Abstract
A major ceramide monohexoside (CMH) was purified from lipidic extracts of Cryptococcus neoformans. This molecule was analyzed by high-performance thin-layer chromatography (HPTLC), gas chromatography coupled with mass spectrometry, and fast atom bombardment-mass spectrometry. The cryptococcal CMH is a β-glucosylceramide, with the carbohydrate residue attached to 9-methyl-4,8-sphingadienine in amidic linkage to 2-hydroxyoctadecanoic acid. Sera from patients with cryptococcosis and a few other mycoses reacted with the cryptococcal CMH. Specific antibodies were purified from patients' sera by immunoadsorption on the purified glycolipid followed by protein G affinity chromatography. The purified antibodies to CMH (mainly immunoglobulin G1) bound to different strains and serological types of C. neoformans, as shown by flow cytofluorimetry and immunofluorescence labeling. Transmission electron microscopy of yeasts labeled with immunogold-antibodies to CMH and immunostaining of isolated cell wall lipid extracts separated by HPTLC showed that the cryptococcal CMH predominantly localizes to the fungal cell wall. Confocal microscopy revealed that the β-glucosylceramide accumulates mostly at the budding sites of dividing cells with a more disperse distribution at the cell surface of nondividing cells. The increased density of sphingolipid molecules seems to correlate with thickening of the cell wall, hence with its biosynthesis. The addition of human antibodies to CMH to cryptococcal cultures of both acapsular and encapsulated strains of C. neoformans inhibited cell budding and cell growth. This process was complement-independent and reversible upon removal of the antibodies. The present data suggest that the cryptococcal β-glucosylceramide is a fungal antigen that plays a role on the cell wall synthesis and yeast budding and that antibodies raised against this component are inhibitory in vitro.
Cryptococcus neoformans is a pathogenic fungus commonly associated with immunocompromised hosts. The cryptococcal infection follows the inhalation of fungal basidiospores (63) or of poorly encapsulated yeasts (30). Infection is limited to the lung or can disseminate to other tissues. In patients with advanced human immunodeficiency virus (HIV) infection, it gives rise to a most serious, often fatal, meningoencephalitis.
The ability of C. neoformans to escape host defenses and cause disease is closely associated with the production and secretion of capsular polysaccharides, namely, the glucuronoxylomannan (GXM), galactoxylomannan, and mannoprotein antigens. GXM, the major capsular polysaccharide of C. neoformans, is antiphagocytic, inhibits both the recruitment of inflammatory cells and the increased expression of costimulatory molecules, suppresses delayed-type hypersensitivity, and may reduce antibody production in response to fungal infection (reviewed in reference 44). In addition, C. neoformans produces other factors clearly related to pathogenicity, such as mannitol (65) and melanin (64). Additional cryptococcal molecules and enzymatic activities, such as sialic acids (45), phospholipase (5), superoxide dismutase (22), and proteinase (4), have been described with suggested, but still unclear, roles in the infectious process.
Glycosphingolipids (GSLs) are conspicuous membrane constituents of mammalian cells, protozoa, and fungi. Their hydrophobic ceramide moiety is linked to one or more sugars, with the ceramide monohexosides (CMH) commonly having glucose or galactose in both anomeric configurations. GSLs of various sizes are involved in many processes such as cell-cell interaction (19), mediation of apoptotic signaling (29), immunosuppression in cancer patients (28), and adhesion of fungal pathogens to mammalian cells (18, 23). GSLs are also implicated in cell growth, since the inhibitor of glucosylceramide synthase, d-threo-1-phenyl-2-decanoylamine-3-morpholino-propanol, abolished neurite outgrowth in the PC12 cell line (36). The ceramide moiety of GSLs has been reported to modulate growth of human (3) and fungal (14) cells.
Antibodies are highly relevant in the human protection against cryptococcosis (11). In animal hosts, protective, nonprotective, and disease-enhancing monoclonal antibodies (MAbs) against GXM have been described. Protection by anti-GXM antibodies against the experimental infection by different routes with several strains of C. neoformans was obtained when these antibodies were administered alone or in conjunction with antifungal agents (42).
In the present work, we identified a major cryptococcal GSL with a structure similar to CMH described in other fungal pathogens (9, 26, 55, 56, 60). This molecule contains glucopyranose β-linked to the ceramide moiety consisting of 9-methyl-4,8-sphingadienine in amidic linkage to 2-hydroxyoctadecanoic acid and is accumulated mainly on the fungal cell wall. Sera from patients with cryptococcosis recognize the cryptococcal CMH. The reactivity of antibodies to CMH against both acapsular and encapsulated strains of C. neoformans was investigated. Results show that these antibodies arrest fungal growth in vitro presumably by interfering with the cell wall synthesis and yeast budding.
MATERIALS AND METHODS
Chemicals.
Culture media were obtained from Difco Laboratories (Detroit, Mich.). Organic solvents and the chromatographic apparatus were purchased from Merck (Rio de Janeiro, Brazil). Polyvinylidene difluoride (PVDF) membranes, enzyme-linked immunosorbent assay (ELISA) plates, secondary antibodies, and other reagents used for immunofluorescence and flow cytometry were obtained from Sigma Chemical Co. (St. Louis, Mo.). Protein G-Sepharose 4 Fast Flow was purchased from Amersham Pharmacia Biotech. Sera from patients with different mycoses were kindly provided by Marcio Nucci, Hospital Universitario Clementino Fraga Filho, Rio de Janeiro, and Rosely Zancope, Laboratorio de Micologia Médica, Hospital Evandro Chagas, FIOCRUZ, Rio de Janeiro, Brazil.
Fungal strains.
C. neoformans HEC3393 (serotype A, clinical isolate), CN23/10.993 (serotype B, environmental isolate), and HEC40143 (serotype C, environmental isolate) were obtained from Laboratório de Micologia Médica, Hospital Evandro Chagas, FIOCRUZ, Rio de Janeiro, Brazil. The strains ATCC 28597 (serotype D) and cap 67 (acapsular) were obtained from the American Type Culture Collection. Stock cultures were maintained in Sabouraud dextrose agar under mineral oil and kept at 4°C. For lipid extraction, immunofluorescence, electron microscopy, and flow cytometry, C. neoformans cells were cultivated in brain heart infusion (BHI) at room temperature for 5 days and then separated by centrifugation and washed twice in 0.01 M phosphate-buffered saline (PBS; pH 7.2).
Glycolipid extraction and purification.
GSLs from yeast cells of C. neoformans HEC3393 were extracted at room temperature successively with chloroform-methanol 2:1 and 1:2 (vol/vol) (60). The crude lipid extract was partitioned according to the method of Folch et al. (16). The lipids recovered from Folch's lower phase were fractionated on a silica gel column eluted with chloroform, acetone, and methanol. The glycolipid fraction eluted with acetone was purified by another round of silica gel column chromatography. This column was eluted sequentially with the following mixtures: chloroform-methanol (95:5, 9:1, 8:2, and 1:1 [vol/vol]) and finally methanol alone. The purified GSL fraction obtained in the chloroform-methanol 9:1 (vol/vol) fraction was analyzed by high-performance thin-layer chromatography (HPTLC), developed with chloroform-methanol-water 65:25:4 (vol/vol). The spots were visualized with iodine and by spraying with orcinol-H2SO4.
Sugar analysis.
The purified glycosphingolipid fraction was hydrolyzed in 0.5 M sulfuric acid at 100°C for 18 h, and the resulting monosaccharides were identified as their alditol acetates on a Hewlett-Packard 5890 gas chromatograph equipped with a fused silica capillary (25 by 0.22 mm) OV-225 (50% cyanopropylmethyl, 50% phenylmethyl-polysiloxane) column. A temperature program of 180°C held isocratically for the first 15 min and then elevated to 210°C at 2°C/min was used. The analysis was monitored with a flame-ionization detector.
Fatty acid analysis.
