Abstract
The aim of this research was to characterize differences and sources of volatile flavor compounds by using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) and principal component analysis (PCA). Three sweet cherry fruits from different cultivars (cv. Tie, Van, and Lap) and their wines that were produced by the same yeast were detected. The results showed that 27 flavor compounds were identified in cherry fruits, including 10 alcohols, 7 esters, 7 aldehydes, 2 ketones, and 1 organic acid. Twenty-three flavor compounds were identified in cherry wines, including nine esters, eight alcohols, three aldehydes, two organic acids, and one ketone. In cherry fruits, aldehydes, several alcohols, and one ketone were the most prevalent in cv. Tie, and the majority of esters and alcohols in cv. Van. After fermentation, ethanol, butanol, butanal, ethyl propionate, propionaldehyde, 3-hydroxy-2-butanone, and acetic acid increased, whereas 1-hexanol, 3-methyl-3-buten-1-ol, 1-penten-3-ol, ethyl acetate, methyl acetate, (E)-2-hexenal and hexanal decreased. Few differences were detected in the type and content of volatile compounds in cherry wines from cv. Tieton (WT) and cv. Van (WV). Almost all aldehydes are derived from cherry fruits, which cannot be produced during wine-making, and other volatile compounds are almost all produced by saccharomyces cerevisiae. The volatile compounds of cherry wines were determined by row materials and fermentation cultures. Flavor fingerprints were established by HS-GC-IMS and PCA, which provided a theoretical foundation for the evaluation and improvement of flavor quality in cherry wine-making.
Keywords: cherry, cherry wine, volatile compound, HS-GC-IMS, PCA
1. Introduction
Sweet cherry (Prunus avium L.) is popular and well favorite by consumers due to its bright color, particular odor, pleasant taste, and nutritional ingredients [1]. It contains high nutrients and bioactive compounds, such as carbohydrates, amino acids, vitamins, phenolics, organic acids, flavonoids, anthocyanidin, and mineral substances, among which cherry flavonoids show high antioxidant capacity [2,3,4,5,6]. Furthermore, cherries could promote the endogenous production of melatonin and establish high-quality sleep [7]. However, due to its high moisture and sugar content, cherry is a very perishable fruit, which is usually processed into juices and cherry wines to ensure the supply of cherry derivatives throughout the year [8]. Different production processes of fruit wines will also lead to different volatile components. For example, fermentation is a traditional food processing technology that could extend preserving shelf life [9], and it could also promote quality through sensory features and potential health-promoting effects [10].
Flavor is a crucial sensory property of fruits and their fermented drinks, which is still the primary selection criteria for consumers. The cultivars of raw materials may have a significant influence on fruit wines, including sensory properties. Physicochemical characteristics such as weight, dimensions, color, pH, texture, soluble solids content (SSC), and titratable acidity of different cherry cultivars may affect food quality and sensory properties [11,12].
Thereinto, aldehydes, alcohols, and esters were the key volatile compounds in cherry fruits [13,14]. It was found that (E)-2-hexenal, hexanal, benzaldehyde, ethanol, 1-hexanol, benzyl alcohol, ethyl acetate, and ethyl caproate were the key volatile compounds [15,16]. Aldehydes existed throughout the growth process; the contents increased quickly in the color stage and decreased in ripening as to the phytohormone [17]. In contrast, the content of alcohols and esters is raised during the maturation of cherry [18]. The volatile compounds were detected by using gas chromatography-mass spectrometry (GC-MS), and it was found that alcohols and esters were the primary volatile group in cherry wines [19,20,21]. However, there are few reports about differences and sources of volatile flavor compounds of different cultivars, and the source mechanism is still not clear in cherry wines.
This far, there are several ways to measure volatile compounds by using various techniques to enhance and distinguish the characteristics of cherry wines, such as GC-MS, GC-O (Gas chromatography-olfactometry), and coupled with other means [16,19,20]. GC-MS is the most applied tool to differentiate cherries and wines in fruit development, raw materials, cultures, and processing methods [18,19,20,21]. GC-IMS could separate volatile compounds in two dimensions with high separation efficiency with less time and cost compared with GC-MS [22]. IMS could operate without high pressure, and HS means that the samples need no pretreatment. With the advantage of less analysis time, a lower limit of detectability, good separability, and repeatability, the HS-GC-IMS technique could be a powerful way to analyze volatile compounds in multiple samples [23,24,25]. PCA is a key technique to distinguish the differences between multiple samples by summarizing into much fewer variables which are a weighted average of the original variables. The data mining technique and multivariate linear transformation are used to expound the main contributors and analyze the differences in aroma, sensory, and taste properties [26,27,28].
In this research, the volatile flavor of three cherry fruits and their wines were detected. An effective flavor evaluation method was established to characterize the difference and source of volatile flavor compounds in cherry wines by HS-GC-IMS and PCA. As exploratory research, this work may promote the application and quality improvement for the making of cherry alcohol beverages.
2. Materials and Methods
2.1. Materials
In this research, cherry fruits of three different cultivars were collected (May 2021) from Ai Ying Wei ecological manor in Rizhao city (Shandong, China). Commercial active dry wine yeast BV 818 was purchased from Angel Yeast Co., Ltd. (Yichang, China) and Pectinex Ultra SP-L from Novozymes (1000 U/g, China) Biotechnology Co., Ltd. (Tianjin, China). Sucrose was purchased from Rizhao Lingyunhai Sugar Co., Ltd. (Rizhao, China).
