Abstract
Introduction
High-flux hemodialysis membranes may modulate the cytokine storm of SARS-CoV-2, but their impact on chronic hemodialysis (CHD) patients is unknown. The aim of the study was the evaluation of asymmetric cellulose triacetate (ATA) and polymethylmethacrylate (PMMA) dialyzers on inflammatory markers and clinical outcomes in CHD patients with SARS-CoV-2.
Methods
A prospective, observational study on CHD patients with SARS-CoV-2 was carried out. Patients were enrolled from March 2020 to May 2021. Pre- and postdialysis C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) were determined at each session. Patients who underwent on-line hemodiafiltration (OLHDF) with a PMMA dialyzer were compared with those treated with OLHDF with a ATA dialyzer. The primary endpoint was the differences in the reduction ratio per session (RR) of CRP, PCT, IL-6, and IL-6 RR >25%.
Results
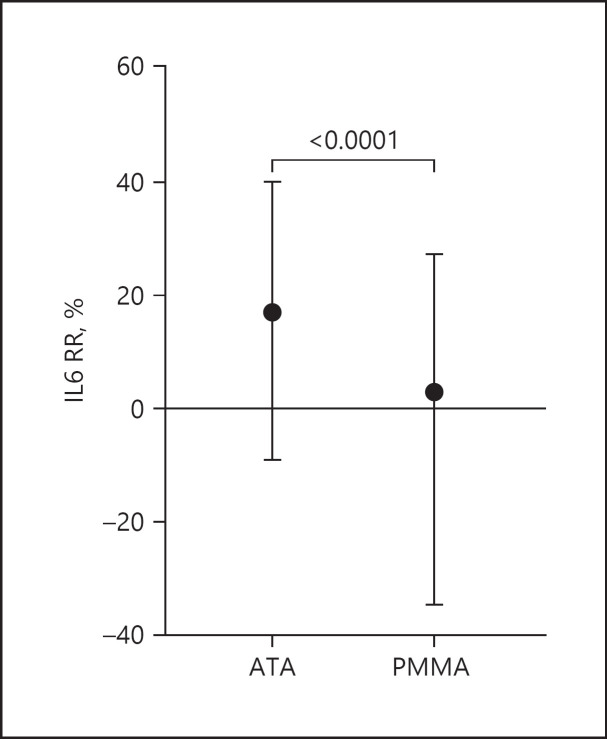
We consecutively enrolled 74 CHD patients with COVID-19, 48 were treated with ATA membrane, and 26 with PMMA. Median IL-6 RR was higher in the ATA group compared to PMMA (17.08%, IQR −9.0 to 40.0 vs. 2.95%, IQR −34.63 to 27.32). Median CRP RR was 7.77% (IQR 2.47–13.77) in the ATA group versus 4.8% (IQR −2.65 to 11.38) in the PMMA group (p = 0.0017). Median PCT-RR% was 77.38% (IQR 70.92–82.97) in ATA group versus 54.59% (IQR 42.62–63.16) in the PMMA group (p < 0.0001). A multiple logistic regression analysis with IL-6 RR >25% as the outcome including the membrane employed, pre-dialysis IL-6, CRP, PCT, and ferritin showed that ATA led to a higher probability to reach the outcome (OR 1.891, 95% CI 1.273–2.840, p = 0.0018) while higher CRP favors the risk of lower IL-6 RR values (OR 0.910, 95% CI 0.868–0.949, p ≤ 0.0001).
Conclusions
In SARS-CoV-2 CHD patients treated with OLHDF, ATA showed a better anti-inflammatory profile, regarding IL-6 RR, compared to PMMA.
Key Words: End stage renal disease, COVID-19, Asymmetric cellulose triacetate, Polymethylmethacrylate, Hemodiafiltration, Cytokine storm
Introduction
Since the start of the pandemic, in December 2019, chronic hemodialysis patients (CHD) have been highly exposed to SARS-CoV-2 infection and experienced high risk of COVID-19-related mortality, independent from known risk factors such as obesity, ischemic heart disease, and lung disease [1, 2, 3]. Although they were among the first who received vaccination against SARS-CoV-2, CHD patients displayed a lower protective effect as compared to the general population [4, 5].
Since COVID-19 can be considered an example of uncontrolled inflammation with unfavorable outcomes, the use of anti-inflammatory drugs is recommended for the general population, while in CHD, extracorporeal procedures that combine high cytokine removal (or low inflammatory generation) with the current uremic toxin removal are also warranted [6, 7, 8, 9]. Since inflammatory mediators belong to the middle molecule uremic toxins, hemodialysis membranes targeted at middle molecule clearance may be useful to mitigate the cytokine storm and to reduce the risk of severe disease progression.
