Abstract
The human body contains a very complex and dynamic ecosystem of bacteria. The bacteriome interacts with the host bi-directionally, and changes in either factor impact the entire system. It has long been known that chronic airway diseases are associated with disturbances in the lung bacteriome. However, less is known about the role of gut bacteriome in the most common respiratory diseases. Here, we aim to summarise the evidence concerning the role of the intestinal bacteriome in the pathogenesis and disease course of bronchial asthma, chronic obstructive pulmonary disease, and obstructive sleep apnea. Furthermore, we discuss the consequences of an altered gut bacteriome on the most common comorbidities of these lung diseases. Lastly, we also reflect on the therapeutic potential of influencing the gut microbiome to improve disease outcomes.
Keywords: microbiome, gut microbiome, bronchial asthma, chronic obstructive pulmonary disease, obstructive sleep apnea, inflammation, hypoxemia
1. Introduction
Despite advances in their management, chronic disorders of the respiratory system, such as asthma, chronic obstructive pulmonary disease (COPD) and obstructive sleep apnoea (OSA) are still common causes of overall morbidity and mortality worldwide. It is becoming widely acknowledged that the development and the course of these disorders are the results of a complex interaction of genetic, epigenetic, and environmental factors. The same factors may also affect the composition of the gut microbiome which in turn may affect the course of these respiratory diseases and associated disorders, such as cardiovascular disease [1]. Understanding the role of gut microbiome in chronic respiratory diseases could be clinically important as gut dysbiosis may serve as a treatable trait.
The microbiome is a complex ecosystem which dynamically interacts with the host cells, immune and metabolic processes. Although microbiome also consists of viruses, fungi, phages, archaea, protists, and helminths [2], most studies focused on the bacteria, especially in the relation of gut-lung axis [1,3]. However, one must note the significant interrelation between different kingdoms, such as the bacteria and fungi; therefore, they cannot be treated in isolation [1]. Nevertheless, as human studies on patients with chronic respiratory disorders overwhelmingly investigated gut bacteria, the current review will focus on this kingdom. The review will focus on studies in humans with some experimental research on animals is also discussed. However, the review did not include all animal studies on this topic which is the main limitation of this paper.
2. Lung-Gut Axis
The effect of gut microbiome on the function of the respiratory system has recently been extensively reviewed [1,3]. However, it is less described that factors contributing to the development of lung diseases (i.e., diet and smoking), or their consequences (i.e., hypoxaemia or sleep fragmentation) may also alter gut microbiome.
Diet strongly influences the composition of gut bacteriome [4]. It is known that due to fragmented sleep and leptin resistance, patients with OSA tend to consume high calorie, carbohydrate- and lipid-reach diet [5]. On the other hand, around 25% of patients with COPD are cachectic [6]. Furthermore, the composition of the rectal and respiratory flora shows a close correlation from the birth [7]. Following up infants with cystic fibrosis, Madan et al., demonstrated that the close link is mainly driven by nutrition [8]. This suggests that dietary interventions may influence the respiratory microbiome and local immunity. Sleep restriction, a hallmark of OSA, may also disrupt gut microbiome most likely via increasing the appetite [9].
Cigarette smoking is the main risk factor for development of COPD and hampers asthma control. It can alter gut microbiome as well. Active smoking is associated with increased abundances of Bacteroidetes, and decreased abundances of Firmicutes [10]. In line with this, smoking cessation increases the abundance of Firmicutes and Actinobacteria and decreases the abundance of Bacteroidetes [11]. However, regarding Proteobacteria the results were contradictory. On one hand Lee et al., reported their lower abundance in active smokers [10]. On the other hand, smoking cessation led to the reduction of Proteobacteria in stool samples [11]. As the latter study did not include never-smokers, the discrepancies between the two investigations cannot be fully explained.
Air pollution, most particularly fine particulate matter (PM2.5), oxides of nitrogen and sulfur, ozone and heavy metals contribute to the development and worsening of COPD [12]. Environmental chemicals may affect gut bacteria through various mechanisms, including modifying their metabolism directly or indirectly following conjugation in liver, causing dysbiosis, and interacting with bacterial products [13]. Most recently, Li et al., have reported that PM2.5 was related to alterations in gut microbiome in healthy subjects [14]. Nitric oxide exposure led to a decrease in the abundance of Clostridium leptum group and Faecalibacterium prausnitzii and an increase in the abundance of the Dialister genus, Escherichia coli, Enterococcus faecalis, and Proteus mirabilis in human fecal samples [15]. Ozone exposure was related to lower bacterial diversity [16]. Finally, the effect of heavy metals on gut microbiome has been extensively reviewed by Claus et al. [13].
Obstructive sleep apnoea and in advanced stage, COPD is characterized by chronic hypoxaemia. Some of the gut bacteria, such as Actinobacteria are oxygen-sensitive [17], and hypoxaemia may lead to reduced growth of such organisms. A significant number of patients with severe to very severe COPD have chronic hypercapnia [18], and some patients with OSA display blunted ventilatory response resulting in hypercapnia. There is some evidence that hypercapnia is associated with gut dysbiosis in a bidirectional way. On one hand, experimental alterations of gut bacteriome with antibiotics or fecal transplantation blunted the respiratory response to hypercapnia in rats [19]. On the other hand, combined hypoxaemia and hypercapnia has led to alternations in gut microbiome in mice [20,21].
Medications may also affect gut microbiome [22]. Most particularly for respiratory disorders, glucocorticosteroids [23] and antibiotics [24] are known to cause dysbiosis. As patients with respiratory diseases, especially COPD and OSA, tend to suffer from multiple comorbidities, the effect of medications needs to be considered when interpreting the results. Unfortunately, most case–control studies have not adjusted for this effect which could be a potential reason for discrepancies.
Gut microbiome directly influences the local, systemic and distal organ immune responses. On one hand, gut bacteria directly interact with immune cells which process is important for immune cell maturation and polarisation [25]. On the other hand, bacterial metabolites, such as lipopolysaccharides (LPS), short-chain fatty acids (SCFA) may enter the systemic circulation and lymphatic system affecting airway inflammation [26,27]. This relationship may either be deleterious or protective. On one hand, segmented filamentous bacteria induce polarisation of lung T helper (Th) cells towards the pro-inflammatory Th17 type [27,28]. On the other hand, germ-free (GF) animals have worse outcomes against respiratory tract infections, suggesting a protective role of gut bacteria [29,30,31]. The process how gut bacteria may alter lung immunity has been extensively reviewed by Bingula et al. [3].
3. Asthma and Gut Microbiome
Asthma affects more than 300 million people worldwide. It is characterised by variable symptoms and airflow limitation that are driven by airway hyperreactivity and chronic airway inflammation [32]. However, the airway inflammation is heterogenous, and different inflammatory processes may lead to the same symptoms [33]. Whilst the type 2 inflammatory pathways are well described and can be addressed by targeted therapies, non-type 2 asthma remains a clinical challenge.
Gut bacteriome may play a role in the development of atopic asthma [34]. According to the hygiene hypothesis, decrease in the infections in the Western world was followed by the rise in allergic and autoimmune diseases [35]. Indeed, GF mice exhibit a type 2 inflammatory phenotype [36] and have less CD4 T cells in the lamina propria with a higher Th2:Th1 ratio [37]. However, this is complicated by the fact that GF mice have lower CXC chemokine receptor 2 (CXCR2) expression and hence reduced mast cell migration towards the intestinal mucosa suggesting more complex mechanisms [38]. Nevertheless, early exposure to intestinal microbiota reduced the levels of invariant natural killer (iNKT) cells which produce interleukin (IL) 4 and IL-13 with a consequent induction of isotype switching to immunoglobulin E (IgE) in GF animals [39]. In addition, gut microbiota acts on the development and function of regulatory T (Treg) cells by expressing an outer membrane pili-like protein. This protein induces Tregs which suppress the excessive activation of Th2 cells involved in asthma [40].
