Abstract
Diffusely adhering Escherichia coli (DAEC) strains expressing adhesins of the Afa/Dr family bind to epithelial cells in a diffuse adherence pattern by recognizing a common receptor, the decay-accelerating factor (CD55). Recently, a novel CD55-binding adhesin, named Dr-II, was identified from the pyelonephritogenic strain EC7372. In this report, we show that despite the low level of sequence identity between Dr-II and other members of the Afa/Dr family, EC7372 induces pathophysiological effects similar to those induced by other Afa/Dr DAEC strains on the polarized epithelial cell line Caco-2/TC7. Specifically, the Dr-II adhesin was sufficient to promote CD55 and CD66e clustering around adhering bacteria and apical cytoskeleton rearrangements. Unlike other Afa/Dr DAEC strains, EC7372 expresses a functional hemolysin that promotes a rapid cellular lysis. In addition, cell death by apoptosis or necrosis was observed in EC7372-infected Caco-2/TC7 cells, depending on infection time. Our results indicate that EC7372 harbors a pathogenicity island (PAI) similar to the one described for the pyelonephritogenic strain CFT073, which carries both hly and pap operons. Cumulatively, our findings indicate that strain EC7372 can be considered a prototype of a subclass of Afa/Dr DAEC isolates that have acquired a PAI harboring several classical uropathogenic virulence genes.
Urinary tract infections (UTIs) are among the most common bacterial infections in humans. Escherichia coli, the dominant etiologic pathogen in UTIs, accounts for more than 80% of all cases (3). Epidemiological studies show that diffusely adhering E. coli (DAEC) strains, defined by Scaletsky et al. (53), are involved in 30 to 50% cystitis in children, 30% pyelonephritis in pregnant women, and recurrent UTIs in young adult women (22, 33, 48). In addition, a subset of DAEC strains has been found associated with diarrhea. The DAEC family consists of a heterogeneous group of E. coli strains whose virulence factors, except for their adhesin, remain largely unknown. Some DAEC strains may be evolutionarily close to enteroaggregative E. coli (EAEC) (14). On the other hand, a subset of diffuse adhering strains have been renamed diffusely adhering enteropathogenic E. coli (DA-EPEC) because they contain a homologue of the locus of enterocyte effacement pathogenicity island (PAI) and exhibit pathogenic properties characteristic of enteropathogenic strains (4). DAEC strains express adhesins of the Afa/Dr family, which include the afimbrial adhesins AfaE-I (34) and AfaE-III (35), the Dr adhesin (42), and the fimbrial F1845 adhesin (9). The structural assembly genes coding for Afa/Dr adhesins are similar in organization, consisting of operons of at least five genes. Genes A to D, encoding accessory proteins, are highly conserved between the family members, whereas the gene E encoding the adhesin molecule itself is more divergent.
Afa/Dr adhesins mediate bacterial adhesion in a diffuse adherence pattern to erythrocytes (44) and epithelial cells (5, 6) by binding to a common receptor, the decay-accelerating factor (CD55), a complement regulatory protein (36). The CD55 molecule has four contiguous short consensus repeat (SCR) domains, followed by a serine/threonine-rich C-terminal domain. A glycosylphosphatidylinositol (GPI) anchor attaches the molecule to the outer leaflet of the cell membrane. The Afa/Dr adhesins bind preferentially to the SCR3 domain on the CD55 molecule (43). CD55 is present in several tissues, including renal tissue (Bowman's capsule and basement membranes) and the uroepithelium of the urinary tract. Afa/Dr adhesins (F1845 and Dr) recruit the brush border-associated GPI proteins CD55 and carcinoembryonic antigen (CD66e) around adhering bacteria (23, 24), suggesting that GPI-associated signal transduction is important in DAEC pathogenesis. In addition, Afa/Dr adhesins induce F-actin disorganization resulting from activation of a GPI-linked Ca2+-dependent signal pathway in intestinal epithelial cell lines (46).
Recently, a novel CD55-binding adhesin (termed Dr-II) was cloned from E. coli strain EC7372, which was recovered from an acute gestational pyelonephritis patient (49). Dr-II is 96% identical to the nonfimbrial adhesin NFA-1, an adhesin associated with UTI whose receptor has not been identified (2). Interestingly, although NFAs have not previously been considered part of the Afa/Dr family, they are very similar in genetic organization. Although it shows only 20% identity to the Afa/Dr adhesins, Dr-II adhesin displays receptor specificity for the SCR3 domain of the CD55 molecule. To gain further insights into the mechanism(s) of pathogenicity of the uropathogenic DAEC strain EC7372, we have examined its interaction with polarized human epithelial cells which express CD55 (5). We show that EC7372 induces adhesin-mediated cellular events similar to those previously observed with other Afa/Dr DAEC strains. In addition, EC7372 induces cellular lysis and promotes cell death by necrosis or apoptosis. We establish that the pyelonephritis-associated strain EC7372 contains the hemolysin-encoding gene hlyA and our results strongly suggest that hlyA is located on a known PAI, that of strain CFT073 (PAICFT073). We present here the first detailed study on an Afa/Dr DAEC strain in which virulence factors other than the adhesin have been identified. Our findings indicate that EC7372 can be considered a prototype of a subclass of uropathogenic Afa/Dr DAEC isolates that have acquired a PAI harboring several classical UTI virulence genes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. All bacterial strains were maintained on Luria-Bertani plates; prior to infection, bacteria were grown in Luria broth (Difco Laboratories) at 37°C for 18 h with appropriate antibiotics.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| EC7372 | DAEC clinical strain isolated from patient with gestational pyelonephritis, expressing Dr-II adhesin | 49 |
| TP411 | DH5α recombinant strain carrying pTP411 (Ampr), encoding Dr-II adhesin | 49 |
| IH11128 | DAEC clinical strain isolated from patient with pyelonephritis, expressing Dr adhesin | 56 |
| J96 | UPEC clinical strain isolated from patient with pyelonephritis, hly+ cnf1+ pap+ | 54 |
| DH5α/pOMEO | DH5α recombinant strain carrying pOMEO (Carbr), encoding cytolethal distending toxin (cdt+) | 47 |
| SE124 | C600 recombinant strain carrying pSE124 (Kanr), encoding hemolysin (hly+) | 17 |
| MG1655 | K-12 derivative | 10 |
Cell lines and culture conditions.
The Caco-2/TC7 clone (13) was established from the parental human colonic adenocarcinoma cell line Caco-2 (20), which spontaneously differentiates in culture (50). Cells were grown in Dulbecco's modified Eagle's minimal essential medium (DMEM; 4.5 g of glucose/liter; Life Technologies) supplemented with 15% fetal calf serum (Boehringer) and 1% nonessential amino acids (Life Technologies). The cells were maintained at 37°C in a 10% CO2–90% air atmosphere. Differentiated cells at late postconfluence were used for infection assays (15 days postseeding).
