Abstract
Aged or fermented garlic extract (FGE) is a natural remedy that improves vascular function through increasing vascular nitric oxide (NO) bioavailability. This is because nitrite (NO2−), a NO metabolite, can be produced through bioconversion with macrobacteria during the fermentation of foods like garlic. We aimed to evaluate the effects of NO2− in FGE on blood flow (BF), blood pressure (BP), velocity of the common carotid artery (CCA) and internal carotid artery (ICA), regional cerebral BF (rCBF), and peripheral BF (PBF). The study was divided into two parts: (1) Thirty healthy adults were divided into FGE and placebo groups to compare BP and velocity of the CCA and ICA; and (2) Twenty-eight healthy adults were divided into FGE and placebo groups to compare rCBF and PBF and determine changes before/after ingestion. Significant changes were noted in BP and the velocity of both CCA 30–60 min after FGE ingestion. FGE ingestion resulted in significant increases in rCBF and increases in body surface temperature through alterations in PBF. No detectable clinical side effects were noted. Overall, oral administration of NO2− containing FGE demonstrated acute positive effects in upregulating BF, including the CCA, BP, rCBF, and PBF. Follow-up studies with larger sample sizes and long-term ingestion may be needed.
Keywords: fermented garlic extract, blood flow, blood pressure, nitric oxide
1. Introduction
Cardiovascular disease (CVD) is the leading cause of mortality worldwide [1], and age is a major risk factor. CVD related mortality increases in older adults, due in large part to adverse changes occurring in arteries associated with vascular dysfunction. Age-related declines in cardiovascular functions may impair cerebral blood flow (BF) regulation, leading to the disruption of neuronal micro-environmental homeostasis [2]. As the brain requires a large amount of energy to sustain neuronal metabolism, cerebral BF is essential in maintaining normal brain functions. Moreover, the impairment of cerebrovascular or neurovascular functions can increase the incidence of neurological disorders, such as vascular cognitive impairment and Alzheimer’s disease [3].
Nitric oxide (NO) is a gaseous signal molecule that is generated from the endothelial cells and causes relaxation of vascular smooth muscle. In the aging process, progressive declines in NO production or NO bioavailability are associated with decreased antioxidants in the vessels [4]. Additionally, impaired NO bioavailability is characterized by disturbed vasodilator and anticoagulant function, increased inflammation, and a breakdown of barrier function, which leads to atherosclerosis formation [5,6]. This NO is produced through nitrate (NO3−)—nitrite (NO2−)—in the NO pathway [7,8,9]. Alternatively, when NO3− is aged or fermented, it is converted to NO2−. NO2− is converted to NO by combining with glutathione present in the human body, or combining with flavonoids derived from plants, or in response to intestinal microorganisms [7,8,9]. The NO3− –NO2−– NO pathway is a major alternative source of NO and is essential for NO dependent physiological functions in the body [7,8,9]. In particular, NO2− is emerging as an endogenous signaling molecule, with potential therapeutic implications for CVD [10,11,12].
Many plants contain NO3− [13]. NO3− can naturally change to NO2− through digestion in the human body, but most of it is discharged without switching [14]. We hypothesized that nitric oxide-related effects would be greater in the body if plants were fermented and consumed in the NO2− state. We previously developed a new bioconversion technique that allows for the production of high concentrations of NO2− from various plant materials, including garlic, lettuce, and beans. This occurs during the fermentation process and requires the long-term stabilization of NO2− in fermented fluid [15,16,17]. In earlier studies, fermented garlic extract (FGE) has shown to decrease BP in hypertensive rodent models through the activation of intracellular NO signaling in the artery [18]. Additionally, FGE attenuated monocrotaline-induced pulmonary hypertension by decreasing pulmonary endothelial injury via the NO-sGC-PKG pathway [19]. Direct application of FGE into the brain surface induced the upregulation of cerebral BF through activation of intracellular NO signaling [20].
In previous animal studies, we confirmed that FGE containing NO2− may result in effective NO signaling in the vascular system. However, more research is needed to confirm the effectiveness and safety of FGE in humans. Therefore, in this study, we aimed to confirm whether FGE contributed to changes in BF in healthy adults, including regional cerebral blood flow (rCBF), carotid artery (CA) BF, peripheral blood flow (PBF), and BP and to confirm safety.
2. Materials and Methods
2.1. Preparation and Fermentation of Garlic Fermented Broth
The garlic fermented broth used in this study was supplied by HumanEnos (Wanju-gun, Jeonbuk, Republic of Korea) and was prepared similarly to previous studies [18,21]. The manufacturing process included first peeling the skin from raw garlic. The garlic was then washed, sterilized, and ground for 24 hours, after which, the mixture was diluted with water at a constant ratio (1:9 [w/v]). Activated Bacillus subtilis was then inoculated and aerobic fermentation was carried out at 37 °C for one month. Fermentation was stopped when NO2− in the fermentation broth reached a concentration of 150 ppm or more. The supernatant was separated from the suspension by centrifuge, and the separated fermented garlic solution was concentrated using an evaporator to dry FGE up to at least 2,000 ppm of the concentration of NO2− [18,19].
2.2. Determination of NO2− Ions in the Garlic Fermentation Broth and NaNO2 Solution
NO2− levels in the fermentation broth or dried FGE were quantified using the NO3−/NO2− Colorimetric Assay Kit (Cayman Chemical Co., Ann Arbor, USA). The same amounts of reaction solution and Griess reagent (2.5% [v/v] phosphoric acid, 1% [w/v] sulfanilamide, and 0.1% [w/v] naphylethylenediamine) were mixed. After reacting at room temperature for ten min, the absorbance was measured at 540 nm using a UV spectrophotometer (Ultrospec 2100 Pro, Amersham Pharmacia Biotech, Cambridge, UK). In order to quantify the NO2− amount in the garlic fermentation broth, NaNO2 (Sigma-Aldrich® Co., St. Louis, MO, USA) as a standard material was diluted with distilled water. After reacting the diluted NaNO2 solution with Griess reagent, the standard curve was generated by using the UV spectrophotometer configured for UV absorbance measurements. NO2− levels were then quantified by applying the absorbance value of garlic fermentation broth to the standard curve.
2.3. Determination of NO Release in Simulated Gastric Fluid
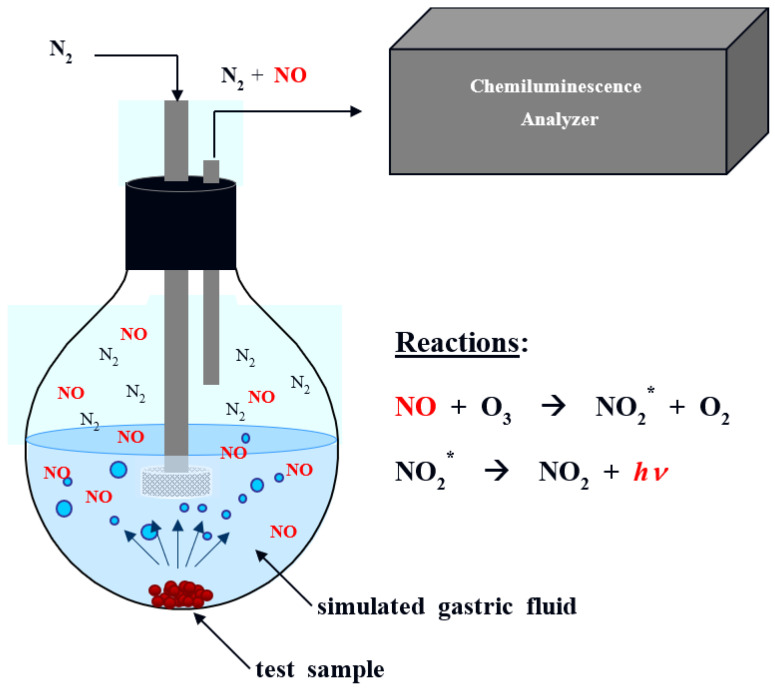
NO generation profiles from FGE in simulated gastric fluid (SGF) were collected using a Sievers 280 chemiluminescent NO analyzer (Boulder, CO, USA). SGF is composed of 50 mM HCl, 75 mM NaCl, and 13 mM KCl (pH 1.3). The instrument was calibrated with an atmospheric sample that had been passed through an NO zero filter and a 24.1 ppm NO gas standard (balance N2). One FGE tablet was finely ground, dissolved in phosphate buffered saline (1 mL), and then immersed in deoxygenated SGF at 37 °C. Liberated NO was provided from the SGF to the analyzer with a stream of N2 bubbled into the solution at a flow rate of 80 mL/min. NO was detected via a chemiluminescent reaction with ozone (Figure 1). As control, one tablet of Neo40® (Humann, Austin, TX, USA), which is a representative NO-increasing supplement, was finely ground, dissolved in the buffer (1 mL), and put in the SGF to quantify the amount of NO release.
