Abstract
Tuberculosis remains one of the most significant diseases of humans and animals. The only currently available vaccine against this disease is a live, attenuated vaccine, bacillus Calmette-Guérin (BCG), which was originally derived from Mycobacterium bovis and despite its variable efficacy is the most widely administered vaccine in the world. With the advent of the human immunodeficiency virus-AIDS pandemic concern has been raised over the safety of BCG. Moreover, since BCG sensitizes vaccinated individuals to the tuberculin test, vaccination with BCG prevents diagnosis of infection in vaccinated individuals. Recently, auxotrophic strains of BCG have been generated by insertional mutagenesis which have been shown to be safer than the parent BCG strain following administration to mice with severe combined immunodeficiency disease. These strains have also been shown to give comparable protection against intravenous and intratracheal challenge of BALB/c mice with M. tuberculosis relative to conventional BCG. Here we report that one of these mutants, a leucine auxotroph of BCG, conferred significant protection of the lungs and spleens of guinea pigs infected with M. bovis and protection of the spleens of guinea pigs infected with M. tuberculosis in the absence of a cutaneous hypersensitivity reaction to tuberculin. Therefore, protective immunity to tuberculosis may, at least in part, be achieved without sensitization to the tuberculin skin test. These results indicate that it may be possible to develop a new generation of vaccines based on BCG that are protective, are safe for use in the immunocompromised, and do not preclude the use of the tuberculin skin test in both humans and animals.
Tuberculosis (TB) caused by infection with Mycobacterium tuberculosis or Mycobacterium bovis has been the scourge of humans and animals for centuries (4, 28) and continues to inflict a huge cost, in terms of both human and animal health and financially (5, 22, 26, 29). This is particularly disappointing since for over 70 years a vaccine has been available for TB: bacillus Calmette-Guérin (BCG). BCG possesses many of the qualities of an ideal vaccine: it is cheap to produce and administer, it is safe and has been shown to be efficacious in many circumstances, especially against severe and fatal tuberculosis in children (reviewed in reference 2). However, BCG has been found to give variable efficacy in a number of clinical trials. In the Medical Research Council trial in the United Kingdom, BCG imparted 77% protection (12), while, at the other end of the spectrum, in the largest clinical trial in India it exhibited zero protective efficacy (30). This lack of protection has resulted in increased efforts to develop a new generation of TB vaccines. Nevertheless, although BCG generally gives poor protection against pulmonary TB in adults, it remains the “gold-standard” against which candidate TB vaccines with improved efficacy are measured and is currently the only available vaccine for the prevention of TB.
With the advent of the human immunodeficiency virus-AIDS pandemic, concern has been raised over the safety of BCG. Since BCG can be pathogenic in situations of compromised or deficient immunity (reviewed in reference 35), vaccination with BCG can be contraindicated for those very individuals most at risk of contracting TB. This problem has been addressed recently using molecular genetic tools to generate mutants of BCG that are further attenuated (10, 17, 24). Using this approach, deletion of genes involved in amino acid and purine biosynthesis resulted in auxotrophic mutants of BCG that were unable to persist in both immunocompetent (17, 24) and severely immunocompromised mice (10). However, the mutants were able to persist long enough to engender a degree of protective immunity, and it has been suggested that such mutants of BCG could be used to vaccinate individuals at risk of developing compromised or deficient immunity (10).
A further limitation to the widespread use of BCG is its tendency to sensitize for the tuberculin skin test. BCG is not administered routinely to individuals in the United States, so that tuberculin may be used to test for exposure to M. tuberculosis, e.g. (23). Similarly, BCG cannot be used as a vaccine for bovine TB in countries that have a tuberculin test-and-slaughter policy for the control of TB in cattle (36). Unless diagnostic reagents are developed that can differentiate between vaccination with BCG and exposure to M. tuberculosis or M. bovis, the use of BCG in its present form will remain restricted (32).
There is, therefore, a need for the development of novel TB vaccines that do not compromise use of the tuberculin test in humans and animals. However, it will not be possible to develop such vaccines if the cutaneous delayed-type hypersensitivity (DTH) reaction to tuberculin is a conditional manifestation of a protective immune response to TB. As early as 1967, the protection conferred by BCG vaccination was found to be unrelated to the degree of tuberculin sensitivity (11). Further studies of animals and humans have established that tuberculin hypersensitivity is not a prerequisite for protective immunity to TB (8, 20, 27, 37).