The fatty acid portion of the cryptococcal GSL was characterized by gas chromatography coupled with mass spectrometry (GC-MS). Fatty acid methyl esters were obtained by incubating the purified GSL overnight, at 70°C, in the presence of toluene-methanol (1:1, vol/vol) containing 2.5% concentrated sulfuric acid. The sample was diluted in water and extracted twice with hexane-chloroform (4:1, vol/vol). The combined extracts were dried by vacuum centrifugation, and the trimethylsilyl derivatives were prepared with bis(trimethylsilyl)trifluoroacetamide-pyridine (1:1, vol/vol) for 30 min at 60°C. Samples were dried by vacuum centrifugation and analyzed in a Kratos MS80 RFA spectrometer directly interfaced to a Carlo Erba 5160 chromatograph. Samples were introduced by splitless injection (splitless time, 30 s) into a BPX-5 fused silica column (25 m by 0.2 mm; SGE, Milton Keynes, United Kingdom). The injector and interface oven were maintained at 250°C. One minute after injection, the oven temperature was programmed from 60 to 200°C at 40°C/min and then at a rate of 3°C/min to 230°C, with a final 8°C ramp to 265°C. This temperature was maintained for 10 min. Electron ionization spectra were recorded at an ionization energy of 70 eV, trap current of 100 μA, and a source temperature of 220°C.
Structural elucidation of the cryptococcal glycolipid.
For structural analysis of the cryptococcal glycolipid, fast atom bombardment-mass spectrometry (FAB-MS) was used. The underivatized cryptococcal GSL and its peracetyl derivative were analyzed in a Kratos MS80 spectrometer as previously described by Duarte and coworkers (9).
Reactivity of CMH with patients' sera.
The reactivity of the cryptococcal glucosylceramide with sera from patients with cryptococcosis, aspergillosis, histoplasmosis, and paracoccidioidomycosis was evaluated by using ELISA as described by Villas-Boas et al. (61) with minor modifications. The GSL was dissolved in methanol (200 μg/ml), and 50 μl of this solution was added per well to a flat-bottomed polystyrene microtiter plate. The plate was dried, and blocked with PBS containing 10% bovine serum albumin (BSA) for 1 h at 37°C. After a washing with PBS, 50 μl of normal human sera or sera from patients, diluted 1:50 in PBS, was added to each of the coated wells and incubated at 37°C for 1 h. The plate was washed three times prior to incubation with peroxidase-labeled goat anti-human immunoglobulin (1:2,000) for 1 h at 37°C. The plate was again washed three times with PBS, and 50 μl of o-phenylenediamine in citrate-phosphate buffer containing H2O2 was added to each well, followed by incubation in the dark and reading at 492 nm.
The relative participation of antibodies to CMH in the reaction of cryptococcal antigens and sera of patients with cryptococcosis was evaluated by comparing the reactivities of a pool of five sera with a crude fungal extract before and after depletion of antibodies to CMH. The crude extract of strain HEC3393 was obtained from a thick suspension of C. neoformans cells in a lysis buffer (0.01 M PBS, pH 7.4; 1% Triton X-114; 1 mM EDTA; 1 mM E-64; 5 mM dithiothreitol; 1 μg of aprotinin per ml; 1 μg of pepstatin per ml). An equivalent volume of glass beads (0.3 mm in diameter) was then added to the suspension, and yeasts were broken in a cell disrupter (type 853023/8; B. Braun Biotech International, Germany) by alternating 1-min shaking periods and 2-min cooling intervals. After removal of the glass beads, the suspension was centrifuged (10,000 × g, 30 min, 4°C). The total protein of the crude extract was determined by the method of Lowry et al. (27) and adjusted to 200 μg/ml for use in ELISA reactions.
Purification of antiglucosylceramide antibodies.
The purified cryptococcal glucosylceramide (500 μg) was dissolved in 50 μl of methanol and spotted onto a strip of PVDF membrane, which was blocked with PBS containing 0.1% Tween 20 and 10% BSA for 2 h at room temperature. The membrane was washed four times in PBS-Tween and then incubated overnight at 4°C in the presence of a pool of five sera (at 1:2) obtained from patients with cryptococcosis. After repeated washing to remove unbound proteins, bound antibodies were eluted using 2 ml of 100 mM glycine acid buffer (pH 3.0) and immediately neutralized with 1 M Tris-HCl (pH 9.0). This process was repeated three times, and the unbound depleted fraction contained antibodies that gave absorbance readings by ELISA similar to those with normal human serum (NHS) at the same low dilution. Eluted samples were further purified in protein G-Sepharose 4 Fast Flow, according to the manufacturer's protocol. Fractions containing antibodies to CMH were ultrafiltered in a Centricon-10 micropartition system from Amicon and concentrated by vacuum centrifugation. The recovery of antiglucosylceramide antibodies was monitored by analyzing the eluted samples by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with a monoclonal peroxidase-labeled anti-human antibody. As a control, the CMH-bound PVDF membrane was incubated overnight at 4°C in the presence of normal human immunoglobulin G (IgG). Bound antibodies, eluted with glycine buffer (pH 3.0), were immediately neutralized with 1 M Tris-HCl (pH 9.0) and then combined with the unbound antibody fraction and purified in protein G-Sepharose.
Flow cytometry analysis.
Capsulated (HEC3393) and noncapsulated (cap 67) C. neoformans cells were fixed in 4% paraformaldehyde cacodylate buffer (0.1 M, pH 7.2) for 1 h at room temperature. Fixed yeast cells were washed twice in PBS and incubated sequentially for 30 min in PBS containing 150 mM NH4Cl and then in 1% BSA in PBS (PBS-BSA) for 1 h. Yeast cells (106/ml) were washed in PBS and sequentially incubated with antibodies to CMH (at 1:10 dilution) and fluorescein isothiocyanate (FITC)-labeled anti-human IgG (at 1:100 dilution) for 1 h at room temperature. Yeasts were again washed, and 5,000 cells were analyzed in an EPICS ELITE flow cytometer (Coulter Electronics, Hialeah, Fla.) equipped with 15-mW argon laser emitting at 488 nm. Data were obtained using listmode, which makes further analysis possible. Control cells were incubated with the fluorescein conjugate but not with antiglucosylceramide antibodies.
Immunofluorescence analysis.
Cryptococcal yeasts from different serotypes (HEC3393, CN23/10.993, HEC40143, ATCC 28597, and cap 67 strains) were fixed and blocked as described for flow cytometry and incubated in the presence of the antibody preparations at 1:10 dilution for 1 h (acapsular cells) or 12 h (encapsulated cells) at room temperature. After incubation, the cells were washed three times in PBS and incubated with FITC-labeled anti-human IgG at 1:100 for 1 h at room temperature (acapsular cells) or 24 h at 4°C (encapsulated cells). Control cells, which had not been incubated with antiglucosylceramide antibodies, were also prepared. Cells were washed and, for microscopic examination, 5 μl of a cell suspension at 107 yeasts/ml containing FITC-labeled-antibody-treated C. neoformans was placed on glass slides and observed with Axioplan 2 (Zeiss) fluorescence microscope. Acapsular cells, which showed the most intense reaction, were also evaluated by confocal microscopy in a Zeiss LSM 410 Inverted confocal laser-scanning microscope, with the LSM software. Computer-generated images were finally edited using Adobe Photoshop version 4.0, which included color, brightness, and contrast adjustment.
HPTLC-immunostaining.
C. neoformans var. neoformans (serotype D) or gattii (serotype B) were extracted and partitioned as described above. The Folch's inferior phases were then separated by HPTLC, the plate being air dried, soaked in 0.5% polymethacrylate in diethyl ether, and blocked for 2 h with 10% skimmed milk in PBS. The plate was then incubated for 2 h in presence of the preparation of antibodies to CMH (at 1:10 dilution), followed by sequential incubation with peroxidase-conjugated anti-human antibody and diaminobenzidine, as described elsewhere (51). As a positive control, the purified glucosylceramide from whole cryptococcal cells (strain HEC3393) was used.
Lipid extraction from cell wall preparations.
