Abstract
Bordetella pertussis is readily killed after uptake by professional phagocytes, whereas its close relative Bordetella bronchiseptica is not and can persist intracellularly for days. Phagocytosis of members of either species by a mouse macrophage cell line results in transport of the bacteria to a phagosomal compartment positive for the lysosome-associated membrane protein 1, the protease cathepsin D, and the late endosomal vacuolar proton-pumping ATPase but negative for the early endosome antigen 1 and the early endosomal transferrin receptor. In addition, we demonstrate that Bordetella-containing phagosomes rapidly acidify to pH 4.5 to 5.0. Taken together, these data demonstrate that Bordetella-containing phagosomes rapidly mature to an acidic late endosomal/lysosomal compartment. Following up on this observation, we determined that B. pertussis does not survive in bacterial growth media adjusted to a pH of 4.5, whereas this pH has only minor effects on the growth of B. bronchiseptica. Raising the intracellular pH in infected macrophages by the addition of bafilomycin A1, ammonium chloride, or monensin increases the survival of acid-sensitive B. pertussis but, surprisingly, decreases that of acid-tolerant B. bronchiseptica. In summary, we hypothesize that the differential survival of B. pertussis and B. bronchiseptica in macrophages is, at least in part, due to the differences in their acid tolerance.
Bordetella pertussis is the causative agent of whooping cough (27). Its close relative Bordetella bronchiseptica causes infections of the respiratory tract in a variety of mammals and occasionally in humans (18, 63). Although they were previously considered to be extracellular pathogens, several recent reports have indicated significant cell invasive properties of these bacteria, e.g., for various typically nonphagocytic epithelial cell types (11, 32, 54, 55). However, the bacterial factors involved in the uptake of either species appear to be different, because invasion by B. pertussis depends on the presence of factors transcriptionally activated by the BvgAS two-component system (11, 32), the master regulator of virulence in these bacteria (5), whereas invasion by B. bronchiseptica was shown to occur independently of these factors (54, 55).
While the invasion of epithelial cells requires dedicated bacterial features, these are not necessary for the uptake by professional phagocytes, such as macrophages. When macrophages ingest bacteria, they wrap them with their plasma membrane and incorporate the newly formed so-called phagosomes. Phagosomes are not static organelles, but structures which undergo several maturation steps that transform the newly formed phagosomes into phagolysosomes. In detail, phagosome maturation is characterized by the sequential acquisition and loss of early endosomal, late endosomal, and lysosomal structural and compositional features (9, 10). The transition of an “early phagosome” into a phagolysosome is also accompanied by the exposure of the ingested bacteria to a number of potentially bactericidal mechanisms, such as the generation and release of reactive oxygen metabolites (superoxide and nitric oxide radicals) into the phagosome, acidification of the phagosome to a pH of below 5.0, and release of lysosomal hydrolases into the phagosomal space (9, 10, 24, 25, 46). A low pH may be toxic by itself but, in addition, it enhances the efficiency of other bactericidal mechanisms. For instance, spontaneous dismutation of O2− within the phagosome is maximal at a pH of 4.8 (15), and many lysosomal proteins such as acidic hydrolases have their optimal activity at a low pH (25).
Several intracellular pathogens have developed mechanisms to survive this hostile environment (reviewed in reference 24): for example, Legionella pneumophila and Mycobacterium tuberculosis inhibit the maturation of their phagosomes to phagolysosomes. Other pathogens, such as Listeria monocytogenes and Shigella flexneri, escape from the phagosome into the cytoplasm early after uptake. Salmonella spp. and other members of the Enterobacteriaceae developed the so-called acid tolerance response (ATR) system activating more than 50 acid shock proteins, which enables them to withstand the low pH encountered in maturing phagosomes (14). Salmonella enterica serovar Typhimurium even requires acidification of phagosomes for the transcriptional induction of a subset of virulence genes that enables it to multiply in macrophages (42, 52).
The interaction of B. pertussis and B. bronchiseptica with professional phagocytes was the subject of several recent reports (2, 12, 16, 23, 31, 57, 58, 60). Both Bordetella species secrete several well-characterized factors which can impair cellular defense mechanisms (27, 62). For example, the adenylate cyclase toxin was shown to inhibit phagocytosis of the bacteria and can even induce apoptosis of phagocytic cells (20, 31, 45). In spite of this toxin equipment, it is not clear whether the bordetellae, once engulfed by phagocytic cells, will be quantitatively eliminated. Our current knowledge about the intracellular fate of these bacteria is very limited, and only some conflicting results have been published. For example, although it was reported that B. pertussis may survive at least for several hours after uptake by certain phagocytes, including human macrophages and polymorphonuclear leukocytes (16, 57, 58), efficient killing of B. pertussis was observed in other phagocytes, such as murine J774.A1 macrophage-like cells and mouse bone marrow-derived macrophages (BMMs) (2). B. bronchiseptica, in marked contrast to B. pertussis, can survive in various cell types, including macrophages and dendritic cells, for several days (2, 12, 23). It was shown that the uptake of either Bordetella species by phagocytic cells induces a significant oxidative burst activity (19, 57). The intracellular compartments to which the bacteria localize are not well characterized, although evidence was reported indicating that B. pertussis may interfere with the maturation of its phagosomes, whereas B. bronchiseptica may not (2, 23, 58).
To be able to compare the intracellular fates of B. pertussis and B. bronchiseptica directly, we investigated the intracellular survival of the two Bordetella species after uptake by murine MH-S alveolar macrophage-like cells and analyzed the maturation of Bordetella-containing phagosomes. We show that, in contrast to B. pertussis, B. bronchiseptica is insensitive to an acidic pH as low as 4.5 and that the acidic environment in phagolysosomes contributes to increased survival of this pathogen.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are described in Table 1. Bordetella isolates were grown at 37°C on either Bordet-Gengou agar plates (Difco) containing defibrinated horse blood (20% [vol/vol]) or charcoal agar plates (Difco). Liquid cultures were cultivated in Stainer Scholte (SS) broth (56). Salmonella and Escherichia coli were grown on Luria Bertani (LB) agar or in LB liquid culture. Antibiotics were used at the following concentrations: streptomycin sulfate, 100 μg/ml; and gentamicin, 50 μg/ml.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain and plasmid | Relevant features | Source or reference |
|---|---|---|
| B. pertussis Tohama I | Wild type | 29 |
| B. pertussis TI Δtox | As Tohama I but Δptx | 48 |
| B. pertussis BP347 | As Tohama I but bvgS::Tn5 | 61 |
| B. pertussis W28 | Wild type | 33 |
| B. pertussis 18323 | Wild type and type strain | 47 |
| B. bronchiseptica BB7865 | Wild type but Strr | 55 |
| B. bronchiseptica BB7866 | As BB7865 but ΔbvgS | 55 |
| B. bronchiseptica Rem1 | Wild type | 22 |
| B. bronchiseptica MUCOB4 | Wild type | 38 |
| Serovar Typhimurium LT2 | 64 | |
| E. coli DH5α | GIBCO |
Determination of the pH-dependent growth limits and of the acid tolerance response.
