Abstract
Pseudomonas aeruginosa, an important nosocomial pathogen of humans, expresses a type III secretion system that is required for virulence. Previous studies demonstrated that the lung-virulent strain PA103 has the capacity to be either cytotoxic or invasive. Analyses of mutants suggest that PA103 delivers a negative regulator of invasion, or anti-internalization factor, to host cells via a type III secretion system. In this work we show that the type III secreted protein ExoT inhibits the internalization of PA103 by polarized epithelial cells (Madin-Darby canine kidney cells) and J774.1 macrophage-like cells. ExoS, which is closely related to ExoT but has additional ADP-ribosylating activity, can substitute for ExoT as an anti-internalization factor. ExoT contains a signature arginine finger domain found in GTPase-activating proteins. Mutation of the conserved arginine in ExoT diminished its anti-internalization activity and altered its ability to disrupt the actin cytoskeleton. Cell fractionation experiments showed that ExoT is translocated into host cells and that mutation of the arginine finger did not disrupt translocation. In a mouse model of acute pneumonia, PA103ΔUΔT reached the lungs as efficiently as PA103ΔU but showed reduced colonization of the liver. This finding suggests that the ability to resist internalization may be important for virulence in vivo.
Pseudomonas aeruginosa is an important nosocomial pathogen with an impressive arsenal of secreted and cell-associated virulence factors (42). Although considered a primarily extracellular pathogen, some clinical and laboratory isolates are internalized by epithelial cells (11, 12, 14). Invasive strains cause disease (11), but the role of invasion in the pathogenic process is unclear. Bacterial internalization may actually benefit the host by serving as a defense mechanism. Shedding of epithelial cells harboring intracellular P. aeruginosa may help clear the bacteria from the site of infection (40), and phagocytosis by macrophages may help eliminate the pathogen and stimulate adaptive immune responses.
Initial analyses suggested that P. aeruginosa strains were either exclusively cytotoxic or exclusively invasive (14), but further investigations have revealed that at least one strain, lung isolate PA103, has the capacity to be either cytotoxic or invasive (24). Wild-type PA103 is cytotoxic and noninvasive, but isogenic mutants defective in type III secretion are no longer cytotoxic and are efficiently internalized by multiple eukaryotic cell types (8, 24). PA103 expresses the type III secreted effector proteins ExoT and ExoU but not ExoS and ExoY, which have been identified in other P. aeruginosa strains (52). An isogenic PA103 mutant lacking the type III secreted effector ExoU is not cytotoxic and is not internalized (24). These results prompted the hypothesis that ExoT or an additional unknown type III secreted effector protein negatively regulates invasion of eukaryotic cells (8, 24).
P. aeruginosa joins a growing list of gram-negative bacteria that regulate their internalization by delivering effector proteins to the host cell via a type III secretion system. Examples of other bacteria with these properties include enteropathogenic Escherichia coli (EPEC), Yersinia species, and Salmonella enterica serovar Typhimurium. EPEC inhibits its own uptake by macrophages by a type III secretion-dependent mechanism that coincides with phosphotyrosine dephosphorylation of a subset of host proteins. The dephosphorylation activity was inhibited by pervanadate (an inhibitor of tyrosine phosphatases), but no tyrosine phosphatase activity could be detected (18). In Yersinia species, the invasin protein interacts with high affinity with the β1 integrin receptors on the host cell, resulting in bacterial uptake (26, 27). Two Yersinia type III secreted effectors, YopE and YopH, function as anti-internalization factors (38, 41). YopH is a potent tyrosine phosphatase whose targets include the focal adhesion protein p130cas and focal adhesion kinase (4, 19, 20, 38). The invasion of epithelial cells by S. enterica serovar Typhimurium is exquisitely regulated by the type III secreted effectors SopE and SptP. SopE catalyzes the conversion of the inactive GDP-bound forms of CDC42 and Rac to the active GTP-bound forms by virtue of its guanine nucleotide exchange activity (21). The increases in CDC42 and Rac activities lead to a disruption of the actin cytoskeleton (ruffling), which promotes bacterial internalization (5). The SopE-induced ruffling is terminated and thus tightly regulated by SptP. The N-terminal portion of SptP shares homology with YopE of Yersinia and with the type III secreted effector proteins ExoS and ExoT of P. aeruginosa; all four effectors have a conserved arginine finger domain characteristic of GTPase-activating proteins (GAPs) (16).
While this work was in progress, Cowell and coworkers demonstrated that ExoT inhibits internalization of P. aeruginosa by corneal epithelial cells (6). The closely related protein ExoS has been shown to function as an anti-internalization factor (6, 15). Bacteria secreting either of these proteins also induce rounding and/or detachment of some epithelial cell types in culture (48). We build upon these results and demonstrate that ExoT can inhibit the internalization of P. aeruginosa by polarized epithelial cells and by macrophage-like cells in culture. We hypothesize that the anti-internalization activity of ExoT results from its ability to act as a GAP. In support of this hypothesis, we demonstrate that the arginine finger domain present in ExoT and characteristic of GAPs contributes to its anti-internalization activity as well as its ability to alter the actin cytoskeleton. Cell fractionation experiments showed that ExoT is translocated into host cells and that mutation of the arginine finger does not disrupt translocation. Finally, we demonstrate that the virulence of PA103ΔUΔT is abrogated compared to that of PA103ΔU, revealing that the ability of P. aeruginosa to regulate its internalization is important for its pathogenic potential.
MATERIALS AND METHODS
Bacterial strains and media.
Bacterial strains were routinely cultured in Luria-Bertani (LB) broth or Vogel-Bonner minimal medium (VBM) with antibiotics as needed for cloning purposes. The following antibiotics and concentrations were used: ampicillin, 100 μg/ml for E. coli; carbenicillin, 200 μg/ml for P. aeruginosa; and gentamicin, 15 μg/ml for E. coli and 100 μg/ml for P. aeruginosa. All antibiotics and chemicals were purchased from Sigma (St. Louis, Mo.) except where noted. A list of strains and constructs used in this study is shown in Table 1. All cloning steps were performed with XL2-Blue ultracompetent E. coli (Stratagene). Primer bases shown in lowercase letters are not homologous to the chromosomal sequence.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference(s) or source |
|---|---|---|
| Strains | ||
| PA103 | Virulent lung isolate of P. aeruginosa; known type III secreted effector proteins are ExoT and ExoU | 1, 36 |
| PA103pscJ::Tn5 | Tn5 Gmr cassette inserted into pscJ; defective in type III secretion | 29 |
| PA103exoU::Tn5 | Tn5 Gmr cassette inserted into exoU; referred to as mutant 8 in previous publications | 23, 29 |
| PA103ΔT | PA103 with an xylE aacC1 cassette replacing aa 36–348 of exoT; Gmr | This study |
| PA103ΔU | PA103 with an in-frame deletion of aa 330–571 of exoU | This study |
| PA103ΔU+pUCP20 | PA103ΔU transformed with pUCP20; Cbr | This study |
| PA103ΔU+pExoS | PA103ΔU transformed with pUCP20/exoS; Cbr | This study |
| PA103ΔUΔT | PA103ΔU with an xylE aacC1 cassette replacing aa 36–348 of exoT; Gmr | This study |
| PA103ΔUΔT+pUCP20 | PA103ΔUΔT transformed with pUCP20; Gmr Cbr | This study |
| PA103ΔUΔT+pExoS | PA103ΔUΔT transformed with pUCP20/exoS; Gmr Cbr | This study |
| PA103ΔUΔT+pExoT | PA103ΔUΔT transformed with pUCP20/exoT; Gmr Cbr | This study |
| PA103ΔUΔT+pExoT(R149G) | PA103ΔUΔT transformed with pBK162; Gmr Cbr | This study |
| PA103ΔUΔT+pExoT(R149K) | PA103ΔUΔT transformed with pBK151; Gmr Cbr | This study |
| PA103ΔU/T(R149G) | PA103ΔU with a point mutation in ExoT(R149G) | This study |
| PA103ΔU/T(R149K) | PA103ΔU with a point mutation in ExoT(R149K) | This study |
| 388 | P. aeruginosa laboratory strain; known type III secreted effector proteins are ExoS, ExoT, and ExoY | 2, 25 |
| S17.1 | E. coli strain used for mating constructs into P. aeruginosa; thi pro hsdR recA RP4-2 (Tet::Mu) (Km::Tn7) | 46 |
| XL2-Blue | E. coli strain used for cloning; recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr) Amy Camr] | Stratagene |
| Plasmids | ||
| pAH806 | pBR322 containing a 3.5-kb genomic fragment from PA103 including the exoU gene | 23 |
| pBK151 | pUCP20 with ExoT(R149K) point mutation | This study |
| pBK152 | pEX100T with XmaI fragment from pBK151 | This study |
| pBK162 | pUCP20 with ExoT(R149G) point mutation | This study |
| pBK163 | pEX100T with XmaI fragment from pBK162 | This study |
| pEX100T | Allelic replacement suicide plasmid; Apr (Cbr)bsacB oriT | 45 |
| pEX100T/exoTgent | pEX100T with a 3,055-bp ApaI-NotI fragment of pGEM-T/exoTgent cloned into the SmaI site; Gmr | This study |
| pEX100T/exoU | pEX100T with a 3.2-kb SspI fragment from pAH806 including the exoU gene cloned into the SmaI site | This study |
| pEX100T/ΔexoU | pEX100T/exoU with a 726-bp BclI fragment encoding aa 330–571 of ExoU removed | This study |
| pGEM-T | Vector for cloning PCR products; Apr | Promega |
| pGEM-T/exoS | pGEM-T containing a 1,503-bp PCR product and bearing the exoS gene and upstream ExsA binding site from strain 388 | This study |
| pGEM-T/exoT | pGEM-T containing a 1,494-bp PCR product and bearing the exoT gene from strain PA103 | This study |
| pGEM-T/exoT+ | pGEM-T with a 1,619-bp PCR product and bearing the exoT gene and upstream ExsA binding site from PA103 | This study |
| pGEM-T/exoTgent | pGEM-T/exoT with a 939-bp SmaI fragment encoding ExoT with aa 36–348 replaced with a 2,446-bp SmaI fragment encoding the xylE aaC1 cassette from pX1918GT | This study |
| pUCP20 | P. aeruginosa expression vector; Apr (Cbr)b | 51 |
| pUCP20/exoS | pUCP20 with a 1,570-bp SacI-SphI fragment containing exoS from pGEM-T/exoS | This study |
| pUCP20/exoT | pUCP20 with a 1,666-bp NcoI-NdeI fragment containing exoT from pGEM-T/exoT | This study |
| pX1918GT | Plasmid containing xylE aacC1 insertional Gmr cassette; Apr | 45 |
aa, amino acids; Tetr, tetracycline resistance; Camr, chloramphenicol resistance; Apr, ampicillin resistance.
