Abstract
Aim
To explore pituitary–gonadal hormone concentrations and assess their association with inflammation, severe respiratory failure, and mortality in hospitalized men and women with COVID-19, and compare these to hormone concentrations in hospitalized patients with bacterial community-acquired pneumonia (CAP) and influenza virus CAP and to concentrations in a reference group of healthy individuals.
Methods
Serum concentrations of testosterone, estrone sulfate, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and interleukin-6 (IL-6) were measured within 4 days of admission. Associations were assessed by logistic regression analysis in patients with COVID-19, and results were reported as odds ratio with 95% CI per two-fold reduction after adjustment for age, comorbidities, days to sample collection, and IL-6 concentrations.
Results
In total, 278 patients with COVID-19, 21 with influenza virus CAP, and 76 with bacterial CAP were included. Testosterone concentrations were suppressed in men hospitalized with COVID-19, bacterial and influenza virus CAP, and moderately suppressed in women. Reductions in testosterone (OR: 3.43 (1.14–10.30), P = 0.028) and LH (OR: 2.51 (1.28–4.92), P = 0.008) were associated with higher odds of mehanical ventilation (MV) in men with COVID-19. In women with COVID-19, reductions in LH (OR: 3.34 (1.02–10-90), P = 0.046) and FSH (OR: 2.52 (1.01–6.27), P = 0.047) were associated with higher odds of MV.
Conclusion
Low testosterone and LH concentrations were predictive of severe respiratory failure in men with COVID-19, whereas low concentrations of LH and FSH were predictive of severe respiratory failure in women with COVID-19.
Keywords: COVID-19, testosterone, estrone-sulfate, LH, FSH, mortality, morbidity, hypogonadism
Background
Male sex is a risk factor for severe disease and mortality in patients hospitalized with COVID-19 (1). Concentrations of gonadal hormones differ between sexes, and gonadal and pituitary hormones have been suggested to play a role in disease progression. Studies have reported association of the hypothalamic–pituitary–gonadal (HPG) axis in men hospitalized with COVID-19 with severely suppressed concentrations of testosterone and increased concentrations of estradiol with a reported association to mortality and respiratory failure (2, 3, 4, 5, 6, 7, 8). Additionally, severe COVID-19 is driven by hyperinflammation as reflected by high circulating levels of inflammatory biomarkers such as C-reactive protein (CRP) and interleukin (IL)-6. Animal and human studies have shown association between low testosterone concentrations and higher pro-inflammatory cytokines (e.g. IL-6) and lower anti-inflammatory cytokines (e.g. IL-10), and thus, low testosterone concentrations may increase disease severity in COVID-19 (9). Progressive age and comorbidities are associated with hospitalization with lower respiratory tract illnesses (LRTI), including COVID-19, as well as a high prevalence of hypogonadism among males (up to 25% at age>70 years) (10). In this context, we studied concentrations of testosterone, estrone sulfate, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) among patients hospitalized with COVID-19, and their impact on mortality and disease severity after adjustment for other factors associated with a poor outcome including the level of inflammation. Further, we hypothesized that the changes in HPG axis hormones were similar in patients with severe LRTI of other causes. Therefore, we studied hormone concentrations in patients with COVID-19 community-acquired pneumonia (CAP), bacterial CAP, and influenza virus CAP and in healthy individuals.
Methods
The study was a multicentric prospective observational cohort study of patients hospitalized with COVID-19 and CAP caused by bacteria or influenza virus with retrospective analysis of biobank blood samples. Patients with COVID-19 were admitted to the Emergency Department at Copenhagen University Hospital – Amager and Hvidovre – and transferred to the Department of Infectious Diseases or to the intensive care unit as previously described (11, 12). Patients who were hospitalized from March 16 to June 16, 2020 (first COVID-19 cohort) and from July 25 to February 3, 2021 (second COVID-19 cohort) were included. The two cohorts were separated by those who were introduced to remdesivir and dexamethasone as standard-of-care. Inclusion criteria for patients with COVID-19 were (i) presence of SARS-CoV-2 RNA confirmed by reverse-transcriptase polymerase-chain-reaction from either naso-/oropharyngeal swab, sputum, or endotracheal aspirate, (ii) age ≥ 18 years, and (iii) COVID-19 illness requiring hospitalization. Exclusion criteria were blood sample collected >4 days of admission. Patients with CAP were admitted to the Emergency Department of Copenhagen University Hospital – North Zealand, Hillerød and transferred to the Department of Pulmonary and Infectious Diseases as previously described (13). Inclusion criteria were (i) age ≥ 18 years, (ii) radiographically confirmed pulmonary infiltrate, (iii) respiratory illness requiring hospitalization, and (iv) either confirmed etiology with a bacterium or with influenza virus. The reference groups were men and women aged >70 years old (Health2006) (14, 15) and adults 30–60 years (Health2008) (16).
Data collection
Data including demographic variables, comorbidities, use of remdesivir and/or dexamethasone, vital parameters (peripheral oxygen saturation, temperature), plasma CRP, disease severity at admission (baseline oxygen requirements), and clinical outcome (intensive care unit (ICU), extracorporeal membrane oxygenation, mechanical ventilation, discharge, and/or death) were manually extracted from electronic health records. Baseline oxygen requirements were ascertained as the highest level of respiratory support within 24 h of admission. Mortality within 90 days was measured from hormonal sample size measurement. Respiratory failure was defined as the need for mechanical ventilation.
Biochemical analysis
Blood from patients with COVID-19 was drawn between 07:00 and 09:00 h within the first 4 days of admission. Blood from patients with CAP was drawn within 24 h of admission. Serum was stored at −20°C for later analysis at the Department of Growth and Reproduction, Copenhagen University Hospital – Rigshospitalet. Hormones were tested once in all patients. Concentrations of testosterone and estrone sulfate were determined for all groups by isotope-dilution TurboFlow-liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously reported (17). Limits of quantification (LOQ) were 0.026 nmol/L for estrone sulfate and 0.012 nmol/L for testosterone, and inter-assay CVs were ≤7% for both analytes. Concentrations of LH and FSH were measured for all disease groups except the second COVID-19 cohort using time-resolved fluoroimmunometric assays (AutoDELFIA, Perkin Elmer) with limits of detection of 0.05 IU/L and inter-assay coefficients of variations (CVs) below 9%.
