Abstract
We identified a new oligopeptide permease operon in the pathogen Listeria monocytogenes. This opp operon consists of five genes (oppA, oppB, oppC, oppD, and oppF) and displays the same genetic organization as those of several bacterial species. The first gene of this operon, oppA, encodes a 62-kDa protein sharing 33% identity with OppA of Bacillus subtilis and is expressed predominantly during exponential growth. The function of oppA was studied by constructing an oppA deletion mutant. The phenotype analysis of this mutant revealed that OppA mediates the transport of oligopeptides and is required for bacterial growth at low temperature. The wild-type phenotype was restored by complementing the mutant with oppA. We also found that OppA is involved in intracellular survival in macrophages and in bacterial growth in organs of mice infected with L. monocytogenes, although the level of virulence was not altered in the mutant. These results show the major role of OppA in the uptake of oligopeptides and the pleiotropic effects of this oligopeptide-binding protein on the behavior of this pathogen in the environment and in its host.
Listeria monocytogenes is a facultative intracellular pathogen that induces sporadic severe food-borne infections in humans and many animals (16). This gram-positive bacterium is widely spread in the environment, including soil, decaying vegetation, and food (10). Outbreaks of listeriosis are due to the contamination of food products, like raw vegetables, meat, and dairy products (10). Acquisition of nutrients from food products as well as from the cytoplasm of host cells therefore appears to be crucial for the survival and propagation of this pathogen. Growth in food products is dependent upon several characteristics of L. monocytogenes: (i) it is a psychrotrophic species, slowly growing at temperatures as low as −0.1°C (45); (ii) it is a multiple-amino-acid auxotroph species requiring several amino acids as carbon and nitrogen sources for growth (34); and (iii) it is apparently unable to hydrolyze proteins, and its growth depends upon other proteolytic systems that allow degradation of food proteins. It is believed that peptides and free amino acids present in foods result from the activity of indigenous proteinases (11) and/or proteinases from diverse populations of microorganisms, such as lactic acid bacteria (24, 40). The growth of L. monocytogenes was found to be enhanced to a large extent by Pseudomonas fragi and Bacillus cereus in a medium containing casein as the sole source of nitrogen (43).
Peptide metabolism and transport have been extensively characterized for gram-negative and gram-positive bacteria (30). The most common peptide transporters are binding-protein-dependent permeases, which are multicomponent transport systems and members of the ATP-binding cassette (ABC) transporter-channel superfamily (17). The process of peptide transport involves the extracytoplasmic binding of the substrate, transfer to one or two membrane-bound permeases for translocation across the cytoplasmic membrane, and ATP hydrolysis by one or two proteins located on the cytoplasmic side of the membrane (17). The best-documented transport systems are those for dipeptides (Dpp), tripeptides (Tpp), and oligopeptides (Opp) from Escherichia coli (26) and Salmonella enterica serovar Typhimurium (19, 20). Among these transport systems, the oligopeptide permease Opp systems possess one of the most versatile binding proteins, since they transport a large variety of peptides composed of various natural and/or modified residues (30). The Opp systems of these bacteria are involved in nutrient uptake but also in recycling the cell wall peptides for synthesis of new peptidoglycan (15), cytoadherence in some Streptococcus spp. (6, 7), sensing of extracellular signaling molecules (called pheromones) required for initiation of competence and sporulation in Bacillus subtilis (31, 36, 39), or induction of conjugation in Enterococcus faecalis (25). For L. monocytogenes, there is evidence that auxotrophic mutants may utilize intracellular peptides as a source of amino acids during intracytoplasmic growth (27). Subsequently, biochemical studies demonstrated that L. monocytogenes possesses two different peptide transport systems allowing internalization of peptides of up to eight residues, which are ultimately hydrolyzed by internal peptidases to serve as sources of amino acids essential for growth: (i) a proton motive force-dependent di- and tripeptide transport system with a broad substrate specificity, supplying bacteria with amino acids (42), and (ii) an oligopeptide transport system, presumably requiring ATP for peptide translocation (43).
In this work, we identified the oligopeptide permease (Opp) operon of L. monocytogenes and showed that the first gene of this operon encodes OppA, an oligopeptide-binding protein involved in the transport of oligopeptides. OppA is required for bacterial growth at low temperature and favors intracellular survival of L. monocytogenes in macrophages.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and transformation.
The bacterial strains used in this work are listed in Table 1. We used L. monocytogenes reference strain LO28 and E. coli K-12 strain TG1. All strains were routinely grown in brain heart infusion (BHI) medium. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml for E. coli; erythromycin, 8 μg/ml for L. monocytogenes and 200 μg/ml for E. coli; kanamycin, 50 μg/ml; colistin, 10 μg/ml; and nalidixic acid, 50 μg/ml. Constructs were introduced into Listeria strains by conjugation or electroporation as previously described (33). Bacterial growth and phenotype analysis of strains were performed as described previously (35). The metabolic profiles were determined on API strip (50 substrates) (Biomérieux, Marcy l'Etoile, France). For growth experiments with peptides, we used a chemically defined minimal medium (modified Welshimer's broth [MWB]) prepared as previously described (34), supplemented with histidine (0.01%), which is essential for growth of LO28 strains. For cultures with valine-containing peptides, valine was omitted from the medium, and peptides were used at a concentration of 0.1 mM. All peptides (V-P-L, V-G-D-L, R-K-D-V-Y, S-Q-N-Y-P-I-V) were provided by Sigma (St. Louis, Mo.). The toxicity of bialaphos was tested by spreading bacteria (fresh liquid cultures washed three times in MWB) onto MWB agar plates with bialaphos (10 μg) spotted on a filter disc in the center of the plate. After 24 h of incubation at 37°C, the zone of inhibition was measured.
TABLE 1.