Apr in E. coli; Cbr in P. aeruginosa.
For analysis of ExoT secretion, translocation, anti-internalization activity, cell rounding activity, and disruption of the actin cytoskeleton, all strains were grown without shaking in 2 ml of LB broth (containing 200 μg of carbenicillin per ml for strains carrying plasmids) for 18 h at 37°C. Although growth in minimal medium maximizes type III secretion in vitro, plasmid-carrying strains could not be grown in minimal medium in the presence of carbenicillin (data not shown). In order to accomplish our add-back experiments, the culturing conditions described above were adopted and used uniformly for strains not containing plasmids as well. Numbers of bacteria used for specific assays were determined by assessment of optical density and confirmed by plating serial dilutions for colony counts.
The PA103ΔU in-frame deletion mutant was created as follows. A 3.2-kb SspI fragment of plasmid pAH806 containing the entire exoU gene from PA103 was cloned into the SmaI site of the sacB-based gene replacement vector pEX100T (45). Digestion of this construct with BclI, followed by self-ligation, removed a 726-bp fragment encoding amino acids 330 to 571 of ExoU, creating pEX100T/ΔexoU. E. coli S17.1 carrying the pEX100T/ΔexoU construct was mated with P. aeruginosa PA103. Exconjugants were selected on VBM with 200 μg of carbenicillin per ml and then streaked to VBM with 5% sucrose to select for loss of vector sequences through a second recombination event. Sucrose-resistant, carbenicillin-susceptible (Cbs) colonies were tested for the exoU deletion by PCR, and the deletion was confirmed to be in frame by sequence analysis of the PCR product (data not shown).
The PA103ΔT and PA103ΔUΔT mutants were constructed as follows. The exoT gene was amplified by PCR using primers ExoT3 (5′-aagaattCACGGCCAATCCTGATAGGCGGAGG-3′) and ExoT4 (5′-aagaattCTTGGGAGTGTCCGTCTCTGCCGTC-3′), with PA103 chromosomal DNA as a template. The 1,494-bp PCR product was cloned into pGEM-T (Promega), creating construct pGEM-T/exoT. Amino acids 36 to 348 of ExoT were removed by digestion of pGEM-T/exoT with SmaI and replaced by ligation with a 2,446-bp SmaI fragment of pX1918GT encoding the xylE aacC1 gentamicin-resistant (Gmr) cassette. The resulting pGEM-T/exoTgent construct was digested with ApaI and NotI to release a 3,055-bp fragment from the polylinker containing exoT with the Gmr cassette inserted (exoTgent). The 3,055-bp fragment was treated with T4 polymerase to obtain blunt ends and cloned into the SmaI site of pEX100T, creating pEX100T/exoTgent. E. coli S17.1 carrying the pEX100T/exoTgent construct was mated with PA103 and PA103ΔU. Exconjugants were selected on VBM with 200 μg of carbenicillin per ml and 100 μg of gentamicin per ml and then streaked to VBM with 5% sucrose and 100 μg of gentamicin per ml. Sucrose-resistant, Cbs Gmr exconjugants were checked for the exoTgent gene replacement by PCR.
The exoS gene was cloned for episomal expression by PCR with primers ExoSN1 (5′-ggggtaCCGGAGAGACTGTTAATC-3′) and ExoSC1 (5′-gccctAGGTGTCCGTTCGTGAC-3′) using chromosomal DNA from P. aeruginosa strain 388 as a template. We obtained a 1,503-bp product that included 142 bp upstream of the 5′ end of the complete exoS open reading frame, including the consensus ExsA binding site. This fragment was cloned into pGEM-T to form the construct pGEM-T/exoS. The exoS gene was subcloned by digestion of pGEM-T/exoS with SphI and SacI to release a 1,570-bp fragment from the polylinker that was ligated to pUCP20 digested with SphI and SacI to form the construct pUCP20/exoS. PA103ΔU and PA103ΔUΔT were transformed with pUCP20/exoS by electroporation and selection for Cbr to create strains PA103ΔU+pExoS and PA103ΔUΔT+pExoS.
The exoT gene was cloned for episomal expresson by PCR with primers ExoT4 (sequence above) and ExoT5 (5′-aagaattcATATCCATCGGGTTCTCCGCCCCGG-3′) using chromosomal DNA from PA103 as a template. We obtained a product of 1,619 bp that included 200 bp upstream of the 5′ end of the complete exoT open reading frame, including the consensus ExsA binding site. The product was cloned into pGEM-T to form the construct pGEM-T/exoT+. pGEM-T/exoT+ was digested with NcoI and NdeI to release a 1,666-bp fragment containing the exoT gene from the polylinker. The 1,666-bp fragment was treated with T4 polymerase to generate blunt ends and ligated to SmaI-cut pUCP20 to form pUCP20/exoT. pUCP20/exoT was used to transform PA103ΔUΔT by electroporation, followed by selection for Cbr, to create strain PA103ΔUΔT+pExoT.
The conserved arginine (position 149) of the predicted arginine finger motif found in ExoT was mutated to glycine and lysine as follows. PCR primers were designed to create a unique restriction enzyme site (BamHI for R149G, AflII for R149K) at the point of mutation. The R149G mutation was introduced by amplifying PA103 exoT with primers BAKA22 (5′-CGGTGTAGGCGCACGGGAG-3′) and BAKA24 (5′-GGCCAGgGAtCcCAGtGCGCCGTCGCCGCTG-3′) to yield a 625-bp product. A second 934-bp product was amplified using primers BAKA23 (5′-CGTCGACCGGTCAGGCCAG-3′) and BAKA25 (5′-GCaCTGgGaTCcCTGGCCACCGCCCTGGTCG-3′). The 625- and 934-bp products were cut with BamHI and ligated, which yielded a final 1,559-bp product. The gel-purified 1,559-bp product served as a template for PCR with internal primers BAKA32 (5′-CTGGCGGGGAAACATCAGG-3′) and BAKA33 (5′-AGGTGGAGAGATAGCCGGC-3′), yielding a 1,075-bp product, which was cloned into pGEM-T. An NsiI-NgoMIV fragment comprising the first 1,024 bp of the ExoT coding sequence and containing the R149G mutation was swapped with the corresponding fragment of pUCP20/exoT to create pBK162. In order to introduce the R149G point mutation into the PA103ΔU chromosome, an internal 939-bp XmaI fragment of pBK162 was subcloned into the XmaI site of pEX100T to create pBK163. E. coli S17.1 carrying pBK163 was mated with PA103ΔU, and exconjugants were screened for the point mutation by PCR with primers BAKA32 and BAKA33. Exconjugants whose PCR products could be digested by BamHI were predicted to contain the R149G mutation, and this was confirmed by sequencing the PCR product. The resulting mutant strain was named PA103ΔU/T(R149G). A similar strategy was used to create the ExoT R149K point mutant. In this case the initial PCR was performed with primers BAKA22 and BAKA26 (5′-GGCCAGCGActtaAGtGCGCCGTCGCCGCTG-3′) to amplify the 625-bp product. Primers BAKA23 and BAKA27 (5′-GCaCTtaagTCGCTGGCCACCGCCCTGG-3′) were used to amplify the 934-bp product. The two products were digested with AflII in this case and ligated to form the expected 1,559-bp product. The correct ligation product was amplified with BAKA32 and BAKA33 and subcloned into pGEM-T. The R149K mutation was swapped into pUCP20/exoT as described above to create pBK151. Similarly, the internal XmaI fragment was subcloned into pEX100T to form pBK152, and this construct was used to create the PA103ΔU/T(R149K) mutant. Of note, the subcloned PCR product contains a second mutation, A211V, which was judged to be a conservative change; this mutation persists in pBK151 and is also found in the chromosome of PA103ΔU/T(R149K).
Immunoblot analysis.
Five milliliters of cultured bacteria was centrifuged at 6,000 × g at 4°C for 20 min. Proteins were recovered from the supernatants by ammonium sulfate precipitation (final concentration of 55%). After incubation on ice for 18 h, precipitated proteins were concentrated by centrifugation at 13,000 × g at 4°C for 20 min. The pellet was boiled in a solution containing 50 μl of 10 mM NaCl and 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 5 min, and 20 μl of each sample was electrophoresed on an SDS–8% polyacrylamide gel (33). Proteins were electrotransferred to nitrocellulose for immunoblot analysis, and ExoT was detected using a rabbit polyclonal ExoT antiserum (24). Goat anti-rabbit immunoglobulin G (IgG) horseradish peroxidase conjugate (Gibco BRL, Gaithersburg, Md.) diluted 1:5,000 was used as a secondary antibody. Detection was performed using the enhanced chemiluminescence (ECL) system (Amersham Corp., Arlington Heights, Ill.).
HeLa cell rounding assay.