Normal ranges according to sex and age (70 years) for men and women for LH were 4.5 IU/L (1.7–19.0) and 22.7 IU/L (5.5–51.4), respectively (18) and for FSH were 7.19 IU/L (2.8–44.8) and 61.0 IU/L (13.0–122.4), respectively (18). Primary hypogonadism among men was defined as testosterone <9 nmol/L and LH ≥ 9 IU/L. Secondary hypogonadism among men was defined as testosterone concentrations <9 nmol/L and LH concentrations <9 IU/L.
Serum IL-6 was measured in the first COVID-19 cohort as previously described for the first cohort (11). For the second COVID-19 cohort, serum IL-6 was measured by microfluidic ELISA (Ella, R&D Systems).
To account for measurement bias, the protocol for measurement of gonadal hormones was the same regardless of cohort and etiology.
Statistics
The study population was characterized using descriptive statistics where categorical variables were reported as counts (%), parametric continuous variables were summarized using means with s.d., and non-parametric continuous variables with medians with interquartile ranges (IQR). Comparison of baseline characteristics between the four cohorts was performed using χ2-test, one-way ANOVA, and Kruskal–Wallis test, as appropriate. Comparisons of hormonal concentrations within men and women, respectively, were performed using non-parametric Kruskal–Wallis test, followed by Dunn’s post hoc test using the Holm method. Correlations between hormones and CRP or IL-6 were performed by linear regression model with Pearson rho. Skewed data were log2-transformed prior to analysis. The association between gonadal hormones and mechanical ventilation or 90-day mortality was assessed by logistic regression analysis in crude and adjusted models in men and women with COVID-19 and expressed as odds ratio (OR) with 95% CI per two-fold reduction of the gonadal hormone. The adjusted variables (<10% missing) were predefined known confounders that included age, presence or absence of comorbidity (hypertension, cancer, COPD, asthma, diabetes, or cardiovascular disease), time-to-sample collection, and IL-6. For sensitivity analysis, we added cortisol as a confounder in the outcome analysis. Additionally, in men with COVID-19, we performed a logistic regression analysis of the association between primary or secondary hypogonadism and 90-day mortality. Data processing and statistical analysis were performed using R version 1.2.5001 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics
The study was approved by the Regional Data Protection Center (P-2020-260) and by the Danish Regional Committee on Health Research Ethics (H-20040649 and H-18024256). The measurements from Health2006 and Health2008, used for references, were approved by the Danish Regional Committee on Health Research Ethics (H-20059668 and H-KA-20060011). Requirement of individual informed consent was exempted by the committee.
Results
A total of 389 patients were examined for eligibility, 14 patients with COVID-19 were excluded due to blood samples collected >4 days of admission yielding 375 patients including 278 (74%) with COVID-19, 21 (6%) with influenza virus CAP, and 76 (20%) with bacterial CAP. Baseline characteristics are given in Table 1. Patients in the first COVID-19 cohort were significantly older than those in the second COVID-19 cohort (72 years old (59–81) vs 61 years old (49–73), P <0.001), and the majority in the two COVID-19 cohorts were men. The two COVID-19 cohorts were otherwise comparable at baseline. More patients with bacterial CAP and influenza virus CAP had chronic obstructive pulmonary disease compared to patients in the two COVID-19 cohorts but otherwise comparable. Patients in both COVID-19 cohorts were moderately to severely ill at admission as indicated by the frequent use of low- or high-flow oxygen (>80% in each cohort), and four patients (3%) in the second COVID-19 cohort required mechanical ventilation at admission making them eligible for early admittance to the intensive care unit. Among patients in COVID-19 cohort, 40 were intubated during hospitalization. No patients with bacterial CAP or influenza virus CAP were intubated. The mortality proportion within 90 days was 30.7% in the first COVID-19 cohort, 10.1% in the second COVID-19 cohort, 5.3% in patients with bacterial CAP, and 0.0% in patients with influenza virus CAP.
Table 1.
Baseline characteristics, hospitalization, ICU admission, and mortality in patients with COVID-19 (first and second cohort), influenza virus CAP, and bacterial CAP.
| COVID-19 1st cohort, n = 130 | COVID-19 2nd cohort, n = 148 | Bacterial CAP, n = 76 | Influenza virus CAP, n = 21 | |
|---|---|---|---|---|
| Period | March 16 to June 16, 2020 | July 25, 2020 to February 3, 2021 | January 15, 2019 to March 27, 2020 | January 23, 2019 to February 15, 2020 |
| Age, years (IQR) | 72 (59–81) | 61 (49–73) | 72 (63–83) | 74 (68–79) |
