13046-021-02197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tao Y., Zhao Z., Ma J., Dong L., Liang Y., Li S., Mao Y., Li Y., Zhang Y. MiR-214-3p regulates the viability, invasion, migration and EMT of TNBC cells by targeting ST6GAL1. Cytotechnology. 2019;71:1155–1165. doi: 10.1007/s10616-019-00352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Q., Ma H., Sun X., Liu B., Xiao Y., Pan S., Zhou H., Dong W., Jia L. The regulatory ZFAS1/miR-150/ST6GAL1 crosstalk modulates sialylation of EGFR via PI3K/Akt pathway in T-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2019;38:199. doi: 10.1186/s13046-019-1208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Minami A., Shimono Y., Mizutani K., Nobutani K., Momose K., Azuma T., Takai Y. Reduction of the ST6 β-Galactosamide α-2,6-Sialyltransferase 1 (ST6GAL1)-catalyzed Sialylation of Nectin-like Molecule 2/Cell Adhesion Molecule 1 and Enhancement of ErbB2/ErbB3 Signaling by MicroRNA-199a. J. Biol. Chem. 2013;288:11845–11853. doi: 10.1074/jbc.M112.405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia L., Luo S., Ren X., Li Y., Hu J., Liu B., Zhao L., Shan Y., Zhou H. miR-182 and miR-135b Mediate the Tumorigenesis and Invasiveness of Colorectal Cancer Cells via Targeting ST6GALNAC2 and PI3K/AKT Pathway. Dig. Dis. Sci. 2017;62:3447–3459. doi: 10.1007/s10620-017-4755-z. [DOI] [PubMed] [Google Scholar]
- 110.Liu B., Liu Y., Zhao L., Pan Y., Shan Y., Li Y., Jia L. Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway. Mol. Carcinog. 2017;56:2669–2680. doi: 10.1002/mc.22710. [DOI] [PubMed] [Google Scholar]
- 111.Miao X., Jia L., Zhou H., Song X., Zhou M., Xu J., Zhao L., Feng X., Zhao Y. miR-4299 mediates the invasive properties and tumorigenicity of human follicular thyroid carcinoma by targeting ST6GALNAC4. IUBMB Life. 2016;68:136–144. doi: 10.1002/iub.1467. [DOI] [PubMed] [Google Scholar]
- 112.Bai L., Luo L., Gao W., Bu C., Huang J. miR-182 modulates cell proliferation and invasion in prostate cancer via targeting ST6GALNAC5. Braz. J. Med. Biol. Res. 2021;54:e9695. doi: 10.1590/1414-431x2020e9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shan Y., Liu Y., Zhao L., Liu B., Li Y., Jia L. MicroRNA-33a and let-7e inhibit human colorectal cancer progression by targeting ST8SIA1. Int. J. Biochem. Cell Biol. 2017;90:48–58. doi: 10.1016/j.biocel.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 114.Xing P., Wang Y., Zhang L., Ma C., Lu J. Knockdown of lncRNA MIR44352HG and ST8SIA1 expression inhibits the proliferation, invasion and migration of prostate cancer cells in vitro and in vivo by blocking the activation of the FAK/AKT/β-catenin signaling pathway. Int. J. Mol. Med. 2021;47:93. doi: 10.3892/ijmm.2021.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen M., Wang F., Fan L., Wang H., Gu S. Long Noncoding RNA TUG1 Aggravates Cerebral Ischemia/Reperfusion Injury by Acting as a ceRNA for miR-3072-3p to Target St8sia2. Oxid. Med. Cell. Longev. 2022;2022:9381203. doi: 10.1155/2022/9381203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Ma X., Dong W., Su Z., Zhao L., Miao Y., Li N., Zhou H., Jia L. Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4. Cell Death. Dis. 2016;7:e2561. doi: 10.1038/cddis.2016.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang N., Cao S., Wang X., Zhang L., Yuan H., Ma X. lncRNA MALAT1/miR26a/26b/ST8SIA4 axis mediates cell invasion and migration in breast cancer cell lines. Oncol. Rep. 2021;46:181. doi: 10.3892/or.2021.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma W., Zhao X., Liang L., Wang G., Li Y., Miao X., Zhao Y. miR-146a and miR-146b promote proliferation, migration and invasion of follicular thyroid carcinoma via inhibition of ST8SIA4. Oncotarget. 2017;8:28028–28041. doi: 10.18632/oncotarget.15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu W., Yu G., Liang J., Fan P., Dong K., Zhang B., Chen X., Zhu H., Chu L. miR-144-5p and miR-451a Inhibit the Growth of Cholangiocarcinoma Cells Through Decreasing the Expression of ST8SIA4. Front. Oncol. 