To determine whether the cryptococcal CMH is cell wall associated, cell wall preparations of C. neoformans (cap 67) were obtained as previously described (43). Cryptococcal yeasts were mixed with glass beads and broken in a cell disrupter (type 853023/8; B. Braun Biotech International). The glass beads were removed, and the suspension centrifuged at 2,000 × g for 10 min at 4°C. The pellet containing the cell wall fragments was suspended in distilled water, homogenized, and centrifuged at 16,000 × g for 15 min. This step was repeated 50 times, and the final cell wall preparation extracted with chloroform-methanol at 2:1 and at 1:2 (vol/vol) (60). The cell wall lipid extract was partitioned according to the method of Folch et al. (16), and the inferior phase was analyzed by HPTLC or HPTLC-immunostaining, as described above.
Immunogold labeling of cryosections.
Cryptococcal yeasts (strain cap 67) were fixed for 1 h in 0.1 M sodium cacodylate buffer (pH 7.2) containing 4% paraformaldehyde and 2% glutaraldehyde. Cells were then infiltrated for 2 h in a solution containing 25% polyvinylpyrrolidone and 2.1 M sucrose and rapidly frozen by immersion in liquid nitrogen. They were transferred to a cryoultramicrotome (Ultracut Reichert), and cryosections were obtained in a temperature range of −70 to −90°C. The material was collected with a sucrose loop and transferred to Formvar-carbon-coated grids. For immunolabeling the cryosections were incubated for 15 min in PBS containing 3% BSA, quenched for 30 min in 50 mM NH4Cl, and subsequently incubated overnight in the presence of purified antibodies to CMH (1:20 dilution). Grids were then washed twice with PBS-BSA and incubated for 1 h in the presence of 15-nm (particle size) immunogold-labeled anti-human IgG (1:100 dilution). Samples were then washed in PBS containing decreasing concentrations of BSA (3, 2, and 1%) and thinly embedded in 3% polyvinyl alcohol-uranile acetate (9:1, vol/vol). Specimens were finally observed in a Zeiss 900 transmission electron microscope operating at 80 kV.
Antibody specificity.
The possibility of cross-reactions between antibodies to CMH and fungal cell wall components was evaluated by ELISA. Cryptococcal CMH was added to a flat-bottomed polystyrene microtiter plate as described above and incubated in the presence of antibodies to CMH. The following components and preparations were tested as inhibitors of the reaction between CMH and antibodies to this glycolipid: (i) an alkaline-soluble extract from lipid-free C. neoformans cell walls prepared as previously described (2), (ii) N,N,N,N-tetraacetylchitotetraose ([GlcNAc]4), (iii) mannan from Saccharomyces cerevisiae, (iv) β-gentibiose (glucosyl-β-1,6-glucose); (v) laminarin (mostly linear β-1,3-glucan); and (vi) zymosan A; all were used at 1 mg/ml. Control systems consisted of protozoan cruzipain (a gift from Julio Scharfstein)–anti-cruzipain MAb and human IgG–anti-human IgG antibody.
Cell growth.
To evaluate the influence of antibodies to CMH on cell growth and budding, 106 yeast cells from strains HEC3393 or cap 67 of C. neoformans were inoculated in sterile microcentrifuge tubes containing 500 μl of BHI supplemented with 20% of NHS or 20% of heat-inactivated NHS (triplicate sets). Purified antibodies to CMH were added at 10 μg/ml, 10-μl aliquots from each culture were taken at 12-h intervals, and the total numbers of cells in these suspensions as well as the numbers of budding cells were counted in a Neubauer chamber. Control systems had either no antibodies added or normal human IgG (100 μg/ml), prepared as described above. To evaluate the ability of antibodies to CMH to kill cryptococci, HEC3393 cells were first cultivated in the presence of these antibodies for 72 h in BHI supplemented with normal or heat-inactivated NHS. The antibody-containing culture medium was then replaced by BHI, and the yeasts were cultivated for 168 h. At 24-h intervals, the cell numbers in 10-μl aliquots of the cultures were counted in a Neubauer chamber.
RESULTS
Purification and identification of GSL.
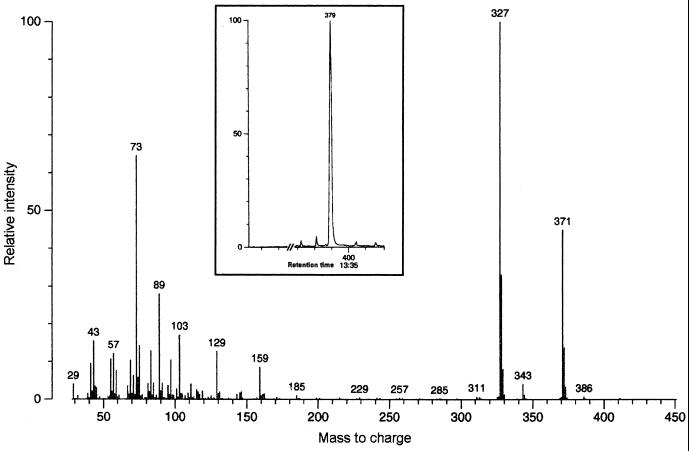
The purification steps of GSLs from C. neoformans cells (strain HEC3393) are shown in Fig. 1. A single orcinol-reactive band was detected by HPTLC, with an Rf similar to that of a standard CMH from bovine brain. The monosaccharide was identified as glucose by gas chromatography (data not shown). For fatty acid analysis, the cryptococcal CMH was methanolysed, trimethylsilylated, and examined by GC-MS. A single peak (Fig. 2) provided a mass spectrum with a weak molecular ion (M) at a mass-to-charge (m/z) of 386, an ion at m/z 371 (M-15), and a base peak at m/z 327 (M-29). The latter fragment, which originated from cleavage between the carboxyl group and carbon 2, is characteristic of 2-hydroxy fatty acid methyl esters (10, 46), which allowed us to identify the compound as 2-hydroxy-octadecanoic acid.
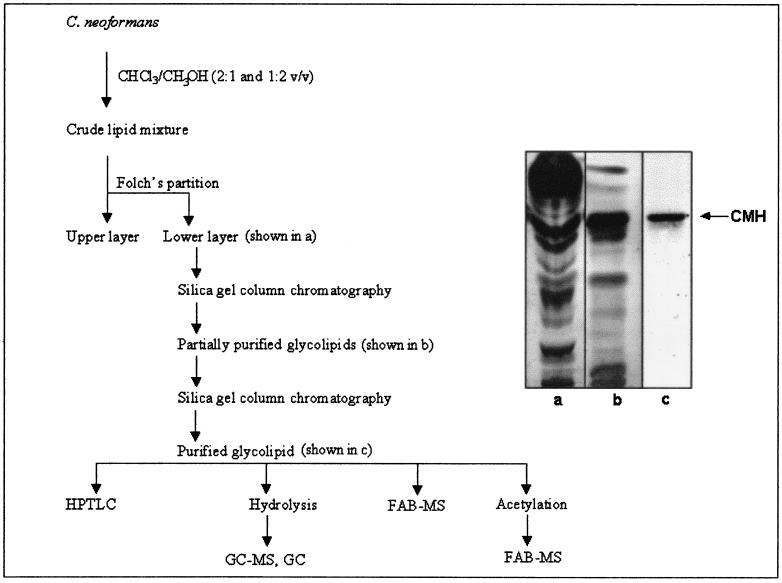
FIG. 1.
Isolation and purification of C. neoformans glucosylceramide. Steps of purification (left) and their corresponding fractions (right) are shown. Abbreviations are as defined in the text.
FIG. 2.
GC-MS analysis of the fatty acid moiety obtained from hydrolysis of the cryptococcal glucosylceramide. A single peak corresponding to a hydroxylated fatty acid was detected (inset), identified as 2-hydroxy-octadecanoic acid. For interpretation of the fragmentation, see Results.