| Age, years (range) | 28–93 | 19–97 | 20–97 | 19–87 |
| Sex, n (%) | ||||
| Men | 75 (58) | 92 (62) | 42 (55) | 9 (43) |
| BMI, kg/m2 (s.d.) | 28.9 (6.6) | 31.1 (16.8) | 25.5 (6.0) | 28.4 (8.3) |
| Missing | 18 | 19 | ||
| Plasma CRP, mg/L (IQR) | 100 (49–55) | – | 138 (72–202) | 75 (34–128) |
| Serum IL-6, pg/mL (IQR) | 77 (39–168) | 18 (9–46) | – | – |
| Comorbidity, n (%) | ||||
| Hypertension | 60 (47) | 50 (34) | 3 (4) | 0 (0) |
| Diabetes | 41 (32) | 37 (25) | 12 (16) | 6 (29) |
| Cardiovascular disease | 9 (13) | 51 (35) | 17 (22) | 6 (29) |
| Missing | 59 | 0 | ||
| Cancer | 22 (17) | 13 (9) | 10 (13) | 1 (5) |
| Chronic obstructive pulmonary disease | 10 (8) | 11 (7) | 24 (32) | 10 (48) |
| Asthma | 17 (13) | 17 (12) | 0 (0) | 0 (0) |
| Vitals | ||||
| Respiratory rate per minute | 21 (18–28) | 22 (20–28) | 22 (19–25) | 20 (20–24) |
| Peripheral oxygen saturation, % | 94.9 (3.2) | 90.4 (6.2) | 94.4 (3.7) | 94.2 (4.3) |
| Temperature, ˚C | 38.1 (37.0–38.8) | 38.1 (37.5–39.0) | 38.0 (37.3–38.7) | 38.2 (37.4–38.6) |
| Baseline oxygen requirement, n (%) | ||||
| No oxygen | 23 (18) | 19 (13) | – | – |
| Low flow | 61 (47) | 74 (50) | – | – |
| High flow | 45 (35) | 51 (35) | – | – |
| Mechanical ventilation | 0 | 4 (3) | – | – |
| Days with symptoms | 7 (5–11) | 7 (5–9) | – | – |
| Missing | 26 | 3 | ||
| Treatment | ||||
| Remdesivir, n (%) | 0 (0) | 132 (89) | – | – |
| Dexamethasone, n (%) | 0 (0) | 129 (87) | – | – |
| Intensive care, n (%) | 20 (15) | 17 (12) | 3 (4) | 0 (0) |
| Intubation, n (%) | 19 (15) | 16 (11) | 0 (0) | 0 (0) |
| ECMO, n (%) | 7 (5) | 4 (3) | – | – |
| 90-day mortality, n (%) | 40 (30.7) | 15 (10.1) | 4 (5.3) | 0 (0.0) |
CAP, community acquired pneumonia; ECMO, extra-corporal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Pituitary–gonadal hormones in patients with COVID-19, bacterial CAP, and influenza virus CAP and in the healthy reference group
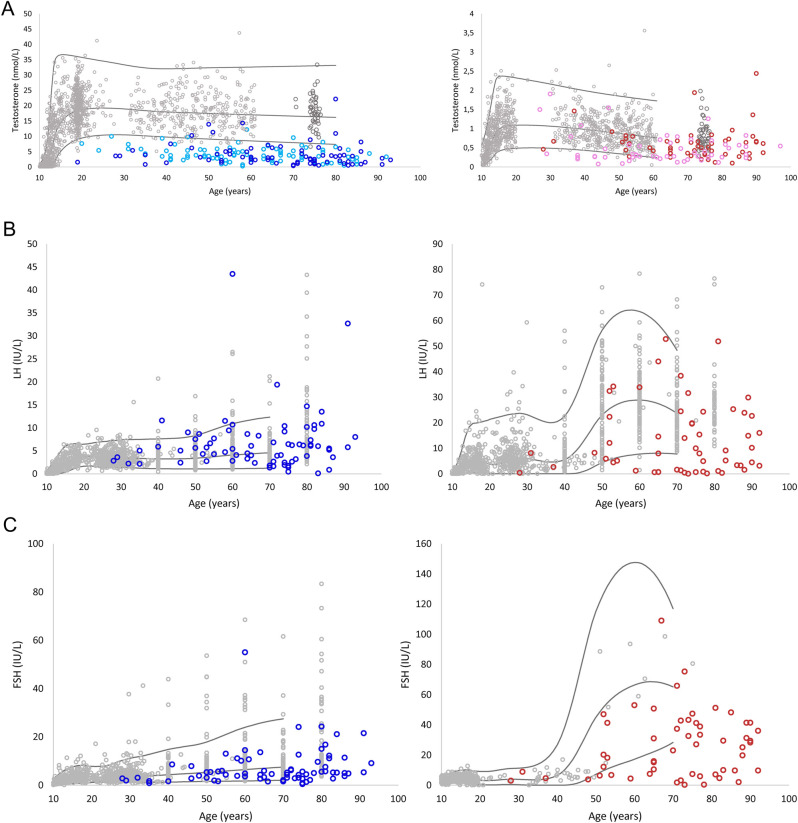
Pituitary–gonadal hormone concentrations among men and women are given in Table 2 and Fig. 1.
Table 2.
Reproductive serum hormone concentrations in men and women hospitalized with COVID-19, bacterial CAP, or influenza virus CAP compared to a healthy reference group.
| First cohort | Second cohort | Influenza virus CAP | Bacterial CAP | Reference group | |
|---|---|---|---|---|---|
| Men | |||||
| (n = 75) | (n = 92) | (n = 9) | (n = 42) | (n = 50) | |
| Women | |||||
| (n = 55) | (n = 56) | (n = 12) | (n = 34) | (n = 51) | |
| Days until sampling, median and range | 2 (1–3) | 1 (1–2) | |||
| Testosterone, nmol/L | 3.2 (1.6–5.2)† | 3.7 (2.4–5.5)† | 2.1 (1.9–4.2)† | 3.8 (2.2–6.8)† | 18.0 (15.0–21.2) |
| Estrone sulfate, nmol/L | 2.9 (1.2–5.7)† | 3.4 (2.2–5.9) | 2.4 (1.7–5.4) | 2.7 (1.2–5.9) | 2.3 (1.6–2.9) |
| LH, IU/L | 5.6 (2.9–8.2) | – | 3.3 (2.4–5.9) | 4.2 (2.5–7.7) | – |
| FSH, IU/L | 5.0 (2.9–9.0) | – | 3.8 (2.3–8.7) | 4.3 (2.5–7.6) | – |
| Days until sampling, median and range | 2 (2–3) | 2 (1–2) | |||
| Testosterone, nmol/L | 0.5 (0.4–0.7)† | 0.3 (0.3–0.6)† | 0.3 (0.2–0.4)† | 0.5 (0.3–0.8)† | 0.8 (0.6–1.0) |
| Estrone sulfate, nmol/L | 2.0 (1.0–3.5) | 1.9 (0.9–3.6) | 2.2 (0.9–2.5) | 1.0 (0.5 -1.2) | 0.9 (0.5–1.2) |
| LH, IU/L | 8.1 (1.6–22.5) | – | 9.7 (3.7–20.4) | 9.2 (3.7–20.4) | – |
| FSH, IU/L | 23.0 (7.2–41.2) | – | 20.7 (12.5–26.3) | 17.7 (6.3–31.9) | – |
†Significantly different from reference group as assessed by non-parametric Kruskal–Wallis test followed by Dunn’s post hoc test using the Holm method.
CAP, community-acquired pneumonia; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Figure 1.
Concentrations of testosterone (A), luteinizing hormone (LH) (B), and follicle-stimulating hormone (FSH) (C) in healthy men and women hospitalized with COVID-19 plotted against normal ranges (mean, +2 s.d. and −2 s.d.) (dark blue: first cohort men; light blue: second cohort men; dark red: first cohort women; pink: second cohort women; grey: healthy reference range).
Men
Patients in the first and second COVID-19 cohort, as well as patients with influenza virus CAP and bacterial CAP, had lower concentrations of testosterone compared to the reference group. No difference in testosterone concentrations was found between disease groups. Patients with bacterial CAP but not COVID-19 or influenza virus CAP had significantly higher concentrations of estrone sulfate compared to the reference group. No difference in estrone sulfate concentrations was found between disease groups. Concentrations of LH and FSH were similar for all groups (Table 2).
14 patients (19%) in the first COVID-19 cohort and 6 patients (14%) with bacterial CAP had primary hypogonadism, whereas 95 (73%) in the first COVID-19 cohort, 31 patients (74%) with bacterial CAP, and 9 patients (100%) with influenza virus CAP had secondary hypogonadism.