HeLa cells (American Type Culture Collection) were grown in Dulbecco's modified Eagle medium with 4.5 g of glucose per liter (DME-H21) obtained from the University of California, San Francisco, Cell Culture Facility (UCSF-CCF) and 10% heat-inactivated fetal bovine serum (FBS) (Gibco BRL). Cells were plated at 2 × 105 cells/well in 24-well plates and grown overnight at 37°C in the presence of 5% CO2. Cells were washed twice with minimal essential medium-Eagle's medium containing Hanks' buffered saline solution (HBSS) (MEM; Sigma Chemical Co.), 20 mM HEPES buffer (pH 8.0), 3.5% sodium bicarbonate, and 0.6% bovine serum albumin (MEM-etc.). HeLa cell wells were inoculated with 5 × 106 bacteria and incubated for 6 h at 37°C in room air. Rounding was assessed by visual inspection of the wells at ×100 and ×200 magnifications on a Nikon Eclipse TE200 microscope. Images were captured using a charge-coupled device (CCD) camera and SPOT imaging software (Diagnostic Instruments).
Fluorescence microscopy of infected HeLa cells.
HeLa cells were infected by the same procedure used for the rounding assay described above, except that the wells contained 12-mm-diameter acid-etched glass coverslips. After 6 h of infection, wells were washed twice with ice-cold phosphate-buffered saline (PBS). Cells were fixed by rocking them in 0.4% paraformaldehyde in PBS at room temperature for 30 min. Wells were rinsed once with PBS, and Quench (PBS with 75 mM NH4Cl and 20 mM glycine) was applied for 10 min, with rocking at room temperature. Fixed cells were stored in PBS for 18 h at 4°C. Cells were permeabilized in PBS with 0.7% (wt/vol) fish scale gelatin, 0.005% (wt/vol) saponin, and 100 μg of RNase A per ml (PBS-FSG-SAP) for 30 min with rocking at 37°C. Coverslips were placed cells down on a 50-μl drop of PBS-FSG-SAP with unpurified anti-ExoU polyclonal antiserum (diluted 1:500) in a sealed humid container at 37°C for 2 h. Although the strains used in these experiments do not produce ExoU, the antiserum contains additional antibodies, likely to lipopolysaccharide, which result in sufficient labeling of the bacteria. Coverslips were returned to their original wells and washed with PBS-FSG-SAP for 10 min four times, with rocking at 37°C. Coverslips were placed face down on 50-μl drops of PBS-FSG-SAP with a 1:500 dilution of goat anti-rabbit–fluorescein isothiocyanate antiserum (Jackson Laboratories, Bar Harbor, Maine) and a 1:200 dilution of Texas Red X-phalloidin (Molecular Probes, Eugene, Oreg.) in a sealed humid container at 37°C for 45 min. Coverslips were returned to their original wells and washed with PBS-FSG-SAP twice for 10 min with rocking at 37°C. The coverslips were then washed with PBS containing 0.1% (wt/vol) Triton X-100 twice for 5 min with rocking at 37°C. Coverslips were rinsed once with PBS and dipped in distilled H2O before being mounted on glass slides with a ProLong Antifade Kit (Molecular Probes). Cells were observed, and images were captured at ×1,000 magnification under oil immersion using a Nikon Eclipse E800 fluorescence microscope. Images were captured using a CCD camera and SPOT imaging software.
MDCK cell invasion assay.
MDCK cells were cultured in minimal essential medium-Eagle's medium with Earle's buffered saline solution (UCSF-CCF) with 5% FBS. Polarized monolayers were prepared by adding 106 MDCK cells to 12-mm-diameter, 0.4-μm-pore-size Transwell filter supports (Corning Costar Corp., Cambridge, Mass.) and incubating them for 3 days at 37°C with 5% CO2. MDCK cell monolayers were washed once with MEM-etc. and 107 bacteria were added to the upper chamber of the transwell. The bacteria were incubated with the MDCK cells for 2 h at 37°C with 5% CO2 to allow for invasion. The MDCK cell monolayers were then washed once with MEM-etc. and incubated for 2 h at 37°C with 5% CO2 in the presence of 400 μg of amikacin per ml to kill extracellular bacteria. Monolayers were then washed with MEM-etc., and the filters were excised and placed in Falcon 2059 tubes, where the cells were lysed by vortexing them with glass beads in Ca2+- and Mg2+-free HBSS (UCSF-CCF) with 0.25% (wt/vol) Triton X-100 (Sigma). Lysates were plated on LB agar and incubated for 18 h at 37°C to quantify the CFU of internalized bacteria. Invasion counts for the various mutants were normalized to that for PA103pscJ::Tn5 for strains without plasmids and to that for PA103ΔUΔT+pUCP20 for strains with plasmids.
J774.1 invasion assays.
J774.1 macrophage-like cells were cultured in DME-H21 with 10% FBS. Cells were plated in 24-well culture plates at 1.25 × 105 cells/well and incubated for 18 h at 37°C with 5% CO2. The J774.1 cells were washed once with DME-H21 with 10% FBS, 107 bacteria were used to inoculate each well, and the plate was incubated at 37°C with 5% CO2 for 2 h. Amikacin was added to the infected wells to a final concentration of 400 μg/ml, and the plates were incubated an additional 2 h at 37°C with 5% CO2. We have noted marked detachment of the J774.1 cells when infecting with ExoS- or ExoT-expressing bacteria; thus, removal of the antibiotic-containing medium from the wells prior to release of intracellular bacteria was performed as follows. Infected J774.1 cells were scraped off the bottom of the wells using a cell scraper, and the suspension was placed in an Eppendorf tube. The cells were microcentrifuged at 14,000 rpm in an Eppendorf 5417C centrifuge for 2 min, the supernatant was removed, and the pellet was resuspended in Ca2+- and Mg2+-free HBSS with 0.25% Triton X-100. Suspensions were returned to their original wells and incubated for 30 min at room temperature. Serial dilutions were plated for counts of internalized bacteria on LB agar. An aliquot of the lysed cell suspension from each well was assayed for total lactate dehydrogenase (LDH) activity by measuring pyruvate reduction as instructed by the manufacturer (Sigma, procedure 500). The total LDH activity per well was used as a measure of total J774.1 cells left in the well at the end of the assay. The number of internalized bacteria counted for each well was divided by the total LDH units measured for that well. These ratios were normalized to that obtained with PA103pscJ::Tn5 for non-plasmid-containing strains or normalized to that obtained with PA103ΔUΔT+pUCP20 for plasmid-containing strains. The average percentages of invasion and standard errors of the means are shown.
Fractionation of infected HeLa cells.
HeLa cells were plated at approximately 106 cells per 10-cm-diameter dish in DME-H21 with 10% FBS and incubated at 37°C in the presence of 5% CO2 for 36 h. On the day of the assay, an extra plate was trypsinized and a count of 4 × 106 cells per plate was obtained using a hemacytometer. Cells were washed once with MEM-etc. without albumin, and each plate was infected with 2 × 107 bacteria, a multiplicity of infection (MOI) of approximately 5. The infected cells were incubated for 1.5 h at 37°C in the presence of 5% CO2; this time point was chosen to minimize HeLa cell detachment due to the cell rounding effects of ExoT.
Fractionation of the infected HeLa cells into medium (supernatant and pellet) and cell (supernatant and pellet) was performed as described by Lee et al. (34, 35) with the following modifications. The medium in each infected dish was collected and centrifuged at 10,000 × g for 20 min. For the medium supernatant fraction, 1 ml of the supernatant was precipitated on ice for 30 min by the addition of 110 μl of trichloroacetic acid. Following microcentrifugation at 14,000 rpm in an Eppendorf 5417 centrifuge at 4°C for 15 min, the supernatant was removed and the pellet was washed with 1 ml of acetone. The samples were microcentrifuged at 12,000 rpm in an Eppendorf 5417 centrifuge at 4°C for 15 min, and the pellet was air dried and resuspended in 250 μl of 2× SDS-PAGE sample buffer. For the medium pellet fraction, the pellet from the initial medium spin was rinsed once with 2 ml of PBS containing Ca2+ and Mg2+ and centrifuged for 10 min at 10,000 × g. The final pellet was resuspended in 50 μl of 10% SDS and boiled for 5 min, and 200 μl of 2× sample buffer was added.
To fractionate the adherent HeLa cells into cytosolic lysate and membrane-bound bacterial pellet fractions, following removal of the medium, the infected monolayers were rinsed twice with 10 ml of cold PBS and lysed with 5 ml of PBS containing 1% Triton X-100, 50 mM EDTA, and 10 μg of aprotinin per ml. Cells were gently scraped off the dish and transferred to a chilled centrifuge tube. The tubes were incubated on ice for 20 min with vortexing for 15 s every 5 min and then centrifuged for 15 min at 32,500 × g at 4°C to separate into supernatant cell lysate and cell pellet fractions. Three milliliters of the supernatant was placed in a 30-ml Corex tube, the remaining supernatant was discarded, and the pellet was resuspended in 5 ml of 1% SDS in PBS. Three milliliters of the resuspended pellet was placed in a 30-ml Corex tube. Proteins from both the supernatant and the resuspended pellet fractions were precipitated at 25°C by the addition of 4 volumes of methanol (12 ml), 1 volume of chloroform, and 3 volumes of H2O (9 ml). Following centrifugation for 5 min at 12,000 × g, the top phase, but not the interface, was discarded and 9 ml of methanol was added to the lower phase. The tubes were then centrifuged for 5 min at 12,000 × g. The supernatants were removed, and the pellets were dried under nitrogen gas and resuspended in 250 μl of 2× SDS-PAGE sample buffer.
Fractionated proteins were electrophoresed on both SDS–8% and SDS–13% PAGE gels. Gels were transferred to nitrocellulose and immunoblotted as described above with rabbit polyclonal anti-ExoT antiserum for 8% gels and rabbit polyclonal anti-sigma E antiserum for 13% gels. The anti-sigma E antiserum was raised against the E. coli protein and was a generous gift of Carol Gross, UCSF Department of Microbiology and Immunology.
Animal experiments.
