Abstract
Gastrointestinal disease is prevalent and broad, manifesting itself in a variety of ways, including inflammation, fibrosis, infection, and cancer. However, historically, diagnostic technologies have exhibited limitations, especially with regard to diagnostic uncertainty. Despite development of newly emerging technologies such as optoacoustic imaging, many recent advancements have focused on improving upon pre-existing modalities such as ultrasound, computed tomography, magnetic resonance imaging, and endoscopy. These advancements include utilization of machine learning models, biomarkers, new technological applications such as diffusion weighted imaging, and new techniques such as transrectal ultrasound. This review discusses assessment of disease processes using imaging strategies for the detection and monitoring of inflammation, fibrosis, and cancer in the context of gastrointestinal disease. Specifically, we include ulcerative colitis, Crohn’s disease, diverticulitis, celiac disease, graft vs. host disease, intestinal fibrosis, colorectal stricture, gastric cancer, and colorectal cancer. We address some of the most recent and promising advancements for improvement of gastrointestinal imaging, including unique discussions of such advancements with regard to imaging of fibrosis and differentiation between similar disease processes.
Keywords: imaging, gastrointestinal tract, inflammation, fibrosis, cancer
1. Introduction
Gastrointestinal disease includes a host of pathologies, often associated with inflammation, infection, fibrosis, hemorrhage, malignancy, or some combination thereof. Gastrointestinal disease is prevalent, resulting in an estimated annual 3.8 million hospitalizations and 255,407 deaths [1], with hemorrhage being the leading cause of associated hospitalization and colorectal cancer being the leading cause of associated death [2]. Shared signs and symptomology present challenges with regard to diagnosis and definitive treatment of gastrointestinal diseases. Priorities in the development of new diagnostic modalities and techniques should therefore include improved differentiation between similar disease processes, early diagnosis, and minimizing invasiveness of diagnostic technologies, ultimately leading to improved outcomes and minimal complications. Gastrointestinal disease diagnostics rely heavily on imaging modalities to provide insight into the macroscopic structural abnormalities associated with these pathologies. Colonoscopy has supplanted barium enema as a mainstay for assessment and diagnosis of lower gastrointestinal diseases. However, it nonetheless presents several challenges including risk of complications from anesthesia and bowel perforation, especially in pediatric patients and those with co-morbidities [3], as well as issues with patient compliance [4]. This has driven increases in use of technologies such as computed tomography (CT) and magnetic resonance imaging (MRI) which have shown recent potential through greater optimization of equipment settings, increasing their effectiveness and applicability. Further advancements in molecular imaging of gastrointestinal disease provide greater diagnostic information on a cellular and molecular level to aid in clinical decision-making. Utilizing advanced imaging modalities and applications is likely to reduce subjectivity in diagnostics, reduce the risk of observing a small area not representative of the affected region of the gastrointestinal tract, and maximize safety. In this review, we address many of the recent advancements regarding imaging of the gastrointestinal tract, including novel technologies or applications which allow for fibrosis identification and differentiation between similar disease processes.
2. Inflammation
Inflammation is caused by autoimmunity or an immune response to infection, injury, irritants, and other triggers. Inflammation and infection account for approximately one quarter of cancer-causing factors, impacting pathophysiology and progression through their participation in cell growth, survival, and metastasis [5,6]. A significant challenge faced by the field of inflammatory gastrointestinal disease diagnostics is differentiation between similar disease processes, such as ulcerative colitis and Crohn’s disease. Machine learning technologies, biomarkers, and other recent advancements discussed here aim to address this. While great strides have been made in gastrointestinal inflammation diagnostics, there is tremendous room for improvement to maximize safety, specificity, and sensitivity while minimizing subjectivity in the diagnostic process.
2.1. Inflammatory Bowel Disease
Inflammatory bowel disease refers to chronic gastrointestinal inflammation, encompassing ulcerative colitis and Crohn’s disease. While IBD can occur at any age, the peak age of onset occurs from 15–30 years of age [7]. It is estimated that up to 20% of people with IBD are diagnosed during childhood [8], and the incidence and prevalence of pediatric IBD is increasing worldwide [9]. Ulcerative colitis (UC) is a chronic and often intermittent or relapsing inflammatory disease, characterized by a host of non-specific symptoms. These include nausea, fatigue, weight loss, bowel obstruction, diarrhea, and abdominal pain [10]. Endoscopic signs include bleeding, ulcerations, granularity, and abnormal vascularity, among others [11]. The etiology of UC is suspected to be a combination of heritable, environmental, and immune factors with intestinal microbiota [12] and disruption by bacterial infection [13] also potentially playing a role. Additionally, cytomegalovirus increases severity of inflammation and other symptoms in the context of UC [14,15]. Currently, UC is primarily diagnosed by clinical signs and symptoms, exclusion of infection, and endoscopy with more definitive confirmation by biopsy and histopathology [16,17].
Crohn’s disease (CD) generally presents similarly to UC, with the primary distinction being the potential presence of lesions anywhere along the alimentary canal in CD [18] as opposed to the relative localization in the colon in UC. Although, disease is localized exclusively to the colon in 25% of CD patients [19]. The transmural nature of CD is also unique, predisposing CD patients to penetrating lesions or stenosis of the bowel, and indicating benefit of cross-sectional imaging modalities such as computed tomography and magnetic resonance imaging which can better assess this transmural nature [20]. In addition, cross-sectional imaging allows for assessment of small intestine not amenable to endoscopic evaluation.
Differences in treatment standards require differentiation between UC and CD, though this is not achieved in approximately 5% of patients with chronic inflammatory bowel disease who ultimately remain classified as “indeterminate colitis” [21]. A recently developed machine learning model may allow for more definitive diagnosis through utilization of RNA sequencing to differentiate between the two disease processes [22]. The disadvantage to this technique is the biopsy-associated risk, though biopsy is generally already a component of standard of care diagnostics. Nonetheless, there is certainly potential for development of a less invasive means of diagnosis and differentiation of CD and UC which is not reliant on biopsy. Aside from the biopsy-related risks associated with anesthesia or potential for bowel perforation, there is a tremendously increased risk associated with obtaining a biopsy on patients who potentially have bowel infections or are passing large volumes of diarrhea. Novel molecular biomarkers may provide a strong alternative, relying on samples such as blood and fecal matter which are non-invasive [23], and imaging technologies may hold promise in this area as well.
2.2. Diverticulitis
Diverticulitis, diverticular inflammation, is the leading cause of colon operations and gastrointestinal-related hospitalization [24,25]. It can be classified as uncomplicated or complicated, with complicated diverticulitis being characterized by abscess, phlegmon, perforation, obstruction, or bleeding [26]. In addition, there exists a potential for fistula or stricture formation. Due to the microbial nature of diverticulitis, antibiotics are often utilized in medical management of acute complicated diverticulitis, though surgical management is also common.
Diverticulitis patients often present with persistent left lower quadrant abdominal pain or tenderness, abdominal distention, and a host of non-specific abdominal symptoms. Diagnosis of the disease is generally through clinical signs and basic laboratory testing, including a complete blood count, C-reactive protein measurement, metabolic panel, and urinalysis, with computed tomography being used to further determine disease severity [27]. Diverticulitis is frequently misdiagnosed, demanding improvement in diagnostic capabilities. A combination of biomarkers, i.e., fecal calprotectin [28] and elevated C-reactive protein levels [29], with symptomatic presentation may reduce misdiagnosis [30]. To better overcome misdiagnosis of diverticulitis and IBD, use of newly emerging imaging technologies may also be helpful.
2.3. Celiac Disease
Celiac disease is a prevalent disease which is caused by genetic determinants and triggered by gluten consumption, impacting 0.5–1% of the population globally [31]. While multifaceted, celiac disease most often presents as an immune-mediated enteropathy triggered by development of gluten peptides subsequent to gluten consumption [32]. These immune responses ultimately result in small intestinal mucosal inflammation and damage as well as malabsorption in genetically predisposed individuals [33].
While celiac disease has effective diagnostic biomarkers, tremendous gaps in effective treatment remain. Diagnosis of celiac disease is generally achieved through serological results prior to and following a change to a gluten-free diet and through duodenal biopsy [31]. However, serology may be sufficient due to its prediction accuracy and minimal invasiveness, potentially eliminating the need for biopsy [34]. Novel biomarkers identify potential informants of celiac disease in response to gluten exposure, such as inflammatory plasma cytokines, with IL-2 being the most prominent and longstanding [35,36]. Overall, diagnosis of celiac disease largely depends upon biomarker assessment, eliminating the need for imaging modality use.
2.4. Imaging Inflammation
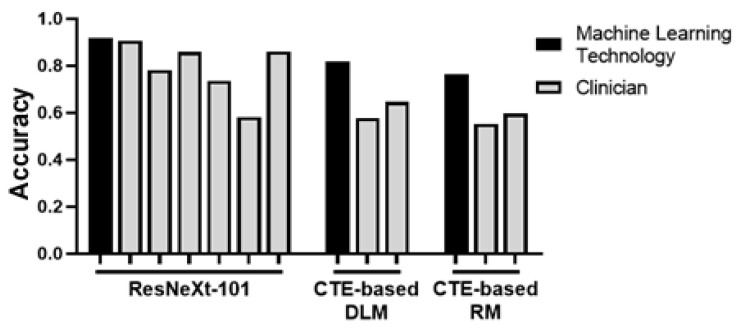
Colonoscopy is perhaps the most common and important mechanism by which inflammatory bowel disease is imaged and pathologically diagnosed [37]. However, extraordinary expertise and precision are required for proper interpretation of and differentiation between images from UC and CD patients. Recently, a convolutional neural network was established to mitigate this issue. ResNeXt-101 classified endoscopic images of CD, UC, and healthy bowel at an accuracy rate of 90.91% in per-patient analysis, superior to rates of a majority of clinicians, indicating its potential for future clinical application [38] (Table 1; Figure 1).
Table 1.
