Abstract
Chronic lung disease (CLD) of prematurity is an inflammatory disease with a multifactorial etiology. The importance of Ureaplasma urealyticum in the development of CLD is debated, and steroids produce some improvement in neonates with this disease. In the present study, the capability of U. urealyticum to stimulate rat alveolar macrophages to produce nitric oxide (NO), express inducible nitric oxide synthase (iNOS), and activate nuclear factor κB (NF-κB) in vitro was characterized. The effect of NO on the growth of U. urealyticum was also investigated. In addition, the impact of dexamethasone and budesonide on these processes was examined. We found that U. urealyticum antigen (≥4 × 107 color-changing units/ml) stimulated alveolar macrophages to produce NO in a dose- and time-dependent manner (P < 0.05). This effect was further enhanced by gamma interferon (100 IU/ml; P < 0.05) but was attenuated by budesonide and dexamethasone (10−4 to 10−6 M) (P < 0.05). The mRNA and protein levels of iNOS were also induced in response to U. urealyticum and inhibited by steroids. U. urealyticum antigen triggered NF-κB activation, a possible mechanism for the induced iNOS expression, which also was inhibited by steroids. NO induced by U. urealyticum caused a sixfold reduction of its own growth after infection for 10 h. Our findings imply that U. urealyticum may be an important factor in the development of CLD. The host defense response against U. urealyticum infection may also be influenced by NO. The down-regulatory effect of steroids on NF-κB activation, iNOS expression, and NO production might partly explain the beneficial effect of steroids in neonates with CLD.
Chronic lung disease (CLD) is a major problem in the care of very-low-birthweight infants (1), often leading to prolonged ventilator care and sometimes to yearlong oxygen dependency. The development of CLD is characterized by an initial increase of inflammatory cells and mediators (12, 32). Monocytes/macrophages, airway epithelial cells, endothelial cells, T lymphocytes, B lymphocytes, NK cells, leukocytes, and fibroblasts seem to contribute to the inflammatory reaction (19). Extensive release of proinflammatory cytokines (tumor necrosis factor, interleukin-1 [IL-1], IL-6), chemokines (IL-8, macrophage inflammatory protein-2), lipid mediators (leukotriene B4, platelet-activating factor, and prostaglandins), platelet factor 4, and platelet-derived growth factor in the alveolar space of the neonates seem to play an important role in the inflammatory response. Alteration in the balance of the complex network of the inflammatory response normally changes the inflammation process into a healing and reparative process. If CLD develops there is a predominance of lung fibrosis during the later phases.
The etiology of CLD is multifactorial, and infections are thought to be one of the major causes of neonatal lung injury (19). There is evidence supporting the theory that vertically transmitted colonization and infection with Ureaplasma urealyticum is an important risk factor for CLD (21–23, 34, 35). However, the contribution of U. urealyticum to the development of CLD is still controversial (33). U. urealyticum has been isolated from blood, cerebrospinal fluid, tracheobronchial aspirate fluid, and lung tissue (34), and evidence exists that it can cause acute bronchiolitis, pneumonia, and CLD in preterm neonates (1, 23, 35). A recent metaanalysis also supported an independent role for U. urealyticum in the development of CLD (36). These findings suggest that U. urealyticum can elicit an inflammatory response in preterm infants.
Administration of steroids to infants who are oxygen or ventilator dependent produces an improvement in pulmonary mechanics and gas exchange, facilitating the discontinuation of mechanical ventilation and possibly reducing the duration of oxygen therapy and the incidence of severe CLD (4). Steroids are thought to be effective by controlling inflammation (19), and they can be administered either systemically (dexamethasone) or by inhalation (budesonide).
Nitric oxide (NO) is generated from l-arginine by three different NO synthases. Of these, two are constitutive isoforms; the third, inducible and Ca2+-independent NO synthase (iNOS), is expressed only following transcriptional activation of its gene (16, 41), as occurs in acute and chronic inflammation (10). Biosynthesis of NO has been increasingly recognized as an important intra- and intercellular messenger molecule in vascular relaxation, platelet activation, and immune responses (27) in human mononuclear cells. It plays important roles in the pathogenesis of septic shock caused by gram-negative bacteria and of other infectious disease sequelae (37).
NF-κB is a ubiquitous transcription factor that governs the expression of genes coding for cytokines, chemokines, growth factors, cell adhesion molecules, and some acute phase proteins. Presently, five mammalian NF-κB family members have been identified. These include NF-κB1 (p50/p105), NF-κB2 (p52/p100), p65(RelA), RelB, and c-Rel. NF-κB is activated by several agents, including bacterial and viral products (6).
In order to investigate the potential pulmonary pathogenicity of U. urealyticum, we characterized the production of NO, the expression of iNOS, and the activation of NF-κB in direct response to heat-killed U. urealyticum antigen in a rat alveolar macrophage cell line and evaluated the effects of dexamethasone and budesonide. We also examined the influence of the induced NO on the growth of U. urealyticum.
MATERIALS AND METHODS
Cell culture.
A rat alveolar macrophage cell line (ATCC 8383, Rockville, Md.) was maintained in Ham's F-12 medium (Gibco Bethesda Research Laboratories [BRL], Gaithersburg, Md.) and supplemented with 15% heat-inactivated fetal bovine serum (Myoclone; Gibco BRL).