Cells were initially grown overnight, pelleted (room temperature, 5,000 rpm; 15 min; Eppendorf microfuge), and diluted to an optical density at 595 nm (OD595) of 0.2 in SS broths of various pHs between 4.0 and 6.25. The growth rate was determined in log phase. To analyze the ATR, cells were initially grown to stationary phase in SS broth (Bordetella) or LB broth (Salmonella) and harvested. Unadapted cells were directly resuspended to 3 × 108 cells per ml in SS or LB broth of various pHs. Adapted cells were first resuspended in an adequate medium with a pH that was 0.75 pH units higher than the minimal growth pH of the organism for 4 h (pH 5.75 for B. pertussis, pH 5.25 for B. bronchiseptica, and pH 5.50 for serovar Typhimurium) and then shifted to otherwise lethal pH (pH 4.50, 4.25, and 4.00 for B. pertussis; pH 4.50, 3.50, 3.25, and 3.00 for B. bronchiseptica; and pH 3.50 for serovar Typhimurium). Numbers of surviving bacteria were determined at various times via plate counts. Each experiment was carried out independently at least three times.
Phagocytosis assay.
Phagocytosis assays were carried out as previously described (2). The mouse alveolar macrophage cell line MH-S (36) was cultivated at 37°C and 5% CO2 in RPMI 1640 medium with l-glutamine (Gibco) and the following additives per 500 ml: 50 ml of heat-inactivated fetal bovine serum (Gibco), 0.75 g of NaHCO3, 2.25 g of glucose, 1.19 g of HEPES, 1.7 μl of β-mercaptoethanol (14.3 M), and 55 mg of pyruvate. One milliliter of this culture medium containing 105 macrophages was added to each well of a 24-well plate. Macrophages were permitted to adhere and grow overnight. Infection was carried out with freshly harvested bacteria at a multiplicity of infection (MOI) of 100. After 1 h at 37°C, macrophages were washed thrice with phosphate-buffered saline (PBS), and fresh medium containing 100 μg of gentamicin/ml was added. After 2 h, cells were washed thrice with PBS, and the gentamicin concentration was reduced to 20 μg/ml for the remaining time of the assay. Macrophages were lysed with deionized water at various times after three washing steps with PBS to remove gentamicin. Numbers of surviving bacteria were determined via plate counts. Where indicated, 100 nM bafilomycin A1 (Calbiochem Inc.), 25 mM NH4Cl, or 5 μM monensin (ICN Biochemicals) was added. Bafilomycin A1 was added 15 min prior to infection, as described elsewhere (49, 52). None of these inhibitors interfered directly with the viability of the bordetellae (data not shown).
Fluorescence labeling of bacteria.
Bacteria were surface labeled with either the succinimidyl ester of carboxyfluorescein (Molecular Probes Inc.) or the succinimidyl ester of Oregon green 488 carboxylic acid (Molecular Probes Inc.). Cells were grown overnight and then harvested. In some experiments, bacteria were heat killed by incubation at 60°C for 10 min. Bacteria corresponding to 7 OD595 units were then resuspended in 190 μl of bicarbonate buffer (100 mM; pH 8.0). Ten microliters of labeling reagent in dimethyl sulfoxide was added to a final concentration of 1 mg/ml. Cell suspensions were incubated at room temperature for 20 min and washed twice with 10 mM bicarbonate–100 mM Tris-HCl (pH 8.0) to quench nonreacted label. The pellet was resuspended in PBS. Survival rates were determined by plate counts and were greater than 90% compared to those of the unlabeled control bacteria. Recently it was reported that fluorescence labels may influence the interaction of B. pertussis with eukaryotic cells (60). Therefore, we determined the rate of intracellular survival of fluorescence-labeled bordetellae in MH-S macrophages, but no significant difference from the nonlabeled bacteria could be observed (data not shown).
Determination of phagosomal pH.
Infection was carried out with 6 × 105 MH-S macrophages per well in 6-well plates at an MOI of 1,000 with fluorescence-labeled bacteria. Oregon green was used to determine pH values below 6.0, and fluorescein was used to determine those above pH 6.0. At various times, macrophages were washed thrice with PBS and resuspended in 3 ml of PBS. Macrophages were counted microscopically in a Fuchs-Rosenthal chamber. Fluorescence excitations at 495 and 450 nm (λEm = 520 nm) were determined with a Spex FluoroMax fluorescence photometer. Phagosome pH calibration graphs were obtained by equilibrating intracellular and extracellular pH with the ion transporters 28 μM nigericin and 5 μM monensin in the presence of 120 mM KCl. To correct for the fluorescence intrinsic to the macrophages used, fluorescence was calculated for 3 × 105 macrophages per ml by subtracting the intrinsic fluorescence of macrophages from each measured value, yielding the fluorescence produced by phagosomal bacteria only. Using the calibration graphs (see above), phagosomal pH could be determined from the relevant pH/fluorescence intensity data set. Because the intrinsic fluorescence of macrophages was found to be pH dependent, different calibrations were performed for each pH. All experiments were repeated at least three times, and values for B. bronchiseptica were calculated from the means of three calibration graphs.
Fluorescence microscopy.
MH-S macrophages (2 × 105) were grown on glass coverslips in 24-well plates overnight. Infection was carried out as described above with fluorescein-labeled bacteria. One hour after infection, macrophages were washed thrice with PBS, and fresh medium without gentamicin was added. Four hours after infection, macrophages were washed twice with PBS and then fixed with PLPS fixative (51) for 10 min. Cells were washed twice with PBS, once with PBS-50 mM NH4Cl, and then permeabilized with ice-cold methanol for 2 min. Again the cells were washed twice with PBS and then were covered with blocking solution (1:10 diluted horse serum in PBS–0.05% saponin [Sigma]) for at least 10 min. Glass coverslips were incubated with antibody solutions at the appropriate dilutions in blocking solution for 1 h at room temperature and then washed with blocking solution. Incubation with secondary antibodies labeled with lissamine-rhodamine was carried out likewise. After a last washing step with blocking solution, glass coverslips were mounted using Mowiol (Calbiochem Inc.) according to the protocol of Ojcius and coworkers (44). Analysis was done with a confocal laser scanning microscope (Leica Lasertechnik, Heidelberg, Germany), and frequencies of label colocalizations were determined. The antibodies to compartmental marker proteins were as follows: a rabbit polyclonal antibody to vacuolar ATPase holoenzyme from Dictyostelium (kindly provided by T. L. Steck, University of Chicago [41]), a rabbit polyclonal antibody to cathepsin D from murine liver (kindly provided by J. S. Mort, Shriner's Hospital, Montreal, Quebec, Canada [1]), a rabbit polyclonal antibody to a polypeptide fragment of early endosome antigen 1 (EEA1) (kindly provided by M. J. Clague and I. G. Mills, University of Liverpool [39]), monoclonal antibody 1D4B (developed by T. August [7]; obtained from the Developmental Study Hybridoma Bank; developed under the auspices of the NICHD and maintained by the University of Iowa), and monoclonal murine antibody H68.4 raised to the cytoplasmic tail of the human transferrin receptor (Zymed Laboratories Inc., San Francisco, Calif.).
