Abstract
Currently, functional dairy products pave a promising way for the prophylaxis of essential hypertension, and the search for new strains capable of producing such products is a constant challenge for scientists around the world. In this study, the antihypertensive properties of milk fermented with several strains of traditional yogurt starters (Lactobacillus delbrueckii strains Lb100 and Lb200; Lactococcus lactis strains dlA, AM1 and MA1; Streptococcus thermophilus strains 159 and 16t) and one strain of non-conventional probiotic starter (Lacticaseibacillus paracasei ABK) were assessed. The in vitro assessment using angiotensin-converting enzyme inhibition assay was performed for all fermentation products, and the best performed products were tested in vivo using Spontaneously Hypertensive Rat (SHR) animal model. In addition, for the best performed products the fatty acid (FA) composition and FA-related nutritional indices were determined. As a result, the milk fermented with two strains (Lb. delbrueckii LB100 and Lc. lactis AM1) demonstrated significant antihypertensive effect during both in vitro and in vivo experiments. Moreover, the milk fermented with Lb. delbrueckii Lb100 demonstrated significantly better FA-related nutritional indexes and lowered total cholesterol in SHRs upon regular consumption. The obtained results can be used in the future to develop new starter cultures producing effective functional antihypertensive dairy products.
Keywords: lactic acid bacteria, milk fermentation, angiotensin-converting enzyme inhibition, fatty acids, nutritional indices, spontaneously hypertensive rats, blood cholesterol
1. Introduction
According to the recently published study, which analyzed the prevalence of hypertension and progress in its detection, treatment and control from 1990 to 2019 for 200 countries and territories, the number of people with hypertension has nearly doubled in the past 30 years—from 650 million to 1.28 billion worldwide [1]. The experts of the World Health Organization (WHO, Geneva, Switzerland) constantly emphasize that high blood pressure significantly increases the risk of cardiovascular diseases, as well as diseases of the brain and kidneys. Tens of millions of people die every year due to hypertension-related causes [2].
Currently, the use of inhibitors of the renin-angiotensin-aldosterone system (RAAS) is recommended first-line evidence-based therapy for patients with arterial hypertension [3,4,5]. The RAAS is one of the evolutionary oldest hormonal systems that serve primarily to balance blood pressure and electrolyte homeostasis [6]. In short, the functioning of the RAAS can be described as follows [7]: Upon a reduction in blood volume or a drop in blood pressure, the kidneys start to secrete a specific enzyme, renin, into the bloodstream. In the bloodstream, renin converts 12 aa peptide angiotensinogen (secreted by liver) into decapeptide angiotensin I. The angiotensin I is further converted by the angiotensin-converting enzyme (ACE) locating on the surface of vascular endothelial cells (mainly the lungs) into octopeptide angiotensin II. The action of angiotensin II causes narrowing of the blood vessels and increases the reabsorption of water into the blood which in turn causes an increase in blood pressure.
The most well-known RAAS inhibitors are synthetic drugs such as captopril, enalapril and perindopril, which tend to inhibit ACE activity leading to the relaxation of blood vessels and lowering of blood pressure [8]. These synthetic drugs are widely used in medicine for the treatment of hypertension; however, their unpleasant side effects (e.g., abnormal taste, skin rashes and coughing) and hepatotoxicity have currently prompted the development of natural, safe, and novel alternatives such as “ACE inhibiting (ACE-I) foods” [9]. In their composition ACE-I foods contain specific peptides that can interact with an active site of ACE and concurrently inhibit conversion of angiotensin I into angiotensin II [10]. However, it should be especially emphasized that at the present state of development consumption of the ACE-I food can be regarded only as a prophylaxis but not as a treatment of hypertension [11].
Currently, one of the best studied ACE-I foods are dairy products produced by the fermentation with lactic acid bacteria (LAB) [12,13]. During the fermentation, proteolytic system of LAB generates small peptides from milk’s proteins (mainly α, β and κ-caseins), which may have various beneficial properties including ACE-I [14]. However, the proteolytic systems in various LAB are extremely diverse and vary not only between different LAB species but also between different strains of the same species [15,16,17]. Consequently, the selection of conventional starter strains producing fermented milk with higher ACE-I properties or the inclusion of non-conventional starter strains into the fermentation in order to enrich final product with ACE-I peptides is extremely important and active direction of research [18].
Analyzing the enrichment of fermented dairy products with ACE-I properties, it is especially worth paying attention to the fact that in some cases, there is no correlation between the ACE-I activity of fermented milk measured in vitro and its hypotensive effect in vivo [19]. On the one hand, the in vivo antihypertensive effect can be less than expected due to the fact that peptides in the gastrointestinal tract may be further degraded during digestion, which may lead to a decrease in their bioavailability and beneficial effects [20]. On the other hand, besides ACE-I peptides fermented milk products contain LAB cells and their metabolites that upon ingestion can exert an additional hypotensive effect via induction of eubiosis, reduction in oxidative stress and improvement of endothelial function [21,22,23,24,25]. In addition, the fermentation of milk changes composition of its matrix components (e.g., calcium, peptides, phosphorus, amino and fatty acids) and drastically influences health outcomes of its consumption [26]. Hence, it is of paramount importance not only test ACE-I of products in vitro, but also to substantiate their antihypertensive action in vivo using suitable animal models.
In this article, several strains of traditional yogurt starters (Lactobacillus delbrueckii, Lactococcus lactis and Streptococcus thermophilus) and one strain of non-conventional starter (Lacticaseibacillus paracasei) were tested on their ability to produce fermented dairy products with ACE-I properties. The milk fermented by the best performed strains was further analyzed and its fatty acid (FA) content as well as FA-related nutritional indices were determined. In addition, the antihypertensive properties of the fermented milks were assessed in vivo using Spontaneously Hypertensive Rat (SHR) animal model.
2. Materials and Methods
2.1. Strains and Cultivation Conditions
The strains of LAB were obtained from the Collection of the All-Russian Research Institute of the Dairy Industry (VNIMI, Moscow, Russia), where they were kept at −80 °C in skim milk containing 10% (v/v) glycerol. For each strain, the GeneBank accession of the 16S rRNA sequence as well as optimal growth temperature used during all cultivations are presented in Table 1.
Table 1.
Strains of lactic acid bacteria that were used in this study.
Upon reception, stock cultures of Lactobacillus strains were streaked on de Man, Rogosa and Sharpe (MRS) medium (HiMedia Laboratories, Mumbai, India) and grown overnight at the optimal temperature (Table 1) under anaerobic conditions using Anaero Bag System 24 (HiMedia Laboratories, Mumbai, India). Stock cultures of Lactococcus and Streptococcus strains were streaked on M17 medium (HiMedia Laboratories, Mumbai, India) and grown overnight at the optimal temperature (Table 1) under aerobic and anaerobic conditions, respectively. To obtain a preculture, the Lactobacillus colonies were inoculated in MRS broth and Lactococcus and Streptococcus colonies in M17 broth and grown at the optimal temperature (Table 1) to the 106–107 CFU∙mL−1, depending on the strain. To obtain a working culture, commercial skim milk powder grade “Standard” (Complimilk, Slutsk cheese-making plant, Slutsk, Belarus) was reconstituted (12%, w/v), sterilized (110 °C, 10 min), cooled to approximately 30 °C, inoculated with the preculture (3%, v/v) and incubated overnight at the optimal growth temperature (Table 1).
For milk fermentation, one liter of the reconstituted skim milk (RSM), prepared as described above, was pasteurized (80–85 °C for 30 min), cooled to approximately 30 °C and inoculated with the working culture (1%, v/v). The fermentation was performed at the optimal growth temperature (Table 1). Samples were taken for analysis at 0, 6, 16, 24, 48, and 72 h of fermentation. For all strains but Lb. paracasei ABK, the samples from the 16 h of fermentation were used in the animal experiments, for which they were cooled and stored at 4 °C until use. For Lb. paracasei ABK, the samples from the 48 h of fermentation were used in the animal experiments.
2.2. Proteolytic, Antioxidant, and Angiotensin-I-Converting Enzyme Inhibitory Activities
The fermented milk samples with pH 4.6 and below were centrifuged at 3000× g for 15 min at 4 °C (Eppendorf centrifuge 5430 R, Hamburg, Germany). If the pH of the samples was higher than 4.6, the samples were preliminarily titrated with trichloroacetic acid (TCA, 0.75%, w/v) to pH 4.6. The supernatant was stored at −20 °C in 2 mL-aliquots. Prior to use, the supernatant was thawed at 4 °C, centrifuged at 10,000× g for 3 min at room temperature, and filtered through a 0.45 µm syringe filter with hydrophilic membrane (Merk Millipore, Darmstadt, Germany).