Preparation of U. urealyticum antigen.
U. urealyticum serotype standard strain 8 (T960) (ATCC) was cultured at 37°C in 1.5 liters of ureaplasma broth medium containing 22.5 g of trypticase soy broth/liter, 16.5% horse serum, a 7.5% solution of 25% fresh yeast extract, 0.36% urea, 380,000 U of penicillin G/liter, and phenol red. Cells were harvested in late log phase by centrifugation at 30,000 × g for 90 min at 4°C. The pellet was washed three times by resuspension in phosphate-buffered saline and centrifugation as described above for 30 min. After the final wash, the ureaplasmas were resuspended in a total volume of 2 ml of phosphate-buffered saline. The number of color-changing units (CCUs) of the concentrated suspension was determined in duplicate by a 10-fold titration in ureaplasma broth. The remaining U. urealyticum suspension was heat killed by incubation in a water bath at 56°C for 20 min. Complete killing was assured by incubating 25 μl of the suspension on ureaplasma agar as well as incubation in ureaplasma broth without urea supplement and subsequent subculturing on agar on days 1, 3, and 7. The latter procedure was used since the heat-killed suspension of U. urealyticum produced a prompt color change in ureaplasma broth due to urease activity. The Limulus amebocyte lysate (Charles River Endosafe, Charleston, S.C.) test showed that the endotoxin level was less than 20 pg/ml in 4 × 108 CCU of U. urealyticum antigen per ml. The U. urealyticum antigen was then stored at −80°C in 0.1-ml aliquots until use.
Study protocol.
Rat alveolar macrophages were distributed into 24-microwell plates at a concentration of 106 cells/ml in serum- and phenol-free medium and stimulated with 4 × 106 to 4 × 108 CCU of U. urealyticum antigen/ml or 100 ng of lipopolysaccharide/ml (LPS, O55:B5) (Sigma, St. Louis, Mo.) or in combination with 100 IU of gamma interferon (IFN-γ) (Genzyme, Cambridge, Mass.) per ml for 24 h at 37°C with 5% CO2. To evaluate the role of steroids, rat macrophages were incubated with 4 × 108 CCU of U. urealyticum/ml in the presence of dexamethasone (10−4 to 10−6 M) or budesonide (10−4 to 10−6 M). To examine if U. urealyticum could induce NO production directly, the macrophages were incubated with 4 × 108 CCU of U. urealyticum/ml in combination with the protein synthase inhibitor cycloheximide (CHX; Sigma) at a concentration of 1 μg/ml for 24 h. The experiments were repeated four to eight times.
Effects of NO on U. urealyticum growth.
Rat alveolar macrophages were distributed into 24-microwell plates at a concentration of 106 cells/ml in serum- and phenol-free medium and incubated with 1 × 105 and 2 × 105 CFU of live U. urealyticum (ATCC 1484)/ml alone or in combination with 3 mM concentrations of the NO synthase inhibitor NG-monomethyl-l-arginine (l-NMMA) or its inactive enantiomer, NG-monomethyl-d-arginine (d-NMMA), for 10, 14, and 24 h, respectively. The supernatant was collected and 100 μl was cultured on U. urealyticum culture agar plates (which contained 29.1 g of trypticase soy broth, 242 ml of horse serum, 12 ml of IsoVitaleX enrichment, 12 ml of 25% yeast extract, 1.2 ml of 20% urea, and 12 ml of penicillin [100,000 IU] per ml). After 4 days, the CFU of U. urealyticum growth was determined and cultures were photographed using a light microscope (Nikon, Tykuo, Japan). The experiments were repeated four times.
Nitrite assay.
All the supernatants were collected after stimulation and stored at −70°C for analysis of NO. The accumulation of NO2−, a stable end product of NO formation, in conditioned media was measured as an indicator of NO production. A 100-μl aliquot of cell-free conditioned medium was incubated for 10 min with 100 μL of Griess reagent at room temperature, and the absorbance at 540 nm was measured using a microplate reader. The concentration of NO2− in the samples was calculated from a standard curve of sodium nitrite.
Western blot analysis.
Macrophages were lysed with Laemmli sample buffer and denatured by boiling for 5 min. The protein concentration was determined using a bicinchoninic acid kit (Pierce, Oud Beijerlands, The Netherlands). For Western blot analysis, 10 μg of protein per lane was separated on sodium dodecyl sulfate–7.5% polyacrylamide gels and electroblotted on hydrophobic polyvinylidene difluoride (PVDF) membranes (Amersham, Little Chalfont, Buckshire, United Kingdom). The membrane was blocked in 5% nonfat dry milk dissolved in TTBS (150 mM NaCl, 10 mM Tris-HCl, and 0.1% Tween 20 [pH 7.4]) and subsequently incubated for 1 h at room temperature with a monoclonal antibody against macrophage iNOS (Transduction Lab, Lexington, Ky.) followed by incubation for 1 h with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin (Amersham). Immunoreactive bands were visualized by using an enhanced chemiluminescence kit (Amersham).
Reverse transcription (RT)-PCR.