L. monocytogenes strains and plasmids used in this study
| Listeria plasmid or strain | Genotype or description | Source or reference |
|---|---|---|
| Plasmids | ||
| pAUL-A | Shuttle vector, thermosensitive, Em | 5 |
| pAUL-oppAΩaphA3′ | pAUL-A carrying oppAΩaphA3′ (2,331-bp fragment) | This study |
| pAT18 | Shuttle vector, Em | 41 |
| pAT18PdltA/oppA | pAT18 carrying oppA under control of the dltA promoter (2,035-bp fragment) | This study |
| Strains | ||
| LO28 | Virulent wild type, clinical isolate | 44 |
| LO28/pAT18 | LO28 harboring pATl8 (Em) | This study |
| LO28-oppA ΩaphA3′ | oppA mutant (Km) | This study |
| LO28-oppA ΩaphA3′/ pAT18 | oppA mutant harboring pAT18 (Em Km) | This study |
| LO28-oppA ΩaphA3′/ pAT18-PdltA/ oppA | oppA mutant harboring pAT18 with oppA under control of dltA promoter (Em Km) | This study |
DNA manipulations, RNA extraction, Northern blot analysis, and RT-PCR.
Chromosomal DNA preparation, plasmid extraction, electrophoresis, restriction enzyme analysis, hybridizations, and amplification by PCR were performed by standard protocols (37). DNA sequencing was performed with an ABI-Prism 310 sequencer (Perkin-Elmer Corp, Norwalk, Conn.). Total RNA was extracted from L. monocytogenes cultures grown in BHI broth at different temperatures (5 or 37°C) and phases (exponential or stationary), and Northern blotting performed as previously described (4). The oppA, oppB, oppC, oppD, and oppF probes (1,513, 875, 700, 836, and 831 bp, respectively) were obtained by PCR from chromosomal DNA of L. monocytogenes LO28, using the following primers: oppA1 (5′-CTTGGTAGCATGCGGAGGCGG-3′) and oppA2 (5′-AGCTACATCATCCGTAAGAAGG-3′), oppB1 (5′-CATCATTGCTTCGGTTACG-3′) and oppB2 (5′-CTACCTCCAGACACACGG-3′), oppC1 (5′-CAGCCAGCACACATTCTGG-3′) and oppC2 (5′-CCTAAAGTCATGGAAGCC-3′), oppD1 (5′-CATTCCACACATATGCCGG-3′) and oppD2 (5′-GTGCAGCAAATGCGTCCCC-3′), and oppF1 (5′-CTGCAAGTGAAGTACGTGC-3′) and oppF2 (5′-CCAGGAGCAATCTCGCGC-3′). These primers were also used to amplify opp mRNA by reverse transcription-PCR (RT-PCR), as described in the kit (SuperScript One-Step RT-PCR System; Life Technologies, Paisley, Scotland). Prior to RT-PCR, total RNA samples were incubated for 1 h at 37°C with DNaseI-RNase-free (Boehringer, Mannheim, Germany) to eliminate any DNA contamination. For amplifications of up to 3 kb, Elongase (Life Technologies) was added into the RT-PCR mixture. For amplification of the cspB and cspL probes from chromosomal DNA of LO28, we used primers cspB1 (5′-ATGCAAACAGGTACAGTTAAATGG-3′) and cspB2 (5′-GTTTAGTAACTTTTTCTGCTTGTGGG-3′) and primers cspL1 (5′-ATGAACATGGAACAAGGTACAG-3′) and cspL2 (5′-TTACGCTTTTTGAACGTTAGCTGC-3′). The GenBank accession numbers for the cspL and cspB genes from L. monocytogenes ATCC 23074 are X91789 and U90213, respectively.
Cloning and sequencing of the opp operon.
A 3-kb XbaI chromosomal DNA fragment from LO28, hybridizing with mecA of B. subtilis, was previously cloned into pUC19 and sequenced (3). The 5′ extremity of the insert was very similar to the 3′ portion of the opp operon of B. subtilis. The complete opp operon of LO28 was then cloned and sequenced by chromosome walking (37).
Construction of a deletion mutant and complementation.
An oppA mutant (LO28 oppAΩaphA3) was constructed by deletion of a 27-bp internal fragment of oppA (nucleotides 801 to 827) and insertion of a promoterless aphA-3 gene conferring resistance to kanamycin (28) by double recombination. The deletion-replacement mutant of oppA was constructed by inserting a 727-bp KpnI-BamHI LO28 DNA fragment (+92 to +800), a 855-bp BamHI E. faecalis DNA fragment carrying aphA-3, and a 749-bp BamHI-XbaI LO28 DNA fragment (+828 to +1,560), between the KpnI and XbaI sites of the thermosensitive shuttle vector pAUL-A (5) to give plasmid pAUL-oppAΩaphA3. Positions are given relative to the translation initiation codon of oppA. These three DNA fragments were generated by PCR using the following primers: Mut1 (5′-GGGGTACCCCGACAAAAAAGGCTCAGATTCAGG-3′) and Mut 2 (5′-CGGGATCCCGTCCAGTACCGGAGTCTTGAAC-3′), Km1 (5′-CGGGATCCCGACTAACTAGGAGGAATA-3′) and Km2 (5′-CGGGATCCCGGGTCATTATTCCCTCC-3′), and Mut3 (5′-CGGGATCCCGTACTGTATTGAGTGCAGAC-3′) and Mut4 (5′-GCTCTAGAGCTACATCATCCGTAAGAAGG-3′). pAUL-oppAΩaphA3 was introduced into LO28 by electroporation, and transformants were selected for erythromycin resistance at 30°C. We used a previously described gene replacement procedure (5) to obtain an isogenic mutant carrying the disrupted oppA gene on the chromosome. The genotype of the mutant was confirmed by PCR sequencing and Southern and Northern blot analyses.
For complementation of the LO28 oppA strain, we used a previously characterized promoter of dltA (PdltA [GenBank accession number AJ012255]) (E. Abachin and P. Trieu-Cuot, unpublished data) amplified from LO28 and cloned between the EcoRI and BamHI sites of plasmid pAT18 (41). The complete oppA gene (1,865 bp) and its terminator of transcription was amplified with primers 5′-GGATCCAGAAAAATAAAAAAGGGAGGTCTA-3′ and 5′-TCTAGAAGACTAGAAAAGGATA-3′ and inserted between the BamHI and XbaI sites of plasmid pAT18-PdltA to give pAT18-PdltA/oppA. The recombinant plasmid pAT18-PdltA/oppA was introduced by conjugation into the oppA mutant. The transconjugants were selected on BHI agar plates containing colistin, nalidixic acid, and erythromycin. As controls, pAT18 was introduced by conjugation into LO28 and the oppA mutant.