The proteolytic activity in the obtained supernatant was measured using the TNBS (2,4,6-trinitrobenzenesulfonic acid) method according to Adler-Nissen [27]. The 0.25 mL of the diluted in SDS (1% w/v) sample was mixed with 2 mL of sodium phosphate buffer (0.2 M, pH 8.2). Then, 2 mL of TNBS (Sigma-Aldrich, St. Louis, MO, USA) reagent (0.1% w/v in water) was added. Test tubes were mixed and incubated at 50 °C for 60 min, and the reaction was stopped by the addition of 4 mL of 0.1 N HCl. Absorbance was measured at 340 nm using a Synergy 2 microplate photometer–fluorimeter (BioTek, Winooski, VT, USA). A calibration curve was prepared using L-leucine (L-Leu) as a standard (0.1–2.0 mM). The proteolytic activity was expressed as L-Leu molar equivalents, mM (Leu).
The antioxidant activity in the obtained supernatant was determined by the oxygen radical absorbance capacity fluorescence method (ORAC) with generation of the peroxyl radical according to Ou, Hampsch-Woodill, and Prior [28] with slight modifications as described in Nikolaev et al. [29]. The antioxidant capacity of samples against peroxyl radicals was expressed as an amount of Trolox molar equivalents, mM (Trolox).
The ACE-I activity in the obtained supernatant was determined in terms of half maximal inhibitory concentration (IC50), as described in Torkova et al. [30]. ACE activity was measured using o-Aminobenzoyl-Phe-Arg-Lys(dinitrophenyl)-Pro (Sigma-Aldrich, St. Louis, MO, USA) as a substrate with internal fluorescence quenching. The measurements were carried out on a Synergy 2 microplate photometer–fluorometer (BioTek, Winooski, VT, USA).
2.3. Fatty Acid Profile of Fermented Milk
The FAs extraction from the samples of fermented milk was performed according to the Folch method. After the extraction, FAs were derivatizedusing 3 M methanolic HCl (Supelco, Bellefonte, PA, USA), according to the manufacturer’s protocol. Derivatized FAs were separated using GC 2010 chromatograph (Shimadzu, Kyoto, Japan) equipped with an MDN-5 column (30 m × 0.25 mm; Bellefonte, PA, USA) and analyzed using a mass detector GCMS-QP 2010 in the regime of temperature gradient. The PUFA-2 (Supelco, Bellefonte, PA, USA) kit was used as a standard. The identification of FAs was carried out as described in Moiseenko et al. [31]. The relative intensities (further relative abundances) of FA were obtained by normalization on the total intensity of the assigned peaks. All experiments were performed in triplicate.
The FA-related nutritional indices were calculated according to Chen et al. [32]:
| PUFA/SFA = ∑PUFA/∑SFA | (1) |
| IA = [C12:0 + (4 × C14:0) + C16:0]/[∑MUFA + ∑PUFA] | (2) |
| HPI = [∑MUFA + ∑PUFA]/[C12:0 + (4 × C14:0) + C16:0] | (3) |
| IT = [C14:0 +C16:0 + C18:0]/[0.5 × (∑MUFA + ∑PUFA{n − 6})] | (4) |
| HH = [C18:1 + ∑PUFA]/[C12:0 + C14:0 + C16:0] | (5) |
| UI = 1 × (%monoenoics) + 2 × (%dienoics) + 3 × (%trienoics) + 4 × (%tetraenoics) | (6) |
where SFA stands for saturated fatty acids; MUFA—monounsaturated fatty acids; PUFA—polyunsaturated fatty acids; PUFA/SFA—ratio of total PUFA to total SFA; IA—index of atherogenicity; HPI—health-promoting index (which is the reciprocal of IA and mainly used in research on dairy products); IT—index of thrombogenicity; HH—hypocholesterolemic/hypercholesterolemic ratio; UI—unsaturation index.
2.4. Spontaneously Hypertensive Rat (SHR) Animal Model
The hypotensive effect of selected fermented milks was studied using SHR animal model. A total of thirty male SHRs (27 weeks old; 286 ± 15 g body weight; 165 ± 15 mmHg systolic (Psyst) and 107 ± 18 mmHg diastolic (Pdiast) blood pressures, respectively) were obtained from Puschino Kennel of Laboratory Animals (Pushchino, Russia).
For the experiment, the SHRs were randomized into six groups of five animals each. In addition to the standard ration (Laboratormkorm, Moscow, Russia), the groups ad libitum received: (1) distilled water (the intact group); (2) 20 mL per day per animal of RSM (the control group); (3)–(6) 20 mL per day per animal of RSM, fermented with Lb. delbrueckii Lb100, Lb. paracasei ABK, Lc. lactis AM1, and Str. thermophilus 159, respectively. Each SHR group was housed in a separate cage at 22 ± 1 °C and 12 h:12 h light-dark cycles. The experiment was carried out for four weeks, and all the cages were cleaned from the remaining feed residues 12 h before the end of the experiment. At the end of the experiment, the SHRs were euthanized in a carbon dioxide chamber (VetTech, Congleton, UK). The samples of blood serum and aorta were collected and stored at −80 °C for further analysis. The blood serum was obtained by centrifugation of blood using Eppendorf 5702 R centrifuge (Eppendorf, Hamburg, Germany) at 4 °C and 2000× g for 10 min.
2.5. Measurements of Blood Pressure
The Psyst and Pdiast of SHRs were measured with a CODA Monitor Rat-Cuff KIT (Kent Scientific, Torrington, CT, USA) using a tail-cuff. At least ten measurement cycles were performed for each animal, and the results were averaged.
2.6. Measurments of Biochemical Parameters
The ACE activity in the aorta and blood serum samples was measured as described in Section 2.2 but without adding of ACE in the reaction mixture. Prior to measurements the aorta samples were homogenized with Silent Crusher M homogenizer (Heidolph, Schwabach, Germany) in the buffer used for ACE activity measurements.
To determine the enzymatic activities in blood serum of alanine aminotransferase (ALT), aspartic aminotransferase (AST) and lactate dehydrogenase (LDH), as well as to measure concentrations of total cholesterol (Chl), triglycerides (TG), cholesterol in high-density lipoproteins (HDLc), diagnostic kits for clinical chemistry (High Technology Inc., Attleboro Falls, MA, USA) were used. The analysis was performed on a BioChem FC-360 automatic chemistry analyzer (High Technology Inc., Attleboro Falls, MA, USA). The cholesterol concentration in low-density lipoproteins (FR-LDLc) was calculated according to the Friedewald’s equation [33]:
| FR-LDLc = Chl − HDLc − TG/2.2 | (7) |
The analysis of antioxidant capacity of blood serum was performed using trolox equivalent antioxidant capacity (TEAC) assay as described in Kruchinin et al. [34]. The antioxidant capacity of samples against ABTS radical was expressed as an amount of Trolox molar equivalents, mM (Trolox). Lipid peroxidation in blood serum samples was quantified by 2-thiobarbituric acid reactive substance (TBARS) assay according to method of Jentzsch et al. [35] with minor modifications as described in Kruchinin et al. [34]. The results were expressed as an amount of malondialdehyde (MDA) molar equivalents, µM (MDA).
2.7. Statistical Analysis
All statistical comparisons were firstly performed using one-way ANOVA omnibus F-Test. When a significant (p < 0.05) value of F-statistics was found, differences between means were evaluated using Tukey’s HSD (honestly significant difference) multiple comparison test (p < 0.05).
3. Results
3.1. The Fermentation Perfomance of Different Strains of Lactic Acid Bacteria (LAB): Selection of the Most Promissing, in Terms of the In Vitro Antioxidant and Antihypertensive Properties of the Fermented Milk, LAB Strains
For the original screening, seven traditional yogurt starter strains of Lb. delbrueckii, Lc. lactis and Str. thermophilus and one non-conventional starter strain of Lb. paracasei were chosen. The general fermentation parameters such as: the dynamic of changes for the viable cell count (i.e., CFU) during the fermentation; the development of the pH in the fermented milk; and the degree of proteolysis of the fermented milk—are presented in Figure 1A. The dynamic of changes for the antioxidant and ACE-I activities of the fermented milk are presented in Figure 1B.
Figure 1.
