Abstract
Initiation and perpetuation of the inflammatory intestinal responses in inflammatory bowel disease (IBD) may result from an exaggerated host defense reaction of the intestinal epithelium to endogenous lumenal bacterial flora. Intestinal epithelial cell lines constitutively express several functional Toll-like receptors (TLRs) which appear to be key regulators of the innate response system. The aim of this study was to characterize the expression pattern of TLR2, TLR3, TLR4, and TLR5 in primary intestinal epithelial cells from patients with IBD. Small intestinal and colonic biopsy specimens were collected from patients with IBD (Crohn's disease [CD], ulcerative colitis [UC]) and controls. Non-IBD specimens were assessed by immunofluorescence histochemistry using polyclonal antibodies specific for TLR2, TLR3, TLR4, and TLR5. Primary intestinal epithelial cells (IEC) of normal mucosa constitutively expressed TLR3 and TLR5, while TLR2 and TLR4 were only barely detectable. In active IBD, the expression of TLR3 and TLR4 was differentially modulated in the intestinal epithelium. TLR3 was significantly downregulated in IEC in active CD but not in UC. In contrast, TLR4 was strongly upregulated in both UC and CD. TLR2 and TLR5 expression remained unchanged in IBD. These data suggest that IBD may be associated with distinctive changes in selective TLR expression in the intestinal epithelium, implying that alterations in the innate response system may contribute to the pathogenesis of these disorders.
The two major forms of idiopathic inflammatory bowel diseases (IBDs), ulcerative colitis (UC) and Crohn's disease (CD), are distinguished by a complex chronic inflammatory process. Although there is increasing evidence that IBD results from the combined effects of environmental agents in the genetically susceptible host, neither the specific genes nor the environmental triggers have been definitively identified yet. A central role for specific immune responses to discrete antigens has been presumed, and circumstantial evidence in humans and direct studies of mutant murine models implicate lumenal bacteria as necessary cofactors for initiating and perpetuating inflammation.
Bacteria contain toxic compounds which are potent stimuli of innate immune responses, as exemplified by the gram-negative bacterial cell wall component lipopolysaccharide (LPS). However, the mechanistic basis of the interaction between the lumenal flora and the intestinal mucosa remains to be fully defined. It is possible that bacterial products penetrate the epithelial barrier, either due to damage or via paracellular pathways, to directly stimulate the underlying constituents of the mucosal immune system. However, alternatively, it is possible that products may interact at the apical surface and induce responses in the intestinal epithelial cell which in turn produces cytokines, chemokines, and other mediators inducing inflammatory activation of the mucosal immune system (4, 9, 11, 30, 35, 36).
A variety of studies have provided increasing evidence that the surface epithelium serves a critical function as the defensive front line of the mucosal innate immune system in the gastrointestinal tract (23). We and colleagues have previously demonstrated that various intestinal epithelial cell lines constitutively express several members of a novel family of transmembrane receptors designated Toll-like receptors (TLRs) which may serve as a major link between innate and adaptive mucosal immune responses (5). LPS is able to elicit several immediate stress responses in intestinal epithelial cell lines in vitro which result in secretion of numerous proinflammatory cytokines and chemokines via distinct signaling pathways through TLRs (5, 19, 42).
The TLR family comprises at least eight human homologues of the Drosophila Toll protein which appear to be key regulators in differential cellular recognition of conserved molecular patterns associated with microbial pathogens (25, 33, 40). Recent studies have demonstrated that TLRs may act as transmembrane coreceptors with CD14 in the cellular response to LPS, a glycolipid derived from the outermost membrane of pathogenic gram-negative bacteria (18). Among this family of receptors, TLR2 and TLR4 have been most extensively studied to date, and these studies have variably suggested that both TLRs may serve as potential main mediators of LPS signaling (15, 16, 39, 41). Downstream, LPS-induced signaling through TLRs rapidly leads to NF-κB activation and cytokine expression in monocytes (7, 10). However, the functional roles of the other TLRs and the possible interactions between different TLRs and other nonbacterial ligands, as well as the details of the TLR-induced cellular signal transduction pathways, have not been fully defined. Moreover, it remains unclear whether dysregulation of TLR-mediated microbial recognition is present in infectious and inflammatory diseases. However, two murine mutant models suggest that TLR dysregulation may be associated with increased or decreased susceptibility to infection: TLR4 point mutation renders C3H/HeJ mice endotoxin hyporesponsive, whereas variant alleles of the TLR5 gene may make MOLF/Ei mice susceptible to Salmonella enterica serovar Typhimurium (32, 38).
Recent studies reinforce earlier observations suggesting that the overlying epithelial mucous surface may be severely impaired in patients with IBD (37). This may increase direct exposure of the intestinal epithelium to large amounts of lumenal bacteria. We speculate that immune imbalance in IBD might result from an exaggerated activation of the mucosal innate immune system in response to the bacterial products of the lumen initiated through dysregulation of TLRs in the intestinal epithelium. However, it remains unclear whether TLRs are expressed in primary intestinal epithelial cells in vivo and how expression may be altered in association gastrointestinal inflammation. Thus, the aim of this study was to characterize expression of the potential LPS-signaling receptors TLR2, -3, -4, and -5 in intestinal mucosa of IBD patients compared with normal controls.