Infection of macrophages.
Bone marrow-derived macrophages from C57/BL6 mice were cultured and infected as described previously (12). After 15 min of bacterial adherence on ice, macrophages were exposed for 15 min at 37°C at bacterium/macrophage ratios of 1:1 and 15:1 for growth curves and microscopic studies, respectively. The numbers of intracellular bacteria in cell lysates were estimated at selected intervals (from 0 to 10 h postinfection). Double fluorescence labeling of F-actin and bacteria was performed as described previously (23) using phalloidin coupled to Oregon green 488 (Molecular Probes, Eugene, Oreg.) and a rabbit anti-Listeria O antigen (J. Rocourt, Institut Pasteur, Paris, France) revealed with an anti- immunoglobulin antibody coupled to Alexa 546 (Molecular Probes). Images were scanned on a Zeiss LSM 510 confocal microscope.
Processing for electron microscopy.
Macrophages were infected for 3 h at a bacterium/macrophage ratio of 20:1, fixed for 1 h at room temperature, and processed as described previously (8). The percentage of intraphagosomal or intracytoplasmic bacteria was determined in 50 to 100 different cell profiles (about 100 bacteria were examined).
Mouse virulence assay.
Six- to eight-week-old female Swiss mice (Janvier, Le Geneset St. Isle, France) were inoculated intravenously (i.v.) with various doses of bacteria. Mortality was monitored over a 14-day period for groups of five mice. The 50% lethal doses were determined by the probit method. Bacterial growth in organs (spleen and liver) of mice infected i.v. with 8 × 105 bacteria was monitored as previously described (29).
Nucleotide sequence accession number.
The sequence determined in this study has been assigned GenBank accession no. AF103793.
RESULTS
Cloning and sequence analysis of the opp operon of L. monocytogenes.
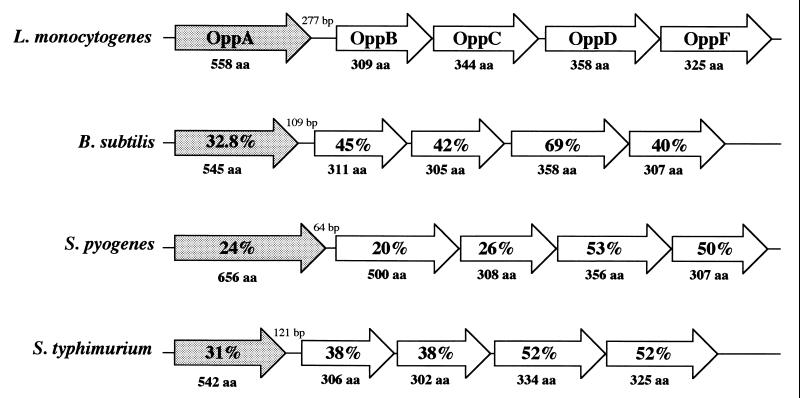
A 3-kb XbaI DNA fragment from L. monocytogenes LO28 containing the yjbD and mecA genes was previously cloned into pUC19 (3). The 5′ end of the insert showed high similarities with the 3′ ends of the oppF genes of B. subtilis and other bacteria. The oppF gene is the last gene of the oligopeptide permease (opp) operon, which is present in several bacterial species From the oppF sequence, we used inverse PCR to identify the entire opp operon of L. monocytogenes. Sequencing of the amplified DNA fragments revealed the existence of five predicted open reading frames (ORFs), with significant sequence identity with known Opp proteins, and a conserved gene order (oppA, oppB, oppC, oppD, oppF). The highest similarities in terms of peptide identity and operon organization were found with the oligopeptide permease opp operon of B. subtilis. The genetic organizations of the opp operons of L. monocytogenes, B. subtilis, Streptococcus pyogenes, and S. enterica serovar Typhimurium are presented in Fig. 1. There is a 277-bp noncoding sequence between oppA and oppB of L. monocytogenes, which contains a potential stem-loop structure (−30.5 kJ mol−1), located 30 bp downstream from the oppA stop codon. An similar loop is responsible, at least in serovar Typhimurium (19) and S. pyogenes (32), for expression of oppA alone. A second stem-loop structure (−43.9 kJ mol−1) was found 5 bp downstream of the last gene, oppF, presumably corresponding to the termination of transcription of the opp operon. For the other genes of the operon, the intercistronic regions are short or absent, with two overlapping regions between oppB and oppC and between oppD and oppF.
FIG. 1.
Genetic organization of the opp operons of L. monocytogenes, B. subtilis, S. pyogenes, and S. enterica serovar Typhimurium. Similarities between the Opp proteins are given as percent amino acid (aa) identities.
The first ORF, designated oppA, starts with a GTG initiation codon and encodes a putative protein of 558 amino acids. The deduced polypeptide chain starts with a N-terminal peptide leader of 27 amino acids which contains a transmembrane helix (from residue 7 to 24), a signal peptidase recognition site [(−3)-GGSDS-(+2)], and a lipoprotein attachment site at position 23. Finally, the predicted protein possesses a bacterial extracellular solute-binding protein signature (from residue 96 to 116). Thus, OppA of L. monocytogenes is presumably a lipoprotein attached to the external part of the cytoplasmic membrane. The putative OppA protein revealed homologies with several substrate-binding proteins of bacterial oligopeptide transport systems (∼32% identity), with the pheromone-binding proteins (TraC) of several conjugative plasmids of E. faecalis (37% identity), and with DppE (dipeptide-binding protein) from B. subtilis (31% identity).
Downstream from oppA, we found four ORFs with the highest similarity to genes encoding the core domains of the oligopeptide transport system. OppB and OppC were two predicted integral membrane proteins of 309 and 344 residues, with five and six transmembrane-spanning segments, respectively. The oppC gene from L. monocytogenes strain ScottA was previously sequenced (W. He and J. B. Luchansky, GenBank accession number U78885), and the deduced protein showed 99% peptide identity with LO28 OppC. The two last ORFs encode putative OppD (358 amino acids) and OppF (325 amino acids) proteins, with high similarities with several ATP-binding proteins. These gene products possess an ATP-binding motif and the ABC transporter signature sequence. The highest scores were found with proteins that are part of the oligopeptide transport systems. Peptide similarities between L. monocytogenes Opp proteins and those from other bacterial species are presented in Fig. 1.