(A)—The general fermentation parameters of the screened strains of lactic acid bacteria; (B)—The development of antioxidant and ACE-I activities in the milk fermented by the screened strains of LAB. Strains of Lactobacillus delbrueckii and Lacticaseibacillus paracasei are abbreviated as Lb; strains of Lactococcus lactis and Streptococcus thermophilus are abbreviated as Lc and Str, respectively. For all datapoints the standard error from three biological replicas did not exceed 5%.
In terms of the general fermentation parameters (Figure 1A), the maximally attainable viable cell count was observed at 16–24 h of fermentation for all studied strains, after which it was steadily decreasing until 72 h. The exceptions were Lc. lactis MA1 and Lb. paracasei ABK; demonstrating significantly lower growth rate and a prominent lag-phase, these strains achieved the maximally attainable viable cell count only at 48 h of fermentation. For all strains of Str. thermophilus and Lc. lactis the maximally attainable viable cell count comprised 7.0 × 108–1.1 × 109 CFU·mL−1, and for both Lb. delbrueckii strains it comprised 1.1 × 108 CFU·mL−1. For all Str. thermophilus and Lc. lactis strains, the pH values reached its minimum of approximately 4.5 after 16 h of fermentation. Similarly, for both Lb. bulgaricus strains, the minimal pH of approximately 3.7–3.9 was detected after 16 h of fermentation. The Lb. paracasei ABK demonstrated the slowest rate of pH decrease reaching the pH of 4.5 at approximately 48 h of fermentation. For all studied strains, the observed degrees of milk proteolysis during the first 16 h of fermentation were comparable. However, the final degrees of proteolysis, achieved after 24 h of fermentation, appeared to be strain specific. The exceptions were two strains of Str. thermophilus and two strains of Lc. lactis (dlA and MA1) for which the final degrees of proteolysis were similar.
For the antioxidant activity (Figure 1B), its development was comparable for all studied strains during the first 16 h of milk fermentation. After which, its value increased in a similar manner for almost all studied strains reaching approximately 1150 mM (Trolox) at the end of fermentation. As an exception, for both strains of Str. thermophilus the antioxidant activity did not change after 16 h of fermentation remaining at the same level of 700 mM (Trolox). It should be noted, that both strains of Str. thermophilus produced the smallest degree of milk proteolysis in the current experiment (Figure 1A). Previously we already discussed, that strains’ proteolytic activity generally correlates with antioxidant activity of milk fermented by them [36]. The main reason for this correlation is not very stringent requirements which peptides must meet in order to possess reasonable antioxidant activity [37,38,39].
In contrast to the antioxidant activity, the dynamic of changes for ACE-I activity does not always correlate with the degree of milk proteolysis, since ACE-I peptides are very sequence-specific [39]. In our study, Str. thermophilus strains having similar values of proteolytic activity demonstrated different ACE-I activity. On the contrary, while Lb. delbrueckii Lb100 had lower proteolytic activity than Lb. delbrueckii Lb200, it demonstrated higher ACE-I activity.
Among all studied strains of Lb. bulgaricus the highest ACE-I activity (i.e., the lowest IC50 value) was demonstrated by the milk fermented with Lb. bulgaricus Lb100—IC50 values of 1–1.5 mg·mL−1. Among the strains of Str. thermophilus the highest ACE-I activity was demonstrated by the milk fermented with Str. thermophilus 159—IC50 values of 1–1.5 mg·mL−1, similar with Lb. bulgaricus Lb100. Among the strains of Lc. lactis the higher ACE-I activity was demonstrated by the milk fermented with L. lactis AM1—IC50 values of 1.7–2.0 mg·mL−1. The IC50 value of the milk fermented with Lb. paracasei ABK comprised 2.1–2.5 mg·mL−1. Consequently, the milk fermented with Lb. bulgaricus Lb100, Str. thermophilus 159, Lc. lactis AM1 and Lb. paracasei ABK were chosen for further investigations.
3.2. Profile of Fatty Acids (FA) and FA Nutritional Indices for the Milk Fermented by the Selected LAB Strains
The qualitative composition and relative abundances (percentage from total FA) of FA for the milk fermented by the chosen LAB strains are shown in Table 2. In total, in all fermentation products, 38 different FA were detected, 18 of which belonged to the group of saturated fatty acids (SFA), seven to monounsaturated fatty acids (MUFA), three to polyunsaturated fatty acids (PUFA, two “n-6” and one “n-3”), three to branched chain fatty acids (BCFA), four to hydroxyl saturated fatty acids (OH-SFA) and three to 2-hydroxy branched chain fatty acids (2OH-BCFA). Qualitatively, the greatest number of different FA were detected in the milk fermented with Lb. paracasei ABK (16—SFA, six—MUFA, one—PUFA, one—BCFA, four—OH-SFA and three—2OH-BCFA), followed by the milk fermented by Lc. lactis AM1 (18—SFA, seven—MUFA, two—PUFA and three—2OH-BCFA), Lb. delbrueckii Lb100 (14—SFA, five—MUFA, one—PUFA and two—2OH-BCFA) and Str. termophilus 159 (13—SFA, seven—MUFA, two—PUFA, three—BCFA). In terms of overall FA contents, in the milk fermented with Lb. delbrueckii Lb100 the proportion of MUFA and 2OH-BCFA (23% and 2.7%, respectively) was significantly higher than in other fermentation products. The BCFA was totally absent in the milk fermented with Lb. paracasei ABK and Lc. lactis AM1, and in the milk fermented with Str. termophilus 159 their content was significantly higher than that in the milk fermented by Lb. paracasei ABK (1.9 vs. 0.06%,). The OH-SFA were detected only in the milk fermented with Lb. paracasei ABK (2.7%) and n-3 PUFA only in the milk fermented with Lc. lactis AM1 (0.3%). The proportion of SFA was statistically the same for all fermentation products (approximately 65%).
Table 2.
Fatty acids (FA) composition of the milk fermented by the chosen strains of lactic acid bacteria.
| Fatty Acid | Relative Abundance, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Abbreviation | Lb. delbrueckii Lb100 | Lb. paracasei ABK | Lc. lactis AM1 | Str. termophilus 159 | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Saturated Fatty Acids (SFA) | |||||||||
| Pentanoic acid | C5:0 | ND | - | 0.03 | 0.01 | 0.03 | 0.01 | ND | - |
| Hexanoic acid | C6:0 | 5.06 a | 0.32 | 2.99 b | 0.14 | 3.29 b | 0.23 | 3.42 b | 0.13 |
| Heptanoic acid | C7:0 | 0.16 | 0.08 | 0.09 | 0.13 | 0.11 | 0.03 | ND | - |
| Octanoic acid | C8:0 | 8.09 a | 0.16 | 4.84 b | 0.17 | 5.53 b | 0.39 | 6.38 b | 0.09 |
| Nonanoic acid | C9:0 | 0.32 | 0.03 | 0.28 | 0.03 | 0.22 | 0.08 | 0.24 | 0.08 |
| Decanoic acid | C10:0 | 10.36 a | 1.12 | 6.49 b | 1.01 | 7.43 b | 0.91 | 8.11 b | 1.80 |
| Undecanoic acid | C11:0 | ND | - | 0.17 | 0.07 | 0.23 | 0.05 | 0.21 | 0.15 |