The BCG auxotrophs mc2798 and mc2789 have an absolute requirement for leucine and methionine, respectively (24). These auxotrophic strains differ from one another, and from BCG, in the length of time they persist in mice (24). We have tested these mutants for their ability to sensitize for tuberculin hypersensitivity and confer protective immunity in a guinea pig low-dose aerosol challenge model (34). We report that the leucine auxotroph of BCG conferred significant protection from infection with M. bovis and protection against hematogenous spread of M. tuberculosis in the absence of a cutaneous DTH reaction to tuberculin. This result indicates that a new generation of vaccines based on BCG may be developed that are protective, are safe for use in the immunocompromised, and do not preclude the use of the tuberculin skin test in both humans and animals.
MATERIALS AND METHODS
Bacterial strains and media.
Lyophilized M. bovis BCG Pasteur strain (obtained from the Statens Serum Institut, Copenhagen, Denmark) was cultured in 10 ml of M-ADC-TW broth (18) for 7 days and stored at −80°C in seed lots. Methionine and leucine auxotrophic mutants of BCG Pasteur (vaccines BCG Met− and BCG Leu−, respectively) have been described previously (24). Seed lots of M. tuberculosis H37Rv (NCTC 7416) were grown on Middlebrook 7H10 agar (Difco Laboratories, Detroit, Mich.) containing 0.2% (vol/vol) glycerol and 10% (vol/vol) Middlebrook OADC enrichment, harvested and stored at −70°C as a dense suspension in deionized water. The strain of M. bovis used in this study (1692/96) was isolated from a tuberculin test reactor cow in 1996 and cultured at VLA Weybridge. For enumeration, M. tuberculosis H37Rv and BCG Pasteur were plated on Middlebrook 7H10 agar containing 0.2% (vol/vol) glycerol and 10% (vol/vol) Middlebrook OADC enrichment; the auxotrophic BCG mutants were plated on the same medium supplemented with 0.5% Casamino Acids (Difco) and kanamycin (20 μg/ml); and M. bovis strain 1692/96 was plated on Middlebrook 7H10 agar containing sodium pyruvate (4.16 mg/ml) and 10% (vol/vol) Middlebrook OADC enrichment. Where necessary, serial dilution of bacterial suspensions was made in water containing 0.05% (vol/vol) Tween 80 to maintain dispersion.
Preparation of bacteria for infection of guinea pigs.
Cultures of BCG Pasteur and the BCG auxotrophs were grown in 50 ml of M-ADC-TW broth supplemented with 0.5% Casamino Acids (Difco) in 490-cm2 roller bottles at 37°C with the addition of kanamycin (20 μg/ml) for the auxotrophs. M. bovis strain 1692/96 was grown without agitation in 100 ml of M-ADC-TW broth supplemented with sodium pyruvate (4.16 mg/ml) in place of glycerol. When the cells reached densities of >5 × 107 CFU/ml, the cells were resuspended in phosphate-buffered saline (PBS) and frozen in 1-ml aliquots. A seed stock of M. tuberculosis H37Rv was plated and grown on Middlebrook 7H10 agar containing 0.2% (vol/vol) glycerol and 10% (vol/vol) Middlebrook OADC enrichment for 3 weeks at 37°C and then harvested into sterile deionized water. The suspension was left for 30 min to allow clumps to fall out of solution. The cleared suspension was then frozen as 1-ml aliquots. Titers of all frozen cells were determined from a thawed sample. Immediately prior to injection, the concentration of the BCG vaccines was adjusted to 2 × 105 CFU/ml and the cells were dispersed by brief sonication using a CV18 converter fitted with a 3-mm-diameter tip attached to a Vibracell control unit set at 20% power (Sonics & Materials Inc., Danbury, Conn.). Female Dunkin-Hartley guinea pigs weighing between 350 and 450 g and free of intercurrent infection were obtained from Charles River UK Ltd., Margate, United Kingdom. For protection studies, guinea pigs were injected subcutaneously in the nape with 250 μl of each vaccine preparation (representing an inoculum of approximately 5 × 104 CFU) in groups of six animals. Twelve control animals received 250 μl of PBS each.
Persistence of vaccines in guinea pigs.
Additional guinea pigs (housed separately from those challenged) were used to determine the persistence of BCG Pasteur and the leucine auxotroph in the draining lymph node and spleen. Two groups of 15 guinea pigs were injected intramuscularly in the biceps femoris with 5 × 103 CFU of BCG Pasteur or 104 CFU of a leucine auxotroph. Three animals per group were sacrificed by peritoneal overdose of sodium pentobarbitone (Euthatal; Rhone Merieux) at days 1, 13, 21, 28, and 37 after inoculation. The spleen and lymph node(s) draining the site of bacterial inoculation (deep inguinal lymph node) were removed for bacterial enumeration.
Bovine standard PPD.