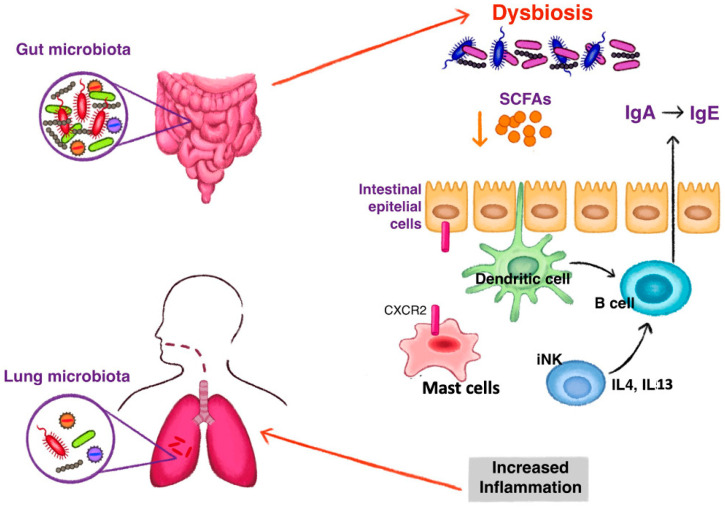
Bacteroidetes are known to ferment dietary fibres into SCFA which seem to be key mediators in the gut-lung axis (Figure 1). SCFA increase IgA production through stimulating dendritic cells in the Peyer’s patches to activate B cells to switch classes [41]. In their elegant experiment, Trompette et al., treated mice with high- and low-fibre diet. They reported an increase in circulating SCFA levels following high-fibre diet which resulted in dampening of the type 2 inflammation [26]. Importantly, the challenge also resulted in decrease in airway hyperresponsiveness [26].
Figure 1.
The mechanism of gut dysbiosis leading to the development of type 2 inflammation in asthma. Reduction of short chain fatty acids (SCFAs) induce a class switching of immunoglobulin (Ig) with an increase of fecal IgE acting on dendritic cells. Switching to IgE production is also stimulated by high levels of interleukin (IL) 4 and IL 13, produced by invariant natural killer (iNK) cells under dysbiosis stimuli. Dysbiosis influences the homing of mast cells to the intestine by the expression of CXCR2. Fewer intestinal mast cells and increased blood levels stimulate an inflammatory state observed in asthma.
In the Canadian Healthy Infant Longitudinal Development Study, it has been demonstrated that infants who develop atopy and wheeze had lower levels of Faecalibacterium, Lachnospira, Veillonella and Rothia genera during the first 100 days of life. In addition, lower levels of SCFA were measured in these children [42]. As a follow up of this observation, the authors inoculated GF mice with these bacteria and reported reduction in airway inflammation [42]. Investigating 690 children, Stokholm et al., reported that at 1 year age, the relative abundance of Faecalibacterium, Bifidobacterium, Roseburia, Alistipes, Lachnospiraceae incertae sedis, Ruminococcus and Dialister was inversely related, whilst the abundance of Veillonella was directly correlated with the development of asthma at age 5 years but only in children of asthmatic mothers. This suggests that specific alterations of gut bacteriome during the first year of life can trigger the inherited asthma risk [43]. Investigating gut microbiome in 2–12 months children in the Protection against Allergy: Study in Rural Environments cohort, bacteria producing butyrate were in an inverse relationship with asthma risk at school age. The study highlighted the protective effect of farm exposure for asthma development as well [44].
A few case–control studies investigated gut microbiome in asthma. Chiu et al., evaluated 35 children with asthma and 26 healthy controls. They found lower abundance of Firmicutes in asthma, but there was no difference in bacterial diversity [45]. Demirci et al., investigated 92 children with asthma and 88 healthy controls. In their targeted analysis, they reported lower abundances of Akkermansia muciniphila and Faecalibacterium prausnitzii [40]. These bacteria previously shown to be associated with regulatory T cell polarization [46] and therefore the findings of Demirci are suggestive for enhanced inflammatory response. Focusing on 24 asthmatic and 8 non-asthmatic adults, Begley reported decreased Bacteroidetes/Firmicutes ratio, most particularly reduced abundance of Bacteroides, Enterobacteriaeceae and higher abundance of Bifidobacterium and Lachnospiraceae in asthma. These results related to lung function values [47]. In 80 adult volunteers with and 40 without asthma, Okba described higher Lactobacilli and E. coli abundances; however these results were not related to disease outcomes [48]. In a metagenome-wide association study, Wang et al., compared 36 patients with asthma and 185 control subjects. The authors reported that Faecalibacterium prausnitzii, Sutterella wadsworthensis and Bacteroides stercoris were depleted, whereas Clostridiums with Eggerthella lenta were over-represented in individuals with asthma. In overall, the authors concluded an increase in SCFA-producing bacteria in asthma [49]. Investigating 47 patients with asthma and 20 healthy subjects, Zou et al., reported that patients with asthma have higher abundance of Ruminococcus gnavus, Bacteroides plebeius, and Clostridium clostridioforme and lower abundance of Roseburia inulinivorans and Clostridium disporicum. The authors also reported that the gut bacteriome was different in allergic and non-allergic subjects [50]. Comparing 15 patients with severe asthma to 14 with non-severe asthma and 15 healthy controls, Wang et al., demonstrated lower abundance of Acidaminococcaceae and higher abundance of Veillonellaceae and Prevotellaceae in severe asthma. The abundance of Veillonellaceae related to lung function [51]. In a very recent clinical trial, Van Engelen et al., modulated gut microbiome by administering antibiotics in patients with asthma. Changes in gut microbiome did not result in any alterations in markers of airway inflammation [24].
In overall, although gut dysbiosis may affect the development of asthma, the results of individual case–control studies were not replicated by other studies. This could be due to significant biological and clinical heterogeneity of asthma. On the other hand, standardization of microbiome analyses is warranted to understand the role of gut bacteriome in asthma.
4. COPD and Gut Microbiome
Chronic obstructive pulmonary disease is a chronic, usually progressive disorder of the airways, lung parenchyma and vasculature caused by exposure to fumes and noxious particles, most commonly cigarette smoke [12]. The gut microbiome may alter in patients with COPD due to smoking, environmental exposure, and diet (see Section 2). In addition, patients with severe COPD are particularly susceptible to bacterial infections [52]. An animal study demonstrated that respiratory infections may alter the gut bacteriome, namely, the instillation of intrapulmonary lipopolysaccharide significantly increases the total bacterial count in mice cecum [53].
Unfortunately, the number of studies investigating the gut bacteriome in COPD is low. Comparing 28 patients with COPD to 29 healthy controls, no difference was noticed in the gut bacteriome diversity. However, patients with COPD had an increased abundance of Streptococcus, Rothia, Romboutsia and Intestinibacter and decreased abundance of Bacteroides, Roseburia and Lachnospira. The abundance of the members of Streptococci and Lachnospiraceae inversely related to lung function [54]. Analysing 60 patients with COPD, Chiu et al., reported increased abundances of Fusobacterium and Aerococcus in more advanced disease. The abundance of Bacteroides was related to blood eosinophilia and lung function [55]. In their follow-up study, Chiu et al., reported that patients with rapid lung function decline have a higher abundance of Firmicutes and a lower abundance of Bacteroidetes and Alloprevotella. Along with worsening lung function, the authors reported an increasing abundance of Acinetobacter and Stenotrophomonas [56].
The gut microbiome may play a role in the occurrence of adverse outcomes during acute exacerbations of COPD. Sprooten et al., have reported an increase in small intestinal permeability during flare-ups suggesting that bacterial products may more likely enter the circulation [57]. In line with this, in patients with COPD exacerbations, increased levels of trimethylamine N-oxide (TMAO), a molecule which is metabolized by the liver from the bacterial product trimethylamine (TMA), were associated with increased long-term mortality [58]. Following up 15 patients with exacerbations during their recovery, Sun et al., have reported significant alterations in individual microbes without any change in their diversity [59].
Due to the low number and cross-sectional nature of the studies in COPD, it is not clear how gut dysbiosis affects the course of COPD. As mentioned in Section 3, dysbiosis may alter airway inflammation; however, this needs to be proven in patients with COPD. On the other hand, gut bacteriome could lead to accelerated lung ageing [60]. Most importantly, changes in the gut microbiome contribute to the development and worsening of comorbidities (see Section 6) which frequently accompany COPD and contribute to its burden [61].