| Dodecanoic acid | C12:0 | 4.01 a | 0.95 | 6.06 b | 0.14 | 6.82 b | 0.23 | 6.54 b | 0.43 |
| Tridecanoic acid | C13:0 | 0.30 | 0.22 | 0.27 | 0.02 | 0.23 | 0.03 | ||
| Tetradecanoic acid | C14:0 | 6.15 a | 1.30 | 9.64 b | 0.42 | 8.82 b | 1.01 | 8.90 b | 0.18 |
| Pentadecanoic acid | C15:0 | 1.42 | 0.52 | 2.07 | 0.68 | 2.10 | 0.23 | 2.16 | 0.25 |
| Hexadecanoic acid | C16:0 | 15.96 | 1.80 | 14.77 | 4.23 | 15.52 | 3.20 | 16.24 | 4.01 |
| Heptadecanoic acid | C17:0 | ND | - | 1.21 | 0.11 | 1.07 | 0.08 | 0.91 | 0.12 |
| Octadecanoic acid | C18:0 | 13.29 | 3.12 | 13.18 | 5.20 | 12.75 | 0.74 | 12.69 | 3.25 |
| Eicosanoic acid | C20:0 | 0.61 a | 0.18 | ND | - | 1.17 b | 0.01 | 0.88 a | 0.11 |
| Docosanoic acid | C22:0 | 1.09 a | 0.06 | 0.61 b | 0.14 | 0.64 b | 0.22 | ND | - |
| Tricosanoic acid | C23:0 | 0.78 a | 0.13 | ND | - | 0.44 b | 0.22 | ND | - |
| Tetracosanoic acid | C24:0 | 0.72 a | 0.07 | 0.28 b | 0.08 | 0.30 b | 0.08 | ND | - |
| Total SFA | 68.05 | 4.16 | 63.02 | 6.84 | 66.77 | 3.61 | 66.91 | 5.50 | |
| Monounsaturated fatty acids (MUFA) | |||||||||
| 4-Decenoic acid | C10:1 (n-6) | 2.45 a | 0.33 | 1.48 b | 0.21 | 1.81 b | 0.41 | 1.99 b | 0.22 |
| Dodecenoic acid | C12:1 (n-10) | ND | - | 0.36 | 0.06 | 0.41 | 0.17 | 0.42 | 0.20 |
| 9-Tetradecenoic acid | C14:1 (n-5) | 0.41 a | 0.09 | 1.75 b | 0.73 | 1.13 b | 0.24 | 0.91 b | 0.11 |
| 9-Hexadecenoic acid | C16:1 (n-7) | 1.41 | 0.25 | 3.64 | 1.03 | 2.45 | 0.38 | 2.08 | 0.23 |
| 9-Octadecenoic acid | C18:1 (n-9) | 16.07 | 1.98 | 12.45 | 2.23 | 13.63 | 3.01 | 17.67 | 2.35 |
| 11-Octadecenoic acid | C18:1 (n-7) | 2.91 a | 0.63 | 9.19 b | 1.02 | 5.62 a | 2.01 | 3.59 a | 0.98 |
| 11-Eicosenoic acid | C20:1 (n-9) | ND | - | ND | - | 3.25 a | 1.02 | 0.78 b | 0.09 |
| Total MUFA | 23.25 a | 2.12 | 28.87 b | 2.77 | 28.28 b | 3.81 | 27.44 b | 2.58 | |
| Polyunsaturated fatty acids (PUFA) | |||||||||
| 9,12-Octadecadienoic acid | C18:2 (n-6) | 5.99 a | 1.22 | 4.17 b | 2.03 | 4.14 b | 1.56 | 3.35 b | 0.69 |
| 5,8,11,14-Eicosatetraenoic acid | C20:4 (n-6) | ND | - | ND | - | ND | - | 0.41 | 0.10 |
| 4,7,10,13,16,19-Docosahexaenoic acid | C22:6 (n-3) | ND | - | ND | - | 0.33 | 0.05 | ND | - |
| Total PUFA | 5.99 | 1.22 | 4.17 | 2.03 | 4.48 | 1.56 | 3.77 | 0.70 | |
| Branched chain fatty acids (BCFA) | |||||||||
| Tetradecanoic acid, 9-methyl | 9Me-C14:0 | ND | - | 0.63 | 0.11 | ND | - | 0.76 | 0.11 |
| Hexadecanoic acid, 15-methyl- | 15MeC16:0 (iso-C17:0) | ND | - | ND | - | ND | - | 0.46 | 0.15 |
| Hexadecanoic acid, 14-methyl- | 14MeC16:0 (anteiso-C17:0) | ND | - | ND | - | ND | - | 0.68 | 0.09 |
| Total BCFA | ND | - | 0.63 a | 0.11 | ND | - | 1.89 b | 0.21 | |
| Hydroxy saturated fatty acids (OH-SFA) | |||||||||
| Octanoic acid, 3-hydroxy- | 3OH-C8:0 | ND | - | 0.05 | 0.20 | ND | - | ND | - |
| Decanoic acid, 3-hydroxy- | 3OH-C10:0 | ND | - | 0.07 | 0.22 | ND | - | ND | - |
| Tetradecanoic acid, 3-hydroxy- | 3OH-C14:0 | ND | - | 0.22 | 0.11 | ND | - | ND | - |
| Octadecanoic acid, 10-hydroxy- | 10OH-C18:0 | ND | - | 2.39 | 1.03 | ND | - | ND | - |
| Total OH-SFA | ND | - | 2.73 | 1.08 | ND | - | ND | - | |
| 2-hydroxy branched chain fatty acids (2OH-BCFA) | |||||||||
| Butyric acid, 2-hydroxy-3-methyl- | 2OH-3MeC4:0 (2OH-iso-C5:0) | ND | - | 0.09 | 0.10 | 0.10 | 0.08 | ND | - |
| Pentanoic acid, 2-hydroxy-4-methyl- | 2OH-4MeC5:0 (2OH-iso-C6:0) | 1.80 a | 0.32 | 0.43 b | 0.13 | 0.20 b | 0.26 | ND | - |
| Pentanoic acid, 2-hydroxy-3-methyl- | 2OH-3MeC5:0 (2OH-anteiso-C6:0) | 0.91 a | 0.26 | 0.06 b | 0.02 | 0.18 b | 0.03 | ND | - |
| Total 2OH-BCFA | 2.72 a | 0.41 | 0.58 b | 0.17 | 0.48 b | 0.10 | ND | - | |
ND: not detected; SD: standard deviation. a,b Means within the same row with different superscripts are significantly different (p < 0.05).
It is well known that the consumption of certain dietary fats, which are generally FA, may exert either positive or negative effects on human health [40,41,42]. To evaluate the potential role of FA content of food in the treatment and prevention of cardiovascular diseases, several FA-related nutritional indices have been developed. Currently, the most frequently used FA-related nutritional indices are [32]: PUFA/SFA—ratio of total PUFA to total SFA; IA—index of atherogenicity; HPI—health-promoting index (which is the reciprocal of IA and mainly used in research on dairy products); IT—index of thrombogenicity; HH—hypocholesterolemic/hypercholesterolemic ratio; UI—unsaturation index. The calculation of mentioned indices for the studied dairy beverages as well as their general interpretation are presented in Table 3. Generally, all of the calculated indices were in the range previously reported for different dairy products such as yogurt, kefir, ryazhenka and amasi [31,32]. The comparison of studied fermentation products in terms of the mentioned FA-related nutritional indices did not detect any statistically-significant differences (p > 0.05). The exception was IA, HPI and HH indices by which milk fermented with Lb. delbrueckii Lb100 significantly outperformed (p < 0.05) all other fermentation products.
Table 3.
Fatty acid (FA) related nutritional indices of the milk fermented by the chosen strains of lactic acid bacteria.
| Index | General Interpretation | Lb. delbrueckii Lb100 | Lb. paracasei ABK | Lc. lactis AM1 | Str. termophilus 159 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| PUFA/SFA | The higher—The better | 0.09 | 0.02 | 0.07 | 0.03 | 0.07 | 0.02 | 0.06 | 0.01 |
| IA | The lower—The better | 1.53 a | 0.15 | 1.80 b | 0.23 | 1.76 b | 0.24 | 1.87 b | 0.21 |
| HPI | The higher—The better | 0.66 a | 0.07 | 0.56 b | 0.07 | 0.57 b | 0.08 | 0.53 b | 0.06 |
| IT | The lower—The better | 2.42 | 0.20 | 2.28 | 0.27 | 2.15 | 0.26 | 2.42 | 0.22 |
| HH | The higher—The better | 0.84 a | 0.12 | 0.55 b | 0.12 | 0.58 b | 0.11 | 0.68 b | 0.09 |
| UI | The higher—The better | 35.22 | 2.45 | 37.21 | 3.43 | 38.56 | 4.12 | 35.79 | 2.67 |
ND: not detected; SD: standard deviation. a,b Means within the same row with different superscripts are significantly different (p < 0.05).
3.3. The In Vivo Assessment of the Antihypertensive Properties for the Milk Fermented by the Selected LAB Strains in the SHR Animal Model
The in vivo assessment of the antihypertensive properties for the milk fermented by the chosen LAB strains was performed in SHR animal model. Currently, SHR is one of the most popular animal models of essential (or primary) hypertension [43,44,45]. The experimental setup included six groups of SHRs: one Intact, one Control and four experimental. The Intact animals received only a standard daily ration; the ration of the Control group was supplemented with milk; and the ration of the experimental groups Lb100, ABK, AM1 and St159 was supplemented with milk fermented by Lb. bulgaricus LB100, Lb. paracasei ABK, L. lactis AM1 and Str. thermophilus 159, respectively.