Low-flux hemodialysis guarantees small molecule clearance, but it is not adequate for middle molecule removal. Although more effective, removal of toxins with molecular weights greater than β2-microglobulin is limited even with high-flux hemodialysis [10]. Nonetheless the Work Group of the KDOQI clinical practice guideline for hemodialysis adequacy thought that high-flux dialyzers should be used preferentially [11]. On-line hemodiafiltration (OLHDF), which associates convective transport to diffusion, is promising in enhancing middle molecules clearance, although the effects on clinical outcomes in stable CHD patients are still uncertain [12, 13, 14, 15]. In COVID-19 CHD patients, data on the effect of dialysis technique as well as dialysis membrane type on cytokine clearance and clinical outcomes are lacking: medium-cut-off hemodialysis (MCO) had been proposed, but no conclusive data are available [16, 17].
Nonetheless, polymethylmethacrylate (PMMA) membrane seems adequate in COVID-19 CHD because it allows both effective convective transport and solute removal by adsorption, although the process is self-limiting due to the progressive membrane saturation [6]. The adsorption capacity of PMMA allows clearance of large middle molecules, normally not removed by filtration such as circulating free light chains (22 and 45 kDa) [7, 18].
Recently, a new generation filter in asymmetric cellulose triacetate (ATA) has been developed with the advantage of high hydraulic permeability, allowing high-volume OLHDF like synthetic materials [19]. The aim of our study was to compare the effects of two highly biocompatible dialysis membranes (ATA and PMMA) on IL-6, inflammatory markers, and clinical outcomes in CHD patients affected by COVID-19.
Patients and Methods
Patients and Study Design
A prospective, single-center, observational study on CHD patients (age ≥18) affected by SARS-CoV-2 infection was carried out. SARS-CoV-2 infection was confirmed by positive reverse transcriptase polymerase chain reaction on a nasopharyngeal swab. From March 15, 2020, to March 15, 2021, patients who required hospitalization to the Nephrology Dialysis and Renal Transplantation Unit of S. Orsola University Hospital, Bologna, Italy as well as to other COVID units of the same hospital consecutively recruited into the present study. In the period considered the Dialysis Unit was designed to provide the care of the SARS-CoV-2 CHD patients in the Bologna metropolitan area that comprises about 800,000 inhabitants. None of the patients underwent vaccination because the anti-SARS-CoV-2 vaccination campaign started after March 15, 2021. All the patients required chronic HD with a three-times-weekly schedule. All the hemodialysis treatments from March 15, 2020, to May 30, 2021, were considered. Patients with acute kidney injury, renal transplant recipients, and those who required mechanical ventilation at diagnosis were excluded. Until May 2020, all the patients underwent hydroxychloroquine therapy with dose adjusted according to ESRD (400 mg b.i.d. the first day, then 200 mg/day for 5 days) and azithromycin until the definitive demonstration of their futility [20]. Considering the well-known potential of hydroxychloroquine for increasing QTc, a baseline EKG was carried out before therapy commencement and afterward every 2 days. Subsequently standard treatment was performed with dexamethasone. Antibiotics were administered according to the clinical needs for bacterial infections combined with the SARS-CoV-2 infection. The low molecular weight heparin was administered every other day during non-dialysis days at the same dialysis dose according to the body weight of the patient (see below) to prevent the thromboembolic risk of SARS-CoV-2 infection. The Dialysis Unit provided noninvasive ventilatory support, namely the Venturi mask or continuous positive airway pressure support, according to the pneumologist prescription. Respiratory failure was diagnosed when the ratio between PaO2 (partial pressure of oxygen in arterial blood) and FiO2 (inspired oxygen fraction) fell below 200 mm Hg.
Demographic and clinical baseline features were recorded at enrollment. Clinical parameters (body temperature, blood pressure, heart rate, peripheral oxygen saturation, respiratory rate) were recorded twice during each dialysis session before and after dialysis in the Dialysis ward.
Baseline laboratory evaluation consisted in the determination of complete blood cells count, lymphocytes count (L), a panel of acute phase reactant including interleukin-6 (IL-6), serum ferritin, C-reactive protein (CRP), and procalcitonin (PCT). Laboratory tests were performed before and after each dialysis session including CRP, PCT, and IL-6. Laboratory values were obtained by commercially available tests (https://ambo.ausl.bologna.it/metro/som/lum/il-lum-per-i-medici-di-medicina-generale-e-gli specialisti/lum-standard-di-prodotto-27_2_17.pdf/view. Accessed April 2020).
The values measured during dialysis were corrected for hemoconcentration due to the patient's weight loss assuming a unicompartimental behavior solutes described by the Bergström and Wehle following formula [21]:
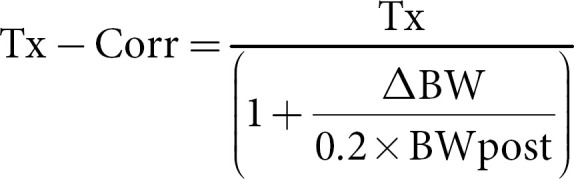
where Tx is the blood solute concentration and Tx-corr is the concentration of solutes corrected for the hemoconcentration, ΔBW (body weight) is the intradialytic weight loss, and BWpost is the body weight at the end of dialysis. The stop dialyzate flow method that involves also the slow blood flow before blood drawing, was used to avoid access recirculation [22].
