Abstract
Background:
This study aimed to demonstrate the effectiveness of transcutaneos tibial nerve stimulation (TTNS) in the treatment of bladder pain syndrome (BPS).
Materials and methods:
The data of 16 female patients, diagnosed with BPS in our clinic between 2019 and 2021 and had TTNS twice a week for 12 weeks, were retrospectively analyzed.
Results:
The mean age of the patients was 46.00 ± 13.11 years, and the mean body mass index was 26.43 ± 3.07 kg/m2. After the treatment, the median day time frequency of the patients decreased from 13.37 (3.69) to 10.25 (4.56) (p < 0.001). Nocturia also decreased after treatment from 4.37 (1.81) to 3.00 (1.94) (p = 0.001). The median voiding volume increased by 26.5 mL (p = 0.001). The median of the patients’ visual analog scale scores decreased after treatment (median of visual analog scale score changed from interquartile range 8 [1] to 7 [4]) (p = 0.001). In addition, the median interquartile range interstitial cystitis symptom index scores decreased from 17 (4) to 15 (10) (p = 0.002).
Conclusions:
In this study it was demonstrated that TTNS is an alternative method that can be successfully applied before invasive methods in the treatment of BPS.
Keywords: Cystitis, Interstitial cystitis, Transcutaneous electric nerve stimulation
1. Introduction
Bladder pain syndrome (BPS) is a symptom-based condition in which there is at least 1 voiding complaint in addition to pain, pressure, and discomfort in the pelvic region and with no other pathology to explain these findings.[1] There are serious differences regarding the definition and diagnosis of BPS in the European Association of Urology (EAU), American Urological Association (AUA), and International Continence Society (ICS) guidelines. It is thought that these differences are due to the fact that the etiology of the disease cannot be clearly revealed and is multifactorial.
BPS is one of the chronic pelvic pain (CPP) syndromes affecting the urogenital and rectal regions.[2] Other causes of CPP include dysmenorrhea, prostadynia, chronic scrotal pain, CPP secondary to endometriosis, and vestibulodynia. ICS divided the diagnosis of BPS into 2 sub-groups according to their cystoscopic and histological features as Hunner's lesion and nonlesion.[2] Many factors are thought to be involved in the etiology of BPS, including infectious agents, immunological mechanisms, and defects in the glycosaminoglycan layer of the urothelium.
Another possible etiology is chronic perineuritis and neuro-proliferation in the bladder wall.[3] As a result of this complex nature of the disease, no effective curative treatment has been found. Therefore, symptomatic treatments are primarily given in the management of the disease. Along with behavioral and medical treatments, electrical stimulation methods are also recommended before invasive treatments.[4]
Many forms of electrical nerve stimulation have been used in the treatment of chronic pain. However, the exact mechanism by which neuromodulation provides pain control is unknown. According to the gate control theory, the main mechanism of action is that stimulation of larger myelinated afferent nerve fibers reduces pain by inhibiting conduction in smaller nociceptive fibers.[5] On the other hand, newer neurostimulation techniques suggest that other mechanisms may also play a role. Nevertheless, it has been shown many times that electrical stimulation therapy can be beneficial in the treatment of CPP.[6] Our study aimed to reveal the results and effectiveness of transcutaneous tibial nerve stimulation (TTNS) applied in patients diagnosed with BPS.
2. Materials and methods
The data of 16 female patients treated with TTNS for BPS between June 2019 and June 2021 were retrospectively reviewed. The study was approved by Ethics Committee of Kutahya Health Science University on June 30, 2021 with November 13, 2021 as the decision number.
2.1. Patient selection
Patients over the age of 18 and meeting the BPS syndrome criteria of Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) were included in the study.[7] All patients underwent cystoscopy. Those with glomerulation after bladder distension and/or histological findings together with Hunner's lesion in cystoscopy performed under anesthesia were included in the study. Patients with a neurological deficit, history of bladder or gynecological malignancy, history of radiotherapy to the pelvic region, overactive bladder, stress urinary incontinence, urinary infection, or pelvic organ prolapse were excluded from the study. Also, pregnant patients and patients with a history of peripheral nerve damage, heart disease, or cardiac pacing were not included in the study. In addition, patients with missing data were not included in the study.