The bovine standard purified protein derivative (PPD) used in this study was the Biological 1st International Standard antigen—tuberculin, PPD, bovine—currently held and distributed by the National Institute for Biological Standards and Control, Potters Bar, United Kingdom. Each ampoule contains 58,500 IU (approximately 1.8 mg) of PPD derived from cultures of M. bovis strain AN5.
Cutaneous DTH assay.
Three additional groups of five guinea pigs (housed separate from those challenged) were assayed for cutaneous DTH five weeks after subcutaneous injection with approximately 5 × 104 CFU of the BCG vaccines. A further seven PBS control animals were also tested for cutaneous DTH. An area of approximately 10 to 13 cm by 5 cm on the flank of each animal was shaved to remove the fur. The bovine standard PPD was diluted in isotonic PBS plus Tween to give a working concentration of 250 IU/ml. Of this PPD solution, 0.2 ml (50 IU) was injected into the shaved dermis of each animal using a 1-ml syringe fitted with a 25-gauge needle. The extent of the reaction was measured 24 and 48 h later using digital calipers. Two measurements were taken at right angles to one another in order to calculate the induration size in square millimeters.
Aerosol infection of guinea pigs.
Separate groups of BCG-vaccinated guinea pigs were challenged with M. tuberculosis H37Rv or M. bovis strain 1692/96 via the aerosol route 5 weeks after vaccination. Batches of eight animals (two from each treatment group) were exposed for 5 min to bacterial aerosols containing particles mostly less than 5 μm in diameter (diameter range, 0.5 to 7 μm; mean, 2 μm). The aerosol was generated from the suspensions of mycobacteria with a three-jet Collison nebulizer in conjunction with a modified version of the mobile Henderson apparatus as described previously (6, 34). The apparatus allows controlled delivery of aerosols directly to the snouts of the animals without contamination of fur or eyes. A suspension containing approximately 106 CFU/ml was used in order to obtain an inhaled retained dose in the lungs of approximately 10 organisms. A control group of eight animals vaccinated with BCG Pasteur were unexposed to aerosol.
Postmortem examination of guinea pigs.
Aerosol-infected and control animals were killed 4 weeks after challenge by peritoneal overdose of sodium pentobarbitone. Examination was carried out immediately after death. External assessment of body condition was followed by gross internal examination of the neck region and thoracic and abdominal cavities. The lungs, with the trachea, bronchus, heart, and tracheobronchial lymph nodes attached, were removed to 10% formal-buffered saline for later examination. The whole spleen was removed aseptically and placed into 5 ml of sterile distilled water for bacteriology.
Determination of pulmonary disease from formalin-fixed tissue.
The fixed lungs from each animal were examined in detail, and the number of lesions on the dorsal surface that were large enough to be counted was recorded on a diagram of the lungs with their position and size, together with whether caseation or consolidation was present. A numerical value representing the extent of pulmonary disease was assigned to the lungs of each animal based on the following criteria: confluent foci where individual foci could not be enumerated reliably scored a 5; foci of 3 mm or more in diameter scored a 3; foci of 2 mm in diameter scored a 2; and foci of 1 mm in diameter scored a 1. Foci smaller than 1 mm in diameter were not scored. Discrete, but not confluent, foci containing caseated pus scored double. The ventral surface of the lung was also examined and occasionally scored for comparison. Since no significant differences were found between the scores from either surface, the score from the dorsal surface was taken as representative of the whole lung pathology.
Bacterial enumeration.
Spleens and lymph nodes were homogenized in 5 ml of sterile distilled water using a rotating-blade macerator system. Viable counts were performed on serial dilutions of the macerate and examined after 4 weeks of incubation at 37°C for growth of mycobacteria.
Statistical analyses.
Appropriate statistical tests were chosen, and all data were analyzed using the InStat software package (version 3.00; GraphPad, San Diego, Calif.). Where possible, the unpaired t test was applied unless any data set under analysis could not be assumed to come from a population that followed Gaussian distribution, in which case the nonparametric Mann-Whitney test was used. Welch's correction was applied to the unpaired t test where the data sets under analysis had significantly different standard deviations.
RESULTS
The leucine auxotroph of BCG does not sensitize for a cutaneous DTH reaction against tuberculin.