2.2. Fermentation Process of Cherry Wines
Cherry fruits of different cultivars were selected as the main material. Cherry was washed, manually removed the peduncle, destemmed, and crushed. Pectin was degraded by pectinase (0.5 mL/L), then about 13% of sucrose (m/m) was added to the cherry pulp. The SSC could attain 25 (°Brix). Commercial yeast (300 mg/L) was inoculated for fermentation promoter, and alcohol fermentation process was implemented at 23 ± 1 °C for about 160 h throughout static fermentation. During fermentation, every fermented broth was stirred twice or thrice a day to keep the pulp uniformity. After fermentation, all resulting wines were separated with centrifugalization at 2808× g for 10 min (room temperature) to terminate the fermentation.
2.3. HS-GC-IMS Analytical Conditions
Analyses of volatile compounds were performed with an automatic HS sampling unit (CTC Analytics AG, Zwingen, Switzerland). GC unit (Agilent 490, Agilent Technologies, Palo Alto, CA, USA) and IMS instrument (FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) were combined. The conditions of the automatic sampler and GC-IMS are shown in Table 1.
Table 1.
Analysis conditions for cherry fruits and their wines by HS-GC-IMS.
| Gas Phase-Ion Mobility Spectrometry Unit | |
|---|---|
| Analysis time | 30 min |
| Column type | MXT-5, 15 m, 0.53 mm ID, 1 μm FT |
| Column temperature | 60 °C |
| Carrier gas/drift gas | N2 |
| IMS temperature | 45 °C |
| Automatic Headspace Sampling Unit | |
| Injection volume | 500 μL |
| Incubation time | 20 min |
| Incubation temperature | 40 °C |
| Syringe temperature | 85 °C |
| Incubation speed | 500 rpm |
2.4. Statistical Analysis
The identification of the volatile compound was according to the retention index (RI) and drift time (RIP relative) in the GC-IMS library. The spectrogram was analyzed by its accompanied software, including VOCal and three plugins (Reporter, Gallery Plot, and Dynamic PCA plugins). By using the topographic plots, Reporter plugin can analyze the spectral differences in different samples. The fingerprint comparison was shown by the Gallery plot plugin of cherry fruits and their wines derived from three cultivars. The data were processed with the peak intensity of detected volatile compounds by using the dynamic PCA plugin. The PCA data matrix of cherry fruits had 42 columns (names of volatile compounds) and nine rows (three samples with three replicates). In cherry wines, there were 35 columns and nine rows. Then, the peak intensity was scaled and mapped to appropriate intervals from the matrix automatically. The principal components were determined, and the corresponding contribution rate calculations were performed. Finally, scores and loading plots were drawn to detect the regularity and difference between cherry fruits and their wines. All the samples were tested in triplicate.
3. Results and Discussion
3.1. Characterization of Sweet Cherry Fruit from Three Cultivars
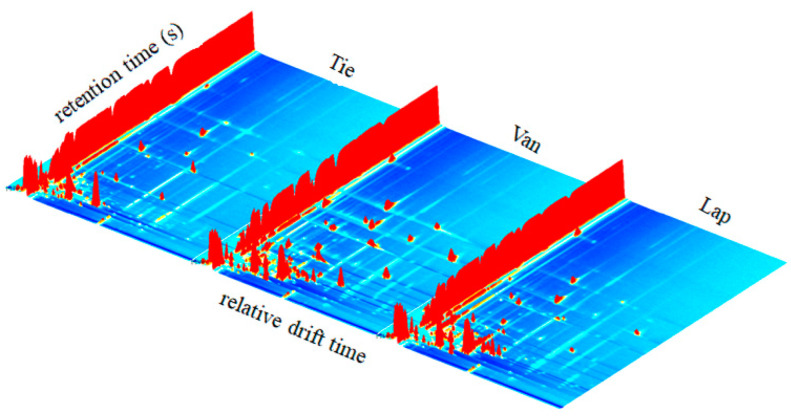
As shown in Figure 1, volatile compounds intuitively differ in three sweet cherry cultivars. The three-dimensional (3D) respectively represented the ion drift time, gas phase retention time, and peak intensity. Each peak signal represented one type of volatile compound. The results demonstrated visualized differences in different cultivars, which also inferred differences in their wines.
Figure 1.
Differences in the three-dimensional (3D)-topographic of volatile compounds in cherry fruits from three cultivars (Tie: cherry cultivar Tieton; Van: cherry cultivar Van; Lap: cherry cultivar Lapins).
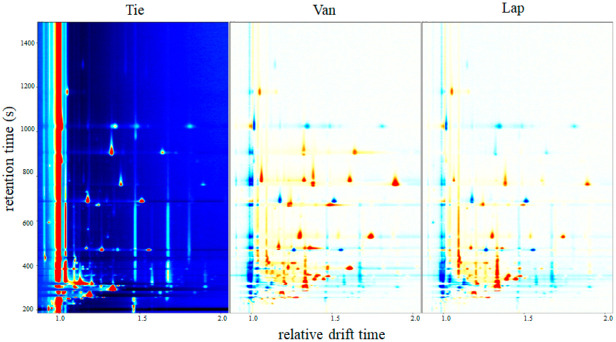
GC-IMS detection results were shown in a pseudo-color map (Figure 2) to differentiate the topographic plot of cherry fruits from three cultivars intuitively. In the two-dimensional (2D) topographic map of different cultivars, X and Y axis meant the drift time and retention time, respectively. Each point represented one type of volatile compound or a dimer in the spectrum. The grayscale image represented the normalized difference. The red or blue point indicated that the signal was strong or weak, which meant that the relative content was higher or lower. The integration parameters of volatile compounds were detected in Table 2. Moreover, it could be observed that some flavor compounds appeared as proton-bound monomers and dimers.
Figure 2.
Differences in the two-dimensional (2D)-topographic of volatile compounds in cherry fruits from three cultivars.