Dialysis Prescription
All patients enrolled received three dialysis sessions per week through OLHDF. On the basis of their high biocompatibility and potential immunomodulatory properties, we employed two different dialyzers.
The first dialyzer was a high-flux PMMA filter (Filtryzer BG-UTM, Toray, Tokyo, Japan) with a surface area of 2.1 m2, a membrane cut-off value of 20,000 Da and a KUF of 43 mL/h/mm Hg. The second dialyzer was a high-flux ATA membrane (SolaceaTM, Nipro), with a surface area of 2.1 m2, a membrane cut-off value of 45,000 Da and a KUF of 72 mL/h/mm Hg.
The ultrafiltration rate was established according to the need for dehydration of the patient. Dialyzate flow was 500 mL/min. Low molecular weight heparin (enoxaparin sodium InhixaTM; Techdow Pharma, Milan, Italy) was used for anticoagulation of the extracorporeal circuit. The dose administered was 2,000 IU for patients <50 kg of body weight, 4,000 IU for patients between 50 and 90 kg of body weight, and 6,000 IU for patients >90 kg of body weight. Enoxaparin was administered in a single bolus on starting dialysis in the venous bubble catcher.
Endpoints
The primary endpoint was to evaluate the effects of dialysis treatment on inflammatory markers, in particular IL-6 removal. Secondary endpoints were to evaluate effects of different dialysis membranes on CRP and PCT blood levels and on IL-6 RR >25% according to Quiroga et al. [23]. Diagnosis of SARS-CoV-2 pneumonia was obtained through reverse transcriptase-polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 on a respiratory tract sample tested by our laboratory in accordance with the protocol established by the World Health Organization (WHO; Geneva, Switzerland).
The study was approved by our Institutional Ethics Committee (AVEC, nr. 596/2019/Oss/AOUBo) and was in accordance with the Helsinki Declaration. Patients enrolled have given their written informed consent.
Statistical Analysis
Continuous variables with normal distribution were reported as means with standard deviation (SD). Results for variables with skewed distributions were presented as median and interquartile range (IQR). Data comparison was made using the paired or unpaired sample t test for continuous variables with normal distribution, Mann-Whitney, and Wilcoxon test for variables with non-normal distribution and Fisher test for percentage variables. A p value <0.05 was considered significant. Calculations were performed using GraphPad PrismTM (version 8 for Windows; GraphPad Software Inc., San Diego, CA, USA).
Results
We consecutively enrolled 74 CHD patients with SARS-CoV-2; mean age was 68.30 years (SD 15.71), 50 (71%) were male, dialysis vintage was 43.50 months (IQR 14.25–88.00) and the median Charlson Comorbidity Index (CCI) was 4.50 (IQR 3.00–6.00). Sixty-one (82%) patients had a history of cardiovascular disease, 28 (37%) had diabetes mellitus, 9 (12%) had cancer, and 10 (14%) were obese. Forty-nine (66%) patients had interstitial pneumonia, 37 (50%) developed respiratory failure, and 13 (18%) died. Sixty-one (82%) were hospitalized while the remaining were managed as dialysis outpatients. Among hospitalized patients, 7 (9%) were admitted to the ICU, and the median hospitalization length was 14 days (IQR 5.00–25.00). A total of 612 post-dilution OLHDF were performed with a median blood flow of 300 mL/min (IQR 250–300) and a median convective volume of 18.30 L (IQR 15.70–20.70).
Among the whole population, 48 patients were treated with OLHDF with ATA membrane and 26 with OLHDF with PMMA membrane. Subjects were well matched in terms of age, sex, and CCI. No difference in baseline IL-6 and CRP levels between the two groups were detected, while PCT levels were higher in the ATA group (1.60 pg/mL, IQR 0.72–2.77 vs. 0.95 pg/mL IQR 0.53–1.48). Median pre-dialysis IL-6 blood levels were comparable between ATA and PMMA sessions (14.50 pg/mL, IQR 5.75–41.43 vs. 13.90 pg/mL, IQR 5.80–34.10, p = 0.6386). Detailed demographic, clinical, and laboratory features are presented in Table 1.
Table 1.