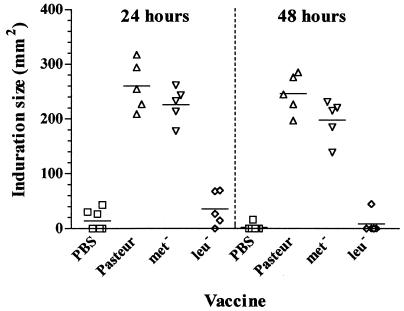
We tested the BCG vaccines for their ability to sensitize guinea pigs to the cutaneous DTH reaction against tuberculin. Both BCG Pasteur and the methionine auxotroph sensitized guinea pigs for cutaneous DTH reactivity (Fig. 1). At neither 24 nor 48 h after intradermal PPD injection was there any significant difference between the induration caused by BCG Pasteur or BCG Met− (unpaired t test). No significant induration was seen in the animals inoculated with the leucine auxotroph (Fig. 2). At both the 24- and 48-h time points, the difference in induration between BCG Pasteur- and BCG Leu−-sensitized animals was extremely significant (P < 0.0001 [unpaired t test]).
FIG. 1.
Abilities of BCG vaccines to sensitize guinea pigs to cutaneous DTH. Data are mean induration size 24 and 48 h after intradermal injection of bovine PPD. Animals were inoculated with 5 × 104 CFU of BCG Pasteur, BCG methionine auxotroph (Met−), BCG leucine auxotroph (Leu−), or PBS and 5 weeks later were tested by intradermal injection of 50 IU of bovine standard PPD.
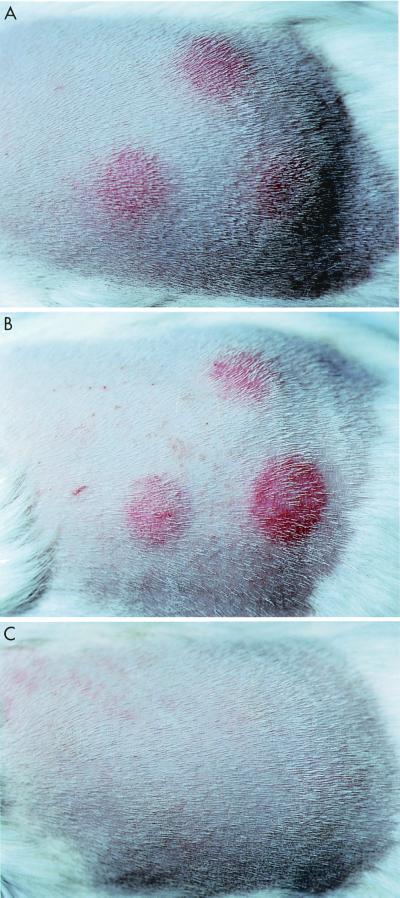
FIG. 2.
Ability of BCG vaccines to sensitize guinea pigs to cutaneous DTH. Shown is the appearance of the flank of representative guinea pigs 48 h after intradermal injection of bovine PPD. Animals were inoculated with 5 × 104 CFU of BCG Pasteur (top panel), a BCG methionine auxotroph (middle panel), or a BCG leucine auxotroph (bottom panel) and 5 weeks later were tested by intradermal injection of 50 IU of bovine standard PPD.
The leucine auxotroph of BCG does not persist in guinea pigs.
Since the BCG auxotroph did not sensitize guinea pigs for a cutaneous DTH reaction against tuberculin, we decided to test whether it was able to persist in guinea pigs following vaccination. The persistence of the leucine auxotroph in spleen and lymph nodes draining the site of bacterial inoculation was compared with that of its parental strain of BCG following intramuscular injection with vaccination level doses of organisms. The results for the draining lymph node are shown in Table 1. The parental strain of BCG was detected in the deep inguinal lymph node throughout the experiment (37 days). In contrast, we were unable to detect the leucine auxotroph even 1 day postvaccination. In addition, BCG Pasteur (log10 = 2.6) was detected in the spleen of one animal at day 28 postvaccination. The leucine auxotroph was not detected in the spleens of vaccinated animals.
TABLE 1.
Bacterial load in the deep inguinal lymph node draining the site of intramuscular injection of BCG Pasteur or BCG leucine auxotroph (Leu−)
| Days postvaccination | Log10 total CFU
|
|
|---|---|---|
| BCG Pasteur | BCG Leu− | |
| 1 | 1.7 | <1.7a |
| 13 | 1.9 | <1.7 |
| 21 | 3.0 | <1.7 |
| 28 | 2.1 | <1.7 |
| 37 | 2.2 | <1.7 |
Represents limit of detection.
BCG vaccines protect guinea pigs against hematogenous spread of aerogenic M. bovis better than against similar spread of M. tuberculosis.