Women
Compared to the reference group, testosterone concentrations were significantly lower in all disease groups with no difference between the groups. Concentrations of estrone sulfate, LH, and FSH were also comparable between disease groups (Table 2).
Correlations between reproductive hormones and inflammatory markers
In the first COVID-19 cohort, there was a low-to-moderate inverse correlation between testosterone and CRP (R = −0.32, P = 0.041) or IL-6 (R = −0.49, P = <0.001), LH and CRP (R = −0.27, P = 0.092) or IL-6 (R = −0.28, P = 0.018), FSH and CRP (R = −0.38, P = 0.014) or IL-6 (R = −0.31, P = 0.008) and a correlation between estrone sulfate and CRP (R = 0.29, P = 0.074) or IL-6 (R = 0.41, P = <0.001). In men with bacterial CAP, there was an inverse correlation between testosterone and CRP (R = −0.5, P = 0.001), LH and CRP (R = −0.4, P = 0.009), FSH and CRP (R = −0.4, P = 0.009) and a correlation between estrone sulfate and CRP (R = 0.49, P = <0.001). In the second COVID-19 cohort, there was no correlation between testosterone/estrone sulfate and CRP but a low inverse correlation between testosterone and IL-6 (R = −0.35, P=0.001) and a low correlation between estrone sulfate and IL-6 (R = 0.22, P = 0.037).
There was no correlation between reproductive hormones and either CRP or IL-6 in women with COVID-19. In women with bacterial CAP, there was a correlation between testosterone and CRP (R = 0.30, P = 0.087) and between estrone sulfate and CRP (R = 0.40, P = 0.020).
Pituitary–gonadal hormones and risk of mechanical ventilation and 90-day mortality
Men
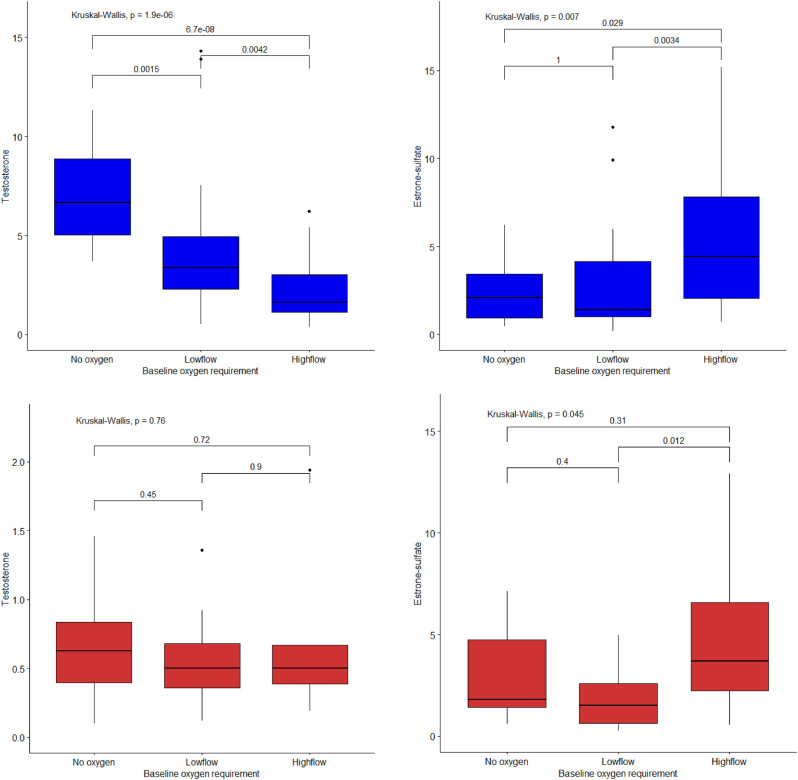
Hormonal concentrations in men and women with COVID-19 according to 90-day survival status are provided in Table 3. Men in the first COVID-19 cohort who died within 90 days were older (77 years (71–82) vs 64 years (53–75), P = 0.002), had comparable BMI (29.9 (6.5) vs 28.0 (4.6), P = 0.215), had lower testosterone concentrations, higher estrone sulfate concentrations, and lower LH concentrations compared to men who survived. Patients who were mechanically ventilated during hospitalization had lower concentrations of testosterone, LH, FSH, and higher concentrations of estrone sulfate compared to patients who were not mechanically ventilated (Supplementary Table 1, see section on supplementary materials given at the end of this article). Similarly, men in need of high-flow oxygen at admission (severe disease) had lower testosterone concentrations compared to patients in need of low flow (moderate disease) or ambient air (mild disease) (1.6 nmol/L (1.1–3.0), 3.4 nmol/L (2.3–4.9), and 6.7 nmol/L (5.0–8.9), respectively, P < 0.001), as well as higher estrone sulfate concentrations (4.4 nmol/L (2.0–7.8), 1.4 nmol/L (1.0–4.1), and 2.1 nmol/L (0.9–3.4), respectively, P = 0.007) (Fig. 2). Men with bacterial CAP who died within 90 days of hospitalization did not significantly differ in age, BMI, or hormonal concentrations except for higher FSH concentrations (11.7 IU/L (9.1–18.1) vs 4.1 (2.4–7.2), P = 0.043) (Supplementary Table 2). Adjusted associations between pituitary–gonadal hormones and 90-day mortality or mechanical ventilation in patients with COVID-19 are given in Tables 4 and 5 with further adjustment for IL-6 in separate column. In the first COVID-19 cohort, a two-fold reduction of testosterone was associated with higher odds of mechanical ventilation and 90-day mortality in the main adjusted analysis, but the associations were attenuated once further adjusted for IL-6. In the second COVID-19 cohort, there was an association between testosterone and mechanical ventilation but not mortality. However, the association did not remain once adjusted for IL-6. A two-fold reduction of estrone sulfate was associated with lower odds of mechanical ventilation and 90-day mortality in the first COVID-19 cohort, but the associations did not remain once adjusted for IL-6. In the first COVID-19 cohort, two-fold reductions of LH were associated with mechanical ventilation and 90-day mortality; however, once adjusted for IL-6, the association only remained between mechanical ventilation and LH. FSH was associated with higher odds of mechanical ventilation, but no associations were found once adjusted for IL-6. Cortisol concentrations did not affect any of the associations.
Table 3.