MATERIALS AND METHODS
Population and tissue samples.
Tissue samples (n = 31) were obtained from 25 patients (Table 1) undergoing complete colonoscopy at the Massachusetts General Hospital (MGH), Boston, Mass. Informed consent was obtained from all patients prior to the procedure, and the protocol was approved by the Human Studies Committee of the MGH. In each case, the diagnosis was confirmed by standard endoscopic and histological criteria (additional hematoxylin and eosin staining of each sample). Specimens were taken from macroscopically “involved” and “noninvolved” mucosa in patients with active IBD. Specimens were taken from all areas of the colon, including the terminal ileum. All 31 tissue samples were processed to be stained with the anti-TLR antisera or preimmune sera, respectively, and then evaluated. Thus, reported findings show representative results of all samples examined from each subgroup (non-IBD, CD, and UC), if not otherwise specified in Results.
TABLE 1.
Study population
| Characteristic | Non-IBD | CD | UC | Total |
|---|---|---|---|---|
| No. of patients | 9 | 8 | 8 | 25 |
| No. of samples | 12 | 11 | 8 | 31 |
| Clinical disease activity (no. of patients) | ||||
| Active | 6 | 6 | 12 | |
| Inactive | 2 | 2 | 4 | |
| Tissue distribution (no. of samples) | ||||
| Terminal ileum | 3 | 6 | 0 | 9 |
| Colon | 9 | 5 | 8 | 22 |
| Endoscopic evaluation of area of biopsies in active disease (no. of samples) | ||||
| Involved | 5 | 6 | 11 | |
| Noninvolved | 6 | 0 | 6 |
Four patients (two CD and two UC) were in remission and had no clinically identifiable, active disease at the time of sample acquisition. Control specimens were taken from patients with normal endoscopic findings and without macroscopic evidence for inflammatory or neoplastic disease. This group included, predominantly, patients who underwent regular colon cancer screening examinations and/or polypectomy. Fresh tissue samples were immediately snap-frozen in OCT compound (Miles Laboratories, Elkhart, Ind.) and stored at −80°C until further processing, as described below.
Antibodies.
Human TLR3 protein (GI no. 2459626) was plotted using the ExPASy Proteomics Tools website (www.expasy.ch) and the SeqWeb interface (helix.mgh.harvard.edu:8080/gcg-bin/seqweb.cgi) (predictions of secondary structure according to Garnier, Osguthorpe, and Robson, hydrophilicity according to Kyte and Doolittle, and antigenicity index according to Jameson and Wolf), and the area including amino acids 444 to 469 in the extracellular domain was subsequently chosen for peptide synthesis (GQELTGQEWRGLENIFEIYLSYNKYL). Peptides of the extracellular domains of human TLR2 (FRASDNDRVIDPGKVETLTIRRLHIPR) and human TLR4 (FKEIRLHKLTLRNNFDSLNVMKT) were selected, as previously described (43).
Possible homologies of all three peptide sequences with other known TLRs or similarities with other peptides were excluded by conducting BLASTP (2.0.10) alignments through the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). Peptides were synthesized at the MGH Peptide Core Facility, Boston, Mass.
Rabbits were immunized (Covance, Denver, Pa.) as follows. An initial single boost of 500 μg of peptide was administered (intradermal back, multiple sites), followed by individual 250-μg boosts of each peptide (subcutaneous nodal area, dorsal or neck at multiple sites), which were repeated every 10 to 14 days. After 10 weeks of injections, production bleeds (20 ml per rabbit) were pursued every 14 to 21 days. The antisera were affinity purified (NAb protein A spin chromatography kit; Pierce Chemical Co., Rockford, Ill.), tested, and stored in aliquots at −20°C. Protein concentration was determined by the colorimetric (595 nm) Bradford protein assay (Bio-Rad, Hercules, Calif.). A specific goat polyclonal antibody to the human TLR5 NH2-terminal domain was obtained from Santa Cruz Biotechnology, Santa Cruz, Calif.
Testing of antisera: specificity by Western blotting.
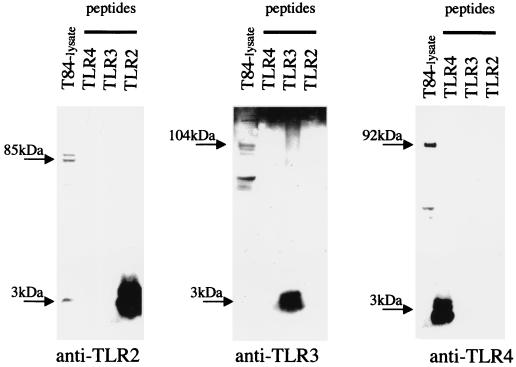
Cross-reactivity of the antisera with each other was excluded, and the specificity of the newly generated antibodies to TLR2, TLR3, and TLR4 antigens was confirmed by Western blotting (Fig. 1). The human colonic cancer cell line T84 constitutively expresses TLR2, TLR3, and TLR4 mRNA, as previously shown by this laboratory (5), and was therefore used as a positive control for correct molecular weight sizing of the TLR proteins.