Total RNA was extracted from cells with RNAzol B (Biotecx Laboratories, Houston, Tex.) after the different treatments, according to the manufacturer's instructions. First-strand cDNA synthesis of total RNA was performed using SuperScript RNase H− reverse transcriptase (Gibco BRL) and random hexamer primers [pd(N)6; Amersham Pharmacia Biotech, Uppsala, Sweden]. Specific oligonucleotide primers were synthesized for rat iNOS (Clontech, Palo Alto, Calif.). The sequences of the 3′ and 5′ primers used are CCCTTCCGAAGTTTCTGGCAGCAG and GGGCTCCTCCAAGGTGTTGCCC (25). The rat G3PDH primers were obtained from Innovagen (Lund, Sweden). The sequences are CTCAAGATTGTCAGCAATGC and CAGGATGCCCTTTAGTGGGC (39). The PCR using Taq polymerase (final concentration, 0.025 U/μl; Gibco BRL) was performed in a final volume of 25 μl containing 2 μl of cDNA for iNOS and G3PDH in a DNA Thermocycler 480 (Perkin-Elmer, Norwalk, Conn.) for 33 cycles for rat iNOS under the following conditions: 1 min denaturation at 94°C, 1 min annealing at 60°C, and 2 min extension at 72°C. PCR was conducted for rat glyceraldehyde-3-phosphate dehydrogenase with 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. The PCR products were separated on a 1.5% agarose gel (Gibco BRL). The ethidium bromide-stained gel was photographed under UV light with a DC120 digital zoom camera (Eastman Kodak Company, Rochester, N.Y.), and the net intensities of the PCR products were analyzed with the Kodak Digital Science Electrophoresis Documentation and Analysis System 120 (Eastman Kodak).
Electrophoretic mobility shift assay (EMSA).
Cells grown in serum-free medium were stimulated with 4 × 108 CCU of U. urealyticum antigen/ml for 30 min or pretreated with dexamethasone (10−4 M) or budesonide (10−4 M) for 1 h. Nuclear extracts were prepared as described previously (43), and nuclear protein concentrations were determined using the bicinchoninic acid method (Pierce, Rockford, Ill.). The nuclear extract (3 μg of protein) was preincubated for 10 min in the reaction buffer [10 mM HEPES (pH 7.9), 10% glycerol, 60 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 2 mg of poly(dI-dC)], followed by incubation for 30 min at room temperature with 50,000 cpm of 32P-labeled NF-κB probe (double-stranded oligonucleotides containing an NF-κB consensus binding site, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; Promega, Madison, Wis.). After 30 min at room temperature, samples were separated on 4% native polyacrylamide gels in a low-ionic-strength buffer (22.3 mM Tris borate, 0.5 mM EDTA; pH 8). Dried gels were autoradiographed with intensive screens at −80°C. In some cases, the incubation of nuclear extracts with 32P-labeled NF-κB probe was performed in the presence of a 25- or 50-fold excess of unlabeled NF-κB probe or unlabeled irrelevant oligonucleotide probe for AP-1 (Promega). For the supershift analysis, rabbit anti-p50 and anti-p65 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) were incubated with the nuclear extracts for 15 min prior to the addition of radiolabeled probe.
Immunolocalization of NF-κB and iNOS.
Cells (3,000/well) were plated on glass coverslips and incubated with 4 × 108 CCU of U. urealyticum/ml alone or in combination with dexamethasone (10−4 M) or budesonide (10−4 M) for 24 h for evaluating iNOS and for 30 min for evaluating NF-κB (additional cells were also pretreated with steroids for 1 h). After treatment, the cells were fixed with cold methanol and acetone. Intracellular p65 and iNOS were visualized by indirect immunofluorescence using polyclonal rabbit-anti-p65 antibody (Santa Cruz Biotechnology) and polyclonal rabbit-antimacrophage iNOS antibody (Affiniti BioReagents, Golden Colo.) followed by fluorescein isothiocyanate-labeled goat-anti-rabbit immunoglobulin G (Daco, Copenhagen, Denmark).
Statistical analysis.
Data from pooled experiments were reported as the mean nitrite concentrations ± the standard errors of the means. Data were analyzed by a one-way analysis-of-variance test followed by the Newman-Keuls test for two-mean comparison. A P value of less than 0.05 was considered to be significant.
RESULTS
NO production and iNOS expression after treatment with U. urealyticum antigen.
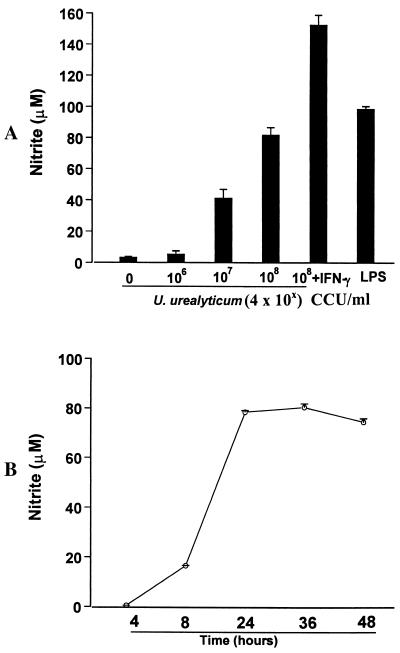
U. urealyticum stimulated alveolar macrophage production of NO in a dose- and time-dependent manner (Fig. 1). U. urealyticum at concentrations of ≥4 × 107 CCU/ml induced the production of NO (P < 0.05) at 24 h. A concentration of 4 × 108 CCU of U. urealyticum/ml induced the production of NO to levels (81.9 ± 4.8 μM) similar to those in cultures stimulated by LPS (100 ng/ml) (98.6 ± 1.8 μM). This effect could be further enhanced in the presence of IFN-γ (100 IU/ml; P < 0.05). The induced NO production could be detected in the conditioned media after 4 h of stimulation with U. urealyticum and reached peak levels at 36 h.