Bacteria were grown for 24 h in MINS medium (25 mM KH2O4, 95 mM NH4Cl, 50 mM monosodium glutamate, 110 mM disodium succinate, 10 mM trisodium nitrilotriacetic acid, 2.5% glycerol, 5 mM MgSO4, 18 μM FeSO4) or LB broth at 37°C with shaking and then washed and resuspended in PBS; the growth conditions of the bacteria do not affect the outcome of the virulence experiments (J. Engel, unpublished results). Equal numbers of bacteria of PA103 and PA103ΔT (5.0 × 106 CFU of each strain), PA103ΔU and PA103ΔUΔT (5.0 × 107 CFU of each strain), or PA103exoU::Tn5 and PA103ΔU/T(R149K) (5.0 × 107 CFU of each strain) were resuspended in a total of 50 μl of PBS and instilled into the nares of anesthetized 6- to 8-week-old female BALB/c mice. Twenty-four hours later, the animals were sacrificed, at which time the animals were near death. The right lobes of the lung and of the liver were removed under sterile conditions, placed in 1 ml of LB broth, and homogenized. Serial dilutions were plated onto LB agar plates containing no antibiotic or 50 μg of gentamicin per ml [PA103 and PA103ΔU carry no antibiotic resistance markers, whereas PA103ΔT and PA103ΔUΔT carry a Gmr gene that replaces part of the exoT gene; PA103ΔU/T(R149K) does not carry a resistance marker, whereas PA103exoU::Tn5 has a transposon carrying a Gmr gene inserted into the exoU gene]. A competitive index was calculated by obtaining the ratio of the number of cells of the ExoT mutant strain to that of the wild type (with respect to ExoT expression) recovered from the lung or liver and comparing it to the same ratio obtained with the infecting inoculum (roughly 1.0 but calculated precisely for each experiment). A competitive index greater than 1.0 indicates that the ExoT mutant strain colonized better than the wild type, and a competitive index less than 1.0 indicates that the ExoT mutant strain was less efficient than the wild type in colonization. All animal experiments were carried out in accord with the policies of the Committee on Animal Research at UCSF.
Statistical analysis.
Statistical analysis was performed using InStat 1.12 software.
RESULTS
ExoT is required for inhibition of internalization of PA103 by multiple cell types.
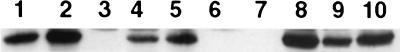
Because bacterial invasion is difficult to assess in a cytotoxic strain, the anti-invasive properties of ExoT were studied in an exoU mutant background. A strain containing an in-frame deletion of the exoU gene, PA103ΔU, was constructed as described in Materials and Methods. Reverse transcriptase-PCR analysis demonstrated that the mutation did not affect transcription of the downstream spcU gene (data not shown and reference 9). This mutant was then used to construct an exoU exoT double mutant (PA103ΔUΔT) (see Materials and Methods). Figure 1 demonstrates that ExoT secretion was not detected by immunoblot analysis of PA103ΔUΔT (Fig. 1, lane 3) but was restored by complementation with a vector carrying the exoT gene cloned from PA103 (strain PA103ΔUΔT+pExoT) (Fig. 1, lane 8).
FIG. 1.
Immunoblot analysis of secreted ExoT. Bacterial strains were cultured under the same conditions used for phenotypic assays (LB broth with 200 μg of carbenicillin per ml for plasmid-containing strains, standing for 18 h at 37°C). Proteins secreted into the culture medium were precipitated with ammonium sulfate, electrophoresed on SDS-polyacrylamide gels, transferred to nitrocellulose, and hybridized with rabbit anti-ExoT polyclonal antiserum. Goat anti-rabbit IgG horseradish peroxidase conjugate was used as a secondary antibody, and detection was performed using the ECL system. Lane 1, PA103; lane 2, PA103ΔU; lane 3, PA103ΔUΔT; lane 4, PA103ΔU/T(R149G); lane 5, PA103ΔU/T(R149K); lane 6, PA103pscJ::Tn5; lane 7, PA103ΔUΔT+pUCP20; lane 8, PA103ΔUΔT+pExoT; lane 9, PA103ΔUΔT+pExoT(R149G); lane 10, PA103ΔUΔT+pExoT(R149K).
The requirement for ExoT to inhibit invasion was tested in MDCK cells, which form polarized epithelial monolayers similar to the host tissues P. aeruginosa encounters in vivo. As seen in Fig. 2A, the ExoT-producing strain PA103ΔU is internalized approximately 10-fold less efficiently than the isogenic type III secretion mutant PA103pscJ::Tn5 (P < 0.0001, Student's two-tailed t test). The isogenic mutant PA103ΔUΔT, which is still competent for type III secretion, showed an increase in invasion of more than 15-fold that of PA103ΔU (P < 0.0001, Student's two-tailed t test). This finding suggests that the failure to deliver ExoT to the host cell is responsible for the increased internalization observed in a type III secretion mutant of PA103. Internalization of PA103ΔUΔT also reproducibly surpassed that of PA103pscJ::Tn5 (P < 0.001, Student's two-tailed t test). The significance of this finding is unclear, but it suggests that there may be other type III secretion-dependent factors that promote internalization.
FIG. 2.
ExoT inhibits internalization of P. aeruginosa by MDCK cells. Polarized monolayers of MDCK cells were infected apically at an MOI of 10 to 20 with the isogenic mutants of P. aeruginosa strain PA103 shown. (A) For strains containing no episomal vectors, the number of internalized bacteria for each well was normalized to the average number of internalized PA103pscJ::Tn5 bacteria. The normalized values were averaged to calculate the percentages of invasion, and the standard errors of the means for 17 to 18 experimental points are shown. The actual average numbers of internalized bacteria per well for PA103pscJ::Tn5 and standard errors of the means for the three experimental rounds shown were 691.67 ± 64.16, 955.00 ± 134.46, and 591.83 ± 119.14. (B) For strains containing episomal vectors, the number of internalized bacteria for each well was normalized to the average number of internalized PA103ΔUΔT+pUCP20 bacteria. The normalized values were averaged to calculate the percentages of invasion, and the standard errors of the means for 10 to 12 experimental points are shown. The actual average numbers of internalized bacteria per well for PA103ΔUΔT+pUCP20 and standard errors of the means for the two experimental rounds shown were 1,911.67 ± 484.49 and 3,873.33 ± 292.64.
The ability of ExoT expressed from an episome to restore anti-internalization activity is shown in Fig. 2B. For these experiments, internalization is normalized to PA103ΔUΔT harboring the control vector (PA103ΔUΔT+pUCP20) for the following reason. Under the conditions that we established to maximize internalization efficiency (growth of bacteria overnight in LB broth without shaking), carbenicillin was required for stable maintenance of the plasmids but resulted in filamentous growth of the bacteria (see Fig. 5H, J, L, N, and P). In contrast, bacteria without episomal vectors were grown in the absence of carbenicillin (all the chromosomal mutants) (Fig. 2A) and exhibited very little filamentous growth (see Fig. 5D and F). No difference in growth rates of any of the plasmid-containing strains was noted under the conditions of the invasion assay (growth in MEM-etc. for 4 h) (data not shown).
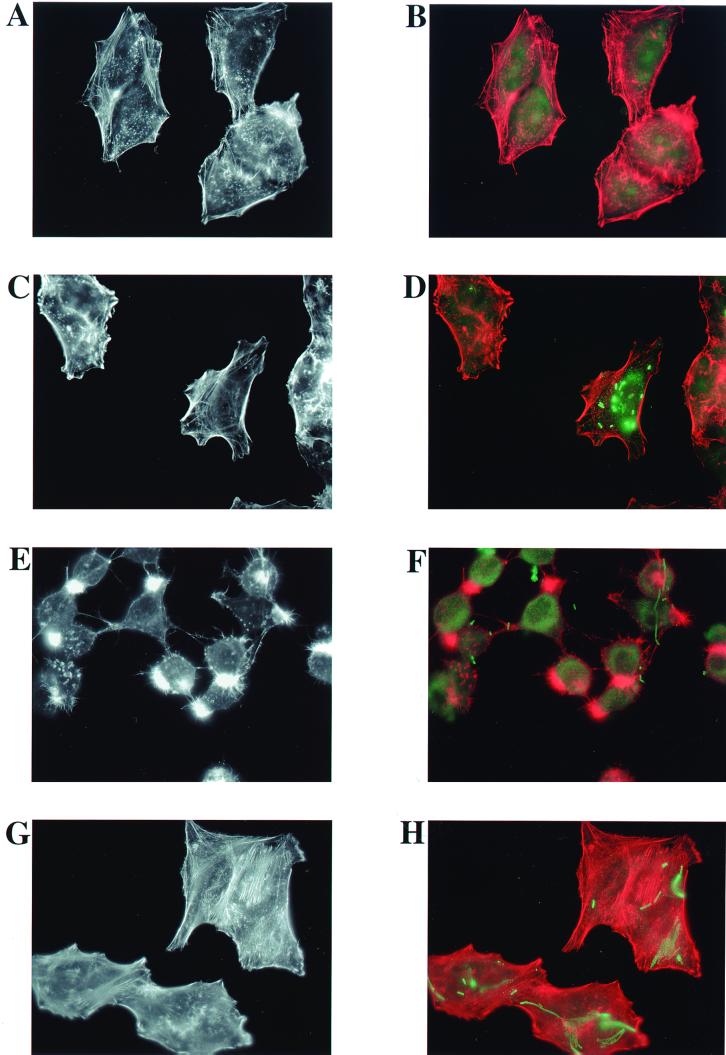
FIG. 5.