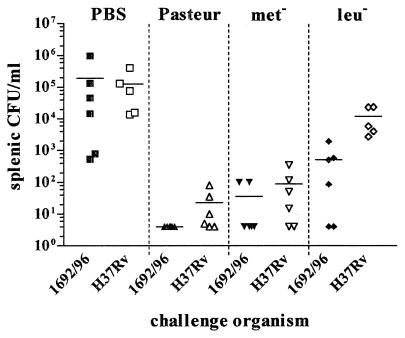
Since it had previously been observed that the leucine and methionine auxotrophs conferred protection against challenge with M. tuberculosis in mice (10), we tested whether these auxotrophic strains of BCG could also protect guinea pigs against aerosol challenge with M. tuberculosis and M. bovis. Five weeks after BCG vaccination, guinea pigs were exposed to a low dose of either M. bovis or M. tuberculosis via the aerogenic route. Four weeks after aerogenic challenge, the spleens of all animals were removed and cultured for the presence of mycobacteria (Fig. 3). The spleen culture from one animal vaccinated with the leucine auxotroph and challenged with M. tuberculosis was contaminated, precluding determination of mycobacterial load in this one animal. One guinea pig mock vaccinated with PBS and challenged with M. tuberculosis died before the end of the experiment. Postmortem examination revealed disseminated TB, which was considered to be the cause of death. The lungs from this animal were fixed in formal-buffered saline, enabling the disease severity to be scored, but the spleen was not tested for bacterial load. A consistent finding was that greater protection from hematogenous spread was observed against M. bovis challenge than M. tuberculosis challenge (Fig. 3). For challenge with M. bovis, compared with the PBS control, parental BCG gave 4.7 log protection, the methionine auxotroph gave 3.7 log protection, and the leucine auxotroph gave 2.6 log protection. Most strikingly, no organisms could be recovered from the spleen of any animal vaccinated with BCG Pasteur and challenged with M. bovis, with the lower limit of detection being 5 CFU/ml. For challenge with M. tuberculosis, compared with the PBS control, parental BCG Pasteur gave 3.8 log protection, the methionine auxotroph gave 3.2 log protection, and the leucine auxotroph gave 1.0 log protection (still significant: P < 0.05 [unpaired t test]), with the lower limit of detection being 5 CFU/ml. Equivalent bacterial burdens were observed in the spleens of unvaccinated (PBS control) animals irrespective of whether the animals were infected with M. bovis or M. tuberculosis (P = 0.40 [unpaired t test with Welch's correction]). Neither of the auxotrophic vaccines grew on the unsupplemented medium used to culture the spleen macerate. Additionally, no mycobacteria were cultured from the spleens of control animals inoculated with BCG Pasteur and left unchallenged, indicating that any colonies grown from the spleens of infected guinea pigs were solely derived from the challenge organism.
FIG. 3.
Ability of BCG vaccines to protect guinea pigs from hematogenous spread following aerogenic challenge with M. bovis or M. tuberculosis. Data are numbers of CFU per milliliter recovered from the spleens of animals 4 weeks after challenge via the aerogenic route with M. bovis strain 1692/96 or M. tuberculosis strain H37Rv (limit of detection for both = 5 CFU/ml) at a retained dose of 10 organisms. Five weeks before challenge, animals were vaccinated with 5 × 104 CFU of BCG Pasteur, a BCG methionine auxotroph (Met−), or a BCG leucine auxotroph (Leu−).
Protection from hematogenous spread is not the same as protection from pulmonary disease.
Recent evaluation of novel vaccines for M. tuberculosis in a guinea pig aerogenic challenge model revealed that protection was expressed in terms of a reduction in the pathology, rather than the bacterial load, in the lungs (1). For this reason, we assessed the ability of the BCG vaccines in this study to protect against pulmonary pathology.
The lungs and associated lymph tissue were examined after fixation in formalin, and an assessment was made of the gross lesions. No significant lesions were seen in the lungs of control animals inoculated with BCG Pasteur and left unchallenged. For the remaining animals, a disease severity score was determined on the basis of gross lung pathology (Fig. 4). The most severe pathology was seen in the unvaccinated groups challenged with M. bovis or M. tuberculosis. Although the scores obtained with each organism were equivalent, differences were observed in the nature of the pulmonary lesions. Equivalent numbers of caseated lesions were seen in animals infected with either organism, but larger (diameter, >3 mm) discrete caseated lesions were seen in animals infected with M. bovis. In contrast, significantly more confluent lesions were seen in animals infected with M. tuberculosis (P = 0.008 [unpaired t test]). BCG Pasteur and the methionine auxotroph gave equivalent and significant protection against pulmonary disease following challenge with M. bovis or M. tuberculosis (P < 0.02 [unpaired t test with Welch's correction]) (Fig. 4). In contrast, the leucine auxotroph conferred significant protection against M. bovis only (P < 0.02 [unpaired t test with Welch's correction]). As implied by the disease severity score, vaccine protection from pulmonary disease was expressed in a reduction in the number and size of lesions and in the number of caseated lesions.
FIG. 4.