2.2. Transcutaneous tibial nerve stimulation procedure
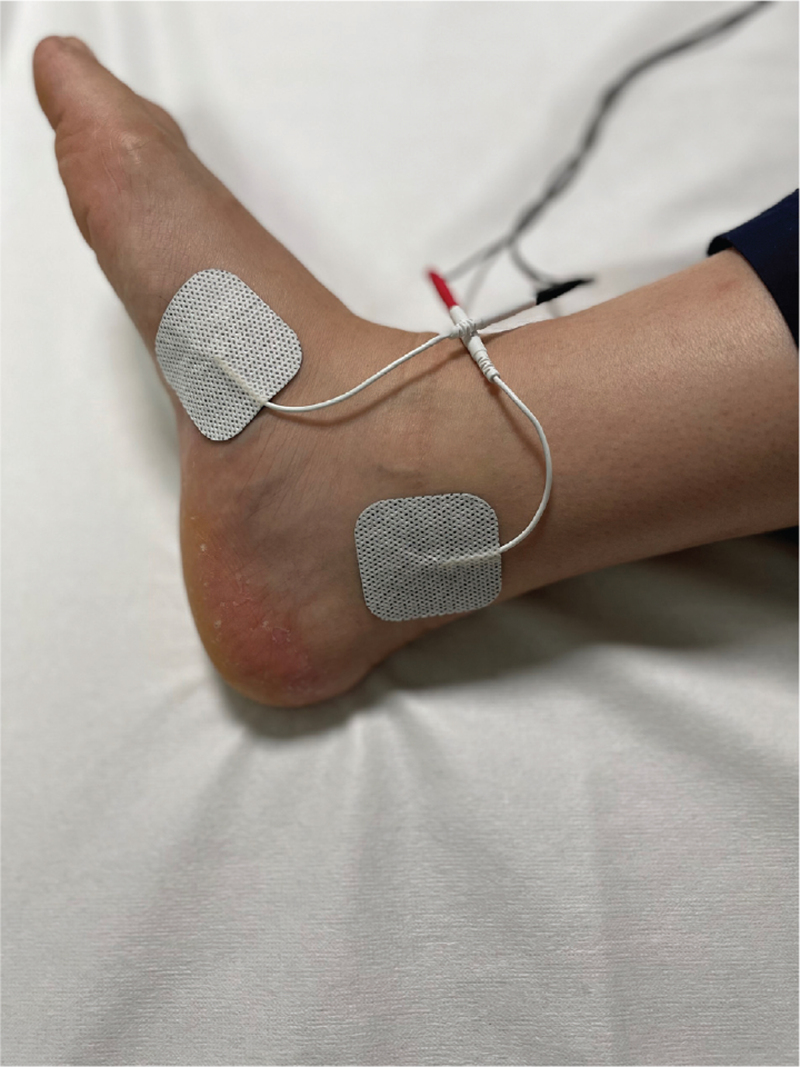
TTNS was performed with a transcutaneous electrical nerve stimulation device (NeuroTrac, Verity Medical, UK) and electrode pads. One of the electrode pads was placed approximately 5 cm above and behind the medial malleolus. The other electrode pad was placed medial to the foot (Fig. 1). The electrodes were connected to the stimulator. Bipolar continuous stimulation was set at a frequency of 10 Hz and a pulse width of 200 μs. Each session was carried out by the same investigator in the hospital for 30 minutes. Treatment sessions were applied to each patient twice a week for 12 weeks.
Figure 1.
Electrode positions.