Table 2.
GC-IMS integration parameters of volatile compounds detected in cherry fruits and their wines.
| No. | Compounds | CAS | Formula | MW | RI | RT [sec] |
DT [a.u.] |
|---|---|---|---|---|---|---|---|
| 1 | Propionic acid | C79094 | C3H6O2 | 74.1 | 1705.6 | 1534.95 | 1.10999 |
| 2 | Acetic acid | C64197 | C2H4O2 | 60.1 | 1511.2 | 1181.04 | 1.1571 |
| 3 | Ethyl octanoate-M | C106321 | C10H20O2 | 172.3 | 1455.8 | 1080.20 | 1.48292 |
| 4 | Ethyl octanoate-D | C106321 | C10H20O2 | 172.3 | 1455.8 | 1080.20 | 2.02662 |
| 5 | 1-Hexanol-M | C111273 | C6H14O | 102.2 | 1361.3 | 908.19 | 1.32393 |
| 6 | 1-Hexanol-D | C111273 | C6H14O | 102.2 | 1360.9 | 907.54 | 1.64526 |
| 7 | 3-Methyl-1-butanol-M | C123513 | C5H12O | 88.1 | 1212.9 | 673.05 | 1.24442 |
| 8 | 3-Methyl-1-butanol-D | C123513 | C5H12O | 88.1 | 1210.4 | 669.48 | 1.50227 |
| 9 | Ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1238.5 | 708.71 | 1.79654 |
| 10 | Hexyl acetate-M | C142927 | C8H16O2 | 144.2 | 1281.7 | 769.01 | 1.3894 |
| 11 | Hexyl acetate-D | C142927 | C8H16O2 | 144.2 | 1277.8 | 763.52 | 1.89309 |
| 12 | (E)-2-hexenal-M | C6728263 | C6H10O | 98.1 | 1225.9 | 691.14 | 1.18288 |
| 13 | (E)-2-hexenal-D | C6728263 | C6H10O | 98.1 | 1234.8 | 703.62 | 1.51499 |
| 14 | 3-Methyl-3-buten-1-ol | C763326 | C5H10O | 86.1 | 1253.6 | 729.77 | 1.17268 |
| 15 | 3-Hydroxy-2-butanone-M | C513860 | C4H8O2 | 88.1 | 1291.3 | 782.35 | 1.06755 |
| 16 | 3-Hydroxy-2-butanone-D | C513860 | C4H8O2 | 88.1 | 1291.7 | 782.92 | 1.33107 |
| 17 | Benzaldehyde-M | C100527 | C7H6O | 106.1 | 1577 | 1300.77 | 1.14883 |
| 18 | Butanol-M | C71363 | C4H10O | 74.1 | 1146.3 | 564.09 | 1.18197 |
| 19 | Butanol-D | C71363 | C4H10O | 74.1 | 1147.8 | 566.62 | 1.38972 |
| 20 | Isoamyl acetate-M | C123922 | C7H14O2 | 130.2 | 1128.4 | 532.65 | 1.30544 |
| 21 | Isoamyl acetate-D | C123922 | C7H14O2 | 130.2 | 1127.9 | 531.92 | 1.74943 |
| 22 | 2-Methyl-1-propanol-M | C78831 | C4H10O | 74.1 | 1099.6 | 482.38 | 1.17133 |
| 23 | 2-Methyl-1-propanol-D | C78831 | C4H10O | 74.1 | 1098.3 | 480.17 | 1.37408 |
| 24 | 1-Propanol | C71238 | C3H8O | 60.1 | 1039.7 | 413.82 | 1.26147 |
| 25 | Isobutyl acetate | C110190 | C6H12O2 | 116.2 | 1015.7 | 387.03 | 1.61493 |
| 26 | Ethyl propionate | C105373 | C5H10O2 | 102.1 | 958.9 | 339.85 | 1.44949 |
| 27 | Propyl acetate | C109604 | C5H10O2 | 102.1 | 951 | 334.50 | 1.47663 |
| 28 | Hexanal-M | C66251 | C6H12O | 100.2 | 1090.6 | 470.71 | 1.26538 |
| 29 | Hexanal-D | C66251 | C6H12O | 100.2 | 1090.7 | 470.79 | 1.55908 |
| 30 | Ethanol | C64175 | C2H6O | 46.1 | 927.5 | 318.59 | 1.13044 |
| 31 | 1-Penten-3-ol | C616251 | C5H10O | 86.1 | 1163.2 | 593.48 | 0.94008 |
| 32 | Heptanal | C111717 | C7H14O | 114.2 | 1190.1 | 640.59 | 1.33915 |
| 33 | Ethyl acetate | C141786 | C4H8O2 | 88.1 | 892.7 | 295.00 | 1.33551 |
| 34 | Methyl acetate | C79209 | C3H6O2 | 74.1 | 855.8 | 270.00 | 1.19134 |
| 35 | Butanal | C123728 | C4H8O | 72.1 | 837 | 257.28 | 1.28121 |
| 36 | 2-Methylbutanal | C96173 | C5H10O | 86.1 | 918.3 | 312.37 | 1.39874 |
| 37 | Propionaldehyde | C123386 | C3H6O | 58.1 | 831.9 | 253.80 | 1.14343 |
| 38 | 2-Methyl-1-butanol | C137326 | C5H12O | 88.1 | 1212.2 | 672.02 | 1.23289 |
| 39 | Butyl acetate | C123864 | C6H12O2 | 116.2 | 1075.5 | 453.80 | 1.23674 |
| 40 | Trans-2-methyl-2-butenal-M | C497030 | C5H8O | 84.1 | 1051.5 | 426.957 | 1.09627 |
| 41 | Trans-2-methyl-2-butenal-D | C497030 | C5H8O | 84.1 | 1047.4 | 422.414 | 1.33819 |
| 42 | Butan-2-ol | C78922 | C4H10O | 74.1 | 1024 | 396.256 | 1.14746 |
| 43 | 4-Methyl-2-pentanone | C108101 | C6H12O | 100.2 | 1013.7 | 384.747 | 1.17867 |
MW: molecular weight; RI: retention index; RT: retention time; DT: drift time; D: Dimer; M: Monomer.