Figure 1.
NO, nitric oxide; O3, ozone; NO2*, excited nitrogen dioxide; O2, oxygen; hv, photon; Schematic illustration of the chemiluminescence technique for measurement of NO gas generated from the test sample in simulated gastric fluid (pH 2). NO reacts with O3 to create NO2*, and NO is quantified by measuring the hv emitted when NO2* converted to stable NO2.
2.4. Formulation of FGE Tablets
One tablet of encapsulated FGE consisted of 180 mg of dried FGE containing 7 mg of NO2− and 270 mg of other food grade ingredients. The dose of NO2− ions in one FGE tablet was determined based on our previous animal study where the oral, single administration of FGE solution (10 mg of NO2−/mL) showed dose-dependent decrease in systolic BP in hypertensive rats [18]. The dose was also determined by other clinical studies that demonstrated decreased BP due to inorganic NO2− supplements without clinical side effects [22,23]. FGE and placebo groups were administered single-dose 450 mg encapsulated FGE and 450 mg placebo tablets, respectively. The placebo tablet was prepared with a food ingredient that was harmless to the human body that did not contain garlic, and its weight and shape were almost the same compared with the FGE tablet.
2.5. Clinical Study
2.5.1. Experimental Design and Participants
This study was conducted from October 2020 to March 2021. This study was approved by the Ethics Committee of Pusan National University Yangsan Hospital (approval no. 04-2020-038, 04-2020-036), and registered on ClinicalTrials.gov (accessed on 1 November 2022) (NCT05349604, NCT05349253).
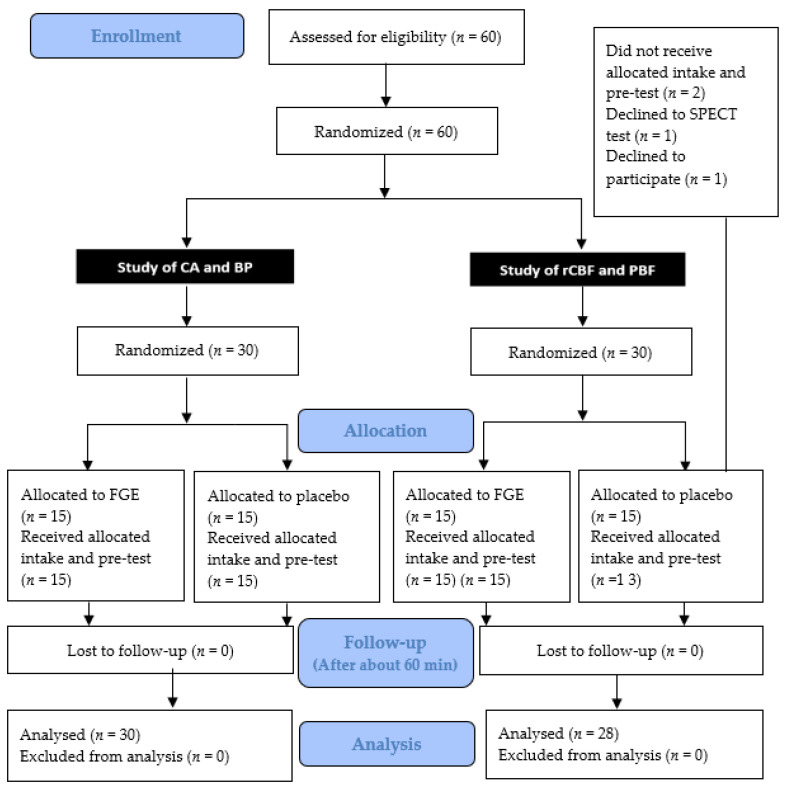
Informed consent was obtained from all interested and eligible participants. A total 60 participants were initially included in the experiment and were divided into each study: (1) a study of CA and BP (04-2020-038, NCT05349604) and (2) a study of rCBF and PBF (04-2020-036, NCT05349253). Two participants were ultimately excluded, as one declined to participate and one canceled the evaluations. Therefore, 58 participants were enrolled in this experiment. The characteristics of the participants (n = 58) in each experimental group are summarized in Table 1. Figure 2 is an overview of the study progress according to the CONSORT guidelines.
Table 1.
Characteristics of participants.
| Experiment Set | Groups | Age | Sex | Total Number | |
|---|---|---|---|---|---|
| M ± SD | Male | Female | |||
| Study of CA and BP (04-2020-038, NCT05349604) |
FGE | 61.33 ± 4.85 | 8 | 7 | 15 |
| Placebo | 61.20 ± 12.91 | 4 | 11 | 15 | |
| Study of rCBF and PBF (04-2020-036, NCT05349253) |
FGE | 54.27 ± 10.91 | 8 | 7 | 15 |
| Placebo | 55.92 ± 13.91 | 3 | 10 | 13 | |
CA, carotid artery; BP, blood pressure, FGE, fermented garlic extract; rCBF, regional cerebral blood flow; PBF, peripheral blood flow; M, mean; SD, standard deviation.
Figure 2.
Flowchart of the study progress according to the CONSORT guidelines.
This experiment was an investigator-led, randomized, double-blind, placebo-controlled study. We determined the experiment group by: (1) screening for BP; (2) surveying for demographic data, including sex, age, height, and weight; (3) determining the past medical history and concomitant diseases present; (4) smoking and drinking history intake; (5) drug administration history intake; and (6) fertility, pregnancy, and lactation history.
We recruited adults aged ≥19 years who had not participated in any other clinical trials in the past 3 months. Pregnant women, individuals taking medications or food that can affect BP, and individuals with severe comorbidities, such as hypertension, cardiovascular disease, autoimmune diseases, and liver or kidney disease were excluded from the study.
2.5.2. Doppler Ultrasonography (CA and BP)
Thirty healthy individuals were recruited and equally and randomly divided into FGE and placebo groups. On the day of the test, participants visited the Department of Neurology, Pusan National University Yangsan Hospital and had carotid Doppler ultrasounds performed to measure the changes in the velocity of the CA. The CA velocity was measured by subdividing the artery into the common carotid artery (CCA) and internal carotid artery (ICA). The BP was measured using a BP measurement device (BPBIO320, Inbody, Seoul, Republic of Korea).
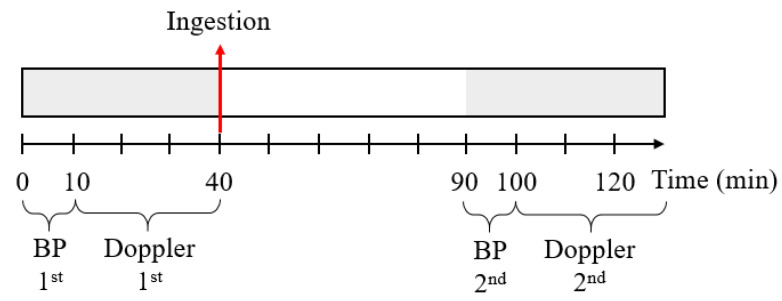
Before ingesting FGE or placebo tablets, the Doppler ultrasounds of the bilateral CA and BP measurements were performed to get baseline values. After, tablets were administered to the participants, and CA velocity and BP measurements were performed again approximately 30–60 min after ingestion [24] (Figure 3).
Figure 3.
Timeline of the first part of the clinical study to measure carotid artery velocity and blood pressure (BP).