Summary of pre-existing and newly developed imaging modalities for inflammation, fibrosis, and cancer in the GI tract.
| Disease | Modality | Citation | |
|---|---|---|---|
| Inflammation | IBD | Endoscopy and Convolution Neural Network (CNN) | [38] |
| Endoscopy | [39] | ||
| Endoscopy and Deep Learning CNN | [40] | ||
| Computed Tomography (CT) Enterography | [41] | ||
| Magnetic Resonance (MR) Enterography | [42] | ||
| MR Enterography: Diffusion Weighted Imaging | [43] | ||
| Chromoendoscopy | [44] | ||
| Transabdominal Ultrasound | [45] | ||
| 18F-FDG Positron Emission Tomography (PET)/MR Enterography | [46] | ||
| Multispectral Optoacoustic Tomography (MSOT) | [47] [48] |
||
| Diverticulitis | Ultrasound | [49] | |
| Colonoscopy | [50] | ||
| Magnetic Resonance Imaging (MRI) | [51] | ||
| CT | [52] | ||
| Celiac Disease | Endoscopy | [53] | |
| CT | [54] | ||
| Ultrasound | [55] | ||
| Fibrosis | Graft versus Host Disease | 18F-FDG PET | [56] |
| MRI | [57] | ||
| 18F-FDG PET and MRI | [58] | ||
| Ultrasound | [59] | ||
| CT | [60] | ||
| Intestinal Fibrosis |
MRI: Diffusion Kurtosis Imaging | [61] | |
| CT Enterography: Radiomic Model |
[62] | ||
| CT Enterography: Deep Learning Model | [63] | ||
| Magnetization Transfer Imaging and Native T1 Mapping | [64] | ||
| Cancer | Gastric Cancer | Multidetector Row Computed Tomography | [65] |
| MRI | [66] | ||
| Endoscopic Ultrasound | [67] | ||
| 18F-FDG-PET/CT and Laparoscopy | [68] | ||
| 18F-FDG-PET/CT | [69] | ||
| Colorectal Cancer |
Colonoscopy and Sigmoidoscopy | [70] | |
| 18F-FDG-PET/CT | [71] | ||
| CT | [72] | ||
| CT + Artificial Intelligence (AI) and MRI + AI | [73] | ||
| MRI | [74] | ||
Similarly, a deep learning convolutional neural network-based diagnosis system has been developed based on endoscopic images and videos to score and predict gastrointestinal inflammatory activity [40]. However, applications of these deep learning advancements reach beyond endoscopy. Another recently developed system utilized multiple deep learning networks to classify intestinal inflammation based on micro-ultrasonography, allowing detection prior to human detectability by micro-ultrasound or endoscopy [75]. Machine learning opportunities to minimize inconsistency and remove subjectivity from the diagnosis process are becoming increasingly abundant (Figure 1).
Figure 1.
Machine learning- and clinician-derived data [38,62,63] were compiled into the depicted graph to demonstrate accuracy as measured by either percent or AUC. In each case, machine learning algorithms performed on par or better. This is anticipated to reduce human error in accurate diagnosis (original graph).
The associated hallmarks of inflammation provide several surrogates for measuring inflammatory disease processes. In addition to endoscopy, CT enterography (CTE) is often used as a means of imaging IBD to identify increased thickening of the intestinal wall [11,41]. Similar parameters can also be measured using intestinal ultrasonography [76] and magnetic resonance enterography [77]. A recent development in the use of the latter involves utilization of very small superparamagnetic iron oxide nanoparticles (VSOPs) as a contrast agent to detect intestinal inflammation and extracellular matrix composition changes. The mechanism depends on the altered abundance of glycosaminoglycans such as hyaluronic acid in response to changes to the extracellular matrix and inflammation. These factors impact VSOP binding and, thus, MRI image enhancement by VSOPs [78]. 18F-fluorodeoxyglucose (FDG)-PET/CT and ultrasonography techniques can also be used to determine molecular information about the gastrointestinal tract including protein dysregulation [79], immune cell presence [80], or biochemical activity [81] through use of targeted contrast, all suggesting future improvements in these fields.
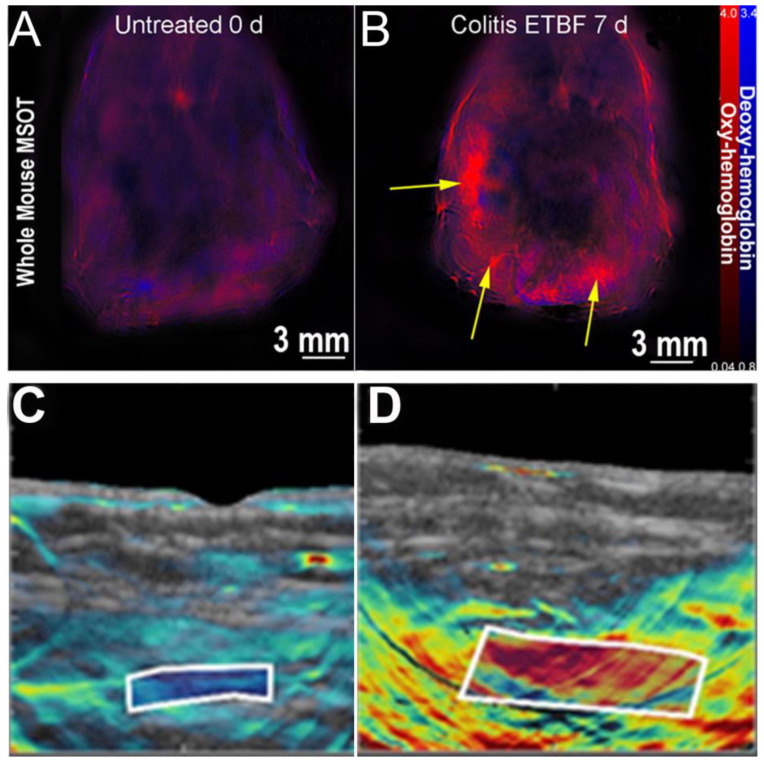
Multispectral optoacoustic tomography (MSOT) is a non-invasive imaging technology which utilizes near infrared light to reduce photon scatter compared to optical imaging modalities [82]. It thus provides increased imaging depth without the sacrifice of resolution observed in optical imaging modalities. Exogenous agents are utilized to visualize select chromophores in the NIR-II window for high-resolution imaging of ulcerative colitis [83] or microparticles within the gastrointestinal tract [84]. In addition to use of exogenous contrast agents, MSOT can provide visualization and quantification of endogenous contrast agents such as oxygenated and deoxygenated hemoglobin which serve as proxies for perfusion and, thus, inflammation [85]. MSOT has been shown to be effective in preliminary clinical and pre-clinical models of CD [48,86] and colitis [47,87] (Figure 2). While no studies to date have directly investigated the efficacy of MSOT in diverticulitis assessment, the properties and advantages of the optoacoustic effect suggest potential benefit for diagnostic application in determining diverticular inflammation.
Figure 2.
MSOT can visualize colitis (A,B) and Crohn’s disease (C,D) by utilizing differential levels of oxy- and deoxyhemoglobin. (A) Control murine model with no detectable areas of colitis. (B) Murine model treated with enterotoxigenic Bacteroides fragilis, resulting in visually concentrated areas of colitis (yellow arrows). (C) Intestinal wall of a patient in remission from Crohn’s disease, showing previous inflammation seen as deoxyhemoglobin in the white box. (D) Intestinal wall of a patient with active Crohn’s disease, showing inflammation seen as oxy-hemoglobin in the white box. Panels A and B are adapted with permission from [47]. Panels C and D adapted with permission from [86].
3. Fibrosis
Fibrosis is the accumulation of extracellular matrix components, primarily collagen, in tissues of various organ systems, often leading to organ dysfunction and increased mortality. Fibrosis generally occurs in response to inflammation, cancer, and trauma, as well as heritable diseases such as cystic fibrosis. Gastrointestinal fibrosis and stricture often play a role in diseases such as Crohn’s disease [88,89]. Meanwhile, other gastrointestinal pathologies such as graft versus host disease (GvHD) or post-endoscopic submucosal dissection stricture are almost exclusively characterized by fibrosis. Historically, gastrointestinal fibrosis has been difficult to identify. However, recent imaging advancements provide promise to improvement of diagnostics and thus patient outcomes in this area.
3.1. Graft Versus Host Disease
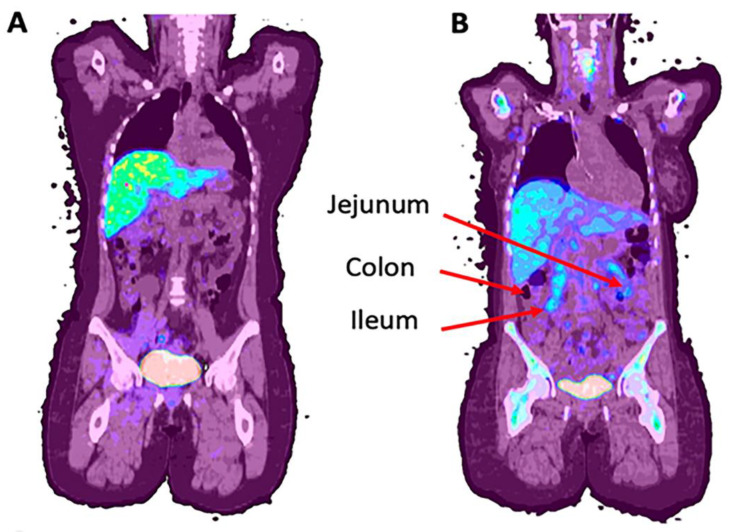
Graft versus host disease is a life threatening disease process in allogeneic hematopoietic stem cell transplantation as a result of donor T lymphocyte rejection of recipient tissue [90]. The risk of this increases in cases of greater HLA disparity between the donor and recipient, with other risk factors also having an impact [91]. GvHD is notably different from graft failure, the rejection of donor tissue by the host. Briefly, the pathophysiology of GvHD includes pre-transplant host tissue damage, followed by activation of donor T-cells and inflammatory factor release which results in amplified tissue damage [92,93]. This ultimately results in widespread inflammation and multiorgan system fibrosis [94]. One of the greatest challenges of GvHD is its nearly identical symptomatic presentation and appearance on most imaging devices to colitis. Further, the immune suppressants required for bone marrow transplantation put patients at greater risk of bacterial or viral-mediated colitis, such as cytomegalovirus-driven colitis. As both diagnoses are therefore likely and have divergent treatments, this diagnostic ambiguity can be associated with significant morbidity and mortality. A relatively new and currently accepted diagnostic approach, The Mount Sinai Acute GvHD International Consortium algorithm probability (MAP), utilizes serum biomarkers ST2 and REG3α to determine damage to intestinal crypts in the context of GvHD [95]. MAP is believed by many to be a superior prognostic indicator to previous clinical standards, Glucksberg criteria and the International Bone Marrow Transplant Registry severity index [96], which rely on patterns of organ involvement and, in the case of Glucksberg criteria, clinical performance [97,98]. However, while the MAP test may provide significant value in predicting non-relapse mortality, the acuity of the disease in many patients calls for a more rapidly and readily available means of obtaining diagnostics which can inform acute treatment. Recently, we have aimed to address this through use of 18F-fluorothymidine (18F-FLT) for GvHD assessment (Figure 3).