A total of thirty-six signal peaks were identified in cherry fruits, corresponding with twenty-seven volatile compounds, including ten alcohols, seven esters, seven aldehydes, two ketones, and one organic acid. Most of the signals were concentrated in 200–1000 s (retention time) in cherry fruits. Compared with cv. Tie and Lap, cv. Van had more red points. It was demonstrated that cv. Van had the most abundant content of volatile compounds.
Benzaldehyde, (E)-2-hexenal, hexanal, and heptanal showed the most abundant signals of volatile compounds in cv. Tie, and alcohols, esters, and organic acid showed the most abundant signals in cv. Van. There were only 2-methyl-1-butanol, ethyl propionate, and trans-2-methyl-2-butenal showed abundant in cv. Lap. Alcohols may derive from the infection by wild yeast when the cherry was on the trees [29], which is also a source of cultures from spontaneous fermentation. Alcohols possibly promoted the generation of esters, whereas they decreased aldehydes.
Figure 2 and Table 2 show the main volatile compounds of different cultivars. Aldehydes such as benzaldehyde, (E)-2-hexenal, hexanal, trans-2-methyl-2-butenal, butanal and propionaldehyde, esters such as ethyl acetate, hexyl acetate, isoamyl acetate, and methyl acetate, alcohols such as ethanol, 1-hexanol, and 1-penten-3-ol, and an organic acid such as acetic acid were abundant in all three cultivars. The results correspond to some researchers’ reports that benzaldehyde, (E)-2-hexenal, hexanal, ethanol, 1-hexanol, and ethyl acetate were key volatile compounds in cherry fruits [15,16].
The fingerprint analysis was given to qualitatively characterize the volatile compounds in different cherry cultivars (Figure 3). In the fingerprint, every row represents one kind of sample, and every column represents one kind of volatile compound. The light of each square roughly represents the content of each volatile compound. As described above, the aldehydes were the most prevalent in cv. Tie, for example, benzaldehyde, (E)-2-hexenal, hexanal, and heptanal. Furthermore, 3-methyl-3-buten-1-ol, 1-penten-3-ol, butan-2-ol, and 4-methyl-2-pentanone also showed the most abundant. Almost all esters and alcohols showed the most prevalent in cv. Van. Except for trans-2-methyl-2-butenal and ethyl propionate, there was no volatile compound that had more prevalent in cv. Lap.
Figure 3.
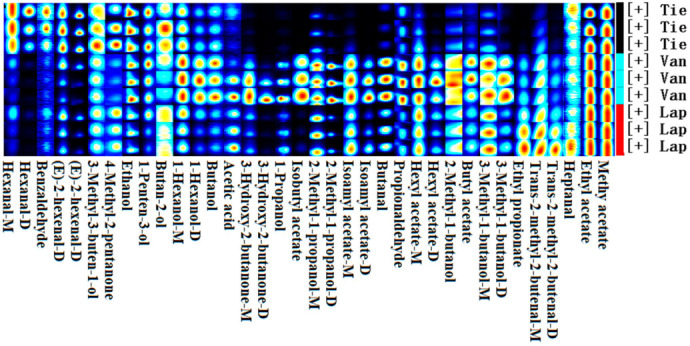
The Gallery Plot of volatile compounds in cherry fruits from three cultivars.
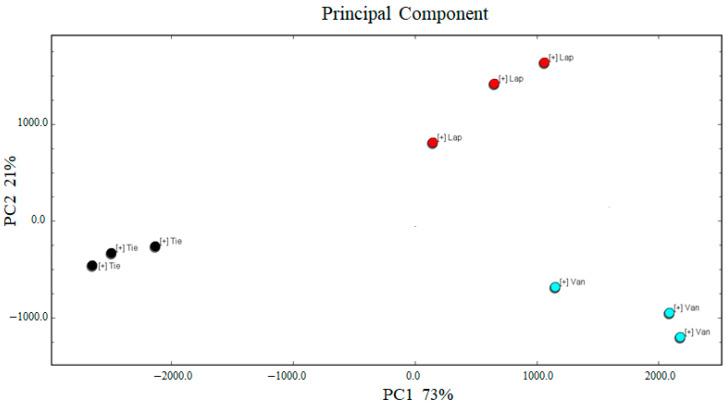
The scores plot of PCA in three cherry cultivars was shown in Figure 4, the accumulative variance contribution rate was 73% and 21%, and the total cumulative contribution ratio exceeded 60%, so the PCA model can be regarded as a well-separated instrument [27]. These components showed good repeatability in cherry fruits of different cultivars. There was a significantly different composition and content of volatile compounds of Tie, Van and Lap. The main contributing volatile compounds of cv. Tie to the differentiation were 4-methyl-2-pentanone heptanal, (E)-2-hexenal, 3-methyl-3-buten-1-ol, benzaldehyde, butan-2-ol, ethyl acetate, methyl acetate (Figure S1 in Supplementary Materials). 2-methyl-1-propanol, trans-2-methyl-2-butenal, ethyl propionate, 3-methyl-1-butanol and acetic acid contributed to cv. Lap. Hexyl acetate, isoamyl acetate, 3-methyl-1-butanol, 2-methyl-1-propanol, 3-hydroxy-2-butanone, propionaldehyde, isobutyl acetate, 1-hexanol, and butanal contributed to cv. Van. Hence compared with cv. Lap, the odor type of cv. Tie were fruity and green, and cv. Van were fruity and ethereal. Combing with the results of Figure 3 and Figure 4, HS-GC-IMS and PCA could be ways to distinguish different cherry cultivars.