2.5.3. Measurement of rCBF and PBF
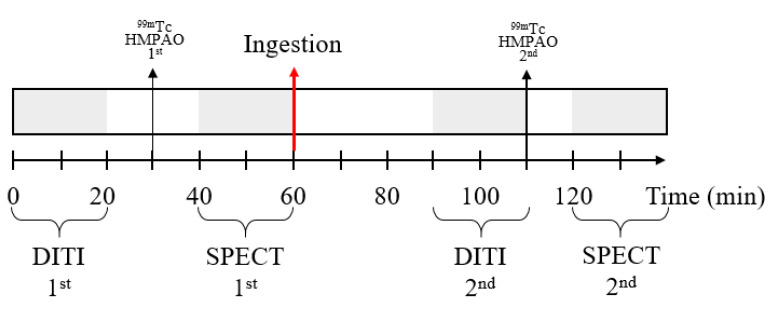
Twenty-eight healthy individuals were recruited and were randomly divided into groups of 15 and 13 participants for the FGE and placebo groups, respectively. Before ingesting the FGE or placebo tablet, 28 participants were examined using single photon emission computed tomography (SPECT, General Electric NM 830 SPECT, USA) and digital infrared thermographic imaging (DITI, Iris-XP, Medi-core, Seoul, Republic of Korea) scanning. Then, the test product was orally administered to the participants. Thereafter, they underwent SPECT and DITI measurements again around 30–60 min after ingestion (Figure 4). The primary outcome assessment was made by rCBF using SPECT [25,26]. Changes in rCBF were measured through Tc-99 m HMPAO SPECT imaging, which was then converted into a Z-score using Tc-99 m HMPAO SPECT [27]. The standard set of the volume that defines each cortex area was analyzed by a p value < 0.0001 (cluster size 50 voxel), based on the Automated Anatomical Labeling Atlas [28]. In this study, the body surface temperature (BST) test was performed to confirm the PBF. The BST was measured using DITI (Iris-XP, Medi-core, Seoul, Republic of Korea).
Figure 4.
Timeline of the second part of the clinical study to measure rCBF and PBF. SPECT, single photon emission computed tomography; DITI, digital infrared thermographic imaging; rCBF, regional cerebral blood flow; PBF, peripheral blood flow.
2.6. Safety Variable Analysis
Safety evaluations were conducted on all participants who participated in the study and participants who ingested the product at least once. The NCI Common Terminology Criteria for Adverse Events (CTCAE, ver4.0) was utilized, with responses of “none (0)”, “mild (1)”, “moderate (2)”, and “severe (3)”.
2.7. Statistical Analysis
The data were analyzed within each study, and no statistical analyses were performed between the two studies. The Wilcoxon signed rank test is a nonparametric statistical hypothesis test used to evaluate whether population means differ in rank by comparing repeated measures of two related, matched, or single samples. Efficacy was evaluated using Wilcoxon signed rank tests and before-after differences were evaluated using Mann–Whitney U tests. Ranked analysis of covariance (Quade’s test) was also used to control for baseline differences in age and covariates as well as to compare the outcome before and after ingestion. A p-value of <0.05 was considered to be statistically significant, and statistical analyses were performed using R statistical software (version 4.0.3; The R Foundation). The SPECT image data were analyzed using a 3D voxel-based statistical analysis (SPM) procedure and an ROI-based method (p FWE-corr < 0.001, uncorrected for multiple comparison with cluster extent threshold Ke = 50 voxels).
3. Results
3.1. Induction of NO from FGE under Artificial Gastric Juice
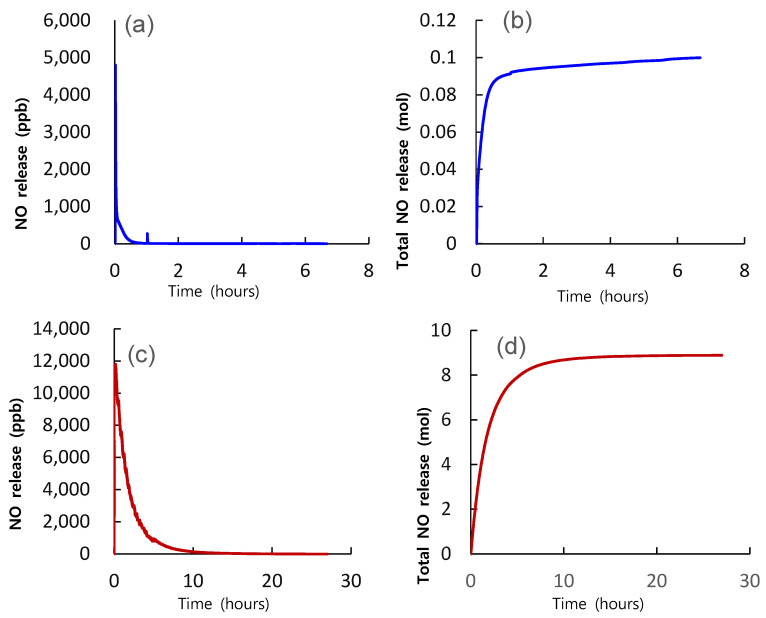
Compared to the Neo40® tablet, the FGE tablet in this study had a higher amount of NO release and a longer duration of NO release time in the artificial gastric juice (pH 1.5). Based on the serving size, the total mass of NO produced by one tablet of Neo40® was approximately 0.1 mol, and the total mass of NO produced by one tablet of FGE was approximately 8.9 mol. Moreover, the initial flux of NO was approximately 4800 and 11,800 ppb in the Neo40® and FGE tablets, respectively. In addition, the durations of NO until the time when NO release was finished were < 6 h and 24 h for the Neo40® and FGE (Figure 5) tablets, respectively.
Figure 5.
Representative line histograms showing time-dependent changes of NO release of the single FGE tablet and Neo40® tablet on the artificial gastric juice (pH 1.5), respectively. Panels (a,b) present the data of Neo40® tablets, while panels (c,d) present the data of FGE tablets. Panels (a,c) show the concentration (ppb) of NO over time, while panels (b,d) present the mass (mol) of NO over time.
3.2. Changes in BP and CA Velocity
Thirty participants completed the study and did not drop out. The results of the test of equal variances for the two sexes were F = 0.083, p = 0.934. The difference between the FGE and placebo groups had a p-value > 0.05, indicating that there was no statistically significant difference. Therefore, no significant differences existed in the sex distribution between the two groups, indicating that homogeneity was secured. Additionally, the FGE and placebo groups had a mean (±standard deviation) age of 61.33 (±4.85) and 61.20 (±12.91) years, respectively. The results of the test of equal variances for the two groups was F = 1.466, p = 0.217. The difference between the FGE and placebo groups had a p-value > 0.05, indicating that there was no statistically significant difference in the age distribution between the two groups and that homogeneity was secured.
Table 2 shows the result of the changes in BP over time in each group. In the placebo group (n = 15), baseline systolic and diastolic BPs were 133.4 ± 23.83 mm Hg and 84.20 ± 17.68 mm Hg, respectively, and the baseline heart rate was 80 ± 10 bpm. Slight reductions in systolic (9.20 mm Hg) and diastolic (9.07 mm Hg) pressures were noted after ingestion of the placebo tablet; however, the changes were not statistically significant. In contrast, in the FGE group (n = 15), the average baseline systolic and diastolic BPs were 124.0 ± 13.7 mm Hg and 79.4 ± 12.3 mm Hg, respectively. A marked reduction of systolic (16.93 mm Hg) and diastolic (12.34 mm Hg) BPs 30 min after the ingestion of the FGE tablet was observed, which was significantly statistically different than the before values (p = 0.001).
Table 2.
Comparison of changes in arterial BP before and after FGE intake.
| Systolic BP | Diastolic BP | |||||||
|---|---|---|---|---|---|---|---|---|
| before | after | Z | p | before | after | Z | p | |
| FGE | 124 ± 13.76 | 107.07 ± 15.29 | −3.325 | * 0.001 | 79.47 ± 12.30 | 67.13 ± 12.47 | −3.355 | * 0.001 |
| Placebo | 133.40 ± 23.83 | 124.20 ± 26.19 | −1.665 | 0.096 | 84.20 ± 17.68 | 75.13 ± 11.77 | −1.877 | 0.061 |
* p < 0.05, Data are expressed as mean ± standard deviation. BP, blood pressure; FGE, fermented garlic extract.