HeLa cells were cultured at 37°C in 5% CO2–95% air in RPMI 1640 medium (Life Technologies) supplemented with 2 mM l-glutamine (BioWhittaker) and 10% fetal calf serum.
Cell infection assay.
Prior to infection, cells were washed twice with phosphate-buffered saline (PBS). Infecting E. coli cells were suspended in culture medium, and 1 ml of this suspension was added to each tissue culture plate in order to have a multiplicity of infection of 100:1. The infection assay was conducted in the presence of 1% mannose to prevent type 1 fimbria-mediated binding. The plates were incubated at 37°C in 10% CO2–90% air for the indicated time. Infected cells were then washed three times with sterile PBS to remove nonadhering bacteria.
Antibodies.
The mouse monoclonal antibody CY-CD55 directed against human CD55 was obtained from Valbiotech (Paris, France). The polyclonal anti-CD66e rabbit antibody was from Dako (Tebu, France). Fluorescein isothiocyanate (FITC)-conjugated goat anti-immunoglobulin G was from Institut Pasteur Productions (Paris, France). Staining for F-actin was performed with FITC-labeled phalloidin (Molecular Probes, Inc.). The polyclonal anti-CD55 antibody used to prevent Dr-II–CD55 interaction was kindly provided by D. M. Lublin (Washington University, St. Louis, Mo.).
Immunofluorescence.
Cultured cells were prepared on glass coverslips in 24-well tissue culture plates (Corning Glass Works, Corning, N.Y.). Preparations were fixed for 10 min at room temperature in 3.5% paraformaldehyde in PBS.
CD55 and CD66e clustering around adhering bacteria was detected on unpermeabilized cell layers by indirect immunofluorescence as described previously (24). Cell monolayers were incubated with antibodies directed against human CD55 or human CD66e for 45 min at room temperature, washed, and then incubated with FITC-labeled secondary antibody.
To visualize F-actin, coverslips were permeabilized with 0.2% Triton X-100 in PBS for 4 min at room temperature before incubation with fluorescein-phalloidin for 45 min at room temperature. The coverslips were then washed three times with PBS.
Specimens were mounted in Citifluor antifade mounting medium (Citifluor Laboratories, Birmingham, United Kingdom). Specimens were examined by epifluorescence using a Leitz Aristoplan microscope. All photographs were taken on Kodak T-MAX 400 black-and-white or color film (Eastman Kodak Co., Rochester, N.Y.).
Hemolysin assay.
For qualitative evaluation of hemolysin production, bacterial strains were inoculated onto Columbia agar plates (Biomérieux, Dardilly, France) containing 5% sheep blood. Hemolysis was defined as a clear zone around or under bacterial colonies after 18 h of culture at 37°C.
Measurement of cell lysis and sugar protection.
Cell lysis was determined by measuring the release of lactate dehydrogenase (LDH) from epithelial cells in the culture medium postinfection (Enzyline LDH kit; Biomérieux). For each bacterial strain, assays were performed in triplicate.
Sugars of different molecular weights and molecular radius were used at 30 mM in DMEM-PBS (1:1) as protectants as previously described (8). Sucrose and raffinose were purchased from Sigma Chemical Co. Dextran 4 and dextran 8 were purchased from Serva Laboratories.
PCR, Southern hybridization, and DNA sequencing.
Colony PCR was carried out using PCR Beads Ready To Go (Amersham Pharmacia) according to the manufacturer's protocol. PCRs were performed with a Gene Amp PCR system 2400 (Perkin-Elmer/Applied Biosystems), and PCR products were examined on 1% agarose gels. PCR for detection of hemolysin (hlyA), cytotoxic necrotizing factor 1 and 2 (cnf1 and -2), and cytolethal distending toxin (cdt) sequences were performed as follows. After an initial denaturation (5 min at 94°C), samples were subjected to 30 cycles of amplification, each of which consisted 30 s at 94°C, 30 s at 57°C, and 1 min at 72°C. A final extension of 10 min at 72°C was performed. Primers hlyA1 (5′-CTC ATT GGC CTC ACC GAA CGG-3′) and hlyA2 (5′-GCT GGC AGC TGT GTC CAC GAG-3′), which are conserved between hlyA sequences (GenBank accession numbers M10133 and AF037572 to AF037579) were designed to amplify a 299-bp internal fragment from the hlyA gene. Primers cnfA (5′-CTG AGC GGC ATC TAC TAT GAA G-3′) and cnfB (5′-CCT GTC AAC CAC AGC CAG TAC-3′), which are conserved between cnf1 and cnf2, were used to amplify a 626-bp internal fragment from cnf genes. Degenerate primers cdt1 (5′-GTW GCR ACY TGG AAY YTK CAR GG-3′) and cdt2 (5′-KCM GGY KMR CGR TTR AAA TCW CC-3′) were designed by comparing four cdt sequences (GenBank accession numbers U03293, U04208, U89305, and U53215) to amplify a 500-bp internal fragment from the cdt gene. The specificity of hlyA, cnf, and cdt primers was tested with both positive and negative controls (see Results).
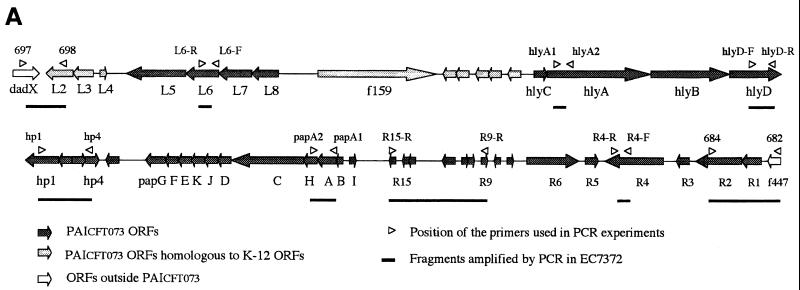
Detection in EC7372 of sequences encoded by PAICFT073 (Fig. 7A) was conducted by PCR on colonies as described above, except that annealing temperature was 55°C and elongation time was 3 min. Positions of the different primers are shown in Fig. 7A. The left junction of the PAI was amplified with primers 697 and 698 (32); the right junction of the PAI was amplified with primers 682 and 684 (32). Primers from PAICFT073 were designed according to the published sequence (GenBank accession numbers AF081283, AF081284, and AF081285). Primers L6-R (5′-TTC ACG AAG TAA CGC CAG-3′) and L6-F (5′-AGA TGT TAA CTA CCC TGG-3′) were used to amplify a 200-bp internal fragment from L6. Primers hlyD-F (5′-CTG AAG AGG AAG TAC TGC-3′) and hlyD-R (5′-AGA GCA GTA ACC TCC AGC-3′) were used to amplify a 575-bp internal fragment from hlyD. Primers hp1-R (5′-TAC TGA GAT GGC TTC ATC-3′) and hp4-R (5′-GCT GTC GCC AGT CGA TAC-3′) were used to amplify a 1,840-bp fragment extending from hp1 to hp4. Primers papA1 and papA2 (11) were used to amplify a 856-bp fragment from papA. Primers R15-R (5′-CCA GCC TTC CCA GCA ATC-3′) and R9-R (5′-ACC TAA CAG CAG CAC ATC-3′) were used to amplify a 4,400-bp fragment extending from R9 to R15. Primers R4-R (5′-GTA TCA CAT ATC CTG TTG-3′) and R4-F (5′-ATT CGT CAC TGA GCG CTG-3′) were used to amplify a 242-bp fragment from R4. Primers specific for each class of papG alleles have been described previously (29).