Ability of BCG vaccines to protect guinea pigs from pulmonary disease. Disease severity score based on the number and size of lesions, together with the presence of caseation, on the dorsal surface of formalin-fixed lungs removed 4 weeks after challenge via the aerogenic route with M. bovis strain 1692/96 or M. tuberculosis strain H37Rv at a retained dose of 10 organisms. Five weeks before challenge, animals were vaccinated with 5 × 104 CFU of BCG Pasteur, a BCG methionine auxotroph (Met−), or a BCG leucine auxotroph (Leu−).
DISCUSSION
In this paper we report that a mutant strain of BCG (mc2798), auxotrophic for leucine (24), was able to confer statistically significant protection from aerogenic infection with M. bovis (reduction in lung pathology and hematogenous spread) and M. tuberculosis (hematogenous spread only) in guinea pigs, in the absence of a cutaneous DTH reaction to tuberculin. In contrast, the parental strain of BCG (Pasteur) and a methionine auxotroph of BCG (24) conferred a greater degree of protection against challenge but sensitized for tuberculin hypersensitivity. Although protection against challenge with M. tuberculosis had been reported for these vaccine strains in mice (10), the vaccines were further evaluated in a low-dose aerosol challenge guinea pig infection model (34) for a number of reasons (reviewed in reference 25). In particular, guinea pigs are highly susceptible to infection with low doses of M. tuberculosis or M. bovis, providing a large window in which to evaluate vaccine efficacy. Furthermore, guinea pigs are the animal of choice for the routine evaluation of tuberculin potency due to the reliability of their hypersensitivity response to a cutaneously administered antigen(s) (7, 13, 19, 31).
We tested the BCG vaccines for their ability to induce cutaneous DTH. Both BCG Pasteur and the methionine auxotroph sensitized guinea pigs for cutaneous DTH reactivity. In marked contrast, no significant induration was seen in the animals inoculated with the leucine auxotroph. At least two reports (3, 16) have linked the ability and extent of BCG strains to sensitize for cutaneous DTH with the persistence of the organism in the spleen and peripheral lymphoid tissue. The ability of the auxotrophs used in this study to persist in mice has been examined previously (24). The methionine auxotroph (referred to as mc2789 in that study) showed growth characteristics similar to those of BCG Pasteur, with an initial decrease in bacterial numbers, a growth phase, and slow decline. In contrast, the leucine auxotroph (referred to as mc2798 in that study) showed no evidence of growth in vivo. Similarly, we were able to detect the parental BCG but not the leucine auxotroph in the lymph nodes draining the site of inoculation over a 5-week period following vaccination. This suggests that, as for mice (15, 24), the host leucine supplies within the intracellular environment that BCG occupies postvaccination in guinea pigs are limiting for growth. Moreover, our results are consistent with the hypothesis that the ability of the vaccine to sensitize for DTH is related to the length that it persists in the host. Recently, Wedlock et al. correlated the ability of virulent and attenuated strains of M. bovis to induce DTH in cattle with the strains' ability to grow in macrophages (33).
All the BCG vaccines tested in this study conferred a statistically significant degree of protection against spleen infection following challenge of the guinea pigs with M. bovis or M. tuberculosis. Protection was defined as a reduction in the mean CFU count compared with that of the PBS control. Significance was defined as P < 0.05, by the unpaired t test. The degree of protection was related to both the challenge organism and the vaccine. In every case, the BCG vaccines conferred less protection to challenge with M. tuberculosis than to challenge with M. bovis. This was consistent with previous studies (34). These differences were not related to discrepancies in the administered dose of M. bovis compared with M. tuberculosis nor to differing abilities of the strains to colonize the spleen, since the unvaccinated (PBS control) animals displayed equivalent levels of spleen infection whether infected with M. bovis or M. tuberculosis. Since BCG is derived from M. bovis, the ability of BCG to protect better against challenge with M. bovis suggests that there may be protective antigens in M. bovis that are absent from, or expressed differently in, M. tuberculosis (14, 21).
In a recent study evaluating subunit protein and DNA vaccines in guinea pigs infected aerogenically with M. tuberculosis, both types of vaccine were reported to prevent the onset of caseating disease and to prolong the survival of animals without significantly reducing the pulmonary bacterial load (1). We therefore assessed the abilities of the BCG vaccines in this study to protect against pulmonary pathology. The trends in protection from pulmonary disease mirrored protection from hematogenous spread. However, although the leucine auxotroph gave significant protection from hematogenous spread of both M. bovis and M. tuberculosis, it was only able to confer significant protection from pulmonary tuberculosis caused by M. bovis. Thus, not only may the protective antigens differ between M. tuberculosis and M. bovis, but different antigens may be the target for preventing pulmonary disease versus hematogenous spread of mycobacteria. It is possible that the leucine auxotroph was not sufficiently metabolically active to synthesize the antigens required for protection against M. tuberculosis in the lung.
