Abstract
Although degenerative disc disease (DDD) and associated low back pain (LBP) are growing public health problems, however the underlying disease mechanisms are unclear. Various investigations implicated the increase in vascular endothelial growth factor (VEGF) levels in DDD, our goal was to determine the role of VEGF receptors (VEGFrs) in DDD, using the mouse model of DDD. Progressive DDD was induced by anterior stabbing of lumbar intervertebral discs in wild type (WT) and VEGFR-1 tyrosine-kinase deficient mice (vegfr-1TK−/−). Pain assessments were performed weekly for 12 weeks. Histological and immunohistochemical assessments were made for discs, dorsal root ganglions and spinal cord. Both vegfr-1TK−/− and WT mice presented with similar pathological changes in discs with an increased expression of inflammatory cytokines and matrix-degrading enzymes. Despite these similar pathological patterns, vegfr-1TK−/− mice showed insensitivity to pain compared to WT mice. This insensitivity to discogenic pain was associated with lower levels of the pain factors in discs and peripheral sensory neurons as well as with spinal glial activation in vegfr-1TK−/− compared to WT mice. Exogenous stimulation of bovine disc cells with VEGF increased inflammatory and cartilage degrading enzyme. Silencing vegfr-1 by small-interfering-RNA (siRNA) decreased VEGF-induced expression of pain markers, while silencing vegfr-2 decreased VEGF-induced expression of inflammatory and metabolic markers without changing pain markers. This suggests the involvement of VEGFR-1 signaling specifically in pain transmission. Collectively, our results suggest that VEGF signaling plays an important role in DDD and in particular, VEGFR-1 is critical for discogenic LBP transmission independent of the degree of disc pathology.
Keywords: Intervertebral disc degeneration, Discogenic low back pain, Vascular endothelial growth factor receptor-1, Vascular endothelial growth factor, Mouse disc degeneration disease model
1. Introduction
Low back pain (LBP) is a major crippling disorder, both physically and socioeconomically, affecting up to 80% of adults, at least once during their lifetime. In the United States alone, the cost due to LBP exceeds $100 billion per year (Dagenais et al., 2008). Despite the major negative impact on quality of life, health care cost and management, the etiology of LBP and the molecular mechanisms driving this pathogenesis are poorly understood, and thus, there is no therapeutic approach yet to cure or prevent LBP. How LBP develops to a painful and debilitating disease is unclear and represents key gaps in our knowledge.
Degenerative disc disease (DDD) is thought to contribute greatly to LBP pathogenesis and accounts for an estimated 40% of the chronic LBP cases (Schwarzer et al., 1994; Zhang et al., 2009). Many studies have been conducted in the past few decades to address the pathogenesis and the molecular mechanisms of DDD and the associated LBP. It was recognized that the formation of new blood vessels during the progression of DDD is a common and important pathological phenomenon (Karamouzian et al., 2010; Pai et al., 1999; Ratsep et al., 2013; Tolonen et al., 2006). Vascular endothelial growth factors (VEGFs), potent angiogenic factors, promote neovascularization in intervertebral discs (IVDs). Cadaveric and animal studies found that VEGF expression is significantly increased in degenerative discs and correlated not only with the degree of degeneration but also with the severity of LBP (Binch et al., 2014; Haro et al., 2002). However, VEGF-induced disc degeneration triggers pain signal transduction is yet to be elucidated.
VEGFs exert their effects through the cognate receptors (Ferrara et al., 2003; Hamilton et al., 2016), leading to a wide range of different biological functions (Meyer et al., 1999; Ogawa et al., 1998; Shibuya et al., 2006) and well-known pathophysiologic effects, including contributions to tumor angiogenesis, rheumatoid arthritis, psoriasis, macular degeneration, as well as knee osteoarthritis (OA), in which anti-VEGF treatment has already achieved preliminary success. In most of the arthritic researches, VEGF-A was targeted to alleviate pathological progression (Hamilton et al., 2016; Nagai et al., 2014). However, considering redundant and compensatory roles among VEGF ligands, targeting their functional receptors may be more efficacious. It has long been believed that VEGFR-2 is the primary receptor mediating the biological effects of VEGF, and that VEGFR-1/Flt-1 may act as a negative regulator of VEGFR-2. However, there is also evidence for VEGFR-1 in transferring biological signals and enhancing the activity of VEGFR-2 (Olsson et al., 2006). Based on recent publications, it appears that the VEGF signaling may play an important role in pain transmission (Lin et al., 2010; Sato et al., 2017; Selvaraj et al., 2015). For example, Selvaraj et al. demonstrated that VEGFR-1/Flt-1 could directly modulate the excitability of sensory neurons and induce the expression of pain-related neuropeptides in dorsal root ganglia (DRG) (Selvaraj et al., 2015). More recently, Das et al., showed the distinct role of VEGFR-1, but not VEGFR-2, in knee OA pain transmission (Das et al., 2018). Therefore, we hypothesize that VEGFR-1 contributes to the nociceptive pain signal transduction during IVD degeneration. In this study, we investigated the precise roles for VEGFR-1 in disc degeneration and associated LBP transmission in vivo by using VEGFR-1 kinase domain mutant mice (vegfr-1TK−/− mice) (Hiratsuka et al., 1998). We further employed in-vitro approaches to elucidate the detailed molecular mechanism and signaling pathway of VEGF and its cognate receptors by using small interfering RNAs (siRNAs) targeting vegfr-1/2 in bovine disc cell culture.