FIG. 1.
The specificity of newly generated anti-TLR antisera is confirmed using Western blotting against peptides. The human colon cancer cell line T84 was used as a presumed positive control for TLR protein expression.
TLR2, TLR3, and TLR4 peptides were completely dissolved in 1 M Tris-HCl, pH 7.5 (1 μg/μl), by thorough vortexing (10 min at room temperature). Differentiated, nonstimulated T84 cells (grown on filters) were rinsed in cold phosphate-buffered saline (PBS) and then lysed in a mixture of 1% Triton X-100, 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 10 mM dithiothreitol, including protease inhibitor cocktail tablets (“complete mini”; Roche) and 2 mM phenylmethylsulfonyl fluoride. The lysate was centrifuged (12,000 × g, 15 min at 4°C), and the resulting supernatant was diluted in 4× LDS sample buffer (Invitrogen-Novex, San Diego, Calif.) and heated (85°C, 2 min). T84 whole-cell lysate proteins (25 μg per lane) and TLR peptides (4 μg per lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (100 V, 90 min; 4 to 12% N,N-methylenebisacrylamide [BIS]–Tris; MES running buffer; Invitrogen-Novex) and then transferred (25 V, 90 min) onto a polyvinylidene difluoride membrane (Millipore). The specific TLR proteins were detected using the different anti-TLR antisera (1:500) and a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech). Immune complexes were detected using the ECL detection system (NEN Life Science). As shown in Fig. 1, the TLR2 peptide (3.2 kDa) was specifically detected by anti-TLR2 antisera and not by anti-TLR3 or anti-TLR4 antisera. Similar results were obtained for anti-TLR3 and anti-TLR4 antisera against the TLR3 (3.1 kDa) and TLR4 (2.9 kDa) peptides, respectively. The specificity of the newly generated antibodies to the whole TLR2, TLR3, and TLR4 proteins was additionally confirmed by showing clear bands at the approximate sizes of 85, 104, and 92 kDa, respectively, in T84 cells. This experiment has been reproduced twice.
Immunoblots were then stripped with 62.5 mM Tris-HCl [pH 6.8]–2% sodium dodecyl sulfate containing 100 mM 2-mercaptoethanol at 50°C for 30 min and then excessively washed with TBST. Subsequent reprobing with rabbit preimmune serum showed no specific bands at the appropriate molecular sizes, validating the specificity of the antisera (data not shown).
Immunohistochemistry.
In order to facilitate permeabilization, all biopsy specimens were pretreated with freshly made, ice-cold 2% paraformaldehyde (pH 7.0) containing 0.1% Triton X-100 for 30 min at 4°C and then further fixed in 4% buffered formalin, embedded in paraffin wax, sectioned, mounted on coated glass slides, and baked for 15 min. Each sample was also stained with hematoxylin and eosin and subsequently analyzed histologically for the presence of inflammatory, neoplastic, and fibrotic changes in order to confirm the macroscopic diagnosis. After deparaffinization (three times, each 5 min; Hemo-De; Fisher Scientific, Pittsburgh, Pa.), sections were rehydrated in graded ethanol (100% twice, 90% once, 80% once, and 70% once, each for 5 min), washed in distilled water (twice, 5 min each), and then exposed to microwave pretreatment (in 10 mM sodium citrate, pH 6.0, at 900 W for two periods of 5 min) to enhance antigenicity. After cooling to room temperature for 20 min and subsequently washing with distilled water (three times, 2 min each), sections were incubated for 10 min in ice-cold 3% H2O2 (Sigma). After washing with PBS (two times, 5 min each), nonspecific binding was blocked with normal goat or rabbit serum (1:100 in PBS, Vector Laboratories) for 60 min at room temperature. After removal of the blocking solution, sections were then incubated in a moist chamber with anti-TLR2, anti-TLR3, anti-TLR4, or anti-TLR5 serum (1:100 to 1:500 in PBS) or—as negative controls—with normal rabbit or goat immunoglobulin G (IgG) (Santa Cruz) and preimmune serum (equivalent dilutions) overnight at 4°C. Fluorescein-conjugated goat anti-rabbit IgG or rabbit anti-goat IgG antibodies (Vector Laboratories, Burlingame, Calif.) were used as secondary antibodies (1:250, 60 min, room temperature). While protected from direct light exposure, samples were washed three times in PBS and mounted (Vectashield mounting media; Vector Laboratories). Control experiments were performed omitting the primary antibody.
Image acquisition and analysis.