Statistical analysis.
All experiments were evaluated for their statistical significance using the Sigma Plot program package.
RESULTS
B. bronchiseptica survives in MH-S macrophages, whereas B. pertussis is readily killed.
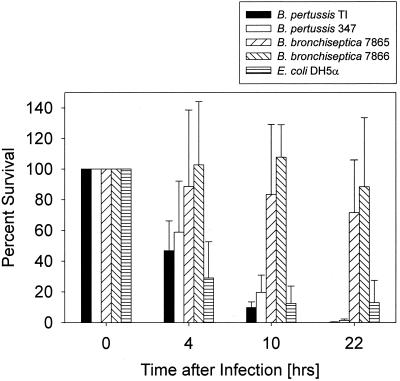
It has been reported that, in contrast to B. pertussis, B. bronchiseptica survives well in several types of professional phagocytes (2, 23). In the present study, we analyzed the intracellular survival of these two Bordetella species in a mouse alveolar macrophage cell line (MH-S). Our choice of MH-S cells as host macrophages was based on their close relatedness to in vivo target cells, as alveolar macrophages are encountered by the bacteria early during infection of the respiratory tract (27, 61) and because these cells apparently share several functional features with freshly harvested alveolar macrophages (28, 36). Figure 1 shows that after ingestion by the macrophages, B. bronchiseptica strains survived for more than 24 h without much reduction in viability whereas B. pertussis was eradicated within several hours. We reasoned that this could have been due to the expression of pertussis toxin, which is produced only by B. pertussis (27). However, deletion of the ptx operon in B. pertussis did not have any significant effect on intracellular survival in our system (data not shown). In agreement with previous studies using other macrophages (2), bvg mutants of B. pertussis and B. bronchiseptica survived better in the MH-S cells than the corresponding wild-type strains (Fig. 1).
FIG. 1.
B. bronchiseptica survives in MH-S macrophages 20 h after phagocytosis, whereas B. pertussis does not. The columns represent the percentage of surviving bacteria. The time scale refers to the time after elimination of the extracellular bacteria by gentamicin treatment and extensive washing of the cells, when the number of intracellular bacteria of each strain was set as 100%. Each experiment was carried out at least three times in duplicate. The vertical bars indicate the standard deviations.
B. pertussis and B. bronchiseptica are localized to a late endosomal/lysosomal compartment soon after phagocytosis.
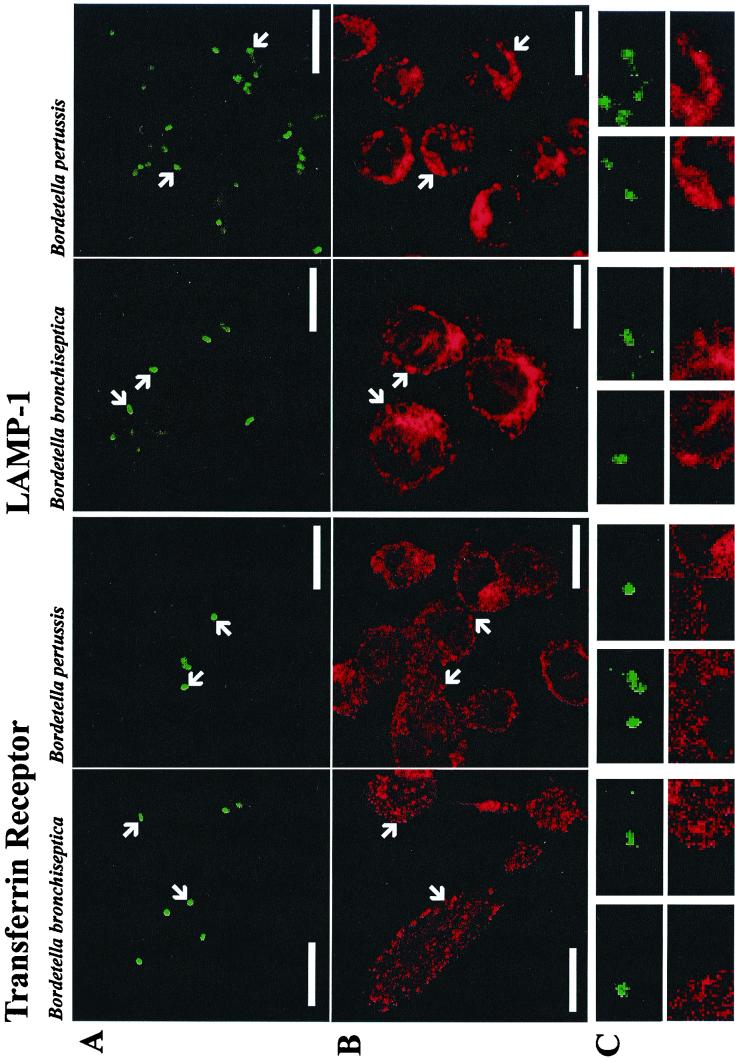
In the past few years, it has become clear that phagosome maturation is very similar to endosome maturation (9, 10, 46) and that at least some of the same molecular key players that regulate fusion of, e.g., early endosomes with each other, also regulate the fusion of early endosomes with “early phagosomes” (reviewed in reference 24). To describe the intracellular compartmentation of the bacteria, we determined the occurrence of various marker proteins of the host's endosome-lysosome continuum in compartments containing phagocytosed bacteria. The colocalization frequency of fluorescein-labeled bordetellae 4 h after infection was analyzed using fluorescence microscopy. Most bacteria clearly colocalized with late endosomal/lysosomal markers, independent of the Bordetella species investigated (Fig. 2 and Table 2). E. coli DH5α was used as a control organism that does not interfere with phagosome maturation and, accordingly, colocalized to a similar extent (data not shown). Markers characteristic of early endosomes, such as the transferrin receptor or the EEA1, however, did not colocalize significantly with the bacteria (Fig. 2 and Table 2). These data demonstrate that both Bordetella species are transported to a late endosomal/lysosomal compartment during the first 4 h after engulfment. Frequent colocalization of bacteria with v-ATPases was observed (Table 2), indicating that significant acidification of the phagosomes may have occurred (see below).