Figure 4.
The scores plot of PCA analysis in cherry fruits from three cultivars.
3.2. Characterization of Cherry Wines Derived from Three Cultivars
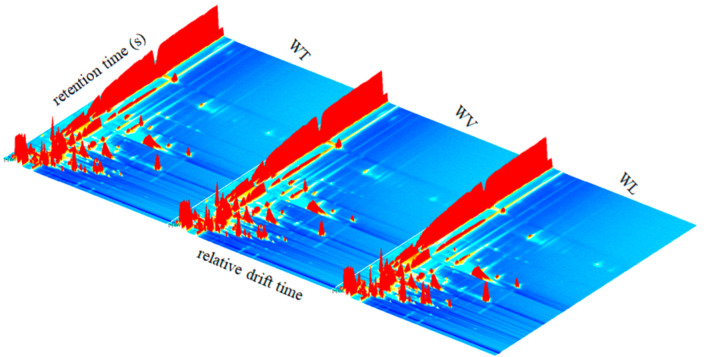
As shown in Figure 5 and Figure 6, there were a few differences in WT and WV, and the contents of some volatile compounds were higher or lower in the cherry wine (WL) from cv. Lapins. In primary fermentation, more alcohols and esters were produced by yeast, which constituted the dominating volatile flavor compounds in cherry wines. Combined with Table 2, twenty-nine signal peaks were identified in cherry wines derived from different cultivars, corresponding with twenty-three volatile compounds. Among them, twenty-three compounds were composed of eight alcohols, nine esters, three aldehydes, two organic acids, and one ketone.
Figure 5.
Differences in the three-dimensional (3D)-topographic of volatile compounds in cherry wines derived from three cultivars (WT: cherry wine from cultivar Tieton; WV: cherry wine from cultivar Van; WL: cherry wine from cultivar Lapins).
Figure 6.
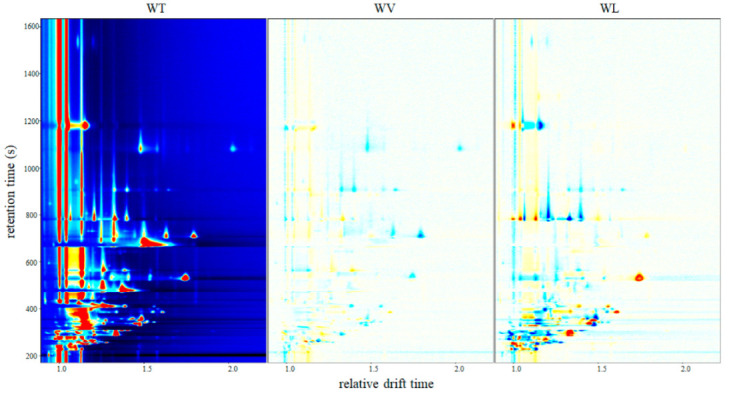
Differences in the two-dimensional (2D)-topographic of volatile compounds in cherry wines derived from three cultivars.
There were few differences in the content of alcohols (1-hexanol, 3-methyl-1-butanol, 3-methyl-3-buten-1-ol, 1-propanol, and ethanol) of all three samples. The content of alcohols may be determined by fermentation cultures, which corresponded to some research [30]. Except for ethanol, higher alcohols such as 3-methyl-1-butanol, 2-methyl-1-propanol, 1-hexanol, and butanol might be generated through glycolysis and the Ehrlich pathway in the yeast [31]. The content of higher alcohols is mainly relative to ferment factors and the content of amino acids [32], which means that the three cherry samples shared a similar constitution of amino acids. Moreover, WT and WV hold the similar type and content of some volatile elements, including isoamyl acetate, ethyl propionate, propyl acetate, ethyl acetate, butanal, 2-methylbutanal, propionaldehyde, and propionic acid, as shown in the Gallery Plot (Figure 7). Compared with WT and WV, isoamyl acetate, isobutyl acetate, ethyl propionate, ethyl hexanoate, ethyl acetate, and methyl acetate showed relatively high content in WL, whereas two esters (propyl acetate, butyl acetate), all aldehydes, organic acids and ketone showed the relatively lower content. The content of ethyl octanoate, ethyl hexanoate, and methyl acetate in WT was higher than in WV, while isobutyl acetate, butyl acetate, and 3-hydroxy-2-butanone were lower.
Figure 7.
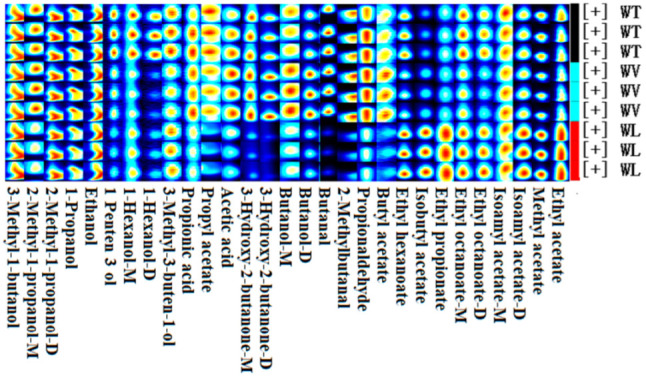
The Gallery Plot of volatile compounds in cherry wines derived from three cultivars.