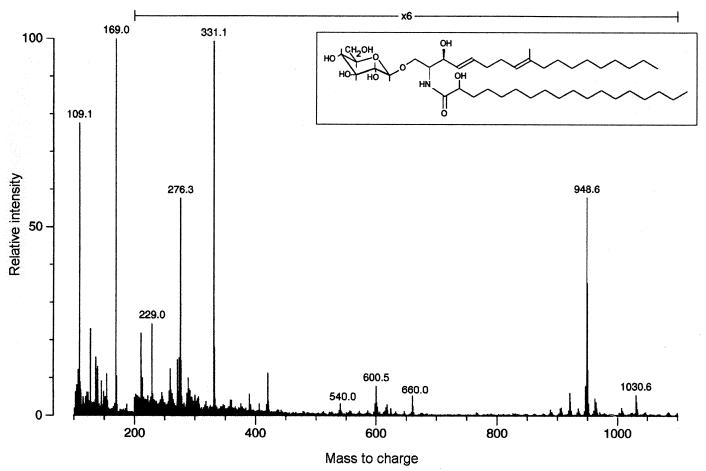
The negative-ion FAB spectrum of the underivatized CMH from C. neoformans (not shown) revealed an abundant ion at m/z 754, which is consistent with the deprotonated molecule of a monohexosylceramide containing hydroxyoctadecanoic acid and C19 sphingadienine. A fragment at m/z 592 represents loss of hexose via a Y-type process (8). These data were confirmed by FAB-MS of the peracetylated glycolipid (Fig. 3). The [M + Na]+ signal observed at m/z 1030 indicated the addition of six acetyl groups to the mass of the underivatized glycolipid, which is consistent with a hydroxy acid containing CMH. Another ion was detected at m/z 948 (M + H-HOAc [HOAc, acetic acid]). The abundant ion detected at m/z 331 indicated the presence of a terminal hexose; the fragment at m/z 229 was attributed to the loss of ketene plus acetic acid from m/z 331, and further elimination of acetic acid gave m/z 169. Peaks at m/z 660 (CerAc2+ [diacetylated ceramide]), 600 (CerAc2+ − HOAc), and 540 (CerAc2+ − 2HOAc) identified the ceramide moiety. The fragment at m/z 276, which is diagnostic of a C19 sphingadiene (9), originates from the long-chain base.
FIG. 3.
FAB-MS of the peracetylated glucosylceramide from C. neoformans and its corresponding native structure. For interpretation of fragmentation, see Results.
Reactivity of the cryptococcal glucosylceramide with patients' antibodies.
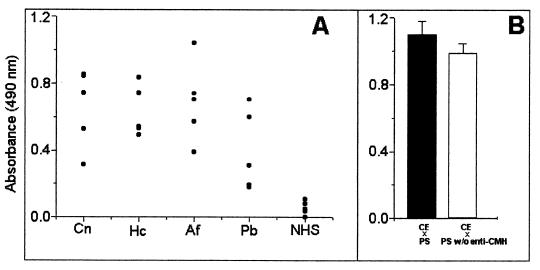
The purified cryptococcal CMH reacted with sera from five patients with cryptococcosis (Fig. 4A). Sera from patients with histoplasmosis, aspergillosis, and paracoccidioidomycosis also recognized the cryptococcal CMH, indicating that antibodies against similar structures are produced during the course of these mycoses. The reactivity of crude multiantigenic extracts of C. neoformans with patients' sera or with sera that had been depleted of antibodies to CMH by immunoadsorption showed that the latter represented ca. 10% of the total serum reactivity (Fig. 4B).
FIG. 4.
(A) Reactivity of CMH with patients' antibodies. Sera from individuals with cryptococcosis (Cn), histoplasmosis (Hc), aspergillosis (Af), and paracoccidioidomycosis (Pb) recognize the cryptococcal CMH, while sera from normal individuals (NHS) do not. (B) Reactivity of crude extracts (CE) of C. neoformans with a pool of patients' serum (■) and patients' serum depleted of antibodies to CMH (□).
Immunofluorescence.
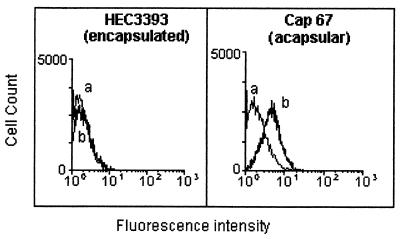
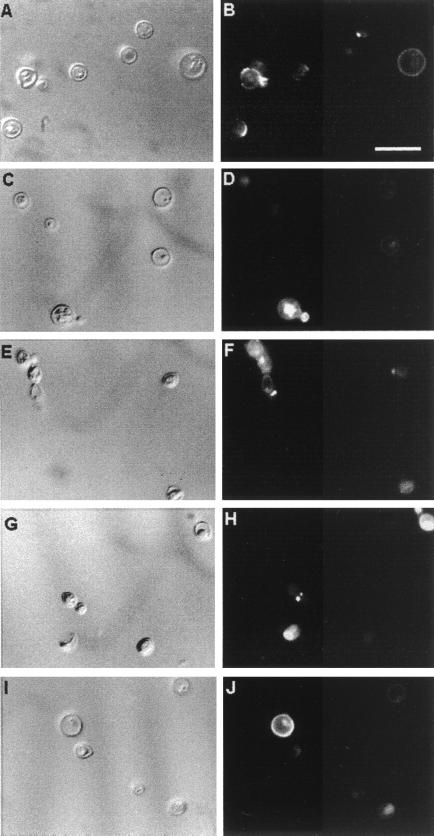
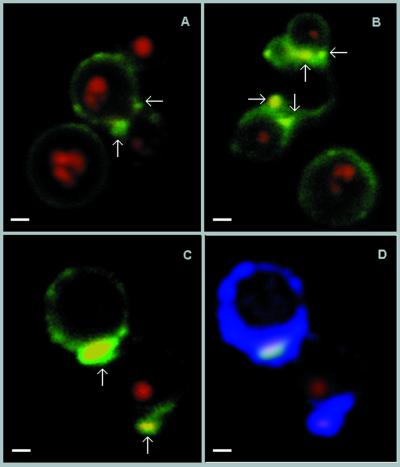
Antiglucosylceramide antibodies were obtained from the sera of patients with cryptococcosis, which were pooled, immunoadsorbed on the solid-phase fixed glucosylceramide, and finally purified by affinity chromatography. Acid-eluted antibodies were mainly IgG, as monitored by SDS-PAGE and Western blotting. The predominant isotypes were IgG1 ≫ IgG4 > IgG3 (not shown). These antibodies were incubated with encapsulated and acapsular C. neoformans cells and analyzed by flow cytofluorimetry, as shown in Fig. 5. On short incubation (1 h), the reaction of encapsulated cells with antiglucosylceramide antibodies was absent or very weak. In contrast, the acapsular population readily reacted with the antiglucosylceramide antibodies, with a shift of the fluorescence peak. Immunofluorescence microscopy (Fig. 6), under the same conditions as those described above, also showed no detectable reaction of encapsulated cells with antibodies to CMH (data not shown), whereas the acapsular yeasts showed uniform surface labeling of mature nondividing cells. The fluorescent labeling was rather concentrated at the sites of budding in dividing cells (Fig. 6B). Encapsulated cells were then incubated with the antibodies for longer periods, which allowed the detection of positive fluorescent reactions at the yeast budding sites and on young daughter and nondividing cells (Fig. 6D, F, H, and J). Cells were heterogeneously immunostained, with intense and poor labeling being observed on the same microscopic field. Acapsular cells, which were more strongly reactive, were examined by confocal microscopy, which showed that the distribution of the glucosylceramide in mature cells was in small aggregates on the cell surface (Fig. 7A and B). In dividing cells the budding sites seemed to accumulate most of the antibody-reactive CMH as if the glycolipid clusters were part of the underlying structure where the cell wall is synthesized for bud formation. The use of the LSM software allowed us to overlay the specific fluorescent reaction of CMHs with antibodies to CMH and the autofluorescence of the thickened cryptococcal cell wall at the site of bud emergence (Fig. 7C and D). In general, the CMH reactions seemed to coincide with cell wall thickening.
FIG. 5.
Flow cytometric analysis of encapsulated (HEC3393) and acapsular (cap 67) C. neoformans yeast cells incubated with antiglucosylceramide antibodies. Data curves: a, control cells (no antiglucosylceramide antibodies were added prior to incubation with FITC-labeled anti-human IgG); b, incubation of C. neoformans with antiglucosylceramide antibodies. After 1 h of exposure, the acapsular but not the encapsulated cells were reactive.
FIG. 6.