The conserved residue R149 contributes to the ability of ExoT to disrupt the actin cytoskeletons of infected cells. HeLa cells were infected with isogenic strains of P. aeruginosa at an MOI of 25 for 6 h. (A and B) No bacteria; (C and D) PA103pscJ::Tn5; (E and F) PA103ΔU+pUCP20; (G and H) PA103ΔUΔT+pUCP20; (I and J) PA103ΔUΔT+pExoS; (K and L) PA103ΔUΔT+pExoT; (M and N) PA103ΔUΔT+pExoT(R149G); (O and P) PA103ΔUΔT+pExoT(R149K). Infected cells were fixed, permeabilized, and stained with Texas Red-labeled phalloidin to visualize actin (red). In addition, the fixed and permeabilized cells were labeled with rabbit anti-ExoU antiserum and a secondary goat anti-rabbit–fluorescein isothiocyanate antibody to visualize P. aeruginosa (green). Note that although the bacterial strains shown do not express ExoU, the anti-ExoU antiserum contains additional antibodies, likely to lipopolysaccharide, which result in sufficient labeling of the bacteria. Labeled cells were observed by fluorescence microscopy, and the images shown were captured at a ×1,000 magnification with a CCD camera and SPOT imaging software. (A, C, E, G, I, K, M, and O) Actin staining only; (B, D, F, H, J, L, N, and P) merged image of both actin (red) and bacterial (green) staining.
As seen in Fig. 2B, expression of ExoT from a plasmid (strain PA103ΔUΔT+pExoT) was sufficient to restore anti-internalization activity; invasion was decreased nearly 20-fold (P < 0.0001, Student's two-tailed t test) compared to that of PA103ΔUΔT carrying the empty expression plasmid pUCP20. These results confirm that the mutation of ExoT, not a polar effect on a downstream gene, is responsible for the increase in internalization seen in the PA103ΔUΔT mutant. A possible explanation for an apparent decrease in the numbers of internalized bacteria is the detachment or death of infected MDCK cells. To evaluate this possibility, cell loss was assessed by fluorescence microscopy examination of infected monolayers that were fixed, permeabilized, and stained for actin with Texas Red-conjugated phalloidin and for nuclei with DAPI (4′,6′-diamidino-2-phenylindole). Quantitation of cellular LDH release into the medium during infection was used as a measure of cytotoxicity. Virtually no increase in cytotoxicity or cell loss that could account for differences in numbers of internalized bacteria was observed during MDCK cell infections with any of our mutant strains (data not shown).
Consistent with a previous report (6), expression of ExoS also abrogated internalization (ninefold, P < 0.0001, Student's two-tailed t test), although not as efficiently as ExoT did in PA103ΔUΔT (Fig. 2B). Whether ExoS has inherently less anti-internalization activity or if simply less of it was translocated into the MDCK cells cannot be distinguished in these experiments. Interestingly, expression of ExoS carried on an episome in PA103ΔU resulted in a small but reproducible stimulation of invasion (Fig. 2B), raising the possibility that its expression may antagonize the endogenous ExoT anti-internalization activity.
ExoT inhibits bacterial internalization by macrophages.
In addition to contacting epithelial cells, P. aeruginosa is likely to interact with macrophages in vivo (31, 43). The ability of the bacterium to prevent its internalization by professional phagocytes, thus avoiding destruction and antigen presentation, likely provides a survival benefit and enhances pathogenesis. Bacterial uptake into macrophages may occur by pathways alternate or additional to those employed by epithelial cells. We tested the ability of ExoT to inhibit internalization of P. aeruginosa by the J774.1 macrophage-like cell line. Preliminary experiments demonstrated that ExoS and ExoT cause rounding and detachment of J774.1 cells. J774.1 cells are also susceptible to type III secretion-dependent apoptotis-like damage (22), potentially confounding quantitation of bacterial internalization. To correct for this, following cocultivation of the bacteria with the adherent cells, the medium was spun to collect any detached cells. The cell pellet was combined with the remaining adherent cells prior to lysis of the cells to release internalized bacteria. In addition, the amount of internalized bacteria calculated for each well was normalized to the total number of J774.1 cells in that well as measured by total LDH activity released during the lysis step.
Figure 3A demonstrates that PA103ΔUΔT uptake by J774.1 cells was enhanced compared to the uptake of the isogenic ExoT-producing strain PA103ΔU. The increase in internalization in this case was approximately sixfold (P < 0.0001, Student's two-tailed t test) and, in contrast to the results with MDCK cells, closely matched that of the type III secretion mutant PA103pscJ::Tn5. Inhibition of internalization was restored by the expression of ExoT from an episome carried by PA103ΔUΔT (strain PA103ΔUΔT+pExoT) (Fig. 3B). However, the difference in internalization between PA103ΔU and PA103ΔUΔT, each carrying a control vector (PA103ΔU+pUCP20 and PA103ΔUΔT+pUCP20) and grown in the presence of carbenicillin, was only twofold (Fig. 3B) (P < 0.0001, Student's two-tailed t test).
FIG. 3.
ExoT inhibits internalization of P. aeruginosa by J774.1 macrophage-like cells. J774.1 cells were infected with isogenic mutants of P. aeruginosa strain PA103, shown at an MOI of 40 to 60. For each infected well, the number of internalized bacteria was divided by the value for total LDH activity released upon lysis of the cells in that well to normalize for J774.1 cell detachment from the culture plate during infection (internalized CFU/LDH). Averages ± the standard errors of the means for five to six experimental points are shown. (A) For strains containing no episomal vectors, the internalized CFU/LDH value for each well was normalized to the average internalized CFU/LDH value for PA103pscJ::Tn5 in experiments performed with J774.1 cells of the same passage. The normalized CFU/LDH values were averaged to calculate the percentages of invasion, and the standard errors of the means are shown. The actual average number of internalized CFU per well ± the standard error of the mean for PA103pscJ::Tn5 in the representative experimental results shown was 3.87 × 106 ± 4.44 × 104, and the average LDH units per well was 9.05 × 104 ± 3.91 × 103, yielding an average internalized CFU/LDH of 43.1 ± 1.35. (B) For strains containing episomal vectors, the internalized CFU/LDH for each well was normalized to the average internalized CFU/LDH for PA103ΔUΔT+pUCP20. The actual average number of internalized CFU per well ± the standard error of the mean for PA103ΔUΔT+pUCP20 in the representative experimental results shown was 1.26 × 106 ± 6.91 × 104, and the average value for LDH units per well was 4.96 × 104 ± 2.33 × 103, yielding an average internalized CFU/LDH and standard error of the mean of 25.54 ± 1.65.
Unlike our results with epithelial cells, expression of ExoS from an episome in PA103ΔUΔT was at least as efficient as that of ExoT at preventing internalization into J774.1 cells (PA103ΔUΔT+pExoS) (Fig. 3B). ExoS did not appear to antagonize the endogenous ExoT anti-internalization activity of PA103ΔU in macrophage infections (PA103ΔU+pExoS) (Fig. 3B). This may reflect uptake by alternate pathways by macrophages, although control experiments demonstrated that the presence or absence of serum during the J774.1 macrophage invasion assays did not affect either the absolute levels or the overall efficiency of bacterial internalization (data not shown).
The arginine finger domain of ExoT is important for anti-internalization activity.
The N-terminal region of ExoT is homologous to those of ExoS, YopE, and SptP (16). The region of homology lies in a motif, GXXRXSG, characteristic of and required for GTPase activity in GAPs. In particular, the invariant arginine residue is critical to GAP activity (54). ExoS, YopE, and SptP have demonstrable GAP activities which require the invariant arginine residue in biochemical assays utilizing purified substrates in vitro (3, 16, 50). Mutation of the conserved arginine in SptP abolishes the ability of S. enterica serovar Typhimurium to restore the normal appearance of the actin cytoskeletons of epithelial cells following infection (16). We hypothesized that ExoT might also exert effects on the host cell actin cytoskeleton through GAP activity. In accordance with our hypothesis, it has been recently reported that a truncated version of ExoT (amino acids 78 to 237) possesses GAP activity toward RhoA, Rac1, and CDC42 in vitro (32).
The conserved arginine residue of ExoT was changed to a glycine or lysine and used to replace the wild-type exoT gene in PA103ΔU by allelic exchange to generate strain PA103ΔU/T(R149G) or PA103ΔU/T(R149K), respectively. As shown in Fig. 2A, strains PA103ΔU/T(R149G) and PA103ΔU/T(R149K) were internalized by MDCK cells at a level intermediate to that observed with PA103ΔUΔT and PA103ΔU (twofold decrease compared to the level observed with PA103ΔUΔT; P < 0.002, Student's two-tailed t test). Complementation of PA103ΔUΔT with ExoT(R149G) or ExoT(R149K) expressed from a plasmid likewise resulted in only partial restoration of anti-internalization activity in MDCK cells as shown in Fig. 2B (threefold compared to sixfold with pExoT; P < 0.01 compared to results with PA103ΔUΔT+pExoT, by Student's two-tailed t test). Similar trends were observed for internalization of the arginine finger mutants into J774.1 cells. As seen in Fig. 3A, PA103ΔU/T(R149G) and PA103ΔU/T(R149K) were internalized half as much as PA103ΔUΔT (P < 0.06, Student's two-tailed t test) and approximately threefold more than the ExoT-producing strain PA103ΔU (P < 0.0002, Student's two-tailed t test) (Fig. 3A). Western blot analysis showed that ExoT(R149G) and ExoT(R149K) were secreted into the growth medium, though at slightly lower levels than that of wild-type ExoT (Fig. 1, lanes 4 and 5, compared to wild-type level in lane 1). As shown in a later section, mutation of the invariant arginine residue did not decrease the translocation of ExoT into eukaryotic cells. Our results suggest that the arginine finger domain of ExoT is essential for full anti-internalization activity but that other residues or other type III secreted proteins may also contribute. The important function of the arginine finger motif further implies that the GAP activity of ExoT is likely to be involved in the ability to inhibit internalization of P. aeruginosa by epithelial cells and macrophages.
The arginine finger domain of ExoT is required for cell rounding and cytoskeleton disruption.
Previous reports have implicated both ExoS and ExoT in cell rounding and in the disruption of the actin cytoskeleton in CHO cells; these biological effects might be predicted in a protein with GAP activity towards the small GTPases Rho, Rac, and/or Cdc42. PA103ΔUΔT expressing either ExoT or ExoS caused cell rounding when it was incubated with CHO cells (48). Similarly, transfection of ExoS into CHO cells altered the cytoskeletal architecture (37).