Figure 3.
Examples of 18F-FLT uptake in patients with (A) no GvHD and (B) jejunum and ileum uptake c/w GvHD (NCT01338987). (Original data).
3.2. Intestinal Fibrosis and Colorectal Stricture
Intestinal fibrosis, specifically including colorectal strictures, can provide an extreme degree of patient discomfort, potentially detrimental to quality of life [99]. Due to the relationship between inflammation and fibrosis, colorectal strictures are not uncommonly observed in patients with inflammatory bowel disease or diverticulitis. Strictures can also occur in response to surgical intervention or ischemic events [100]. Yet another potential cause of intestinal stricture is procedural intervention. A prime example is that which occurs in response to endoscopic submucosal dissection (ESD), known as post-ESD stricture. ESD is a relatively recent advancement in gastrointestinal neoplastic therapy. Stenosis, among other complications, occurs much more frequently in ESD relative to endoscopic mucosal resection, the comparable technique [101]. However, these risks may be outweighed by the decreased recurrence rates following ESD procedures relative to endoscopic mucosal resection [102]. Nonetheless, the fibrosis resulting from the procedure can result in strictures. ESD procedures involving circumferential ESD or subtotal dissection of ≥90% of the rectal circumference serve as an independent risk factor for strictures [103]. This calls for an efficient and non-invasive means of monitoring stenosis of post-ESD lesions, as well as gastrointestinal tissue in patients with other risk factors, to allow for medical management when appropriate. Imaging may provide that monitoring mechanism.
3.3. Imaging Fibrosis
Though the gap in adequate technologies suitable for imaging fibrosis has historically been vast, a host of recent developments address this issue. While computed tomography enterography (CTE) is not a novel imaging modality for gastrointestinal disease diagnostics, various recent developments provide potential for improving its effectiveness as CTE was not previously a reliable technology for imaging fibrosis. In 2021, a radiomic model was developed which provided significantly greater accuracy in characterizing enteric fibrosis than human radiologist capability [62] (Figure 1). However, while development of a radiomic model made great strides in providing a method of evaluating intestinal fibrosis, it has limitations which were addressed by the investigators through another machine learning-based approach. As in intestinal inflammation, recent development of a deep learning system has also provided increased accuracy and objectivity in interpretation of intestinal fibrosis. Accuracy of fibrosis severity assessment by this novel CTE-based deep learning model was greater than CTE assessment by two radiologists [63] (Figure 1). Another recent study aimed to further characterize and resolve intestinal fibrosis grading, also utilizing CTE. The authors developed a nomogram which combined clinical markers and CTE-derived mesenteric abnormality findings, resulting in a successful differentiation model between severity levels of intestinal stenosis [104].
As with CTE, MRI has long been used for gastrointestinal imaging. Benefits of MRI include the ability to observe the bowel transmurally and from various perspectives. Recent advancements have made MRI increasingly effective for imaging fibrosis. For example, magnetization transfer imaging, a contrast mechanism sensitive to intestinal collagen, and native T1 mapping, a quantitative technique capable of identifying fibrosis characteristics, have both been established as promising advancements in the field of magnetic resonance with regard to bowel fibrosis detection and differentiation [64]. Another example involves use of diffusion weighted imaging. Diffusion weighted imaging capitalizes on the fact that when tissues are inflamed, diffusion of water molecules is restricted [43]. Mapping of water molecule diffusion is utilized, as quantified by the apparent diffusion coefficient (ADC). With regard to fibrosis, the apparent diffusion coefficient has been found to be significantly correlated with histopathologically derived inflammation and fibrosis scores, as well as percent gain. Further, based on an established cutoff value, the apparent diffusion coefficient correctly distinguished fibrosis with 72% sensitivity and 94% specificity, proving its potential usefulness as a non-invasive technology contributing to fibrosis identification [105]. Building upon this is diffusion kurtosis imaging which allows for identification of tissue diffusional heterogeneity through dimensionless quantification of deviation from Gaussian behavior [106]. A study which confirmed significant ADC correlation with histologically derived inflammation grades also showed that ADC and apparent diffusional kurtosis were significantly correlated to histologically derived fibrosis grades. The authors further showed that apparent diffusional kurtosis was able to differentiate absence of fibrosis or mild fibrosis from moderate to severe fibrosis with a sensitivity of 95.9% and specificity of 78.1%, indicating its potential for beneficial application to MRI use in bowel fibrosis assessment [61]. Additionally, the aforementioned MSOT imaging technology has potential for future intestinal fibrosis diagnostics due to the capability to detect collagen as a result of exhibited optoacoustic signal [107]. Overall, relatively new technologies, as well as numerous improvements and developments building on pre-existing technologies, have potential to revolutionize the field of imaging fibrosis, improving diagnostic outcomes for countless patients.
4. Cancer
Cancer is a disease marked by the toxic over-proliferation of cells within the body. Gastric cancer and colorectal cancer are both devastating diseases with complex, multifactorial etiologies including causal influences such as diet, bacterial infection, and pre-existing medical history. The biggest prognostic indicator for cancer is the stage at diagnosis, making it imperative that the disease is identified early and that the stage of disease is precisely determined. This staging uses a combination of biomarker testing and imaging techniques.
4.1. Gastric and Colorectal Cancer
Gastric cancer ranks fifth among cancers in diagnostic prevalence and third in mortality worldwide [108]. Anatomically, gastric cancer is observed as cardia or non-cardia by location in the uppermost and more distal portions of the stomach, respectively [109]. While there are some similarities between the etiologies of cardia and non-cardia gastric cancer, there are differences as cardia risk factors include obesity and gastroesophageal reflux disease [110], while non-cardia risk factors include dietary factors, atrophic gastritis, and Helicobacter pylori infection [110,111]. Gastric cancer patients commonly present with upper abdominal pain and weight loss, as well as potential nausea, melaena, or dysphagia [112]. Biomarker testing and pathology reports are often used as diagnostic tools along with traditional imaging technologies. Despite use of multiple standard imaging techniques, in many instances poor molecular understanding and inter-reader variability result in ultimate reliance on biopsy for improved diagnostic accuracy. Current diagnostic limitations result in approximately 50% of patients presenting with advanced stages of the disease at time of diagnosis and therefore having poor prognoses [108]. As technologies and contrast agents are developed further, improvements in diagnosis and monitoring of gastric cancer should follow.
Colorectal cancer (CRC) is the third most prevalent with the second highest mortality rate of cancers worldwide [113]. While the disease is decreasing in overall incidence due to improved screening, CRC is rising in the younger patient population [114]. CRC patients often present with abdominal pain, lack of appetite, constipation, diarrhea, abdominal distention, intestinal bleeding, and a host of non-specific symptoms [115]. There are many additional non-invasive biomarker screenings which can be performed using stool samples, including the fecal immunochemical test (FIT), high-sensitivity guaiac fecal occult blood testing (gFOBT), and multitarget stool DNA (mtsDNA) tests [116]. Using biomarkers in conjunction with one another, i.e., a combination of FIT and Fusobacterium nucleatum tests, provides improved accuracy of diagnosis with regard to sensitivity and specificity [117].
More specifically, rectal cancer is defined as adenocarcinoma cases arising within 15 cm from the anal verge. Rectal cancer comprises around 30–40% of colorectal cancer cases [118]. Successful management following endoscopic and radiographic staging is achieved through a multidisciplinary approach including appropriate surgical intervention. The prognosis is directly related to tumor extent, mesorectal infiltration, and achievement of disease-free circumferential surgical margins [119].
4.2. Imaging Gastrointestinal Tract Cancers
Colonoscopy is a standard of care technology in the area of colorectal cancer (CRC) screening, primarily due to its high sensitivity and specificity for detecting cancerous and pre-cancerous lesions [120]. Colonoscopy utilizes a flexible endoscope to evaluate the entire colon and rectum. Both colorectal cancer and adenomas ≥10 mm can be detected by colonoscopy with a sensitivity of approximately 95% [121]. Sigmoidoscopy, a technology which generally utilizes a similar preparation and mechanism to colonoscopy, can be performed without anesthesia or oral bowel preparation when necessary [122], circumventing some of the limitations of colonoscopy. However, as this modality is limited by failure to evaluate the proximal colon, patients with positive sigmoidoscopy results generally will require a colonoscopy follow up [123].
In gastric cancer, endoscopic ultrasound is a preferred modality for differentiation between submucosal and mucosal lesions due to its enhanced ability to distinguish distinct layers of the gastric mucosa relative to other modalities [124]. Notably, EUS has been demonstrated to distinguish T1 and T2 from T3 and T4 gastric cancers with 86% sensitivity and 90% specificity [125] and detect N stage with an accuracy of 76.2% for staging and 88.5% for restaging, significantly higher than that of PET-CT [67]. However, despite its strong ability to distinguish between the different gastric layers, EUS does have limitations, specifically with regard to invasiveness and potential for human error.
In addition to conventional endoscopic imaging methods, MSOT has the potential to expand into an endoscopic technique through development of specialized probes as evidenced by related applications in gynecological disease [126]. MSOT not only benefits from the aforementioned advantages related to circumventing photon scatter, but this technique may offer advanced functional information compared to EUS. As stated, MSOT has shown potential for imaging diseases that include fibrosis and inflammation due to differential oxy/deoxyhemoglobin and collagen concentrations, the most viable endogenous optoacoustic contrast agents [82]. This includes GI defects such as Crohn’s disease [48] and ulcerative colitis [87]. As evidenced by MSOT imaging in dermatologic cancer [127], breast cancer [128,129,130], and thyroid cancer [131], development of specific exogenous contrast agents will facilitate application of MSOT in a clinical setting for gastrointestinal cancer imaging.
Computed tomography remains a first line imaging modality in cancer patients and others presenting with abdominal pain. This is due in large part to its availability and ability to identify a wide variety of pathological changes [28], despite risks associated with ionizing radiation [132] and requirement for adequate gastric distention [133]. Recent improvements to the spatial resolution of CT, specifically with multidetector computed tomography (MDCT), prove to be beneficial in improving diagnostic accuracy, notably evident in locoregional staging. MDCT demonstrated a 76.9% diagnostic accuracy for T staging of gastric cancer compared to 74.7% by EUS in a comparative study, though EUS outperformed MDCT in N staging accuracy [134].