FIG. 1.
U. urealyticum antigen-induced NO production by alveolar macrophages. Macrophages were stimulated with U. urealyticum (4 × 106 to 4 × 108 CCU/ml) or LPS (100 ng/ml), or in combination with IFN-γ (100 IU/ml). NO production was assessed by determining the NO2− concentration in conditioned medium. (A) A dose-related induction of NO was seen after 24 h of stimulation with U. urealyticum. (B) Kinetics of NO production by macrophages in response to 4 × 108 CCU of U. urealyticum/ml.
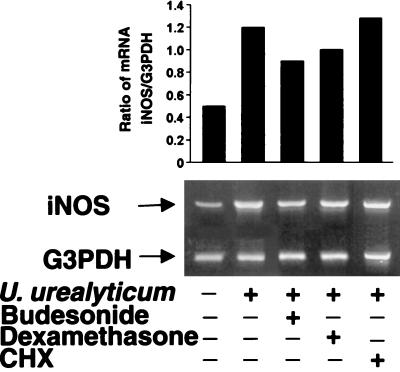
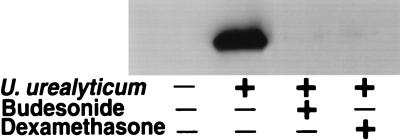
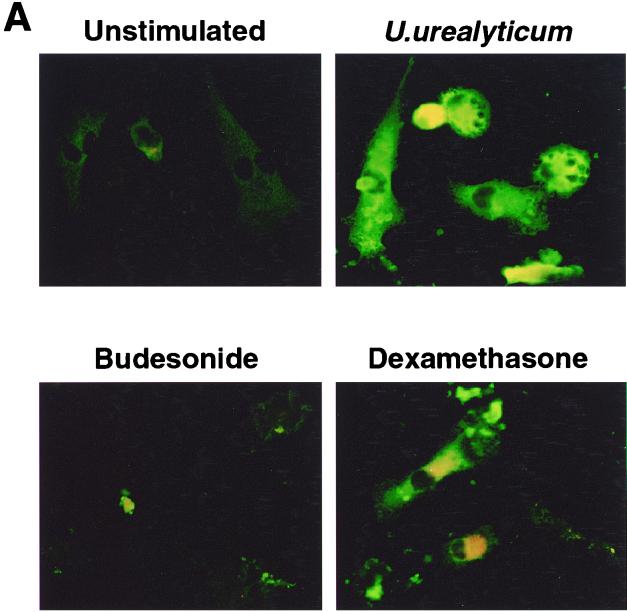
Because iNOS is regulated mainly at the transcriptional level, we next examined iNOS transcripts by using RT-PCR. Abundant iNOS mRNA was found in the U. urealyticum-stimulated cells at 24 h compared to untreated cells (Fig. 2). The level of iNOS protein showed the same pattern in the U. urealyticum-stimulated cells, as determined by Western blotting (Fig. 3) and immunostaining (Fig. 4A).
FIG. 2.
U. urealyticum antigen-induced expression of iNOS mRNA in alveolar macrophages. Macrophages were stimulated with U. urealyticum at a concentration of 4 × 108 CCU/ml for 24 h and mRNA was determined by RT-PCR. iNOS mRNA was inhibited in the presence of budesonide (10−4 M) and dexamethasone (10−4 M). U. urealyticum (4 × 108 CCU/ml) antigen-induced iNOS mRNA expression was not affected in the presence of CHX (1 μg/ml).
FIG. 3.
Expression of iNOS protein in alveolar macrophages was assessed by Western blot analysis with a monoclonal anti-iNOS antibody. U. urealyticum at a concentration of 4 × 108 CCU/ml stimulated iNOS protein expression, compared to unstimulated alveolar macrophages. iNOS protein expression was inhibited by budesonide (10−4 M) and dexamethasone (10−4 M).
FIG. 4.
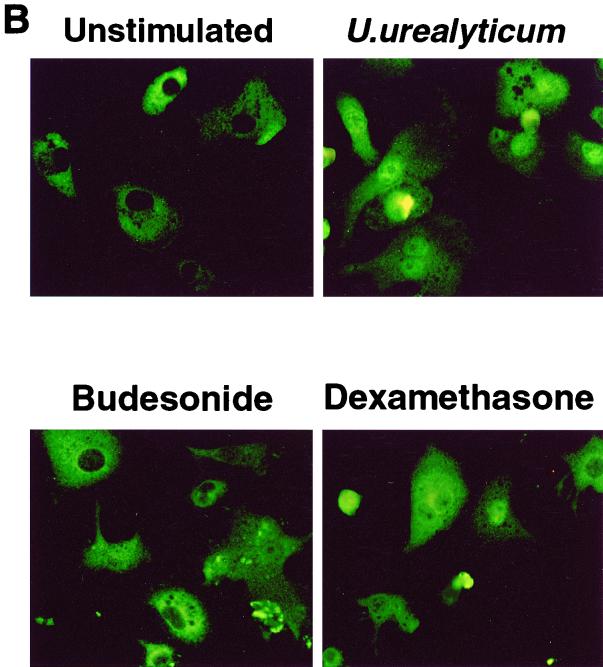
(A) Expression of iNOS protein in alveolar macrophages was assessed by immunostaining. U. urealyticum at a concentration of 4 × 108 CCU/ml stimulated iNOS protein expression, compared to unstimulated alveolar macrophages. iNOS protein expression was inhibited by budesonide (10−4 M) and dexamethasone (10−4 M). Magnification, ×500. (B) U. urealyticum antigen (4 × 108 CCU/ml) activated NF-κB expression after 30 min of incubation, compared to unstimulated alveolar macrophages. NF-κB expression was inhibited by budesonide (10−4 M) and dexamethasone (10−4 M). The intracellular location of p65 was detected by indirect immunofluorescence with an anti-p65 antibody. Magnification, ×500.