5. OSA and Gut Microbiome
Obstructive sleep apnoea (OSA) is common disease which is characterised by the repetitive collapse of the upper airways during sleep. These respiratory events lead to chronic intermittent hypoxaemia and sleep fragmentation and ultimately OSA is a risk factor for cardiovascular, metabolic and cognitive disease [62]. Experimental animal studies show that both hypoxaemia and sleep fragmentation may lead to alterations in gut microbiome [63]. In addition, the effect of Western diet needs to be considered (please see Section 3).
Only a limited number of studies have investigated gut microbiome in OSA so far. In a cohort of two-year old children, Collado et al., have reported that the ratio of Firmicutes/Bacteroides was higher, whilst the ratio of Actinobacteria/Proteobacteria was lower in snorers. Snorers also showed lower microbial diversity compared to non-snorers [64]. In line with this, in a limited cohort of children of 2–12 years, Valentini et al., concluded a lower microbial diversity and higher abundance of Proteobacteria in patients with OSA [65]. Ko et al., analysed stool samples of 93 adult patients with OSA and 20 controls. They found significant differences only at genus level with decreased relative abundances of a few SCFA-producing bacteria which could lead to epithelial barrier disruption [66]. A further analysis of the same cohort revealed a significant relationship between the Prevotella enterotype and OSA [67]. The same group compared 60 patients with OSA and 12 control subjects. In this cross-sectional study, the abundance of Megamonas, Gemmiger, Dialister, and Oscillibacter genera were lower in OSA and SCFA-producing bacteria were in an inverse relationship with the presence of hypertension [68]. Investigating 19 adult patients with OSA and 20 controls, Bikov et al., reported a lower abundance of Actinobacteria phylum. Although, the abundance of Proteobacteria, Gammaproteobacteria, Lactobacillae, and Lactobacillus were related to disease severity, dyslipidaemia and cardiovascular disease, following adjustment on cardiometabolic risk factors, these associations became insignificant. This highlights the importance of adjusting for external factors on gut bacteria [69]. Investigating 32 patients with OSA and 14 healthy controls, Wang et al., concluded that OSA was associated with increased ratio of Firmicutes/Bacteroides. At genus level, the authors reported that the abundance of Rikenellaceae and Alistipes increased and Clostridium_XlVa decreased in OSA [70]. In patients with type 2 diabetes mellitus (2TDM), the presence of OSA was not associated with altered diversity, and there were only subtle differences, such as increased abundance of Oscillibacter and decreased abundance of Phascolarctobacterium in patients with OSA [71]. Finally, in patients with OSA, carotid atherosclerosis was associated with lower abundance of Peptostreptococcaceae [72].
6. Consequences of Altered Gut Microbiome on Frequent Comorbidities of Lung Diseases
The physiological role of human microbiome has been extensively reviewed previously [73,74]. In the current review we will focus on results identified by studies described in Section 2, Section 3 and Section 4 and how these relate on comorbidities.
One of the functions of the healthy microbiome is to maintain the metabolism of the epithelial cells in the gut. In line with this, dysbiosis may lead to disruption in the epithelial barrier. Through the disrupted intestinal barrier, gut microbiota dependent metabolites, bacterial toxins, and other bacterial products can enter the systemic circulation [75]. These bacterial products and metabolites may induce a proinflammatory state in the host, increasing the risk of cardiovascular diseases (CVD), and potentially worsening the inflammation associated with airway diseases.
One of the most important gut-microbiota dependent metabolites is TMA, a product of bacterial choline/phosphatidylcholine metabolism [76]. TMA produced in the intestines enters the host circulation and is oxidised in the liver via flavin monooxygenases into TMAO [73]. Previous studies have confirmed that high blood TMAO levels are associated with heart diseases [77,78] through enhancing atherosclerosis [76], promoting platelet reactivity [79,80], worsening vascular inflammation and increasing inflammasome activation [81,82], and augmenting oxidative stress [83,84]. These pathophysiological processes assessed in vitro and in vivo explain the results of human clinical studies reporting an association between circulating TMAO levels and adverse outcomes in peripheral and coronary artery disease [85], acute coronary syndrome [86], and heart failure [87].
Phenylacetylglutamine (PAG), a derivate of phenylalanine metabolism, is also a gut-microbiota dependent product which is associated with major adverse cardiac events, e.g., heart attack, ischemic stroke, and death [88]. PAG induces platelet hyperresponsiveness and thus increases thrombosis potential through the activation of G-protein-coupled receptors (GPCR), including α- and β-adrenergic receptors [88]. Moreover, Nemet et al., has also proven in the same study that in mice the β-blocker carvedilol reverses the pro-thrombotic effect of PAG [88].
Additionally, besides enhanced production of microbiota-dependent metabolites, dysbiosis can also modify the production and absorption of other intestinal molecules, including primary and secondary bile acids, and SCFA. Secondary bile acids (SBA) are synthetised by gut bacteria via bile salt hydrolysis or 7α-dehydroxylation of primary bile acids, and the production is mainly regulated by the interaction between the host and the gut microbiota. SBAs absorbed in the circulation impact the host physiology in several ways, including GPCRs, farnesoid-X receptors, liver-X receptors and pregnane-X receptors [75]. As SBAs have hormone-like functions, they have been linked to metabolic disorders, for example type 2 diabetes mellitus [89]. In addition, SCFAs (e.g., acetate, propionate, butyrate) are also a product of bacterial processes with physiologic functions, but the disturbances in the absorbed SCFA milieu may have pathologic consequences, such as hypertension, adiposity [90], or worsening of the airway inflammation [18]. SCFAs are produced from dietary fibres through anaerobic fermentation in the intestines, and they act as an energy source for intestinal epithelial cells and have an important role in the regulation of blood pressure and glucose and lipid metabolism. The receptors that mediate the effects in blood pressure regulation are the olfactory receptor 78 (Olfr78) [91] and the G-protein receptor 41 (Gpr41) [92]. The activation of Olfr78 causes hypertension through the renin-angiotensin pathway, while Gpr41 has the opposite effect by relaxing the vascular smooth muscle cells. The role of these receptors and SCFA generation was proven in multiple animal studies, either via SCFA administration in genetic knock-out mice [91] or by fecal transplantation in germ-free mice from human hypertensive or control normotensive donors [93]. However, it is likely that the overall effect of SCFAs on blood pressure is influenced by genetic variations and the cross-talk with other microbiota-dependent plasma metabolites [75].
Furthermore, Gram-negative bacteria, such as Proteobacteria produce LPS which can enter the circulation if the host barrier function is not intact. LPS may then induce vascular, systemic and pulmonary inflammation through the activation of surface Toll-like receptors of immune cells [94]. Several mechanistic models performed in cell cultures, animals, and humans proved that the administration of LPS triggers an inflammatory response in the host [95,96,97], and causes dysfunction in certain cells, such as cardiomyocytes [98]. Additionally, a human observational clinical study also confirmed the relationship between gut-derived systemic LPS concentration and CVD risk, namely, the LPS level was found to be predictive for major adverse cardiovascular events in a cohort of patients with atrial fibrillation [99].
Gut bacteria may produce neurotransmitters or their precursors which could affect mood, cognitive function and sleep quality [100]. For instance, Actinobacteria, Lactobacilli and Bifidobacteria can produce gamma-aminobutyric acid (GABA) which is a sleep promoting neurotransmitter [101]. In line with this, Smith et al., have reported an inverse relationship between Actinobacteria and the number of awakenings in healthy volunteers [102]. Very interestingly, feces transplanted from mice exposed to intermittent hypoxaemia induced sleepiness in recipient mice, suggesting that hypoxaemia-induced sleepiness in OSA may be partially mediated by gut microbiome [103]. It has also been reported, that increasing the gut microbiota diversity and the abundance of certain bacterial phyla, such as Actinobacteria and Firmicutes, is associated with increased cognitive function [104]. Furthermore, psychological factors, e.g., emotional stress or sleep deprivation can also influence the microbiota profile potentially leading to dysbiosis, intestinal inflammation, and increased intestinal permeability [105,106]. The interplay between mental health and the gut microbiome is thus bi-directional, which proposes further research and possible therapeutic potential [107].
7. Therapeutic Aspects of Influencing the Gut Microbiome
A number of different preclinical and clinical studies have explored the use of probiotics, prebiotics, dietary components and faecal microbiota transplantation (FMT) to modulate the gut microbiota as therapeutic strategies to influence the gut-lung axis [3,108]. However, the specific efficacy of these supplements in the treatment of respiratory diseases, including asthma, COPD, lung cancer and respiratory infections, has not been clearly defined.