The specificity of CT in detection of regional and distal lymph node metastasis can be enhanced upon integration with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG- PET). As CT detects lymph node metastasis based on anatomical abnormality, the presence of enlarged inflammatory nodes and minimally enlarged tumor-harboring lymph nodes can impair accuracy of detection [133]. Therefore, 18F-FDG PET/CT is able to further inform the diagnostic process as compared to routine CT alone. Though less sensitive than conventional CT, the increased specificity provided by 18F-FDG PET/CT has potential to reduce unnecessary interventions [69].
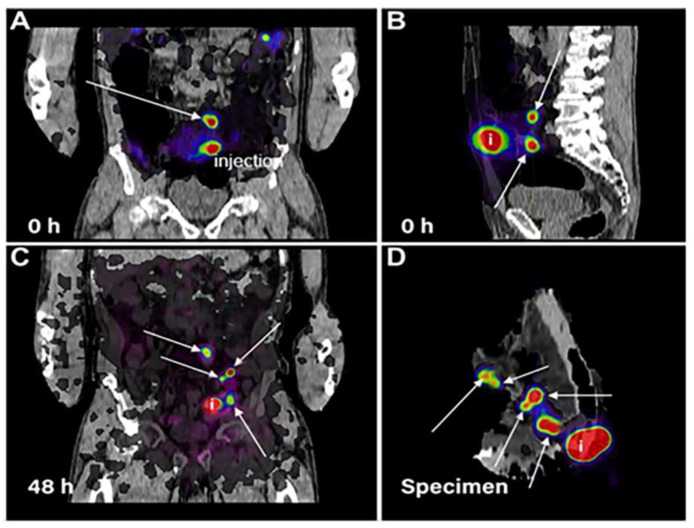
In addition to 18F-FDG PET, other PET techniques have been explored for gastric imaging. A recent alternative method for assessment of tumor-positive sentinel lymph nodes includes a colloidal solution of albumin which has been labeled with 89Zr for preferential uptake in sentinel lymph nodes, detectable with PET/CT. The study yielded clear visualization of cancerous foci, providing the surgeon with valuable information pre-operatively to ensure complete removal of cancerous lesions (Figure 4) [135].
Figure 4.
PET-CT identification of sentinel lymph nodes following injection of colloidal albumin radiolabeled with 89Zr. Arrows indicate positive disease. Top images of the frontal (A) and sagittal (B) plane immediately following injection of radiotracer show foci. By 48 h post-injection (C), lymph node foci are clearly visible, prior to surgery. Static PET-CT of the specimen following surgery corroborates pre-operative images (D). i represents location of injection. Reprinted/adapted with permission from [135].
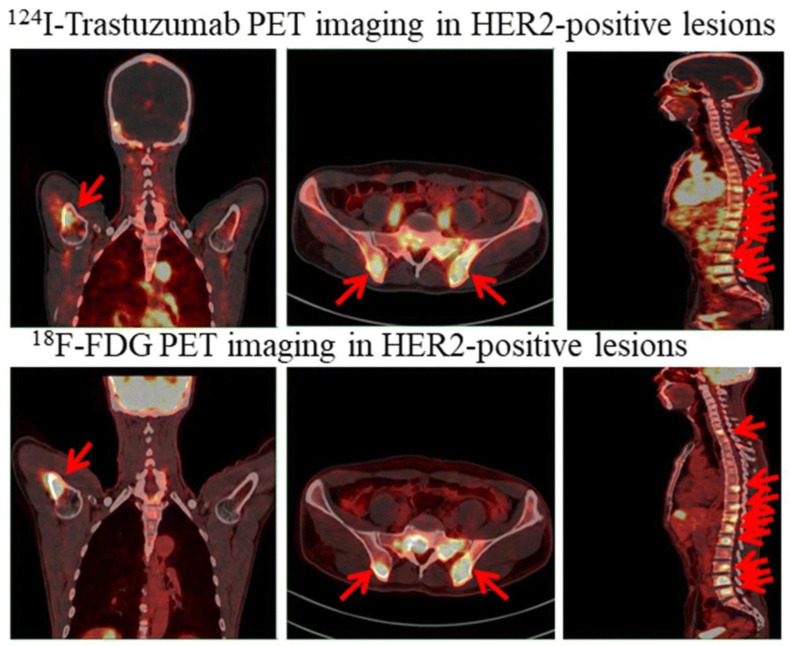
An additional example of PET imaging that differs from conventional 18F-FDG-based contrast is the use of radiolabeled I124-trastuzumab. I124-trastuzumab undergoes preferential uptake in HER2-positive tumors relative to HER2-negative ones, allowing for visualization of HER2 positivity in both primary and metastatic lesions in gastric cancer patients using PET imaging [136] (Figure 5). Yet another PET application, the aforementioned 18F-FLT imaging, has been utilized in imaging of proliferative processes, specifically in cancer and to identify repopulation in proliferative systems such as the hematopoietic and lymphocyte systems [137,138,139,140]. Touted as a major advantage over 18F-FDG, 18F-FLT does not identify predictably inflammatory events in differentiated hematopoietic cells such as neutrophils [141]. However, 18F-FLT does identify certain disease processes that have been considered inflammatory systems. Furthermore, in the explosion of therapies that modulate the immune system, including drugs such as checkpoint inhibitors, 18F-FLT imaging has been useful to identify strong lymphocyte proliferation associated with immunologic response [138,142,143]. Utilization of 18F-FLT as a modality to evaluate and monitor response of lymphocytic systems continues to expand and 18F-FLT could be utilized as an imaging biomarker of lymphocytic inflammatory response via identification of lymphocyte proliferation [144]. Overall, while PET imaging is not without its disadvantages, namely high expense and low sensitivity, it provides unique benefits to the diagnostic process [132,145].
Figure 5.
Detection of HER2-positive lesions with 124I-trastuzumab and standard 18F-FDG PET imaging in humeral, bilateral ilium, and spinal metastases. Arrows point to HER2-positive gastric cancer lesions. Reprinted/adapted with permission from Ref. [136].
With regard to rectal cancer specifically, the initial local staging after endoscopic evaluation is performed using either magnetic resonance imaging (MRI) with rectal protocol, using thin-section MRI with pelvic phased-array coil, or transrectal ultrasound (TRUS) which is also known as endorectal ultrasound [119,146]. While, overall, TRUS represents a strong staging option, it is limited by the patient’s active symptoms, the tumor characteristics and bulkiness, tumor location [147,148], and the inherent operator-dependent nature of the procedure [149]. Other modalities such as MDCT may also be used to image rectal cancer. However, although MDCT is often used in evaluation of metastatic rectal lesions, its applicability for local rectal cancer staging has been challenged due to its limited sensitivity in resolving bowel wall layers compared to other modalities. Therefore, it is considered to be “usually not appropriate” as a modality for locoregional rectal cancer staging based on the American College of Radiology (ACR) appropriateness criteria for pre-treatment staging [150].
Radiomics is an increasingly attractive artificial intelligence-based imaging application for rectal cancer in particular, and can be applied to multiple different imaging modalities [151]. Use of radiomic features on MRI images improves sensitivity and specificity to 100% and 91%, respectively, while demonstrating a positive predictive value of 72–92% and negative predictive value of 96–100% when used to assess treatment response [152,153]. Radiomics offers a non-invasive option and the ability to obtain high-quality imaging, improving upon conventional MRI use in terms of lesion characterization, detection of pre-treatment rectal cancer pathological feature biomarkers [154], and post-treatment surveillance [152].
5. Conclusions
Gastrointestinal disease, specifically with regard to its inflammatory, cancerous, and fibrotic manifestations, is a significant issue with regard to hospitalizations, patient wellbeing, financial implications, and more. The increasing prevalence of inflammatory gastrointestinal diseases such as IBD is particularly striking, as observed in both adult and pediatric patients. An ongoing clinical challenge involves refining the diagnostics of these disease processes, related to both detection and, in many instances, differentiation. This is further complicated by the co-presentation of many of these disease processes, making both development and use of these differential diagnostic mechanisms difficult. Use of biomarkers and up-and-coming applications of imaging technologies prove to be promising in mitigating this issue. In the future, the imaging technologies discussed in this review, among others, could be combined in a multimodal context to provide a better picture of a given pathology. Further, multiplexed diagnostics with these imaging technologies and biomarkers or other diagnostic methods could provide an even greater comprehensive analysis of patients’ individual disease processes.