Sections were viewed within 12 h on inverted and upright immunofluorescence microscopes (10× or 40× objective, model IX70 [TLR2, TLR4] or AX70 [TLR3, TLR5]; Olympus, New Hyde Park, N.Y.). Fluorescence images were viewed in a blinded fashion and obtained using standardized camera settings (Olympus PM-C35DX) between positive sample and negative control. Digitized images were then cropped into Adobe Photoshop 5.0.2 (Adobe Systems, Inc.). The pictures were imported to PowerPoint (Microsoft) for assembly and labeling in a standardized way.
All images shown are representatives of the common result in each disease subgroup. Each picture shown represents a different patient.
Statistical analysis.
The measurement of the automatic exposure time (full image area to a final standard) was taken as an indirect reflection of the staining intensity of TLR3 (P value; t test [heteroscedastic, two tailed]).
RESULTS
TLR2.
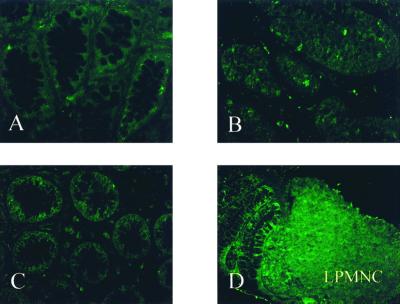
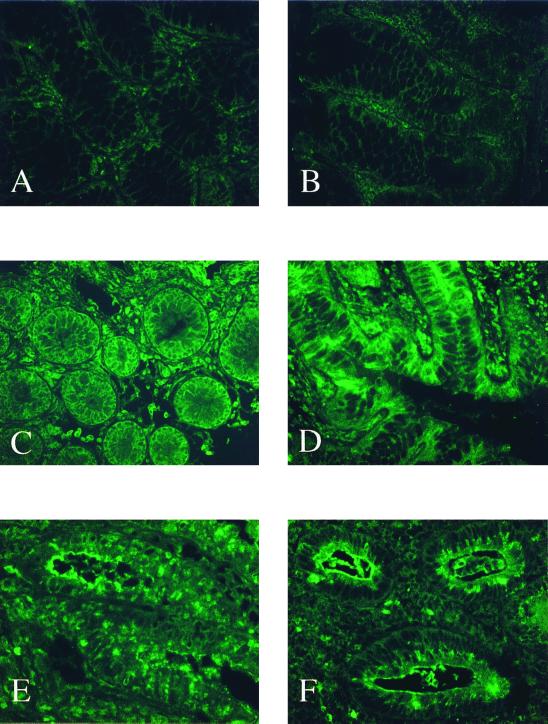
TLR2 expression was barely detectable in primary intestinal epithelial cells throughout the normal terminal ileum and colon in all samples examined (Fig. 2A). Isolated cells within the lamina propria, presumably monocytes and macrophages, were only weakly positive for TLR2 in the normal mucosae examined. The intensity of the staining for TLR2 was not significantly increased in the ileal or colonic epithelium from either UC or CD patients compared to normal tissue (Fig. 2B and C). Significant upregulation of TLR2 protein expression in active IBD was observed in scattered inflammatory cells of the lamina propria (Fig. 2D). Similar results were obtained for all patients examined.
FIG. 2.
TLR2 expression is minimally detectable in IECs and remains unchanged in active IBD. (A) Normal, nondiseased mucosa. (C) UC, inactive. (B and D) CD, active colon involved. (Olympus IX70; original magnification, ×40; standardized exposure time, 1.039 s; full image area, gain 16; gamma adjust, 1.20; noise filter, no background subtraction.)
TLR3.
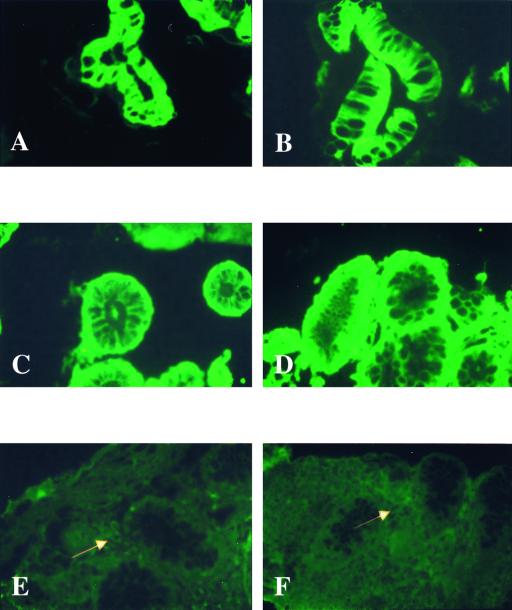
In all normal small intestine and colon specimens, intense staining for cytoplasmic TLR3 was consistently present on intestinal epithelial cells (Fig. 3A and B). Subepithelial blood vessels and muscle cells of the submucosa also showed substantial TLR3 protein expression (not shown). A similar pattern of TLR3 expression was present in colonic specimens from all UC patients. There were no apparent differences in expression level at different sites or between noninflamed and inflamed mucosae in tissues from UC patients (Fig. 3C and D). TLR3 expression was mostly present on basolateral surfaces of intestinal epithelial cells in colonic specimens from UC patients. Inflammatory cell infiltrates of the lamina propria also expressed abundant TLR3 on cell surfaces in UC (not shown).