7.1. Oral Supplementations for the Treatment of Respiratory Diseases
According to the WHO nutritional guidelines, probiotics are useful supplements containing live microorganisms that, when taken properly, provide important health benefits for an individual [109].
7.1.1. Oral Supplementations in Asthma
Mice fed with high fibre diet had increased circulating levels of SCFA and were protected against allergic airway disease [26]. Similarly in mice, gut inoculation with Lactobacillus johnsonii significantly reduced the Th2 response in the lungs [110]. Supplementation of Ligilactobacillus salivarius LS01 and Bifidobacterium breve B632 on a paediatric population with asthma resulted in a significant reduction in the number, frequency and severity of asthma flare-ups [111]. A study conducted in mouse models argues that a combined nutritional intervention with Bifidobacterium lactis BB-12 and the supplementation of nutrients with antioxidant and anti-inflammatory properties (docosahexanoic acid, vitamin C and E) is a rational option to alleviate air pollution-related lung inflammation [112]. Recently, a 3-month randomized controlled study investigated the efficacy of co-administration of Bifidobacterium lactis Probio-M8 with conventional therapy in the management of asthma, observing an improvement in asthma symptoms and reduction in exhaled and alveolar nitric oxide levels [113]. More importantly, the therapeutic synergy with the probiotic increased the resilience of the gut microbiome showing significant increases in the potentially beneficial species of Bifidobacterium animalis, Bifidobacterium longum and Prevotella, and decreases in Parabacteroides distasonis and Clostridiales, compared to the placebo group [113].
Administration of Lactobacillus rhamnosus mitigated airway inflammation and bronchial reactivity in an asthma mice model [114]. In addition, obese mice fed with Lactobacillus gasseri exhibited better anti-microbial response [115]. Moreover, it was observed that Probio-M8 reduced the duration of flu-like symptoms compared with more prevalent anti-inflammatory effects against lower respiratory tract infections [116].
7.1.2. The Effect of Oral Supplementations on the Upper Respiratory Tract
A randomised, double-blind, parallel, placebo-controlled study evaluated the effects of Bifidobacterium longum BB536 on diarrhoea and/or upper respiratory tract disease in 520 children aged 2–6 years [117]. Although BB536 did not exert significant effects against diarrhoea, this multifunctional probiotic was shown to reduce the duration of common upper respiratory tract infections (URTI) by modulating the gut microbiota. Analysis of the gut microbiota at the genus level revealed a significantly higher abundance of bacterial genera with immunomodulatory and anti-inflammatory properties, such as Faecalibacterium in the treated group compared to the placebo group, illustrating the potential protective effects of BB536 against respiratory diseases [117]. Improvements in the duration of nasal symptoms and the frequency of URTIs were obtained following daily administration for 12 weeks of Lactobacillus plantarum DR7 [118], a strain isolated from cow’s milk that acts on the activation and phosphorylation of protein kinase AMP (AMPK) [119], exerting a certain immunomodulatory protective role against URTIs and influenza virus infections through activation of macrophages [120]. Reduced plasma levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), and an enhancement of anti-inflammatory cytokines (IL-4, IL-10) were also observed, accompanied by reduced levels of oxidative stress, lower expression of CD4 and CD8 cells, and a higher presence of NK cells in adults who received DR7 compared to placebo [118]. Similar results were reported following oral administration of Lactobacillus casei Zhang (9 log CFU per day) against URTIs and gastrointestinal disorders, through potential anti-oxidative and immune-modulatory effects [121].
7.1.3. Oral Supplementations in COPD
There is evidence that the use of probiotics may have an effect on COPD thus confirming its connection with the gut microbiota [122,123]. Verheijden showed that intragastric supplementation with Lactobacillus rhamnosus and Bifidobacterium breve in mice mitigated airway inflammation and alveolar damage [122]. Furthermore, Mortaz et al., revealed that the same two probiotics had analogous anti-inflammatory effect on cigarette smoke-induced inflammation in human macrophages [123]. The intake of a multi-strain probiotic on faecal microbiota composition and bowel movements in patients with COPD treated with antibiotics showed a modest probiotic effect on bacterial subgroups, i.e., an increase in yeasts and a decrease in Bifidobacteria, in the treated group compared to placebo [124].
7.1.4. Oral Supplementations in Sleep Disorders
Lactobacillus fermentum strain PS150 improved sleep quality in mice [125] by promoting non-rapid eye movement (nREM) sleep [126]. On the other hand, ergothioneine, a metabolite of Lactobacillus reuteri, increased REM sleep duration in rats [127]. A prebiotic diet has prolonged both REM and nREM sleep in another study in rats [128]. A recent review on this topic concluded that these alterations could lead to improvement in mental disorders in patients with sleep disorders [129]. Finally, administration of probiotics improved coronary artery changes induced by intermittent hypoxaemia but could not fully mitigate coronary artery disease [130].
7.2. Preliminary Evidence on Fecal Microbiota Transplantation on the Gut-Lung Microbiota Axis
Given that intestinal bacterial flora plays an important role in the physiology of the human body, among the possibilities of actively intervening on the intestinal microbiota, either for health or therapeutic purposes, faecal microbiota transplantation (FMT) is gaining popularity, not least because of its apparent ‘radicality’ [131]. As reported earlier, gut dysbiosis influences lung disease via the gut-lung axis. Preliminary studies on FMT have explored the mechanism of rebuilding gut flora on respiratory diseases. Experimentally, it was observed that the intervention of a diet rich in fibre and FMT reduced the severity of pulmonary emphysema in mice with COPD, while also increasing the abundances of SCFAs-producing gut bacteria, such as Bacteroidaceae and Lachnospiraceae [132]. In another study on smoking induced COPD mice model, fecal transplantation ameliorated COPD. The authors highlighted the protective role of Parabacteroides goldsteinii [133]. Similarly, a research group explored the mechanism of gut flora regulation in the host defence against LPS-induced acute lung injury by constructing a model of gut microflora dysbiosis with antibiotic administration and reconstruction of the gut ecology via FMT. Following FMT intervention, a down-regulation of the TLR4/NF-kB signaling pathway was observed in the lung and a reduction in the levels of inflammation and oxidative stress in animals with acute lung injury by restoring the gut microbiota composition [134]. Another study on the gut-lung axis showed that the process of restoring commensal flora by FMT in C57BL/6 mice deprived of the gut microbiota and intranasally infected with S. pneumoniae resulted in normalisation of lung bacterial counts and the levels of TNF-α and IL-10 six hours after infection. The study concluded that the gut microbiome is a protective factor against pneumococcal pneumonia by regulating alveolar macrophage function and inflammatory response [135].
It is, however, a more complex method than it may seem, which in fact selectively transfers some strains and others do not and should be precisely defined in terms of procedure, treatment of the bacterial material, selection of the donor and assessment of the risk of pathogenic bacterial contamination. Further future research will be needed to evaluate the efficacy and safety of FMT in modulating the gut microbiota in patients with respiratory diseases.
8. Summary
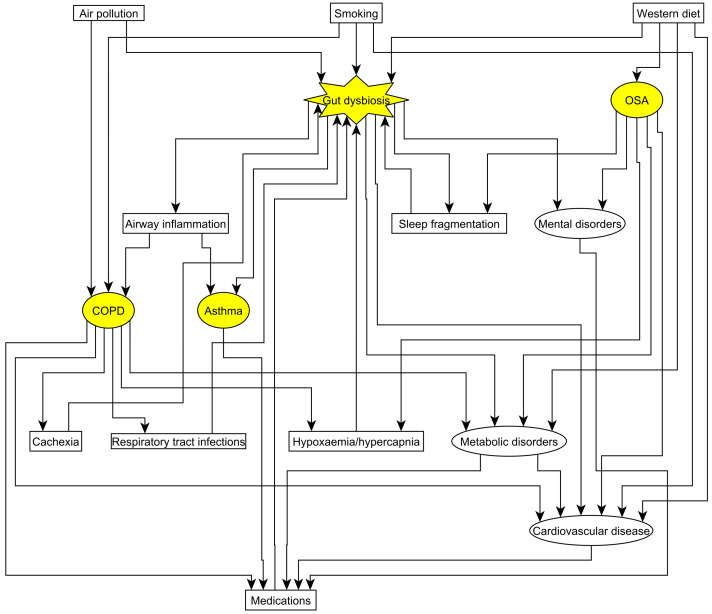
The interplay between the respiratory system and the gut bacteriome is multifactorial (Figure 2). On one hand, it opens an avenue to study gut bacteriome as a treatable trait, especially when reducing cardiovascular risk. On the other hand, it brings a complexity when interpreting and comparing the results of human studies. The effect of respiratory pharmaceutical treatment on gut bacteriome was rarely investigated, whilst non-pharmaceutical approaches, such as oxygen or positive airway pressure were not studied at all. Although oral supplementation and fecal transplantation are promising in animals, they still need to be tested in large randomized controlled trials in humans.