FIG. 7.
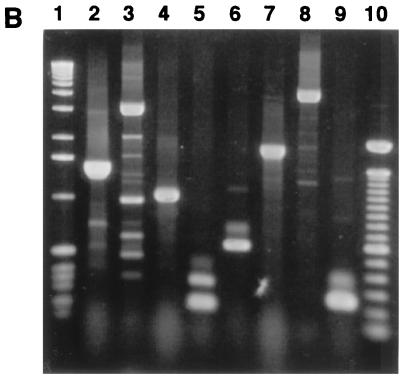
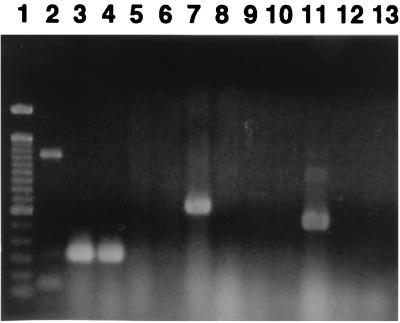
Detection by PCR of PAICFT073 sequences in strain EC7372. (A) Schematic representation of PAICFT073 (26) and positions of the primers complementary to PAICFT073 sequences used to amplify DNA fragments in EC7372. ORFs, open reading frames. (B) Amplification in EC7372 of DNA fragments using primers complementary to PAICFT073 sequences. Lane 1; 1-kb DNA ladder. PCR fragments of the expected sizes were amplified from EC7372 with PAICFT073 right-junction primers (lane 2), PAICFT073 left-junction primers (lane 3), papA primers (lane 4), L6 primers (lane 5), hlyD primers (lane 6), hp1-hp4 primers (lane 7), R9-R15 primers (lane 8), and R4 primers (lane 9). Lane 10, 100-bp DNA ladder.
Southern hybridization analysis was carried out using chromosomal DNA digested by BamHI, size fractionated in 1% agarose gels, and transferred to a nylon membrane by capillarity as described elsewhere (52). Labeling of PCR-generated fragments (hlyA fragment or hp1-hp4 fragment) and hybridization (at 42°C) were performed using the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia) according to the manufacturer's protocol.
DNA sequencing was performed on purified PCR products (Qiagen gel extraction kit) using a dye terminator cycle sequencing kit with AmpliTaq DNA polymerase (Perkin-Elmer) and an ABI PRISM 310 system. The length of DNA sequenced from each PCR product was approximately 300 bp.
Apoptosis assay.
Caco-2/TC7 cells in culture plates (Corning Glass Works) were infected with bacteria for 1.5 h. Cells were then washed five times with sterile PBS to remove nonadherent bacteria, treated by gentamicin (100 μg/ml) for 1 h to kill extracellular bacteria, and incubated for 8 h at 37°C in 10% CO2–90% air. As a positive control, cells were cultivated in presence of the apoptosis inducer NaBt (5 mM; Sigma) as described by Kamitani et al. (31).
For morphological assessment of cells undergoing apoptosis, cells were stained with Hoechst 33258 (5 μg/ml; Sigma) for 1 h at 37°C in 10% CO2–90% air and costained with ethidium bromide (EB; 5 μg/ml; Eurobio, Les Ulis, France) added just before observation. Immunofluorescent staining and cell morphology were examined by epifluorescence with filters for UV excitation (<350 nm) and phase-contrast microscopy using a Leitz Aristoplan microscope. All photographs were taken on Kodak T-MAX 400 color film (Eastman Kodak).
For flow cytometry analysis, trypsinized cells were pelleted, permeabilized in 70% cold ethanol, and stored at 4°C for at least 24 h. Immediately prior to analysis, cells were pelleted and resuspended in PBS. Propidium iodide was added to reach a final concentration of 50 μg/ml. Cells were analyzed for DNA content on a FACScalibur (Becton Dickinson) flow cytometer after selection on the basis of light-scattering properties to eliminate cell debris. Due to reduced DNA stainability, apoptotic cells accumulate in a characteristic sub-G1 peak in the DNA content profile and can thus be quantified (15). Results of one experiment, representative of three, are shown.
Statistical analysis.
Values are the means ± standard errors of the means from three separate experiments, each performed in triplicate.
RESULTS
E. coli EC7372 promotes the characteristic Afa/Dr adhesin-induced cellular responses via the Dr-II adhesin.
We have previously reported that infection of cultured intestinal Caco-2/TC7 cells by Afa/Dr DAEC strain C1845 or IH11128, bearing F1845 or Dr adhesin, respectively, induces the recruitment of CD55 and CD66e GPI-anchored proteins around adhering bacteria (24). Recombinant E. coli strains expressing F1845 or Dr adhesin also display such rearrangements. To investigate whether strain EC7372, bearing the Dr-II adhesin, induces a similar phenotype, we examined the distribution of CD55 and CD66e upon infection of Caco-2/TC7 cells. Immunofluorescence experiments using anti-CD55 and anti-CD66e antibodies revealed recruitment of CD55 and CD66e proteins around adhering EC7372 bacteria at 1.5 h postinfection (p.i.) (Fig. 1C and D, respectively). Similar clustering of CD55 and CD66e was observed around adhering TP411 bacteria, which are DH5α derivatives carrying the Dr-II adhesin operon on a plasmid (Fig. 1E and not shown, respectively), indicating that CD55 and CD66e recruitment is directly triggered by the Dr-II adhesin. As expected, no clustering was observed with the nonadherent strain E. coli DH5α (Fig. 1F).