Clinical, demographic, laboratory features and outcomes of the whole population and of ATA versus PMMA group
| Variables | Total (74) | ATA (48) | PMMA (26) | p value |
|---|---|---|---|---|
| Age, year, mean (SD) | 68.30 (15.71) | 67.67 (15.48) | 69.46 (16.37) | 0.6421 |
| Male, n (%) | 51 (70) | 34 (71) | 17 (66) | 0.7930 |
| HD age, months, median (SD) | 43.50 (14.25–88.00) | 47.00 (13.75–89.75) | 27.50 (14.25–71.50) | 0.3653 |
| Charlson Comorbidity Index, median (IQR) | 4.50 (3.00–6.00) | 4.00 (3.00–5.00) | 5 (3.00–7.25) | 0.2549 |
| Vascular access, n (%) | ||||
| Arteriovenous fistula | 50 (67) | 36 (75) | 14 (54) | 0.0744 |
| Central venous catheter | 24 (33) | 12 (25) | 12 (46) | |
| Comorbidity | ||||
| CV disease, n (%) | 61 (82) | 39 (81) | 22 (84) | >0.9999 |
| DM, n (%) | 28 (37) | 15 (31) | 13 (50) | 0.1364 |
| Cancer, n (%) | 9 (12) | 5 (10) | 4 (15) | 0.7113 |
| BMI > 30 | 10 (14) | 4 (8) | 6 (23) | 0.1583 |
| Dialysis sessions, n | 611 | 420 | 191 | NA |
| Blood flow, mL/min, median (IQR) | 300 (250–300) | 300 (280–300) | 300 (250–300) | 0.1281 |
| Convective volume, L, median (IQR) | 18.30 (15.70–20.70) | 18.30 (15.35–21.20) | 18.30 (15.95–20.50) | 0.6747 |
| Respiratory failure, n (%) | 37 (50) | 22 (46) | 15 (58) | 0.8108 |
| Interstitial pneumonia, n (%) | 49 (66) | 29 (60) | 20 (77) | 0.2008 |
| Antibiotic therapy at diagnosis, n (%) | 35 (49) | 27 (56) | 8 (31) | 0.0512 |
| IL-6 RR%, median (IQR) | 14.55 (–15.13 to 36.71) | 17.08 (–9.0 to 40.0) | 2.95 (–34.63 to 27.32) | <0.001 |
| Pre-HD IL-6, pg/mL, median (IQR) | 14.30 (5.80–39.00) | 14.50 (5.75–41.43) | 13.90 (5.80–34.10) | 0.6386 |
| IL-6 RR% based on pre-dialysis IL-6 level | ||||
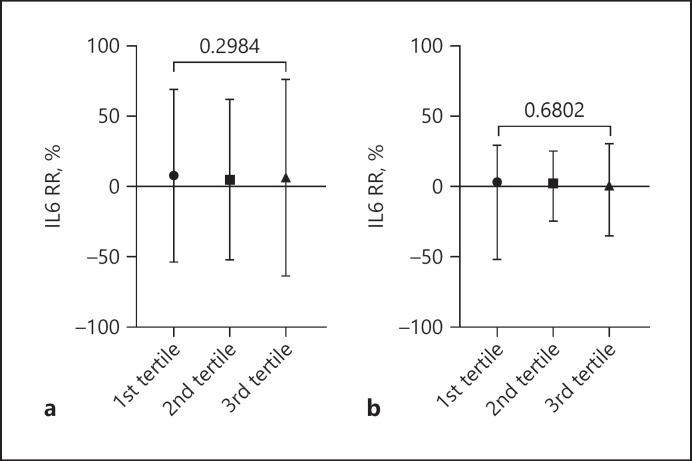
| 1st tertile, median (IQR) | 17.21 (–21.93 to 39.59) | 23.55 (–8.96 to 47.40) | 3.72 (–51.66 to 30.08) | 0.0013 |
| 2nd tertile median (IQR) | 13.13 (–14.40 to 34.91) | 16.69 (–9.79 to 39.39) | 2.18 (–24.03 to 25.95) | 0.0405 |
| 3rd tertile, median (IQR) | 12.10 (–14.63 to 34.76) | 12.99 (–8.73 to 35.75) | 1.14 (–34.70 to 31.33) | 0.0501 |
| CRP RR%, median (IQR) | 7.20 (0.15–13.04) | 7.77 (2.47–13.77) | 4.80 (–2.65 to 11.38) | 0.0017 |
| PCT-RR%, median (IQR) | 72.75 (58.86–80.18) | 77.38 (70.92–82.97) | 54.59 (42.62–63.16) | <0.0001 |
| Laboratory variables at diagnosis | ||||
| Hb, g/dL, median (IQR) | 10.90 (9.6–11.90) | 11.00 (9.70–12.10) | 10.30 (9.17–11.60) | 0.5985 |
| PLT, 109/L, median (IQR) | 186 (140–217) | 178 (144–221) | 191 (131–216) | 0.9363 |
| WBC, 109/L, median (IQR) | 5.17 (3.99–7.11) | 5.00 (3.90–6.57) | 6.50 (4.13–7.92) | 0.0974 |
| Lymphocytes, 109/L, median (IQR) | 0.65 (0.50–0.99) | 0.64 (0.48–0.90) | 0.75 (0.59–1.38) | 0.2064 |
| Eosinophils, 109/L, median (IQR) | 0.10 (0.03–0.33) | 0.09 (0.03 – 0–19) | 0.17 (0.04–0.60) | 0.3934 |
| IL-6, pg/mL, median (IQR) | 21.25 (9.22–56.48) | 20.30 (9.10–62.10) | 22.60 (9.80–56.35) | 0.8763 |
| CRP, mg/dL, median (IQR) | 3.30 (0.54–12.68) | 3.88 (0.77–143.70) | 3.30 (0.33–9.98) | 0.4134 |
| PCT, pg/mL, median (IQR) | 1.40 (0.63–2.58) | 1.60 (0.72–2.77) | 0.95 (0.53–1.48) | 0.0388 |
| Albumin, g/dL, median (IQR) | 32.20 (22.10–36.60) | 32.60 (28.70–37.40) | 32.00 (29.65–34.55) | 0.7105 |
CV, cardiovascular; DM, diabetes mellitus; BMI, body mass index; CRP, C-reactive protein; PCT, procalcitonin.