Throughout this study, the efficacy of the three BCG vaccines was related to their ability to sensitize for DTH and by inference, to their persistence (3, 16). Although from the results observed for the leucine auxotroph of BCG and its parental strain there was a correlation among protection, DTH, and persistence, the leucine auxotroph still conferred significant protection from challenge in the absence of a DTH reaction to tuberculin, especially in the case of M. bovis. This supports the notion that there may be two separate immunological mechanisms responsible for protection from pathogenic mycobacteria and cutaneous sensitization to tuberculin. In fact, numerous studies support this contention (8, 11, 27, 37). The biological rules that will enable these two processes to be teased apart are yet to be determined in any detail, but ICAM-1 would appear to have a part to play (20). However, given the overall reduced protection conferred by the leucine auxotroph, especially from M. tuberculosis challenge, it is possible that the inability of this auxotroph to sensitize for cutaneous hypersensitivity may merely reflect a lower level of immune activation. Recently it has been reported that a leucine auxotroph of M. tuberculosis both was attenuated and conferred significant protection against death from M. tuberculosis infection in mice (15). However, this auxotroph was cleared more rapidly and was less effective than live BCG in reducing organ burdens and tissue pathology. These findings, along with those reported here for the BCG leucine auxotroph, emphasize the challenge of achieving the optimal balance of attenuation and immunogenicity for live vaccines against TB. Vaccination with booster doses may improve the immunogenicity of the leucine auxotroph and should serve to determine whether the ability to sensitize for DTH is related to persistence of the vaccine or the overall level of immune activation induced by it. Such studies are currently under way in our laboratory.
As more live attenuated vaccines for TB are evaluated for protective efficacy and ability to sensitize for tuberculin, it may be possible to identify candidates that give optimum protection, are safe for the immunosuppressed, and do not compromise use of the tuberculin skin test. If such strains can be identified they may allow the development of models with which to dissociate the mechanisms of protective immunity from those of DTH.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Agriculture, Fisheries, and Food, Great Britain.
We thank the Animal Services Units at VLA Weybridge and CAMR.
REFERENCES
- 1.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenicity, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 531–557. [Google Scholar]
- 3.Brandely M, Hurtrel B, Lagrange P H. Comparison between immunopotency tests and specific active or passive acquired resistance against Mycobacterium tuberculosis in mice induced with three different preparations of BCG pasteur vaccine. Clin Exp Immunol. 1983;54:143–150. [PMC free article] [PubMed] [Google Scholar]
- 4.Crubezy E, Ludes B, Poveda J D, Clayton J, Crouau-Roy B, Montagnon D. Identification of Mycobacterium DNA in an Egyptian Pott's disease of 5,400 years old. C R Acad Sci III. 1998;321:941–951. doi: 10.1016/s0764-4469(99)80009-2. [DOI] [PubMed] [Google Scholar]
- 5.Dankner W M, Waecker N J, Essey M A, Moser K, Thompson M, Davis C E. Mycobacterium bovis infections in San Diego: a clinicoepidemiologic study of 73 patients and a historical review of a forgotten pathogen. Medicine (Baltimore) 1993;72:11–37. [PubMed] [Google Scholar]
- 6.Druett H A. A mobile form of the Henderson apparatus. J Hyg. 1969;67:437–448. doi: 10.1017/s0022172400041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Pharmacopoeia. European pharmacopoeia. 3rd ed. Strasbourg, France: European Pharmacopoeia; 1997. pp. 1680–1681. [Google Scholar]
- 8.Fine P E, Sterne J A, Ponnighaus J M, Rees R J. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344:1245–1249. doi: 10.1016/s0140-6736(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 9.Grange J M, Collins C H. Bovine tubercle bacilli and disease in animals and man. Epidemiol Infect. 1987;99:221–234. doi: 10.1017/s0950268800067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guleria I, Teitelbaum R, McAdam R A, Kalpana G, Jacobs W R, Jr, Bloom B R. Auxotrophic vaccines for tuberculosis. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 11.Hart P D, Sutherland I, Thomas J. The immunity conferred by effective BCG and vole bacillus vaccines, in relation to individual variations in induced tuberculin sensitivity and to technical variations in the vaccines. Tubercle. 1967;48:201–210. [Google Scholar]