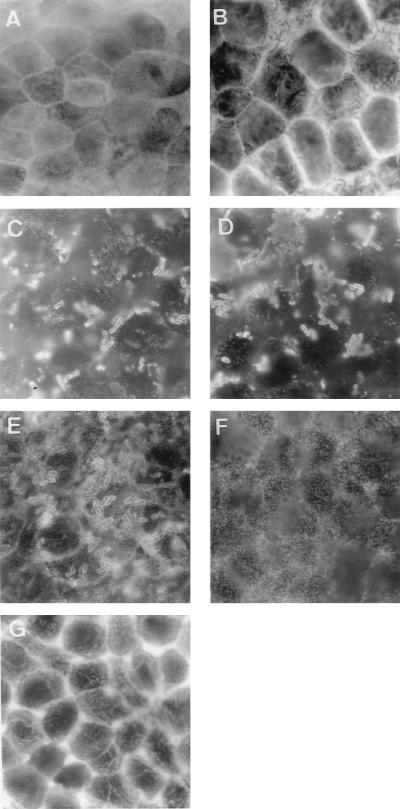
FIG. 1.
Changes in the distribution of apical F-actin, CD55, and CD66e in Caco-2/TC7 cells upon infection by EC7372 and TP411 (Dr-II+). Cells were infected for 1.5 h at 37°C. Fixed cells were stained with fluorescein-phalloidin for F-actin labeling (A, B, and G) or with polyclonal antibodies anti-CD55 (C, E, and F) or anti-CD66e (D). Apical domains of the cells were examined by epifluorescence microscopy. (A) Uninfected cells show the fine flocculated F-actin labeling which represents F-actin in the microvilli on the apical surface. (B) EC7372-infected cells show the typical Afa/Dr-induced F-actin disassembly. (C) CD55 clustering around adhering EC7372 bacteria. (D) CD66e clustering around adhering EC7372 bacteria. (E) CD55 clustering around adhering TP411 (Dr-II+) bacteria. (F) Lack of CD55 clustering with nonadherent DH5α bacteria. (G) TP411-infected cells show the typical Afa/Dr-induced F-actin disassembly. Magnifications, ×100.
We have previously demonstrated that Afa/Dr DAEC strains induce F-actin disorganization in intestinal epithelial cell lines due to the Afa/Dr adhesin-CD55 interaction (6). At 1.5 h p.i., the adhering EC7372 strain promoted disorganization of the apical F-actin, with the appearance of dense F-actin at the perijunctional ring of infected cells (Fig. 1B), whereas noninfected cells displayed a fine flocculated F-actin labeling at the apical surface (Fig. 1A). This EC7372-induced F-actin disassembly is identical to effects previously observed with Afa/Dr strain C1845 and IH11128 (6). As shown in Fig. 1G, TP411 (Dr-II+) also induced F-actin disorganization, indicating that the Dr-II adhesin is sufficient to mediate F-actin rearrangement.
Taken together, these results demonstrate (i) that E. coli EC7372 promotes cellular responses similar to those previously observed upon infection of Caco-2/TC7 cells with other members of the Afa/Dr DAEC family and (ii) that the Dr-II adhesin is sufficient to promote these responses.
E. coli EC7372 induces a rapid hemolysin-dependent cell lysis.
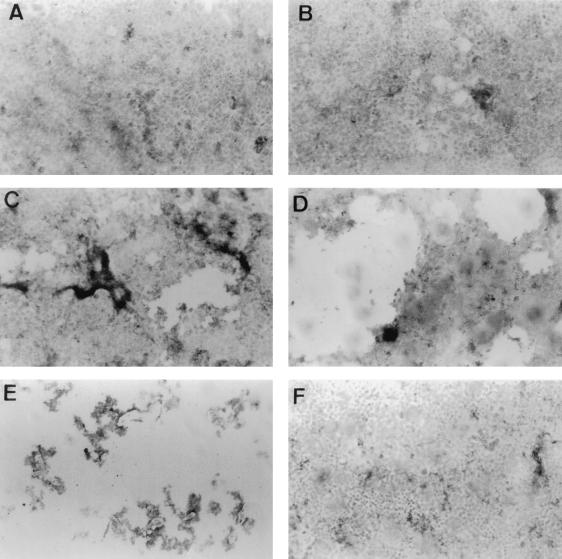
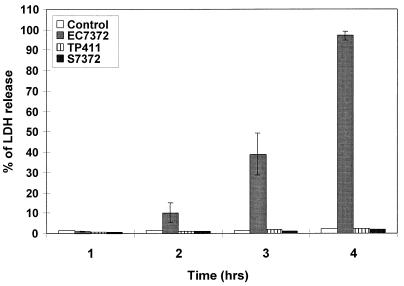
During the experiment reported above, we noticed that increasing infection time of Caco-2/TC7 cells resulted in cell lysis. Using phase-contrast light microscopy, we showed that EC7372 induced lysis in a time-dependent manner (Fig. 2A to E): cell lysis became apparent at 2 h p.i. (Fig. 2C), and at 4 h p.i. the cell monolayer was entirely destroyed (Fig. 2E). The extent of cell lysis was assessed by measuring LDH release from EC7372-infected Caco-2/TC7 cells. The kinetic of LDH release in the cell culture medium correlated with the morphological analysis of cell lysis (Fig. 3). We observed similar effects of EC7372 infection on the HeLa cell line, demonstrating that the EC7372-induced cell lysis was not specific to the Caco-2/TC7 cell line. On the other hand, neither cell lysis (Fig. 2F) nor LDH release (Fig. 3) occurred when the cells were infected with the recombinant strain TP411 (Dr-II+), indicating that the Dr-II adhesin is not sufficient to promote cell lysis. Furthermore, no cell lysis was observed at 4 h p.i. with the DAEC strain IH11128 harboring the Dr adhesin (not shown). Taken together, these results indicate that strain EC7372 promotes cell lysis in different cell lines by a virulence factor distinct from the Dr-II adhesin.
FIG. 2.
Detection of cell lysis by phase-contrast microscopy in EC7372-infected Caco-2/TC7 cells as a function of the time. (A) Uninfected cells; (B to E) EC7372-infected cells at 1, 2, 3, and 4 h p.i., respectively; (F) TP411 (Dr-II+)-infected cells at 4 h p.i. Magnifications, ×25.
FIG. 3.
Release of intracellular LDH from Caco-2/TC7 cells infected with EC7372 as a function time. S7372 is the spent culture supernatant of EC7372 (18 h in culture). Data are presented as the percentage of LDH released from the infected cells; 100% release (3,000 U of LDH/ml) was obtained by lysing the cells with distilled water. Values are the means ± standard errors from a minimum of three experiments.
To identify the virulence factor expressed by strain EC7372 which promotes cell lysis, we investigated by PCR analysis the presence of genes encoding known cytotoxins such as hemolysin (hlyA), cytotoxic necrotizing factors (cnf1 and -2), and cytolethal distending toxin (cdt). As shown in Fig. 4, the use of specific hlyA primers allowed amplification of a DNA fragment of the same size with strain EC7372 and with the positive control J96. In contrast, no amplification was found with DAEC strain IH11128 or the nonpathogenic E. coli strain MG1655. No amplification was detected using cnf and cdt primers in strains EC7372 and IH11128, whereas amplified DNA fragments were obtained with positive control strains (J96 and DH5α/pOMEO).