2.3. Measurement
The 24-hour voiding frequency, the number of nocturia, the number of urges, and the mean voided urine volume data were recorded in the 3-day voiding diary given to the patients before and after the treatment. Visual analog scale (VAS) scores and Turkish-validated Interstitial Cystitis Symptom Index (ICSI) scores of the patients were also recorded before and after the treatment.[8]
2.4. Statistical analysis
Statistical analysis was performed with SPSS 22 software (SPSS Inc., Chicago, IL). Descriptive analyses were performed using means and standard deviations for normally distributed data, medians, and interquartile range (IQR) values for non-normally distributed and ordinal variables. The paired sample t test was used for normally distributed data and the Wilcoxon test for nonnormally distributed data to compare differences in urgency, nocturia, daytime frequency, and voided volume obtained from the pretreatment bladder diary data, as well as VAS and ICSI scores. p < 0.05 was considered statistically significant.
3. Results
The mean age of the 16 patients who were included in the study with the diagnosis of BPS and received the 12-week TTNS treatment was 46.00 ± 13.11 years. The mean body mass index was 26.43 ± 3.07 kg/m2. The mean symptom duration of the patients until the time of diagnosis was 3 years (Table 1). Table 1 shows pretreatment day time frequency, nocturia, mean voiding volume, VAS score, and ICSI score data of the patients.
Table 1.
Patients’ demographics and characteristics.
| Demographics and characteristics | n = 16 |
|---|---|
| Age (mean + SD), yr | 46.00 ± 13.11 |
| BMI (mean + SD), kg/m2 | 26.43 ± 3.07 |
| Duration of symptoms, (median [IQR]), yr | 3.0 (1) |
| Baseline day time frequency (median [IQR]) | 13.37 (3.69) |
| Baseline nocturia (median [IQR]) | 4.37 (1.81) |
| Baseline AVV (median [IQR]), mL | 133.50 (28.75) |
| Baseline VAS score (median [IQR]) | 8 (1) |
| Baseline ICSI score (median [IQR]) | 17 (4) |
AVV = avarage voided volume; BMI = body mass index; ICSI = Interstitial Cystitis Symptom Index; IQR = interquartile range; VAS = visual analog scale.
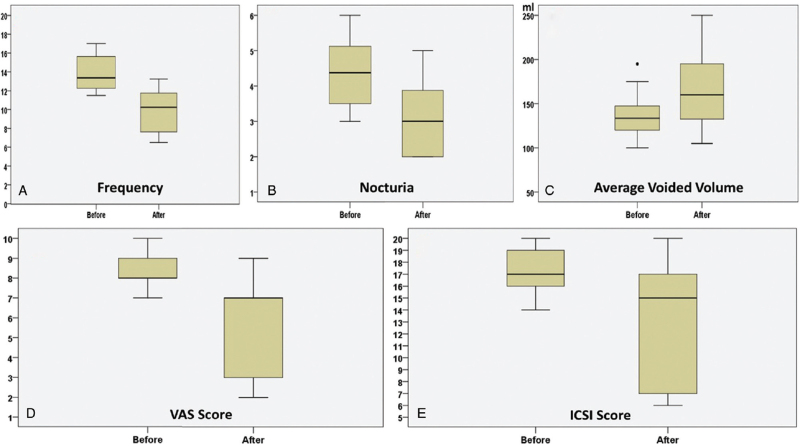
Comparison of day time frequency, nocturia, mean voiding volume, VAS score, and ICSI score of the patients before and after TTNS treatment showed that the median day time frequency (IQR) decreased from 13.37 (3.69) to 10.25 (4.56) (p < 0.001). Nocturia was also found to statistically significantly decrease after treatment [median of change (IQR) from 4.37 (1.81) to 3.00 (1.94)] (p = 0.001). After treatment, the median voiding volume (IQR) increased from 133.50 (28.75) mL to 160 (66.25) mL (p = 0.001). The median of VAS scores, which reveal the severity of pain of the patients, also statistically significantly decreased after treatment [median of VAS score changed from (IQR) 8 (1) to 7 (4)] (p = 0.001). The median of ICSI scores (IQR), which indicates the severity of BPS symptoms, decreased from 17 (4) to 15 (10) (p = 0.002) (Table 2). The graphs of change in day time frequency, nocturia, mean voiding volume, VAS score, and ICSI score of the patients before and after TTNS treatment are presented in Figure 2.
Table 2.