The data on the absolute values of the Psyst, Pdias and Weight for each animal group at the beginning and end of the experiment are present in Figure 2A, and the changes of these parameters (ΔPsyst, ΔPdias and ΔWeight) are presented Figure 2B. For all animal groups but Lb100 and AM1, Psyst increased at the end of the experiment and reached approximately 182 mmHg, and ΔPsyst was approximately 21 mmHg. For Lb100 and AM1, the Psyst decreased down to 154 mmHg at the end of experiment, and ΔPsyst was approximately −17 mmHg. While no statistically-significant changes (p > 0.05) in Pdias for all studied groups were detected, for Lb100 and AM1 the downward trend in Pdias was observed. In case of the Weight, the was no statistically-significant difference (p > 0.05) between ΔWeight of the Intact, Control, ABK, and AM1 groups. Animals from these groups gained on average 25 g at the end of the experiment, reaching the final weight of 315 g. In comparison, the weight gains of Lb100 and St159 were significantly smaller (p < 0.05) and comprised 7 g, reaching the final weight of 285 g.
Figure 2.
The in vivo assessment of antihypertensive properties for the milk fermented by the chosen strains of lactic acid bacteria using Spontaneously Hypertensive Rat (SHR) animal model: (A)—the absolute values of the Psyst, Pdias and Weight. The black bars correspond to the Mean ± SE at the beginning of the experiment, and the blue bar correspond to the Mean ± SE at the end (4 weeks) of the experiment; (B)—the changes of the Psyst, Pdias and Weight. The bars (Mean ± SE) for the groups between which no statistically-significant differences were detected are depicted in the same color.
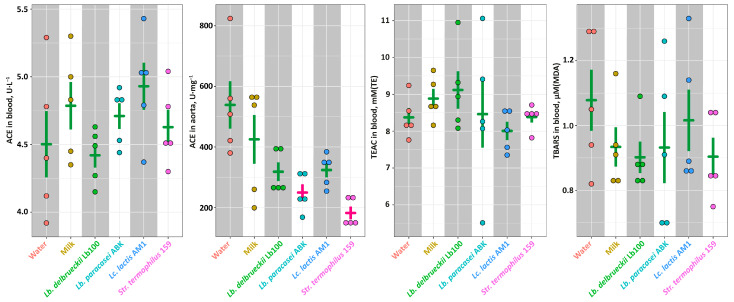
At the end of the experiment all animals were euthanized, and several health-relevant biochemical parameters were assessed. No statistically-significant differences (p > 0.05) of the ACE activity were observed in the animals’ blood (Figure 3). In the animals’ aorta, the ACE activity was significantly lower (p < 0.05) in the ABK and St159 groups compared to the Intact and Control groups, and for the Lb100 and AM1 groups only the downward trends were observed. For all animals, the level of the oxidative stress was assessed by the measuring of TEAC and TBARS concentration in blood (Figure 3). The TEAC value represent an overall antioxidant capacity of biological sample [46], and TBARS value corresponds to the advancement of lipid peroxidation under the oxidative stress. For all animal groups, the values of both TEAC and TBARS were not statistically-significantly different (p > 0.05). However, there was a trend for the milk fermented with Lb. delbrueckii Lb100 to have higher TEAC and lower TBARS than in other fermented products.
Figure 3.
The ACE activity in blood and aorta, as well as the markers of the oxidant-antioxidant status for studied animal groups. The bars (Mean ± SE) for the groups between which no statistically-significant differences were detected are depicted in the same color.
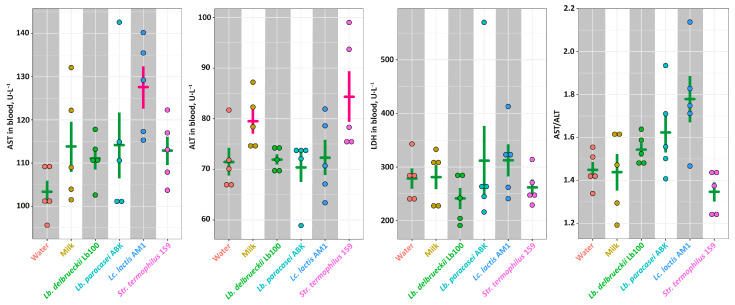
The function of liver was assessed by measuring widespread biomarkers of liver health—AST, ALT and LDH (Figure 4). Although the significantly elevated (p < 0.05) level of AST was detected in the AM1 group, and the significantly elevated (p < 0.05) level of ALT in the Milk and St159 groups, the AST/ALT ratios were not statistically-significantly different (p > 0.05) for all studied groups. Similarly, the level of LDH in blood was the same for all studied groups.
Figure 4.
The markers of the liver health for studied animal groups. The bars (Mean ± SE) for the groups between which no statistically-significant differences were detected are depicted in the same color.
The lipid profile (i.e., Chl, TG, FR-LDLc and HDLc) for each animal group as well as the value of atherogenic coefficient (AC) calculated according to Nimmanapalli et al. [47] are presented in Figure 5. Among studied groups, there was no statistically-significant differences (p > 0.05) detected by all measured parameters. The exceptions were levels of Chl and HDLc that were significantly lower (p < 0.05) in the Lb100 group.
Figure 5.
The markers of the lipid metabolism for studied animal groups. The bars (Mean ± SE) for the groups between which no statistically-significant differences were detected are depicted in the same color.
4. Discussion
Hypertension is a serious medical condition that significantly increases the risks of cardiovascular and other diseases [48]. The extreme widespread of hypertension in modern world forces scientists to look for new ways of its prophylaxis, among which antihypertensive functional food, especially fermented dairy products, is the most promising one [49]. Therefore, the objective of the current study was to compare different strains of LAB commonly used in dairy industry on their ability to produce fermented milk with antihypertensive properties. At the first stage of the investigation, the in vitro assessment of ACE-I properties of milk fermented by different LAB strains was carried out, and the best performed strains were selected. At the second stage, the antihypertensive properties of the milk fermented by the selected strains were tested in vivo using SHR animal model. Additionally, the FA composition of the fermented milk was investigated, and FA-related nutritional indices linked with cardiovascular health were calculated.
At the first stage of the investigation, two strains of Lb. delbrueckii (Lb100 and Lb200), three strains of Lc. lactis (dlA, AM1 and MA1), two strains of Str. thermophilus (159 and 16t) and the strain Lb. paracasei ABK were tested (Figure 1). As a result, the strains Lb. bulgaricus Lb100, Lc. lactis AM1, Str. thermophilus 159, and Lb. paracasei ABK demonstrated ACE IC50 of 1–1.5 mg·mL−1, 1.8–2.0 mg·mL−1, 1–1.5 mg·mL−1, and 2.1–2.5 mg·mL−1, respectively, were chosen for further investigations. Currently, milks fermented with Lactobacillus spp. are the most commonly studied for ACE-I activity, while data about Lactococcus spp. and Streptococcus spp. are relatively scarce. The typically reported diapason of IC50 for the different strains of Lb. bulgaricus (and for Lactobacillus spp. in general) is 0.5–10 mg·mL−1 [9,19,50,51,52,53]. The typically reported diapason of IC50 for the different strains of Lc. lactis is 0.1–8 mg·mL−1 [51,52,53,54,55], and for the different strains of Str. thermophilus is 0.2–0.8 mg·mL−1 [50,51,52]. Thus, although our strains did not show outstanding results in terms of IC50 of fermented milk, they demonstrated values close to the lower end of the previously reported range.
At the second stage of the investigation, SHRs were fed by milk fermented with the selected LAB strains and changes in systolic and diastolic blood pressure were measured. Additionally, several biochemically relevant parameters such as: ACE activity in aorta; ACE, AST, ALT and LDH activities in blood; TEAC, TBARS, Chl, TG, HDLc and FR-LDLc concentration in blood—were assessed. Out of the four selected fermentation products, which demonstrated substantial ACE-I activity in vitro, only the milks fermented by Lb. bulgaricus Lb100 and Lc. lactis AM1 demonstrated pronounced in vivo antihypertensive effect (Figure 2). While the consumption of the milk fermented with Lc. lactis AM1 stabilized Psyst of SHRs at the initial (at the beginning of the experiment) level, the consumption of the milk fermented with Lb. bulgaricus Lb100 lowered Psyst of SHRs during the experiment. The observed poor agreement between in vitro (using the ACE-I assay) and in vivo (using appropriate animal models) methods for evaluation of antihypertensive properties of fermented milk is well known. The main reason of this poor agreement is that in vitro tests reflect only the interaction of the inhibitors (e.g., peptides) with ACE, and in vivo tests account for many other possible physiological factors involved in the manifestation of hypertension [20].