Figure 2.
The complex interrelation between asthma, COPD, OSA and gut dysbiosis. For the mechanism in detail, please check the text.
Acknowledgments
Andras Bikov is supported by the NIHR Manchester Biomedical Research Centre (BRC). Balazs Csoma is supported by the Hungarian Respiratory Society and by the Hungarian State Eötvös Scholarship of the Tempus Public Foundation.
Author Contributions
Conceptualization, A.B.; methodology, A.B.; writing—original draft preparation, A.B., S.D., B.C., C.M., P.F., G.R., L.P., M.G. and S.S.; writing—review and editing, A.B., B.C., S.D. and S.S.; visualization, C.M. and A.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enaud R., Prevel R., Ciarlo E., Beaufils F., Wieërs G., Guery B., Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingula R., Filaire M., Radosevic-Robin N., Bey M., Berthon J.Y., Bernalier-Donadille A., Vasson M.P., Filaire E. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J. Oncol. 2017;2017:5035371. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gileles-Hillel A., Kheirandish-Gozal L., Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat. Rev. Endocrinol. 2016;12:290–298. doi: 10.1038/nrendo.2016.22. [DOI] [PubMed] [Google Scholar]
- 6.Wagner P.D. Possible mechanisms underlying the development of cachexia in COPD. Eur. Respir. J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 7.Grier A., McDavid A., Wang B., Qiu X., Java J., Bandyopadhyay S., Yang H., Holden-Wiltse J., Kessler H.A., Gill A.L., et al. Neonatal gut and respiratory microbiota: Coordinated development through time and space. Microbiome. 2018;6:193. doi: 10.1186/s40168-018-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madan J.C., Koestler D.C., Stanton B.A., Davidson L., Moulton L.A., Housman M.L., Moore J.H., Guill M.F., Morrison H.G., Sogin M.L., et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: Interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3:e00251-12. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poroyko V.A., Carreras A., Khalyfa A., Khalyfa A.A., Leone V., Peris E., Almendros I., Gileles-Hillel A., Qiao Z., Hubert N., et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.H., Yun Y., Kim S.J., Lee E.J., Chang Y., Ryu S., Shin H., Kim H.L., Kim H.N., Lee J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018;7:282. doi: 10.3390/jcm7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biedermann L., Zeitz J., Mwinyi J., Sutter-Minder E., Rehman A., Ott S.J., Steurer-Stey C., Frei A., Frei P., Scharl M., et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. [(accessed on 2 December 2022)]. Available online: www.goldcopd.org.
- 13.Claus S.P., Guillou H., Ellero-Simatos S. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T., Fang J., Tang S., Du H., Zhao L., Wang Y., Deng F., Liu Y., Du Y., Cui L., et al. PM(2.5) exposure associated with microbiota gut-brain axis: Multi-omics mechanistic implications from the BAPE study. Innovation. 2022;3:100213. doi: 10.1016/j.xinn.2022.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclerc M., Bedu-Ferrari C., Etienne-Mesmin L., Mariadassou M., Lebreuilly L., Tran S.L., Brazeau L., Mayeur C., Delmas J., Rué O., et al. Nitric Oxide Impacts Human Gut Microbiota Diversity and Functionalities. mSystems. 2021;6:e0055821. doi: 10.1128/mSystems.00558-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouladi F., Bailey M.J., Patterson W.B., Sioda M., Blakley I.C., Fodor A.A., Jones R.B., Chen Z., Kim J.S., Lurmann F., et al. Air pollution exposure is associated with the gut microbiome as revealed by shotgun metagenomic sequencing. Environ. Int. 2020;138:105604. doi: 10.1016/j.envint.2020.105604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albenberg L., Esipova T.V., Judge C.P., Bittinger K., Chen J., Laughlin A., Grunberg S., Baldassano R.N., Lewis J.D., Li H., et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csoma B., Vulpi M.R., Dragonieri S., Bentley A., Felton T., Lázár Z., Bikov A. Hypercapnia in COPD: Causes, Consequences, and Therapy. J. Clin. Med. 2022;11:3180. doi: 10.3390/jcm11113180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor K.M., Lucking E.F., Golubeva A.V., Strain C.R., Fouhy F., Cenit M.C., Dhaliwal P., Bastiaanssen T.F.S., Burns D.P., Stanton C., et al. Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. eBioMedicine. 2019;44:618–638. doi: 10.1016/j.ebiom.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi A., Melnik A.V., Xue J., Poulsen O., Meehan M.J., Humphrey G., Jiang L., Ackermann G., McDonald D., Zhou D., et al. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems. 2018;3:e00020-18. doi: 10.1128/mSystems.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allaband C., Lingaraju A., Martino C., Russell B., Tripathi A., Poulsen O., Dantas Machado A.C., Zhou D., Xue J., Elijah E., et al. Intermittent Hypoxia and Hypercapnia Alter Diurnal Rhythms of Luminal Gut Microbiome and Metabolome. mSystems. 2021;6:e00116-21. doi: 10.1128/mSystems.00116-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vich Vila A., Collij V., Sanna S., Sinha T., Imhann F., Bourgonje A.R., Mujagic Z., Jonkers D., Masclee A.A.M., Fu J., et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020;11:362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang E.Y., Inoue T., Leone V.A., Dalal S., Touw K., Wang Y., Musch M.W., Theriault B., Higuchi K., Donovan S., et al. Using corticosteroids to reshape the gut microbiome: Implications for inflammatory bowel diseases. Inflamm. Bowel Dis. 2015;21:963–972. doi: 10.1097/MIB.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Engelen T.S.R., Yang J., Haak B.W., Bonta P.I., Van Der Poll T., Wiersinga W.J. Gut Microbiome Modulation by Antibiotics in Adult Asthma: A Human Proof-of-Concept Intervention Trial. Clin. Gastroenterol. Hepatol. 2022;20:1404–1407.e4. doi: 10.1016/j.cgh.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Elson C.O., Alexander K.L. Host-microbiota interactions in the intestine. Dig. Dis. 2015;33:131–136. doi: 10.1159/000369534. [DOI] [PubMed] [Google Scholar]
- 26.Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 27.McAleer J.P., Nguyen N.L., Chen K., Kumar P., Ricks D.M., Binnie M., Armentrout R.A., Pociask D.A., Hein A., Yu A., et al. Pulmonary Th17 Antifungal Immunity Is Regulated by the Gut Microbiome. J. Immunol. 2016;197:97–107. doi: 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley C.P., Teng F., Felix K.M., Sano T., Naskar D., Block K.E., Huang H., Knox K.S., Littman D.R., Wu H.J. Segmented Filamentous Bacteria Provoke Lung Autoimmunity by Inducing Gut-Lung Axis Th17 Cells Expressing Dual TCRs. Cell Host Microbe. 2017;22:697–704.e4. doi: 10.1016/j.chom.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagundes C.T., Amaral F.A., Vieira A.T., Soares A.C., Pinho V., Nicoli J.R., Vieira L.Q., Teixeira M.M., Souza D.G. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J. Immunol. 2012;188:1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 30.Fox A.C., McConnell K.W., Yoseph B.P., Breed E., Liang Z., Clark A.T., O’Donnell D., Zee-Cheng B., Jung E., Dominguez J.A., et al. The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock. 2012;38:508–514. doi: 10.1097/SHK.0b013e31826e47e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown R.L., Sequeira R.P., Clarke T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017;8:1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Global Initiaitve for Asthma. Global Strategy for Asthma Management and Prevention. 2022. [(accessed on 1 July 2022)]. Available online: www.ginasthma.org.