FIG. 2.
B. pertussis and B. bronchiseptica colocalize with proteins of late endosomal/lysosomal but not with early endosomal macrophage compartments. Exemplarily, double labeling of bacteria and the transferrin receptor or lysosome-associated membrane protein 1 (LAMP-1) are shown. (A) Fluorescein-labeled bacteria. (B) Position of the subcellular markers. (C) A magnification of the areas of panels A and B indicated by arrows. The length of the bars in panels A and B corresponds to 10 μm.
TABLE 2.
B. pertussis and B. bronchiseptica colocalize with late endosomal/lysosomal macrophage proteins 4 h postinfection
| Antibody directed againstb: | % Colocalizationa
|
|
|---|---|---|
| B. pertussis | B. bronchiseptica | |
| LAMP-1 | 75 ± 5 | 74 ± 4 |
| Cathepsin D | 79 ± 7 | 83 ± 11 |
| v-ATPase | 69 ± 14 | 75 ± 10 |
| EEA1 | 3 ± 1 | 3 ± 2 |
| Transferrin receptor | 5 ± 2 | 4 ± 1 |
Numbers indicate the percentage of colocalization of vacuoles (stained with the respective antibody) and bacteria ± the standard deviation. The average of three experiments is shown, and 50 macrophages were counted each time.
LAMP-1, lysosome-associated membrane protein 1.
Phagosomes containing Bordetella spp. are acidic.
The above data suggested that bordetellae would not interfere with maturation of their phagosomes, notably not with acidification. To directly investigate this, phagosome acidification was determined in situ using bacteria labeled with either fluorescein or Oregon green, whose emission intensities are dependent on pH. These two different dyes were needed to cover the whole pH spectrum of interest (pH 4 to 8), because the pH-dependent fluorescence of Oregon green is linear only in the acidic range whereas that of fluorescein is linear in the slightly acidic to neutral range (59). To measure acidification of the phagosomes, the bacteria were surface labeled with a reactive derivative of Oregon green and used to infect MH-S macrophages. The fluorescence at 450 nm is invariant (isosbestic point) and can therefore be used as an internal indicator for the number of bacteria present in the sample, whereas fluorescence at 495 nm is pH dependent. Therefore, the overall fluorescence of the infected macrophages was determined, and 495 nm/450 nm ratios (λEM = 520 nm) were calculated. The phagosomal pH can be directly determined by comparison with a calibration graph. In the experiments that involved the use of acidification inhibitors (see Fig. 6), the bacteria were labeled with fluorescein only, and phagosomal pH was determined as described above.
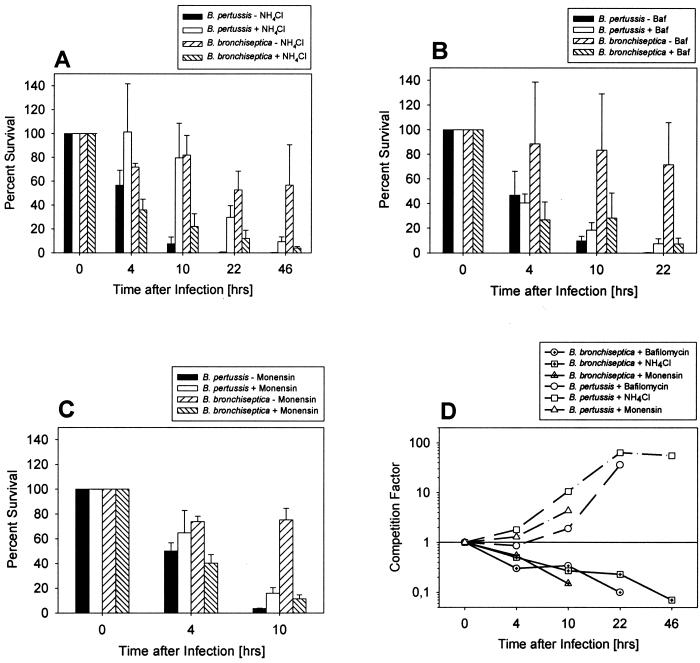
FIG. 6.
B. pertussis survives better in neutralized phagosomes, whereas elimination of B. bronchiseptica is enhanced. Bordetella-containing phagosomes were neutralized by ammonium chloride (A), bafilomycin A1 (B), or monensin (C). The time scale refers to the time after elimination of the extracellular bacteria by gentamicin treatment and extensive washing of the cells. At this time, the number of intracellular bacteria of each strain was set as 100%. Each experiment was carried out at least three times in duplicate, and the means of these data are presented. (D) For direct comparison of the effects of the various drugs, the fraction of surviving bacteria in the presence of the neutralizing drugs compared to surviving bacteria in the absence of these drugs (competition factor) is shown for each time.
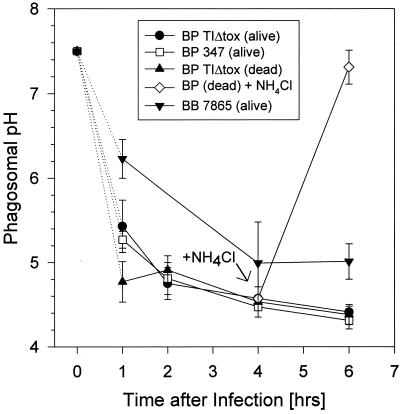
In the case of wild-type B. pertussis, the pH dropped to 5.1 within 1 h after infection and reached a pH of 4.5 after an additional 3 h (Fig. 3). Considering the fact that the fluorometric method integrates all phagosomes present in the sample and considering that the phagolysosomal pH is typically between 4.5 and 5.0 (44, 49), these data indicate that acidification is accomplished in most phagosomes within 1 h. The same data were obtained when a bvg mutant of B. pertussis or heat-killed bacteria were used (Fig. 3). As a control, we added 50 mM NH4Cl to one sample 4 h after infection (see the arrow in Fig. 3). Ammonium chloride permeates cell membranes and, as a weak base, should accumulate in acidic compartments and neutralize them. This was also true for Bordetella-containing phagosomes when we used this method of pH determination (Fig. 3) and hence validated our methodology. The determination of the pH of B. bronchiseptica-containing vacuoles was hampered by the low surface-labeling efficiency of these bacteria, with the fluorescent dyes leading to greater variations than in the data obtained with B. pertussis. Nevertheless, the data clearly show that 4 h after infection, the pH of these vacuoles is acidic, although with a mean value of 5.0 (± 0.5), the vacuoles appear to be slightly less acidic than in the case of B. pertussis.