Regarding the primary endpoint, median IL-6 RR was significantly higher in the ATA group compared to PMMA (17.08%, IQR −9.0 to 40.0 vs. 2.95%, IQR −34.63 to 27.32) (Fig. 1). After dividing the groups into tertiles based on IL-6 pre-dialysis levels, ATA membrane determined higher IL-6 RR in all the tertiles. Neither ATA nor PMMA showed a difference in IL-6 RR from higher to lower tertiles (Table 1; Fig. 2).
Fig. 1.
IL-6 RR% in ATA versus PMMA group. Data expressed as median (IQR).
Fig. 2.
IL-6 RR% comparison between tertiles on the basis of IL-6 pre-diaysis level in ATA (a) and in PMMA group (b). Data expressed as median (IQR).
Pre-dialysis IL-6 levels were similar in ATA and PMMA across the first 6 HD sessions, as described in Table 2. Beyond IL-6, both CRP and PCT showed higher median reduction ratios for ATA membrane (Table 1).
Table 2.
IL-6 (pg/mL) blood level before dialysis session
| OLHDF sessions | ATA | PMMA | p value |
|---|---|---|---|
| 1st | 19.70 (9.00–58.25) | 22.60 (11.20–56.35) | 0.6974 |
| 2nd | 24.70 (10.05–69.00) | 24.10 (7.20–51.60) | 0.4888 |
| 3rd | 25.25 (6.10–70.45) | 10.90 (5.50–36.40) | 0.3094 |
| 4th | 22.90 (5.10–55.00) | 20.80 (5.60–99.10) | 0.6809 |
| 5th | 15.30 (4.67–65–15) | 16.20 (8.23–37.58) | 0.8557 |
| 6th | 22.90 (5.10–55.00) | 20.80 (5.60–99.10) | 0.6809 |
Results expressed as median (IQR).
We performed a multiple logistic regression analysis with IL-6 RR >25% as outcome including the membrane employed, pre-dialysis IL-6, CRP, PCT, and ferritin. ATA led to a higher probability to reach the outcome (OR 1.891, 95% CI 1.273–2.840, p = 0.0018) while higher PCR favors the risk of lower IL-6 RR values (OR 0.910, 95% CI 0.868–0.949, p ≤ 0.0001) (Table 3).
Table 3.
Multiple logistic regression with IL-6 RR >25% as outcome
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| ATA | 1.891 | 1.273–2.840 | 0.0018 |
| IL-6 pre-HD | 1.003 | 1.001–1.007 | 0.0123 |
| CRP pre-HD | 0.9101 | 0.8682–0.9496 | <0.0001 |
| PCT pre-HD | 0.9528 | 0.8644–1.008 | 0.2270 |
| Ferritin | 1.000 | 0.9998–1.000 | 0.7697 |
Discussion/Conclusion
The present study was an observational prospective single-center study, aimed at investigating the effects of two different dialyzers, ATA and PMMA, on clinical outcomes and cytokine removal in CHD affected by SARS-CoV-2. The high mortality related to SARS-CoV-2 in such patients justifies the efforts to optimize HD treatment to counteract the cytokine storm that characterizes this disease, leading to ARDS and multiorgan failure [1, 24]. The 18% of our patients died confirming the high SARS-CoV-2-related mortality rate in such population although slightly inferior compared to the 23% recently described by the analysis of the European Renal Association SARS-CoV-2 Database, keeping into account that our population was largely a “second wave” SARS-CoV-2 cohort treated with more standardized therapeutic protocols [1].
Given the role of IL-6 as a main driver of the CRS in SARS-CoV-2, we focused on such a molecule to evaluate the inflammatory effect of the two membranes. Nonetheless, IL-6 belongs to the middle molecular uremic toxins (21 kDa), PCT has a molecular weight of 14 kDa and CRP a molecular weight of 24 kDa. Consequently, only few specific membranes can technically remove these molecules and in particular high-flux dialyzers, which have cut-off values of about 20 kDa and up to 45 kDa [25]. We choose the OLHDF technique and two high-flux dialyzers with high KUF and high biocompatibility to increase middle molecule clearance and to minimize blood-membrane proinflammatory interactions. Selecting these dialyzers, we compared a membrane with high MWCO and high hydraulic permeability (ATA) and a membrane with high adsorptive properties (PMMA) [26]. Recently, the risk of albumin loss after 4 weeks and 12 weeks of MCO-HD as well as the lack of IL-6 improvement after 24 weeks of treatment were reported: as a consequence, we considered the MCO dialyzer not suitable for the study [27, 28]. Cytosorb was not taken into account considering that all dialysis sessions were carried out in the dialysis ward were it is limited to intermittent 4-h length treatment formats [9].