FIG. 4.
Detection of toxin-encoding genes in strain EC7372 by PCR analysis. Lanes 1, 100-bp DNA ladder; 2 to 5, PCR with hlyA primers on MG1655, SE124 (hly+), EC7372, and IH111-28; 6 to 9, PCR with cnf primers on MG1655, J96 (cnf+), EC7372, and IH111-28; 10 to 13, PCR with cdt primers on MG1655, DH5α/pOMEO (cdt+), EC7372, and IH111-28.
In agreement with the detection of hlyA DNA sequence in EC7372, we observed a clear zone of hemolysis under EC7372 colonies cultured on sheep blood agar. Hemolysin cytolysis is known to result from formation of aqueous pores (7, 58). Because of the rapidity of the EC7372-induced lysis, we determined the size of the pores by using sugars of increasing molecular weights as protectants (8). Neither sucrose (effective diameter, 0.9 nm) nor raffinose (molecular diameter, 1.3 nm) was able to completely protect cells against the EC7372-induced lysis (1.5 and 24.6% inhibition of cell lysis, respectively), whereas complete inhibition of cell lysis was obtained when cells were treated with dextran 4 (molecular diameter, ∼3 nm) or dextran 8 (molecular diameter, ∼6 nm). These results indicate that strain EC7372 induces cell lysis by formation of pores of approximately 3 nm, which is consistent with other results obtained with purified E. coli hemolysin (8). Surprisingly, we found that the sterile supernatant of an 18-h culture of strain EC7372 failed to induce cell lysis (Fig. 3). A similar result was obtained with sterile supernatants of cultures grown for 2, 5, or 7 h (data not shown). One could speculate that efficient lysis might be dependent on cell contact mediated by the Dr-II adhesin. However, prevention of interaction of Dr-II with CD55 and CD66e (24) by using polyclonal anti-CD55 and anti-CD66e antibodies did not inhibit cell lysis (94% of LDH release at 4 h p.i.), suggesting that binding of the Dr-II adhesin to its receptors is not required for efficient lysis.
E. coli EC7372 promotes apoptosis and/or necrosis on Caco-2/TC7 cells depending on infection time.
Massive disruption of the host cell membrane by pore-forming toxins overwhelms the cellular homeostasis and results in cell destruction by necrosis. Pore-forming proteins can also induce other subtle biochemical changes, which may result in cell death by apoptosis (39). To determine the mechanism by which Caco-2/TC7 cells died upon EC7372 infection, we investigated nuclear morphologies of the cells by DNA staining and light microscopy. We used the DNA dye Hoechst 33258, which stains normal and apoptotic nuclei blue, whereas EB stains the nuclei of necrotic cells orange-red because staining occurs only when the cell permeability is altered.
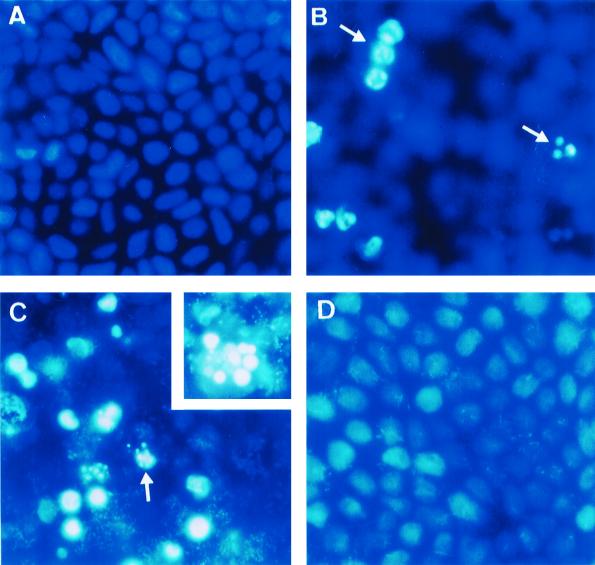
Caco-2/TC7 cells infected with EC7372 for more than 3 h p.i. were detached from the dishes and stained orange-red, indicating cytotoxicity by necrosis (data not shown). However, features of apoptosis were detected when the infection time was reduced to 1.5 h. After infection, cells were treated with gentamicin to kill adhering bacteria and were incubated further at 37°C. After 4 h, cells started to display the characteristic features of apoptotic morphology (not shown), but the effect was even clearer after 8 h (Fig. 5C). Intense cytoplasmic vacuolization, reduced size, and enhanced fluorescence of condensed and marginated nuclear chromatin were clearly visible. In these conditions, the number of necrotic cells was not significant. A similar morphology was observed when the apoptosis inducer NaBt was added to Caco-2/TC7 cells (31) (Fig. 5B). In contrast, cells infected with the recombinant strain TP411 (Dr-II+) (Fig. 5D) or with the DAEC strain IH11128 bearing Dr adhesin (data not shown) showed morphologies similar to those observed in noninfected cells (Fig. 5A), indicating that neither apoptosis nor necrosis was induced.
FIG. 5.
Apoptotic cells in EC7372-infected Caco-2/TC7 cells. Cells were infected for 1.5 h, treated for 1 h with gentamicin, incubated for 8 h at 37°C, and then costained with Hoechst 33258 and EB. (A) Uninfected cells; (B) cells treated with the apoptosis-inducer NaBt; (C) EC7372-infected cells. Magnifications, ×40. The insert shows at higher magnification (×100) an apoptotic cell in EC7372-infected cells. (D) TP411 (Dr-II+)-infected cells. Magnification, ×40. Arrows denote cells displaying apoptotic morphologies.
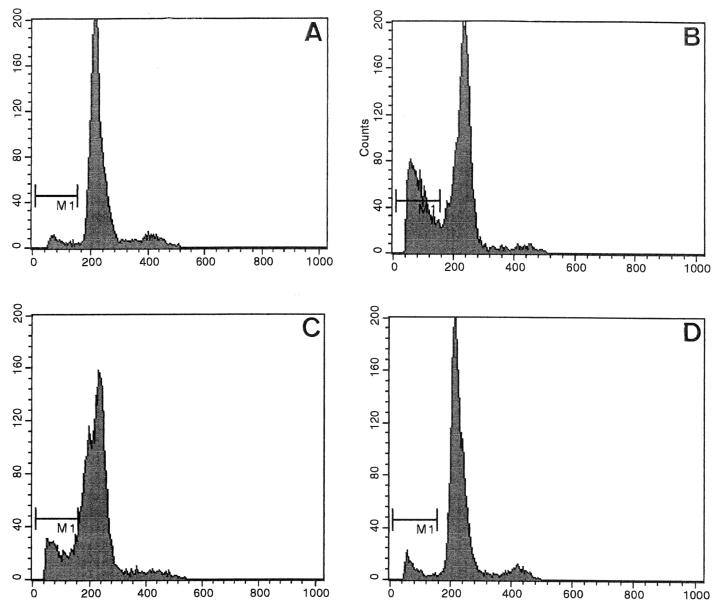
To confirm the EC7372-induced apoptosis and to quantify the process, we performed flow cytometric experiments. A typical flow cytometric hallmark of apoptosis is the appearance of a distinct sub-G1 hypodiploid peak, where dying cells localize due to their reduced amount of DNA (18). Propidium iodide staining generates fluorescence and allows the identification and quantification of cells in different phases of the cell cycle, as well as detection of the sub-G1 peak. In EC7372-infected cells (Fig. 6C), we observed a sub-G1 peak containing 16% apoptotic cells, versus 2% in noninfected cells (Fig. 6A) or in cells infected with the recombinant strain TP411 (Dr-II+) (Fig. 6D). A level of 32% apoptotic cells was found in NaBt-treated cells used as a positive control (Fig. 6B).