Patients’ data before and after treatment.
| Frequency (average) | Nocturia (mean) | Average voided volume (mL) | VAS score | ICSI score | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||||
| Patients | Pre | Post | % | Pre | Post | % | Pre | Post | % | Pre | Post | % | Pre | Post | % | Botox (after TTNS) Benefit from botox | |
| Patient 1 | 16.50 | 13.25 | 19.6 | 6.0 | 4.25 | 29.1 | 128 | 135 | 5.46 | 8 | 7 | 12.5 | 16 | 14 | 12.5 | Yes | No |
| Patient 2 | 15.0 | 7.0 | 53.3 | 4.5 | 2.0 | 55.5 | 140 | 203 | 45.0 | 9 | 3 | 66.6 | 15 | 8 | 46.6 | No | |
| Patient 3 | 11.75 | 7.25 | 38.2 | 3.5 | 2.0 | 42.8 | 195 | 250 | 28.2 | 8 | 2 | 75.0 | 14 | 7 | 50.0 | No | |
| Patient 4 | 16.25 | 8.0 | 50.7 | 4.75 | 2.0 | 57.8 | 135 | 220 | 62.9 | 8 | 3 | 62.5 | 17 | 6 | 64.7 | No | |
| Patient 5 | 17.0 | 9.0 | 47.0 | 5.5 | 2.5 | 54.5 | 105 | 200 | 90.4 | 9 | 2 | 77.7 | 20 | 7 | 65.0 | No | |
| Patient 6 | 14.5 | 9.0 | 37.9 | 4.25 | 3.75 | 11.7 | 130 | 138 | 6.1 | 9 | 9 | 0 | 20 | 18 | 10.0 | Yes | Yes |
| Patient 7 | 15.25 | 12.75 | 16.3 | 5.5 | 5.0 | 9.0 | 150 | 155 | 3.33 | 10 | 8 | 20.0 | 20 | 17 | 15.0 | Yes | Yes |
| Patient 8 | 12.5 | 11.25 | 10.0 | 3.0 | 2.75 | 8.3 | 160 | 165 | 3.12 | 8 | 7 | 12.5 | 16 | 16 | 0 | Yes | No |
| Patient 9 | 11.5 | 10.0 | 13.0 | 3.5 | 3.0 | 14.2 | 175 | 178 | 1.71 | 10 | 7 | 30.0 | 15 | 15 | 0 | Yes | Yes |
| Patient 10 | 13.75 | 12.25 | 10.9 | 4.25 | 4.0 | 5.8 | 120 | 135 | 12.5 | 8 | 7 | 12.5 | 17 | 15 | 11.7 | Yes | No |
| Patient 11 | 12.0 | 11.25 | 6.25 | 3.5 | 3.75 | −7.1 | 115 | 123 | 6.95 | 9 | 8 | 11.1 | 18 | 16 | 11.1 | Yes | No |
| Patient 12 | 13.0 | 10.5 | 19.2 | 4.75 | 4.0 | 15.7 | 120 | 125 | 4.16 | 10 | 7 | 30.0 | 17 | 17 | 0 | Yes | No |
| Patient 13 | 12.5 | 11.25 | 10.0 | 3.5 | 3.75 | −7.1 | 132 | 130 | −1.51 | 8 | 7 | 12.5 | 18 | 17 | 5.5 | Yes | Yes |
| Patient 14 | 12.0 | 7.25 | 39.5 | 4.5 | 2.0 | 55.5 | 145 | 190 | 31.0 | 7 | 3 | 57.1 | 16 | 7 | 56.2 | No | |
| Patient 15 | 12.75 | 6.5 | 49.0 | 3.75 | 2.0 | 46.6 | 137 | 189 | 37.9 | 8 | 3 | 62.5 | 17 | 6 | 64.7 | No | |
| Patient 16 | 16.0 | 12.75 | 20.3 | 5.5 | 3.0 | 45.4 | 100 | 105 | 5.0 | 8 | 7 | 12.5 | 20 | 20 | 0 | Yes | No |
ICSI = Interstitial Cystitis Symptom Index; TTNS = transcutaneous tibial nerve stimulation; VAS = visual analog scale.