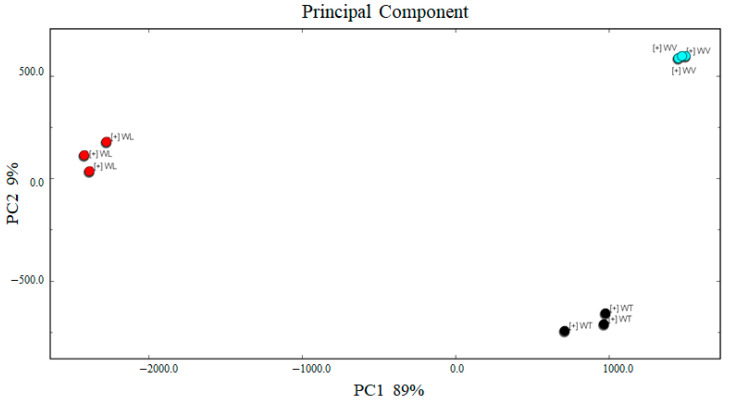
As can be seen from Figure 8, the accumulative variance contribution rate was 89% and 9%, and the total cumulative contribution ratio was 98%. The scores plot of PCA showed good repeatability in cherry wines derived from different cultivars. The main contributing volatile compounds of WT to the differentiation were propyl acetate, 1-penten-3-ol, 2-methyl-1-propanol, and 1-hexanol (Figure S2). Isobutyl acetate, isoamyl acetate, ethyl propionate, ethyl acetate, methyl acetate, ethyl hexanoate, and ethyl octanoate contributed to WL. Butanol, butyl acetate, 1-propanol, butanol, and acetic acid contributed to WV. Those correspond to the result of the Gallery Plot. There were more differences in volatile compounds in WL, which could be distinguished by PC1. Combing with PC2, PCA could apparently separate those cherry wines of different cultivars. Thus, cherry fruits and their wines derived from different cultivars could be distinguished by the Gallery Plot and PCA.
Figure 8.
The scores plot of PCA analysis in cherry wines derived from three cultivars.
On the whole, the main volatile compounds of cherry wines were alcohols and esters. Esters were more abundant in WL compared with WT and WV. The odor type of WT and WV was largely determined by alcohols, while WL was by esters. Hence the odor of volatile compounds was fruity and ethereal in WL. The volatile compounds might be largely determined by the type of fermentation cultures in cherry wines.
3.3. The Source of Volatile Compounds in Cherry Wines
From the perspective of types of volatile compounds, alcohols and esters generally existed in cherry wines. A number of esters were produced by substrate esterification, yeast enzymatic metabolism, and the metabolism of aliphatic acid during fermentation [33]. The main esters were acetate esters, such as isoamyl acetate, isobutyl acetate, propyl acetate, ethyl acetate, methyl acetate, and butyl acetate, which significantly influenced flavor and fragrance. There were several alcohol-O-acetyl (or acyl) transferases (AATases) in yeast; thereinto, Atf1 and Atf2 were in charge of producing acetate esters in Saccharomyces cerevisiae [34]. S. cerevisiae can produce volatile compounds (like esters) through de novo synthesis or biotransformation from the appropriate substrate. The content of acetate esters is usually affected by the existence of higher alcohols and fatty acids during fermentation [35]. Esters were generally regarded as the principal factor of the flavor of alcoholic beverages, bringing about fruity and ethereal odor.
Higher alcohols had a positive-going effect on flavor despite their higher sensory threshold when they held appropriate content [36]. The content of 1-hexanol, 3-methyl-3-buten-1-ol, 2-methyl-1-butanol, and 1-penten-3-ol decreased after fermentation, whereas they showed relatively high content in all three cherry fruits. Furthermore, two esters (hexyl acetate and methyl acetate) were derived from cherry fruit. This meant that these compounds were not produced by yeast. Those alcohols may derive from the maturation process of cherry and infection of wild yeast [18,29]. As to the metabolism of commercial Saccharomyces cerevisiae (BV818), 3-methyl-1-butanol, butanol, 2-methyl-1-propanol, and 1-propanol noteworthily increased and showed relatively high content in all cherry wine samples.
Alcohols may promote the production of esters, whereas decreased aldehydes. The majority of aldehydes, such as hexanal, nonanal, trans-2-methyl-2-butenal, and heptanal, decreased due to the volatilization with fermented CO2 and oxidation that converts aldehydes into their respective acids during fermentation. As the main volatile compounds, aldehydes existed throughout the growth process, while their contents increased quickly in the color stage and decreased in maturation [18]. Thus, those aldehydes derived from cherry fruits cannot be produced during fermentation. Conversely, 2-methylbutanal and propionaldehyde showed low content in cherry fruits and significantly increased in cherry wines, bringing fusel and ethereal aroma. The increasing content of aldehydes may be derived from oxidization during crushing, enzymolysis, and fermentation processes, which may degrade some lipids into aldehydes [5,37]. Meanwhile, the yeast produces a mass of propionic acid, acetic acid, and 3-hydroxy-2-butanone, bringing with acidic and buttery odor. The other volatile compounds are almost produced by saccharomyces cerevisiae.
Some volatile compounds, such as isobutyl acetate, increased in WT and WL but decreased in WV, and the butanal increased in WT and decreased in WV and WL. The starter culture of yeast could regulate the content of some volatile flavor compounds, making cherry wines affected less by raw materials. Therefore, the volatile compounds of cherry wines were regulated and affected by fermentation microorganisms.