- 33.Brusselle G.G., Koppelman G.H. Biologic Therapies for Severe Asthma. N. Engl. J. Med. 2022;386:157–171. doi: 10.1056/NEJMra2032506. [DOI] [PubMed] [Google Scholar]
- 34.Frati F., Salvatori C., Incorvaia C., Bellucci A., Di Cara G., Marcucci F., Esposito S. The Role of the Microbiome in Asthma: The Gut–Lung Axis. Int. J. Mol. Sci. 2018;20:123. doi: 10.3390/ijms20010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada H., Kuhn C., Feillet H., Bach J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbst T., Sichelstiel A., Schär C., Yadava K., Bürki K., Cahenzli J., McCoy K., Marsland B.J., Harris N.L. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y.J., Boushey H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015;135:25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunii J., Takahashi K., Kasakura K., Tsuda M., Nakano K., Hosono A., Kaminogawa S. Commensal bacteria promote migration of mast cells into the intestine. Immunobiology. 2011;216:692–697. doi: 10.1016/j.imbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Akbari O., Stock P., Meyer E., Kronenberg M., Sidobre S., Nakayama T., Taniguchi M., Grusby M.J., DeKruyff R.H., Umetsu D.T. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 40.Demirci M., Tokman H.B., Uysal H.K., Demiryas S., Karakullukcu A., Saribas S., Cokugras H., Kocazeybek B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019;47:365–371. doi: 10.1016/j.aller.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Pascal M., Perez-Gordo M., Caballero T., Escribese M.M., Lopez Longo M.N., Luengo O., Manso L., Matheu V., Seoane E., Zamorano M., et al. Microbiome and Allergic Diseases. Front. Immunol. 2018;9:1584. doi: 10.3389/fimmu.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arrieta M.C., Stiemsma L.T., Dimitriu P.A., Thorson L., Russell S., Yurist-Doutsch S., Kuzeljevic B., Gold M.J., Britton H.M., Lefebvre D.L., et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 43.Stokholm J., Blaser M.J., Thorsen J., Rasmussen M.A., Waage J., Vinding R.K., Schoos A.M., Kunøe A., Fink N.R., Chawes B.L., et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat. Commun. 2018;9:141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Depner M., Taft D.H., Kirjavainen P.V., Kalanetra K.M., Karvonen A.M., Peschel S., Schmausser-Hechfellner E., Roduit C., Frei R., Lauener R., et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020;26:1766–1775. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 45.Chiu C.Y., Chan Y.L., Tsai M.H., Wang C.J., Chiang M.H., Chiu C.C. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ. J. 2019;12:100021. doi: 10.1016/j.waojou.2019.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottman N., Reunanen J., Meijerink M., Pietilä T.E., Kainulainen V., Klievink J., Huuskonen L., Aalvink S., Skurnik M., Boeren S., et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begley L., Madapoosi S., Opron K., Ndum O., Baptist A., Rysso K., Erb-Downward J.R., Huang Y.J. Gut microbiota relationships to lung function and adult asthma phenotype: A pilot study. BMJ Open Respir. Res. 2018;5:e000324. doi: 10.1136/bmjresp-2018-000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okba A.M., Saber S.M., Abdel-Rehim A.S., Amin M.M., Mohamed D.A. Fecal microbiota profile in atopic asthmatic adult patients. Eur. Ann. Allergy Clin. Immunol. 2018;50:117–124. doi: 10.23822/EurAnnACI.1764-1489.48. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q., Li F., Liang B., Liang Y., Chen S., Mo X., Ju Y., Zhao H., Jia H., Spector T.D., et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018;18:114. doi: 10.1186/s12866-018-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou X.L., Wu J.J., Ye H.X., Feng D.Y., Meng P., Yang H.L., Wu W.B., Li H.T., He Z., Zhang T.T. Associations Between Gut Microbiota and Asthma Endotypes: A Cross-Sectional Study in South China Based on Patients with Newly Diagnosed Asthma. J. Asthma Allergy. 2021;14:981–992. doi: 10.2147/JAA.S320088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z., Lai Z., Zhang X., Huang P., Xie J., Jiang Q., Zhang Q., Chung K.F. Altered gut microbiome compositions are associated with the severity of asthma. J. Thorac. Dis. 2021;13:4322–4338. doi: 10.21037/jtd-20-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurst J.R., Vestbo J., Anzueto A., Locantore N., Mullerova H., Tal-Singer R., Miller B., Lomas D.A., Agusti A., Macnee W., et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 53.Sze M.A., Tsuruta M., Yang S.W., Oh Y., Man S.F., Hogg J.C., Sin D.D. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS ONE. 2014;9:e111228. doi: 10.1371/journal.pone.0111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowerman K.L., Rehman S.F., Vaughan A., Lachner N., Budden K.F., Kim R.Y., Wood D.L.A., Gellatly S.L., Shukla S.D., Wood L.G., et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020;11:5886. doi: 10.1038/s41467-020-19701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu Y.C., Lee S.W., Liu C.W., Lin R.C., Huang Y.C., Lan T.Y., Wu L.S. Comprehensive profiling of the gut microbiota in patients with chronic obstructive pulmonary disease of varying severity. PLoS ONE. 2021;16:e0249944. doi: 10.1371/journal.pone.0249944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu Y.C., Lee S.W., Liu C.W., Lan T.Y., Wu L.S. Relationship between gut microbiota and lung function decline in patients with chronic obstructive pulmonary disease: A 1-year follow-up study. Respir. Res. 2022;23:10. doi: 10.1186/s12931-022-01928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprooten R.T.M., Lenaerts K., Braeken D.C.W., Grimbergen I., Rutten E.P., Wouters E.F.M., Rohde G.G.U. Increased Small Intestinal Permeability during Severe Acute Exacerbations of COPD. Respir. Int. Rev. Thorac. Dis. 2018;95:334–342. doi: 10.1159/000485935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ottiger M., Nickler M., Steuer C., Bernasconi L., Huber A., Christ-Crain M., Henzen C., Hoess C., Thomann R., Zimmerli W., et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition. 2018;45:135–141.e1. doi: 10.1016/j.nut.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Sun Z., Zhu Q.L., Shen Y., Yan T., Zhou X. Dynamic changes of gut and lung microorganisms during chronic obstructive pulmonary disease exacerbations. Kaohsiung J. Med. Sci. 2020;36:107–113. doi: 10.1002/kjm2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saint-Criq V., Lugo-Villarino G., Thomas M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2021;66:101235. doi: 10.1016/j.arr.2020.101235. [DOI] [PubMed] [Google Scholar]
- 61.Bikov A., Horváth A., Tomisa G., Bártfai L., Bártfai Z. Changes in the Burden of Comorbidities in Patients with COPD and Asthma-COPD Overlap According to the GOLD 2017 Recommendations. Lung. 2018;196:591–599. doi: 10.1007/s00408-018-0141-7. [DOI] [PubMed] [Google Scholar]
- 62.Bikov A., Frent S., Pleava R., Kunos L., Bokhari S., Meszaros M., Mihaicuta S. The Burden of Associated Comorbidities in Patients with Obstructive Sleep Apnea-Regional Differences in Two Central-Eastern European Sleep Centers. J. Clin. Med. 2020;9:3583. doi: 10.3390/jcm9113583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F., Zou J., Xu H., Huang W., Zhang X., Wei Z., Li X., Liu Y., Zou J., Liu F., et al. Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology. Front. Endocrinol. 2022;13:820939. doi: 10.3389/fendo.2022.820939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collado M.C., Katila M.K., Vuorela N.M., Saarenpää-Heikkilä O., Salminen S., Isolauri E. Dysbiosis in Snoring Children: An Interlink to Comorbidities? J. Pediatr. Gastroenterol. Nutr. 2019;68:272–277. doi: 10.1097/MPG.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 65.Valentini F., Evangelisti M., Arpinelli M., Di Nardo G., Borro M., Simmaco M., Villa M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: A pilot study. Sleep Med. 2020;76:140–147. doi: 10.1016/j.sleep.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Ko C.Y., Liu Q.Q., Su H.Z., Zhang H.P., Fan J.M., Yang J.H., Hu A.K., Liu Y.Q., Chou D., Zeng Y.M. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: Disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 2019;133:905–917. doi: 10.1042/CS20180891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko C.Y., Fan J.M., Hu A.K., Su H.Z., Yang J.H., Huang L.M., Yan F.R., Zhang H.P., Zeng Y.M. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9:e01287. doi: 10.1002/brb3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko C.Y., Su H.Z., Zhang L., Zeng Y.M. Disturbances of the Gut Microbiota, Sleep Architecture, and mTOR Signaling Pathway in Patients with Severe Obstructive Sleep Apnea-Associated Hypertension. Int. J. Hypertens. 2021;2021:9877053. doi: 10.1155/2021/9877053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bikov A., Szabo H., Piroska M., Kunos L., Szily M., Ligeti B., Makra N., Szabo D., Tarnoki D.L., Tarnoki A.D. Gut Microbiome in Patients with Obstructive Sleep Apnoea. Appl. Sci. 2022;12:2007. doi: 10.3390/app12042007. [DOI] [Google Scholar]