Comparing of our SHR experiments with previously published, it should be noted that most of the works are devoted to either products obtained using combined starter cultures [56,57,58,59,60] or products artificially enriched with antihypertensive peptides [61,62], and only few studies have tested hypotensive effect of milk fermented with individual strains. Previously, the in vivo effect of long-term intake of milk fermented with individual LAB strains was studied for the milk fermented with Lc. lactis [63,64], Lactobacillus helveticus [60,61,65], Lb. delbrueckii [63], and Str. thermophilus [63]. In most cases, the blood pressure of SHRs gradually increased during the experiment, and fermented milk only slowed down the development of hypertension (i.e., the Psyst at the end of experiments was higher than at their beginning). Only two studies, as well as our results, demonstrated a reduction in pressure not only relative to the control but also relative to the starting point of the experiment [58,65].
To find out whether the observed in vivo antihypertensive effect is the result of ACE inhibition, we measured the ACE activity in the blood and aorta of SHRs (Figure 3). While ACE activity in the blood was not statistically-significantly different (p > 0.05) for all studied groups, the decrease of the ACE activity in the aorta of SHRs consuming fermented milk products was detected. This is consistent with the data of other researchers – in the works of Nakamura et al. [56] and Kim et al. [57], when animals were fed with hypotensive fermented milk, the ACE activity in the blood plasma did not change, while the ACE activity in the aorta was significantly reduced. However, consumption of milks fermented with Lb. paracasei ABK and Str. thermophilus 159 statistically-significantly reduced (p < 0.05) the activity of ACE in the aorta, but did not affect blood pressure (Figure 2 and Figure 3). At the same time, milks fermented with Lb. bulgaricus Lb100 and L. lactis AM1, for which only a trend (p > 0.05) for a decrease in ACE activity in the aorta was detected, caused significant (p < 0.05) improvement of Psyst (Figure 2 and Figure 3). This once again emphasizes that the development of primary hypertension is a complex process for which a causal relationship is not always clear.
It is generally recognized that hypertension is closely associated with endothelial dysfunction, which leads to a decrease in the bioavailability of nitric oxide, one of the main vasodilators in the human body, due to an increased level of oxidative stress (nitric oxide reacts with the reactive oxygen species, ROS). In addition, several cross-sectional studies suggested a link between dyslipidemia and hypertension, and proposed that dyslipidemia can cause endothelial damage [66,67,68,69]. Hypertension occurs more frequently for hypercholesterolemic subjects, as compared to normolipid [70].
In our work, we evaluated the level of oxidative stress by measuring TEAC and TBARS in the blood, as well as the blood lipid profile (Figure 3 and Figure 5). Unfortunately, at this stage, we cannot put forward any hypotheses about the reasons for the decrease in Psyst upon the consumption of milk fermented with Lc. lactis AM1. However, the effect of reducing blood pressure upon the consumption of milk fermented with Lb. bulgaricus Lb100 can be related to its two additional to ACE-I properties—antioxidant and hypocholesterolemic. Compared to other animal groups, there was a strong trend for SHRs consuming the milk fermented with Lb. bulgaricus Lb100 to have increased level of TEAC and decreased level of TBARS in the blood, which suggests their better oxidant/antioxidant status. Moreover, the milk fermented with Lb. bulgaricus Lb100 statistically-significantly decreased the level of blood cholesterol (p < 0.05).
Since the consumption of certain FAs was associated with the risks of cardiovascular and other diseases for several decades [71,72,73,74], for each in vivo tested fermented milk the analysis of FA profile was performed. It is generally accepted that substitution of SFA with n-3 PUFAs and MUFAs in diet can exert positive cardiovascular effects via improvement of plasma membrane fluidity, decreasing the risk of inflammation, improving oxidant/antioxidant status etc. [75]. Currently, several FA-related nutritional indices that take into account both types of SFAs and their ratio to n-3 PUFAs and MUFAs in different ways (Equations (1)–(6)) have been developed; however, the potential usage of these indices in disease prevention and treatment is still a controversial issue [32]. Interestingly, the milk fermented with Lb. bulgaricus Lb100 demonstrated a number of improved FA-related nutritional indices (i.e., IA, HPI and HH) compared to other products (Table 2). Hence, our results suggested that at least in case of fermented dairy products such FA-related nutritional indices as IA, HPI and HH can have a potential predictive value for prevention of primary hypertension.
5. Conclusions
In the present study, the eight dairy products obtained via the fermentation with different starter strains of LAB were tested in vitro for their ACE-I activity, and four best performed products were further tested in vivo in SHR animal model for their antihypertensive effect. Only two out of four products demonstrated pronounced in vivo antihypertensive effect, which highlights the poor agreement between the in vitro (using the ACE-I assay) and in vivo (using SHR animal model) methods for evaluation of antihypertensive properties of fermented milk. Moreover, the most prominent in vivo antihypertensive effect was demonstrated for the milk fermented with Lb. bulgaricus Lb100, consumption of which did not significantly reduced (p > 0.05) the ACE activity in the aorta of SHRs (although, the trend for its reduction was observed), suggesting a more complex reason of its antihypertensive action than just ACE inhibition. It was further shown that consumption of milk fermented by Lb. bulgaricus Lb100 significantly (p < 0.05) reduced the level of total cholesterol in the blood of SHRs, and improved (as a trend, p > 0.05) their oxidant/antioxidant status (i.e., increased level of TEAC and decreased level of TBARS in their blood). It can be hypothesized that the pronounced antihypertensive effect of the milk fermented with Lb. bulgaricus Lb100 can be a result of synergy between decrease of ACE activity and improvement of endothelial functions via lowering of cholesterol and improvement of oxidant/antioxidant status. Additionally, the milk fermented with Lb. bulgaricus Lb100 demonstrated a number of improved FA-related nutritional indices (i.e., IA, HPI and HH) that were previously linked with cardiovascular health, although their predictive value is still debatable. Therefore, Lb. bulgaricus Lb100 can be regarded as promising starter strain for production of functional fermented milk or yogurt with antihypertensive and hypocholesterolemic properties without additional ingredients in its recipe, although the exact mechanism of its health promoting action is still remains to be discovered.