Analyzing a high number of HD sessions (420 for ATA and 191 for PMMA), we found that the median IL-6 RR% was greater for ATA than with PMMA (Fig. 1). The pre-HD IL-6 levels were similar between the ATA and PMMA groups across the disease span (Table 2). Such result was confirmed also after dividing the population into tertiles of pre-HD IL-6 levels. Moreover, higher IL-6 pre-HD levels did not influence the RR neither for ATA nor for PMMA. The multiple logistic regression analysis showed that the probability to obtain an IL-6 RR major than 25% was higher with ATA than with PMMA dialyzer, while active pre-HD inflammatory status reduced the likelihood to reach significant RR, suggesting a role of IL-6 overproduction during HD treatment itself.
The threshold of 25% was the median value of IL-6 RR found by Quiroga et al. [23] in HD COVID-19 patients who survived; the value was found during the first hemodialysis session after COVID-19 diagnosis. We obtained a median IL-6 RR of 2.95% (IQR −34.63 to 27.32) with our PMMA BGU dialyzer that was much lower than that reported by Quiroga who used the PMMA NF dialyzer with a KUF of 55 mL/h/mm Hg [23]. Nonetheless some other methods differences are present: first, all HD sessions across SARS-CoV-2 duration were considered; second, controversies exist about combining a PMMA dialyzer with high adsorptive properties and convection during OLHDF [26]. It is hard to assess whether IL-6 concentration decline is determined only by the extracorporeal therapy or if it reflects, at least in part, the dynamic nature of the cytokine storm, the improvement of underlying disease or the response to treatment [29]. It is also worth noting that in some sessions, IL-6 blood levels increased after HD as found also by Quiroga et al. [23]. We hypothesize that this phenomenon is not due to a lack of removal but to a huge excess of production caused by disease activity or to intradialytic cytokine release due to the enhancement of monocyte activation [30, 31, 32, 33]. Moreover, cytokine half-lives are short and their endogenous production might be more rapid than clearance during extracorporeal therapy, leading to a potential underestimation of the effective RR, especially in the acute phase of infection [34].
Beyond IL-6, we observed an elevated PCT RR in the whole population that was significantly higher in ATA group. Elevated PCT blood levels are observed in SARS-CoV-2, are associated with poor prognosis, and are reliable in diagnosis of overlapped bacterial infection [35, 36]. It is well known that PCT is not solely an inflammatory marker but also exerts a complex immunomodulatory role in sepsis that consists of proinflammatory effects (neutrophil and lymphocyte activation, increased cytokine production) and ones that may contribute to “immunoparhalysis” [37, 38]. Although with no statistical significance, patients belonging to the ATA group displayed a higher rate of overlapped bacterial infection, testified by more frequent antibiotic administration as well as higher PCT levels at diagnosis, suggesting a relationship between the two parameters in our caseload. However, given the low median PCT level, we believe that the main driver of the inflammatory response in our population was SARS-CoV-2 infection.
Few data on cytokines removal are available for CHD patients affected by SARS-CoV-2. Esposito et al. [17] performed a prospective trial on 29 CHD patient with SARS-CoV-2 randomized to MCO or PMMA: no difference in mortality risk was found. However, this study was focused on inflammatory mediator changes: pre- and post-HD IL-6 blood levels were measured only in the first session (HD with MCO) at diagnosis and no significant differences were detected [17]. Yalin et al. [39] did not find any difference in mortality in a retrospective study that examined 60 CHD patients affected by SARS-CoV-2 treated with MCO or low-flux membranes. Tarakcioǧlu et al. [40] evaluated cytokines removal in 21 stable CHD patients treated with low-flux HD and did not find any difference as to IL-1 beta, IL-6, TNF-alpha, and sIL-2R concentrations between pre- and post-HD specimens. De la Flor et al. [41] published a case series of 10 CHD patients affected by SARS-CoV-2 who underwent post-dilution OLHDF with ATA. The authors found a higher IL-6 decrease after 14 days in survivors, although no IL-6 RR was determined and there was not a control group [41].
In conclusion, a reduced inflammatory pattern can be assessed using ATA in comparison to PMMA. Nevertheless, at the best of our knowledge, these are the first data on the comparison of ATA versus PMMA in CHD patients in SARS-CoV-2, and the strength of our study are the high number of HD sessions analyzed. The present study has some limitations: its observational nature, the limited pattern of inflammatory parameters assessed, and the small population analyzed that do not allow conclusions on mortality as well as on other clinical outcomes.
Statement of ethics
The study was approved by our Institutional Ethics Committee (AVEC, nr. 596/2019/Oss/AOUBo) and was in accordance with the Helsinki Declaration. Patients enrolled have given their written informed consent.
Conflict of interest statement
The authors have no conflict of interest to declare.
Funding sources
The authors declare no funding.