FIG. 3.
Bordetella-containing phagosomes in MH-S cells are acidic. The pH was determined with Oregon green-labeled bacteria in the acidic range and with fluorescein-labeled bacteria in the neutral range, as described in Materials and Methods. As an example, the kinetics of acidification of phagosomes containing B. pertussis strains are shown. Each experiment was performed three times, and the means of these data are presented with the vertical bars representing the standard deviation.
B. pertussis and B. bronchiseptica differ substantially in their acid tolerance in vitro.
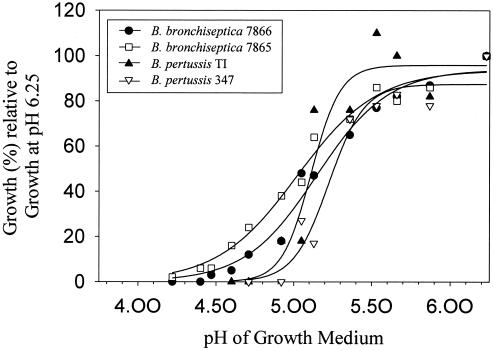
To investigate the impact of low pH on the survival of Bordetella species, we compared the growth rate of B. pertussis Tohama I and B. bronchiseptica BB7865 strains at different pHs. Interestingly, as shown in Fig. 4, these two strains differ significantly in their minimal growth pH. B. pertussis can multiply only at a pH above 5.0 whereas B. bronchiseptica is still able to multiply at a pH as low as 4.5. The corresponding bvg mutants of these strains behaved essentially like their wild-type strains. To exclude the possibility that these differences in acid tolerance are characteristic only of these two Bordetella isolates, we analyzed two more independent isolates of each (Table 1). All B. bronchiseptica strains exhibited a high level of acid tolerance whereas all B. pertussis strains were equally acid sensitive (data not shown). This suggested that the differential survival of B. bronchiseptica and B. pertussis in macrophages may have been due to their different sensitivities to acidic pH.
FIG. 4.
B. bronchiseptica still grows at an acidic pH which inhibits the growth of B. pertussis. The pH-dependent growth rate (in percent) of B. bronchiseptica and B. pertussis wild-type strains and the bvg mutants are shown relative to the growth rate at pH 6.25. The graphs were fitted sigmoidally (three parameters) with the Sigma Plot program.
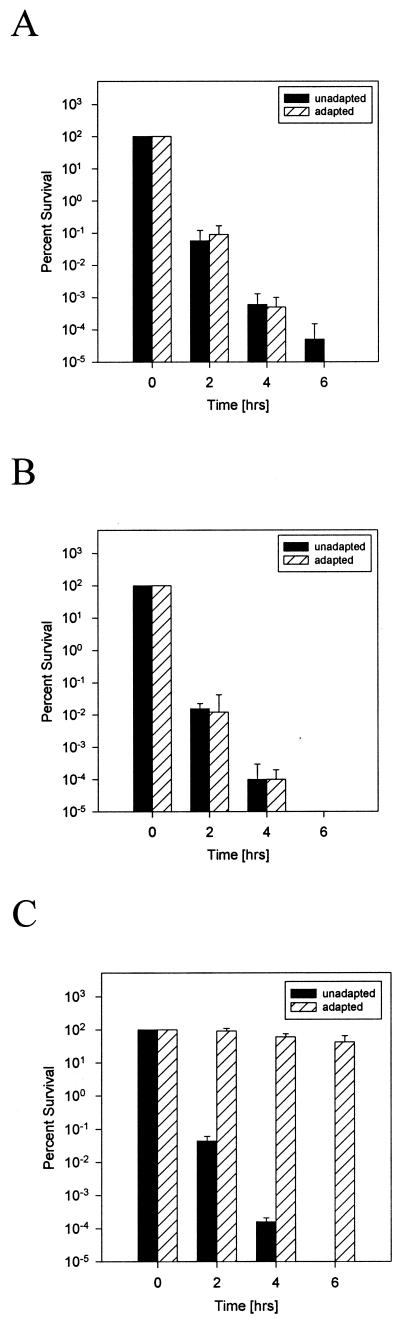
Some bacteria, including Salmonella spp., are protected from very low pH through the products induced by their ATR system, which allows their survival at a pH close to the minimal growth pH if the ATR is first induced at a nonlethal low pH (14). To investigate whether Bordetella species may also be endowed with an ATR, we determined the survival rate of the bacteria either previously exposed for a short period of time or not exposed to acidic pH and compared these data with acid-induced Salmonella serovar Typhimurium as a positive control. None of the Bordetella strains tested showed any significant difference in their survival whether or not they were preincubated at low pH, whereas, as described previously (14), pH adaptation led to a dramatic increase in the survival of serovar Typhimurium (Fig. 5). We conclude that the analyzed Bordetella strains do not possess a stationary phase ATR system functionally similar to that of Salmonella spp. In addition, these data provide further evidence for the significant difference in acid resistance of B. pertussis and B. bronchiseptica, because the same killing rate of the two organisms is achieved with a difference of an entire pH unit (Fig. 5).
FIG. 5.
Absence of an ATR system in Bordetella strains. Time-dependent survival of the microorganisms in acidic media is shown. (A) B. bronchiseptica at pH 4.25. (B) B. pertussis at pH 3.25. (C) Serovar Typhimurium at pH 3.50. Unadapted bacteria were directly resuspended in the acidic media, whereas the adapted microorganisms were first resuspended and incubated in an adequate medium with a pH that was 0.75 pH units greater than the minimal growth pH of the respective organism for 4 h. Surviving bacteria were quantified at the time points indicated. Each experiment was repeated at least three times, and the means of these data are presented.
In order to further affirm the observed differences in acid sensitivity, B. pertussis and B. bronchiseptica were challenged at various pHs, and percent survival was determined after 4 h. Table 3 shows that B. bronchiseptica tolerates acidic pH much better than B. pertussis, which is eradicated at pH 4.00. In contrast, we found surviving B. bronchiseptica even at pH 3.00.
TABLE 3.
Percent survival after 4 h of challenge at the given pH
| Bacterial strain | % Survival after 4 h ata:
|
|||||
|---|---|---|---|---|---|---|
| pH 4.50 | pH 4.25 | pH 4.00 | pH 3.50 | pH 3.25 | pH 3.00 | |
| B. pertussis TIΔtox | 1.07 ± 0.94 | 0.0001 ± 0.0001 | 0 ± 0 | ND | ND | ND |
| B. bronchiseptica BB 7865 | 55.9 ± 8.1 | ND | ND | 17.4 ± 10.6 | 0.0006 ± 0.0006 | 0.0001 ± 0.0001 |
Presented values are the means of three independent experiments with the standard deviations. ND, not determined.