Author Contributions
Writing—original draft preparation, K.M.H., W.M.M., J.H.-C., K.H., A.X.A., M.S., S.C., J.G.-G., and L.R.M.; data curation, K.M.H., J.H.-C., and K.W.; writing—review and editing, K.M.H., J.H.-C., G.M., S.C., J.G.-G., G.S., and L.R.M.; project administration, L.R.M.; funding acquisition, W.M.M., J.H.-C., J.G.-G., and L.R.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Cancer Institute and the National Heart, Lung, and Blood Institute, grant numbers F31CA261044, R01CA205941, R01CA212350, R01HL46668, and the United States Department of Defense W81XWH2210295.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peery A.F., Crockett S.D., Murphy C.C., Jensen E.T., Kim H.P., Egberg M.D., Lund J.L., Moon A.M., Pate V., Barnes E.L., et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2021;162:621–644. doi: 10.1053/j.gastro.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peery A.F., Crockett S.D., Barritt A.S., Dellon E.S., Eluri S., Gangarosa L.M., Jensen E.T., Lund J.L., Pasricha S., Runge T., et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731–1741.e3. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lightdale J.R., Liu Q.Y., Sahn B., Troendle D.M., Thomson M., Fishman D.S. Pediatric Endoscopy and High-risk Patients: A Clinical Report from the NASPGHAN Endoscopy Committee. J. Pediatr. Gastroenterol. Nutr. 2019;68:595–606. doi: 10.1097/MPG.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen M.J., Dhawan A., Saeed S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053–1060. doi: 10.1001/jamapediatrics.2015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata M. Inflammation and cancer. Environ. Health Prev. Med. 2018;23:1–8. doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Y., Manne S., Treem W.R., Bennett D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates from Large National Databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020;26:619–625. doi: 10.1093/ibd/izz182. [DOI] [PubMed] [Google Scholar]
- 8.Sýkora J., Pomahačová R., Kreslová M., Cvalínová D., Štych P., Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018;24:2741–2763. doi: 10.3748/wjg.v24.i25.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K.T., Ehrlich O.G., Allen J.I., Meadows P., Szigethy E.M., Henrichsen K., Kim S.C., Lawton R.C., Murphy S.M., Regueiro M., et al. The Cost of Inflammatory Bowel Disease: An Initiative from the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020;26:1–10. doi: 10.1093/ibd/izz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.-F. Ulcerative colitis. Lancet. 2016;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajendran M., Loganathan P., Jimenez G., Catinella A.P., Ng N., Umapathy C., Ziade N., Hashash J.G. A comprehensive review and update on ulcerative colitis. Dis-a-Mon. 2019;65:100851. doi: 10.1016/j.disamonth.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Roberts-Thomson I.C., Bryant R.V., Costello S.P. Uncovering the cause of ulcerative colitis. JGH Open. 2019;3:274–276. doi: 10.1002/jgh3.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki M., Klapproth J.-M.A. The Role of Bacteria in the Pathogenesis of Ulcerative Colitis. J. Signal Transduct. 2012;2012:1–6. doi: 10.1155/2012/704953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourad F.H., Hashash J.G., Kariyawasam V.C., Leong R.W. Ulcerative Colitis and Cytomegalovirus Infection: From A to Z. J. Crohn’s Colitis. 2020;14:1162–1171. doi: 10.1093/ecco-jcc/jjaa036. [DOI] [PubMed] [Google Scholar]
- 15.Wada Y., Matsui T., Matake H., Sakurai T., Yamamoto J., Kikuchi Y., Yorioka M., Tsuda S., Yao T., Yao S., et al. Intractable ulcerative colitis caused by cytomegalovirus infection: A prospective study on prevalence, diagnosis, and treatment. Dis. Colon Rectum. 2003;46 doi: 10.1097/01.DCR.0000087486.21981.C6. [DOI] [PubMed] [Google Scholar]
- 16.Feuerstein J.D., Cheifetz A.S. Ulcerative Colitis: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2014;89:1553–1563. doi: 10.1016/j.mayocp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kaenkumchorn T., Wahbeh G. Ulcerative Colitis: Making the Diagnosis. Gastroenterol. Clin. N. Am. 2020;49:655–669. doi: 10.1016/j.gtc.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Veauthier B., Hornecker J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician. 2018;98:661–669. [PubMed] [Google Scholar]
- 19.Gajendran M., Loganathan P., Catinella A.P., Hashash J.G. A comprehensive review and update on Crohn’s disease. Dis.-a-Mon. 2018;64:20–57. doi: 10.1016/j.disamonth.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Kilcoyne A., Kaplan J.L., Gee M.S. Inflammatory bowel disease imaging: Current practice and future directions. World J. Gastroenterol. 2016;22:917–932. doi: 10.3748/wjg.v22.i3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geboes K. Crohn’s disease, ulcerative colitis or indeterminate colitis—How important is it to differentiate? Acta Gastro-Enterol. Belg. 2001;64:197–200. [PubMed] [Google Scholar]
- 22.Park S.-K., Kim S., Lee G.-Y., Kim S.-Y., Kim W., Lee C.-W., Park J.-L., Choi C.-H., Kang S.-B., Kim T.-O., et al. Development of a Machine Learning Model to Distinguish between Ulcerative Colitis and Crohn’s Disease Using RNA Sequencing Data. Diagnostics. 2021;11:2365. doi: 10.3390/diagnostics11122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alghoul Z., Yang C., Merlin D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines. 2022;10:1492. doi: 10.3390/biomedicines10071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peery A.F. Management of colonic diverticulitis. BMJ. 2021;372:n72. doi: 10.1136/bmj.n72. [DOI] [PubMed] [Google Scholar]
- 25.Simianu V.V., Flum D.R. Rethinking elective colectomy for diverticulitis: A strategic approach to population health. World J. Gastroenterol. 2014;20:16609–16614. doi: 10.3748/wjg.v20.i44.16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkins T., Embry K., George R. Diagnosis and management of acute diverticulitis. Am. Fam. Physician. 2013;87:612–620. [PubMed] [Google Scholar]
- 27.Bailey J., Dattani S., Jennings A. Diverticular Disease: Rapid Evidence Review. Am. Fam. Physician. 2022;106:150–156. [PubMed] [Google Scholar]
- 28.Frickenstein A.N., Jones M.A., Behkam B., McNally L.R. Imaging Inflammation and Infection in the Gastrointestinal Tract. Int. J. Mol. Sci. 2019;21:243. doi: 10.3390/ijms21010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laméris W., Van Randen A., Van Gulik T.M., Busch O.R.C., Winkelhagen J., Bossuyt P.M.M., Stoker J., Boermeester M.A. A Clinical Decision Rule to Establish the Diagnosis of Acute Diverticulitis at the Emergency Department. Dis. Colon Rectum. 2010;53:896–904. doi: 10.1007/DCR.0b013e3181d98d86. [DOI] [PubMed] [Google Scholar]
- 30.Jones M.A., MacCuaig W.M., Frickenstein A.N., Camalan S., Gurcan M.N., Holter-Chakrabarty J., Morris K.T., McNally M.W., Booth K.K., Carter S., et al. Molecular Imaging of Inflammatory Disease. Biomedicines. 2021;9:152. doi: 10.3390/biomedicines9020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gujral N., Freeman H.J., Thomson A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012;18:6036–6059. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindfors K., Ciacci C., Kurppa K., Lundin K.E.A., Makharia G.K., Mearin M.L., Murray J.A., Verdu E.F., Kaukinen K. Coeliac disease. Nat. Rev. Dis. Prim. 2019;5:3. doi: 10.1038/s41572-018-0054-z. [DOI] [PubMed] [Google Scholar]
- 33.Villanacci V., Vanoli A., Leoncini G., Arpa G., Salviato T., Bonetti L.R., Baronchelli C., Saragoni L., Parente P. Celiac disease: Histology-differential diagnosis-complications. A practical approach. Pathologica. 2020;112:186–196. doi: 10.32074/1591-951X-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beig J., Rostami K., Hayman D.T.S., Hassan S., Gerred S., Ogra R. Is duodenal biopsy always necessary for the diagnosis of coeliac disease in adult patients with high anti-tissue transglutaminase (TTG) antibody titres? Front. Gastroenterol. 2021;13:287–294. doi: 10.1136/flgastro-2020-101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel G., Tye-Din J.A., Qiao S.-W., Russell A.K., Mayassi T., Ciszewski C., Sarna V.K., Wang S., Goldstein K.E., Dzuris J.L., et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv. 2019;5:eaaw7756. doi: 10.1126/sciadv.aaw7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarna V.K., Lundin K.E., Mørkrid L., Qiao S.-W., Sollid L.M., Christophersen A. HLA-DQ–Gluten Tetramer Blood Test Accurately Identifies Patients With and Without Celiac Disease in Absence of Gluten Consumption. Gastroenterology. 2018;154:886–896.e6. doi: 10.1053/j.gastro.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Hundorfean G., Pereira S.P., Karstensen J.G., Vilmann P., Saftoiu A. Modern Endoscopic Imaging in Diagnosis and Surveillance of Inflammatory Bowel Disease Patients. Gastroenterol. Res. Pract. 2018;2018:5738068. doi: 10.1155/2018/5738068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Chen L., Wang X., Liu K., Li T., Yu Y., Han J., Xing S., Xu J., Tian D., et al. Development of a Convolutional Neural Network-Based Colonoscopy Image Assessment Model for Differentiating Crohn’s Disease and Ulcerative Colitis. Front. Med. 2022;9:980. doi: 10.3389/fmed.2022.789862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danese S., Fiorino G., Angelucci E., Vetrano S., Pagano N., Rando G., Spinelli A., Malesci A., Repici A. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: A pilot study. World J. Gastroenterol. 2010;16:2396–2400. doi: 10.3748/wjg.v16.i19.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y., Mu R., Xu H., Xie C., Zhang Y., Liu L., Wang L., Shi H., Hu Y., Ren J., et al. Novel deep learning–based computer-aided diagnosis system for predicting inflammatory activity in ulcerative colitis. Gastrointest. Endosc. 2022 doi: 10.1016/j.gie.2022.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Baker M.E., Hara A.K., Platt J.F., Maglinte D.D.T., Fletcher J.G. CT enterography for Crohn’s disease: Optimal technique and imaging issues. Abdom. Imaging. 2015;40:938–952. doi: 10.1007/s00261-015-0357-4. [DOI] [PubMed] [Google Scholar]