Surface distribution of the cryptococcal glucosylceramide. Acapsular (A and B) and encapsulated (C to J) C. neoformans yeast cells were incubated in the presence of antiglucosylceramide antibodies, followed by incubation with FITC-labeled anti-human IgG and observed in a fluorescence microscope. In control systems, in which no antiglucosylceramide antibodies were added prior to incubation with FITC-labeled anti-human IgG, no detectable fluorescence was observed (not shown). The left panels show C. neoformans yeasts using differential interferential contrast microscopy. Panels: A and B, acapsular cells; C and D, cryptococci of serotype A; E and F, serotype B cells; G and H, serotype C cells; and I and J, serotype D cells. Bar, 10 μm.
FIG. 7.
Confocal microscopy of CMH at the cell surface of acapsular C. neoformans cells. (A to C) Budding cells show the CMH (arrows), mainly concentrated at the budding region, while mature nondividing cells have some weakly labeled clusters on the cell surface. (D) Cells shown in Fig. 7C were analyzed by overlaying the specific fluorescent reaction of surface CMH with antibodies to them (arrows) and the autofluorescence of the cryptococcal cell wall (blue). This technique shows that the polar concentration of CMH colocalizes with a thickened cell wall to the site of bud formation. Autofluorescence of the nucleus is shown in red. Bars, 1 μm.
Immunostaining.
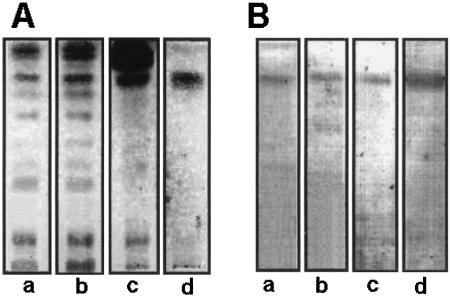
Lipid extracts from whole C. neoformans cells, as well as from cell wall preparations, were obtained and partitioned according to the method of Folch and coworkers (16). The lower layers were then analyzed by HPTLC and HPTLC-immunostaining. Orcinol-reactive bands with Rf values similar to that of the purified CMH were detected in extracts from C. neoformans var. neoformans (Fig. 8A) or gattii and from cell wall preparations. These bands were recognized by antibodies to CMH (Fig. 8B), confirming that CMH is produced by different varieties and serotypes of C. neoformans. Additionally, this analysis suggested that CMH molecules make part of the fungal cell wall components.
FIG. 8.
HPTLC (A) and HPTLC-immunostaining (B) of glycolipids obtained from serotype B (lanes a) and D (lanes b) cells or from cell wall preparations from cap 67 cells (lanes c) compared with the purified glucosylceramide from HEC3393 cells (lanes d). Orcinol-reactive bands with Rf values similar to that of the purified cryptococcal glucosylceramide were detected by HPTLC. Immunostaining showed that antibodies to CMH recognized components with migration similar to that of CMH.
Cryptococcal CMH is cell wall associated.
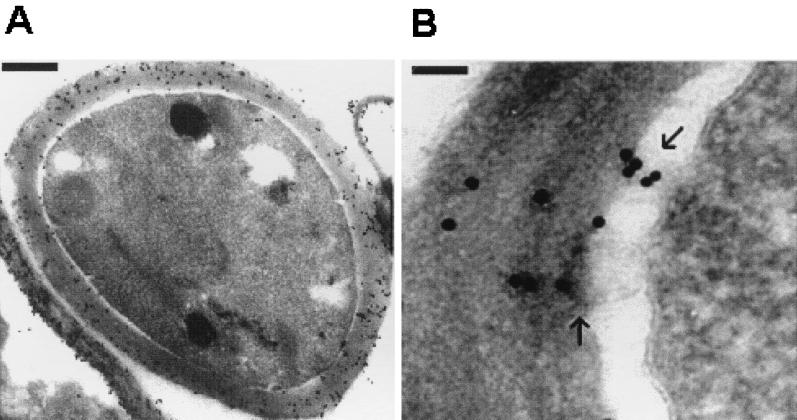
To confirm the subcellular sites of CMH accumulation, cryosections of C. neoformans cells (cap 67) were processed for transmission electron microscopy using immunogold labeling. By using this technique we showed extensive antibody binding to the cryptococcal wall (Fig. 9A), including the cell wall structures that had detached from the cell during processing. Only a few gold particles were seen on the membrane and a few more were found in the periplasmic space. Points of transport of the presumed CMH-containing vesicles from the plasma membrane to the cell wall were also suggested (Fig. 9B). To exclude the possibility that the strong reaction with antibodies to CMH was due to cross-reaction with known cell wall components, delipidated cell wall extracts and molecules commonly found in fungal walls were tested as inhibitors of the antigen-antibody interactions in ELISA. Of these, only laminarin was partially effective in inhibiting the reaction of CMH and antibodies to CMH (Table 1). The specificity of this inhibition was, however, doubtful, since laminarin also inhibited binding of a monoclonal anticruzipain antibody to protozoan cruzipain as well as that of anti-human IgG antibodies to human IgG, which were used as control systems.
FIG. 9.
Cryptococcal CMH are located at the fungal cell wall. (A) Transmission electron microscopy showed an extensive binding of antibodies to CMH to the cryptococcal cell wall, integrated or detached from the cells. Bar, 0.5 μm. (B) Possible CMH-containing vesicles are seen (arrows), which can move across the periplasmic space and deposit cell membrane constituents on the cell wall. Bar, 0.1 μm.
TABLE 1.
Reactivity of cryptococcal CMH with human antibodies to CMH in the presence of fungal cell wall componentsa
| Cell wall componentsb at 1 mg/ml | % Decrease in reactivity |
|---|---|
| N,N′,N′′,N′′′-Tetraacetylchitotetraose | 6.6 |
| Mannan (S. cerevisiae) | 7.2 |
| β-Gentibiose | 0 |
| C. neoformans cell wall alkaline extract (neutralized to pH 7.0) | 0 |
| Zymosan A | 14.6 |
| Laminarin | 40.8 (anti-CMH), 43.3 (anti-cruzipain)c, 57.3 (anti-IgG)c |
ELISA of β-glucosylceramide at 10 μg/well and patients' antibodies to CMH diluted 1:50.
Chitin and β-glucan-derived oligosaccharides, polysaccharides, and soluble cell wall extracts incubated with the antibodies to CMH and added to the CMH-coated plates.
Inhibition by laminarin of the homologous systems with cruzipain–anti-cruzipain MAbs and IgG–anti-IgG antibodies.
Antibodies to CMH inhibit cell budding and growth.
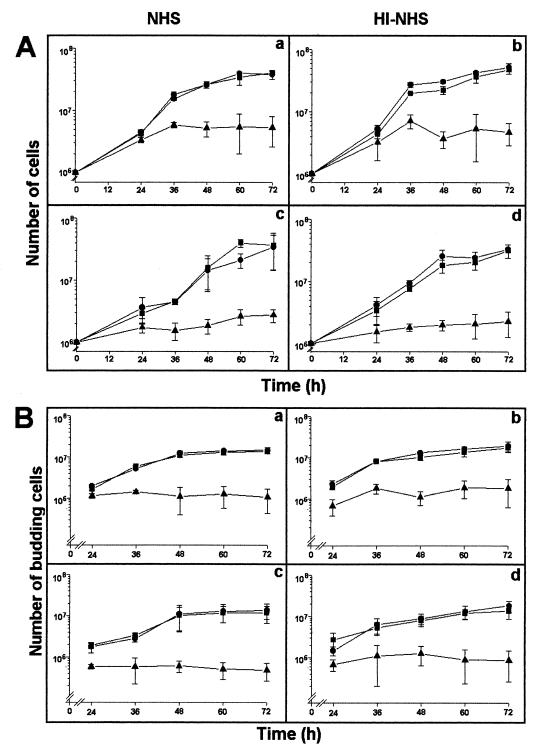
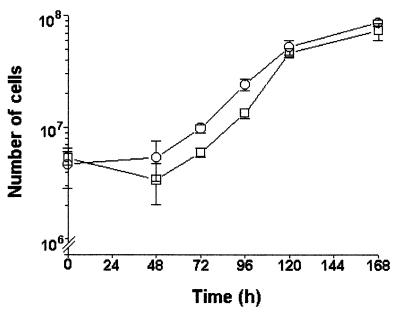
To clarify the involvement of CMH in the cell budding of C. neoformans, antibodies to CMH were added to cryptococcal cultures, and the number of cryptococci determined at 12-h intervals. The growth of C. neoformans cells in the presence of antibodies to CMH was quantified and compared to the cryptococcal growth in the absence of antibodies or in the presence of normal human IgG, used as a negative control. The addition of antibodies to CMH to the cultures clearly inhibited fungal growth (Fig. 10A) either of acapsular or encapsulated cells. The inhibitory effect was observed after 24 h in the culture of the acapsular strain, whereas a longer period, 36 h, was necessary for a similar inhibition of the encapsulated strain. Seemingly, the capsule in C. neoformans retards the diffusion of antibodies to the cell surface of this fungus. The number of budding cells, as well as the number of buds per cell, in both acapsular and encapsulated cells was similarly reduced by reaction of the CMH-rich structures with antibodies to CMH (Fig. 10B). The inhibitory effect of these antibodies was not complement dependent, since similar growth rates were observed when C. neoformans cells were cultivated in the presence of NHS or heat-inactivated NHS. Additionally, antibodies raised against CMH did not kill C. neoformans, since yeast growth resumed after replacement of the antibody-containing media by BHI (Fig. 11).