Authors contributions
Gabriele Donati, Anna Scrivo, Fulvia Zappulo, Angelo Rigotti, and Gaetano La Manna: conceptualization, writing, original draft preparation, and draft revision; they all agree to the published version of the manuscript and to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Lorenzo Gasperoni: conceptualization, data collection, data interpretation, writing, original draft preparation, and draft revision; he agrees to the published version of the manuscript and to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Marianna Napoli, Chiara Abenavoli, Lilio Hu, Andrea Angelini, Miriam Di Nunzio, Edoardo Tringali, Alessandra Cingolani, and Beatrice Claudia Marchegiani: conceptualization, writing, original draft preparation, draft revision, and data collection; they all agree to the published version of the manuscript and to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Gabriele Donati and Lorenzo Gasperoni contributed equally to the paper.
References
- 1.Goffin E, Candellier A, Vart P, Noordzij M, Arnol M, Covic A, et al. COVID-19-related mortality in kidney transplant and haemodialysis patients a comparative, prospective registry-based study. Nephrol Dial Transplant. 2021 Nov;36((11)):2094–2091. doi: 10.1093/ndt/gfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584((7821)):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordio M, Reboldi G, Di Napoli A, Quintaliani G, Alberici F, Postorino M, et al. Risk factors and action thresholds for the novel coronavirus pandemic. Insights from the Italian Society of Nephrology COVID-19 Survey. J Nephrol. 2021 Apr;34((2)):325–335. doi: 10.1007/s40620-020-00946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Hanoy M, Laurent C, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021 Sep;32((9)):2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021 Jun;99((6)):1490–1492. doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro A, Guadagni G., Dialysis membrane from convection to adsorption. Clin Kidney J. 2010 May;3((Suppl 1)):i36–i39. doi: 10.1093/ndtplus/sfq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoike I. Clinical significance of protein adsorbable membranes long-term clinical effects and analysis using a proteomic technique. Nephrol Dial Transplant. 2007 Jul;22((Suppl 5)):v13–v19. doi: 10.1093/ndt/gfm295. [DOI] [PubMed] [Google Scholar]
- 8.Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020 Oct;26((10)):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rampino T, Gregorini M, Perotti L, Ferrari F, Pattonieri EF, Grignano MA, et al. Hemoperfusion with CytoSorb as adjuvant therapy in critically ill patients with SARS-CoV2 pneumonia. Blood Purif. 2021;50((4–5)):566–571. doi: 10.1159/000511725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maduell F, Navarro V, Cruz MC, Torregrosa E, Garcia D, Simon V, et al. Osteocalcin and myoglobin removal in on-line hemodiafiltration versus low- and high-flux hemodialysis. Am J Kidney Dis. 2002 Sep;40((3)):582–589. doi: 10.1053/ajkd.2002.34918. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney. Foundation. KDOQI clinical practice guideline for hemodialysis adequacy 2015 update. Am J Kidney Dis. 2015;66((5)):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Grooteman MPC, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AHA, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012 Jun;23((6)):1087–1096. doi: 10.1681/ASN.2011121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ok E, Asci G, Toz H, Ok E, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013 Jan;28((1)):192–202. doi: 10.1093/ndt/gfs407. [DOI] [PubMed] [Google Scholar]
- 14.Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017 Jun;91((6)):1495–1495. doi: 10.1016/j.kint.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013 Mar;24((3)):487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco C, Reis T, Cozzolino M. Rationale for medium cutoff membranes in COVID-19 patients requiring renal replacement therapy. Nephron. 2020;144((11)):550–554. doi: 10.1159/000509807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito P, Cipriani L, Verzola D, Grignano MA, De Amici M, Testa G, et al. Effects of different dialysis strategies on inflammatory cytokine profile in maintenance hemodialysis patients with COVID-19 a randomized trial. J Clin Med. 2021 Mar;10((7)):1383. doi: 10.3390/jcm10071383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchison CA, Cockwell P, Reid S, Chandler K, Mead GP, Harrison J, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma in vitro and in vivo studies. J Am Soc Nephrol. 2007 Mar;18((3)):886–895. doi: 10.1681/ASN.2006080821. [DOI] [PubMed] [Google Scholar]
- 19.Sunohara T, Masuda T., Fundamental characteristics of the newly developed ATATM membrane dialyzer . Contributions to nephrology. In: Kawanishi H, Takemoto Y, editors. S. Karger AG. 2017. pp. p. 215–221. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Sonpar A. In adults exposed to COVID-19 hydroxychloroquine did not reduce confirmed or probable COVID-19 trial stopped for futility. Ann Intern Med. 2020 Oct;173((8)):JC41. doi: 10.7326/ACPJ202010200-041. [DOI] [PubMed] [Google Scholar]