FIG. 3.
TLR3 is constitutively expressed in the IECs of healthy controls and UC patients but significantly decreased in the IECs of CD patients. Shown are normal, nondiseased mucosa of the terminal ileum (A) and colon (B); inactive (C) and active (D) UC cells; and active, noninvolved CD colon cells (E) and active, involved CD colon cells (F). White arrows indicate scattered lamina propria mononuclear cells. Olympus AX70; original magnification, ×40; exposure times: 8 s (A), 10 s (B, C, and F), 9 s (D), and 16 s (E); Elitechrome Kodak Select series ISO100, Olympus exposure control unit PM-30, Superfluorescence 30 (exposure adjust setting, auto 2).
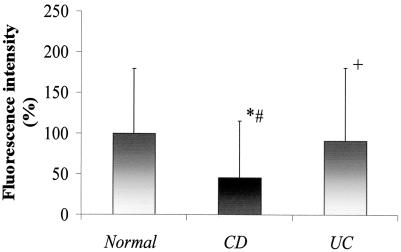
In contrast to UC, epithelial expression of TLR3 was significantly decreased in active CD specimens compared with specimens from UC patients and normal controls (Fig. 3E and F versus A to D). With the exception of one patient with inactive disease, staining for TLR3 was markedly diminished in both noninvolved and involved mucosae from patients with CD (Fig. 3E versus F). Cells within the lamina propria did stain positively for TLR3 in active CD (see arrows). However, the overall amount and intensity of TLR3 expression in the lamina propria were also less than those observed in healthy controls (Fig. 3E and F versus A and B). This observation was consistent in all samples examined and was confirmed by standardized measurements of the fluorescence intensity supporting the significant visual decrease of the staining intensities in CD compared to UC (43%) or control (55%) patients (Fig. 4).
FIG. 4.
The fluorescence intensity of TLR3 staining is decreased on average by 55 or 43% in CD samples compared to healthy controls or UC patients, respectively. Average automatic exposure times of representative samples (n = 7 per disease subgroup) were plotted as an indirect measurement of the fluorescence intensity of TLR3. t test (two tailed, heteroscedastic) results show the following: ∗, CD/Normal, P < 0.01; #, CD/UC, P < 0.03; +, UC/Normal, P > 0.7.
TLR4.
While only minimally detectable in small intestinal and colonic epithelial cells of normal, non-IBD mucosa (Fig. 5A and B), TLR4 was abundantly expressed by epithelial cells of all UC and CD patients (Fig. 5C to F). Both microscopically inflamed and noninflamed mucosae throughout the colon and terminal ileum showed intense intestinal epithelial cell expression of TLR4 (Fig. 5C to F). This result was reproducible in all samples examined. Of note, the subcellular distribution of TLR4 in the epithelial compartment differed between CD and UC. Intense staining was mostly present at basolateral surfaces in mucosal sections of UC patients (Fig. 5C and D). In contrast, staining of TLR4 was intense at the apical pole of intestinal epithelial cells in most CD samples (Fig. 5E and F). It is noteworthy that TLR4-positive intestinal epithelial cells were also found in inactive disease (Fig. 5C). Scattered inflammatory cells in the lamina propria were weakly positive for TLR4 in normal controls (Fig. 5A and B). Enhanced expression of TLR4 was not limited to epithelial cells, and a significant increase of the expression intensity was also present in the lamina propria of IBD specimens (Fig. 5C to F).
FIG. 5.
TLR4 expression is minimal in the IECs of healthy controls but significantly increased in the IECs of both UC and CD. Shown are normal, nondiseased mucosae of the terminal ileum (A) and colon (B); inactive (C) and active (D) UC cells; and active, noninvolved CD terminal ileum cells (E) and active, involved CD colon cells (F). (Olympus IX70; original magnification, ×40; standardized exposure time, 1.039 s; full image area, gain 16; gamma adjust, 1.20; noise filter, no background subtraction.)
TLR5.
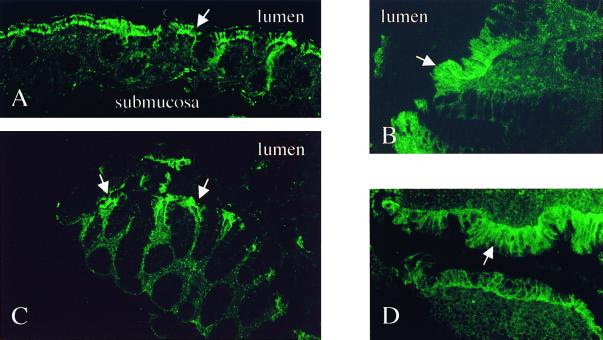
TLR5 was expressed on epithelial cells throughout the normal lower gastrointestinal tract (Fig. 6A and B). There was no evident difference in expression between normal controls and IBD patients (Fig. 6A and B versus C and D). Of note, TLR5 appeared to be selectively expressed by the surface epithelium and was not present in either crypt epithelium or cell populations within the underlying lamina propria and submucosa (Fig. 6A and C). All samples showed similar results.