Figure 2.
Graphs of changes in day time frequency, nocturia, mean voiding volume, VAS score, and ICSI score of the patients before and after TTNS treatment. ICSI = Interstitial Cystitis Symptom Index; TTNS = transcutaneous tibial nerve stimulation; VAS = visual analog scale.
No local or systemic side effects were observed in the patients during and after the treatment. While 6 of the patients (37.5%) reported satisfaction after 12weeks of TTNS treatment, intravesical Botulinum toxin-A (BoNT/A) was administered to the other 10 patients because their symptoms did not improve. While 4 out of 10 patients who received intravesical BoNT/A had a significant benefit, the symptoms of the other 6 patients continued and so these patients were evaluated for further treatment options.
4. Discussion
Our study demonstrated that TTNS can be used as a successful method before invasive steps in the treatment of BPS, which is an important subgroup of CPP syndrome. Also, TTNS, which is easily applicable, noninvasive, and has no known significant side effects, may be an effective method in the treatment of BPS in future studies.[6] We think that it can be evaluated alone or often in combination with lifestyle changes and various oral treatments in primary care. Electrical stimulation therapy such as TTNS, posterior tibial nerve stimulation (PTNS), and sacral neuromodulation has been used many times in the treatment of CPP.[6,9] However, there is no study to our knowledge in which TTNS was applied in the treatment of BPS. In the literature, most studies use PTNS in the treatment of BPS.[10,11]
Diagnosing BPS can be quite difficult due to the lack of objective diagnostic criteria. There are different opinions for diagnosis in urology guidelines. When all guidelines are considered, methods that provide objective data such as identification of symptoms and exclusion of other possible diagnoses that may cause these symptoms, urodynamics, cystoscopy and, if necessary, histological sampling are recommended. Also, symptom inquiries validated in the EAU and AUA guidelines are recommended for diagnosis.[4,12] In our study, we diagnosed patients with BPS according to the AUA/SUFU diagnostic criteria. We performed cystoscopy and hydrodistension in all patients with the VAS score and ICSI questionnaire to support our diagnosis.
Pharmacological agents such as antibiotics, anti-inflammatories, antidepressants, and alpha-blockers, physical therapy methods such as pelvic floor exercises and biofeedback, intravesical treatments, and surgical methods are used in the treatment of BPS. Due to the complex nature of the disease, combination therapies generally provide more benefits.[6,13] Electrical nerve stimulation is used alone in the treatment of BPS in different ways (PTNS, TTNS, and sacral neuromodulation).[14] Sudol et al. found a 30% reduction in symptoms after 12 weeks of PTNS.[8] Other studies have also shown a statistically significant decrease in VAS scores.[15–17] On the other hand, Ragab et al. found that PTNS treatment did not provide a statistically significant benefit.[11] They stated that they thought that this was because PTNS was only applied once a week.
There are many studies in which TTNS was used in the treatment of CPP along with PTNS. Van Balken et al. achieved a 42% improvement in CPP symptoms.[18] Lauretti et al. achieved a significant improvement in symptoms in 14 of 20 female patients who underwent transcutaneous electrical nerve stimulation for CPP (dysmenorrhea).[19] In our study, we successfully applied TTNS treatment in 16 female patients diagnosed with BPS. We achieved a significant benefit in 37.5% of patients. Our study is the first study in which TTNS was applied in BPS. Other studies were performed either with all CPP subgroups or for other causes such as dysmenorrhea, vulvodynia, or chronic prostatitis.
Bladder diary findings were used as well as the VAS score and ICSI score to evaluate treatment success in our study. A bladder diary is recommended in the AUA and EAU guidelines to evaluate the diagnosis and treatment results and it is suggested to do it for at least 3 days.[20–23] Since bladder diary data are based on self-measurement of the patient, they can sometimes produce nonobjective results. Also, parameters such as air temperature, daily workload, and activity of the patient depend on the season in which the bladder diary is kept and can affect the fluid intake of the patients and change the results. Considering that the treatment period in our study was 12 weeks, it was thought that bladder diary data may have significant differences, which can be among the limitations of our study.