To determine if U. urealyticum has a direct effect on iNOS expression, we used the protein synthase inhibitor CHX (26) to block the de novo synthesis of cytokines. As shown in Fig. 2, CHX at 1 μg/ml did not affect U. urealyticum-induced iNOS expression, indicating that induction of macrophage iNOS expression by U. urealyticum is not dependent on cytokine production.
Down-regulation of NO production and iNOS expression by steroids.
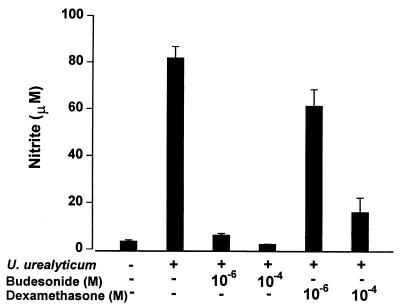
Budesonide (10−4 to 10−6 M) and dexamethasone (10−4 to 10−6 M) significantly inhibited the NO production stimulated by U. urealyticum in rat alveolar macrophages (P < 0.05) (Fig. 5), and the effect was regulated at the transcript level, as determined by RT-PCR (Fig. 2), Western blotting (Fig. 3), and immunostaining (Fig. 4A).
FIG. 5.
Down-regulation of U. urealyticum (4 × 108 CCU/ml) antigen-stimulated NO production in the rat alveolar macrophage cell line by different doses of budesonide and dexamethasone.
NF-κB activation.
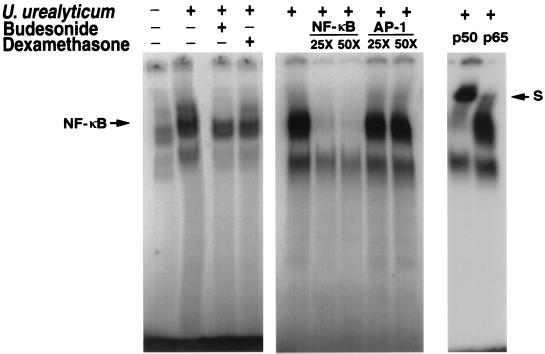
The effect of U. urealyticum on the NF-κB signal transduction pathway in alveolar macrophages was determined by using EMSA. After treatment of macrophages with U. urealyticum for 30 min, a substantially enhanced NF-κB binding complex was observed in the nuclear extract of macrophages (Fig. 6). The specificity of the NF-κB-DNA complex was ascertained by a competition study. As shown in Fig. 6, the indicated NF-κB-DNA complexes were substantially removed by excessive cold NF-κB probe but were not affected by excessive unlabeled AP-1 probe. To identify components in the NF-κB-DNA complex, nuclear extracts from U. urealyticum-stimulated cells were incubated with antibodies against two NF-κB members, p50 and p65. This resulted in a shift of bands, as indicated in Fig. 6. Our results suggest that NF-κB-DNA complexes are at least composed of p50 and p65 in nuclear extracts derived from U. urealyticum-stimulated cells.
FIG. 6.
EMSA of NF-κB-DNA complexes. U. urealyticum antigen (4 × 108 CCU/ml) activated NF-κB expression after 30 min of incubation, compared to unstimulated alveolar macrophages. NF-κB expression was inhibited by budesonide (10−4 M) and dexamethasone (10−4 M). The 32P-labeled oligonucleotide corresponding to a consensus κB site, the excessive cold NF-κB probe, and the AP-1 probe were incubated with 3μg of nuclear protein and antibodies to the p50 and p65 subunits of NF-κB. S, supershift bands.
Activation of NF-κB was also evaluated in terms of the intracellular translocation of p65 after 30 min of treatment with U. urealyticum. In untreated macrophages, p65 was sequestered in the cytoplasm, while treatment with U. urealyticum resulted in the translocation of p65 into the nuclei of the macrophages (Fig. 4B).
The down-regulatory effects on the expression of NF-κB by budesonide and dexamethasone were observed by EMSA (Fig. 6) and immunostaining (Fig. 4B).
Effects of NO on growth of U. urealyticum.