FIG. 6.
Constitutive TLR5 is predominantly expressed on surface IECs in healthy controls and remains unchanged in IBD. Shown are an overview (A) and detail (B) of normal, nondiseased mucosa of the colon; active, noninvolved CD colon cells (C); and active UC cells (D). White arrows indicate surface epithelial staining. Olympus AX70; original magnifications, ×40 (B and D) and ×10 (A and C); exposure times: 4 s (A), 3 s (B), and 5 s (C and D); Elitechrome Kodak Select series ISO400, Olympus exposure control unit PM-30, Superfluorescence 30 (exposure adjust setting, auto 2).
DISCUSSION
The present study demonstrates that TLRs are expressed in normal human intestinal mucosa. This same study demonstrates that epithelial cells rather than macrophages and other lamina propria populations are the predominant cells expressing TLRs. Further, this pilot study suggests that there is differential expression of different members of this receptor family. Thus, primary intestinal epithelial cells (IECs) of normal, nondiseased mucosa constitutively express TLR3 and TLR5, whereas TLR2 and TLR4 are present in much lower amounts as assessed by immunohistochemistry.
The intestinal epithelium serves as an essential barrier between microbes of the lumen and inflammatory cells of the lamina propria. It also plays a critical role in regulating the host immune defense reaction by recognizing and subsequently responding to invading pathogens by secretion of proinflammatory cytokines and chemokines (19, 42). We have recently demonstrated that various intestinal epithelial cell lines constitutively express several TLRs in vitro (5). This expression is consistent with the emerging consensus that these receptors act as key mediators of host defense to bacterial challenges, linking innate and adaptive immune responses (9). Thus these receptors appeared to be deployed to the true site of interface with lumenal bacteria and their products, the surface epithelium. The detection of TLRs at the mucosal surface in vivo is consistent with the detection of the TLRs on the apical surface of intestinal epithelial cell lines in vitro (unpublished observation).
Although the various TLRs seem to differ in their recognition of diverse bacterial products, TLRs' induced cell responses may be mediated by a common signaling pathway which shares many features with the IL1R pathway, including the involvement of MyD88, IRAK, TRAF6, and NF-κB. Stimulation of this pathway leads to production of inflammatory cytokines and costimulatory molecules (43). It is very likely that LPS-induced TLR signals via a similar pathway in IECs, as intestinal epithelial activation of these proteins has recently been shown in response to IL-1β stimulation (2).
In addition to the demonstration of TLR expression by normal intestinal epithelium, the present unicenter study suggests that expression of TLRs may be selectively altered in association with IBD and further that some of these alterations may be specific to the form of IBD, whether UC or CD. Thus, TLR3 and TLR4 are differentially modulated in the intestinal epithelium of patients with IBD. While TLR3 expression by IECs of UC patients is comparable to that of normal controls, TLR3 expression is significantly downregulated in CD. Of interest, reduced expression of TLR3 on IECs seems to be consistent in CD, irrespective of location or inflammatory activity. At a minimum, this implies that reduced TLR3 does not simply reflect the local effect of some inflammatory mediator. Inflammatory cells of the lamina propria remain positive for cell surface expression of TLR3, suggesting that the decrease of TLR3 may reflect a distinct intestinal epithelial cell-specific impairment in active CD but not UC. Thus, deficient TLR3 expression in the intestinal epithelium may be a distinctive feature of CD but not UC and could reflect a divergent dimension of pathophysiological mechanisms involved in these two disorders.
It is important to note that the functional role of TLR3 in mediating innate immune responses to specific microbes and their toxic constituents has not yet been definitively established. TLR3 mRNA may be significantly downregulated in response to LPS in mature dendritic cells (26). In contrast, preliminary studies from this laboratory reveal that TLR3 protein is significantly upregulated in IECs in response to LPS in vitro (unpublished observation). Others have also recently demonstrated that expression of TLR3 mRNA may be upregulated by LPS and also tumor necrosis factor alpha in mature Langerhans cells (12). Collectively, these divergent findings suggest that TLR3 can mediate cell-specific responses to LPS (26, 27). Further studies are needed to clarify the functional role of TLR3 as a specific pattern recognition receptor, its interaction with other receptor molecules, its regulation by cytokines in the intestinal epithelium as well as inflammatory cells, and finally, its causal relevance in the differential pathogenesis of inflammatory bowel diseases.
Interestingly, TLR3 is localized on chromosome 4 (q35) (33) at the border of a large linkage region of a recently described IBD susceptibility gene, suggesting a potential pathogenic association of IBD with the TLR3 gene (14). Thorough assessments of novel mutant polymorphisms in the TLR3 gene may provide insight into IEC-specific dysregulation of the receptor in CD.