FIG. 6.
Apoptosis in EC7372-infected Caco-2/TC7 cells revealed by flow cytometric analysis. Cells were labeled with propidium iodide before cell cycle analysis by flow cytometry. Nuclear propidium iodide fluorescence intensity was measured on a linear scale, and histograms were derived from analysis. The area marked M1 contains the apoptotic cell population. (A) Noninfected cells; (B) NaBt-treated cells; (C) EC7372-infected cells; (D) TP411 (Dr-II+)-infected cells. NaBt-treated and EC7372-infected cells exhibited a distinct sub-G1 peak.
Taken together, these results indicate that strain EC7372 induced apoptosis in Caco-2/TC7 cells infected for a short period or necrosis when the infection time was increased. In contrast, the recombinant strain TP411 (Dr-II+) and other DAEC strains failed to promote apoptosis or necrosis.
EC7372 harbors a PAI similar to that of the pyelonephritogenic strain CFT073.
Southern analysis indicated that the hlyA gene of EC7372 is present as a single copy and is chromosomally encoded (not shown). In uropathogenic E. coli (UPEC) strains, hly genes are often part of large virulence chromosomal clusters called PAIs (16). For example, the pyelonephritogenic strain CFT073 harbors a 58-kb PAI that contains both hly and pap operons (26, 32) (Fig. 7A). Primers specific for the left and right junctions of this PAI have been designed to amplify fragments of 1.4 and 3.1 kb, respectively, from CFT073 genomic DNA (32). Using these specific primers, we found that similar fragments could be amplified from strain EC7372 (Fig. 7B, lanes 2 and 3). As expected, such fragments could not be amplified from the K-12 derivative MG1655 (data not shown). In addition, PCR products were amplified from strain EC7372 using primers specific to the papA gene (Fig. 7B, lane 4). The PapG adhesin occurs in three known molecular variants (classes I to III). Using oligonucleotides specific for each class (29), we demonstrated by PCR experiments that, similarly to CFT073, EC7372 carries the class II papG allele (data not shown). Because PCR fragments corresponding to hlyA, pap, and the left and right junctions of PAICFT073 could be amplified from EC7372, we hypothesized that EC7372 contains a PAI analog of PAICFT073.
We investigated the extent of similarity between EC7372 sequences and PAICFT073 by performing PCR with several couples of primers, covering different areas of the island (Fig. 7A). All five sets of primers tested gave amplification of DNA fragments with the expected size when EC7372 was used as the template (Fig. 7B, lanes 5 through 9), whereas no such fragment was amplified from the MG1655 control (not shown). In addition, DNA sequencing of four of these PCR fragments (L6, hlyD, hp1-hp4, and R4) showed that sequence identity between EC7372 and CFT073 ranges from 99.3 to 100%. To determine whether the hlyA gene is included in the PAI, we performed a Southern hybridization analysis of EC7372 chromosomal DNA digested with BamHI. We used as probes a DNA fragment from the hlyA gene and a DNA fragment which is linked to hlyA in PAICFT073, hp1-hp4 (Fig. 7A). Both hlyA and hp1-hp4 probes hybridized to a BamHI restriction fragment of approximately 8 kb (data not shown). By analogy with the CFT073 genetic organization (Fig. 7A), it is likely that hlyA and hp1-hp4 are carried by the same restriction fragment in EC7372. Taken together, these results strongly suggest that the hlyA gene of strain EC7372 is encoded by a PAI similar to the one described for the UPEC strain CFT073.
DISCUSSION
In this study, we investigated the pathogenicity of the pyelonephritogenic DAEC strain EC7372, which expresses a novel Afa/Dr adhesin, Dr-II (49). Despite the low level of homology between Dr-II and other members of the Afa/Dr family of adhesins, Dr-II binds to the SCR3 domain of the CD55 molecule. Interaction of Afa/Dr adhesins with the CD55 molecule, whose major function is to protect the cells against lysis by autologous complement, can be considered as a prototypic example of the cross-talk between a pathogen and the host cells (1) in which interaction of bacterial adhesin with a membrane-associated receptor leads to a signal transduction promoting cellular responses. Binding to the CD55 molecules by Afa/Dr adhesins triggers clustering of CD55 and CD66e molecules around adhering bacteria and F-actin disassembly (6, 24). CD55 binding is necessary but not sufficient to promote these responses: we have shown that a mutant of the Dr adhesin, in which the aspartic acid at position 54 is replaced by a cysteine, retained the ability to bind CD55 but failed to induce CD55 and CD66e clustering (24) or actin reorganization (45). Despite the fact that Dr-II has a proline at the position corresponding to Asp54, we found that interaction of EC7372 with cultured human intestinal Caco-2/TC7 cells induces cellular responses similar to those displayed by other Afa/Dr adhesins. These phenotypes are promoted by the Dr-II adhesin, because identical results are observed with a recombinant strain carrying only the Dr-II adhesin. Therefore, the low level of homology between Dr-II and other Afa/Dr adhesins allows both recognition of the SCR3 domain of the CD55 molecule and induction of CD55-mediated cellular responses. This result is in agreement with the hypothesis that the Afa/Dr DAEC strains develop a common mechanism of pathogenicity through interaction of their adhesins with the CD55 GPI-anchored protein.