Differential effect of drugs inhibiting the acidification of phagosomes on intracellular survival of B. pertussis and B. bronchiseptica.
As shown above, the pH of Bordetella-containing phagosomes decreases to a level critical for the survival of B. pertussis but not of B. bronchiseptica. Differences in the acid tolerance between the two species may, therefore, contribute to their different intracellular survival. To test this hypothesis, we infected MH-S cells and simultaneously blocked phagosome maturation and/or acidification by the addition of bafilomycin A1, ammonium chloride, or monensin. Bafilomycin is a membrane-permeant macrolide antibiotic specifically inhibiting vacuolar-type proton translocating ATPases (v-ATPases) which are involved in phagosome acidification (6). We confirmed the inhibition of the proton pumps by demonstrating that the phagosomal pH of macrophages treated with the antibiotic did not drop below a value of 7.0, at least during the first 6 h after infection (done as in Fig. 3; data not shown). Ammonium chloride not only neutralizes acidic compartments (Fig. 3) but can also interfere with the maturation of phagosomes and inhibit phagosome lysosome fusion (26). Monensin acts as an ion carrier equilibrating extra- and intracellular pH (50) and, due to this property, is frequently used to establish calibrations for pH determination (see Materials and Methods).
As would be expected, all three compounds had a protective effect on the intracellular survival of B. pertussis, and a significant increase (P < 0.05) in the viable counts could be observed 10 h after infection in their presence. In marked contrast, the number of viable B. bronchiseptica wild-type bacteria had already decreased significantly (P < 0.05) in macrophages treated with either bafilomycin, monensin, or ammonium chloride 4 h after infection compared to untreated cells (Fig. 6). Due to the cytotoxic effects of constantly present monensin, experiments with this drug could be performed only for 10 h postinfection (Fig. 6). The survival rates of a bvg mutant of B. bronchiseptica did not reveal any differences with or without bafilomycin, whereas the bvg mutant of B. pertussis showed an increased survival rate in the presence of the compound compared to the wild type (data not shown).
DISCUSSION
Several recent reports demonstrated that B. bronchiseptica can survive in professional phagocytes (2, 12, 23). However, the specific properties of this pathogen leading to resistance against the otherwise bactericidal host cell defense mechanisms are not characterized. In this study, we investigated the intracellular fate of B. pertussis and B. bronchiseptica in a murine macrophage line and began to characterize differences between the two species relevant for intracellular survival. We demonstrate that B. bronchiseptica is much better adapted to acidic environments than B. pertussis. In fact, B. bronchiseptica strains are still able to slowly multiply at pH 4.5 and survive even lower pH. This surprisingly places B. bronchiseptica in one line with those of the very distantly related Enterobacteriaceae known to be especially acid resistant, such as serovar Typhimurium (pH 4.0), E. coli (pH 4.4), and S. flexneri (pH 4.8) (34). B. pertussis tolerates only relatively mild acidic pH values (pH > 5), similar to many soil bacteria (34). However, we demonstrate that in contrast to the Enterobacteriaceae, neither Bordetella species is endowed with an ATR system similar to that of serovar Typhimurium, which would allow them to adapt efficiently to very low pH environments (14). In addition, the virulence-regulating BvgAS two-component system did not affect acid tolerance in our experiments. The adaptation to acidic pH is particularly important for those facultative intracellular members of the Enterobacteriaceae, which not only encounter the extremely acidic environment of the stomach but also survive and multiply subsequently in acidic compartments in professional phagocytes. Since we have shown here that B. bronchiseptica survives well in macrophages, its acid tolerance may also be relevant for pathogenicity.
Uptake of either B. bronchiseptica or B. pertussis by MH-S macrophages leads to efficient and fast fusion of the bacterium-containing phagosomes with lysosomes to form phagolysosomes, as demonstrated by a highly significant colocalization of their phagosomes with the late endosomal/lysosomal markers lysosome-associated membrane protein 1, cathepsin D, and functional v-ATPase, whereas no colocalization of the bacteria could be observed with early endosomal markers such as the transferrin receptor and the EEA1. No differences were observed between heat-killed and viable bordetellae and a nonpathogenic E. coli control strain. These data agree with previous experiments which indicated fusion of at least some B. bronchiseptica-containing phagosomes with lysosomes in dendritic cells and in BMMs (2, 23). We did not obtain any indication of an inhibition of phagosome-lysosome fusion by B. pertussis in the MH-S macrophages, although evidence for the inhibition of phagosome-lysosome fusion in B. pertussis-infected human polymorphonuclear leukocytes was reported by others previously (58). At present, it is not clear whether this discrepancy is caused by the use of different phagocytic cells. We find that in the phagolysosomal compartment of MH-S cells, B. pertussis is readily killed but B. bronchiseptica strains are able to withstand the macrophage attacks, and although they do not multiply, they remain viable for several days.
The presence of v-ATPases in the bacterium-containing compartments suggested that these phagosomes were acidified. To determine the phagosomal pH, we labeled the bacteria with the pH-dependent fluorescent dyes Oregon green and fluorescein. Soon after phagocytosis, the pH of phagosomes containing B. pertussis or B. bronchiseptica dropped to about 4.5 and 5.0, respectively (Fig. 3). Such low pH values are usually considered to be a typical feature for mature lysosomal compartments (24, 44, 49). The low pH reached in the Bordetella-containing phagosomes is clearly critical for the killing of B. pertussis in vitro but, as described above, not of B. bronchiseptica (Fig. 4 and Table 3). Acidification of phagosomes and differences in acid tolerance between B. pertussis and B. bronchiseptica may therefore explain, in part, the different survival rates of these species in macrophages. This assumption is further supported by experiments in which we inhibited the acidification of endosomal compartments by the addition of bafilomycin A1, ammonium chloride, or monensin. Bafilomycin A1 selectively acts on v-ATPases and is able to block acidification in phagosomes. Similarly, the other two inhibitors are known to raise the phagosomal pH (26, 50). In infection experiments with macrophages treated with these compounds, we observed an increase in survival of the acid-sensitive B. pertussis strains 4 h after infection for all compounds but bafilomycin. A statistically significant increase in survival was found at later time points. This demonstrates that acidification is in fact involved in efficient killing of intracellular B. pertussis and may be of increasing relevance with time. However, the protective effect of ammonium chloride and monensin on B. pertussis survival was stronger than that of bafilomycin, although bafilomycin inhibited acidification completely. Acidification is one of the factors that contributes to the clearance of bacteria, though other factors contribute as well or may even predominate since B. pertussis is killed even when acidification is blocked. In this case, however, long-term survival is significantly higher. Surprisingly, treatment of the macrophages with either of the three inhibitors caused a significant decrease in the viability of wild-type B. bronchiseptica soon after infection (Fig. 6). This unexpected result is reminiscent of recent reports about the intracellular growth rates of Brucella suis and Francisella tularensis, which were also decreased by blocking the endosome acidification (13, 49). In the case of F. tularensis, the acidity is required to release iron from transferrin essential for the growth of the bacteria (13). It is possible that, similar to the situation for serovar Typhimurium (14), acidic pH is a signal for B. bronchiseptica which leads to a better adaptation to an intracellular environment. However, this effect was not seen with B. bronchiseptica bvg mutants, which survive better intracellularly than the isogenic wild type (2). As the BvgAS system does not affect acid tolerance in vitro and as no ATR system could be identified, the significance of this phenomenon remains unknown, although it may indicate that other signal transduction systems are engaged in the adaptation to hostile intracellular compartments.