Transcriptional analysis of the oligopeptide permease opp operon.
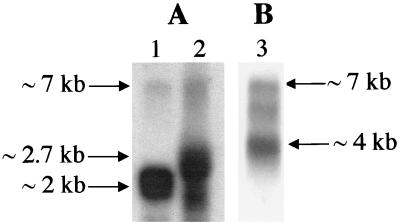
A Northern blot analysis of the five L. monocytogenes opp genes was performed on total RNA prepared from LO28 grown in BHI medium at 37°C to mid-log exponential phase, using intragenic probes of each gene. With the oppA probe (Fig. 2A, lane 1), we found a major ∼2-kb transcript corresponding in size to oppA, which is consistent with the presence of a putative terminator downstream from oppA, and a very weak ∼7-kb transcript, presumably corresponding to a polycistronic transcript of the oppA-to-oppF genes. As a control for the oppA mutant described in the next section, we analyzed the oppA transcription in this strain (Fig. 2A, lane 2). We found a major transcript of ∼2.7 kb, corresponding to oppA and the aphA-3 insertion into the oppA gene. With the oppB probe (Fig. 2B), a weak ∼7-kb transcript was also detected, together with a major ∼4-kb transcript, in agreement with a polycistronic transcription starting from the intergenic noncoding region between oppA and oppB. The same pattern with two large transcripts was detected with each of the other probes (oppC, oppD, and oppF) (data not shown). The polycistronic transcription of the opp genes was confirmed by RT-PCR by amplifying the entire opp operon mRNA using primers corresponding to the 5′ region of oppA and the 3′ region of oppF (data not shown). Taken together, these results indicate that although all opp genes are transcribed as part of an operon, oppA is predominantly expressed alone.
FIG. 2.
Northern blot analysis of the opp operon of L. monocytogenes LO28. Bacterial strains were cultured at 37°C to mid-log exponential phase, and total RNA was extracted and hybridized with an oppA probe (A) or an oppB probe (B). Lanes 1 and 3, LO28 (wild type); lane 2, the oppA mutant.
OppA is essential for uptake of oligopeptides.
We constructed an oppA mutant from strain LO28 by deletion of an internal fragment of oppA and insertion of a kanamycin resistance cassette (aphA-3′) (see Materials and Methods). To complement this mutant, the multicopy plasmid pAT18 (41) carrying oppA under control of PdltA, a strong promoter of LO28 (pAT18-PdltA/oppA) (see Materials and Methods), was then introduced by conjugation into the oppA mutant. We also introduced pAT18 alone into the oppA mutant and the wild-type strain.
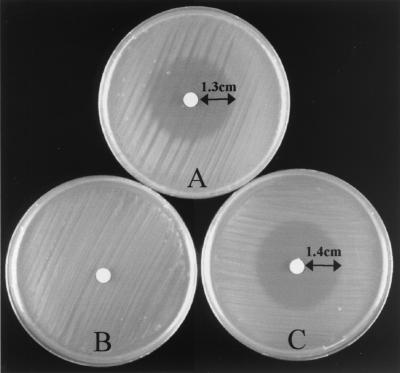
There was no difference between the oppA mutant and the wild-type LO28 with respect to the morphology, aspect of colonies, motility at 22°C, growth in BHI broth at 30 and 37°C, metabolic profiles on an API strip, and hemolytic activity on horse red blood cells at 37°C. The function of OppA was then studied by testing resistance to bialaphos, a toxic peptide derivative. The toxicity of bialaphos was tested in LO28, the oppA mutant, and the transformed strains cultivated on a solid defined minimal medium (MWB). As expected, wild-type LO28 and LO28/pAT18 were highly susceptible to bialaphos (Fig. 3A). In constrast, the oppA and oppA/pAT18 mutants were fully resistant to the toxic peptide (Fig. 3B), and susceptibility was restored in an oppA-complemented mutant (oppA mutant/pAT18-PdltA/oppA) (Fig. 3C). These results demonstrate that OppA is functional and mediates the transport of bialaphos, like other bacterial OppA proteins (18, 31, 32).
FIG. 3.
The oppA mutant is resistant to bialaphos. Bacteria were grown overnight in BHI broth and washed three times in minimal defined medium before being spread onto solid minimal defined medium plates at 37°C, with bialaphos (10 μg) spotted on a filter disc in the center of the plate. After 24 h, the zone of inhibition was measured. (A) LO28; (B) oppA mutant; (C) oppA-complemented mutant.
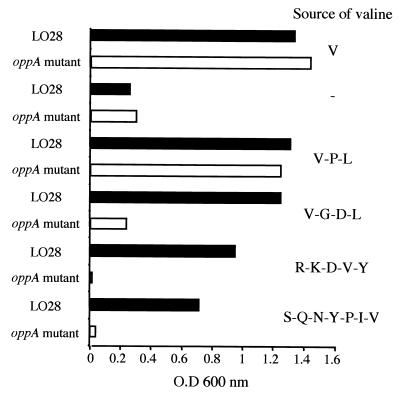
Previous functional studies have revealed that the L. monocytogenes Scott A strain possessed a di- and tripeptide transport system (42) and an oligopeptide transport system (43). To analyze the role of the peptide permease encoded by the opp operon in the utilization of peptides, we took advantage of the fact that valine is an amino acid essential for growth of the auxotrophic species L. monocytogenes. Growth of wild-type LO28 and the oppA mutant was strictly dependent upon the addition of valine in defined minimal medium (Fig. 4). Bacterial growth was then tested in the same medium except that valine was replaced by valine-containing peptides of various sizes (V-P-L, V-G-D-L, R-K-D-V-Y, and S-Q-N-Y-P-I-V). As illustrated in Fig. 4, we found that wild-type and oppA mutant strains grew in the presence of a valine-containing tripeptide (V-P-L), a result consistent with the finding that L. monocytogenes possesses a distinct system for the transport of tripeptides (42). In contrast, the oppA mutant was unable to use peptides longer than three residues, whereas the parental strain grew on all peptides provided (Fig. 4). Similar results were obtained with the wild-type strain or the complemented mutant (data not shown). These data demonstrate that OppA of L. monocytogenes is a functional homologue of the other bacterial OppA proteins and mediates the transport of oligopeptides into the cell.