In contrast to TLR3, TLR4 is significantly increased in IECs throughout the lower gastrointestinal tract regardless of whether assessed in active or inactive disease of both CD and UC patients. However, the subcellular distribution of TLR4 differed between CD and UC epithelia (apical versus basolateral). Recent findings from animal models and genetic complementation studies have suggested that TLR4 can serve as a major transducing subunit of the LPS receptor complex (31, 39). Lumenal LPS is usually well tolerated in large quantities within the healthy intestine. This tolerance could result from TLR4 downregulation minimizing LPS recognition, given that primary IECs in normal tissue appear to express very little, if any, TLR4 (28). However, in IBD, host tolerance towards lumenal bacterial toxins may be broken (8, 17, 21), which could reflect increased LPS recognition as a result of TLR4 upregulation. Acute injury of the intestinal mucosa may also lead to recruitment of TLR4-positive macrophages into the mucosa. These inflammatory cells highly express the TLR4 coreceptor CD14, which could play an important linking role in enhancing hyperresponsiveness of the intestinal mucosa to LPS in IBD (1, 13, 34).
Spontaneous mutations of TLR4 could prime individuals experiencing acute infections to develop especially severe disease (3). It has previously been shown that C3H/HeJ mice which have a single point mutation of TLR4 (16, 32) are highly susceptible to developing a more severe form of dextran sodium sulfate-induced colitis (9). Interestingly, the TLR4 gene is localized on chromosome 9 (q32-33) (33), another genomic region in which a CD susceptibility gene has been implicated (6). In active IBD, variant alleles in the TLR4 gene could induce functional dysregulation of the receptor to LPS. In this study, we also found that during long-standing, quiescent disease, IECs constitutively overexpress TLR4 compared to normal controls. This observation could result from a “gain-of-function” mutation in this receptor which could functionally exhibit proinflammatory effects in response to physiological concentrations of LPS. However, it remains to be shown whether upregulated TLR4 confers functional hyperresponsiveness of the intestinal epithelium to LPS or rather reflects a loss of response. Moreover, TLR4 upregulation could also result from the effects of ligands other than LPS (20, 29). The factors and mechanisms regulating TLR4 expression in IECs in IBD remain to be further elucidated.
Our study suggests that TLR2 and TLR5 expression in IECs remain unchanged in active IBD. Neither control nor IBD tissues exhibit significant TLR2 expression in IECs. Upregulation of TLR2 is restricted to scattered inflammatory cells of the lamina propria in active IBD. While TLR4 appears to be important for LPS signaling, recent in vitro studies suggest that TLR2 mainly transduces signals by gram-positive ligands such as lipoteichoic acid, peptidoglycan, and lipopeptides (22, 24). The lack of any significant alteration in intestinal epithelial expression of TLR2 in IBD suggests that such bacterial cell wall components of gram-positive microbes may not play a major role in modulation of innate immune responses in these disorders.
Similar to TLR2, TLR5 also appears not to be significantly regulated in acute intestinal inflammation in IBD. TLR5 is constitutively expressed on all surface IECs, regardless of whether derived from normal or IBD mucosae. However, the functional role of TLR5 in the gastrointestinal immune system needs to be further defined.
This is the first report suggesting that TLR expression may be altered in disease. However, the conclusiveness of this preliminary report is limited by the fact that it has been performed in a unicenter setting. It is evident that larger multicenter studies are needed to further specify differences in TLR expression between these entities of IBD and, more importantly, other inflammatory diseases of the gastrointestinal tract. So far only 10 members of the TLR superfamily have been identified. It is expected that more than 30 different TLRs are expressed in mammals; hence, at this point we cannot exclude the possibility that our newly generated antibodies might cross-react with any other, so far unknown TLR which might show homologies in the extracellular domains with TLR2, TLR3, or TLR4.
Based on the results of this initial study, we note that epithelial cells may be the predominant site of TLR expression in intestinal mucosa and postulate that IBD may be associated with distinctive changes in selective TLR expression in the intestinal epithelium. However, it remains unclear whether immune imbalance in IBD may either lead to or result from TLR dysregulation in IEC. Further studies are needed to focus on the direct pathogenetic relevance and immune consequences of TLR dysregulation in active IBD.
ACKNOWLEDGMENTS
This work was supported by grants DK41557 and DK43351 from the National Institutes of Health (to D.K.P.) and Ca226/2-1 from the Deutsche Forschungsgemeinschaft (to E.C.).