FIG. 10.
Influence of antibodies to CMH on the cell growth (A) and budding (B) of encapsular (a and b) and acapsulated (c and d) C. neoformans. The culture medium was supplemented with NHS (a and c) or heat-inactivated NHS (HI-NHS, b and d). Addition of antibodies to CMH to the cell cultures at 10 μg/ml (▴) inhibited fungal growth and budding in both acapsular and encapsulated cells. ●, Untreated cells; ■, cells treated with normal human IgG, processed as for the antibodies to CMH, at 100 μg/ml.
FIG. 11.
Antibodies to CMH are fungistatic but not fungicidal for C. neoformans. Replacement of the antibody-containing medium by BHI restored the fungal growth of encapsulated (strain HEC3393) cells previously cultivated for 48 h in BHI with antibodies to CMH in normal (○) or heat-inactivated NHS (□).
DISCUSSION
GSLs have been characterized in fungal and protozoan pathogens (1, 9, 54–56, 59–61). Although the biological functions of these molecules are not fully understood, they were shown to be antigenic in different infectious agents. For instance, GSLs from Trypanosoma cruzi epimastigotes react with sera from patients with Chagas' disease, and this reactivity is modulated by the ceramide structure (61). Schistosome glycolipids are recognized by IgE, which may have a role in immunity against Schistosoma mansoni (57). A glucosylceramide is the major GSL from the opportunistic pathogen Pseudoallescheria boydii (41) and, as with C. neoformans glycolipid, is recognized by sera from infected individuals. In Paracoccidioides brasiliensis, a galactofuranose-containing GSL is reactive with antibodies from patients with paracoccidioidomycosis (54). Such reactivity was attributed to the nonreducing galactofuranosyl residue in the carbohydrate chain. β-Glucopyranosyl ceramides (GlcCer) with 2′-OH-fatty acids, or GlcCer and β-galactosyl ceramide, were characterized in P. brasiliensis and Aspergillus fumigatus, respectively (56). In C. neoformans, the major sphingolipids described previously consisted of ceramide, inositol, and mannose (62). In a recent work, Franzot and Doering (17) described the synthesis of glycosylphosphatidylinositol (GPI) anchors in membranes of cryptococcal yeasts.
The relevance of the cryptococcal β-glucosylceramide was addressed in the present work. The possible involvement of this molecule in fungal pathogenicity was hypothesized, since human antibodies against this CMH were isolated from sera of patients with cryptococcosis by immunoadsorption on the purified glycolipid and by affinity chromatography. They inhibited C. neoformans budding and growth. The presence of CMH as structural components of the cryptococcal cell wall was demonstrated by electron microscopy of yeast cells labeled with immunogold-antibodies to CMH. Abundant deposition of gold particles was observed on the cryptococcal wall rather than on the plasma membrane. Apparently, the antibody-reactive epitopes of CMH may be sterically accessible only after transfer of the GSLs to the cell wall. Labeling was observed on membrane formations (vesicles?) across the periplasmic space, linking the plasma membrane to the inner face of the cell wall (Fig. 9B). Antibodies located the CMH preferentially to the sites of cell budding, which suggests that this glycolipid is essential for cellular division. A possible relationship of CMH and the cell wall biosynthetic enzymes, particularly at the budding sites, could suggest a specific lipid requirement. Data from another system support this interpretation. Kawai et al. (24, 25) showed that fungal glucocerebrosides had fruiting-inducing activity in bioassays with Schizophyllum commune. The intact 9-methyl-4,8-sphingadienine but not the β-glucopyranosyl residue was essential for this activity.
Binding of lectins or antibodies to cell wall components can interfere with the biosynthesis and organization of the cell wall polymers. In Fusarium sp. (6), treatment with wheat germ agglutinin, which has a known affinity for chitin, resulted in alterations in germ tube formation and caused cell lysis. As a consequence, fungal infection did not spread with lectin-treated Fusarium. The inhibitory activity of antibodies to CMH may involve a different mechanism. GSLs form, with sterols and GPI-anchored proteins, detergent-insoluble lipid rafts on the plasma membrane (35, 48, 67). They are required for the processing of GPI-anchored proteins in yeasts, making part of vesicles that link the RES to Golgi to the plasma membrane (21, 50, 52). For the synthesis of the cell wall structural network it has been proposed that GPI anchors have a pivotal constitutive role (7). A truncated GPI anchor which no longer contains inositol and glucosamine is the substrate for a phosphate-linked β-1,6-glucan extension (49, 58). GPI anchors can be liberated in the periplasmic space by the action of phospholipase C (PI-PLC), as present in S. cerevisiae (15) and abundantly expressed in P. brasiliensis (20), or could be transported to the cell wall in vesicles. This may happen due to the inability of GPI anchor cleavage by PI-PLC, a property of inositol-acylated molecules found in C. neoformans (17), or to a more generalized process in which precursor molecules and enzymes are transferred to the cell wall in vesicles originating from the plasma membrane. Cell wall particles have been described in C. neoformans (47) presumably containing precursors of the capsule. Spherical invaginations which secrete vesicles outside the cell membrane have also been well documented by Takeo et al. (53) in C. neoformans using the freeze-etching technique. Assuming then that GSLs closely associated with GPI precursors as in lipid rafts and presumably also biosynthetic enzymes are transported to the cell wall in vesicles, the action of antibodies to CMH could impair the utilization and reactivity of the carried components. Antibody inhibition of budding can also be correlated with the increased secretion of enzyme-containing vesicles during bud formation (31).
The role of antibody immunity against C. neoformans was reviewed by Pirofski and Casadevall (42). Experiments with polyclonal sera have produced conflicting evidence for and against the importance of antibody immunity in host defense. Accordingly, tests with MAbs to the C. neoformans capsular polysaccharide have revealed the existence of protective, nonprotective, and disease-enhancing MAbs, suggesting that data obtained in experiments with polyclonal preparations may be explained by differences in the relative proportion of protective and nonprotective antibodies in the immune sera. Mechanisms by which protective MAbs would modify the course of infection were proposed based on in vitro experiments, in which these antibodies enhanced effector cell functions against C. neoformans (32–34). The complexity of antibody binding to surface structures of C. neoformans as recently shown, using three MAbs (12), does not warrant the assumption that cell wall-reacting antibodies to CMH will be equally effective when admixed with other anticryptococcal antibodies of different specificity.
The relevance of the anti-CMH effect in vivo is under investigation. Antibodies to CMH should reach a plasma concentration compatible with that minimally effective to inhibit cryptococci in vitro. Purified human antibodies were inhibitory in vitro at 10 μg/ml or at 5 pg/fungal cell, but lower concentrations were not tested. The inhibition was specific since 10-fold-more normal human IgG was inactive. Cryptococcal cells are 100% encapsulated in vivo (M. Feldmesser, personal communication); however, as shown here and previously, with anti-melanin antibodies (38), IgG molecules diffuse through the capsule to reach the cell surface, depending on their concentration and time of exposure. The possibility that C. neoformans can be a facultative intracellular pathogen (13) would represent an efficient escape mechanism against antibodies to CMH.