2020;10:563486. doi: 10.3389/fonc.2020.563486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pepe F., Pagotto S., Soliman S., Rossi C., Lanuti P., Braconi C., Mariani-Costantini R., Visone R., Veronese A. Regulation of miR-483-3p by the O-linked N-acetylglucosamine transferase links chemosensitivity to glucose metabolism in liver cancer cells. Oncogenesis. 2017;6:e328. doi: 10.1038/oncsis.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herzog K., Bandiera S., Pernot S., Fauvelle C., Juhling F., Weiss A., Bull A., Durand S.C., Chane-Woon-Ming B., Pfeffer S., et al. Functional microRNA screen uncovers O-linked N-acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut. 2020;69:380–392. doi: 10.1136/gutjnl-2018-317423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma M., Guo D., Tan Z., Du J., Guan F., Li X. Fucosyltransferase 8 regulation and breast cancer suppression by transcription factor activator protein 2γ. Cancer Sci. 2021;112:3190–3204. doi: 10.1111/cas.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qi Y., Shan Y., Li S., Huang Y., Guo Y., Huang T., Zhao X., Jia L. LncRNA LEF1-AS1/LEF1/FUT8 Axis Mediates Colorectal Cancer Progression by Regulating α1, 6-Fucosylation via Wnt/β-Catenin Pathway. Dig. Dis. Sci. 2022;67:2182–2194. doi: 10.1007/s10620-021-07051-w. [DOI] [PubMed] [Google Scholar]
- 124.Dennis J.W., Laferte S., Waghorne C., Breitman M.L., Kerbel R.S. β 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 125.Ye J., Wei X., Shang Y., Pan Q., Yang M., Tian Y., He Y., Peng Z., Chen L., Chen W., et al. Core 3 mucin-type O-glycan restoration in colorectal cancer cells promotes MUC1/p53/miR-200c-dependent epithelial identity. Oncogene. 2017;36:6391–6407. doi: 10.1038/onc.2017.241. [DOI] [PubMed] [Google Scholar]
- 126.Gao L., He R.Q., Wu H.Y., Zhang T.T., Liang H.W., Ye Z.H., Li Z.Y., Xie T.T., Shi Q., Ma J., et al. Expression Signature and Role of miR-30d-5p in Non-Small Cell Lung Cancer: A Comprehensive Study Based on in Silico Analysis of Public Databases and in Vitro Experiments. Cell Physiol. Biochem. 2018;50:1964–1987. doi: 10.1159/000494875. [DOI] [PubMed] [Google Scholar]
- 127.Pucci M., Gomes F.I., Malagolini N., Ferracin M., Dall’Olio F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020;21:6558. doi: 10.3390/ijms21186558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terraneo L., Avagliano L., Caretti A., Bianciardi P., Tosi D., Bulfamante G.P., Samaja M., Trinchera M. Expression of carbohydrate-antigen sialyl-Lewis a on colon cancer cells promotes xenograft growth and angiogenesis in nude mice. Int. J. Biochem. Cell Biol. 2013;45:2796–2800. doi: 10.1016/j.biocel.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Trinchera M., Aronica A., Dall’Olio F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology. 2017;6:16. doi: 10.3390/biology6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trinchera M., Malagolini N., Chiricolo M., Santini D., Minni F., Caretti A., Dall’Olio F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011;43:130–139. doi: 10.1016/j.biocel.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Ronchetti D., Todoerti K., Vinci C., Favasuli V., Agnelli L., Manzoni M., Pelizzoni F., Chiaramonte R., Platonova N., Giuliani N., et al. Expression Pattern and Biological Significance of the lncRNA ST3GAL6-AS1 in Multiple Myeloma. Cancers. 2020;12:782. doi: 10.3390/cancers12040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang L., Chen X., Meng F., Huang T., Wang S., Zheng Z., Zheng G., Li W., Zhang J., Liu Y. α2,6-Sialylation promotes hepatocellular carcinoma cells migration and invasion via enhancement of nSmase2-mediated exosomal miRNA sorting. J. Physiol Biochem. 2022. Online ahead of print . [DOI] [PubMed]
- 133.Du Q., Xiao R.D., Luo R.G., Xie J.B., Su Z.D., Wang Y. Construction of long non-coding RNA- and microRNA-mediated competing endogenous RNA networks in alcohol-related esophageal cancer. PLoS ONE. 2022;17:e0269742. doi: 10.1371/journal.pone.0269742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Gadwal A., Modi A., Khokhar M., Vishnoi J.R., Choudhary R., Elhence P., Banerjee M., Purohit P. Critical appraisal of epigenetic regulation of galectins in cancer. Int. J. Clin. Oncol. 2021;27:35–44. doi: 10.1007/s10147-021-02048-x. [DOI] [PubMed] [Google Scholar]