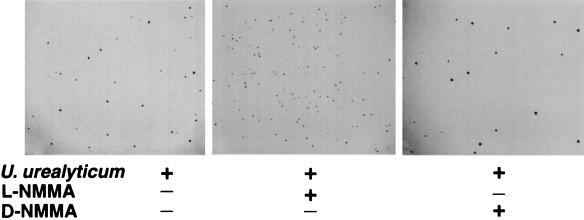
After addition of the NOS inhibitor l-NMMA, the fold increase in CFU of live U. urealyticum were 6.0 ± 0.4 (at 10 h) and 4.6 ± 0.9 (at 14 h), compared to untreated cultures (P < 0.05) (Fig. 7). Unlike l-NMMA, the inactive enantiomer d-NMMA had no significant effect. After a 24-h incubation of live U. urealyticum with macrophages, U. urealyticum grew only on the plates from the samples to which l-NMMA had been added.
FIG. 7.
Inhibition of U. urealyticum growth by macrophages producing NO. U. urealyticum was cultured for 4 days from the supernatant of a 10-h incubation with macrophages alone or in combination with 3 mM l-NMMA or d-NMMA. Magnification, ×45.
DISCUSSION
We demonstrated that heat-killed U. urealyticum antigen could trigger rat alveolar macrophages to express iNOS and thus produce NO in a dose- and time-dependent fashion. This induction was significantly enhanced in the presence of IFN-γ. Moreover, U. urealyticum was capable of activating NF-κB. All these responses were inhibited by steroids. We also found that iNOS induced by live U. urealyticum caused a significant inhibition on the growth of U. urealyticum itself. The protein synthase inhibitor CHX did not influence the iNOS expression stimulated by U. urealyticum, indicating that U. urealyticum directly induced the high-output NO generation when other iNOS gene activation pathways, such as cytokine synthase, were blocked.
Clinical studies have suggested that NO is an important inflammatory mediator in several human infections, especially in fulminant early-onset neonatal pneumonia (2), sepsis, and Leishmania infantum infection (20, 31, 40). Likewise, monocytes and tissue macrophages isolated from patients with rheumatoid arthritis, tuberculosis, and malaria display higher levels of iNOS and generate increased levels of NO in vitro (37). It is known that there are species differences in NO production. Weinberg (37) reviewed reports from 1989 to 1998 regarding NO production and iNOS expression in human mononuclear phagocytes in which some difficulties were reported in detecting NO production, partly depending on the method used. Rodent mononuclear phagocytes have been used for many in vitro studies (18, 30), mainly because these cells seem to be more sensitive.
NF-κB is known as a widespread rapid-response transcription factor that is expressed in a variety of cells (14). Both endogenous and exogenous stimuli induce NF-κB activation. The role of NF-κB in iNOS gene expression has been well elucidated (7). Stimulation of macrophages with LPS or cytokines such as IL-1β leads to activation of NF-κB and subsequent binding to the κB response element of the iNOS promoter (24). Activation of NF-κB is an essential mechanism responsible for LPS- or oxidative stress-induced NO production (42). Our data demonstrate that U. urealyticum is a potent activator of NF-κB, as evidenced by the rapid and intense NF-κB activation in macrophages. This suggests that NF-κB activation may be of great importance for U. urealyticum-induced iNOS expression. In addition, the potential role of NF-κB in inflammation and immune modulation in U. urealyticum infection is not limited to transcriptional activation of iNOS. In fact, NF-κB has been shown to have a crucial role in the inducible expression of many inflammatory genes encoding transcriptional factors, adhesion molecules, cytokines, and growth factors (6). Therefore, U. urealyticum-induced NF-κB activation in macrophages may represent a key mechanism responsible for the inflammatory reaction associated with CLD.
Although the physiologic production of NO plays a key role in the host defense against various intracellular pathogens, its overproduction may be responsible in part for the pathophysiology of infection (3). Overproduction of NO is likely to contribute to the hemodynamic instability of overwhelming sepsis in humans (8). Animal studies suggest that the high-output NO pathway is responsible for escalating the inflammatory response (11). When the production of NO is left unattenuated, especially under oxidative stress, direct cytotoxic effects of NO can emerge through the formation of peroxynitrite as the result of a reaction between NO and superoxide (5), which are important mediators of tissue injury and organ dysfunction (10). The oxidative stress, DNA damage, and disruption caused by excess NO can lead to cell death by apoptosis or necrosis (17). It is thus likely that when the preterm infant is infected with U. urealyticum in the lungs, alveolar macrophages and lymphocytes will infiltrate and result in elevated levels of NO, cytokines such as tumor necrosis factor alpha, IL-6, and IL-8 (13, 15, 28, 29), and other inflammatory mediators, which may lead to lung tissue injury and fibrosis.
There was a dose-response relationship between the concentrations of U. urealyticum and nitric oxide production. It is difficult to compare in vitro bacterial concentrations with those present in vivo in neonates. Very marked differences in bacterial numbers have been found in vivo. The numbers of bacteria adhering to the airway surface might also be very different to those found in the bronchial fluid.
Another very interesting result in the present study was that the high output of NO induced by U. urealyticum caused a reduction in growth of U. urealyticum, compared to controls. When the iNOS inhibitor l-NMMA was added, U. urealyticum grew significantly more. Thus, the induced NO seems to have dual effects: inhibiting growth of U. urealyticum and causing injury to the airways of the host. Previously, NO was shown to contribute to the host defense response against various intracellular pathogens. It is likely that NO acts in concert with reactive oxygen species to damage microbial DNA, proteins, and lipids (9, 38).