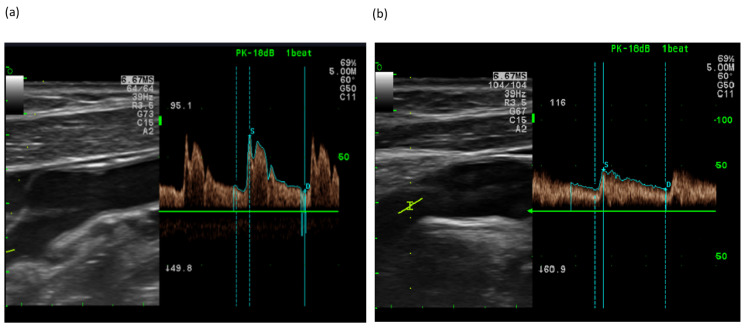
The CA velocity of the bilateral CCA and ICA was measured by Doppler ultrasound before and after ingestion of the test products. In the placebo group, the peak systolic flow velocity (Psv) of the right CCA was 22.2 ± 2.2 cm/sec before and 22.4 ± 4.4 cm/sec 30 min after taking the placebo tablet. In the FGE group the Psv of the right CCA was 24.4 ± 2.2 cm/sec before and 19.9 ± 4.4 cm/sec 30 min after taking the FGE tablet (Figure 6).
Figure 6.
Representative Doppler ultrasound images illustrating changes in blood flow in the common carotid artery before (a) and after (b) the ingestion of a fermented garlic extract tablet.
According to the statistical analysis, the treatment with the FGE tablet in the FGE group caused a marked reductions in the Psv and peak diastolic flow velocity (Edv) in right the CCA (Z = −2.413, p = 0.016, Z = −3.114, p = 0.002), right ICA (Z = −2.480, p = 0.013), and left CCA (Z = −2.204, p = 0.028, Z = −2.240, p = 0.025). In the placebo group, the placebo treatment did not induce a significant change in either the Psv or Edv of the CCA and ICA (Table 3).
Table 3.
Comparison of CABF velocity before and after tablet ingestion.
| Right | Left | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CCA | ICA | CCA | ICA | ||||||
| Psv | Edv | Psv | Edv | Psv | Edv | Psv | Edv | ||
| FGE | Z | −2.413 | −3.114 | −2.480 | −1.819 | −2.204 | −2.240 | −1.822 | −0.377 |
| p | * 0.016 | * 0.002 | * 0.013 | 0.069 | * 0.028 | * 0.025 | 0.068 | 0.706 | |
| Placebo | Z | −1.540 | −1.334 | −0.385 | −0.350 | −0.974 | −0.286 | −0.472 | −1.159 |
| p | 0.124 | 0.182 | 0.701 | 0.726 | 0.330 | 0.775 | 0.637 | 0.246 | |
* p < 0.05. CCA, common carotid arteries; ICA, internal carotid arteries; Psv, peak systolic velocity; Edv, end-diastolic velocity; FGE, fermented garlic extract.
Significant differences between the FGE group and the placebo group regarding the amount of change before and after ingesting FGE were found in systolic BP (Z = −2.263, p = 0.024) and diastolic BP (Z = −2.637, p = 0.008). And Significant differences between the FGE and placebo groups regarding the changes in Psv or Edv before and after ingesting FGE were found in the right CCA Edv (Z = −2.167, p = 0.030) and left CCA Edv (Z = −2.065, p = 0.039) (Table 4).
Table 4.
Comparison of the change in BP and CA velocity before and after FGE intake between the FGE and placebo groups.
| Systolic BP | Diastolic BP | Right | Left | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCA | ICA | CCA | ICA | |||||||
| Psv | Edv | Psv | Edv | Psv | Edv | Psv | Edv | |||
| Z | −2.263 | −2.637 | −0.874 | −2.167 | −1.921 | −1.595 | −0.961 | −2.065 | −1.267 | −0.460 |
| p | * 0.024 | * 0.008 | 0.382 | * 0.030 | 0.055 | 0.111 | 0.336 | * 0.039 | 0.205 | 0.645 |
* p < 0.05. BP, blood pressure; CCA, common carotid arteries; ICA, internal carotid arteries; Psv, peak systolic velocity; Edv, end-diastolic velocity.
3.3. Changes in rCBF and PBF
A total of 30 participants were initially included; however, one declined to be evaluated, and the other cancelled the scheduled participation. Therefore, the results were analyzed for a total of 28 participants. The 15 FGE group participants were composed of eight males and seven females, while the 13 placebo group participants were composed of three males and ten females. The average age of the FGE and placebo groups was 54.3 ± 10.9 years and 55.9 ± 13.9 years, respectively.
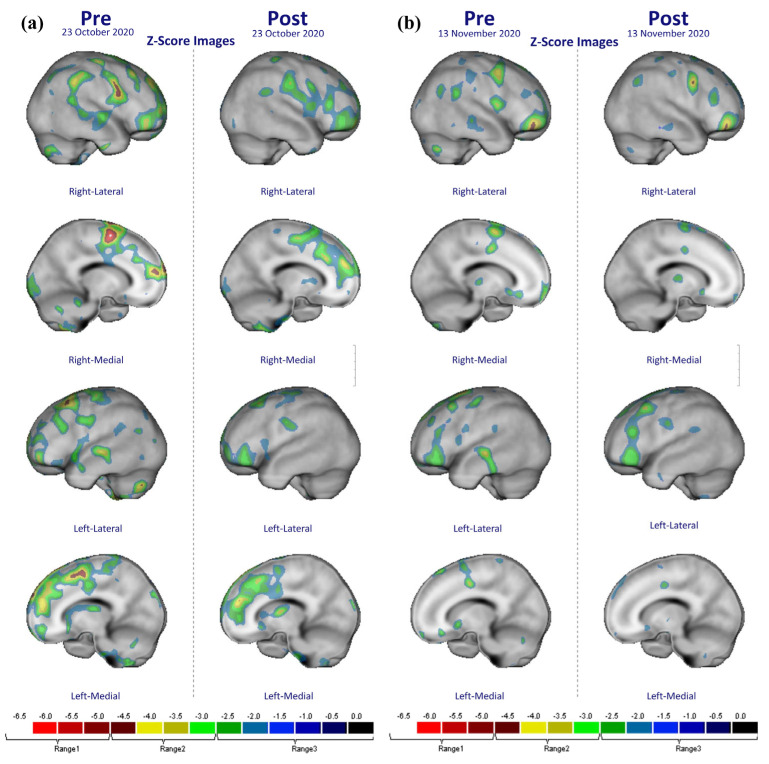
Changes in rCBF in the brain were measured using the Tc-99m HMPAO SPECT imaging technique before and after taking placebo or FGE tablets. A p FWE-corr of <0.001 was considered to be statistically significant based on SPM analysis (50 voxel). The post-test results, excluding the pre-effects of the FGE and placebo groups, were compared. Regarding rCBF, statistically significant trends were found in the right and left frontal cortex (Brodmann area 6, Brodmann area 9, and 10) and the right parietal cortex (Brodmann area 2) (Table 5). Figure 7 shows a statistically significant increase in the rCBF in the brains of the FGE group compared with the placebo group. However, no statistically significant changes in rCBF were observed in the placebo group.
Table 5.
Cortex areas showing upregulation of rCBF in the FGE group in comparison to the placebo group after FGE intake.
| Coordinates (MNI) | Peak Region | Brodmann Area | t | p FWE-corr | ||
|---|---|---|---|---|---|---|
| x | Y | z | ||||
| +34 | −4 | +68 | Rt. Frontal cortex | 6 | 4.145841 | 0.00016 |
| +38 | +2 | +64 | Rt. Frontal cortex | 6 | 4.083884 | 0.000188 |
| +30 | −32 | +72 | Rt. Parietal cortex | 2 | 3.902315 | 0.000301 |
| +46 | +54 | +14 | Rt. Frontal cortex | 10 | 3.960457 | 0.000259 |
| +50 | +52 | +6 | Rt. Frontal cortex | 10 | 3.937369 | 0.000275 |
| +46 | +48 | +24 | Rt. Frontal cortex | 9 | 3.923447 | 0.000285 |
| −32 | +10 | +64 | Lt. Frontal cortex | 6 | 3.734121 | 0.000466 |
| −26 | +26 | +58 | Lt. Frontal cortex | 6 | 3.674036 | 0.000544 |
| −28 | +18 | +62 | Lt. Frontal cortex | 6 | 3.641479 | 0.000591 |
MNI, Montreal Neurological Institute; Rt., right; Lt., left; rCBF, regional cerebral blood flow; FGE, fermented garlic extract.