In the treatment of BPS, lifestyle changes, pelvic floor exercises, oral medical agents, and intravesical treatments are primarily recommended.[4,12] Minimally invasive and surgical methods are used in cases where noninvasive treatments are not successful. Also, we applied TTNS treatment in cases that had not benefited from first-line treatments. Intravesical BoNT/A was applied to the patients with no previous success. Up to 40% of patients benefited from intravesical BoNT/A therapy. Arrom et al. achieved success in 63% of patients with intravesical BoNT/ A.[24] Also, Wang et al. found improvement in 47.5% of patients in their study.[25] Patients who had not benefited from the treatments were evaluated for other invasive methods. Upon the results we obtained in our study, we think that TTNS can be safely applied with or after 1st and 2nd line treatments in patients with BPS.
There are some limitations of our study. Among these are the retrospective nature of the study, the small number of patients, and obtaining the results using partially subjective methods such as the voiding diary and questionnaires. The main weakness of our study is the absence of a control group. Therefore, we could not demonstrate the placebo effect. However, since BPS is a very rare disease, we think that we can still contribute to the literature despite the small number of patients. We think that our results can be supported by randomized placebo-controlled prospective studies in the future.
5. Conclusion
In this retrospective study a significant reduction was achieved in symptoms with 12 weeks of TTNS treatment in 16 female patients with BPS. We think that our findings will contribute to the literature, considering the complex and multifactorial etiology of the disease and that there is no clear standard treatment. Also, TTNS may become routine practice as an alternative treatment method before invasive methods in the treatment of BPS and CPP with prospective randomized controlled urodynamic studies to be conducted in the future.
Acknowledgments
None.
Statement of ethics
The study was approved by Ethics Committee of Kutahya Health Science University. All participants provided consent to the publication of this study. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding source
None.
Author contributions
OA: Project development, data collection, statistical analysis, manuscript writing;
BA: Project development, manuscript writing, editing;
MS: Project development, data collection, editing;
İGK: Project development, data collection, statistical analysis, language editing;
OYS: Project development, data collection;
Hİİ: Project development, data collection, editing.
References
- [1].Malde S, Palmisani S, Al-Kaisy A, Sahai A. Guideline of guidelines: Bladder pain syndrome. BJU Int 2018;122 (5):729–743. [DOI] [PubMed] [Google Scholar]
- [2].Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn 2002;21 (2):167–178. [DOI] [PubMed] [Google Scholar]
- [3].Ghazwani YQ, Elkelini MS, Hassouna MM. Efficacy of sacral neuromodulation in treatment of bladder pain syndrome: Long-term follow-up. Neurourol Urodyn 2011;30 (7):1271–1275. [DOI] [PubMed] [Google Scholar]
- [4].Fall M, Baranowski AP, Elneil S, et al. European Association of Urology. EAU guidelines on chronic pelvic pain. Eur Urol 2010;57 (1):35–48. [DOI] [PubMed] [Google Scholar]
- [5].Melzack R, Wall PD. Pain mechanisms: A new theory. Science 1965;150 (3699):971–979. [DOI] [PubMed] [Google Scholar]
- [6].Cottrell AM, Schneider MP, Goonewardene S, et al. Benefits and harms of electrical neuromodulation for chronic pelvic pain: A systematic review. Eur Urol Focus 2020;6 (3):559–571. [DOI] [PubMed] [Google Scholar]
- [7].Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn 2009;28 (4):274–286. [DOI] [PubMed] [Google Scholar]