- 136.Compagno D., Jaworski F.M., Gentilini L., Contrufo G., Gonzalez P.I., Elola M.T., Pregi N., Rabinovich G.A., Laderach D.J. Galectins: Major signaling modulators inside and outside the cell. Curr. Mol. Med. 2014;14:630–651. doi: 10.2174/1566524014666140603101953. [DOI] [PubMed] [Google Scholar]
- 137.Adams O.J., Stanczak M.A., von Gunten G.S., Laubli H. Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology. 2018;28:640–647. doi: 10.1093/glycob/cwx108. [DOI] [PubMed] [Google Scholar]
- 138.Nagarajah S., Xia S., Rasmussen M., Tepel M. Endogenous intronic antisense long non-coding RNA, MGAT3-AS1, and kidney transplantation. Sci. Rep. 2019;9:14743. doi: 10.1038/s41598-019-51409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagarajah S., Rasmussen M., Hoegh S.V., Tepel M. Prospective Study of Long Noncoding RNA, MGAT3-AS1, and Viremia of BK Polyomavirus and Cytomegalovirus in Living Donor Renal Transplant Recipients. Kidney Int. Rep. 2020;5:2218–2227. doi: 10.1016/j.ekir.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang P., Sun G.B., Dou G.X., Wang B.Q. Long non-coding RNA B3GALT5-AS1 contributes to the progression of gastric cancer via interacting with CSNK2A1. Exp. Ther. Med. 2021;22:927. doi: 10.3892/etm.2021.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Luo M.L., Li J., Shen L., Chu J., Guo Q., Liang G., Wu W., Chen J., Chen R., Song E. The Role of APAL/ST8SIA6-AS1 lncRNA in PLK1 Activation and Mitotic Catastrophe of Tumor Cells. J. Natl. Cancer Inst. 2020;112:356–368. doi: 10.1093/jnci/djz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y., Yang Y., Zhang Y., Liu Z. lncRNA ST8SIA6-AS1 facilitates proliferation and invasion in liver cancer by regulating miR-142-3p. Exp. Ther. Med. 2021;22:1348. doi: 10.3892/etm.2021.10783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Kuai J., Zheng L., Yi X., Liu Z., Qiu B., Lu Z., Jiang Y. ST8SIA6-AS1 promotes the development of hepatocellular carcinoma cells through miR-338-3p/NONO Axis. Dig. Liver Dis. 2021;53:1192–1200. doi: 10.1016/j.dld.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 144.Mou Y., Ding X. LncRNA ST8SIA6-AS1 facilitates hepatocellular carcinoma progression by governing miR-651-5p/TM4SF4 axis. Anticancer Drugs. 2022;33:741–751. doi: 10.1097/CAD.0000000000001326. [DOI] [PubMed] [Google Scholar]
- 145.Qiao Y., Wang B., Yan Y., Niu L. Long noncoding RNA ST8SIA6-AS1 promotes cell proliferation and metastasis in triple-negative breast cancer by targeting miR-145-5p/CDCA3 to inactivate the p53/p21 signaling pathway. Environ. Toxicol. 2022;37:2398–2411. doi: 10.1002/tox.23605. [DOI] [PubMed] [Google Scholar]
- 146.Xu P., Zhang X., Cao J., Yang J., Chen Z., Wang W., Wang S., Zhang L., Xie L., Fang L., et al. The novel role of circular RNA ST3GAL6 on blocking gastric cancer malignant behaviours through autophagy regulated by the FOXP2/MET/mTOR axis. Clin. Transl. Med. 2022;12:e707. doi: 10.1002/ctm2.707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Wang L., Wu S., He H., Ai K., Xu R., Zhang L., Zhu X. CircRNA-ST6GALNAC6 increases the sensitivity of bladder cancer cells to erastin-induced ferroptosis by regulating the HSPB1/P38 axis. Lab. Investig. 2022;102:1323–1334. doi: 10.1038/s41374-022-00826-3. [DOI] [PubMed] [Google Scholar]
- 148.Zhu M., Lian C., Chen G., Zou P., Qin B.G. CircRNA FUT10 regulates the regenerative potential of aged skeletal muscle stem cells by targeting HOXA9. Aging. 2021;13:17428–17441. doi: 10.18632/aging.203233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Joshi H.J., Hansen L., Narimatsu Y., Freeze H.H., Henrissat B., Bennett E., Wandall H.H., Clausen H., Schjoldager K.T. Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases. Glycobiology. 2018;28:284–294. doi: 10.1093/glycob/cwy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.