Figure 7.
Diagrams showing regional cerebral blood flow in the brain of the fermented garlic extract (a) and placebo tablet ingestion groups (b). The closer the z-score is to 0 (black color), the closer it is to the normal value.
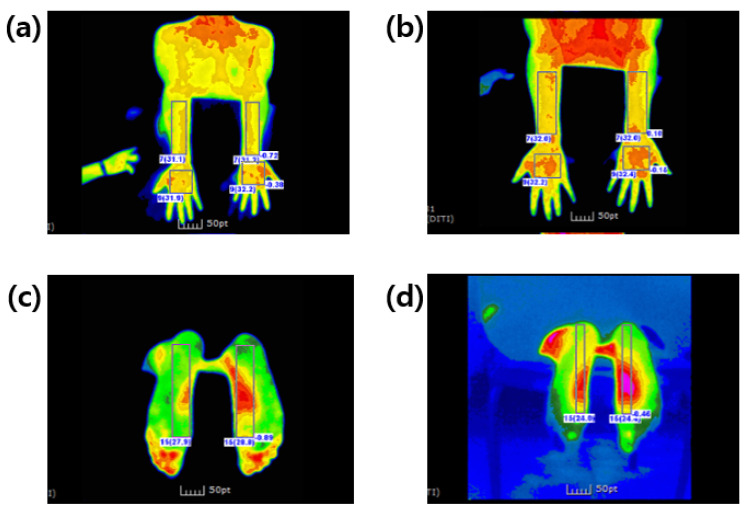
Statistically significant differences in BST between baseline and 30 min after ingestion of FGE tablets were noted in the following extremities: posterior forearm (F = 5.706, p = 0.025), palm (F = 4.864, p = 0.037), and plantar (F = 6.534, p = 0.017) areas. However, significant changes were not observed in these areas in the placebo group (Figure 8).
Figure 8.
Representative photographs showing an increase in body surface temperature before (a,c) and after (b,d) FGE tablet ingestion. Images (a,b) show the posterior forearm, and images (c,d) show the plantar area.
3.4. Evaluation of Adverse Side Effects
Safety evaluations were conducted on all participants who ingested FGE or placebo tablets at least once. The NCI Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) was utilized, with responses of “none (0)”, “mild (1)”, “moderate (2)”, and “severe (3)”. No adverse events, such as pain, low-temperature burns, skin redness, itching, photosensitivity, anxiety, fever, and headache, were observed.
4. Discussion
In the present blinded, placebo-controlled study, we evaluated the acute effects of FGE tablets containing 7 mg of inorganic NO2− ions on BP, velocity of the CA, PBF, and rCBF in 58 adults. A major finding of this study included decreases in both systolic and diastolic BP 30 min after ingestion of the FGE tablet. Furthermore, systolic and diastolic blood velocities in the CCA were significantly reduced following ingestion of the FGE tablet. Additionally, FGE intake resulted in statistically significant increases in the BST in extremities, suggesting that vasodilatation of the peripheral vasculature on the skin may be caused by FGE intake. Similar to our results, a single dose of NO supplementation made of sodium NO2− (20 mg) and phytochemicals resulted in a significant decrease of about 6 mm Hg in the resting systolic and diastolic BP, and also improved vascular stiffness and endothelial function in patients with hypertension [29]. The single oral dose of 80 mg sodium NO2− led to a significant, asymptomatic systolic BP drop of 10/6 mm Hg, with no effect on diastolic pressure. Plasma NO2− levels increased to 3–4 μM, or were approximately ten times higher than the normal steady state within one hour after dosing [30]. Recently, four meta-analyses and two original studies have demonstrated direct clinical evidence for the therapeutic effects of garlic extract in hypertension and in endothelial dysfunction in patients with diabetes [31,32,33]. Aged garlic supplementation can cause an approximately 4 mm Hg and 3 mm Hg decrease in systolic and diastolic BP, respectively, compared with controls [33].
Furthermore, chronic oral sodium NO2− therapy (40 mg/three times daily) has been shown to significantly lower systolic, diastolic, and mean arterial pressures, but tolerance has been observed after 10–12 weeks of therapy in adults with hypertension and metabolic syndrome. Significant improvements in the intima-media thickness of the CA and trends toward improvements in flow-mediated vasodilation of the brachial artery and insulin sensitivity have been reported [23]. In a clinical trial, sodium NO2− increased plasma NO2− and was well tolerated. Additionally, brachial artery flow-mediated dilation (endothelial function) was increased 28% versus baseline after NO2− supplementation (p < 0.05) [11]. A recent meta-analysis revealed that inorganic NO3− intake was found to significantly reduce resting BP (systolic BP: −4.80 mmHg, diastolic BP: −1.74 mmHg), improve endothelial function (flow-mediated dilatation: 0.59%, p < 0.0001), reduce arterial stiffness (pulse wave velocity: −0.23 m/s; augmentation index: −2.1%,), and reduce platelet aggregation by 18.9% [34].
However, there are also differences between previous studies and this study. The first difference is that previous studies used much higher content of NO2− capsules. The reason why a similar effect could be obtained despite the use of a tablet with a much lower NO2− content than in previous studies is considered to be related to the results presented in Section 3.1. In the result, compared to the Neo40® tablet, the FGE tablet in this study showed a longer amount of production and a longer stabilization period on the base of one serving size. A correct reason for a significant difference of NO production between the FGE and the Neo40® under simulated gastric acid is not clear at this point Under acid juice, nitrite is largely reduced to NO in the presence of endogenous reductants, such as thiocyanate and ascorbic acid, whereas in the absence of these species, just 1% is converted to NO [35]. Hirota and Takahama (2014) [36] have reported that polyphenols in apple juice increase NO production from nitrite in acidic buffer solution (pH 2.0). Rocha and colleagues (2009) [37] have demonstrated that the degree of protonation to NO of nitrite from polyphenol/nitrite mixture under acidic juice (pH 2.0) is differs largely according to the type of polyphenol or concentration of polyphenol. For example, Epicatechin-3-O-gallate produces the highest NO production among the polyphenols tested in the study. Furthermore, in vivo NO production induced by the consumption of lettuce in the stomach of healthy volunteers is also different according to ingestion of different polyphenol-containing dietary foods such as apple, berries, cherries, black tea, and red wine 15 min after ingestion of lettuce. Collectively, considering these previous results showing the contribution of polyphenol or reductants to the reduction of nitrite to NO in an acidic medium, it can be postulated that different compositions of polyphenol or reductants are the reason for a significant difference of NO production between the FGE and the Neo40® under the simulated gastric acid. Further research demonstrating the possible mechanisms of NO production from FEG tablets in the human body is warranted. In addition, another difference is that previous studies were focused on patients. We also plan to conduct follow-up studies using the FGE tablets in patients.
Our previous animal study [18] demonstrated that a single oral administration of FGE (10 mg of NO2−/mL) causes a decrease in BP in hypertensive rats, with a short half-life characterized by the peak effect at 30 min and recovery within 2–3 hours after feeding. Moreover, acetylcholine or sodium nitroprusside-induced vasodilatation was more augmented in the thoracic aortic strip of hypertensive rats who were given FGE for 12 days. The chronic feeding of FGE solution resulted in the increased expression of PKG and eNOS proteins in aortic tissue, which was prevented by pretreatment with soluble guanylate cyclase inhibitor (ODQ). In our recent ex vivo study, direct application of FGE with 20 ppm of NO2− into the aortic ring of normotensive rats caused dose-dependent vasodilation. There was a partial inhibition of FGE-induced aortic vasodilatation caused by HXC, a NO scavenger and sodium pyruvate, an H2O2 scavenger, and total suppression of the vasodilation caused by ODQ, an sGC (unpublished data). The chemiluminescent detection method revealed that a marked increase in NO gas was detected when FGE was added into artificial gastric acid, indicating that NO2− in FGE was protonated to NO. Collectively, present and previous results strongly suggest that the possible underlying mechanism of FGE with NO2− is the activation of intracellular molecular events for eNOS and the sGC-cGMP-PKG pathway in the vascular smooth muscle. Additional modulations of intracellular molecular events associated with oxidative stress and inflammation by NO2− ions also can restore vascular functions in aging vessels. For example, inorganic NO2− can inhibit mitochondrial reactive oxygen species or oxidative stress that decreases endothelial function with aging. Sodium NO2− supplementation can reduce blood concentrations of dehydroascorbate, the oxidized form of the superoxide-scavenging metabolite ascorbate, and inflammatory markers [11,38,39].