REFERENCES
- 1.Akashi S, Ogata H, Kirikae F, Kirikae T, Kawasaki K, Nishijima M, Shimazu R, Nagai Y, Fukudome K, Kimoto M, et al. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem Biophys Res Commun. 2000;268:172–177. doi: 10.1006/bbrc.2000.2089. [DOI] [PubMed] [Google Scholar]
- 2.Awane M, Andres P G, Li D J, Reinecker H C. NF-kB-inducing kinase is a common mediator of IL-17-, TNFa-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. [PubMed] [Google Scholar]
- 3.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 4.Bhan A, Mizoguchi E, Smith R N, Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol Rev. 1999;169:195–207. doi: 10.1111/j.1600-065x.1999.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 5.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 6.Cho J H, Nicolae D L, Gold L H, Fields C T, LaBuda M C, Rohal P M, Pickles M R, Qin L, Fu Y, Mann J S, et al. Identification of novel susceptibility loci for inflammatory bowel disease on chromosomes 1p, 3q, and 4q: evidence for epistasis between 1p and IBD1. Proc Natl Acad Sci USA. 1998;95:7502–7507. doi: 10.1073/pnas.95.13.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 8.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde K H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson C O, Sartor R B, Tennyson G S, Riddell R H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 10.Faure E, Equils O, Sieling P A, Thomas L, Zhang F X, Kirschning C J, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of tlr-4 and tlr-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 11.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 12.Gatti E, Velleca M A, Biedermann B C, Ma W, Unternaehrer J, Ebersold M W, Medzhitov R, Pober J S, Mellman I. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J Immunol. 2000;164:3600–3607. doi: 10.4049/jimmunol.164.7.3600. [DOI] [PubMed] [Google Scholar]
- 13.Grimm M C, Pavli P, Van de Pol E, Doe W F. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin Exp Immunol. 1995;100:291–297. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampe J, Schreiber S, Shaw S H, Lau K F, Bridger S, Macpherson A J, Cardon L R, Sakul H, Harris T J, Buckler A, et al. A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet. 1999;64:808–816. doi: 10.1086/302294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cutting edge: cells that carry a null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 16.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 17.Hotta T, Yoshida N, Yoshikawa T, Sugino S, Kondo M. Lipopolysaccharide-induced colitis in rabbits. Res Exp Med. 1986;186:61–69. doi: 10.1007/BF01851834. [DOI] [PubMed] [Google Scholar]
- 18.Ingalls R R, Heine H, Lien E, Yoshimura A, Golenbock D. Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect Dis Clin N Am. 1999;13:341–353. doi: 10.1016/s0891-5520(05)70078-7. [DOI] [PubMed] [Google Scholar]
- 19.Jung H C, Eckmann I, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 21.Lange S, Delbro D S, Jennische E, Mattsby-Baltzer I. The role of the Lps gene in experimental ulcerative colitis in mice. APMIS. 1996;104:823–833. doi: 10.1111/j.1699-0463.1996.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 22.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 23.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccini I. Expression of class II molecules on intestinal epithelial cells in humans: differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100:3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 24.Means T K, Lien E, Yoshimura A, Wang S, Golenbock D T, Fenton M J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 25.Medzhitov R, Preston-Hurlburt P, Janeway C A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 26.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco I P, Allavena P, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 27.Muzio M, Polentarutti N, Bosisio D, Prahladan M K, Mantovani A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leucocytes. J Leukoc Biol. 2000;67:450–456. doi: 10.1002/jlb.67.4.450. [DOI] [PubMed] [Google Scholar]
- 28.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 30.Podolsky D K. Inflammatory bowel disease. N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 31.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between LPS and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–2167. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4. J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock F L, Hardiman G, Timans J C, Kastelein R A, Bazan J F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogler G, Andus T, Aschenbrenner E, Vogl D, Falk W, Scholmerich J, Gross V. Alterations of the phenotype of colonic macrophages in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:893–899. doi: 10.1097/00042737-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Sartor R B. Microbial factors in the pathogenesis of Crohn's disease, ulcerative colitis, and experimental intestinal inflammation. In: Kirsner J B, Shorter R G, editors. Inflammatory bowel disease. 4th ed. Baltimore, Md: Williams & Wilkins; 1995. pp. 96–124. [Google Scholar]
- 36.Sartor R B. Role of enteric flora microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment Pharmacol Ther. 1997;11:17–23. doi: 10.1111/j.1365-2036.1997.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 37.Schultsz C, Van Den Berg F M, Ten Kate F W, Tytgat G N, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117:1089–1097. doi: 10.1016/s0016-5085(99)70393-8. [DOI] [PubMed] [Google Scholar]
- 38.Sebastiani G, Leveque G, Lariviere L, Laroche L, Skamene E, Gros P. Cloning and characterization of the murine toll-like receptor (Tlr5) gene: sequence and mRNA expression studies in salmonella susceptible MOLF/Ei mice. Genomics. 2000;64:230–240. doi: 10.1006/geno.2000.6115. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 40.Wright S D. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R B, Mark M R, Gurney A L, Godowski P J. Signaling events induced by lipopolysaccharide-activated toll-like receptor 2. J Immunol. 1999;163:639–643. [PubMed] [Google Scholar]
- 42.Yang S K, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 43.Zhang F X, Kirschning C J, Mancinelli R, Xu X P, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]