Author Contributions
Conceptualization, O.A.G., K.V.M. and T.V.F.; validation, O.A.G. and T.V.F.; formal analysis, K.V.M.; investigation, O.S.S. and T.V.F.; writing—original draft preparation, O.A.G., K.V.M. and T.V.F.; writing—review and editing, O.A.G., K.V.M. and T.V.F.; visualization, O.A.G. and K.V.M.; supervision, T.V.F.; funding acquisition, T.V.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Experiments were conducted in accordance with the legislation on protection of animals used for scientific purposes in Russia (directive N199n dated 1 April 2016 of the Ministry of Health of Russia) and the EU (directive 1292010/63/EU). Experimental protocols were approved by the Ethics Committee for Animal Research of the Federal Research Center Fundamentals of Biotechnology of the Russian Academy of Sciences (#17 from 25 August 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The article was made with support of the Ministry of Science and Higher Education of the Russian Federation in accordance with agreement No. 075-15-2022-318 date 20 April 2022 on providing a grant in the form of subsidies from the Federal budget of the Russian Federation. The grant was provided for state support for the creation and development of a world-class scientific center: “Agrotechnologies for the Future”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou B., Carrillo-Larco R.M., Danaei G., Riley L.M., Paciorek C.J., Stevens G.A., Gregg E.W., Bennett J.E., Solomon B., Singleton R.K., et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwers S., Sudano I., Kokubo Y., Sulaica E.M. Arterial hypertension. Lancet. 2021;398:249–261. doi: 10.1016/S0140-6736(21)00221-X. [DOI] [PubMed] [Google Scholar]
- 3.Riccio E., Capuano I., Buonanno P., Andreucci M., Provenzano M., Amicone M., Rizzo M., Pisani A. RAAS Inhibitor Prescription and Hyperkalemia Event in Patients with Chronic Kidney Disease: A Single-Center Retrospective Study. Front. Cardiovasc. Med. 2022;9:74. doi: 10.3389/fcvm.2022.824095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari R. RAAS inhibition and mortality in hypertension. Glob. Cardiol. Sci. Pract. 2013;2013:34. doi: 10.5339/gcsp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghazi L., Drawz P. Advances in understanding the renin-angiotensin-aldosterone system (RAAS) in blood pressure control and recent pivotal trials of RAAS blockade in heart failure and diabetic nephropathy. F1000Research. 2017;6:297. doi: 10.12688/f1000research.9692.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steckelings U.M., Rompe F., Kaschina E., Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease—moving from macrovascular to microvascular targets. Fundam. Clin. Pharmacol. 2009;23:693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 7.te Riet L., van Esch J.H.M., Roks A.J.M., van den Meiracker A.H., Danser A.H.J. Hypertension. Circ. Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 8.Aprotosoaie A.C., Costache A.-D., Costache I.-I. Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go? Pharmaceutics. 2022;14:722. doi: 10.3390/pharmaceutics14040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y., Yu J., Xu W. Purification and characterization of angiotensin-I-converting enzyme inhibitory peptides isolated from whey proteins of milk fermented with Lactobacillus plantarum QS670. J. Dairy Sci. 2020;103:4919–4928. doi: 10.3168/jds.2019-17594. [DOI] [PubMed] [Google Scholar]
- 10.Jogi N., Yathisha U.G., Bhat I., Mamatha B.S. Antihypertensive activity of orally consumed ACE-I inhibitory peptides. Crit. Rev. Food Sci. Nutr. 2022;62:8986–8999. doi: 10.1080/10408398.2021.1938508. [DOI] [PubMed] [Google Scholar]
- 11.Das S., Hati S. Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health. Elsevier; Amsterdam, The Netherlands: 2022. Food derived ACE inhibitory peptides; pp. 39–54. [Google Scholar]
- 12.García-Burgos M., Moreno-Fernández J., Alférez M.J.M., Díaz-Castro J., López-Aliaga I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods. 2020;72:104059. doi: 10.1016/j.jff.2020.104059. [DOI] [Google Scholar]
- 13.Ghavami A., Ziaei R., Moradi S., Sharifi S., Reza Moravejolahkami A., Ghaffari S., Irandoost P., Khorvash F., Mokari_yamchi A., Nattagh-Eshtivani E., et al. Potential of favorable effects of probiotics fermented milk supplementation on blood pressure: A systematic review and meta-analysis. Int. J. Food Prop. 2020;23:1925–1940. doi: 10.1080/10942912.2020.1833030. [DOI] [Google Scholar]
- 14.Martin M., Deussen A. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit. Rev. Food Sci. Nutr. 2019;59:1264–1283. doi: 10.1080/10408398.2017.1402750. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi A., Shah N.P. Cell growth and proteolytic activity of Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, and Streptococcus thermophilus in milk as affected by supplementation with peptide fractions. Int. J. Food Sci. Nutr. 2014;65:937–941. doi: 10.3109/09637486.2014.945154. [DOI] [PubMed] [Google Scholar]
- 16.Ji D., Ma J., Xu M., Agyei D. Cell-envelope proteinases from lactic acid bacteria: Biochemical features and biotechnological applications. Compr. Rev. Food Sci. Food Saf. 2021;20:369–400. doi: 10.1111/1541-4337.12676. [DOI] [PubMed] [Google Scholar]
- 17.Szliszka E., Czuba Z.P., Domino M., Mazur B., Zydowicz G., Krol W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules. 2009;14:738–754. doi: 10.3390/molecules14020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai A.K., Sanjukta S., Jeyaram K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit. Rev. Food Sci. Nutr. 2017;57:2789–2800. doi: 10.1080/10408398.2015.1068736. [DOI] [PubMed] [Google Scholar]
- 19.Beltrán-Barrientos L.M., Hernández-Mendoza A., Torres-Llanez M.J., González-Córdova A.F., Vallejo-Córdoba B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016;99:4099–4110. doi: 10.3168/jds.2015-10054. [DOI] [PubMed] [Google Scholar]
- 20.Udenigwe C.C., Mohan A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods. 2014;8:45–52. doi: 10.1016/j.jff.2014.03.002. [DOI] [Google Scholar]
- 21.Mell B., Jala V.R., Mathew A.V., Byun J., Waghulde H., Zhang Y., Haribabu B., Vijay-Kumar M., Pennathur S., Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adnan S., Nelson J.W., Ajami N.J., Venna V.R., Petrosino J.F., Bryan R.M., Durgan D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toral M., Robles-Vera I., Visitación N., Romero M., Sánchez M., Gómez-Guzmán M., Rodriguez-Nogales A., Yang T., Jiménez R., Algieri F., et al. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol. 2019;227:e13285. doi: 10.1111/apha.13285. [DOI] [PubMed] [Google Scholar]
- 25.Beltrán-Barrientos L.M., García H.S., Hernández-Mendoza A., González-Córdova A.F., Vallejo-Cordoba B. Invited review: Effect of antihypertensive fermented milks on gut microbiota. J. Dairy Sci. 2021;104:3779–3788. doi: 10.3168/jds.2020-19466. [DOI] [PubMed] [Google Scholar]
- 26.Paszczyk B., Tońska E. Fatty Acid Content, Lipid Quality Indices, and Mineral Composition of Cow Milk and Yogurts Produced with Different Starter Cultures Enriched with Bifidobacterium bifidum. Appl. Sci. 2022;12:6558. doi: 10.3390/app12136558. [DOI] [Google Scholar]
- 27.Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- 28.Ou B., Hampsch-Woodill M., Prior R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaev I.V., Sforza S., Lambertini F., Ismailova D.Y., Khotchenkov V.P., Volik V.G., Dossena A., Popov V.O., Koroleva O.V. Biocatalytic conversion of poultry processing leftovers: Optimization of hydrolytic conditions and peptide hydrolysate characterization. Food Chem. 2016;197:611–621. doi: 10.1016/j.foodchem.2015.10.114. [DOI] [PubMed] [Google Scholar]
- 30.Torkova A.A., Ryazantseva K.A., Agarkova E.Y., Kruchinin A.G., Tsentalovich M.Y., Fedorova T.V. Rational design of enzyme compositions for the production of functional hydrolysates of cow milk whey proteins. Appl. Biochem. Microbiol. 2017;53:669–679. doi: 10.1134/S0003683817060138. [DOI] [Google Scholar]
- 31.Moiseenko K.V., Glazunova O.A., Savinova O.S., Ajibade B.O., Ijabadeniyi O.A., Fedorova T.V. Analytical Characterization of the Widely Consumed Commercialized Fermented Beverages from Russia (Kefir and Ryazhenka) and South Africa (Amasi and Mahewu): Potential Functional Properties and Profiles of Volatile Organic Compounds. Foods. 2021;10:3082. doi: 10.3390/foods10123082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Liu H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020;21:5695. doi: 10.3390/ijms21165695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 34.Kruchinin A.G., Savinova O.S., Glazunova O.A., Moiseenko K.V., Agarkova E.Y., Fedorova T.V. Hypotensive and Hepatoprotective Properties of the Polysaccharide-Stabilized Foaming Composition Containing Hydrolysate of Whey Proteins. Nutrients. 2021;13:1031. doi: 10.3390/nu13031031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jentzsch A.M., Bachmann H., Fürst P., Biesalski H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 36.Begunova A.V., Savinova O.S., Glazunova O.A., Moiseenko K.V., Rozhkova I.V., Fedorova T.V. Development of Antioxidant and Antihypertensive Properties during Growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow’s Milk: Fermentation and Peptidomics Study. Foods. 2020;10:17. doi: 10.3390/foods10010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: A review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Pihlanto A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006;16:1306–1314. doi: 10.1016/j.idairyj.2006.06.005. [DOI] [Google Scholar]