The cryptococcal β-glucopyranosyl ceramide is very similar to other fungal cerebrosides in that they all contain a 9-methyl-4,8-sphingadienine in combination with N-2′-hydroxy fatty acids that are saturated or unsaturated (9, 26, 55, 56, 60). Hydroxylation at position 2 of the fatty acid is apparently important for antigenicity of the CMH (37, 66), and possible epitopes involve both glucose and the hydroxylated fatty acid, with modulation by the sphingosine-derived base. Conformer 4 of glucosylceramide as studied by Nyholm and Pascher (39, 40), which is allowed in a membrane layer and further stabilized by a hydrogen bond between the 2-OH group on the fatty acid and the 6-OH group on the glucose residue, in addition to the hydrogen bond between glucose O5 and the amide hydrogen, is a candidate for carrying epitopes reactive with antibodies to CMH. Antibodies to CMH elicited in patients with cryptococcosis and also in patients with other mycoses as presently shown could inhibit the growth of pathogenic fungi that express the same GSL, if accessible to the cell wall inner structure and biosynthetic core. Preliminary results from our laboratory indicate that in P. boydii, which also synthesizes a β-glucosylceramide, antibodies to CMH inhibit formation of germinative tubes and growth (E. Barreto-Bergter et al., unpublished results). A general fungistatic effect of antibodies to CMH is thus suggested, which encourages the investigation of their action against other mycopathogens.
ACKNOWLEDGMENTS
This work was supported by Financiadora de Estudos e Projetos (FINEP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa no Estado do Rio de Janeiro (FAPERJ), and Programa de Apoio a Núcleos de Excelência (PRONEX).
We thank Robin Wait for performing the FAB-MS and GC-MS analyses, Pedro Persechini for the use of the flow cytofluorometer, Cristiana Limongi and Venicio F. da Veiga for help with the fluorescence microscopy, and Henrique Lenzi and Marcelo Pelajo-Machado for the confocal microscopy.
REFERENCES
- 1.Barreto-Bergter E, Vermelho A B, Hartmann R, Pohlentz G, Klein R A, Egge H. Structural characterization of neutral glycosphingolipids from Trypanosoma cruzi. Mol Biochem Parasitol. 1992;51:263–270. doi: 10.1016/0166-6851(92)90076-v. [DOI] [PubMed] [Google Scholar]
- 2.Barreto-Bergter E, Travassos L R, Gorin P A J. Chemical structure of the d-galacto-d-mannan component from hyphae of Aspergillus niger and other Aspergillus spp. Carbohydr Res. 1980;86:273–285. [Google Scholar]
- 3.Bielawska A, Linardic C M, Hannun Y A. Modulation of cell growth and differentiation by ceramide. FEBS Lett. 1992;307:211–214. doi: 10.1016/0014-5793(92)80769-d. [DOI] [PubMed] [Google Scholar]
- 4.Chen L C, Blank E S, Casadevall A. Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol. 1996;3:570–574. doi: 10.1128/cdli.3.5.570-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S C A, Muller M, Zhou J Z, Wright L C, Sorrel T C. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175:414–420. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- 6.Ciopraga J, Gozia O, Tudor R, Brezuica L, Doyle R J. Fusarium sp. growth inhibition by wheat germ agglutinin. Biochim Biophys Acta. 1999;1428:424–432. doi: 10.1016/s0304-4165(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 7.De Sampaio G, Bourdineaud J P, Lauquin G J. A constitutive role for GPI anchors in Saccharomyces cerevisiae: cell wall targeting. Mol Microbiol. 1999;34:247–256. doi: 10.1046/j.1365-2958.1999.01585.x. [DOI] [PubMed] [Google Scholar]
- 8.Domon B, Costello C E. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988;27:1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]
- 9.Duarte R S, Polycarpo C R, Wait R, Hartmann R, Barreto-Bergter E. Structural characterization of neutral glycosphingolipids from Fusarium species. Biochim Biophys Acta. 1998;1390:186–196. doi: 10.1016/s0005-2760(97)00179-3. [DOI] [PubMed] [Google Scholar]
- 10.Eglinton G, Hunnerman D H, McCormick A. Gas chromatographic-mass spectrometric studies on long chain hydroxy acids. Org Mass Spectrom. 1968;1:593–611. [Google Scholar]
- 11.Feldmesser M, Casadevall A. Mechanism of action of antibody to capsular polysaccharide in Cryptococcus neoformans infection. Front Biosci. 1998;3:D136–D151. doi: 10.2741/a270. [DOI] [PubMed] [Google Scholar]
- 12.Feldmesser M, Rivera J, Kress Y, Kozel T R, Casadevall A. Antibody interactions with the capsule of Cryptococcus neoformans. Infect Immun. 2000;68:3642–3650. doi: 10.1128/iai.68.6.3642-3650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishbein J D, Dobrowsky R T, Bielawska A, Garrett S, Hannun Y A. Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J Biol Chem. 1993;268:9255–9261. [PubMed] [Google Scholar]
- 15.Flick J S, Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch J, Less M, Tacker T R. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:467–509. [PubMed] [Google Scholar]
- 17.Franzot S P, Doering T L. Inositol acylation of glycosylphosphatidylinositols in the pathogenic fungus Cryptococcus neoformans and the model yeast Saccharomyces cerevisiae. Biochem J. 1999;340:25–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Ghannoum M, Abu el-Teen A K, Radwan S S. Blocking adherence of Candida albicans to buccal epithelial cells by yeast glycolipids, yeast wall lipids and lipids from epithelial cells. Mykosen. 1987;30:371–378. doi: 10.1111/j.1439-0507.1987.tb03631.x. [DOI] [PubMed] [Google Scholar]
- 19.Hakomori S. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochem Soc Trans. 1993;21:583–595. doi: 10.1042/bst0210583. [DOI] [PubMed] [Google Scholar]
- 20.Heise N, Travassos L R, de Almeida M L. Paracoccidioides brasiliensis expresses both glycosylphosphatidylinositol-anchored proteins and a potent phospholipase C. Exp Mycol. 1995;19:111–119. doi: 10.1006/emyc.1995.1013. [DOI] [PubMed] [Google Scholar]
- 21.Horvath A, Sutterlin C, Manning-Krieg U, Movva N R, Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson E S, Jenkins N D, Todd J M. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994;62:4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez-Lucho V, Ginsburg V, Krivan H C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Galβ1-4Glcβ1-1Cer), a possible adhesion receptor for yeasts. Infect Immun. 1990;58:2085–2090. doi: 10.1128/iai.58.7.2085-2090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai G, Ikeda Y. Structure of biologically active and inactive cerebrosides prepared from Schizophyllum commune. J Lipid Res. 1985;26:338–343. [PubMed] [Google Scholar]
- 25.Kawai G, Ohnishi M, Fujino Y, Ikeda Y. Stimulatory effect of certain plant sphingolipids on fruiting of Schizophyllum commune. J Biol Chem. 1986;261:779–784. [PubMed] [Google Scholar]
- 26.Levery S B, Toledo M S, Doong R L, Straus A H, Takahashi H K. Comparative analysis of ceramide structural modification found in fungal cerebrosides by electrospray tandem mass spectrometry with low energy collision-induced dissociation of Li+ adduct ions. Rapid Commun Mass Spectrom. 2000;14:551–563. doi: 10.1002/(SICI)1097-0231(20000415)14:7<551::AID-RCM909>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–272. [PubMed] [Google Scholar]
- 28.Lu P, Sharom F J. Gangliosides are potent immunosuppressors of IL-2-mediated T-cell proliferation in a low protein environment. Immunology. 1995;86:356–363. [PMC free article] [PubMed] [Google Scholar]