FIG. 4.
Growth of L. monocytogenes in minimal defined medium lacking valine and/or supplemented with valine or valine-containing peptides. Wild-type or oppA bacteria were grown at 37°C, and the optical density (O.D.) at 600 nm was measured after 36 h of incubation. Controls include bacteria grown with valine (V) or without valine (−).
OppA is required for growth at low temperature.
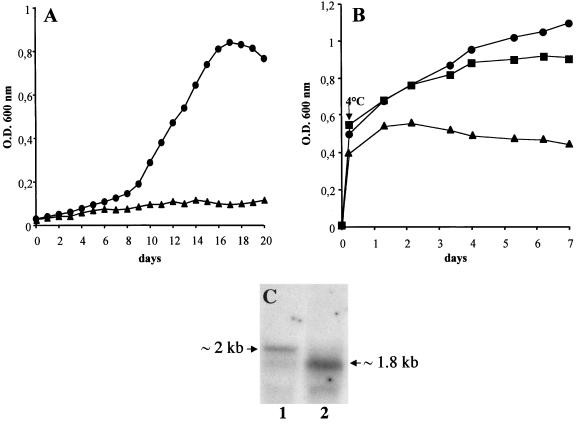
L. monocytogenes grows at temperatures as low as 0°C and can contaminate a variety of refrigerated food products, where bacteria have to find nitrogen sources essential for their development. To evaluate the role of OppA under these conditions, growth curves of the wild-type and mutant strains were obtained in BHI medium at 5°C. The oppA mutant bacteria failed to grow at this temperature during a period of 20 days, whereas the parental strain reached its maximal population density in about 15 days (Fig. 5A). In order to confirm that this phenotype was due to the absence of OppA, we tested the growth of the oppA-complemented mutant at 5°C. In this strain, oppA is carried on plasmid pAT18, whose origin of replication is cryosensitive, indicating that we could not directly test the growth of this strain at low temperature. Thus, LO28/pAT18, the oppA mutant/pAT18, and the oppA-complemented mutant (carrying pAT18-PdltA/oppA) were cultured at 37°C to mid-log exponential phase (optical density of ∼0.5) to allow plasmid replication and the subsequent production of OppA. Cultures were then transferred to 5°C, and bacterial growth was monitored until stationary phase by measuring the optical density. As expected, the oppA mutant could not grow after the cold shock, in contrast to the wild-type strain (Fig. 5B). The growth curve of the oppA-complemented mutant was similar to that of the parental strain, showing that the presence of OppA restores bacterial growth at 5°C. Nevertheless, we observed that the complemented mutant did not reach the same bacterial density as the LO28 strain, presumably due to the progressive loss of the plasmid carrying oppA during the bacterial multiplication at 5°C.
FIG. 5.
The OppA protein of L. monocytogenes is essential for growth at 5°C. Bacteria were cultured in BHI broth, and growth was monitored by measuring optical density (O.D.) at 600 nm. (A) Growth at 5°C. ●, LO28; ▴, oppA mutant. (B) Bacteria were grown to mid-log exponential phase at 37°C and transferred to 5°C until the end of growth. Growth at 5°C is restored in an oppA-complemented mutant. ●, LO28/pAT18; ▴, oppA mutant/pAT18; ▪, oppA-complemented mutant. (C) Northern blot analysis of the oppA gene of L. monocytogenes expressed at 37°C (lane 1) or 5°C (lane 2). LO28 bacteria were cultured at 37 or 5°C to midlog exponential phase, and total RNA was extracted and hybridized with an oppA probe.
We then performed a transcriptional analysis of oppA in LO28 grown at 5°C. We found that oppA was expressed at this temperature to a higher level of transcription than that of bacteria grown at 37°C (Fig. 5C). In addition, the oppA transcript was smaller when expressed at 5°C (∼1.8 kb, versus 2 kb at 37°C), suggesting the presence of a second oppA promoter specifically activated at low temperature.
To understand the mechanisms involved in the OppA-dependent cryotolerance of L. monocytogenes, we analyzed the transcription of the two cold shock genes previously identified in this bacterium, cspL and cspB. Total RNA was prepared from LO28 and oppA mutant strains grown at 37°C (control) and after a cold shock for 30 min at 5°C. We found no difference in the level of expression of cspL or cspB between the two strains tested (data not shown). Although transcription of other, unknown cold shock genes of L. monocytogenes might be affected in the oppA mutant, these results indicate that OppA of L. monocytogenes is required for growth at 5°C, presumably independently from the cold-shock system.
OppA favors intracellular growth of L. monocytogenes in macrophages.