Despite these features shared with the other Afa/Dr DAEC strains, EC7372 was involved in a distinct process leading to rapid cell lysis, as shown by microscopy analysis and LDH release measurements. Our results reveal the presence of hlyA sequence encoding a functional hemolysin in strain EC7372. Hemolysin is a potential virulence factor of UPEC which can cause multiple effects, including release of iron from erythrocytes and direct toxicity to host cells, enhancing inflammatory response and allowing bacteria to penetrate the renal interstitium (28, 38, 55). Hemolysin is a member of the family of RTX toxins, which promote cytotoxicity by pore formation in the cell membrane. The kinetic of cell lysis appears to be faster than for some other UPEC strains encoding hemolysin (38), since the cell monolayer was completely destroyed at 4 h postinfection. Protection experiments using EC7372-infected cells show a predicted pore diameter of approximately 3 nm, which is in agreement with previous results obtained for purified E. coli hemolysin (8). The ability of DAEC strain EC7372 to rapidly destroy polarized epithelial cells evokes the newly characterized pathotype named cell-detaching E. coli (CDEC) (25). This rapid cell-detaching activity, observed on epithelial HeLa cells, is due to a hemolysin similar to the one seen in UPEC (17, 19, 37). Cell detachment has been observed with some diffusely adhering isolates, but it has not been established whether these strains belong to the Afa/Dr DAEC family. Surprisingly, we failed to detect cell lysis using EC7372 supernatant, suggesting that EC7372 does not secrete a functional hemolysin and/or that cell contact is important for efficient cell lysis. Prevention of the interaction between Dr-II adhesin and CD55 and CD66e using polyclonal antibodies did not prevent lysis, suggesting that the binding of Dr-II to its receptors is not required for efficient cell lysis.
Pore-forming toxins, including Staphylococcus aureus alpha-toxin, Actinobacillus leukotoxin, and E. coli hemolysin, can induce cell death by either apoptosis or necrosis, depending on toxin concentration (39). Pores induced by pore-forming toxins result in increasing cytosolic Ca2+, concentration which could be the signal for initiation of apoptosis (12, 41). Fernandez-Prada et al. (19) showed that E. coli hemolysin induces necrosis on human monocyte-derived macrophages and induces apoptosis on the macrophage cell line J774, suggesting that these two cell lines can be more or less sensitive to membrane damage. We investigated the mechanism by which the differentiated intestinal Caco-2/TC7 cells died upon infection by EC7372 and demonstrated host cell death with features of apoptosis or necrosis, depending on infection time. Caco-2/TC7 cells infected with strain EC7372 for more than 2 h undergo necrosis. In contrast, cells exposed to EC7372 for 1.5 h undergo apoptosis, as determined by characteristic morphological changes and emergence of a sub-G1 peak. Our results suggest that Caco-2/TC7 intestinal cells are rather slow to undergo apoptosis, since clear features of apoptosis were detected when cells were incubated for several hours at 37°C after the bacteria have been removed. We present here the first report of induction of apoptosis in polarized epithelial cells by a hemolysin-producing strain. Hemolysin expressed by strain EC7372 is likely to be responsible for apoptosis; however, this inference can be definitively validated only by using an isogenic hly mutant. In addition, we cannot exclude the contribution of some other factor(s). In vivo, it is unclear whether EC7372 would induce necrosis or apoptosis. DAEC strain EC7372 was recovered from a patient with gestational pyelonephritis, which has been defined histologically as a destructive inflammatory process. In vivo, necrotic cells generate a strong inflammatory response. Apoptotic cells could also play a proinflammatory role, since it has been reported that apoptosis promoted by Shigella flexneri or Listeria monocytogenes can initiate an inflammatory response (51, 60). Therefore, both apoptosis or necrosis could contribute to EC7372 pathogenesis in vivo.
Hemolysin genes are often genetically linked to P pilus genes (pap) and grouped with other virulence factors on large DNA regions called PAIs, which have probably been acquired by horizontal gene transfer (16, 21). Five different PAIs that encode hemolysin have been described for UPEC strains. Our results strongly suggest that strain EC7372 carries a PAI similar to the one described for the highly virulent pyelonephritogenic strain CFT073 (26, 32). PAICFT073 carries hly and pap clusters, a putative iron transport system, and several other genes whose functions are unknown. Chromosomal left and right junctions appear to be similar in EC7372 and CFT073, suggesting that the PAI is inserted at the same site in both strains. Sequences derived from PAICFT073 have been found in several clinical isolates and appeared to be significantly associated with acute pyelonephritis and cystitis (26). However, this is the first report of the presence of this PAI in a DAEC strain. This feature is probably not restricted to EC7372, since a recent epidemiological study has described other isolates harboring Afa/Dr adhesin, the pap operon, hlyA, and a marker from PAICFT073 (30), suggesting strongly that those strains harbors a PAI similar to PAICFT073. Therefore, we propose that EC7372 be considered a prototype of a subclass of Afa/Dr DAEC strains that harbor a uropathogenic PAI (PAICFT073). The fact that EC7372 expresses hemolysin and encodes type P pili was not expected because epidemiological studies on uropathogenic strains indicated a lack of association between Afa/Dr adhesins and these classical UPEC-associated virulence factors (3, 21, 59). In the case of EC7372, hemolysin may play a role in the cytotoxic destruction of cells from the luminal side of the renal tubular epithelium, which lacks the CD55 receptor, and allow the bacteria to penetrate the interstitium. Moreover, the association of different adhesins might improve colonization because the P pili and Afa/Dr adhesins display different cellular tropisms (40, 57). EC7372 contains a papG allele from class II, which is the predominant genotype found in acute pyelonephritis strains (27), and it has been proposed that the PapGIA2 type of adhesin may enhance the ability of E. coli to infect the kidneys. The presence of multiple adhesins allows the recognition of various receptors along the urinary tract and may be an important factor in the development of EC7372 pathogenicity.
ACKNOWLEDGMENTS
We are grateful to P. Boquet (INSERM U452, Nice, France), E. Oswald (ENVT-INRA, Toulouse, France), S. Bonacorsi (Université Denis Diderot-Paris 7, Paris, France), and S. Elliott (University of Maryland, Baltimore) for gifts of strains. We thank I. Gaspard (INSERM U461, Châtenay-Malabry, France) for assistance with flow cytometry. We thank E. A. Groisman and anonymous referees for comments on an earlier version of the manuscript.
J. Guignot was supported by a doctoral fellowship from the Ministère de l'Education Nationale de la Recherche et de la Technologie (MENRT). A. L. Servin was supported by a grant from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (MENRT). A.-B. Blanc-Potard was supported by a postdoctoral grant from the Fondation pour la Recherche Médicale (FRM).