- 70.Wang F., Liu Q., Wu H., Tang T., Zhao T., Li Z. The dysbiosis gut microbiota induces the alternation of metabolism and imbalance of Th17/Treg in OSA patients. Arch. Microbiol. 2022;204:217. doi: 10.1007/s00203-022-02825-w. [DOI] [PubMed] [Google Scholar]
- 71.Tang S.S., Liang C.H., Liu Y.L., Wei W., Deng X.R., Shi X.Y., Wang L.M., Zhang L.J., Yuan H.J. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022;28:2320–2333. doi: 10.3748/wjg.v28.i21.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szabo H., Piroska M., Hernyes A., Zoldi L., Juhasz J., Ligeti B., Makra N., Szabo D., Bikov A., Kunos L., et al. The Relationship between Atherosclerosis and Gut Microbiome in Patients with Obstructive Sleep Apnoea. Appl. Sci. 2022;12:11484. doi: 10.3390/app122211484. [DOI] [Google Scholar]
- 73.Rahman M.M., Islam F., Or-Rashid M.H., Mamun A.A., Rahaman M.S., Islam M.M., Meem A.F.K., Sutradhar P.R., Mitra S., Mimi A.A., et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022;12:903570. doi: 10.3389/fcimb.2022.903570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trøseid M., Andersen G., Broch K., Hov J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. eBioMedicine. 2020;52:102649. doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witkowski M., Weeks T.L., Hazen S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang W.H., Wang Z., Fan Y., Levison B., Hazen J.E., Donahue L.M., Wu Y., Hazen S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skye S.M., Zhu W., Romano K.A., Guo C.J., Wang Z., Jia X., Kirsop J., Haag B., Lang J.M., DiDonato J.A., et al. Microbial Transplantation With Human Gut Commensals Containing CutC Is Sufficient to Transmit Enhanced Platelet Reactivity and Thrombosis Potential. Circ. Res. 2018;123:1164–1176. doi: 10.1161/CIRCRESAHA.118.313142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J., Shih D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J. Am. Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X., Li Y., Yang P., Liu X., Lu L., Chen Y., Zhong X., Li Z., Liu H., Ou C., et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-kappaB (Nuclear Factor kappaB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020;40:751–765. doi: 10.1161/ATVBAHA.119.313414. [DOI] [PubMed] [Google Scholar]
- 83.Boini K.M., Hussain T., Li P.L., Koka S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol. Biochem. 2017;44:152–162. doi: 10.1159/000484623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen M.L., Zhu X.H., Ran L., Lang H.D., Yi L., Mi M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017;6:e006347. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Senthong V., Wang Z., Fan Y., Wu Y., Hazen S.L., Tang W.H. Trimethylamine N-Oxide and Mortality Risk in Patients With Peripheral Artery Disease. J. Am. Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan Y., Sheng Z., Zhou P., Liu C., Zhao H., Song L., Li J., Zhou J., Chen Y., Wang L., et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2019;12:e007281. doi: 10.1161/CIRCINTERVENTIONS.118.007281. [DOI] [PubMed] [Google Scholar]
- 87.Troseid M., Ueland T., Hov J.R., Svardal A., Gregersen I., Dahl C.P., Aakhus S., Gude E., Bjorndal B., Halvorsen B., et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 88.Nemet I., Saha P.P., Gupta N., Zhu W., Romano K.A., Skye S.M., Cajka T., Mohan M.L., Li L., Wu Y., et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell. 2020;180:862–877.e22. doi: 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haeusler R.A., Astiarraga B., Camastra S., Accili D., Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 91.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., Berkowitz D.E., Pluznick J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J., Zhao F., Wang Y., Chen J., Tao J., Tian G., Wu S., Liu W., Cui Q., Geng B., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hug H., Mohajeri M.H., La Fata G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients. 2018;10:203. doi: 10.3390/nu10020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kell D.B., Pretorius E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. 2015;7:1339–1377. doi: 10.1039/c5ib00158g. [DOI] [PubMed] [Google Scholar]
- 96.Brooks D., Barr L.C., Wiscombe S., McAuley D.F., Simpson A.J., Rostron A.J. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur. Respir. J. 2020;56:1901298. doi: 10.1183/13993003.01298-2019. [DOI] [PubMed] [Google Scholar]
- 97.Fink M.P. Animal models of sepsis. Virulence. 2014;5:143–153. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yucel G., Zhao Z., El-Battrawy I., Lan H., Lang S., Li X., Buljubasic F., Zimmermann W.H., Cyganek L., Utikal J., et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017;7:2935. doi: 10.1038/s41598-017-03147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pastori D., Carnevale R., Nocella C., Novo M., Santulli M., Cammisotto V., Menichelli D., Pignatelli P., Violi F. Gut-Derived Serum Lipopolysaccharide is Associated with Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Heart Assoc. 2017;6:e005784. doi: 10.1161/JAHA.117.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neroni B., Evangelisti M., Radocchia G., Di Nardo G., Pantanella F., Villa M.P., Schippa S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep Med. 2021;87:1–7. doi: 10.1016/j.sleep.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Yunes R.A., Poluektova E.U., Dyachkova M.S., Klimina K.M., Kovtun A.S., Averina O.V., Orlova V.S., Danilenko V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197–204. doi: 10.1016/j.anaerobe.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 102.Smith R.P., Easson C., Lyle S.M., Kapoor R., Donnelly C.P., Davidson E.J., Parikh E., Lopez J.V., Tartar J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE. 2019;14:e0222394. doi: 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Badran M., Khalyfa A., Ericsson A., Gozal D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp. Neurol. 2020;334:113439. doi: 10.1016/j.expneurol.2020.113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tooley K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients. 2020;12:3009. doi: 10.3390/nu12103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galley J.D., Bailey M.T. Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes. 2014;5:390–396. doi: 10.4161/gmic.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karl J.P., Margolis L.M., Madslien E.H., Murphy N.E., Castellani J.W., Gundersen Y., Hoke A.V., Levangie M.W., Kumar R., Chakraborty N., et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G559–G571. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 107.Morais L.H., Schreiber H.L.t., Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021;19:241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 108.Dumas A., Bernard L., Poquet Y., Lugo-Villarino G., Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018;20:e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 109.Doron S., Gorbach S.L. Probiotics: Their role in the treatment and prevention of disease. Expert Rev. Anti-Infect. Ther. 2006;4:261–275. doi: 10.1586/14787210.4.2.261. [DOI] [PubMed] [Google Scholar]