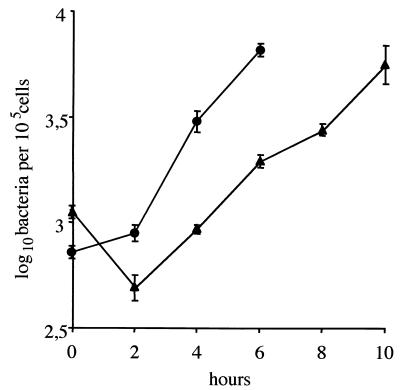
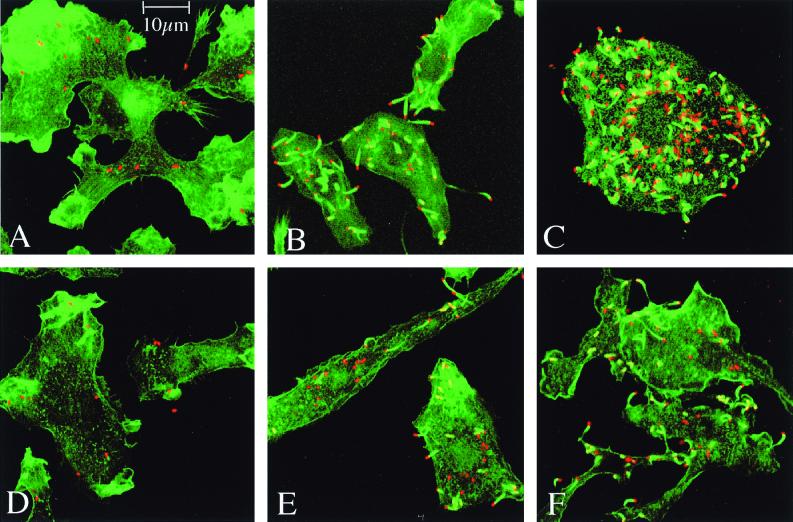
It is known that the intracellular growth of L. monocytogenes is restricted in certain auxotrophic mutants (27). We studied the role of OppA in the intracellular survival of this pathogen. Bone marrow macrophages were exposed for 15 min to wild-type or oppA mutant bacteria (at a bacterium/ cell ratio of 1:1), and intracellular survival of bacteria was monitored for 10 h. The results of a typical experiment are illustrated in Fig. 6. The initial uptake was similar for both strains. Then, wild-type bacteria grew rapidly in macrophages, ultimately inducing cellular lysis after 6 h. After an early drop, the growth of oppA mutant bacteria was delayed, without significant macrophage lysis up to 10 h. At that time, the amount of intracellular mutant bacteria reached that of wild-type bacteria at 6 h. The intracellular fates of wild-type and oppA mutant bacteria (at a bacterium/cell ratio of 15:1) were then examined at various intervals (0, 4, and 8 h) by confocal microscopy after double staining with an anti-Listeria antibody and with β-phalloidin to visualize the F-actin. As shown in Fig. 7A and D, no obvious difference between the two strains was found at time zero postinfection. After 4 and 8 h, most wild-type bacteria were visible inside the cytoplasm associated with typical sheaths of polymerized actin or comet tails with protrusions of bacteria at the surface of macrophages (Fig. 7B and C). For the opp mutant, few comets were visible after 4 h of infection, but a number of bacteria were still confined within phagosomes (Fig. 7E). After 8 h, the number of intracytoplasmic mutant bacteria remained lower than that of wild-type bacteria (Fig. 7F). The escape of wild-type and oppA mutant bacteria from the phagosomes was then examined by quantitative electron microscopy on macrophages infected for 3 h. We determined the phagosomal or cytoplasmic location of bacteria inside infected cells. We found 41% of the wild-type bacteria inside the cytoplasm and 59% in phagosomes. In contrast, 21% of oppA mutant bacteria were located in the cytoplasm and 79% were still confined within phagosomes (data not shown). Taken together, these results suggest that OppA plays a role in the intracellular survival of L. monocytogenes in macrophages, both in the phagosome escape and in the intracytoplasmic multiplication of infected macrophages.
FIG. 6.
Growth of L. monocytogenes in macrophages. Bone marrow-derived macrophages from C57/BL6 mice were exposed for 15 min (time zero) to bacteria (1 bacterium per cell), and bacterial survival was monitored for 10 h after the infection. ●, LO28 (wild type); ▴, oppA mutant. Bacterial growth of the oppA mutant was delayed compared to that of wild-type bacteria. Error bars indicate standard deviations.
FIG. 7.
Confocal microscopy of bone marrow-derived macrophages infected (15 bacteria per cell) with LO28 (A to C) or the oppA mutant (D to F). Macrophages were observed at time zero (A to D), at 4 h (B and E), and at 8 h (C and F) postinfection. F-actin was stained with phalloidin (green). Bacteria were labeled with anti-Listeria antibodies (red). In the absence of OppA, there is a reduction of intracellular growth.
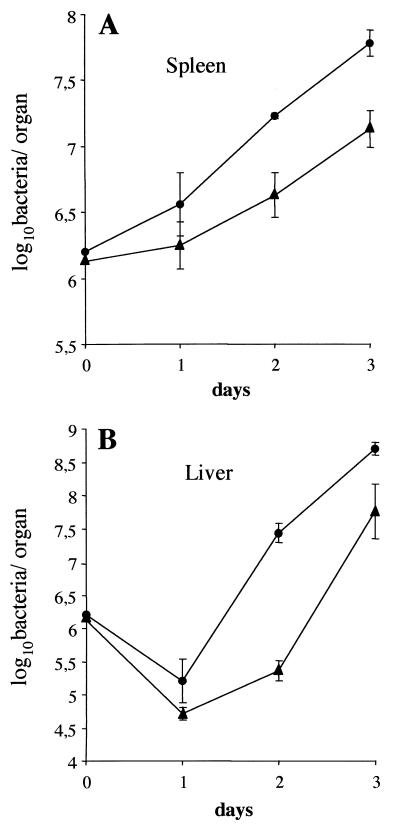
The virulence of the mutant was then studied in mice inoculated i.v. with LO28 or the oppA mutant. The 50% lethal dose of the mutant (105 bacteria per mouse) was similar to that of wild-type bacteria (104.8 bacteria per mouse). This result was confirmed by monitoring the kinetics of bacterial survival over a 3-day period in the livers and spleens of mice inoculated i.v. with 8 × 105 bacteria. The wild-type bacteria grew rapidly in organs (Fig. 8) until death by day 4. The growth of mutant bacteria was delayed in the spleen and the liver, with a 1- to 2-log-unit difference by day 2 to 3 compared to that of the wild-type strain (Fig. 8), but reached a multiplication level similar to that of wild-type bacteria, killing the mice by day 5. These results confirm the role of OppA in the intracellular survival of L. monocytogenes, with the oppA mutant having a growth delay but ultimately being able to infect cells or organs at a high level.
FIG. 8.
Growth of L. monocytogenes LO28 (wild type) (●) or the oppA mutant (▴) in the spleens (A) or livers (B) of mice inoculated i.v. with 8 × 105 bacteria. Error bars indicate standard deviations.