4. Conclusions
In conclusion, aldehydes, alcohols, and esters were prevalent in cherry fruits, and huge diversity in the content of aldehydes, while esters and alcohols were ubiquitous in wines. Distinct differences in volatile compounds could be ways to distinguish different cherry fruits and their wines. Sources of some volatile compounds in cherry wines were from cherry fruits (almost aldehydes, four alcohols and two esters), and the content were determined by cherry cultivars. The content of alcohols showed few differences in three cherry wines. Cherry fruit (Tie) showed relatively low content of esters, but its wine (WT) was similar to WV. This meant that volatile compounds of cherry wines might be largely determined by fermentation cultures. This research may provide a theory foundation for the evaluation and improvement of flavor quality in cherry wines making.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27249056/s1, Figure S1: The loading plot of PCA analysis in cherry fruits from three cultivars; Figure S2: The loading plot of PCA analysis in cherry wines derived from three cultivars.
Author Contributions
B.L.: methodology, validation and writing-original draft; Y.Y.: methodology, validation and writing-original draft; L.R.: methodology, validation; Z.S.: supervision, project administration; X.B.: data curation, methodology and validation; J.F.: editing, methodology and formal analysis; Y.W. and B.H.: methodology and validation; N.Z.: supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This study was supported by the financial support of the National Natural Science Foundation (No. 32072258), Heilongjiang Young Scientific and Technological Talents Promotion Project (2022QNTJ010), the Central financial support for the development of local colleges and Universities, and the Major Innovation Pilot Project of Integration of Science, Education and Industry of Qilu University of Technology (Shandong Academy of Science) (No. 2022JBZ01-08).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ballistreri G., Continella A., Gentile A., Amenta M., Fabroni S., Rapisarda P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013;140:630–638. doi: 10.1016/j.foodchem.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Blando F., Oomah B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019;86:517–529. doi: 10.1016/j.tifs.2019.02.052. [DOI] [Google Scholar]
- 3.Dong W., Chen D., Chen Z., Sun H., Xu Z. Antioxidant capacity differences between the major flavonoids in cherry (Prunus pseudocerasus) in vitro and in vivo models. LWT Food Sci. Technol. 2021;141:110938. doi: 10.1016/j.lwt.2021.110938. [DOI] [Google Scholar]
- 4.Fonseca L.R.S., Silva G.R., Luís A., Cardoso H.J., Correia S., Vaz C.V., Duarte A.P., Socorro S. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules. 2021;26:2941. doi: 10.3390/molecules26102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maria V.V., Anastasia V.B., Stavros K., Michael G.K. Characterization of four popular sweet cherry cultivars grown in Greece by volatile compound and physicochemical data analysis and sensory evaluation. Molecules. 2015;20:1922–1940. doi: 10.3390/molecules20021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., Jiang N., Wang Y., Jiang D.M., Feng X.Y. Characterization of Phenolic Compounds from Early and Late Ripening Sweet Cherries and Their Antioxidant and Antifungal Activities. J. Agric. Food Chem. 2017;65:5413–5420. doi: 10.1021/acs.jafc.7b01409. [DOI] [PubMed] [Google Scholar]
- 7.Pereira N., Naufel M.F., Ribeiro E.B., Tufik S., Hachul H. Influence of dietary sources of melatonin on sleep quality: A review. J. Food Sci. 2019;85:5–13. doi: 10.1111/1750-3841.14952. [DOI] [PubMed] [Google Scholar]
- 8.Abdullah N., Chin N.L. Application of thermosonication treatment in processing and production of high quality and safe-to-drink fruit juices. Agric. Agric. Sci. Procedia. 2014;2:320–327. doi: 10.1016/j.aaspro.2014.11.045. [DOI] [Google Scholar]
- 9.Nur A.R.M.R., Lai K.L. Traditional fermented foods as vehicle of non-dairy probiotics: Perspectives in South East Asia countries. Food Res. Int. 2021;150:110814. doi: 10.1016/j.foodres.2021.110814. [DOI] [PubMed] [Google Scholar]
- 10.Sionek B., Tambor K., Okon A., Szymanski P., Zielinska D., Neffe-Skocinska K., KołozynKrajewska D. Effects of Lacticaseibacillus rhamnosus LOCK900 on development of volatile compounds and sensory quality of dry fermented sausages. Molecules. 2021;26:6454. doi: 10.3390/molecules26216454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maja R., Boris P., Krunoslav D., Nada V., Sinisa SVerica D.-U., Zorica J., Branka L. Quality and sensory study of fresh sour cherry juices upon cultivar, growing area and weather conditions. J. Food Sci. 2019;84:3264–3274. doi: 10.1111/1750-3841.14822. [DOI] [PubMed] [Google Scholar]
- 12.Silva V., Pereira S., Vilela A., Bacelar E., Guedes F., Ribeiro C., Silva A.P., Gonçalves B. Preliminary insights in sensory profile of sweet cherries. Foods. 2021;10:612. doi: 10.3390/foods10030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legua P., Domenech A., Martínez J.J., Sanchez-Rodriguez L., Hernandez F., Carbonell-Barrachina A.A., Melgarejo P. Bioactive and volatile compounds in sweet cherry cultivars. J. Food Nutr. Res. 2017;5:844–851. doi: 10.12691/jfnr-5-11-8. [DOI] [Google Scholar]