- 39.Udenigwe C.C., Aluko R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012;77:R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Cortés P., Juárez M., de la Fuente M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018;81:1–9. doi: 10.1016/j.tifs.2018.08.014. [DOI] [Google Scholar]
- 41.Calder P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015;39:18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 42.Williams C.M. Dietary fatty acids and human health. Ann. Zootech. 2000;49:165–180. doi: 10.1051/animres:2000116. [DOI] [Google Scholar]
- 43.Lerman L.O., Kurtz T.W., Touyz R.M., Ellison D.H., Chade A.R., Crowley S.D., Mattson D.L., Mullins J.J., Osborn J., Eirin A., et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2019;73:e87–e120. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerman L.O., Chade A.R., Sica V., Napoli C. Animal models of hypertension: An overview. J. Lab. Clin. Med. 2005;146:160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Pinto Y. Lessons from rat models of hypertension from Goldblatt to genetic engineering. Cardiovasc. Res. 1998;39:77–88. doi: 10.1016/S0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 46.Horvathova M., Zitnanova I., Kralovicova Z., Balis P., Puzserova A., Muchova J., Kluknavsky M., Durackova Z., Bernatova I. Sex differences in the blood antioxidant defense system in juvenile rats with various genetic predispositions to hypertension. Hypertens. Res. 2016;39:64–69. doi: 10.1038/hr.2015.117. [DOI] [PubMed] [Google Scholar]
- 47.Nimmanapalli H., Kasi A., Devapatla P., Nuttakki V. Lipid ratios, atherogenic coefficient and atherogenic index of plasma as parameters in assessing cardiovascular risk in type 2 diabetes mellitus. Int. J. Res. Med. Sci. 2016;4:2863–2869. doi: 10.18203/2320-6012.ijrms20161966. [DOI] [Google Scholar]
- 48.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.González S., Fernández-Navarro T., Arboleya S., de los Reyes-Gavilán C.G., Salazar N., Gueimonde M. Fermented Dairy Foods: Impact on Intestinal Microbiota and Health-Linked Biomarkers. Front. Microbiol. 2019;10:1046. doi: 10.3389/fmicb.2019.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramchandran L., Shah N.P. Proteolytic Profiles and Angiotensin-I Converting Enzyme and α-Glucosidase Inhibitory Activities of Selected Lactic Acid Bacteria. J. Food Sci. 2008;73:M75–M81. doi: 10.1111/j.1750-3841.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 51.Nejati F., Rizzello C.G., Di Cagno R., Sheikh-Zeinoddin M., Diviccaro A., Minervini F., Gobbetti M. Manufacture of a functional fermented milk enriched of Angiotensin-I Converting Enzyme (ACE)-inhibitory peptides and γ-amino butyric acid (GABA) LWT-Food Sci. Technol. 2013;51:183–189. doi: 10.1016/j.lwt.2012.09.017. [DOI] [Google Scholar]
- 52.Loghman S., Moayedi A., Mahmoudi M., Khomeiri M., Gómez-Mascaraque L.G., Garavand F. Single and Co-Cultures of Proteolytic Lactic Acid Bacteria in the Manufacture of Fermented Milk with High ACE Inhibitory and Antioxidant Activities. Fermentation. 2022;8:448. doi: 10.3390/fermentation8090448. [DOI] [Google Scholar]
- 53.Gobbetti M., Ferranti P., Smacchi E., Goffredi F., Addeo F. Production of Angiotensin-I-Converting-Enzyme-Inhibitory Peptides in Fermented Milks Started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl. Environ. Microbiol. 2000;66:3898–3904. doi: 10.1128/AEM.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pihlanto A., Virtanen T., Korhonen H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010;20:3–10. doi: 10.1016/j.idairyj.2009.07.003. [DOI] [Google Scholar]
- 55.Hagi T., Kobayashi M., Nomura M. Metabolome analysis of milk fermented by γ-aminobutyric acid–producing Lactococcus lactis. J. Dairy Sci. 2016;99:994–1001. doi: 10.3168/jds.2015-9945. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura Y., Masuda O., Takano T. Decrease of Tissue Angiotensin I-Converting Enzyme Activity upon Feeding Sour Milk in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 1996;60:488–489. doi: 10.1271/bbb.60.488. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.M., Park S., Choue R. Effects of fermented milk peptides supplement on blood pressure and vascular function in spontaneously hypertensive rats. Food Sci. Biotechnol. 2010;19:1409–1413. doi: 10.1007/s10068-010-0201-0. [DOI] [Google Scholar]
- 58.Ramchandran L., Shah N.P. Yogurt Can Beneficially Affect Blood Contributors of Cardiovascular Health Status in Hypertensive Rats. J. Food Sci. 2011;76:H131–H136. doi: 10.1111/j.1750-3841.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 59.Kong C.-Y., Li Z.-M., Mao Y.-Q., Chen H.-L., Hu W., Han B., Wang L.-S. Probiotic yogurt blunts the increase of blood pressure in spontaneously hypertensive rats via remodeling of the gut microbiota. Food Funct. 2021;12:9773–9783. doi: 10.1039/D1FO01836A. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y., Liu W., Xue J., Yang J., Chen X., Shao Y., Kwok L., Bilige M., Mang L., Zhang H. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J. Dairy Sci. 2014;97:6680–6692. doi: 10.3168/jds.2014-7962. [DOI] [PubMed] [Google Scholar]
- 61.Sipola M., Finckenberg P., Korpela R., Vapaatalo H., Nurminen M.L. Effect of long-term intake of milk products on blood pressure in hypertensive rats. J. Dairy Res. 2002;69:103–111. doi: 10.1017/S002202990100526X. [DOI] [PubMed] [Google Scholar]
- 62.Jauhiainen T., Collin M., Narva M., Cheng Z.J., Poussa T., Vapaatalo H., Korpela R. Effect of long-term intake of milk peptides and minerals on blood pressure and arterial function in spontaneously hypertensive rats. Milchwissenschaft. 2005;60:358–363. [Google Scholar]
- 63.Yamamoto N., Akino A., Takano T. Antihypertensive Effects of Different Kinds of Fermented Milk in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 1994;58:776–778. doi: 10.1271/bbb.58.776. [DOI] [Google Scholar]
- 64.Rodríguez-Figueroa J.C., González-Córdova A.F., Astiazaran-García H., Hernández-Mendoza A., Vallejo-Cordoba B. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J. Dairy Sci. 2013;96:4094–4099. doi: 10.3168/jds.2012-6014. [DOI] [PubMed] [Google Scholar]
- 65.Ehlers P.I., Kivimäki A.S., Turpeinen A.M., Korpela R., Vapaatalo H. High blood pressure-lowering and vasoprotective effects of milk products in experimental hypertension. Br. J. Nutr. 2011;106:1353–1363. doi: 10.1017/S0007114511001723. [DOI] [PubMed] [Google Scholar]
- 66.Halperin R.O., Sesso H.D., Ma J., Buring J.E., Stampfer M.J., Gaziano J.M. Dyslipidemia and the Risk of Incident Hypertension in Men. Hypertension. 2006;47:45–50. doi: 10.1161/01.HYP.0000196306.42418.0e. [DOI] [PubMed] [Google Scholar]
- 67.Kuwabara M., Kuwabara R., Niwa K., Hisatome I., Smits G., Roncal-Jimenez C., MacLean P., Yracheta J., Ohno M., Lanaspa M., et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients. 2018;10:1011. doi: 10.3390/nu10081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang F., Zhang Y., Guo Z., Yang H., Ren M., Xing X., Cong H. The association of triglyceride and glucose index, and triglyceride to high-density lipoprotein cholesterol ratio with prehypertension and hypertension in normoglycemic subjects: A large cross-sectional population study. J. Clin. Hypertens. 2021;23:1405–1412. doi: 10.1111/jch.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oparil S., Zaman M.A., Calhoun D.A. Pathogenesis of Hypertension. Ann. Intern. Med. 2003;139:761. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 70.Lye H., Kuan C., Ewe J., Fung W., Liong M. The Improvement of Hypertension by Probiotics: Effects on Cholesterol, Diabetes, Renin, and Phytoestrogens. Int. J. Mol. Sci. 2009;10:3755–3775. doi: 10.3390/ijms10093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silva Figueiredo P., Carla Inada A., Marcelino G., Maiara Lopes Cardozo C., de Cássia Freitas K., de Cássia Avellaneda Guimarães R., Pereira de Castro A., Aragão do Nascimento V., Aiko Hiane P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients. 2017;9:1158. doi: 10.3390/nu9101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baum S.J., Kris-Etherton P.M., Willett W.C., Lichtenstein A.H., Rudel L.L., Maki K.C., Whelan J., Ramsden C.E., Block R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012;6:216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuller L.H. Dietary Fat and Chronic Diseases. J. Am. Diet. Assoc. 1997;97:S9–S15. doi: 10.1016/S0002-8223(97)00724-4. [DOI] [PubMed] [Google Scholar]
- 74.Visioli F., Poli A. Fatty Acids and Cardiovascular Risk. Evidence, Lack of Evidence, and Diligence. Nutrients. 2020;12:3782. doi: 10.3390/nu12123782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balta I., Stef L., Pet I., Iancu T., Stef D., Corcionivoschi N. Essential Fatty Acids as Biomedicines in Cardiac Health. Biomedicines. 2021;9:1466. doi: 10.3390/biomedicines9101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.