REFERENCES
- 1.Abraham S N, Jonsson A B, Normark S. Fimbriae-mediated host-pathogen cross-talk. Curr Opin Microbiol. 1998;1:75–81. doi: 10.1016/s1369-5274(98)80145-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahrens R, Ott M, Ritter A, Hoschutzky H, Buhler T, Lottspeich F, Boulnois G J, Jann K, Hacker J. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coli uropathogen. Infect Immun. 1993;61:2505–2512. doi: 10.1128/iai.61.6.2505-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Johnson C E, Rubin R H, Arbeit R D, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beinke C, Laarmann S, Wachter C, Karch H, Greune L, Schmidt M A. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect Immun. 1998;66:528–539. doi: 10.1128/iai.66.2.528-539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38:248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1918–1928. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakdi S, Greulich S, Muhly M, Eberspacher B, Becker H, Thiele A, Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989;169:737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattner F R, Plunkett G, 3rd, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 11.Boyd E F, Hartl D L. Chromosomal regions specific to pathogenic isolates of Escherichia coli have a phylogenetically clustered distribution. J Bacteriol. 1998;180:1159–1165. doi: 10.1128/jb.180.5.1159-1165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buommino E, Morelli F, Metafora S, Rossano F, Perfetto B, Baroni A, Tufano M A. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect Immun. 1999;67:4794–4800. doi: 10.1128/iai.67.9.4794-4800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A, Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Czeczulin J R, Whittam T S, Henderson I R, Navarro-Garcia F, Nataro J P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–2699. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 16.Dozois C M, Curtiss R., III Pathogenic diversity of Escherichia coli and the emergence of 'exotic' islands in the gene stream. Vet Res. 1999;30:157–179. [PubMed] [Google Scholar]
- 17.Elliott S J, Srinivas S, Albert M J, Alam K, Robins-Browne R M, Gunzburg S T, Mee B J, Chang B J. Characterization of the roles of hemolysin and other toxins in enteropathy caused by alpha-hemolytic Escherichia coli linked to human diarrhea. Infect Immun. 1998;66:2040–2051. doi: 10.1128/iai.66.5.2040-2051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferlini C, Di Cesare S, Rainaldi G, Malorni W, Samoggia P, Biselli R, Fattorossi A. Flow cytometric analysis of the early phases of apoptosis by cellular and nuclear techniques. Cytometry. 1996;24:106–115. doi: 10.1002/(SICI)1097-0320(19960601)24:2<106::AID-CYTO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Prada C, Tall B D, Elliott S E, Hoover D L, Nataro J P, Venkatesan M M. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogh J, Fogh J M, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Foxman B, Zhang L, Palin K, Tallman P, Marrs C F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J Infect Dis. 1995;171:1514–1521. doi: 10.1093/infdis/171.6.1514. [DOI] [PubMed] [Google Scholar]
- 22.Foxman B, Zhang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs C F. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 23.Goluszko P, Selvarangan R, Popov V, Pham T, Wen J W, Singhal J. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect Immun. 1999;67:3989–3997. doi: 10.1128/iai.67.8.3989-3997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guignot J, Peiffer I, Bernet-Camard M F, Lublin D M, Carnoy C, Moseley S L, Servin A L. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli infecting the human polarized intestinal Caco-2/TC7 cells. Infect Immun. 2000;68:3554–3563. doi: 10.1128/iai.68.6.3554-3563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunzburg S T, Chang B J, Elliott S J, Burke V, Gracey M. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of Western Australia. J Infect Dis. 1993;167:755–758. doi: 10.1093/infdis/167.3.755. [DOI] [PubMed] [Google Scholar]
- 26.Guyer D M, Kao J S, Mobley H L. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johanson I M, Plos K, Marklund B I, Svanborg C. Pap, papG and prsG DNA sequences in Escherichia coli from the fecal flora and the urinary tract. Microb Pathog. 1993;15:121–129. doi: 10.1006/mpat.1993.1062. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J R, Stapleton A E, Russo T A, Scheutz F, Brown J J, Maslow J N. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect Immun. 1997;65:2153–2159. doi: 10.1128/iai.65.6.2153-2159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson J R, Stell A L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 31.Kamitani H, Geller M, Eling T. Expression of 15-lipoxygenase by human colorectal carcinoma Caco-2 cells during apoptosis and cell differentiation. J Biol Chem. 1998;273:21569–21577. doi: 10.1074/jbc.273.34.21569. [DOI] [PubMed] [Google Scholar]
- 32.Kao J S, Stucker D M, Warren J W, Mobley H L. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labigne-Roussel A, Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988;56:640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labigne-Roussel A F, Lark D, Schoolnik G, Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984;46:251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bouguenec C, Garcia M I, Ouin V, Desperrier J M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lublin D M, Atkinson J P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 37.Marques L R M, Abe C M, Griffin P M, Gomes T A T. Association between alpha-hemolysin production and HeLa cell-detaching activity in fecal isolates of Escherichia coli. J Clin Microbiol. 1995;33:2707–2709. doi: 10.1128/jcm.33.10.2707-2709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mobley H L, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss J E, Aliprantis A O, Zychlinsky A. The regulation of apoptosis by microbial pathogens. Int Rev Cytol. 1999;187:203–259. doi: 10.1016/s0074-7696(08)62419-5. [DOI] [PubMed] [Google Scholar]
- 40.Moulds J M, Nowicki S, Moulds J J, Nowicki B J. Human blood groups: incidental receptors for viruses and bacteria. Transfusion. 1996;36:362–374. doi: 10.1046/j.1537-2995.1996.36496226154.x. [DOI] [PubMed] [Google Scholar]
- 41.Muller A, Gunther D, Dux F, Naumann M, Meyer T F, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowicki B, Barrish J P, Korhonen T, Hull R A, Hull S I. Molecular cloning of the Escherichia coli O75X adhesin. Infect Immun. 1987;55:3168–3173. doi: 10.1128/iai.55.12.3168-3173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiffer I, Guignot J, Barbat A, Carnoy C, Moseley S L, Nowicki B J, Servin A L, Bernet-Camard M-F. Rearrangements of brush border-associated cytoskeletal proteins in human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli is accompanied by modification in distribution of functional proteins. Infect Immun. 2000;68:5979–5990. doi: 10.1128/iai.68.10.5979-5990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peiffer I, Servin A L, Bernet-Camard M F. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;68:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 48.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin D M, Nowicki B J. dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham T Q, Goluszko P, Popov V, Nowicki S, Nowicki B J. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect Immun. 1997;65:4309–4318. doi: 10.1128/iai.65.10.4309-4318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 51.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 53.Scaletsky I C, Silva M L, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swenson D L, Bukanov N O, Berg D E, Welch R A. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trifillis A L, Donnenberg M S, Cui X, Russell R G, Utsalo S J, Mobley H L, Warren J W. Binding to and killing of human renal epithelial cells by hemolytic P-fimbriated E. coli. Kidney Int. 1994;46:1083–1091. doi: 10.1038/ki.1994.370. [DOI] [PubMed] [Google Scholar]
- 56.Vaisanen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984;46:401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Virkola R, Westerlund B, Holthofer H, Parkkinen J, Kekomaki M, Korhonen T K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun. 1988;56:2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Foxman B, Tallman P, Cladera E, Le Bouguenec C, Marrs C F. Distribution of drb genes coding for Dr binding adhesins among uropathogenic and fecal Escherichia coli isolates and identification of new subtypes. Infect Immun. 1997;65:2011–2018. doi: 10.1128/iai.65.6.2011-2018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]