- [8].Esen B, Obaid K, Süer E, et al. Reliability and validity of Turkish versions of the interstitial cystitis symptom index and interstitial cystitis problem index. Neurourol Urodyn 2020;39 (8):2338–2343. [DOI] [PubMed] [Google Scholar]
- [9].Han E, Nguyen L, Sirls L, Peters K. Current best practice management of interstitial cystitis/bladder pain syndrome. Ther Adv Urol 2018;10 (7):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sudol NT, Guaderrama N, Adams-Piper E, Whitcomb E, Lane F. Percutaneous tibial nerve stimulation for the treatment of interstitial cystitis/bladder pain syndrome: A pilot study. Int Urogynecol J 2021;32 (10):2757–2764. [DOI] [PubMed] [Google Scholar]
- [11].Ragab MM, Tawfik AM, Abo El-enen M, et al. Evaluation of percutaneous tibial nerve stimulation for treatment of refractory painful bladder syndrome. Urology 2015;86 (4):707–711. [DOI] [PubMed] [Google Scholar]
- [12].Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193 (5):1545–1553. [DOI] [PubMed] [Google Scholar]
- [13].Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev 2014;(3):CD008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tutolo M, Ammirati E, Heesakkers J, et al. Efficacy and safety of sacral and percutaneous tibial neuromodulation in non-neurogenic lower urinary tract dysfunction and chronic pelvic pain: A systematic review of the literature. Eur Urol 2018;73 (3):406–418. [DOI] [PubMed] [Google Scholar]
- [15].Gokyildiz S, Kizilkaya Beji N, Yalcin O, Istek A. Effects of percutaneous tibial nerve stimulation therapy on chronic pelvic pain. Gynecol Obstet Invest 2012;73 (2):99–105. [DOI] [PubMed] [Google Scholar]
- [16].Istek A, Gungor Ugurlucan F, Yasa C, Gokyildiz S, Yalcin O. Randomized trial of long-term effects of percutaneous tibial nerve stimulation on chronic pelvic pain. Arch Gynecol Obstet 2014;290 (2):291–298. [DOI] [PubMed] [Google Scholar]
- [17].Kabay S, Kabay SC, Yucel M, Ozden H. Efficiency of posterior tibial nerve stimulation in category IIIB chronic prostatitis/chronic pelvic pain: A sham-controlled comparative study. Urol Int 2009;83 (1):33–38. [DOI] [PubMed] [Google Scholar]
- [18].Van Balken MR, Vandoninck V, Messelink BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol 2003;43 (2):158–163. discussion 163. [DOI] [PubMed] [Google Scholar]
- [19].Lauretti GR, Oliveira R, Parada F, Mattos AL. The new portable transcutaneous electrical nerve stimulation device was efficacious in the control of primary dysmenorrhea cramp pain. Neuromodulation 2015;18 (6):522–526. discussion 522–527. [DOI] [PubMed] [Google Scholar]
- [20].Palnaes Hansen C, Klarskov P. The accuracy of the frequency-volume chart: Comparison of self-reported and measured volumes. Br J Urol 1998;81 (5):709–711. [DOI] [PubMed] [Google Scholar]
- [21].Yap TL, Cromwell DC, Emberton M. A systematic review of the reliability of frequency-volume charts in urological research and its implications for the optimum chart duration. BJU Int 2007;99 (1):9–16. [DOI] [PubMed] [Google Scholar]
- [22].Schick E, Jolivet-Tremblay M, Dupont C, et al. Frequency-volume chart: The minimum number of days required to obtain reliable results. Neurourol Urodyn 2003;22:92–96. [DOI] [PubMed] [Google Scholar]
- [23].Bright E, Cotterill N, Drake M, Abrams P. Developing and validating the international consultation on incontinence questionnaire bladder diary. Eur Urol 2014;66 (2):294–300. [DOI] [PubMed] [Google Scholar]
- [24].Mateu Arrom L, Gutierrez Ruiz C, Palou J, Errando-Smet C. Onabotulinumtoxin A injection with or without hydrodistension for treatment of bladder pain syndrome. Int Urogynecol J 2021;32 (5):1213–1219. [DOI] [PubMed] [Google Scholar]
- [25].Wang HJ, Yu WR, Ong HL, Kuo HC. Predictive factors for a satisfactory treatment outcome with intravesical botulinum toxin A injection in patients with interstitial cystitis/bladder pain syndrome. Toxins (Basel) 2019;11 (11):676. [DOI] [PMC free article] [PubMed] [Google Scholar]