Concentrations of reproductive hormones in men and women hospitalized with COVID-19 by 90-day vital status.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Alive, n = 51 | Dead, n = 23 | P-value | Alive, n = 38 | Dead, n = 17 | P-value | |
| Testosterone, nmol/L, (IQR) | 3.8 (2.4–6.2) | 1.4 (0.8–2.5) | 0.001 | 0.5 (0.4–0.7) | 0.6 (0.3–0.7) | 0.799 |
| Estrone sulfate, nmol/L, (IQR) | 2.0 (1.1–4.2) | 7.1 (2.6–11.2) | 0.002 | 1.9 (1.0–3.0) | 2.1 (0.9–4.5) | 0.466 |
| LH, IU/L (IQR) | 5.8 (4.0–8.6) | 3.2 (1.9–6.9) | 0.039 | 11.1 (3.7–24.2) | 3.3 (1.1–8.0) | 0.035 |
| FSH, IU/L (IQR) | 5.2 (3.0–8.2) | 3.2 (2.4–9.3) | 0.430 | 28.9 (9.1–43.1) | 10.0 (4.6–29.4) | 0.038 |
FSH, follicle-stimulating hormone; IQR, interquartile range; LH, luteinizing hormone.
Figure 2.
Concentrations of testosterone and estrone sulfate in men (blue) and women (red) hospitalized with COVID-19 in the first COVID-19 cohort according to baseline oxygen requirements within 24 h of admission. Data are expressed as boxplots. Groups were compared using unpaired, non-parametric Kruskal–Wallis test with Dunn’s post hoc test using the Holm method. P-value annotation legend is provided above the boxplots for the three comparisons and additionally an overall comparison of the groups in top.
Table 4.
Adjusted associations between concentrations of reproductive hormones and mortality within 90-day in men and women hospitalized with COVID-19.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Odds ratio, adjusted* (95% CI) | P-value | Adjusted for IL-6 | Odds ratio, adjusted* (95% CI) | P-value | Adjusted for IL-6 | |
| 1st COVID-19 cohort | ||||||
| Testosterone (per two-fold reduction) | 3.51 (1.77–6.94) | <0.001 | 2.00 (0.97–4.13) | 0.87 (0.38–1.99) | 0.744 | 1.01 (0.37–2.76) |
| Estrone sulfate (per two-fold reduction) | 0.50 (0.32–0.77) | 0.002 | 0.69 (0.41–1.18) | 1.44 (0.86–2.42) | 0.165 | 0.84 (0.48–1.48) |
| LH (per two-fold reduction) | 1.97 (1.14–3.43) | 0.016 | 1.38 (0.80–2.38) | 1.25 (0.90–1.73) | 0.180 | 1.30 (0.88–1.92) |
| FSH(per two-fold reduction) | 1.54 (0.95–2.50) | 0.081 | 0.82 (0.45–1.49) | 1.49 (0.96–2.32) | 0.073 | 1.57 (0.95–2.60) |
| 2nd COVID-19 cohort | ||||||
| Testosterone (per two-fold reduction) | 1.34 (0.70–2.57) | 0.377 | 1.19 (0.60–2.35) | 0.68 (0.20–2.37) | 0.545 | 0.72 (0.12–4.42) |
| Estrone sulfate (per two-fold reduction) | 1.04 (0.61–1.78) | 0.884 | 1.48 (0.77–2.86) | 1.85 (0.77–4.45) | 0.167 | 0.35 (0.10–1.27) |
*Adjusted for age and presence or absence of comorbidity (hypertension, cancer, COPD, asthma, diabetes, or cardiovascular disease).
1st COVID-19 cohort, patients hospitalized with COVID-19 from March 16 to June 16, 2020; 2nd COVID-19 cohort, patients hospitalized with COVID-19 from July 25 2020 to February 3, 2021; FSH, follicle-stimulating hormone; IL-6, interleukin-6; LH, luteinizing hormone.
Table 5.
Adjusted associations between serum concentrations of reproductive hormones and mechanical ventilation in men and women hospitalized with COVID-19.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Odds ratio, adjusted* (95% CI) | P-value | Adjusted for IL-6 | Odds ratio, adjusted* (95% CI) | P-value | Adjusted for IL-6 | |
| 1st COVID-19 cohort | ||||||
| Testosterone (per two-fold reduction) | 3.73 (1.67–8.36) | 0.001 | 3.43 (1.14–10.30) | 2.57 (0.78–8.49) | 0.123 | 2.89 (0.50–16.62) |
| Estrone sulfate (per two-fold reduction) | 0.45 (0.24–0.84) | 0.012 | 0.87 (0.42–1.83) | 1.51 (0.75–3.02) | 0.248 | 0.75 (0.35–1.61) |
| LH (per two-fold reduction) | 2.64 (1.35–5.16) | 0.005 | 2.51 (1.28–4.92) | 1.78 (1.06–3.01) | 0.029 | 3.34 (1.02–10.90) |
| FSH(per two-fold reduction) | 2.59 (1.33–5.03) | 0.005 | 1.91 (0.87–4.23) | 1.81 (1.03–3.19) | 0.040 | 2.52 (1.01–6.27) |
| 2nd COVID-19 cohort | ||||||
| Testosterone (per two-fold reduction) | 2.41 (1.15–5.07) | 0.020 | 2.13 (0.91–5.00) | 1.29 (0.51–3.27) | 0.591 | 1.93 (0.39–9.62) |
| Estrone sulfate (per two-fold reduction) | 0.73 (0.38–1.38) | 0.331 | 0.32 (0.12–0.99 | 1.814 (0.89–3.70) | 0.102 | 0.52 (0.22–1.21) |
*Adjusted for age and presence or absence of comorbidity (hypertension, cancer, COPD, asthma, diabetes, or cardiovascular disease).
1st COVID-19 cohort, patients hospitalized with COVID-19 from March 16 to June 16, 2020; 2nd COVID-19 cohort, patients hospitalized with COVID-19 from July 25, 2020 to February 3, 2020; FSH, follicle-stimulating hormone; IL-6, interleukin-6; LH, luteinizing hormone.
Secondary hypogonadism was not associated with mortality compared to primary hypogonadism (OR = 1.29, 95% CI (0.35–4.68), P = 0.703). No patients with secondary hypogonadism required mechanical ventilation, and thus, we did not test this association.