- 110.Fujimura K.E., Demoor T., Rauch M., Faruqi A.A., Jang S., Johnson C.C., Boushey H.A., Zoratti E., Ownby D., Lukacs N.W., et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc. Natl. Acad. Sci. USA. 2014;111:805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drago L., Cioffi L., Giuliano M., Pane M., Amoruso A., Schiavetti I., Reid G., Ciprandi G., Propam Study G. The Probiotics in Pediatric Asthma Management (PROPAM) Study in the Primary Care Setting: A Randomized, Controlled, Double-Blind Trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706) J. Immunol. Res. 2022;2022:3837418. doi: 10.1155/2022/3837418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Panebianco C., Eddine F.B.N., Forlani G., Palmieri G., Tatangelo L., Villani A., Xu L., Accolla R., Pazienza V. Probiotic Bifidobacterium lactis, anti-oxidant vitamin E/C and anti-inflammatory dha attenuate lung inflammation due to pm2.5 exposure in mice. Benef. Microbes. 2019;10:69–75. doi: 10.3920/BM2018.0060. [DOI] [PubMed] [Google Scholar]
- 113.Liu A., Ma T., Xu N., Jin H., Zhao F., Kwok L.Y., Zhang H., Zhang S., Sun Z. Adjunctive Probiotics Alleviates Asthmatic Symptoms via Modulating the Gut Microbiome and Serum Metabolome. Microbiol. Spectr. 2021;9:e0085921. doi: 10.1128/Spectrum.00859-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spacova I., Van Beeck W., Seys S., Devos F., Vanoirbeek J., Vanderleyden J., Ceuppens J., Petrova M., Lebeer S. Lactobacillus rhamnosus probiotic prevents airway function deterioration and promotes gut microbiome resilience in a murine asthma model. Gut Microbes. 2020;11:1729–1744. doi: 10.1080/19490976.2020.1766345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoda K., He F., Miyazawa K., Kawase M., Kubota A., Hiramatsu M. Orally administered heat-killed Lactobacillus gasseri TMC0356 alters respiratory immune responses and intestinal microbiota of diet-induced obese mice. J. Appl. Microbiol. 2012;113:155–162. doi: 10.1111/j.1365-2672.2012.05316.x. [DOI] [PubMed] [Google Scholar]
- 116.Mageswary M.U., Ang X.Y., Lee B.K., Chung Y.F., Azhar S.N.A., Hamid I.J.A., Bakar H.A., Roslan N.S., Liu X., Kang X., et al. Probiotic Bifidobacterium lactis Probio-M8 treated and prevented acute RTI, reduced antibiotic use and hospital stay in hospitalized young children: A randomized, double-blind, placebo-controlled study. Eur. J. Nutr. 2022;61:1679–1691. doi: 10.1007/s00394-021-02689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lau A.S., Yanagisawa N., Hor Y.Y., Lew L.C., Ong J.S., Chuah L.O., Lee Y.Y., Choi S.B., Rashid F., Wahid N., et al. Bifidobacterium longum BB536 alleviated upper respiratory illnesses and modulated gut microbiota profiles in Malaysian pre-school children. Benef. Microbes. 2018;9:61–70. doi: 10.3920/BM2017.0063. [DOI] [PubMed] [Google Scholar]
- 118.Chong H.X., Yusoff N.A.A., Hor Y.Y., Lew L.C., Jaafar M.H., Choi S.B., Yusoff M.S.B., Wahid N., Abdullah M., Zakaria N., et al. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J. Dairy Sci. 2019;102:4783–4797. doi: 10.3168/jds.2018-16103. [DOI] [PubMed] [Google Scholar]
- 119.Lew L.C., Bhat R., Easa A.M., Liong M.T. Development of probiotic carriers using microbial transglutaminase-crosslinked soy protein isolate incorporated with agrowastes. J. Sci. Food Agric. 2011;91:1406–1415. doi: 10.1002/jsfa.4325. [DOI] [PubMed] [Google Scholar]
- 120.Uematsu T., Fujita T., Nakaoka H.J., Hara T., Kobayashi N., Murakami Y., Seiki M., Sakamoto T. Mint3/Apba3 depletion ameliorates severe murine influenza pneumonia and macrophage cytokine production in response to the influenza virus. Sci. Rep. 2016;6:37815. doi: 10.1038/srep37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hor Y.-Y., Lew L.-C., Lau A.S.-Y., Ong J.-S., Chuah L.-O., Lee Y.-Y., Choi S.-B., Rashid F., Wahid N., Sun Z., et al. Probiotic Lactobacillus casei Zhang (LCZ) alleviates respiratory, gastrointestinal & RBC abnormality via immuno-modulatory, anti-inflammatory & anti-oxidative actions. J. Funct. Foods. 2018;44:235–245. doi: 10.1016/j.jff.2018.03.017. [DOI] [Google Scholar]
- 122.Verheijden K.A.T., van Bergenhenegouwen J., Garssen J., Bezemer G.F.G., Kraneveld A.D., Folkerts G. Treatment with specific prebiotics or probiotics prevents the development of lung emphysema in a mouse model of COPD. Eur. J. Pharmacol. 2011;668:e12–e13. doi: 10.1016/j.ejphar.2011.09.220. [DOI] [Google Scholar]
- 123.Mortaz E., Adcock I.M., Ricciardolo F.L., Varahram M., Jamaati H., Velayati A.A., Folkerts G., Garssen J. Anti-Inflammatory Effects of Lactobacillus Rahmnosus and Bifidobacterium Breve on Cigarette Smoke Activated Human Macrophages. PLoS ONE. 2015;10:e0136455. doi: 10.1371/journal.pone.0136455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koning C.J., Jonkers D., Smidt H., Rombouts F., Pennings H.J., Wouters E., Stobberingh E., Stockbrügger R. The effect of a multispecies probiotic on the composition of the faecal microbiota and bowel habits in chronic obstructive pulmonary disease patients treated with antibiotics. Br. J. Nutr. 2010;103:1452–1460. doi: 10.1017/S0007114509993497. [DOI] [PubMed] [Google Scholar]
- 125.Lin A., Shih C.T., Huang C.L., Wu C.C., Lin C.T., Tsai Y.C. Hypnotic Effects of Lactobacillus fermentum PS150(TM) on Pentobarbital-Induced Sleep in Mice. Nutrients. 2019;11:2409. doi: 10.3390/nu11102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin A., Shih C.T., Chu H.F., Chen C.W., Cheng Y.T., Wu C.C., Yang C.C.H., Tsai Y.C. Lactobacillus fermentum PS150 promotes non-rapid eye movement sleep in the first night effect of mice. Sci. Rep. 2021;11:16313. doi: 10.1038/s41598-021-95659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsuda Y., Ozawa N., Shinozaki T., Wakabayashi K.I., Suzuki K., Kawano Y., Ohtsu I., Tatebayashi Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl. Psychiatry. 2020;10:170. doi: 10.1038/s41398-020-0855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bowers S.J., Summa K.C., Thompson R.S., González A., Vargas F., Olker C., Jiang P., Lowry C.A., Dorrestein P.C., Knight R., et al. A Prebiotic Diet Alters the Fecal Microbiome and Improves Sleep in Response to Sleep Disruption in Rats. Front. Neurosci. 2022;16:889211. doi: 10.3389/fnins.2022.889211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z., Wang Z., Lu T., Chen W., Yan W., Yuan K., Shi L., Liu X., Zhou X., Shi J., et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022;65:101691. doi: 10.1016/j.smrv.2022.101691. [DOI] [PubMed] [Google Scholar]
- 130.Badran M., Khalyfa A., Ericsson A., Puech C., McAdams Z., Bender S.B., Gozal D. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnea: Effect of probiotics. Eur. Respir. J. 2022;60:2200002. doi: 10.1183/13993003.00002-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kazemian N., Kao D., Pakpour S. Fecal Microbiota Transplantation during and Post-COVID-19 Pandemic. Int. J. Mol. Sci. 2021;22:3004. doi: 10.3390/ijms22063004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jang Y.O., Lee S.H., Choi J.J., Kim D.H., Choi J.M., Kang M.J., Oh Y.M., Park Y.J., Shin Y., Lee S.W. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp. Mol. Med. 2020;52:1128–1139. doi: 10.1038/s12276-020-0469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lai H.C., Lin T.L., Chen T.W., Kuo Y.L., Chang C.J., Wu T.R., Shu C.C., Tsai Y.H., Swift S., Lu C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71:309–321. doi: 10.1136/gutjnl-2020-322599. [DOI] [PubMed] [Google Scholar]
- 134.Tang J., Xu L., Zeng Y., Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021;91:107272. doi: 10.1016/j.intimp.2020.107272. [DOI] [PubMed] [Google Scholar]
- 135.Schuijt T.J., Lankelma J.M., Scicluna B.P., de Sousa e Melo F., Roelofs J.J., de Boer J.D., Hoogendijk A.J., de Beer R., de Vos A., Belzer C., et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.