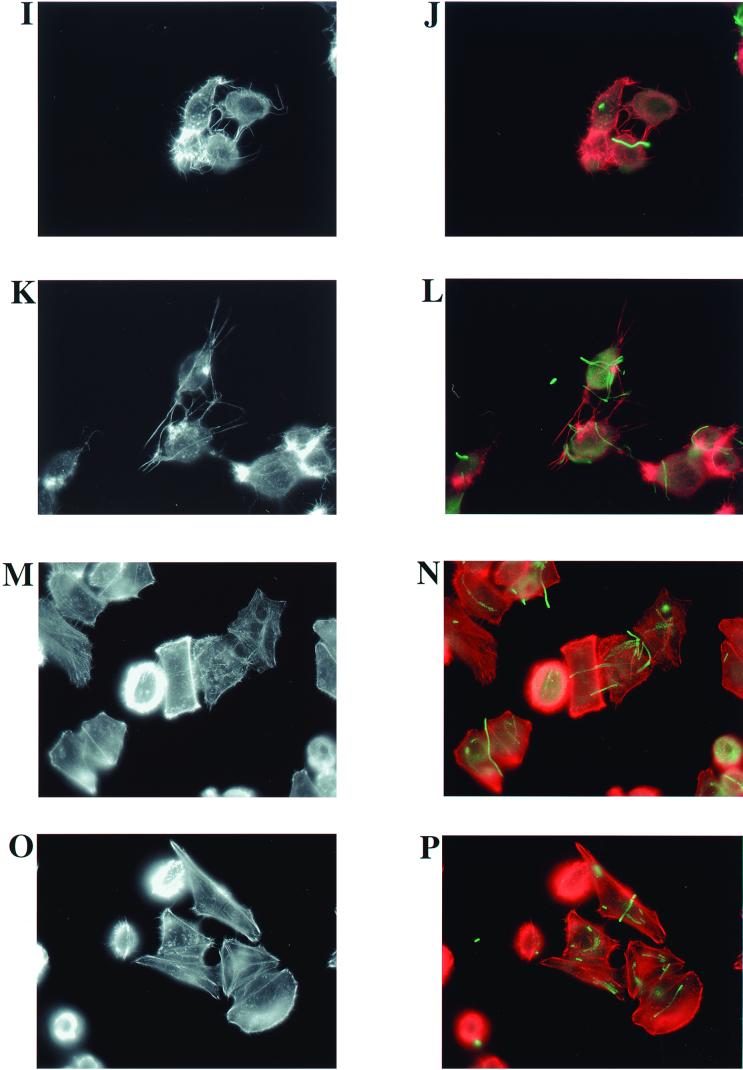
We tested the role of the arginine finger domain of ExoT in cell rounding. HeLa cells were chosen for this assay because polarized MDCK cells are relatively resistant to the rounding effects of ExoS and ExoT (data not shown). Rounding in J774.1 cells is difficult to assess due to their exquisite susceptibility to the additional type III secretion-dependent (but ExoU- and ExoT-independent) apoptosis-like cytotoxicity (reference 22 and data not shown). When incubated with HeLa cells for 6 h, PA103ΔU, PA103ΔUΔT+pExoT, and PA103ΔUΔT+pExoS all caused dramatic rounding of the cells and left vacant areas where cells had clearly detached from the culture plate (Fig. 4C, E, and F). Little cell rounding or detachment was observed with PA103ΔUΔT containing the vector alone (Fig. 4D), and this appearance was similar to that of uninfected HeLa cells (Fig. 4A) or cells cocultivated with PA103pscJ::Tn5 (Fig. 4B). Infection with PA103ΔUΔT+pExoT(R149G) or PA103ΔUΔT+pExoT(R149K) resulted in intermediate amounts of cell rounding (Fig. 4G and H). Similar results were observed with strains in which the ExoT R149 mutations were introduced into the chromosome by allelic exchange [PA103ΔU/T(R149K) and PA103ΔU/T(R149G); data not shown]. For all ExoT- and ExoS-producing strains, changes could be observed as early as 2 h but became more pronounced with longer infection times.
FIG. 4.
The conserved residue R149 contributes to the cell rounding activity of ExoT. HeLa cells were infected with isogenic strains of P. aeruginosa at an MOI of 25 for 6 h. (A) No bacteria; (B) PA103pscJ::Tn5; (C) PA103ΔU+pUCP20; (D) PA103ΔUΔT+pUCP20; (E) PA103ΔUΔT+pExoS; (F) PA103ΔUΔT+pExoT; (G) PA103ΔUΔT+pExoT(R149G); (H) PA103ΔUΔT+pExoT(R149K). Infected cells were observed by phase-contrast microscopy. The larger images were captured at a ×100 magnification, and the inset images were captured at a ×200 magnification using a CCD camera and SPOT imaging software.
Examination of the actin cytoskeleton of infected HeLa cells by phalloidin staining revealed dramatic changes that were dependent on the presence of wild-type ExoT or ExoS (Fig. 5). Figure 5C shows that the pattern of actin staining in HeLa cells infected for 6 h with a type III secretion mutant (PA103pscJ::Tn5) looked indistinguishable from that of the uninfected cells in Fig. 5A. The cells appear flat, with actin stress fibers and punctate regions, suggestive of focal adhesions, prominently revealed by phalloidin staining. As seen in Fig. 5D, where both the actin and bacterial staining are merged, numerous bacteria appear to be associated with the PA103pscJ::Tn5-infected cells. The location of the associated bacteria suggests that they are intracellular, although we note that our staining protocol does not differentiate between intracellular and extracellular bacteria. Figure 5E demonstrates that the ExoT-secreting, but noncytotoxic, strain PA103ΔU caused dramatic cell rounding and polar condensation of intracellular actin. The prominent phalloidin-staining protrusions extending from the HeLa cells may represent remaining points of contact with the substratum as the cells begin to round up and detach. Figure 5F shows that many of the PA103ΔU bacteria associated with the HeLa cells are clearly extracellular, as is predicted from a strain producing ExoT. Interestingly, the foci of actin condensation in the cells do not appear to correlate with the location of associated bacteria. HeLa cells cocultivated with PA103ΔUΔT harboring the vector alone (Fig. 5G) had an appearance similar to that of uninfected (Fig. 5A) and PA103pscJ::Tn5-infected cells (Fig. 5C). Again, we could see numerous bacteria that appeared to be intracellular (Fig. 5H). PA103ΔUΔT expressing ExoS (Fig. 5I) or ExoT (Fig. 5K) from a plasmid resulted in cell rounding, actin condensation, and numerous actin-containing protrusions from the cells. Most of the bacteria appeared to be extracellular (Fig. 5J and L). Figure 5M and O demonstrate that the arginine finger of ExoT is required for full disruption of the actin cytoskeleton. The central four cells of Fig. 5M especially illustrate the intermediate phenotypes observed during infections with PA103ΔUΔT expressing the mutant protein ExoT(R149G). Some of the cells appear minimally affected, with stress fibers readily apparent. In other cells, stress fibers are no longer discernable, peripheral actin staining is more prominent, and some of the cells are rounding up with small spicule-like actin-containing projections. Very few cells infected with strains expressing ExoT(R149G) show the polar collapsed actin structures or exaggerated actin spicules seen with infection by strains expressing wild-type ExoS or ExoT. The bacterial staining suggests that some organisms are extracellular, but others appear to be internalized (Fig. 5N). Similar results were observed in infections with PA103ΔUΔT+pExoT(R149K) (Fig. 5O and P).
Together with the results of the quantitative invasion assays with MDCK and J774.1 cells, these results suggest that the arginine finger domain of ExoT contributes to its ability to both alter the host cell actin cytoskeleton and inhibit bacterial internalization. As mutation of the conserved arginine residue results in loss of GAP function in vivo (B. Kazmierczak and J. Engel, unpublished results), these findings suggest that a second region of ExoT also plays a role in disrupting the actin cytoskeleton and preventing bacterial internalization.
ExoT is translocated into the host cell cytoplasm.
ExoT protein release into the culture medium is known to be type III secretion dependent (48, 52, 53); however, translocation of the protein into the cytoplasm of host cells has not yet been demonstrated. One explanation for the partial loss of the anti-internalization and cell rounding ability of the ExoT R149 mutants is that the mutation causes a defect in translocation to the host cell cytoplasm. In order to test this possibility and to demonstrate translocation of wild-type ExoT, cellular fractionation of infected HeLa cells followed by Western blot analysis was performed.
In preliminary experiments, we were unable to detect translocation of chromosomally encoded ExoT (native or hemagglutinin tagged, using either a polyclonal anti-ExoT antiserum or an anti-HA antiserum, respectively [unpublished results]). Instead, HeLa cells were infected with PA103ΔUΔT+pUCP20 (empty expression plasmid), PA103ΔUΔT+pExoT, PA103ΔUΔT+pExoT(R149G), or PA103ΔUΔT+pExoT (R149K) at an MOI of 5. After incubation for 1.5 h at 37°C in the presence of 5% CO2, infected cells were observed with an inverted microscope and rounding and detachment due to ExoT expression were determined to be at a minimum. The medium was removed and separated into a supernatant fraction, containing any soluble secreted proteins, and a pellet fraction containing nonadherent bacteria. The adherent HeLa cells were washed and lysed to obtain a cell lysate fraction, consisting of the cytoplasm, and a cell pellet, consisting of cell membranes and adherent or internalized bacteria. Equivalent portions of each fraction were precipitated, electrophoresed by SDS-PAGE, and electrotransferred to nitrocellulose membranes, and ExoT was detected by Western blotting as described in Materials and Methods.