Women
Women with severe disease at admission compared to moderate disease had higher concentrations of estrone sulfate (3.7 nmol/L (2.2–6.6), 1.5 nmol/L (0.6–2.6), respectively, P = 0.012). There was no difference in testosterone concentrations between groups based on disease severity (0.5 nmol/L (0.4–0.7), 0.5 nmol/L (0.4–0.7), and 0.6 (0.4–0.8), respectively, P = 0.760) (Fig. 2). Women in the first COVID-19 cohort who died had lower LH and FSH concentrations compared to those who survived (Table 3). Likewise, two-fold reduction of LH and FSH was associated with mortality and mechanical ventilation in the adjusted logistic regression analysis, with even higher odds once adjusted for IL-6. In the first and second COVID-19 cohort, there was no association in the adjusted analysis between testosterone or estrone sulfate and mechanical ventilation or 90-day mortality. Cortisol concentrations did not affect any of the associations.
Discussion
We found that testosterone concentrations were suppressed in men and women hospitalized with either COVID-19, bacterial CAP, or influenza virus CAP. Concentrations of testosterone decreased, while estrone sulfate increased, with disease severity in men with COVID-19. We found that reproductive hormones and inflammatory markers were correlated in men with COVID-19 and bacterial CAP. Low testosterone was predictive of mortality and mechanical ventilation in men with COVID-19 and low LH was predictive of mechanical ventilation. In women with COVID-19, low LH and FSH concentrations were predictive of mechanical ventilation. Once adjusted for IL-6, all associations in men were attenuated, whereas the associations between mortality or mechanical ventilation and LH or FSH concentrations in women strengthened after adjustment for IL-6.
Our study supports the current evidence of testosterone associated with severe respiratory failure in men with COVID-19 before treatment with remdesivir and dexamethasone (2, 3, 4, 5, 6, 7). Testosterone decreased with disease severity at admission as measured by supplemental oxygen requirements. With severe disease, RNA-viraemia increases, which may enhance the risk of peripheral tissue involvement (19). In the testis, angiotensin-converting enzyme-II receptors and transmembrane-serine-protease-2 are abundant, and therefore, it has been speculated that SARS-CoV-2 may directly target the Leydig and Sertoli cells, cause destruction of testicular tissue, and thereby downregulate testosterone (20). However, the evidence of any presence of SARS-CoV-2 in semen and testicular tissue is conflicting (21). 19% of men with COVID-19 and 14% of patients with bacterial CAP fulfilled the criteria for primary hypogonadism (testosterone<9 nmol/L and LH ≥ 9 IU/L), which would support tissue destruction and direct downregulation of testosterone. Three out of four men with COVID-19 and bacterial CAP fulfilled the criteria for secondary hypogonadism (testosterone< 9 nmol/L and LH < 9 IU/L). Male hypogonadism increases with age and comorbidities and is estimated to be up to 25% among men >70 years (10). As the prevalence of hypogonadism in our and other studies is higher, we may assume that suppression of the HPT axis occurs during lower respiratory illnesses. Studies have reported that male hypogonadism is associated with increased cardiovascular disease, COPD, diabetes, and cancer, which coincidentally are risk factors for hospitalization and severe COVID-19 as well as bacterial and influenza virus CAP (22, 23, 24). Therefore, we expect a higher prevalence of secondary hypogonadism in patients hospitalized with COVID-19 or CAP compared to the background population. Testosterone seems to be in a bidirectional relationship with severe disease, which makes it difficult to conclude whether low testosterone induces disease or merely mirrors the disease or both. On one hand, testosterone has long been known for its anti-inflammatory properties through suppression of pro-inflammatory cytokines (e.g. IL-6 and tumor necrosis factor-α) and induction of anti-inflammatory cytokines (e.g. IL-10) (9). As the set-point of inflammation is already altered in patients with low testosterone, we may speculate that these patients are at enhanced risk of severe respiratory illness as is seen in other low-grade inflammatory diseases such as diabetes (25). Furthermore, secondary hypogonadism leads to reduced muscle mass, including respiratory muscles, and low serum testosterone has been associated with reductions in lung function indices, which may enhance respiratory distress and thereby disease severity and hospitalization (26). This is also reflected in our study results, as patients with COVID-19 with need for high-flow oxygen have lower testosterone concentrations compared to patients in need of low-flow oxygen or ambient air. On the other hand, the high prevalence of secondary hypogonadism in all three patient groups could be due to reduced gonadotropin-releasing hormone pulsatility, which is a known phenomenon during acute disease and physiological stress, and may suggest that testosterone concentrations reflect disease severity (27, 28, 29). Spratt et al. studied pituitary–gonadal changes in men undergoing coronary artery bypass graft at pre-admission, during surgery, and the following days post-surgery as a model for acute severe disease (8). As in our study, they found that the study population had decreased concentrations of testosterone, LH, and FSH and increased concentrations of estrone and estradiol as early as the day after surgery. This supports the conclusion that alterations of the HPG axis are a normal physiological response to acute severe disease and systemic inflammation.
In this study, we found that reproductive hormones in men with COVID-19 and bacterial CAP were correlated to CRP and IL-6 with lower testosterone, LH, FSH, and higher estrone sulfate with increasing CRP or IL-6. The correlations between reproductive hormones and CRP were stronger in patients with CAP, whereas correlations between testosterone or estrone sulfate and IL-6 were stronger than with CRP. Severe COVID-19 induces an inappropriate hyperinflammatory response with an upregulation of pro-inflammatory cytokines, and hyperinflammation is linked to morbidity and mortality in patients with COVID-19. IL-6 has been identified as one of the important pro-inflammatory cytokines and IL-6 inhibition is standard-of-care for patients with severe COVID-19 pneumonia (30). Addition of IL-6 to the regression model attenuated associations in men with COVID-19 but strengthened them in women. In men with COVID-19, testosterone, estrone sulfate, and LH were associated with 90-day mortality before adjustment for IL-6, but after adjustment for IL-6, only testosterone remained associated with mortality but with a 95% CI that included 1. Testosterone and LH remained associated with mechanical ventilation after adjustment for IL-6. The correlations of inflammatory markers with reproductive hormones as well as reductions in the associations between reproductive hormones and mortality or mechanical ventilation suggest that reproductive hormones to some extent act as acute-phase reactants in men upon acute systemic inflammation. However, the independent associations with mechanical ventilation after adjustment for inflammation suggest that testosterone or LH may be involved in the pathogenesis of severe respiratory failure in men with COVID-19. In support of our findings, Dhindsa et al. reported inverse correlations between testosterone and a range of pro-inflammatory cytokines including CRP and IL-6 (31). Tsilidis et al. reported that high concentrations of estradiol and low concentrations of testosterone in men with COVID-19 were associated with higher inflammation measured by white blood cell count and C-reactive protein (32). Salonia et al. reported that low testosterone concentrations were associated with severe respiratory failure and increased mortality in men with COVID-19 after adjusting for IL-6 (6). Thus, in the clinical setting, measurements of testosterone and LH may be used as an additional diagnostic tool in the prediction of the disease course among men with COVID-19. Contrary to our results, Schroeder et al. found that high concentrations of estradiol were associated with severe respiratory failure but did not account for IL-6 or other inflammatory markers (5). In our study, the impact of estrone sulfate on mortality and severe respiratory failure was explained by the degree of inflammation as measured by IL-6. However, this study measured estrone sulfate rather than estradiol, which hampers the comparison between the two. As estrone sulfate is considered a more stable buffer capacity for estrogen, this was chosen for analysis in the population.