DISCUSSION
In this work, we identified in the pathogen L. monocytogenes an oligopeptide permease operon adjacent to the recently described mecA locus (3). This operon encompasses five genes whose products are homologous to those of several opp operons identified in gram-negative and gram-positive species, including B. subtilis and S. enterica serovar Typhimurium, and which display the same genetic organization (Fig. 1). The first gene of the L. monocytogenes operon, oppA, is separated by a terminator from the downstream genes (oppB, oppC, oppD, and oppF). Transcriptional analysis revealed that oppA is strongly expressed during exponential growth in nutrient-rich medium (BHI broth) compared to the other genes of the operon (Fig. 2). It encodes a 62-kDa protein of 558 amino acids that is homologous to several substrate-binding proteins of oligopeptide transport systems, with a peptide leader, a lipoprotein attachment site, and a bacterial extracellular solute-binding protein signature sequence. This suggests that OppA is a lipoprotein attached to the external part of the cytoplasmic membrane and required for peptide uptake. This assumption was supported by showing that OppA is an oligopeptide-binding protein involved in the transport of oligopeptides. An oppA mutant was resistant to bialaphos, a toxic peptide derivative known to be transported via the opp system into B. subtilis (31), S. pyogenes (32), E. coli, and serovar Typhimurium (18). The susceptibility of the oppA mutant of L. monocytogenes to bialaphos was restored by complementation (Fig. 3). Further evidence that OppA of L. monocytogenes mediates the transport of oligopeptides into the cell was obtained by testing bacterial growth in a minimal defined medium where valine, an essential amino acid, was replaced by valine-containing peptides of various sizes. As expected, the oppA mutant was unable to use peptides longer than three residues, whereas the parental strain grew on all peptides provided (Fig. 4). We also found that wild-type and oppA mutant bacteria could grow in the presence of a valine-containing tripeptide, which is consistent with the finding that L. monocytogenes possesses a distinct system for the transport of this peptide (42).
An important feature of the epidemiology of L. monocytogenes is its capacity to grow at low temperatures (0 to 4°C). Growth of this pathogen in refrigerated food products depends upon the acquisition of nitrogen sources. A previously undescribed finding of this study was that oppA of L. monocytogenes is required for growth at low temperature. Indeed, the oppA mutant failed to grow at 5°C in BHI broth, in contrast to the case for the wild-type strain. Growth at low temperature was restored by complementation of the mutant with the oppA gene placed on a multicopy plasmid (Fig. 5). Transcriptional analysis revealed that oppA is expressed at 5°C at a higher level than is seen at 37°C (Fig. 5C). The reason for this OppA-dependent growth at low temperature remains unclear. This is probably not related to the expression of cold shock proteins, since the levels of transcription of two known cold shock genes (cspB and cspL) are not altered in the oppA mutant, although this does not rule out the possibility that the function of these or other, unknown cold shock proteins might be affected. It can be speculated that the opp system might transport specific oligopeptides acting as “cold” pheromones activating a transduction signal pathway specific for bacterial replication at low temperature, as the opp system of B. subtilis is required for sensing the competence pheromone CSF (31, 36, 39) or the opp system of E. faecalis is required for sensing the pheromones necessary for the induction of conjugation (25). In this respect, L. monocytogenes OppA shares 37% identity with the pheromone-binding proteins (TraC) of several conjugative plasmids of E. faecalis. An alternative could be that the opp system is the only active transport system for supplying L. monocytogenes with peptides and essential amino acids at low temperature. Finally, the oligopeptide permease might be involved in cryoprotection by accumulation of peptides and/or derived peptides acting, like the osmolyte glycine betaine, in the chill adaptation of L. monocytogenes (2, 14, 21, 22, 38). Glycine betaine is transported by a sodium-driven uptake system (13) and an ATP-driven transporter (Gbu) belonging to the superfamily of ABC transporters, and the transport by Gbu is osmotically and chill activated (14, 21). Indeed, some organic compounds, like glycine betaine or proline betaine, are as effective as osmoprotectants or cryoprotectants in L. monocytogenes (2). In addition, L. monocytogenes accumulates high levels of osmolytes when grown on a variety of processed meats at reduced temperatures (38). Finally, it has been shown that glycine- and proline-containing peptides stimulate growth at high osmolarity and that peptides from the growth medium contribute to osmoregulation (1). Taken together, these data link the salt and cold tolerance of L. monocytogenes with the intracellular accumulation of compounds like the so-called compatible solutes, osmolytes. It is possible that unknown peptides internalized by Opp could be involved in cryoprotection.
Furthermore, the role of the opp transport system of L. monocytogenes might be important in the process of contamination of food products. It has been suggested that casein is degraded in fermented dairy products such as cheese by the cell envelope-located proteinases of lactococci, resulting in the formation of a wide variety of peptides ranging from 4 to at least 18 residues (9). During the fermentation phase, the opp transport system of L. monocytogenes might play a crucial role in supplying bacteria with essential amino acids.
Another important finding of this work is that OppA plays a role in the intracellular survival of L. monocytogenes. In the absence of OppA, bacterial growth was delayed in macrophages in vitro (Fig. 6) as well as in organs of mice during the early phase of infection (Fig. 8). A confocal microscopic study suggests that OppA favors early escape from phagosomes and intracytoplasmic multiplication in macrophages (Fig. 7). A quantitative electron microscopy study confirmed that OppA was implicated in the phagosomal escape. Indeed, only 21% of the oppA mutant bacteria reached the cytoplasm of macrophages after 3 h of infection (versus 41% for the wild-type bacteria). This means that the peptide uptake might play a role at these steps of the intracellular survival of L. monocytogenes. Among the hypotheses to explain the role of OppA, one can speculate that the peptides accumulated by this oligopeptide permease might protect bacteria in phagosomes, as suggested by the initial killing of the oppA mutant inside macrophages (Fig. 6). Alternatively, the peptide uptake might activate an unknown transduction signal pathway, ultimately modulating the kinetics of expression of virulence genes required to escape from phagosomes. The delayed growth in the macrophage cytoplasm might be due to a limitation of nutrients. Indeed, it has been demonstrated that L. monocytogenes utilizes intracellular peptides as a source of amino acids during its intracellular replication (27). However, we found that the oppA mutant could still multiply intracellularly and is fully virulent in the mouse. This is not surprising, since the cytoplasm of eucaryotic cells behaves like a rich medium, explaining the fact that most auxotrophic mutants of L. monocytogenes remain virulent (27). In addition, the other peptide permeases present in L. monocytogenes (42) might compensate the Opp system for the intracytoplasmic uptake of peptides.
In conclusion, we have demonstrated that OppA of L. monocytogenes plays a crucial role in the uptake of peptides, with pleiotropic effects on growth at low temperature and the intracellular survival of this pathogen.