Our secondary major outcome was changes in rCBF, which showed statistically significant increases in the right and left frontal lobe and right parietal lobe following the ingestion of FGE. To the best of our knowledge, this is the first study to show increases in rCBF in local cortical areas following acute ingestion of NO2− supplementation. A previous study observed no changes in the velocities in the large diameter of the middle cerebral artery measured by transcranial doppler ultrasound during continuous infusion of sodium NO2− (0.6 mg/kg/h) [40]. In rats, intravenous infusion of NO2− caused rapid increases in both NO production, by reduction of NO2− in the red blood cells, and cerebral BF measured by laser Doppler flowmetry, which was suppressed by the inhibition of NO synthesis by L-NAME [41]
Supplementation of beet juice containing high NO3− levels (12.4 mmol) in older individuals (aged > 70 years) for four days did not alter global cerebral perfusion, but did lead to increased resting regional cerebral perfusion within the bilateral white matter of the frontal lobes. However, no significant changes were noted in CBF in the cerebral cortex proper [42] using perfusion MRI. According to Wightman and colleague [43], near-infrared spectroscopy (NIRS) showed no changes in total hemoglobin during the abortion period. However, a significant transient increase of total hemoglobin, an index of cerebral BF, was noted during the initial cognitive task following ingestion of beetroot juice (~5.5 mmol of NO3−). The fMRI study demonstrated no basal changes in BF, but increased BOLD responses were observed in the visual cortex during visual stimuli following oral sodium NO3− (0.1 mmol/kg/day) administration for three consecutive days [44]. Considering previous clinical results and our results, inconsistent effects of NO2− or NO3− supplementation on cerebral BF under resting conditions have been observed. These may primarily be due to the strong autoregulation mechanisms of cerebral BF, in place to minimize any changes in the cerebral circulation CBF under various physiological conditions. Various measurement or analysis methods for changes in CFG may also contribute to the inconsistencies.
In this study, the FGE group showed a significant improvement in the right frontal cortex (Brodmann area 6, Brodmann area 9, and 10) and right parietal cortex (Brodmann area 2) compared to the placebo group. Brodmann area 6 is mainly a role in planning a complicated and coordinated movement [45], and Brodmann areas 9 and 10 are mainly involved in functions such as short-term memory, space memory, calculations, language fluency, and problem solving [46,47,48]. Brodmann area 2 belongs to corresponds to the primary body sensory cortex, which is a major sensory receiving area for tactile sense [49]. As there was no study of cortex areas, in which the blood flow was changed after ingestion of FGE or garlic, it is difficult to compare our study results. However, the previous NO study reported that NO significantly increased the blood flow of the frontal cortex through vascular extensions [50]. In addition, previous studies that investigated the change in rCBF using low-level laser therapy (LLLT) reported that LLLT increased the cerebral blood flow of the frontal cortex and prefrontal cortex through increasing the NO level [51,52,53]. Based on these previous studies, we speculate that the reason for the increased rCBF in Brodmann areas 6, 9, and 10 included in the prefrontal cortex in this study may be the effect of NO. However, it is difficult to be certain about the effect on the right parietal cortex.
Garlic (Allium sativum L.) has been widely utilized as an important natural health remedy in improving various diseases in many ancient civilizations [54]. Functional sulfur-containing components presented in garlic include alliin, allicin, sulfides, diallyl trisulfide, and S-allyl-cysteine (SAC) [55]. Raw garlic extract (RGE) basically contains alliin, and when garlic is cut and the parenchyma is broken down, it is converted into allicin by the allinase enzyme. Several other organosulfur compounds, such as N-acetylcysteine, SAC, and S-ally-mercapto cysteine, are derived from alliin through aging or fermentation. In summary, it is known that allicin is a representative component in RGE, and SAC is a representative component in aged garlic extract (AGE) or FGE. Allicin helps with protein interaction and antioxidant activity, and SAC is known to improve antioxidant, anti-inflammatory, regulated redox, pro energetic, anti-apoptotic, and anticancer activities as well as signaling capacities [54,55]. Each company uses different garlic conditions, aging, fermentation, and processing methods. Thus, the ratio of compounds may vary. Previous studies have observed the highest allicin content (0.27%) in RGE, recommending the use of RGE rather than AGE [56]. However, many studies have demonstrated the effectiveness of FGE or AGE. The reason for this is considered to be that allicin, the main component found in fresh raw garlic and garlic powder, is highly volatile and unstable, and SAC, the main active compound, is highly stable [57].
However, in the FGE used in this study, alliin was detected at approximately 0.008 mg, and allicin and SAC were not detected [18]. Therefore, the intrinsic function of garlic compounds and their synergy with NO are considered important. For example, single dose of AGE temporarily increased production of NO metabolites in the plasma by increasing cNOS activity within 1 h of administration to mice [58]. Garlic extract (3–500 microg/mL) or allicin, major metabolites, produced dose- and NO-dependent relaxation under intact endothelium in rat pulmonary arteries [59,60]. Therefore, the action of vascular dilatation through the endothelial NO signaling of major garlic phytoconstituents could be due to the synergism of NO2− induced changes in peripheral and central vascular function in this study. Therefore, it is difficult to ignore the synergistic effect with NO in supplements using garlic.
NO2− ions can be produced as an intermediate through either nitrogen fixation or denitrification [61,62]. Denitrifying bacteria convert NO3− into NO2− using NO3− reductase in the soil or fluid to make atmospheric nitrogen. Many vegetables, such as spinach, lettuce, celery, and red beets, are good sources of NO2− or NO3− for humans. During the fermentation process of these vegetables through denitrifying bacteria, preconverted, or natural NO2−, is made and then rapidly converted into NO3− ions [63]. Vegetables are chewed in the oral cavity, and some are fermented by oral microorganisms. Through such a process, some NO3− ions may be converted to NO2−, some go through digestive organs, and change into NO2− or NO via fermentation by intestinal microorganisms in the stomach [64]. This natural NO2− has been used as a natural curing agent for meat. The production of high concentrations of NO2− in the FGE used in these studies may have been due to the aerobic denitrification or nitrification processed of bacillus strains [61]. However, compared with those of conventional fermentation procedures, the fermentation technique used in this study provided long-term stability of NO2− ions in aqueous fermented solution.
The present study has several limitations. First, even though the data were obtained in a double-blind, randomized controlled study, this was a pilot study with a limited number of participants. Therefore, the findings may not easily be extrapolated to larger populations. Second, this study only looked at the short-term effects of an FGE tablet without a systematic strategy. Third, quality-of-life and hematological parameters in participants were not fully evaluated, and these parameters could have affected the clinical results. Fourth, serum measurements of NO or NO metabolites were not performed. Therefore, whether the FGE supplementation increased concentrations of NO in participants in unknown. However, based on previously published data that described sodium NO2− supplement use and our clinical findings, FGE may increase NO production and availability. Further studies on the temporal profiles of NO metabolites in the blood following the ingestion of an FGE tablet may be needed.
Author Contributions
Conceptualization, Y.I.S. and M.S.K.; methodology, Y.I.S., H.S.C., M.S.K. and J.S.B.; software, J.S.B., S.M.J. and M.S.K.; validation, J.S.B., J.H.M. and S.M.J.; formal analysis, J.S.B. and J.H.A.; investigation, J.S.B. and S.H.K.; resources, H.S.C.; data curation, J.S.B. and S.H.K.; writing—original draft preparation, J.S.B., J.H.M., S.M.J., J.H.A. and M.S.K.; writing—review and editing, J.S.B., J.H.M., S.M.J., Y.I.S. and M.S.K.; visualization, J.S.B. and J.H.M.; supervision, Y.I.S. and M.S.K.; project administration, Y.I.S. and J.S.B.; funding acquisition, H.S.C. and Y.I.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Pusan National University Yangsan Hospital (protocol code 04-2020-036, 04-2020-038 and date of approval).