Inhibiting high-output NO production by blocking iNOS expression or its activity may be a useful strategy for treatment of inflammatory disorders, e.g., CLD. We showed that both budesonide and dexamethasone could down-regulate iNOS expression, probably by decreasing NO production at the inflammatory site. This can perhaps partly explain why steroids have a beneficial effect in the treatment of CLD in neonates.
It seems that steroids had a stronger effect on the iNOS protein than on iNOS mRNA expression. The observed differences could be caused by an influence of steroids on different steps. One influence is to inhibit iNOS at the transcription level through inactivation of the NF-κB pathway. Another influence may be a direct effect on the iNOS protein expression.
We also showed that budesonide and dexamethasone inhibited the activation of NF-κB induced by U. urealyticum. Several mechanisms have been proposed for the inhibition of NF-κB by glucocorticoids (14). One is that glucocorticoids inhibit NF-κB by enhancing the production of IκB-α; the other mechanism is by a direct protein-protein interaction in which glucocorticoids and glucocorticoid receptor complexes directly associate with NF-κB in the cytoplasm and nucleus.
In the clinical situation, inhibition of the iNOS system by steroid treatment might also cause persistence of U. urealyticum in patients. This might help to explain why steroid administration only has a moderate benefit to patients at high risk of developing CLD (4). The consequences and interactions of U. urealyticum infection, iNOS production, and administration of steroids need to be further studied.
In conclusion, our present study implies that U. urealyticum may be an important etiological factor in the development of CLD due to its ability to stimulate the expression of iNOS, which is probably mediated through the activation of NF-κB; also, NO could be involved in the host's defensive response.
ACKNOWLEDGMENTS
This study was supported by Stiftelsen Frimurare Barnhuset, the Founds of Karolinska Institute, Magn. Bergvalls Foundation, the Swedish Medical Research Council (project 6816), the Swedish Heart-Lung Foundation (grant no. 199941318), and Tore Nilsson Foundation.
REFERENCES
- 1.Abele-Horn M, Peters J, Genzel-Boroviczeny O, Wolff C, Zimmermann A, Gottschling W. Vaginal Ureaplasma urealyticum colonization: influence on pregnancy outcome and neonatal morbidity. Infection. 1997;25:286–291. doi: 10.1007/BF01720398. [DOI] [PubMed] [Google Scholar]
- 2.Aikio O, Vuopala K, Pokela M L, Hallman M. Diminished inducible nitric oxide synthase expression in fulminant early-onset neonatal pneumonia. Pediatrics. 2000;105:1013–1019. doi: 10.1542/peds.105.5.1013. [DOI] [PubMed] [Google Scholar]
- 3.Ajizian S J, English B K, Meals E A. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- 4.Bancalari E. Corticosteroids and neonatal chronic lung disease. Eur J Pediatr. 1998;157:S31–S37. doi: 10.1007/pl00014290. [DOI] [PubMed] [Google Scholar]
- 5.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Castranova V, Shi X, Demers L M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 7.Christman J W, Lancaster L H, Blackwell T S. Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24:1131–1138. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans T, Carpenter A, Kinderman H, Cohen J. Evidence of increased nitric oxide production in patients with the sepsis syndrome. Circ Shock. 1993;41:77–81. [PubMed] [Google Scholar]
- 9.Fang F C. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grisham M B, Jourd'Heuil D, Wink D A. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 11.Hierholzer C, Harbrecht B, Menezes J M, Kane J, MacMicking J, Nathan C F, Peitzman A B, Billiar T R, Tweardy D J. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotecha S. Cytokines in chronic lung disease of prematurity. Eur J Pediatr. 1996;155:S14–S17. doi: 10.1007/BF01958074. [DOI] [PubMed] [Google Scholar]
- 13.Kruger T, Baier J. Induction of neutrophil chemoattractant cytokines by Mycoplasma hominis in alveolar type II cells. Infect Immun. 1997;65:5131–5136. doi: 10.1128/iai.65.12.5131-5136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J I, Burckart G J. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981–993. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 15.Li Y-H, Brauner A, Jonsson B, Van der Ploeg I, Söder O, Holst M, Skov Jensen J, Lagercrantz H, Tullus K. Ureaplasma urealyticum induced production of proinflammatory cytokines by macrophages. Pediatr Res. 2000;48:114–119. doi: 10.1203/00006450-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Lorsbach R B, Murphy W J, Lowenstein C J, Snyder S H, Russell S W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 17.Murphy M P. Nitric oxide and cell death. Biochim Biophys Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 18.Orman K L, Shenep J L, English B K. Pneumococci stimulate the production of the inducible nitric oxide synthase and nitric oxide by murine macrophages. J Infect Dis. 1998;178:1649–1657. doi: 10.1086/314526. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir A, Brown M A, Morgan W J. Markers and mediators of inflammation in neonatal lung disease. Pediatr Pulmonol. 1997;23:292–306. doi: 10.1002/(sici)1099-0496(199704)23:4<292::aid-ppul7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Panaro M A, Acquafredda A, Lisi S, Lofrumento D D, Trotta T, Satalino R, Saccia M, Mitolo V, Brandonisio O. Inducible nitric oxide synthase and nitric oxide production in Leishmania infantum-infected human macrophages stimulated with interferon-gamma and bacterial lipopolysaccharide. Int J Clin Lab Res. 1999;29:122–127. doi: 10.1007/s005990050076. [DOI] [PubMed] [Google Scholar]