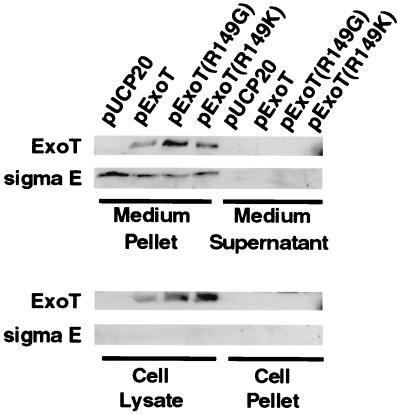
ExoT was clearly present in the cell lysate fraction of HeLa cells infected with PA103ΔUΔT+pExoT, indicating that the protein was translocated across the cell membrane (Fig. 6). ExoT was also detectable in the medium pellet fraction that is expected to contain nonadherent bacteria but was not visible in the medium supernatant or cell pellet. In other experiments with longer infection times, ExoT could also be detected in the medium supernatant and cell pellet fractions (data not shown). As expected, no ExoT protein was detected in any samples from HeLa cells infected with PA103ΔUΔT carrying the vector alone (pUCP20). To evaluate the possibility that the ExoT present in the cell lysate fraction of cells infected with PA103ΔUΔT+pExoT derives from lysis of internalized bacteria rather than from translocation, Western blot analysis was performed on the same samples using an antibody to the bacterial cytosolic protein sigma E. This transcription factor is not secreted and therefore serves as a marker for the presence of bacterial intracellular proteins. As expected, both ExoT and sigma E could be detected in the medium pellet fraction, which contains nonadherent bacteria (Fig. 6). In contrast, sigma E was not detectable in the cell lysate fraction from infection with PA103ΔUΔT+pExoT, yet the amount of ExoT detected in the cell lysate fraction was roughly equivalent to that seen in the medium pellet fraction. These results suggest that the ExoT protein present in the cell lysate fraction was delivered by translocation into the HeLa cell cytoplasm and not simply derived from solubilization of internalized bacteria.
FIG. 6.
ExoT is translocated into the host cell cytoplasm. HeLa cells were infected at an MOI of 5 with PA103ΔUΔT carrying the plasmids shown. After infection for 1.5 h, the medium was removed and separated into supernatant (soluble proteins) and pellet (nonadherent bacteria) fractions. The adherent HeLa cells were lysed and separated into lysate (cytoplasm) and pellet (cell membranes and adherent or internalized bacteria) fractions. Proteins from all fractions were resuspended in SDS-PAGE sample buffer, and 40 μl of each sample (representing 16% of each fraction) was electrophoresed on SDS–8% and SDS–13% polyacrylamide gels. Proteins were electrotransferred to nitrocellulose and hybridized with rabbit polyclonal anti-ExoT antiserum (for 8% gels) or rabbit polyclonal anti-sigma E antiserum (for 13% gels). Goat anti-rabbit IgG–horseradish peroxidase conjugate was used as a secondary antibody, and detection was performed using the ECL system.
Mutation of the R149 residue of ExoT does not prevent translocation of the protein into the host cell cytoplasm (Fig. 6). ExoT could be detected in the medium pellet and cell lysate fractions of HeLa cells infected with PA103ΔUΔT+pExoT(R149G) or PA103ΔUΔT+pExoT(R149K). As with infection with PA103ΔUΔT+pExoT, sigma E could be detected in the medium pellet fractions but not in the cell lysate fractions of infections with bacteria expressing the R149 mutant proteins. Thus, the ExoT detected in the cell lysate fractions was not due to lysis of internalized bacteria. These results demonstrate that mutation of arginine 149 of ExoT does not interfere with translocation of the protein into the host cell cytoplasm.
ExoT is required for full virulence in an animal model of acute pneumonia.
The role of P. aeruginosa internalization in the pathogenesis of disease is unclear. P. aeruginosa strains that are invasive, but not cytotoxic, can cause disease (11). In some cell lines, there is evidence that the cystic fibrosis transmembrane receptor mediates internalization (39). It has been suggested that the loss of internalization by airway epithelial cells contributes to the chronic colonization and recurrent acute pneumonias seen in cystic fibrosis patients and that internalization followed by epithelial cell sloughing is a host defense mechanism (39). The generation of isogenic ExoT mutant strains of wild-type PA103 and PA103ΔU afforded the opportunity to test the role of ExoT in virulence in a mouse model of acute pneumonia.
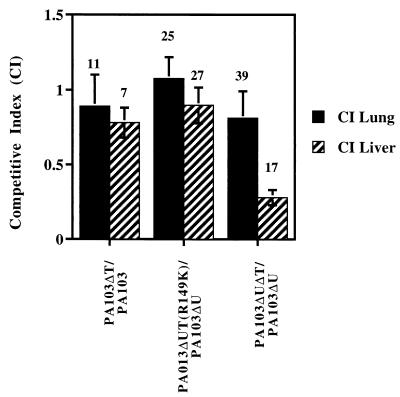
No significant difference in rates of animal death was observed with inocula near the 50% lethal dose of PA103 compared to results with PA103ΔT or with PA103ΔU compared to results with PA103ΔUΔT (data not shown). Therefore, we used the more sensitive competitive index assay that compares the abilities of the various strains to colonize the lung and liver when the animal is coinfected (47) (Materials and Methods). As seen in Fig. 7, the competitive indexes for PA103 and PA103ΔT in both the lung and liver were approximately 0.89 and 0.78 (P = 0.7, Student's two-tailed t test), respectively; thus, these strains colonize the lung and liver with approximately equal efficiencies. In the comparison of PA103ΔU with PA103ΔUΔT, the strains colonized the lung with a competitive index of 0.81, but the competitive index in the liver was 0.28 (P = 0.06, Student's two-tailed t test). Thus, PA103ΔUΔT is defective in its ability to spread to distant organs compared to PA103ΔU. There was no difference in the ability of PA103ΔUΔT(R149K) to colonize the lung or liver compared to that of PA103exoU::Tn5; this is not necessarily surprising since the arginine finger mutant showed residual anti-internalization and cell rounding activity in vitro. We note that the competitive index experiments do not distinguish between the ability of the bacteria to reach the liver and their ability to proliferate within the liver.
FIG. 7.
ExoT is required for full virulence in an animal model of acute pneumonia. Equal amounts of the indicated pairs of strains of bacteria were inoculated into the nares of mice. The lungs and livers of the mice were harvested 24 h later and homogenized, and serial dilutions were plated on selective medium to obtain counts of each bacterial strain. A competitive index was calculated by obtaining the ratio of the ExoT mutant strain counts to wild-type (with respect to ExoT expression) bacterial counts recovered from the lung or liver and comparing it to the same ratio obtained with the infecting inoculum (roughly 1.0 but calculated precisely for each experiment). A competitive index greater than 1.0 indicates that the ExoT mutant strain colonized better than the wild type, and a competitive index less than 1.0 indicates that the ExoT mutant strain was less efficient than the wild type in colonization. The number of animals used for each experiment is shown. Compared to PA103ΔU, PA103ΔUΔT was significantly impaired in its ability to colonize the liver (P = 0.06, Student's two-tailed t test).
DISCUSSION
Like many other gram-negative pathogens, P. aeruginosa can regulate its uptake into host cells. The work presented here, in conjunction with the results of Cowell et al. (6), conclusively identifies ExoT as the anti-internalization factor that can inhibit the internalization of P. aeruginosa by multiple cell types, including polarized and nonpolarized epithelial cells and tissue culture macrophages. Interestingly, of the pathogens examined so far, each one utilizes a unique approach to modulating its internalization. The S. enterica serovar Typhimurium SptP protein is a GAP for Cdc42 and Rac (16), while a type III secreted protein of EPEC may result in the dephosphorylation of one or more host tyrosine-phosphorylated proteins (18). As discussed below, ExoT may function as a Rho GAP to inhibit bacterial internalization.
An additional and intimately related activity of ExoT revealed by these experiments and others (48) is its ability to induce rounding and detachment of some but not all eukaryotic cell types in vitro. Phalloidin staining of HeLa cells incubated with isogenic ExoT mutants reveals that ExoT induces cell rounding, loss of stress fibers, the appearance of peripheral areas of condensed actin, and residual needle-like extensions that may reflect prior sites of host cell-extracellular matrix contact. The actin condensation is polar but does not appear in close proximity to the adherent bacteria. The pathway of actin condensation, the identification of other proteins in the structure, and the cause for the polar localization of the collapsed actin are important biological questions to pursue. In addition, the reason why some cell types are resistant to the cell rounding effects but sensitive to the anti-internalization activity of ExoT, such as polarized MDCK cells, remains to be explored. It is intriguing to speculate that this resistance reflects known differences between cell types in the regulation of cortical actin that contacts tight junctions in polarized epithelium (7).
Our work further suggests a mechanism for the capacity of ExoT to both inhibit bacterial internalization and modify the host cell cytoskeleton. As noted previously, the N-terminal portion of ExoT has an arginine finger domain suggestive of GAP activity. Furthermore, it has recently been shown that the N-terminal portion of ExoT can act as a GAP for Rho, Rac1, and CDC42 in vitro (32). We now demonstrate that the arginine finger domain, and thus GAP activity, is critical to the function(s) of ExoT. Mutation of the conserved arginine to a glycine or lysine partially diminished the anti-internalization activity of ExoT-producing bacteria. Consistent with this partial loss of function, less cell rounding and far less disruption of the actin cytoskeleton were observed in infections with strains expressing the arginine mutant ExoT proteins than in infections with the strain expressing wild-type ExoT.