In women, testosterone concentrations were discretely, albeit statistically significantly, lower in patients in the two COVID-19 cohorts as well as in patients with bacterial CAP and influenza virus CAP compared to the reference group. The pathway for suppression of testosterone concentrations in women differs from men, as the hormone primarily is produced in the adrenal glands. Studies on testosterone, estrone sulfate, LH, and FSH in women with COVID-19 are few and somewhat in conflict with our results. Dhindsa et al. found no differences in hormonal concentrations between women with severe and mild COVID-19 (31). However, they defined severe disease as a composite of mortality, ICU admission, or oxygen requirement of any kind. In our study, we found no difference in testosterone concentrations in mild, moderate, or severe disease, but we found that there was a difference in estrone sulfate concentrations associated with acute respiratory distress, as women receiving high-flow compared to low-flow oxygen had higher concentrations of estrone sulfate. Either way, testosterone and estrone sulfate were not associated with mortality or mechanical ventilation in women with COVID-19 in support of other studies (5, 31). Surprisingly, we found that two-fold reductions of both LH and FSH predicted mechanical ventilation and mortality in women with COVID-19, and we saw that women who died had lower concentrations of the two pituitary hormones. To our knowledge, this study provides novel insight regarding a significant association between the suppression of female pituitary hormones and morbidity in COVID-19.
When we compare the results of the mortality analysis in the second cohort with the first, the associations between testosterone and respiratory failure or mortality did not remain. This may be due to the lower event rate of mortality and mechanical ventilation and thereby reduced statistical power compared to the high event rate in the first COVID-19 cohort (33). Furthermore, nearly nine of ten patients in the second cohort were treated with dexamethasone, which suppresses hormones both centrally at the hypothalamic level, at the pituitary level, and at the adrenal level. Thus, treatment must be taken into consideration in future studies.
Study strengths and limitations
Most other studies have focused on men, and the few studies that have reported hormone concentrations in other CAP etiologies than COVID-19 have not specified the etiology of CAP. The main strength of our study is that it included men and women with different etiologies of CAP for a comprehensive assessment of reproductive hormone changes in patients with COVID-19, bacterial, and influenza virus CAP. The study population of patients with COVID-19 was large with prospective data collection, which offered the opportunity to stratify patients with COVID-19 into groups with different standard-of-care treatment options pre- and post-dexamethasone and remdesivir introduction. Of limitations, the study was a multicenter study with recruitment at different time points and databases, yielding different data sampling (e.g. missing information on baseline oxygen requirement in patients with bacterial and influenza virus CAP). Additionally, blood samples were drawn at different time points during the first 4 days of admission. To account for any effect of varying sample times, survival time was calculated from the day of sampling and not the day of admission. Further, time from admission to sampling was included in the multivariate logistic regression analysis. Lastly, the groups with bacterial and influenza virus CAP were small, especially once stratified by sex, which limited the comparison of hormone concentrations between survivors and non-survivors and outcome analysis.
Conclusion
Testosterone concentrations were severely suppressed in men hospitalized with COVID-19, bacterial, and influenza virus CAP and moderately suppressed in women. The association of reproductive hormones with morbidity in men with COVID-19 was partly induced by inflammatory parameters. However, once adjusted for IL-6, low concentrations of testosterone and LH predicted severe respiratory failure in men with COVID-19, whereas low concentrations of LH and FSH predicted severe respiratory failure and mortality in women with COVID-19.
Supplementary Material
Declaration of interest
TB reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Boehringer Ingelheim, grants and personal fees from Gilead, personal fees from MSD, personal fees from Astra Zeneca, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, personal fees from Pentabase ApS, grants from Erik and Susanna Olesen’s Charitable Fund, outside the submitted work. AJ received unrestricted research grants from Novo Nordisk and Ferring and received speaker fees from Novo Nordisk, Ferring, IPSEN, Takeda and Pfizer, outside the submitted work. All other authors report no conflict of interest.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications 202011 6317. ( 10.1038/s41467-020-19741-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infante M, Pieri M, Lupisella S, D’Amore L, Bernardini S, Fabbri A, Iannetta M, Andreoni M, Morello M. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID-19. European Review for Medical and Pharmacological Sciences 2021255889–5903. ( 10.26355/eurrev_202110_26865) [DOI] [PubMed] [Google Scholar]
- 3.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello Aet al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology 2021988–98. ( 10.1111/andr.12821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng S, Zou Q, Zhang D, Yu F, Bao J, Lou B, Xie G, Lin S, Wang R, Chen Wet al. Serum level of testosterone predicts disease severity of male COVID-19 patients and is related to T-cell immune modulation by transcriptome analysis. Clinica Chimica Acta; International Journal of Clinical Chemistry 2022524132–138. ( 10.1016/j.cca.2021.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder M, Schaumburg B, Mueller Z, Parplys A, Jarczak D, Roedl K, Nierhaus A, de Heer G, Grensemann J, Schneider Bet al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerging Microbes and Infections 2021101807–1818. ( 10.1080/22221751.2021.1969869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AMet al. Severely low testosterone in males with COVID‐19: a case‐control study. Andrology 202191043–1052. ( 10.1111/andr.12993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vena W, Pizzocaro A, Maida G, Amer M, Voza A, Di Pasquale A, Reggiani F, Ciccarelli M, Fedeli C, Santi Det al. Low testosterone predicts hypoxemic respiratory insufficiency and mortality in patients with COVID-19 disease: another piece in the COVID puzzle. Journal of Endocrinological Investigation 202245753–762. ( 10.1007/s40618-021-01700-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Z, Zhong J, Jiang Y, Zhang J. Associations between COVID-19 infection and sex steroid hormones. Frontiers in Endocrinology (Lausanne) 202213 940675. ( 10.3389/fendo.2022.940675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 201922129–140. ( 10.1080/13685538.2018.1482487) [DOI] [PubMed] [Google Scholar]