- 42.Khatri G., Coleman J., Leyendecker J.R. Magnetic Resonance Enterography for Inflammatory and Noninflammatory Conditions of the Small Bowel. Radiol. Clin. N. Am. 2018;56:671–689. doi: 10.1016/j.rcl.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Kim K.-J., Lee Y., Park S.H., Kang B.-K., Seo N., Yang S.-K., Ye B.D., Park S.H., Kim S.Y., Baek S., et al. Diffusion-weighted MR Enterography for Evaluating Crohn’s Disease: How does it add diagnostically to conventional MR enterography? Inflamm. Bowel Dis. 2015;21:101–109. doi: 10.1097/MIB.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 44.Flynn A.D., Valentine J.F. Chromoendoscopy for Dysplasia Surveillance in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018;24:1440–1452. doi: 10.1093/ibd/izy043. [DOI] [PubMed] [Google Scholar]
- 45.Ziech M.L.W., Hummel T.Z., Smets A.M.J.B., Nievelstein R.A.J., Lavini C., Caan M.W., Nederveen A.J., Roelofs J.J.T.H., Bipat S., Benninga M.A., et al. Accuracy of abdominal ultrasound and MRI for detection of Crohn disease and ulcerative colitis in children. Pediatr. Radiol. 2014;44:1370–1378. doi: 10.1007/s00247-014-3010-4. [DOI] [PubMed] [Google Scholar]
- 46.Langhorst J., Umutlu L., Schaarschmidt B.M., Grueneisen J., Demircioglu A., Forsting M., Beiderwellen K., Haubold J., Theysohn J.M., Koch A.K., et al. Diagnostic Performance of Simultaneous [18F]-FDG PET/MR for Assessing Endoscopically Active Inflammation in Patients with Ulcerative Colitis: A Prospective Study. J. Clin. Med. 2020;9:2474. doi: 10.3390/jcm9082474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhutiani N., Grizzle W.E., Galandiuk S., Otali D., Dryden G.W., Egilmez N.K., McNally L.R. Noninvasive Imaging of Colitis Using Multispectral Optoacoustic Tomography. J. Nucl. Med. 2017;58:1009–1012. doi: 10.2967/jnumed.116.184705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knieling F., Neufert C., Hartmann A., Claussen J., Urich A., Egger C., Vetter M., Fischer S., Pfeifer L., Hagel A., et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s disease Activity. N. Engl. J. Med. 2017;376:1292–1294. doi: 10.1056/NEJMc1612455. [DOI] [PubMed] [Google Scholar]
- 49.Nazerian P., Gigli C., Donnarumma E., de Curtis E., Bribani A., Lanzi S., Rovida S., Magazzini S., Grifoni S., Perani C. Diagnostic Accuracy of Point-of-Care Ultrasound Integrated into Clinical Examination for Acute Diverticulitis: A Prospective Multicenter Study. Ultraschall Med. 2021;42:614–622. doi: 10.1055/a-1161-0780. [DOI] [PubMed] [Google Scholar]
- 50.Lee K.-Y., Lee J., Park Y.Y., Oh S.T. Routine colonoscopy may be needed for uncomplicated acute right colonic diverticulitis. BMC Gastroenterol. 2021;21:91. doi: 10.1186/s12876-021-01672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreyer A.G., Fürst A., Agha A., Kikinis R., Scheibl K., Schölmerich J., Feuerbach S., Herfarth H., Seitz J. Magnetic resonance imaging based colonography for diagnosis and assessment of diverticulosis and diverticulitis. Int. J. Color. Dis. 2004;19:474–480. doi: 10.1007/s00384-004-0587-3. [DOI] [PubMed] [Google Scholar]
- 52.Rotert H., Nöldge G., Encke J., Richter G.M., Düx M. The value of CT for the diagnosis of acute diverticulitis. Der Radiol. 2003;43:51–58. doi: 10.1007/s00117-002-0849-4. [DOI] [PubMed] [Google Scholar]
- 53.Cammarota G., Cuoco L., Cesaro P., Santoro L., Cazzato A., Montalto M., La Mura R., Larocca L.M., Vecchio F.M., Gasbarrini A., et al. A highly accurate method for monitoring histological recovery in patients with celiac disease on a gluten-free diet using an endoscopic approach that avoids the need for biopsy: A double-center study. Endoscopy. 2007;39:46–51. doi: 10.1055/s-2006-945044. [DOI] [PubMed] [Google Scholar]
- 54.Eid M., Abougabal A., Zeid A. Celiac disease: Do not miss that diagnosis! Egypt. J. Radiol. Nucl. Med. 2022;44:727–735. doi: 10.1016/j.ejrnm.2013.09.010. [DOI] [Google Scholar]
- 55.Fraquelli M., Colli A., Colucci A., Bardella M.T., Trovato C., Pometta R., Pagliarulo M., Conte D. Accuracy of Ultrasonography in Predicting Celiac Disease. Arch. Intern. Med. 2004;164:169–174. doi: 10.1001/archinte.164.2.169. [DOI] [PubMed] [Google Scholar]
- 56.Stelljes M., Hermann S., Albring J., Köhler G., Löffler M., Franzius C., Poremba C., Schlösser V., Volkmann S., Opitz C., et al. Clinical molecular imaging in intestinal graft-versus-host disease: Mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood. 2008;111:2909–2918. doi: 10.1182/blood-2007-10-119164. [DOI] [PubMed] [Google Scholar]
- 57.Budjan J., Michaely H.J., Attenberger U., Haneder S., Heidenreich D., Kreil S., Nolte F., Hofmann W.-K., Schoenberg S.O., Klein S.A. Assessment of acute intestinal graft versus host disease by abdominal magnetic resonance imaging at 3 Tesla. Eur. Radiol. 2014;24:1835–1844. doi: 10.1007/s00330-014-3224-8. [DOI] [PubMed] [Google Scholar]
- 58.Roll W., Schindler P., Masthoff M., Strotmann R., Albring J., Reicherts C., Weckesser M., Noto B., Stelljes M., Schäfers M., et al. 18F-FDG-PET-MRI for the assessment of acute intestinal graft-versus-host-disease (GvHD) BMC Cancer. 2021;21:1015. doi: 10.1186/s12885-021-08748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calabrese E., Zorzi F., Visconti E., De Angelis G., Cerretti R., Del Vecchio Blanco G., Picardi A., Cudillo L., Postorino M., Franceschini L., et al. Bowel ultrasonography as an aid for diagnosis of intestinal acute graft-versus-host-disease after allogeneic haematopoietic stem cell transplantation. Dig. Liver Dis. 2013;45:899–904. doi: 10.1016/j.dld.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Shimoni A., Rimon U., Hertz M., Yerushalmi R., Amitai M., Portnoy O., Guranda L., Nagler A., Apter S. CT in the clinical and prognostic evaluation of acute graft-vs-host disease of the gastrointestinal tract. Br. J. Radiol. 2012;85:e416–e423. doi: 10.1259/bjr/60038597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du J.-F., Lu B.-L., Huang S.-Y., Mao R., Zhang Z.-W., Cao Q.-H., Chen Z.-H., Li S.-Y., Qin Q.-L., Sun C.-H., et al. A novel identification system combining diffusion kurtosis imaging with conventional magnetic resonance imaging to assess intestinal strictures in patients with Crohn’s disease. Abdom. Radiol. 2020;46:936–947. doi: 10.1007/s00261-020-02765-3. [DOI] [PubMed] [Google Scholar]
- 62.Li X., Liang D., Meng J., Zhou J., Chen Z., Huang S., Lu B., Qiu Y., Baker M.E., Ye Z., et al. Development and Validation of a Novel Computed-Tomography Enterography Radiomic Approach for Characterization of Intestinal Fibrosis in Crohn’s Disease. Gastroenterology. 2021;160:2303–2316.e11. doi: 10.1053/j.gastro.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng J., Luo Z., Chen Z., Zhou J., Chen Z., Lu B., Zhang M., Wang Y., Yuan C., Shen X., et al. Intestinal fibrosis classification in patients with Crohn’s disease using CT enterography–based deep learning: Comparisons with radiomics and radiologists. Eur. Radiol. 2022:1–14. doi: 10.1007/s00330-022-08842-z. [DOI] [PubMed] [Google Scholar]
- 64.Lu B., Lin J., Du J., He S., Cao Q., Huang L., Mao R., Sun C., Li Z., Feng S., et al. Native T1 Mapping and Magnetization Transfer Imaging in Grading Bowel Fibrosis in Crohn’s Disease: A Comparative Animal Study. Biosensors. 2021;11:302. doi: 10.3390/bios11090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen B.-B., Liang P.-C., Liu K.-L., Hsiao J.-K., Huang J.-C., Wong J.-M., Lee P.-H., Shun C.-T., Ming-Tsang Y. Preoperative Diagnosis of Gastric Tumors by Three-dimensional Multidetector Row CT and Double Contrast Barium Meal Study: Correlation with Surgical and Histologic Results. J. Formos. Med. Assoc. 2007;106:943–952. doi: 10.1016/S0929-6646(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 66.Wang C.-K., Kuo Y.-T., Liu G.-C., Tsai K.-B., Huang Y.-S. Dynamic Contrast-Enhanced Subtraction and Delayed MRI of Gastric Tumors: Radiologic–Pathologic Correlation. J. Comput. Assist. Tomogr. 2000;24:872–877. doi: 10.1097/00004728-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Redondo-Cerezo E., Martínez-Cara J.G., Jiménez-Rosales R., Valverde-López F., Caballero-Mateos A.M., Jérvez-Puente P., Ariza-Fernández J.L., Úbeda-Muñoz M., López-De-Hierro M., De Teresa J. Endoscopic ultrasound in gastric cancer staging before and after neoadjuvant chemotherapy. A comparison with PET-CT in a clinical series. United Eur. Gastroenterol. J. 2017;5:641–647. doi: 10.1177/2050640616684697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gertsen E.C., Brenkman H.J.F., van Hillegersberg R., van Sandick J.W., van Berge Henegouwen M.I., Gisbertz S.S., Luyer M.D.P., Nieuwenhuijzen G.A.P., van Lanschot J.J.B., Lagarde S.M., et al. 18F-Fludeoxyglucose–Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC) JAMA Surg. 2021;156:e215340. doi: 10.1001/jamasurg.2021.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smyth E., Schöder H., Strong V.E., Capanu M., Kelsen D.P., Coit D.G., Shah M.A. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012;118:5481–5488. doi: 10.1002/cncr.27550. [DOI] [PubMed] [Google Scholar]
- 70.Ko C.W., Doria-Rose V.P., Barrett M.J., Kamineni A., Enewold L., Weiss N.S. Screening colonoscopy and flexible sigmoidoscopy for reduction of colorectal cancer incidence: A case-control study. PLoS ONE. 2019;14:e0226027. doi: 10.1371/journal.pone.0226027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutman F., Alberini J.-L., Wartski M., Vilain D., Le Stanc E., Sarandi F., Corone C., Tainturier C., Pecking A.P. Incidental Colonic Focal Lesions Detected by FDG PET/CT. Am. J. Roentgenol. 2005;185:495–500. doi: 10.2214/ajr.185.2.01850495. [DOI] [PubMed] [Google Scholar]
- 72.Callstrom M.R., Johnson C.D., Fletcher J.G., Reed J.E., Ahlquist D.A., Harmsen W.S., Tait K., Wilson L.A., Corcoran K.E. CT Colonography without Cathartic Preparation: Feasibility Study. Radiology. 2001;219:693–698. doi: 10.1148/radiology.219.3.r01jn22693. [DOI] [PubMed] [Google Scholar]
- 73.Bedrikovetski S., Dudi-Venkata N.N., Kroon H.M., Seow W., Vather R., Carneiro G., Moore J.W., Sammour T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: A systematic review and meta-analysis. BMC Cancer. 2021;21:1058. doi: 10.1186/s12885-021-08773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao S.-X., Zeng M.S., Xu J.M., Qin X.Y., Chen C.Z., Li R.C., Hou Y.Y. Assessment of T staging and mesorectal fascia status using high-resolution MRI in rectal cancer with rectal distention. World J. Gastroenterol. 2007;13:4141–4146. doi: 10.3748/wjg.v13.i30.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang S., Lemke C., Cox B.F., Newton I.P., Nathke I., Cochran S. A Learning-Based Microultrasound System for the Detection of Inflammation of the Gastrointestinal Tract. IEEE Trans. Med. Imaging. 2021;40:38–47. doi: 10.1109/TMI.2020.3021560. [DOI] [PubMed] [Google Scholar]