- 29.Malisan F, Rippo M R, De Maria R, Testi R. Lipid and glycolipid mediators in CD95-induced apoptotic signaling. Results Probl Cell Differ. 1999;23:65–76. doi: 10.1007/978-3-540-69184-6_4. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS: 100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moor H, Mühlethaler Fine structure in frozen-etched yeast cells. J Cell Biol. 1963;17:609–628. doi: 10.1083/jcb.17.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee J, Pirofski L A, Scharff M D, Casadevall A. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci USA. 1993;90:3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muniz M, Riezman H. Intracellular transport of GPI-anchored proteins. EMBO J. 2000;19:10–15. doi: 10.1093/emboj/19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutoh T, Tokuda A, Inokuchi J, Kuriyama M. Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J Biol Chem. 1998;273:26001–26007. doi: 10.1074/jbc.273.40.26001. [DOI] [PubMed] [Google Scholar]
- 37.Nakakuma H, Arai M, Kawaguchi T, Horikawa K, Hidaka M, Sakamoto K, Iwamori M, Nagai Y, Takatsuki K. Monoclonal antibody to galactosylceramide: discrimination of structural difference in the ceramide moiety. FEBS Lett. 1989;258:230–232. doi: 10.1016/0014-5793(89)81660-6. [DOI] [PubMed] [Google Scholar]
- 38.Nosanchuk J D, Rosas A L, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 39.Nyholm P G, Pascher I. Orientation of the saccharide chains of glycolipids at the membrane surface: conformational analysis of the glucose-ceramide and the glucose-glyceride linkages using molecular mechanics (MM3) Biochemistry. 1993;32:1225–1234. doi: 10.1021/bi00056a005. [DOI] [PubMed] [Google Scholar]
- 40.Nyholm P G, Pascher I. Steric presentation and recognition of the saccharide chains of glycolipids at the cell surface: favoured conformations of the saccharide-lipid linkage calculated using molecular mechanics (MM3) Int J Biol Macromol. 1993;15:43–51. doi: 10.1016/s0141-8130(05)80087-x. [DOI] [PubMed] [Google Scholar]
- 41.Pinto M. Master's thesis. Rio de Janeiro, Brazil: Federal University of Rio de Janeiro; 1997. [Google Scholar]
- 42.Pirofski L A, Casadevall A. Cryptococcus neoformans: paradigm for the role of antibody immunity against fungi? Zentbl Bakteriol. 1996;284:475–495. doi: 10.1016/s0934-8840(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 43.Previato J O, Gorin P A J, Haskins R H, Travassos L R. Soluble and insoluble glucans from different cell types of the human pathogen Sporothrix schenckii. Exp Mycol. 1979;3:92–105. [Google Scholar]
- 44.Rodrigues M L, Alviano C S, Travassos L R. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes Infect. 1999;1:293–301. doi: 10.1016/s1286-4579(99)80025-2. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues M L, Rozental S, Couceiro J N N, Angluster J, Alviano C S, Travassos L R. Identification of N-acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans: Influence on fungal phagocytosis. Infect Immun. 1997;65:4937–4942. doi: 10.1128/iai.65.12.4937-4942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryhage R, Stenhagen E. Mass spectrometry in lipid research. J Lipid Res. 1960;1:361–390. [PubMed] [Google Scholar]
- 47.Sakaguchi N, Baba T, Fukuzawa M, Ohno S. Ultrastructural study of Cryptococcus neoformans by quick-freezing and deep-etching method. Mycopathologia. 1993;121:133–141. doi: 10.1007/BF01104068. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder R J, Ahmed S N, Zhu Y, London E, Brown D A. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 49.Shahinian S, Bussey H. β-1,6-Glucan synthesis in Saccharomyces cerevisiae. Mol Microbiol. 2000;35:477–489. doi: 10.1046/j.1365-2958.2000.01713.x. [DOI] [PubMed] [Google Scholar]
- 50.Skrzypek M, Lester R L, Dickson R C. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straus A H, Levery S B, Jasiulionis M G, Salyan M E, Steele S J, Travassos L R, Hakomori S, Takahashi H K. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993;268:13723–13730. [PubMed] [Google Scholar]
- 52.Sutterlin C, Doering T L, Schimmoller F, Schroder S, Riezman H. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J Cell Sci. 1997;110:2703–2714. doi: 10.1242/jcs.110.21.2703. [DOI] [PubMed] [Google Scholar]
- 53.Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. J Bacteriol. 1973;113:1442–1448. doi: 10.1128/jb.113.3.1442-1448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toledo M S, Suzuki E, Straus A H, Takahashi H K. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J Med Vet Mycol. 1995;33:247–251. doi: 10.1080/02681219580000501. [DOI] [PubMed] [Google Scholar]
- 55.Toledo M S, Levery S B, Straus A H, Takahashi H K. Dimorphic expression of cerebrosides in the mycopathogen Sporothrix schenckii. J Lipid Res. 2000;41:797–806. [PubMed] [Google Scholar]
- 56.Toledo M S, Levery S B, Straus A H, Suzuki E, Momany M, Glushka J, Moulton J M, Takahashi H K. Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry. 1999;38:7294–7306. doi: 10.1021/bi982898z. [DOI] [PubMed] [Google Scholar]
- 57.Van Der Kleij D, Tielens A G, Yazdanbakhsh M. Recognition of schistosome glycolipids by immunoglobulin E: possible role in immunity. Infect Immun. 1999;67:5946–5950. doi: 10.1128/iai.67.11.5946-5950.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Der Vaart J M, te Biesebeke R, Chapman J W, Klis F M, Verrips C T. The β-1,6-glucan containing side-chain of cell wall proteins of Saccharomyces cerevisiae is bound to the glycan core of the GPI moiety. FEMS Microbiol Lett. 1996;145:401–407. doi: 10.1111/j.1574-6968.1996.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 59.Vermelho A B, de Meirelles M, Pereira M C, Pohlentz G, Barreto-Bergter E. Heart muscle cells share common neutral glycosphingolipids with Trypanosoma cruzi. Acta Trop. 1997;64:131–143. doi: 10.1016/s0001-706x(96)00627-4. [DOI] [PubMed] [Google Scholar]
- 60.Villas-Boas M H, Egge H, Pohlentz G, Hartmann R, Barreto-Bergter E. Structural determination of N-2′-hydroxyoctadecenoyl-1-O-beta-d-glucopyranosyl-9-methyl-4,8-sphingadienine from species of Aspergillus. Chem Phys Lipids. 1994;70:11–19. doi: 10.1016/0009-3084(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 61.Villas-Boas M H, da Silva M C, de Oliveira T G, Travassos L R, Barreto-Bergter E. Reactivity of chagasic sera with crude and highly purified glycosphingolipid fractions from Trypanosoma cruzi epimastigotes. J Clin Lab Anal. 1994;8:260–266. doi: 10.1002/jcla.1860080503. [DOI] [PubMed] [Google Scholar]
- 62.Vincent V L, Klig L S. Unusual effect of myo-inositol on phospholipid biosynthesis in Cryptococcus neoformans. Microbiology. 1995;141:1829–1837. doi: 10.1099/13500872-141-8-1829. [DOI] [PubMed] [Google Scholar]
- 63.Wickes B L, Mayorga M E, Edman U, Edman J C. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc Natl Acad Sci USA. 1996;93:7327–7331. doi: 10.1073/pnas.93.14.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williamson P R. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front Biosci. 1997;2:99–107. doi: 10.2741/a231. [DOI] [PubMed] [Google Scholar]
- 65.Wong B, Perfect J R, Breggs S, Wright K A. Production of hexitol d-mannitol by Cryptococcus neoformans in vitro and in rabbits with experimental meningitis. Infect Immun. 1990;58:1664–1670. doi: 10.1128/iai.58.6.1664-1670.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young W W, Jr, Durdik J M, Urdal D, Hakomori S, Henney C S. Glycolipid expression in lymphoma cell variants: chemical quantity, immunologic reactivity, and correlations with susceptibility to NK cells. J Immunol. 1981;126:1–6. [PubMed] [Google Scholar]
- 67.Zhang X, Thompson G A., Jr An apparent association between glycosylphosphatidylinositol-anchored proteins and a sphingolipid in Tetrahymena mimbres. Biochem J. 1997;323:197–206. doi: 10.1042/bj3230197. [DOI] [PMC free article] [PubMed] [Google Scholar]