- 10.Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. International Journal of Endocrinology 20122012 625434. ( 10.1155/2012/625434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen CL, Rasmussen ÅK, Johannsen TH, Hilsted LM, Skakkebæk NE, Szecsi PB, Pedersen L, Benfield T, Juul A. Thyroid function in COVID-19 and the association with cytokine levels and mortality. Endocrine Connections 2021101234–1242. ( 10.1530/EC-21-0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israelsen SB, Kristiansen KT, Hindsberger B, Ulrik CS, Andersen O, Jensen M, Andersen S, Rasmussen C, Jørgensen HL, Østergaard Cet al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March-April 2020. Danish Medical Journal 202067 A05200313. [PubMed] [Google Scholar]
- 13.Ryrsø CK, Dungu AM, Hegelund MH, Jensen AV, Sejdic A, Faurholt-Jepsen D, Krogh-Madsen R, Lindegaard B. Body composition, physical capacity, and immuno-metabolic profile in community-acquired pneumonia caused by COVID-19, influenza, and bacteria: a prospective cohort study. International Journal of Obesity 202246817–824. ( 10.1038/s41366-021-01057-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thuesen BH, Cerqueira C, Aadahl M, Ebstrup JF, Toft U, Thyssen JP, Fenger RV, Hersoug LG, Elberling J, Pedersen Oet al. Cohort profile: the Health2006 cohort, research centre for prevention and health. International Journal of Epidemiology 201443568–575. ( 10.1093/ije/dyt009) [DOI] [PubMed] [Google Scholar]
- 15.Kårhus LL, Thuesen BH, Rumessen JJ, Linneberg A. Symptoms and biomarkers associated with celiac disease: evaluation of a population-based screening program in adults. European Journal of Gastroenterology and Hepatology 2016281298–1304. ( 10.1097/MEG.0000000000000709) [DOI] [PubMed] [Google Scholar]
- 16.Damgaard-Olesen A, Johannsen TH, Holmboe SA, Søeborg T, Petersen JH, Andersson A, Aadahl M, Linneberg A, Juul A. Reference ranges of 17-hydroxyprogesterone, DHEA, DHEAS, androstenedione, total and free testosterone determined by TurboFlow-LC-MS/MS and associations to health markers in 304 men. Clinica Chimica Acta 201645482–88. ( 10.1016/j.cca.2015.12.042) [DOI] [PubMed] [Google Scholar]
- 17.Søeborg T, Frederiksen H, Johannsen TH, Andersson AM, Juul A. Isotope-dilution TurboFlow-LC-MS/MS method for simultaneous quantification of ten steroid metabolites in serum. Clinica Chimica Acta 2017468180–186. ( 10.1016/j.cca.2017.03.002) [DOI] [PubMed] [Google Scholar]
- 18.Ljubicic ML, Jespersen K, Aksglaede L, Hagen CP, Petersen JH, Andersen HR, Linneberg A, Main KM, Andersson AM, Johannsen THet al. The LH/FSH ratio is not a sex-dimorphic marker after infancy: data from 6417 healthy individuals and 125 patients with differences of sex development. Human Reproduction 2020352323–2335. ( 10.1093/humrep/deaa182) [DOI] [PubMed] [Google Scholar]
- 19.Bermejo-Martin JF, González-Rivera M, Almansa R, Micheloud D, Tedim AP, Domínguez-Gil M, Resino S, Martín-Fernández M, Ryan Murua P, Pérez-García Fet al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Critical Care 202024 691. ( 10.1186/s13054-020-03398-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edenfield RC, Easley CA. Implications of testicular ACE2 and the renin–angiotensin system for SARS-CoV-2 on testis function. Nature Reviews. Urology 202219116–127. ( 10.1038/s41585-021-00542-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengupta P, Leisegang K, Agarwal A. The impact of COVID-19 on the male reproductive tract and fertility: a systematic review. Arab Journal of Urology 202119423–436. ( 10.1080/2090598X.2021.1955554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varikasuvu SR, Dutt N, Thangappazham B, Varshney S. Diabetes and COVID-19: a pooled analysis related to disease severity and mortality. Primary Care Diabetes 20211524–27. ( 10.1016/j.pcd.2020.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male 2020231416–1424. ( 10.1080/13685538.2020.1774748) [DOI] [PubMed] [Google Scholar]
- 24.Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, Pivonello R, Salonia A, Sforza A, Maggi M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: systematic review and meta-analysis. Reviews in Endocrine and Metabolic Disorders 202122275–296. ( 10.1007/s11154-021-09630-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wargny M, Potier L, Gourdy P, Pichelin M, Amadou C, Benhamou PY, Bonnet JB, Bordier L, Bourron O, Chaumeil Cet al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia 202164778–794. ( 10.1007/s00125-020-05351-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han YY, Yan Q, Yang G, Chen W, Forno E, Celedon JC. Serum free testosterone and asthma, asthma hospitalisations and lung function in British adults. Thorax 202075849–854. ( 10.1136/thoraxjnl-2020-214875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. Journal of Clinical Endocrinology and Metabolism 1993761548–1554. ( 10.1210/jcem.76.6.8501163) [DOI] [PubMed] [Google Scholar]
- 28.Christeff N, Benassayag C, Carli-Vielle C, Carli A, Nunez EA. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. Journal of Steroid Biochemistry 198829435–440. ( 10.1016/0022-4731(8890254-3) [DOI] [PubMed] [Google Scholar]
- 29.Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. Journal of Clinical Endocrinology and Metabolism 198560444–450. ( 10.1210/jcem-60-3-444) [DOI] [PubMed] [Google Scholar]
- 30.REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk Wet al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. New England Journal of Medicine 20213841491–1502. ( 10.1056/NEJMoa2100433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, Mani K, Randolph GJ, Edwards JR, Mudd PAet al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Network Open 20214 e2111398. ( 10.1001/jamanetworkopen.2021.11398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, Feinleib M, Joshu CE, Kanarek N, Nelson WGet al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology 20131919–928. ( 10.1111/j.2047-2927.2013.00129.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benfield T, Bodilsen J, Brieghel C, Harboe ZB, Helleberg M, Holm C, Israelsen SB, Jensen J, Jensen TØ, Johansen ISet al. Improved survival among hospitalized patients with coronavirus disease 2019 (COVID-19) treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clinical Infectious Diseases 2021732031–2036. ( 10.1093/cid/ciab536) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a