Informed Consent Statement
Informed consent was obtained from all interested and eligible participants.
Data Availability Statement
The data are not publicly available due to privacy of participants.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a Pusan National University 2-Year Research Grant.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Johnson C., Abajobir A., Abd-Allah F., Abera S.F., Abyu G., Ahmed M., Aksut B., Alam T., Alam K., et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing C.Y., Tarumi T., Meijers R.L., Turner M., Repshas J., Xiong L., Ding K., Vongpatanasin W., Yuan L.J., Zhang R. Arterial Pressure, Heart Rate, and Cerebral Hemodynamics Across the Adult Life Span. Hypertension. 2017;69:712–720. doi: 10.1161/HYPERTENSIONAHA.116.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisler K., Nelson A.R., Montagne A., Zlokovic B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sverdlov A.L., Ngo D.T., Chan W.P., Chirkov Y.Y., Horowitz J.D. Aging of the nitric oxide system: Are we as old as our NO? J. Am. Heart Assoc. 2014;3:e000973. doi: 10.1161/JAHA.114.000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimbrone M.A., Jr., García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhoutte P.M., Shimokawa H., Tang E.H., Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 7.Amdahl M.B., DeMartino A.W. Inorganic nitrite bioactivation and role in physiological signaling and therapeutics. Biol. Chem. 2019;401:201–211. doi: 10.1515/hsz-2019-0349. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan N.S., Fernandez B.O., Bauer S.M., Garcia-Saura M.F., Milsom A.B., Rassaf T., Maloney R.E., Bharti A., Rodriguez J., Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 10.Bryan N.S. Functional Nitric Oxide Nutrition to Combat Cardiovascular Disease. Curr. Atheroscler. Rep. 2018;20:21. doi: 10.1007/s11883-018-0723-0. [DOI] [PubMed] [Google Scholar]
- 11.Rossman M.J., Gioscia-Ryan R.A., Santos-Parker J.R., Ziemba B.P., Lubieniecki K.L., Johnson L.C., Poliektov N.E., Bispham N.Z., Woodward K.A., Nagy E.E., et al. Inorganic Nitrite Supplementation Improves Endothelial Function With Aging: Translational Evidence for Suppression of Mitochondria-Derived Oxidative Stress. Hypertension. 2021;77:1212–1222. doi: 10.1161/hypertensionaha.120.16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zand J., Lanza F., Garg H.K., Bryan N.S. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr. Res. 2011;31:262–269. doi: 10.1016/j.nutres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Bouguyon E., Gojon A., Nacry P. Nitrate sensing and signaling in plants. Semin. Cell Dev. Biol. 2012;23:648–654. doi: 10.1016/j.semcdb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal A., Qiang D., Alamzeb M., Xiangru W., Huiping G., Hengheng Z., Nianchang P., Xiling Z., Meizhen S. Untangling the molecular mechanisms and functions of nitrate to improve nitrogen use efficiency. J. Sci. Food Agric. 2020;100:904–914. doi: 10.1002/jsfa.10085. [DOI] [PubMed] [Google Scholar]
- 15.Chun H.S. Manufacturing Method of Fermented Garlic Composition and Fermented Garlic Composition Thereof. KR101609918B1. International Patent. 2014 April 7;
- 16.Chun H.S. Manufacturing Method of Natural Fermented-Composition Containing Fixed Nitric Oxide Precursors and the Natural Fermented-Composition Thereof. KR20190101353A. International Patent. 2021 May 26;
- 17.Chun H.S. Method for Fixing and Stabilizing of Nitric Oxide Metabolites Using Fermentation of Nitrogen-Containing Natural Material. KR102129038B1. International Patent. 2020 July 1;
- 18.Park B.M., Cha S.A., Kim H.Y., Kang D.K., Yua K., Chun H., Chae S.W., Kim S.H. Fermented garlic extract decreases blood pressure through nitrite and sGC-cGMP-PKG pathway in spontaneously hypertensive rats. J. Funct. Foods. 2016;22:10. doi: 10.1016/j.jff.2016.01.034. [DOI] [Google Scholar]
- 19.Park B.M., Chun H., Chae S.W., Kim S.H. Fermented garlic extract ameliorates monocrotaline-induced pulmonary hypertension in rats. J. Funct. Foods. 2017;30:7. doi: 10.1016/j.jff.2017.01.024. [DOI] [Google Scholar]
- 20.Yu H., Rong Z.X., Koo H., Chun H.S., Yoo S.J., Kim M.S. Changes in Cerebral Blood flow Following Fermented Garlic Extract Solution with High Content of Nitrite. Physiol. Soc. Korean Med. Soc. Pathol. Korean Med. 2020;8:326–333. doi: 10.15188/kjopp.2020.12.34.6.326. [DOI] [Google Scholar]
- 21.Lee Y.J., Lee D., Shin S.M., Lee J.S., Chun H.S., Quan F.S., Shin J.H. Potential protective effects of fermented garlic extract on myocardial ischemia-reperfusion injury utilizing in vitro and ex vivo models. J. Funct. Foods. 2017;33:8. [Google Scholar]
- 22.Kapil V., Milsom A.B., Okorie M., Maleki-Toyserkani S., Akram F., Rehman F., Arghandawi S., Pearl V., Benjamin N., Loukogeorgakis S., et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 23.Hughan K.S., Levine A., Helbling N., Anthony S., DeLany J.P., Stefanovic-Racic M., Goodpaster B.H., Gladwin M.T. Effects of Oral Sodium Nitrite on Blood Pressure, Insulin Sensitivity, and Intima-Media Arterial Thickening in Adults With Hypertension and Metabolic Syndrome. Hypertension. 2020;76:866–874. doi: 10.1161/HYPERTENSIONAHA.120.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waaler B.A., Eriksen M. Post-prandial cardiovascular responses in man after ingestion of carbohydrate, protein or fat. Acta Physiol. Scand. 1992;146:321–327. doi: 10.1111/j.1748-1716.1992.tb09426.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara Y. Nuclear medicine for general radiologists: Clinical application of brain SPECT. Nihon Igaku Hoshasen Gakkai Zasshi Nippon Acta Radiol. 2000;60:671–677. [PubMed] [Google Scholar]
- 26.Makino K., Masuda Y., Gotoh S. Comparison of cerebral vasoreactivity to acetazolamide in normal volunteer among 123I-IMP, 99mTc-ECD and 99mTc-HMPAO. Kaku Igaku. Jpn. J. Nucl. Med. 1996;33:551–555. [PubMed] [Google Scholar]
- 27.García-Gómez F., García-Solís D., Luis-Simón F., Marín-Oyaga V., Carrillo F., Mir P., Vázquez-Albertino R. Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane. Rev. Esp. Med. Nucl. Imagen Mol. 2013;32:350–356. doi: 10.1016/j.remnie.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Houston M., Hays L. Acute effects of an oral nitric oxide supplement on blood pressure, endothelial function, and vascular compliance in hypertensive patients. J. Clin. Hypertens. 2014;16:524–529. doi: 10.1111/jch.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenway F.L., Predmore B.L., Flanagan D.R., Giordano T., Qiu Y., Brandon A., Lefer D.J., Patel R.P., Kevil C.G. Single-dose pharmacokinetics of different oral sodium nitrite formulations in diabetes patients. Diabetes Technol. Ther. 2012;14:552–560. doi: 10.1089/dia.2011.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamal S., Cherukuri L., Birudaraju D., Matsumoto S., Kinninger A., Chaganti B.T., Flores F., Shaikh K., Roy S.K., Budoff M.J. Short-term impact of aged garlic extract on endothelial function in diabetes: A randomized, double-blind, placebo-controlled trial. Exp. Ther. Med. 2020;19:1485–1489. doi: 10.3892/etm.2019.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong X.J., Wang P.Q., Li S.J., Li X.K., Zhang Y.Q., Wang J. Garlic for hypertension: A systematic review and meta-analysis of randomized controlled trials. Phytomed. Int. J. Phytother. Phytopharm. 2015;22:352–361. doi: 10.1016/j.phymed.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Varshney R., Budoff M.J. Garlic and Heart Disease. J. Nutr. 2016;146:416S–421S. doi: 10.3945/jn.114.202333. [DOI] [PubMed] [Google Scholar]