- 21.Bergström J, Wehle B. No change in corrected Β2-microglobulin concentration after cuprophane haemodialysis. Lancet. 1987 Mar;1((8533)):628–629. doi: 10.1016/s0140-6736(87)90266-2. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation KDOQI clinical practice guidelines for hemodialysisadequacy update 2006. Am J Kidney Dis. 2006 Jul;48:S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 23.Quiroga B, Muñoz Ramos P, Giorgi M, Santos A, Núñez A, Ortiz A, et al. Dynamic assessment of interleukin-6 during hemodialysis and mortality in coronavirus disease-19. Ther Apher Dial. 2021 Dec;25((6)):908–916. doi: 10.1111/1744-9987.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng Z, Yu H, Chen H, Qi W, Chen L, Chen G, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan China. Crit Care. 2020 Dec;24((1)):525. doi: 10.1186/s13054-020-03255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naka T, Haase M, Bellomo R. “Super high-flux” or “high cut-off” hemofiltration and hemodialysis. Contrib Nephrol. 2010;166:181–189. doi: 10.1159/000314871. [DOI] [PubMed] [Google Scholar]
- 26.Maduell F. Is there an “optimal dose” of hemodiafiltration? Blood Purif. 2015;40((Suppl 1)):17–23. doi: 10.1159/000437409. [DOI] [PubMed] [Google Scholar]
- 27.Cozzolino M, Magagnoli L, Ciceri P, Conte F, Galassi A. Effects of a medium cut-off (Theranova®) dialyser on haemodialysis patients a prospective, cross-over study. Clin Kidney J. 2021;14((1)):382–389. doi: 10.1093/ckj/sfz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner DE, Falzon L, Skoufos L, Bernardo A, Beck W, Xiao M, et al. Efficacy and safety of expanded hemodialysis with the Theranova 400 dialyzer a randomized controlled trial. Clin J Am Soc Nephrol. 2020 Sep 7;15((9)):1310–1319. doi: 10.2215/CJN.01210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atan R, Crosbie D, Bellomo R. Techniques of extracorporeal cytokine removal a systematic review of the literature. Blood Purif. 2012;33((1–3)):88–100. doi: 10.1159/000333845. [DOI] [PubMed] [Google Scholar]
- 30.Girndt M, Heisel O, Kohler H. Influence of dialysis with polyamide vs haemophan haemodialysers on monokines and complement activation during a 4-month long-term study. Nephrol Dial Transplant. 1999 Mar;14((3)):676–682. doi: 10.1093/ndt/14.3.676. [DOI] [PubMed] [Google Scholar]
- 31.Gu Y, Ding F, Qin H, Zhao H, Lin S. Synergetic effect of dialyzer membrane and lipopolysaccharide on peripheral blood mononuclear cell cytokine production in uremic patients. Chin Med J. 2000 Apr;113((4)):315–319. [PubMed] [Google Scholar]
- 32.Poppelaars F, Faria B, Gaya da Costa M, Franssen CFM, van Son WJ, Berger SP, et al. The complement system in dialysis a forgotten story? Front Immunol. 2018 Jan;9:71. doi: 10.3389/fimmu.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angeletti A, Zappulo F, Donadei C, Cappuccilli M, Di Certo G, Conte D, et al. Immunological effects of a single hemodialysis treatment. Medicina. 2020 Feb;56((2)):71. doi: 10.3390/medicina56020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sander A, Armbruster W, Sander B, Daul AE, Lange R, Peters J. Hemofiltration increases IL-6 clearance in early systemic inflammatory response syndrome but does not alter IL-6 and TNFα plasma concentrations. Intensive Care Med. 1997 Aug;23((8)):878–884. doi: 10.1007/s001340050425. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases a systematic literature review and meta-analysis. J Infect. 2020 Aug;81((2)):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houghton R, Moore N, Williams R, El-Bakri F, Peters J, Mori M, et al. C-reactive protein-guided use of procalcitonin in COVID-19. JAC Antimicrob Resist. 2021 Sep;3((4)):dlab180. doi: 10.1093/jacamr/dlab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008 Oct;30((7)):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 38.Wei JX, Verity A, Garle M, Mahajan R, Wilson V. Examination of the effect of procalcitonin on human leucocytes and the porcine isolated coronary artery. Br J Anaesth. 2008 May;100((5)):612–621. doi: 10.1093/bja/aen073. [DOI] [PubMed] [Google Scholar]
- 39.Yalın SF, Altıparmak MR, Dincer MT, Yadigar S, Murt A, Parmaksiz E, et al. Medium cut-off dialysis membranes can they have impact on outcome of COVID-19 hemodialysis patients? Blood Purif. 2021;50((6)):921–924. doi: 10.1159/000513621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarakçıoğlu M, Erbağci AB, Usalan C, Deveci R, Kocabaş R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediators Inflamm. 2003;12((1)):15–19. doi: 10.1080/0962935031000096935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De La Flor JC, Valga F, Marschall A, Monzon T, Albarracín C, Ruiz E, et al. Targeting cytokine storm in COVID-19 a role of online hemodiafiltration with asymmetric cellulose triacetate in maintenance hemodialysis patients − a report of 10 cases. Case Rep Nephrol. 2021 Mar;2021:1–7. doi: 10.1155/2021/5575928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.