- 76.Lalosevic M.S., Milutinovic A.S., Zaric V.M., Lolic I., Toplicanin A., Dragasevic S., Stojkovic M., Stojanovic M., Aleksic M., Stjepanovic M., et al. Intestinal Ultrasonography as a Tool for Monitoring Disease Activity in Patients with Ulcerative Colitis. Int. J. Clin. Pract. 2022;2022:1–6. doi: 10.1155/2022/3339866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biernacka K.B., Barańska D., Matera K., Podgórski M., Czkwianianc E., Szabelska-Zakrzewska K., Dziembowska I., Grzelak P. The value of magnetic resonance enterography in diagnostic difficulties associated with Crohn’s disease. Pol. J. Radiol. 2021;86:143–150. doi: 10.5114/pjr.2021.104581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golusda L., Kühl A.A., Lehmann M., Dahlke K., Mueller S., Boehm-Sturm P., Saatz J., Traub H., Schnorr J., Freise C., et al. Visualization of Inflammation in Experimental Colitis by Magnetic Resonance Imaging Using Very Small Superparamagnetic Iron Oxide Particles. Front. Physiol. 2022;13:1369. doi: 10.3389/fphys.2022.862212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H., Machtaler S., Bettinger T., Lutz A.M., Luong R., Bussat P., Gambhir S.S., Tranquart F., Tian L., Willmann J.K. Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin–targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology. 2013;267:818–829. doi: 10.1148/radiol.13122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aarntzen E.H., Hermsen R., Drenth J.P., Boerman O.C., Oyen W.J. 99mTc-CXCL8 SPECT to Monitor Disease Activity in Inflammatory Bowel Disease. J. Nucl. Med. 2015;57:398–403. doi: 10.2967/jnumed.115.165795. [DOI] [PubMed] [Google Scholar]
- 81.Dmochowska N., Tieu W., Keller M.D., Wardill H.R., Mavrangelos C., Campaniello M.A., Takhar P., Hughes P.A. Immuno-PET of Innate Immune Markers CD11b and IL-1β Detects Inflammation in Murine Colitis. J. Nucl. Med. 2019;60:858–863. doi: 10.2967/jnumed.118.219287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacCUAIG W.M., Jones M.A., Abeyakoon O., McNally L.R. Development of Multispectral Optoacoustic Tomography as a Clinically Translatable Modality for Cancer Imaging. Radiol. Imaging Cancer. 2020;2:e200066. doi: 10.1148/rycan.2020200066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun L., Ouyang J., Zeng F., Wu S. An AIEgen-based oral-administration nanosystem for detection and therapy of ulcerative colitis via 3D-MSOT/NIR-II fluorescent imaging and inhibiting NLRP3 inflammasome. Biomaterials. 2022;283:121468. doi: 10.1016/j.biomaterials.2022.121468. [DOI] [PubMed] [Google Scholar]
- 84.Bhutiani N., Samykutty A., McMasters K.M., Egilmez N.K., McNally L.R. In vivo tracking of orally-administered particles within the gastrointestinal tract of murine models using multispectral optoacoustic tomography. Photoacoustics. 2018;13:46–52. doi: 10.1016/j.pacs.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNally L.R., Mezera M., Morgan D.E., Frederick P.J., Yang E.S., Eltoum I.-E., Grizzle W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016;22:3432–3439. doi: 10.1158/1078-0432.CCR-16-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waldner M.J., Knieling F., Egger C., Morscher S., Claussen J., Vetter M., Kielisch C., Fischer S., Pfeifer L., Hagel A., et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology. 2016;151:238–240. doi: 10.1053/j.gastro.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 87.Knieling F., Hartmann A., Claussen J., Urich A., Atreya R., Rascher W., Waldner M. Multispectral Optoacoustic Tomography in Ulcerative Colitis—A First-in-human Diagnostic Clinical Trial. J. Nucl. Med. 2017;58:1196. [Google Scholar]
- 88.Rieder F., Bettenworth D., Ma C., Parker C.E., Williamson L.A., Nelson S.A., Van Assche G., Di Sabatino A., Bouhnik Y., Stidham R.W., et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment. Pharmacol. Ther. 2018;48:347–357. doi: 10.1111/apt.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alfredsson J., Wick M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020;92:e12990. doi: 10.1111/sji.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeiser R., Blazar B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017;377:2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeiser R., Blazar B.R., Longo D.L. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017;377:2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 92.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol. Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 93.Ferrara J.L., Reddy P. Pathophysiology of Graft-Versus-Host Disease. Semin. Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Socié G. Treating chronic GVHD-induced fibrosis? Blood. 2018;131:1396–1397. doi: 10.1182/blood-2018-02-830505. [DOI] [PubMed] [Google Scholar]
- 95.Gergoudis S.C., DeFilipp Z., Özbek U., Sandhu K.S., Etra A.M., Choe H.K., Kitko C.L., Ayuk F., Aziz M., Baez J., et al. Biomarker-guided preemption of steroid-refractory graft-versus-host disease with α-1-antitrypsin. Blood Adv. 2020;4:6098–6105. doi: 10.1182/bloodadvances.2020003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Srinagesh H.K., Ferrara J.L. MAGIC biomarkers of acute graft-versus-host disease: Biology and clinical application. Best Pract. Res. Clin. Haematol. 2019;32:101111. doi: 10.1016/j.beha.2019.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowlings P.A., Przepiorka D., Klein J.P., Gale R.P., Passweg J.R., Henslee-Downey P.J., Cahn J., Calderwood S., Gratwohl A., Socié G., et al. IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br. J. Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 98.Martino R., Romero P., Subirá M., Bellido M., Altés A., Sureda A., Brunet S., Badell I., Cubells J., Sierra J. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24:283–287. doi: 10.1038/sj.bmt.1701899. [DOI] [PubMed] [Google Scholar]
- 99.Axelrad J.E., Faye A., Slaughter J.C., Harpaz N., Itzkowitz S.H., Shah S.C. Colorectal Strictures in Patients with Inflammatory Bowel Disease Do Not Independently Predict Colorectal Neoplasia. Inflamm. Bowel Dis. 2021;28:855–861. doi: 10.1093/ibd/izab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon Y.H., Jeon S.W., Lee Y.K. Endoscopic Management of Refractory Benign Colorectal Strictures. Clin. Endosc. 2013;46:472–475. doi: 10.5946/ce.2013.46.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y., He S., Zhang Y., Dou L., Liu X., Yu X., Lu N., Xue L., Wang G. Comparing long-term outcomes between endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) for type II esophagogastric junction neoplasm. Ann. Transl. Med. 2021;9:322. doi: 10.21037/atm-20-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang J., Zhang X.H., Ge J., Yang C.M., Liu J.Y., Zhao S.L. Endoscopic submucosal dissection vs. endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J. Gastroenterol. 2014;20:8282–8287. doi: 10.3748/wjg.v20.i25.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohara Y., Toyonaga T., Tanaka S., Ishida T., Hoshi N., Yoshizaki T., Kawara F., Lui K.L., Tepmalai K., Damrongmanee A., et al. Risk of stricture after endoscopic submucosal dissection for large rectal neoplasms. Endoscopy. 2016;48:62–70. doi: 10.1055/s-0034-1392514. [DOI] [PubMed] [Google Scholar]
- 104.Meng J., Mao Y., Zhou J., Chen Z., Huang S., Wang Y., Huang L., Zhang R., Shen X., Lv W., et al. Mesenteric abnormalities play an important role in grading intestinal fibrosis in patients with Crohn’s disease: A computed tomography and clinical marker-based nomogram. Ther. Adv. Gastroenterol. 2022;15 doi: 10.1177/17562848221122504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caruso A., Angriman I., Scarpa M., D’Incà R., Mescoli C., Rudatis M., Sturniolo G.C., Schifano G., Lacognata C. Diffusion-weighted magnetic resonance for assessing fibrosis in Crohn’s disease. Abdom. Radiol. 2020;45:2327–2335. doi: 10.1007/s00261-019-02167-0. [DOI] [PubMed] [Google Scholar]
- 106.Jensen J.H., Helpern J.A., Ramani A., Lu H., Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- 107.Regensburger A.P., Fonteyne L.M., Jüngert J., Wagner A.L., Gerhalter T., Nagel A.M., Heiss R., Flenkenthaler F., Qurashi M., Neurath M.F., et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019;25:1905–1915. doi: 10.1038/s41591-019-0669-y. [DOI] [PubMed] [Google Scholar]
- 108.Ajani J.A., D’Amico T.A., Bentrem D.J., Chao J., Cooke D., Corvera C., Das P., Enzinger P.C., Enzler T., Fanta P., et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 109.Mukaisho K.-I., Nakayama T., Hagiwara T., Hattori T., Sugihara H. Two distinct etiologies of gastric cardia adenocarcinoma: Interactions among pH, Helicobacter pylori, and bile acids. Front. Microbiol. 2015;6:412. doi: 10.3389/fmicb.2015.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansen S., Vollset S.E., Derakhshan M., Fyfe V., Melby K.K., Aase S., Jellum E., McColl K.E.L. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918–925. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thrumurthy S.G., Chaudry M.A., Hochhauser D., Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 113.Lotfollahzadeh S., Recio-Boiles A., Cagir B. Colon Cancer. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 114.Thanikachalam K., Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11:164. doi: 10.3390/nu11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holtedahl K., Borgquist L., Donker G.A., Buntinx F., Weller D., Campbell C., Månsson J., Hammersley V., Braaten T., Parajuli R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021;22:1–13. doi: 10.1186/s12875-021-01452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaukat A., Kahi C.J., Burke C.A., Rabeneck L., Sauer B.G., Rex D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 117.Wong S.H., Kwong T.N.Y., Chow T.-C., Luk A.K.C., Dai R.Z.W., Nakatsu G., Lam T.Y.T., Zhang L., Wu J.C.Y., Chan F.K.L., et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66:1441–1448. doi: 10.1136/gutjnl-2016-312766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayne D., Brown R., McCormack M., Quinn M., Payne H., Babb P. Current Trends in Colorectal Cancer: Site, Incidence, Mortality and Survival in England and Wales. Clin. Oncol. 2001;13:448–452. doi: 10.1053/clon.2001.9311. [DOI] [PubMed] [Google Scholar]