ACKNOWLEDGMENTS
We kindly thank S. Nair for critical reading of the manuscript, D. Mazel for providing bialaphos, P. Trieu-Cuot for the pAT18 vector, E. Abachin for the dltA promoter from LO28, and Y. Goureau for technical assistance in confocal microscopy.
E.B. received a fellowship from the Ministère de l'Education Nationale de la Recherche et de la Technologie. This work was supported by INSERM, The University of Paris V, and two grants from the European Commission (contracts ERBCHRXCT 94-0451 and CT980036).
REFERENCES
- 1.Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;l41:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 2.Bayles D O, Wilkinson B J. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett Appl Microbiol. 2000;30:23–27. doi: 10.1046/j.1472-765x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.Borezée E, Msadek T, Durant L, Berche P. Identification in Listeria monocytogenes of MecA, a homologue of the Bacillus subtilis competence regulatory protein. J Bacteriol. 2000;182:5931–5934. doi: 10.1128/jb.182.20.5931-5934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–117. doi: 10.1046/j.1365-2958.1998.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. JBacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cundell D R, Pearce B J, Sandros J, Naughton A M, Masure H R. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect Immun. 1995;63:2493–2498. doi: 10.1128/iai.63.7.2493-2498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darmstadt G L, Mentele L, Podbielski A, Rubens C E. Role of group A streptococcal virulence factors in adherence to keratinocytes. Infect Immun. 2000;68:1215–1221. doi: 10.1128/iai.68.3.1215-1221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detmers F J, Kunji E R, Lanfermeijer F C, Poolman B, Konings W N. Kinetics and specificity of peptide uptake by the oligopeptide transport system of Lactococcus lactis. Biochemistry. 1998;37:16671–16679. doi: 10.1021/bi981712t. [DOI] [PubMed] [Google Scholar]
- 10.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. . (Erratum, 55:752.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox P F. Indigenous enzymes in milk-proteinases. In: Fox P F, editor. Advanced dairy chemistry. 1. Proteins. London, United Kingdom: Elsevier; 1992. pp. 310–321. [Google Scholar]
- 12.Gaillot O, Pellegrini E, Bregenholt S, Nair S, Berche P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol Microbiol. 2000;35:1286–1294. doi: 10.1046/j.1365-2958.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt P N, Smith L T, Smith G M. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J Bacteriol. 1996;178:6105–6109. doi: 10.1128/jb.178.21.6105-6109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhardt P N, Tombras Smith L, Smith G M. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J Bacteriol. 2000;182:2544–2550. doi: 10.1128/jb.182.9.2544-2550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodell E W, Higgins C F. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1987;169:3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 18.Higgins C F, Gibson M M. Peptide transport in bacteria. Methods Enzymol. 1986;125:365–377. doi: 10.1016/s0076-6879(86)25031-4. [DOI] [PubMed] [Google Scholar]
- 19.Hiles I D, Gallagher M P, Jamieson D J, Higgins C F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987;195:125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- 20.Hiles I D, Powell L M, Higgins C F. Peptide transport in Salmonella typhimurium: molecular cloning and characterization of the oligopeptide permease genes. Mol Gen Genet. 1987;206:101–109. doi: 10.1007/BF00326543. [DOI] [PubMed] [Google Scholar]
- 21.Ko R, Smith L T. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl Environ Microbiol. 1999;65:4040–4048. doi: 10.1128/aem.65.9.4040-4048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 24.Kunji E R, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 25.Leonard B A, Podbielski A, Hedberg P J, Dunny G M. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc Natl Acad Sci USA. 1996;93:260–264. doi: 10.1073/pnas.93.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson M D, Blank V, Brade G, Higgins C F. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature. 1986;321:253–256. doi: 10.1038/321253a0. [DOI] [PubMed] [Google Scholar]
- 27.Marquis H, Bouwer H G, Hinrichs D J, Portnoy D A. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61:3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair S, Frehel C, Nguyen L, Escuyer V, Berche P. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol Microbiol. 1999;31:185–196. doi: 10.1046/j.1365-2958.1999.01159.x. [DOI] [PubMed] [Google Scholar]
- 30.Payne J W, Smith M W. Peptide transport by micro-organisms. Adv Microb Physiol. 1994;36:1–80. doi: 10.1016/s0065-2911(08)60176-9. [DOI] [PubMed] [Google Scholar]
- 31.Perego M, Higgins C F, Pearce S R, Gallagher M P, Hoch J A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 32.Podbielski A, Pohl B, Woischnik M, Korner C, Schmidt K H, Rozdzinski E, Leonard B A. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (opp) and its effect on cysteine protease production. Mol Microbiol. 1996;21:1087–1099. doi: 10.1046/j.1365-2958.1996.661421.x. [DOI] [PubMed] [Google Scholar]
- 33.Poyart C, Abachin E, Razafimanantsoa I, Berche P. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect Immun. 1993;61:1576–1580. doi: 10.1128/iai.61.4.1576-1580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premaratne R J, Lin W J, Johnson E A. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 36.Rudner D Z, LeDeaux J R, Ireton K, Grossman A D. The spoOK locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991;173:1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Smith L T. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl Environ Microbiol. 1996;62:3088–3093. doi: 10.1128/aem.62.9.3088-3093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon J M, Magnuson R, Srivastava A, Grossman A D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 40.Thomas T D, Mills O E. Proteolytic enzymes of starter bacteria. Neth Milk Dairy J. 1981;35:255–273. [Google Scholar]
- 41.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene. 1991;102:99–104. doi: 10.1016/0378-1119(91)90546-n. [DOI] [PubMed] [Google Scholar]
- 42.Verheul A, Hagting A, Amezaga M R, Booth I R, Rombouts F M, Abee T. A di- and tripeptide transport system can supply Listeria monocytogenes Scott A with amino acids essential for growth. Appl Environ Microbiol. 1995;61:226–233. doi: 10.1128/aem.61.1.226-233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verheul A, Rombouts F M, Abee T. Utilization of oligopeptides by Listeria monocytogenes Scott A. Appl Environ Microbiol. 1998;64:1059–1065. doi: 10.1128/aem.64.3.1059-1065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vicente M F, Baquero F, Perez-Diaz J C. Cloning and expression of the Listeria monocytogenes haemolysin in E. coli. FEMS Microbiol Lett. 1985;30:77–79. [Google Scholar]
- 45.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]