- 34.Jackson J.K., Patterson A.J., MacDonald-Wicks L.K., Oldmeadow C., McEvoy M.A. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta-analysis of human evidence. Nutr. Rev. 2018;76:348–371. doi: 10.1093/nutrit/nuy005. [DOI] [PubMed] [Google Scholar]
- 35.Moriya A., Grant J., Mowat C., Williams C., Carswell A., Preston T., Anderson S., Iijima K., McColl K.E. In vitro studies indicate that acid catalysed generation of N-nitrosocompounds from dietary nitrate will be maximal at the gastro-oesophageal junction and cardia. Scand J. Gastroenterol. 2002;37:253–261. doi: 10.1080/003655202317284147. [DOI] [PubMed] [Google Scholar]
- 36.Hirota S., Takahama U. Reactions of Apple Fruit Polyphenols with Nitrite under Conditions of the Gastric Lumen: Generation of Nitric Oxide and Formation of Nitroso Catechins. Food Sci. Technol. Res. 2014;20:439–447. doi: 10.3136/fstr.20.439. [DOI] [Google Scholar]
- 37.Rocha B.S., Gago B., Barbosa R.M., Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265:41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Sindler A.L., Devan A.E., Fleenor B.S., Seals D.R. Inorganic nitrite supplementation for healthy arterial aging. J. Appl. Physiol. 2014;116:463–477. doi: 10.1152/japplphysiol.01100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sindler A.L., Fleenor B.S., Calvert J.W., Marshall K.D., Zigler M.L., Lefer D.J., Seals D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franko E., Ezra M., Crockett D.C., Joly O., Pattinson K. Effect of nitrite on the electroencephalographic activity in the healthy brain. Nitric Oxide Biol. Chem. 2019;90:47–54. doi: 10.1016/j.niox.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Rifkind J.M., Nagababu E., Barbiro-Michaely E., Ramasamy S., Pluta R.M., Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: A role for red cell NO. Nitric Oxide Biol. Chem. 2007;16:448–456. doi: 10.1016/j.niox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Presley T.D., Morgan A.R., Bechtold E., Clodfelter W., Dove R.W., Jennings J.M., Kraft R.A., King S.B., Laurienti P.J., Rejeski W.J., et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide Biol. Chem. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wightman E.L., Haskell-Ramsay C.F., Thompson K.G., Blackwell J.R., Winyard P.G., Forster J., Jones A.M., Kennedy D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015;149:149–158. doi: 10.1016/j.physbeh.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Aamand R., Dalsgaard T., Ho Y.C., Møller A., Roepstorff A., Lund T.E. A NO way to BOLD? Dietary nitrate alters the hemodynamic response to visual stimulation. Neuroimage. 2013;83:397–407. doi: 10.1016/j.neuroimage.2013.06.069. [DOI] [PubMed] [Google Scholar]
- 45.Shah K.B., Hayman L.A., Chavali L.S., Hamilton J.D., Prabhu S.S., Wangaryattawanich P., Kumar V.A., Kumar A.J. Glial tumors in Brodmann area 6: Spread pattern and relationships to motor areas. Radiographics. 2015;35:793–803. doi: 10.1148/rg.2015140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgess P.W., Wu H. Principles of Frontal Lobe Function. Oxford University Press; New York, NY, USA: 2013. Rostral prefrontal cortex (Brodmann area 10) pp. 524–544. [Google Scholar]
- 47.Peng K., Steele S.C., Becerra L., Borsook D. Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Prog. Neurobiol. 2018;161:1–22. doi: 10.1016/j.pneurobio.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uemura K., Shimada H., Doi T., Makizako H., Tsutsumimoto K., Park H., Suzuki T. Reduced prefrontal oxygenation in mild cognitive impairment during memory retrieval. Int. J. Geriatr. Psychiatry. 2016;31:583–591. doi: 10.1002/gps.4363. [DOI] [PubMed] [Google Scholar]
- 49.Stilla R., Sathian K. Selective visuo-haptic processing of shape and texture. Hum. Brain Mapp. 2008;29:1123–1138. doi: 10.1002/hbm.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Vasconcelos A.P., Baldwin R.A., Wasterlain C.G. Nitric oxide mediates the increase in local cerebral blood flow during focal seizures. Proc. Natl. Acad. Sci. USA. 1995;92:3175–3179. doi: 10.1073/pnas.92.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eshaghi E. Effect of Transcranial Low-Level Laser Therapy in Chronic Restraint Stress Induced Depressive-Like Behaviour in Mice. Faculty of Medicine, Tabriz University of Medical Sciences; Tabriz, Iran: 2019. [Google Scholar]
- 52.Hashmi J.T., Huang Y.Y., Osmani B.Z., Sharma S.K., Naeser M.A., Hamblin M.R. Role of low-level laser therapy in neurorehabilitation. PmR. 2010;2:S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hase S.N. Transcranial near Infrared Spectroscopy and Stimulation of Prefrontal Cognitive Functions in Young Adults. The University of Texas at Arlington; Arlington, TX, USA: 2015. [Google Scholar]
- 54.Pittler M.H., Ernst E. Clinical effectiveness of garlic (Allium sativum) Mol. Nutr. Food Res. 2007;51:1382–1385. doi: 10.1002/mnfr.200700073. [DOI] [PubMed] [Google Scholar]
- 55.El-Saber Batiha G., Magdy Beshbishy A., Wasef L.G., Elewa Y.H.A., Al-Sagan A.A., Abd El-Hack M.E., Taha A.E., Abd-Elhakim Y.M., Prasad Devkota H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loghmanifar S., Roozbeh Nasiraie L., Nouri H., Jafarian S. Comparison of Fresh and Aged Garlic Extracts in Terms of Antioxidative Power and Allicin Content. J. Med. Plants Prod. 2022;11:29–35. doi: 10.22092/jmpb.2020.342409.1193. [DOI] [Google Scholar]
- 57.Ried K., Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: Mechanisms of action and clinical relevance. Integr. Blood Press. Control. 2014;7:71–82. doi: 10.2147/IBPC.S51434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morihara N., Sumioka I., Moriguchi T., Uda N., Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71:509–517. doi: 10.1016/S0024-3205(02)01706-X. [DOI] [PubMed] [Google Scholar]
- 59.Ku D.D., Abdel-Razek T.T., Dai J., Kim-Park S., Fallon M.B., Abrams G.A. Garlic and its active metabolite allicin produce endothelium- and nitric oxide-dependent relaxation in rat pulmonary arteries. Clin. Exp. Pharmacol. Physiol. 2002;29:84–91. doi: 10.1046/j.1440-1681.2002.03596.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim-Park S., Ku D.D. Garlic elicits a nitric oxide-dependent relaxation and inhibits hypoxic pulmonary vasoconstriction in rats. Clin. Exp. Pharmacol. Physiol. 2000;27:780–786. doi: 10.1046/j.1440-1681.2000.03333.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim J.K., Park K.J., Cho K.S., Nam S.W., Park T.J., Bajpai R. Aerobic nitrification-denitrification by heterotrophic Bacillus strains. Bioresour. Technol. 2005;96:1897–1906. doi: 10.1016/j.biortech.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 62.Peng Y., Zhu G. Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 2006;73:15–26. doi: 10.1007/s00253-006-0534-z. [DOI] [PubMed] [Google Scholar]
- 63.Qu X.M., Wu Z.F., Pang B.X., Jin L.Y., Qin L.Z., Wang S.L. From Nitrate to Nitric Oxide: The Role of Salivary Glands and Oral Bacteria. J. Dent. Res. 2016;95:1452–1456. doi: 10.1177/0022034516673019. [DOI] [PubMed] [Google Scholar]
- 64.Yong H.I., Kim T.K., Choi H.D., Jang H.W., Jung S., Choi Y.S. Clean Label Meat Technology: Pre-Converted Nitrite as a Natural Curing. Food Sci. Anim. Resour. 2021;41:173–184. doi: 10.5851/kosfa.2020.e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy of participants.