- 12.Hart P D, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;2:293–295. doi: 10.1136/bmj.2.6082.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori H, Yamaguchi F, Wagai N, Kato M, Nomura M. An assessment of antigenic potential of beta-lactam antibiotics, low molecular weight drugs, using guinea pig models. Toxicology. 1997;123:149–160. doi: 10.1016/s0300-483x(97)00118-2. [DOI] [PubMed] [Google Scholar]
- 14.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W J. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 15.Hondalus M K, Bardarov S, Russell R, Chan J, Jacobs W R, Jr, Bloom B R. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishibashi T, Harada Y, Harada S, Yamada H, Takamoto M, Sugiyama K. Mode of immunopotentiating action of BCG: persistence and spread of BCG infection. Jpn J Exp Med. 1978;48:227–232. [PubMed] [Google Scholar]
- 17.Jackson M, Phalen S W, Lagranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray D N, Gicquel B, Guilhot C. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Snapper S B, Udani R A, Jones W, Barletta R G, Bloom B R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 19.Jirova D, Janeckova V, Znojemska S, Pekarek J. Adjuvant activity of muramyldipeptide in a guinea pig model of contact allergy to chromium. J Hyg Epidemiol Microbiol Immunol. 1983;27:395–402. [PubMed] [Google Scholar]
- 20.Johnson C M, Cooper A M, Frank A A, Orme I M. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–1670. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungblut P R, Schaible U E, Mollenkopf H, Zimny-Arndt U, Raupach B, Mattow J, Halada P, Lamer S, Hagens K, Kaufmann S H. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol Microbiol. 1999;33:1103–1117. doi: 10.1046/j.1365-2958.1999.01549.x. [DOI] [PubMed] [Google Scholar]
- 22.Krebs J, Anderson R, Clutton-Brock T, Morrison I, Young D, Donnelly C. Bovine tuberculosis in cattle and badgers—report by the Independent Scientific Review Group. London, United Kingdom: Ministry of Agriculture, Fisheries and Food Publications; 1997. [Google Scholar]
- 23.Lobato M N, Hopewell P C. Mycobacterium tuberculosis infection after travel to or contact with visitors from countries with a high prevalence of tuberculosis. Am J Respir Crit Care Med. 1998;158:1871–1875. doi: 10.1164/ajrccm.158.6.9804106. [DOI] [PubMed] [Google Scholar]
- 24.McAdam R A, Weisbrod T R, Martin J, Scuderi J D, Brown A M, Cirillo J D, Bloom B R, Jacobs W R., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurray D N. Guinea pig model of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenicity, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 135–147. [Google Scholar]
- 26.O'Reilly L M, Daborn C J. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl. 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 27.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salo W L, Aufderheide A C, Buikstra J, Holcomb T A. Identification of Mycobacterium tuberculosis DNA in a pre-Columbian Peruvian mummy. Proc Natl Acad Sci USA. 1994;91:2091–2094. doi: 10.1073/pnas.91.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steele J H. Regional and country status reports—introduction. In: Thoen C O, Steele J H, editors. Mycobacterium bovis infection in animals and humans. Ames: Iowa State University Press; 1995. pp. 47–61. [Google Scholar]
- 30.Tuberculosis Prevention Trial, Madras. Trial of BCG vaccines in South India for tuberculosis prevention. Indian J Med Res. 1980;72(Suppl. 1):1–74. [PubMed] [Google Scholar]
- 31.Vial T, Descotes J. Contact sensitization assays in guinea-pigs: are they predictive of the potential for systemic allergic reactions? Toxicology. 1994;93:63–75. doi: 10.1016/0300-483x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 32.Vordermeier H M, Cockle P C, Whelan A, Rhodes S, Palmer N, Bakker D, Hewinson R G. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–682. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wedlock D N, Aldwell F E, Collins D M, de Lisle G W, Wilson T, Buddle B M. Immune responses induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed-type hypersensitivity with ability of strains to grow in macrophages. Infect Immun. 1999;67:2172–2177. doi: 10.1128/iai.67.5.2172-2177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams A, Davies A, Marsh P D, Chambers M A, Hewinson R G. Comparison of the protective efficacy of bacille Calmette-Guerin vaccination against aerosol challenge with Mycobacterium tuberculosis and Mycobacterium bovis. Clin Infect Dis. 2000;30(Suppl. 3):299–301. doi: 10.1086/313878. [DOI] [PubMed] [Google Scholar]
- 35.Williams D E. Mycobacterium bovis BCG infection in humans. In: Thoen C O, Steele J H, editors. Mycobacterium bovis infection in animals and humans. Ames: Iowa State University Press; 1995. pp. 47–61. [Google Scholar]
- 36.World Health Organization. Joint WHO/FAO Expert Committee on Zoonoses. Technical report series 169. Geneva, Switzerland: World Health Organization; 1959. [Google Scholar]
- 37.Youmans G P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975;111:109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]