- 21.Perzigian R W, Adams J T, Weiner G M, Dipietro M A, Blythe L K, Pierson C L, Faix R G. Ureaplasma urealyticum and chronic lung disease in very low birth weight infants during the exogenous surfactant era. Pediatr Infect Dis J. 1998;17:620–625. doi: 10.1097/00006454-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Pierce M R, Bancalari E. The role of inflammation in the pathogenesis of bronchopulmonary dysplasia. Pediatr Pulmonol. 1995;19:371–378. doi: 10.1002/ppul.1950190611. [DOI] [PubMed] [Google Scholar]
- 23.Rudd P T, Cassell G H, Waites K B, Davis J K, Duffy L B. Ureaplasma urealyticum pneumonia: experimental production and demonstration of age-related susceptibility. Infect Immun. 1989;57:918–925. doi: 10.1128/iai.57.3.918-925.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder R A, Cai C, Kuo P C. Endotoxin-mediated nitric oxide synthesis inhibits IL-1β gene transcription in ANA-1 murine macrophages. Am J Physiol. 1999;277:C523–C530. doi: 10.1152/ajpcell.1999.277.3.C523. [DOI] [PubMed] [Google Scholar]
- 25.Sirsjo A, Soderkvist P, Sundqvist T, Carlsson M, Ost M, Gidlof A. Different induction mechanisms of mRNA for inducible nitric oxide synthase in rat smooth muscle cells in culture and in aortic strips. FEBS Lett. 1994;338:191–196. doi: 10.1016/0014-5793(94)80363-3. [DOI] [PubMed] [Google Scholar]
- 26.Sketelj J, Crne-Finderle N, Strukelj B, Trontelj J V, Pette D. Acetylcholinesterase mRNA level and synaptic activity in rat muscles depend on nerve-induced pattern of muscle activation. J Neurosci. 1998;18:1944–1952. doi: 10.1523/JNEUROSCI.18-06-01944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda M, Kobayashi J, Takezawa M, Miyazaki T, Nakajima T, Shimomura H, Koike K, Satomi A, Ogino H, Omoto R, Komoda T. An assay method for nitric oxide-related compounds in whole blood. Anal Biochem. 1997;247:417–427. doi: 10.1006/abio.1997.2056. [DOI] [PubMed] [Google Scholar]
- 28.Stancombe B B, Walsh W F, Derdak S, Dixon P, Hensley D. Induction of human neonatal pulmonary fibroblast cytokines by hyperoxia and Ureaplasma urealyticum. Clin Infect Dis. 1993;17:S154–S157. doi: 10.1093/clinids/17.supplement_1.s154. [DOI] [PubMed] [Google Scholar]
- 29.Takasaki J, Ogawa Y. Interleukin 8 in the tracheobronchial aspirate of infants acts as a neutrophil chemotactic factor in the development of chronic lung disease. Pediatr Int. 1999;41:78–81. doi: 10.1046/j.1442-200x.1999.01021.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor M J, Cross H F, Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000;191:1429–1435. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiemermann C. Nitric oxide and septic shock. Gen Pharmacol. 1997;29:159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- 32.Tullus K, Noack G W, Burman L G, Nilsson R, Wretlind B, Brauner A. Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. Eur J Pediatr. 1996;155:112–116. doi: 10.1007/BF02075762. [DOI] [PubMed] [Google Scholar]
- 33.van Waarde W M, Brus F, Okken A, Kimpen J L. Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur Respir J. 1997;10:886–890. [PubMed] [Google Scholar]
- 34.Waites K B, Crouse D T, Cassell G H. Systemic neonatal infection due to Ureaplasma urealyticum. Clin Infect Dis. 1993;17:S131–S135. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 35.Walsh W F, Butler J, Coalson J, Hensley D, Cassell G H, deLemos R A. A primate model of Ureaplasma urealyticum infection in the premature infant with hyaline membrane disease. Clin Infect Dis. 1993;17:S158–S162. doi: 10.1093/clinids/17.supplement_1.s158. [DOI] [PubMed] [Google Scholar]
- 36.Wang E E, Ohlsson A, Kellner J D. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg J B. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheeler M A, Smith S D, Garcia-Cardena G, Nathan C F, Weiss R M, Sessa W C. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Invest. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams C M, Coleman J W. Induced expression of mRNA for IL-5, IL-6, TNF-alpha, MIP-2 and IFN-gamma in immunologically activated rat peritoneal mast cells: inhibition by dexamethasone and cyclosporin A. Immunology. 1995;86:244–249. [PMC free article] [PubMed] [Google Scholar]
- 40.Wong H R, Carcillo J A, Burckart G, Kaplan S S. Nitric oxide production in critically ill patients. Arch Dis Child. 1996;74:482–489. doi: 10.1136/adc.74.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Q W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 42.Xie Q W, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 43.Yan Z, Sirsjo A, Bochaton-Piallat M L, Gabbiani G, Hansson G K. Augmented expression of inducible NO synthase in vascular smooth muscle cells during aging is associated with enhanced NF-kappaB activation. Arterioscler Thromb Vasc Biol. 1999;19:2854–2862. doi: 10.1161/01.atv.19.12.2854. [DOI] [PubMed] [Google Scholar]