Interestingly, apart from the BvgAS system, a role was recently attributed to the RisAS two-component system for efficient intracellular survival of B. bronchiseptica controlling the expression of an acid phosphatase. In addition, a urease produced by B. bronchiseptica but not by B. pertussis was reported to contribute to intracellular survival (8, 30, 37). These results are in agreement with our conclusions because only B. bronchiseptica is both ureolytic and produces the acid phosphatase and because both factors are negatively affected in their expression by the BvgAS system. In fact, bvg mutants of B. bronchiseptica expressing these factors constitutively have an advantage over the wild-type strains in long-term intracellular survival (Fig. 1) (2).
B. pertussis and B. bronchiseptica show a remarkable difference in their lipopolysaccharide structures, as only B. bronchiseptica is able to produce O-antigen-specific side chains (27, 38), which make it remarkably resistant to antimicrobial peptides such as the defensins (17). Hence, this difference in their lipopolysaccharide structure may contribute to the different survival properties of the two species (3). However, in the MH-S cells and in BMMs, no difference in intracellular survival between wild-type B. bronchiseptica and mutant derivatives lacking the O-antigen-specific side chains could be observed during the first 24 h after their uptake (data not shown).
A further understanding of the intracellular survival strategies will help to elucidate the relevance of intracellular stages for the pathogenesis by B. bronchiseptica. In this respect it is interesting to note that a significant Th1-type T-cell response is induced after infection of mice with bordetellae, which may indicate the occurrence of relevant intracellular phases of these bacteria during infection (4, 21, 35, 40, 53). Moreover, in agreement with the pronounced intracellular survival of B. bronchiseptica, infections with this organism tend to take a chronic course, e.g., in subclinical purulent pneumonia in pigs, in chronic lung lesions in rabbits, or in chronic bronchopneumonia in humans (22, 63). The identification of factors required for the intracellular survival of these bacteria will permit an evaluation of the significance of intracellular stages during infection and may provide insights into the persistence strategies of Bordetella species in the host organisms.
ACKNOWLEDGMENTS
We thank Claudia Lesch for technical assistance. We are grateful to M. J. Clague, I. G. Mills, J. S. Mort, U. E. Schaible, and T. L. Steck for their generous gifts of antibodies; N. Guiso, N. Preston, and R. Rappuoli for providing strains; and A. Schüttfort for advice regarding the confocal microscope.
This work was supported by grants SFB479-A2 and Ha1929/3-3 from the Deutsche Forschungsgemeinschaft to R.G. and A.H., respectively; by a grant from the Fonds der Chemischen Industrie to R.G.; and through a Heisenberg fellowship of the DFG to A.H.
REFERENCES
- 1.Authier F, Mort J S, Bell A W, Posner B I, Bergeron J J. Proteolysis of glucagon within hepatic endosomes by membrane-associated cathepsins B and D. J Biol Chem. 1995;270:15798–15807. doi: 10.1074/jbc.270.26.15798. [DOI] [PubMed] [Google Scholar]
- 2.Banemann A, Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect Immun. 1997;65:3469–3473. doi: 10.1128/iai.65.8.3469-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banemann A, Deppisch H, Gross R. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect Immun. 1998;66:5607–5612. doi: 10.1128/iai.66.12.5607-5612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard A, Mahon B P, Watkins J, Readhead K, Mills K H. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beier D, Schwarz B, Fuchs T M, Gross R. In vivo characterization of the unorthodox BvgS two-component sensor protein of Bordetella pertussis. J Mol Biol. 1995;248:596–610. doi: 10.1006/jmbi.1995.0245. [DOI] [PubMed] [Google Scholar]
- 6.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J W, Murphy T L, Willingham M C, Pastan I, August J T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chhatwal G S, Walker M J, Yan H, Timmis K N, Guzman C A. Temperature dependent expression of an acid phosphatase by Bordetella bronchiseptica: role in intracellular survival. Microb Pathog. 1996;22:257–264. doi: 10.1006/mpat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins M, Celis J E, van Meer G, Dieplinger H, Jahraus A, Griffiths G, Huber L A. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194–32200. [PubMed] [Google Scholar]
- 10.Desjardins M, Huber L A, Parton R G, Griffiths D. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewanowich C A, Melton A R, Weiss A A, Sherburne R K, Peppler M S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989;57:2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forde C B, Parton R, Coote J G. Bioluminescence as a reporter of intracellular survival of Bordetella bronchiseptica in murine phagocytes. Infect Immun. 1998;66:3198–3207. doi: 10.1128/iai.66.7.3198-3207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortier A H, Leiby D A, Narayanan R B, Asafoadjei E, Crawford R M, Nacy C A, Meltzer M S. Growth of Francisella turlarensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect Immun. 1995;63:1478–1483. doi: 10.1128/iai.63.4.1478-1483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster J W. When protons attack: microbial strategies of acid adaptation. Curr Opin Microbiol. 1999;2:170–174. doi: 10.1016/S1369-5274(99)80030-7. [DOI] [PubMed] [Google Scholar]
- 15.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 16.Friedman R L, Nordensson K, Wilson L, Akporiaye E T, Yocum D E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T, Lehrer R I. Antimicrobial peptides of vertebrates. Curr Opin Immunol. 1998;10:41–44. doi: 10.1016/s0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 18.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graeff-Wohlleben H, Killat S, Banemann A, Guiso N, Gross R. Cloning and characterization of an Mn-containing superoxide dismutase (SodA) of Bordetella pertussis. J Bacteriol. 1997;179:2194–2201. doi: 10.1128/jb.179.7.2194-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gueirard P, Guiso N. Virulence of Bordetella bronchiseptica: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4072–4078. doi: 10.1128/iai.61.10.4072-4078.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueirard P, Minoprio P, Guiso N. Intranasal inoculation of Bordetella bronchiseptica in mice induces long-lasting antibody and T-cell mediated immune responses. Scand J Immunol. 1996;43:181–192. doi: 10.1046/j.1365-3083.1996.d01-30.x. [DOI] [PubMed] [Google Scholar]