There are several possible explanations for the residual anti-internalization activity, induction of cell rounding, and actin cytoskeleton rearrangements observed for the ExoT(R149G) or ExoT(R149K) mutant protein. The mutations may simply interfere with the secretion and/or translocation of ExoT into eukaryotic cells. However, immunoblot analysis demonstrates that plasmid-expressed R149 mutant proteins and wild-type ExoT are secreted when the bacteria are grown under inducing conditions in the absence of eukaryotic cells (Fig. 1). Fractionation of infected HeLa cells demonstrated that the R149 mutant ExoT proteins were translocated into the host cell cytoplasm as efficiently as wild-type ExoT (Fig. 6). Moreover, we have found that when the R149 mutant ExoT proteins are introduced directly into HeLa cells by transient transfection, only minimal cell rounding and actin cytoskeleton disruption are observed and GAP activity is lost (B. Kazmierczak, unpublished data). Similar mutations introduced at the conserved arginine residue of the closely related proteins ExoS and Yersinia YopE or the Salmonella homolog SptP result in loss of GAP activity in vitro (3, 16, 17, 50). Together, these findings suggest that the arginine finger motif is critical to ExoT function but that other domains of ExoT or other type III secreted proteins also contribute to inhibition of internalization and modulation of the actin cytoskeleton. If other type III secreted proteins contribute to this process, they cannot be ExoS or ExoY, as PA103 does not synthesize these proteins. Experiments to test these hypotheses are in progress.
Our demonstration that the putative GAP domain of ExoT is important to its function is consistent with recent investigations into the pathway by which PA103 enters epithelial cells. We have found that P. aeruginosa internalization is likely regulated by small GTPases, as it is inhibited by Clostridium difficile toxin B (B. I. Kazmierczak, K. Mostov, and J. Engel, unpublished data). This bacterial toxin inhibits the Rho, Rac, and CDC42 families of small GTPases that have been shown to effect actin cytoskeleton rearrangements (28). Our work further suggests that Rho is sufficient to stimulate P. aeruginosa internalization but that additional toxin B-sensitive GTPases may also be activated upon P. aeruginosa entry (30). We hypothesize that the target of ExoT GAP activity is one or more toxin B-sensitive GTPases whose activities are required for P. aeruginosa internalization. Support for this notion comes from the observation by others that ExoT exhibits GAP activity towards RhoA, Rac, and CDC42 in vitro (32). Identification of the exact intracellular target(s) of the ExoT anti-internalization factor may provide additional information about the process of internalization itself.
As previously noted by others (6), an interesting paradox is observed in naturally occurring strains that secrete both ExoS and ExoT. While most or all clinical isolates appear to encode the ExoT gene, only a subset of clinical isolates harbor the ExoS gene (13). Such strains thus potentially produce two anti-internalization factors, and yet they appear much more invasive than strains that produce only ExoT, even when the gene encoding the cytotoxin ExoU is inactivated in the ExoT-secreting strains. For example, both the ExoS and ExoT genes encoded by strain 388 confer anti-internalization activity and cause epithelial cell rounding when they are expressed in PA103ΔUΔT (48). Yet strain 388 is highly invasive despite having the ability to secrete both proteins in vitro (52) and to at least translocate ExoS into eukaryotic cells (49) (evidence for translocation of ExoT by strain 388 has not been reported). One possible explanation for these observations is that ExoS can antagonize the activity of ExoT. In fact, this possibility is suggested by our finding that expression of ExoS derived from 388 can antagonize the anti-internalization activity of ExoT in the PA103ΔU+pExoS strain. However, this hypothesis might also predict that deletion of either ExoS or ExoT in the 388 strain background should result in decreased internalization, but this was not observed (L. Garrity-Ryan, unpublished results). Alternatively, ExoS and/or ExoT may be differentially regulated or modified depending upon the strain background. Finally, 388 may be internalized by a different pathway than that of PA103, and this alternate uptake mechanism may be ExoT insensitive.
Why does P. aeruginosa inhibit its internalization into epithelial cells and macrophages? It is likely beneficial to this pathogen to avoid phagocytosis by macrophages, a process that would otherwise result in bacterial destruction and presentation to the immune system. In addition, it has been suggested that epithelial cells that ingest P. aeruginosa are sloughed, thus facilitating bacterial clearance (40). It is equally valid to raise the question of why at least some strains of P. aeruginosa maintain the ability to induce their uptake by nonphagocytic cells such as epithelial cells. Perhaps, as has been suggested for Yersinia species (10), the ability to migrate across an epithelial cell layer by internalization may be important at early stages of infection. Alternatively, invasion of other types of cells may be important in the natural habitat of P. aeruginosa, a soil and waterborne pathogen. Finally, invasive strains of P. aeruginosa are nonetheless capable of causing disease in humans and in animals (44).
Our experiments using a mouse model of acute pneumonia suggest that ExoT plays a role in the pathogenesis of disease caused by strain PA103. Although the PA103ΔUΔT double mutant was as proficient as the PA103ΔU mutant in colonization and proliferation in the lungs, the double mutant was less successful at spreading to distant organs, as manifested by lower colony counts in the liver. This may reflect increased clearance by macrophages. More recently, we have discovered that P. aeruginosa can inhibit wound healing in an ExoT-dependent manner (16a). The decreased ability of an ExoT mutant to antagonize wound healing may prevent its dissemination to distant organs. These possibilities are not mutually exclusive, and the production of ExoT may contribute to the disease process in multiple ways, thus partially explaining the devastating consequences of P. aeruginosa infections once the organism gains access to injured epithelium.
ACKNOWLEDGMENTS
We thank Keith Mostov and Eric Brown and members of the Engel lab for helpful discussions. We thank Carol Gross and Joyce West for providing anti-sigma E antiserum and assistance in immunoblot experiments. We especially acknowledge the expert technical help of Lam Nguyen in the cloning and animal experiments.
This work was supported by grants from the NIH (AI R01 AI42806 to J.N.E., K08 AI01636 to B.K., and K08 AI001524 to A.H.), the American Lung Association (J.N.E.), and the Bank of America Giannini Foundation (L.G.-R. and J.C.). J.N.E. is an established investigator of the American Lung Association.
REFERENCES
- 1.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K, Wiener-Kronish J. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorn M J, Pavlovskis O R, Thompson M R, Iglewski B H. Production of exoenzyme S during Pseudomonas aeruginosa infection in burned mice. Infect Immun. 1979;24:837–842. doi: 10.1128/iai.24.3.837-842.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black D B, Bliska J B. The RhoGAP activity of the Yersinia cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol. 2000;37:515–527. doi: 10.1046/j.1365-2958.2000.02021.x. [DOI] [PubMed] [Google Scholar]
- 4.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L-M, Hobbie S, Galan J E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 6.Cowell B A, Chen D Y, Frank D W, Vallis A J, Fleiszig S M J. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68:403–406. doi: 10.1128/iai.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drubin D G, Nelson W J. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 8.Evans D J, Frank D W, Finck-Barbançon V, Wu C, Fleiszig S M. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finck-Barbançon V, Yahr T L, Frank D W. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J Bacteriol. 1998;180:6224–6231. doi: 10.1128/jb.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig S M, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig S M, Zaidi T S, Pier G B. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K, Kanada D, Sawa T, Yen T S B, Frank D. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Galan J E. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 16a.Geiser, T., B. Kazmierczak, L. Garrity-Ryan, M. Matthay, and J. Engel. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell. Microbiol., in press. [DOI] [PubMed]
- 17.Goehring U M, Schmidt G, Pederson K J, Aktories K, Barbieri J T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 18.Goosney D L, Celli J, Kenny B, Finlay B B. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect Immun. 1999;67:490–495. doi: 10.1128/iai.67.2.490-495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 20.Hamid N, Gustavsson A, Andersson K, McGee K, Persson C, Rudd C E, Fallman M. YopH dephosphorylates cas and fyn-binding protein in macrophages. Microb Pathog. 1999;27:231. doi: 10.1006/mpat.1999.0301. [DOI] [PubMed] [Google Scholar]
- 21.Hardt W-D, Chen L M, Schubel K E, Bustelo X R, Galan J E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–822. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 22.Hauser A, Engel J. Pseudomonas aeruginosa induces type III secretion-mediated apoptosis in macrophages and epithelial cells. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser A R, Kang P J, Engel J. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 24.Hauser A R, Kang P J, Fleiszig S J M, Mostov K, Engel J. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun. 1998;66:1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglewski B H, Sadoff J, Bjorn M J, Maxwell E S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 27.Isberg R R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 28.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 29.Kang P J, Hauser A R, Apodaca G, Fleiszig S, Wiener-Kronish J, Mostov K, Engel J N. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 30.Kazmierczak, B. I., T.-S. Jou, K. Mostov, and J. Engel. Rho-GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell. Microbiol., in press. [DOI] [PubMed]
- 31.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krall R, Schmidt G, Aktories K, Barbieri J T. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect Immun. 2000;68:6066–6068. doi: 10.1128/iai.68.10.6066-6068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu P V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966;116:481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- 37.Pederson K J, Vallis A J, Aktories K, Frank D W, Barbieri J T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 38.Persson C N C, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosqvist R, Forsberg A, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 42.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 43.Sawa T, Corry D, Gropper M, Ohara M, Kurahashi K, Wiener-Kronish J. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–2866. [PubMed] [Google Scholar]
- 44.Sawa T, Ohara M, Kurahashi K, Twining S, Frank D, Doroques D, Long T, Gropper M, Wiener-Kronish J. In vitro cellular cytotoxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun. 1998;66:3242–3249. doi: 10.1128/iai.66.7.3242-3249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schweizer H P, Hoang T T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 46.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 47.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2822–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallis A J, Finck-Barbançon V, Yahr T L, Frank D W. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallis A J, Yahr T L, Barbieri J T, Frank D W. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Pawel-Rammingen U M, Telpnev V, Schmidt G, Aktories K, Wolf-Watz H, Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol Microbiol. 2000;36:737–748. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- 51.West S E, Schweizer H P, Dall C, Sample A K, Runyen-Janecky L J. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;148:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 52.Yahr T, Mende-Mueller L M, Friese M B, Frank D W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yahr T L, Barbieri J T, Frank D W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol. 1996;178:1412–1419. doi: 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B, Zhang Y, Collins C C, Johnson D I, Zheng Y. A built-in arginine finger triggers the self-stimulatory GTPase-activating activity of rho family GTPases. J Biol Chem. 1999;274:2609–2612. doi: 10.1074/jbc.274.5.2609. [DOI] [PubMed] [Google Scholar]