- 14.Wen Y.Q., He F., Zhu B.Q., Lan Y.B., Pan Q.H., Li C.Y., Reeves M.J., Wang J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.) Food Chem. 2014;152:29–36. doi: 10.1016/j.foodchem.2013.11.092. [DOI] [PubMed] [Google Scholar]
- 15.Nategh N.A., Dalvand M.J., Anvar A. Detection of toxic and nontoxic sweet cherries at different degrees of maturity using an electronic nose. J. Food Meas. Charact. 2020;15:1213–1224. doi: 10.1007/s11694-020-00724-6. [DOI] [Google Scholar]
- 16.Sun S.Y., Jiang W.G., Zhao Y.P. Characterization of the aroma-active compounds in five sweet cherry cultivars grown in yantai (China) Flavour Fragr. J. 2010;25:206–213. doi: 10.1002/ffj.1994. [DOI] [Google Scholar]
- 17.Claudio P., Nathalie K., Macarena A., Alson T., Salvatore M., Stefan M., Esther C., Boris S., José M.D., Lee A.M. Differential phenolic compounds and hormone accumulation patterns between early- and mid-maturing sweet cherry (Prunus avium L.) cultivars during fruit development and ripening. J. Agric. Food Chem. 2021;69:8850–8860. doi: 10.1021/acs.jafc.1c01140. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Jiang Y.M., Peng F.T., He N.B., Zhao D.C. Changes of aroma components in hongdeng sweet cherry during fruit development. Agric. Sci. China. 2007;6:1376–1382. doi: 10.1016/S1671-2927(07)60186-2. [DOI] [Google Scholar]
- 19.Li H.M., Jiang D.Q., Dai Z.G., Zhang Y.S., Zhang Y., Sun S.Y., Zhao Y.P. Aromatic property of cherry wine produced by malolactic fermentation of controlled and spontaneous on the bacterial evolution. Int. J. Food Prop. 2019;22:1270–1282. doi: 10.1080/10942912.2019.1640736. [DOI] [Google Scholar]
- 20.Niu Y.W., Wang P.P., Xiao Z.B., Zhu J.C., Sun X.X., Wang R.L. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC-MS, GC-O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019;275:143–153. doi: 10.1016/j.foodchem.2018.09.102. [DOI] [PubMed] [Google Scholar]
- 21.Sun S.Y., Gong H.S., Liu W.L., Jin C.W. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016;55:16–24. doi: 10.1016/j.fm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang S.Q., Chen H.T., Sun B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS) Food Chem. 2020;315:126158. doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- 23.Natalie G., Markus B., Daniel S., Sascha R., Philipp W. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017;409:3933–3942. doi: 10.1007/s00216-017-0338-2. [DOI] [PubMed] [Google Scholar]
- 24.Gao C., Wang R., Zhang F., Sun Z.C., Meng X.H. The process monitors of probiotic fermented sour cherry juice based on the HS-GC-IMS. Microchem. J. 2022;180:107537. doi: 10.1016/j.microc.2022.107537. [DOI] [Google Scholar]
- 25.Yang Y., Wang B., Fu Y., Shi Y.G., Chen F.L., Guan H.N., Liu L.L., Zhang C.Y., Zhu P.Y., Liu Y., et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021;346:128880. doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- 26.Rong Y., Gu X., Li D., Chen L., Wang Z. Characterization of aroma, sensory and taste properties of angelica keiskei tea. Eur. Food Res. Technol. 2021;247:1665–1677. doi: 10.1007/s00217-021-03737-7. [DOI] [Google Scholar]
- 27.Zhang Q., Ding Y.C., Gu S.Q., Zhu S.C., Zhou X.X., Ding Y.T. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020;137:109339. doi: 10.1016/j.foodres.2020.109339. [DOI] [PubMed] [Google Scholar]
- 28.Cozzolino D., Power A., Chapman J. Interpreting and reporting principal component analysis in food science analysis and beyond. Food Anal. Methods. 2019;12:2469–2473. doi: 10.1007/s12161-019-01605-5. [DOI] [Google Scholar]
- 29.Santo D.E., Galego L., Gonalves T., Quintas C. Yeast diversity in the Mediterranean strawberry tree (Arbutus unedo L.) fruits’ fermentations. Food Res. Int. 2012;47:45–50. doi: 10.1016/j.foodres.2012.01.009. [DOI] [Google Scholar]
- 30.Xiao Q., Zhou X., Xiao Z., Niu Y.W. Characterization of the differences in the aroma of cherry wines from different price segments using gas chromatography-mass spectrometry, odor activity values, sensory analysis, and aroma reconstitution. Food Sci. Biotechnol. 2017;26:331–338. doi: 10.1007/s10068-017-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazelwood L.A., Daran J-MMaris A.J.A., Pronk J.T., Dickinson J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M.M., Yang K., Qi Y.M., Zhang J.M., Fan T., Wei X.Y. Fermentation temperature and the phenolic and aroma profile of persimmon wine. J. Inst. Brew. 2018;124:269–275. doi: 10.1002/jib.497. [DOI] [Google Scholar]
- 33.Su Z.B., Liu B.X., Ma C. Analyses of the volatile compounds in cherry wine during fermentation and aging in bottle using HS-GC-IMS. Food Sci. Technol. Res. 2021;27:599–607. doi: 10.3136/fstr.27.599. [DOI] [Google Scholar]
- 34.Cherry J.M., Hong E.L., Amundsen C. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2012;40:700–705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantzouridou F., Paraskevopoulou A. Volatile bio-ester production from orange pulp-containing medium using Saccharomyces cerevisiae. Food Bioprocess Technol. 2013;6:3326–3334. doi: 10.1007/s11947-012-1009-0. [DOI] [Google Scholar]
- 36.Dzialo M.C., Park R., Steensels J., Lievens B., Verstrepen K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017;41:95–128. doi: 10.1093/femsre/fux031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu D.S., Kou C.C., Shen Y.S., Xi P.S., Cao X.H., Liu H., Li J.R. Effects of different processing steps on the flavor and colloidal properties of cloudy apple juice. J. Sci. Food Agric. 2021;101:3819–3826. doi: 10.1002/jsfa.11016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.