- 22.Gueirard P, Weber C, LeCoustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in the host. J Clin Microbiol. 1994;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzman C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas A. Reprogramming the phagocytic pathway—intracellular pathogens and their vacuoles. Mol Membr Biol. 1998;15:103–121. doi: 10.3109/09687689809074522. [DOI] [PubMed] [Google Scholar]
- 25.Haas A, Goebel W. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radic Res Commun. 1992;16:137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- 26.Hart P D, Young M R. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic Mycobacterium and a nonpathogenic yeast. J Exp Med. 1991;174:881–889. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewlett E L. Bordetella species. In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2078–2084. [Google Scholar]
- 28.Horton M R, Shapiro S, Bao C, Löwenstein C J, Noble P W. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162:4171–4176. [PubMed] [Google Scholar]
- 29.Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. Effect of heptakis(2,6-O-dimethyl)β-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect Immun. 1983;41:1138–1143. doi: 10.1128/iai.41.3.1138-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungnitz H, West N P, Walker M J, Chhatwal G S, Guzman C A. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect Immun. 1998;66:4640–4650. doi: 10.1128/iai.66.10.4640-4650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C K, Roberts A L, Finn T M, Knapp S, Mekalanos J J. A new assay for invasion of HeLa229 cells by Bordetella pertussis: effect of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990;58:2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leininger E, Roberts M, Kenimer M G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Lee I S, Frey J, Slonczewski J L, Foster J W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol. 1995;177:4097–4104. doi: 10.1128/jb.177.14.4097-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahon B P, Ryan M S, Griffin F, Mills K H. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mbawuike I N, Hercowitz H B. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol. 1989;46:119–127. doi: 10.1002/jlb.46.2.119. [DOI] [PubMed] [Google Scholar]
- 37.McMillan D J, Shojaei M, Chhatwal G S, Guzman C A, Walker M J. Molecular analysis of the bvg-repressed urease of Bordetella bronchiseptica. Microb Pathog. 1996;21:379–394. doi: 10.1006/mpat.1996.0069. [DOI] [PubMed] [Google Scholar]
- 38.Middendorf B, Gross R. Representational difference analysis identifies a strain-specific LPS biosynthesis locus in Bordetella spp. Mol Gen Genet. 1999;262:189–198. doi: 10.1007/s004380051074. [DOI] [PubMed] [Google Scholar]
- 39.Mills I G, Johnes A T, Clague M J. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- 40.Mills K H, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolta V K, Padh H, Steck T L. An immunocytochemical analysis of the vacuolar pump in Dictyostelium discoideum. J Cell Sci. 1993;105:849–859. doi: 10.1242/jcs.105.3.849. [DOI] [PubMed] [Google Scholar]
- 42.Oh Y K, Alpuche-Aranda C, Berthiaume E, Jinks T, Miller S I, Swanson J A. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect Immun. 1996;64:3877–3883. doi: 10.1128/iai.64.9.3877-3883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojcius D M, Niedergang F, Subtil A, Hellio R, Dautry-Varsat A. Immunology and the confocal microscope. Res Immunol. 1996;147:175–188. doi: 10.1016/0923-2494(96)83169-5. [DOI] [PubMed] [Google Scholar]
- 45.Pearson R D, Symes P, Conboy M, Weiss A A, Hewlett E L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987;139:2749–2754. [PubMed] [Google Scholar]
- 46.Pitt A, Mayorga L S, Stahl P D, Schwartz A L. Alterations in the protein composition of maturing phagosomes. J Clin Investig. 1992;90:1978–1983. doi: 10.1172/JCI116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pittman M. Genus Bordetella. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 388–393. [Google Scholar]
- 48.Pizza M, Bartoloni A, Prugnola A, Silvestri S, Rappuoli Subunit S1 of pertussis toxin: mapping of the regions essential for ADP-ribosyltransferase activity. Proc Natl Acad Sci USA. 1988;85:7521–7525. doi: 10.1073/pnas.85.20.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porte F, Liautard J P, Köhler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prabhananda B S, Kombrail M H. Monensin-mediated transports of H+, Na+, K+ and Li+ ions across vesicular membranes: T-jump studies. Biochim Biophys Acta. 1992;1106:171–177. doi: 10.1016/0005-2736(92)90236-f. [DOI] [PubMed] [Google Scholar]
- 51.Racoosin E L, Swanson J A. Labeling of endocytic vesicles using fluorescent probes for fluid-phase endocytosis. In: Celis J E, editor. Cell biology: a laboratory handbook. New York, N.Y: Academic Press; 1994. [Google Scholar]
- 52.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redhead K, Watkins J, Barnard A, Mills K H. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savelkoul P H M, Kremer B, Kusters J G, van der Zeijst B A M, Gaastra W. Invasion of HeLa cells by Bordetella bronchiseptica. Microb Pathog. 1993;14:161–168. doi: 10.1006/mpat.1993.1016. [DOI] [PubMed] [Google Scholar]
- 55.Schipper H, Krohne G, Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica. Infect Immun. 1994;62:3008–3011. doi: 10.1128/iai.62.7.3008-3011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stainer D W, Scholte M J. A simple chemically defined medium for production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 57.Steed L L, Akporiaye E T, Friedman R L. Bordetella pertussis induces respiratory burst activity in human polymorphonuclear leukocytes. Infect Immun. 1992;60:2101–2105. doi: 10.1128/iai.60.5.2101-2105.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steed L L, Setareh M, Friedman R L. Host-parasite interactions between Bordetella pertussis and human polymorphonuclear leukocytes. J Leukoc Biol. 1991;50:321–330. doi: 10.1002/jlb.50.4.321. [DOI] [PubMed] [Google Scholar]
- 59.Vergne I, Constant P, Laneelle G. Phagosomal pH determination by dual fluorescence flow cytometry. Anal Biochem. 1998;255:127–132. doi: 10.1006/abio.1997.2466. [DOI] [PubMed] [Google Scholar]
- 60.Weingart C L, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss A A, Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984;43:263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 63.Woolfrey F B, Moody J A. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4:234–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinder N D, Lederberg J. Genetic exchange in Salmonella. J Bacteriol. 1952;64:679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]