- 119.Horvat N., Carlos Tavares Rocha C., Clemente Oliveira B., Petkovska I., Gollub M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39:367–387. doi: 10.1148/rg.2019180114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Issa I.A., Noureddine M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017;23:5086–5096. doi: 10.3748/wjg.v23.i28.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Knudsen A.B., Rutter C.M., Peterse E.F.P., Lietz A.P., Seguin C.L., Meester R.G.S., Perdue L.A., Lin J.S., Siegel R.L., Doria-Rose V.P., et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325:1998–2011. doi: 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wright A.P., Piper M.S., Bishu S., Stidham R.W. Systematic review and case series: Flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Aliment. Pharmacol. Ther. 2019;49:1474–1483. doi: 10.1111/apt.15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin B., Lieberman D.A., McFarland B., Andrews K.S., Brooks D., Bond J., Dash C., Giardiello F.M., Glick S., Johnson D., et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008;58:160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 124.Mehmedovi A., Mesihovi R., Saray A., Vanis N. Gastric Cancer Staging: EUS And CT. Med. Arch. 2014;68:34–36. doi: 10.5455/medarh.2014.68.34-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mocellin S., Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst. Rev. 2015;2015:CD009944. doi: 10.1002/14651858.CD009944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basij M., Yan Y., Alshahrani S.S., Helmi H., Burton T.K., Burmeister J.W., Dominello M.M., Winer I.S., Mehrmohammadi M. Miniaturized phased-array ultrasound and photoacoustic endoscopic imaging system. Photoacoustics. 2019;15:100139. doi: 10.1016/j.pacs.2019.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chuah S.Y., Attia A.B.E., Long V., Ho C.J.H., Malempati P., Fu C.Y., Ford S.J., Lee J.S.S., Tan W.P., Razansky D., et al. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Ski. Res. Technol. 2016;23:221–226. doi: 10.1111/srt.12326. [DOI] [PubMed] [Google Scholar]
- 128.Diot G., Metz S., Noske A., Liapis E., Schroeder B., Ovsepian S.V., Meier R., Rummeny E., Ntziachristos V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017;23:6912–6922. doi: 10.1158/1078-0432.CCR-16-3200. [DOI] [PubMed] [Google Scholar]
- 129.Neuschler E.I., Butler R., Young C.A., Barke L.D., Bertrand M.L., Böhm-Vélez M., Destounis S., Donlan P., Grobmyer S.R., Katzen J., et al. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology. 2018;287:398–412. doi: 10.1148/radiol.2017172228. [DOI] [PubMed] [Google Scholar]
- 130.Menezes G.L.G., Pijnappel R.M., Meeuwis C., Bisschops R., Veltman J., Lavin P.T., van de Vijver M., Mann R. Downgrading of Breast Masses Suspicious for Cancer by Using Optoacoustic Breast Imaging. Radiology. 2018;288:355–365. doi: 10.1148/radiol.2018170500. [DOI] [PubMed] [Google Scholar]
- 131.Krönke M., Karlas A., Fasoula N., Markwardt N., Kallmayer M., Eckstein H., Scheidhauer K., Weber W., Ntziachristos V. Multispectral Optoacoustic Tomography (MSOT): A Novel Label-Free Imaging Technique for Thyroid Imaging. Nuklearmedizin. 2019;58:142–143. doi: 10.1055/s-0039-1683583. [DOI] [Google Scholar]
- 132.Borggreve A.S., Goense L., Brenkman H.J., Mook S., Meijer G.J., Wessels F.J., Verheij M., Jansen E.P., Van Hillegersberg R., Van Rossum P.S., et al. Imaging strategies in the management of gastric cancer: Current role and future potential of MRI. Br. J. Radiol. 2019;92:20181044. doi: 10.1259/bjr.20181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tham E., Sestito M., Markovich B., Garland-Kledzik M. Current and future imaging modalities in gastric cancer. J. Surg. Oncol. 2022;125:1123–1134. doi: 10.1002/jso.26875. [DOI] [PubMed] [Google Scholar]
- 134.Hwang S.W., Lee D.H., Lee S.H., Park Y.S., Hwang J.H., Kim J.W., Jung S.H., Kim N.Y., Kim Y.H., Lee K.H., et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J. Gastroenterol. Hepatol. 2010;25:512–518. doi: 10.1111/j.1440-1746.2009.06106.x. [DOI] [PubMed] [Google Scholar]
- 135.Ankersmit M., Hoekstra O.S., van Lingen A., Bloemena E., Jacobs M.A.J.M., Vugts D.J., Bonjer H.J., van Dongen G.A.M.S., Meijerink W.J.H.J. Perioperative PET/CT lymphoscintigraphy and fluorescent real-time imaging for sentinel lymph node mapping in early staged colon cancer. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1495–1505. doi: 10.1007/s00259-019-04284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo X., Zhou N., Chen Z., Liu T., Xu X., Lei X., Shen L., Gao J., Yang Z., Zhu H. Construction of 124I-trastuzumab for noninvasive PET imaging of HER2 expression: From patient-derived xenograft models to gastric cancer patients. Gastric Cancer. 2020;23:614–626. doi: 10.1007/s10120-019-01035-6. [DOI] [PubMed] [Google Scholar]
- 137.Williams K.M., Holter-Chakrabarty J., Lindenberg L., Duong Q., Vesely S.K., Nguyen C.T., Havlicek J.P., Kurdziel K., Gea-Banacloche J., Lin F.I., et al. Imaging of subclinical haemopoiesis after stem-cell transplantation in patients with haematological malignancies: A prospective pilot study. Lancet Haematol. 2018;5:e44–e52. doi: 10.1016/S2352-3026(17)30215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ribas A., Benz M.R., Allen-Auerbach M.S., Radu C., Chmielowski B., Seja E., Williams J.L., Gomez-Navarro J., McCarthy T., Czernin J. Imaging of CTLA4 Blockade–Induced Cell Replication with 18F-FLT PET in Patients with Advanced Melanoma Treated with Tremelimumab. J. Nucl. Med. 2010;51:340–346. doi: 10.2967/jnumed.109.070946. [DOI] [PubMed] [Google Scholar]
- 139.Williams K.M., Chakrabarty J.H. Imaging haemopoietic stem cells and microenvironment dynamics through transplantation. Lancet Haematol. 2020;7:e259–e269. doi: 10.1016/S2352-3026(20)30003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iravani A., Hofman M., Hicks R. Clinical impact of haematopoiesis imaging with 18F-Fluorothymidine (FLT) PET/CT in patients with hematologic disorders or bone marrow compartment involvement. J. Nucl. Med. 2017;58:186. [Google Scholar]
- 141.Shields A.F., Grierson J.R., Dohmen B.M., Machulla H.-J., Stayanoff J.C., Lawhorn-Crews J.M., Obradovich J.E., Muzik O., Mangner T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 142.Volpe A., Adusumilli P.S., Schöder H., Ponomarev V. Imaging cellular immunotherapies and immune cell biomarkers: From preclinical studies to patients. J. Immunother. Cancer. 2022;10:e004902. doi: 10.1136/jitc-2022-004902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yeh R., Trager M.H., Rizk E.M., Finkel G.G., Barker L.W., Carvajal R.D., Geskin L.J., Schwartz G.K., Schwartz L., Dercle L., et al. FLT-PET At 6 Weeks Predicts Response Assessed by CT at 12 Weeks in Melanoma Patients Treated with Pembrolizumab. Clin. Nucl. Med. 2020;45:267–275. doi: 10.1097/RLU.0000000000002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aarntzen E.H.J.G., Srinivas M., De Wilt J.H.W., Jacobs J.F.M., Lesterhuis W.J., Windhorst A.D., Troost E.G., Bonenkamp J.J., van Rossum M.M., Blokx W.A.M., et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3′-fluoro-3′-deoxy-thymidine ([18F]FLT) PET imaging. Proc. Natl. Acad. Sci. USA. 2011;108:18396–18399. doi: 10.1073/pnas.1113045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaneko Y., Murray W.K., Link E., Hicks R.J., Duong C. Improving Patient Selection for 18F-FDG PET Scanning in the Staging of Gastric Cancer. J. Nucl. Med. 2015;56:523–529. doi: 10.2967/jnumed.114.150946. [DOI] [PubMed] [Google Scholar]
- 146.Jhaveri K.S., Hosseini-Nik H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. Am. J. Roentgenol. 2015;205:W42–W55. doi: 10.2214/AJR.14.14201. [DOI] [PubMed] [Google Scholar]
- 147.Ren Y., Ye J., Wang Y., Xiong W., Xu J., He Y., Cai S., Tan M., Yuan Y. The Optimal Application of Transrectal Ultrasound in Staging of Rectal Cancer Following Neoadjuvant Therapy: A Pragmatic Study for Accuracy Investigation. J. Cancer. 2018;9:784–791. doi: 10.7150/jca.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Samee A., Selvasekar C.R. Current trends in staging rectal cancer. World J. Gastroenterol. 2011;17:828–834. doi: 10.3748/wjg.v17.i7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cote A., Graur F., Lebovici A., Mois E., Al Hajjar N., Mare C., Badea R., Iancu C. The accuracy of endorectal ultrasonography in rectal cancer staging. Clujul Med. 2015;88:348–356. doi: 10.15386/cjmed-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fowler K.J., Kaur H., Cash B.D., Feig B.W., Gage K.L., Garcia E.M., Hara A.K., Herman J.M., Kim D.H., Lambert D.L., et al. ACR Appropriateness Criteria ® Pretreatment Staging of Colorectal Cancer. J. Am. Coll. Radiol. 2017;14:S234–S244. doi: 10.1016/j.jacr.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 151.Stanzione A., Verde F., Romeo V., Boccadifuoco F., Mainenti P.P., Maurea S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J. Gastroenterol. 2021;27:5306–5321. doi: 10.3748/wjg.v27.i32.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horvat N., Veeraraghavan H., Khan M., Blazic I., Zheng J., Capanu M., Sala E., Garcia-Aguilar J., Gollub M.J., Petkovska I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology. 2018;287:833–843. doi: 10.1148/radiol.2018172300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Z., Zhang X.-Y., Shi Y.-J., Wang L., Zhu H.-T., Tang Z., Wang S., Li X.-T., Tian J., Sun Y.-S. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Cancer Res. 2017;23:7253–7262. doi: 10.1158/1078-0432.CCR-17-1038. [DOI] [PubMed] [Google Scholar]
- 154.Ma X., Shen F., Jia Y., Xia Y., Li Q., Lu J. MRI-based radiomics of rectal cancer: Preoperative assessment of the pathological features. BMC Med. Imaging. 2019;19:86. doi: 10.1186/s12880-019-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.