2. Methods
2.1. Experimental animals and surgical procedures
C57BL strain background vegfr-1TK−/− mice were prepared previously (Dawson et al., 2009; Hiratsuka et al., 1998; Sawano et al., 1996,1997), in which VEGFR-1/Flt-1 transmit no signal but has intact VEGFR-1 ligand binding capability. Equivalent C57BL wild type mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). vegfr-1TK−/− mice (n=20) and wild type mice (n=20) aged 4 months were used in the experiment according to a protocol that was in agreement with the guidelines of the Rush Institutional Animal Care and Use Committee (IACUC). Disc degeneration was surgically induced using an anterior extraperitoneal approach as described previously (Shi & Das et al., 2018). Briefly, mice were placed in the supine position under inhalation anesthesia. Abdominal hair was shaved, disinfected and draped in sterile fashion. A left paramedian abdominal incision of approximately 1.5 cm in length was made with a #15 scalpel blade. The lumbar spine discs (L4/5, L5/6 and L6/S1) were identified under a surgical microscope, and each disc was stabbed once at their midline with a micro-scalpel at a depth of 0.7 mm. Through the same stabbing incision, another micro-scalpel with a curved point was used to stab once again to take out a part of the nucleus pulposus (NP) tissue. The skin incision was closed with 4-0 vicryl sutures. For sham surgery groups, all aspects of the DDD surgery were duplicated, except that the tip of the micro-scalpel was firmly placed against the surface of the disc without stabbing.
2.2. Animal pain behavioral tests
Behavioral testing for pain in these mice was performed by an investigator who was blinded to the grouping and surgical procedures. Prior to baseline assessment, mice were acclimated to the testing apparatus for 2 hours per day for a total of 5 days. Pain tests were conducted once a week for three weeks to obtain baseline data before the surgery, and then at different time points after surgery until the designated sacrifice time for each mouse. The methods applied for testing included von Frey filament testing for mechanical allodynia, hot plate testing for thermal hyperalgesia and burrowing for spontaneous behavioral changes as described previously (Shi & Das et al., 2018; Shi & Qiu et al., 2018). At the designated time after finishing all the pain behavioral tests, the mice were sacrificed under anesthesia with intra-peritoneal injection of ketamine and xylazine, and perfused transcardially with 0.1M phosphate buffered saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS, pH 7.4).
2.3. Histology
After sacrifice, the mouse lumbar spine samples were harvested, fixed in 4% PFA in PBS, decalcified in 12.5% EDTA in distilled water (pH=7.3) which was changed every 3 days, and then embedded in paraffin. The embedded disc was cut into 5 μm thick slices. Safranin O/Fast green (SO/FG) staining was done according to the protocol to assess histological changes in IVDs, which were analyzed by 2 blinded investigators using a modified Masuda grading scale for scoring (Masuda et al., 2005; Shi & Das et al., 2018).
2.4. Immunofluorescence staining in discs
After deparaffinization and rehydration in downgraded ethanol, the disc sections were incubated in proteinase K solution (20 μg/ml) for 30 min at 37°C for antigen retrieval and then blocked in 5% normal goat serum for 30 minutes at room temperature. The sections were then incubated at 4°C overnight with antibodies against tumor necrosis factor alpha (TNF-α, 1:200, Novus Biological), VEGF (1:200, Abcam), matrix metallopeptidase 13 (MMP-13, 1:200, Abcam), a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5, 1:200, Thermo), and nerve growth factor (NGF, 1:200, Santa Cruz Biotechnology), tropomyosin receptor kinase A (TrkA, 1:100, Abcam) followed by incubation with goat anti-rabbit Alexa 488 fluorescent antibody conjugate (1:250, Invitrogen) for 1 h and counterstained with DAPI for nuclear tests the next morning, followed by fluorescence microscopy. For each section, immunostained images were obtained at low power (40 x magnification) to identify areas of the disc including the NP and annular fibrosus (AF) areas. The percentage of immunoreactive (ir) cells were then counted for an average of 4 fields in the NP and AF areas of each section using 200 x magnification. The same regions were selected for the immunohistological analyses for all samples to ensure consistency.
2.5. Immunofluorescence staining in DRG and spinal cord
After sacrifice, the lumbar enlargement of the spinal cord and bilateral L3-6 DRGs were harvested, fixed and embedded. All slices were cut at 4 μm thickness. After deparaffinization and rehydration, antigen retrieval was done under microwave irradiation in 10 mmol/L sodium citrate solution (pH 6.0). Samples were then permeabilized with 0.5% Triton X-100 solution for 5 minutes and then blocked with 5% normal goat or horse serum for 30 min at room temperature. After that, the DRG sections were processed for double labeling immunofluorescence staining by sequential addition of first primary mouse anti-neuronal nuclei (NeuN) antibody (1:500, Millipore) as a neuronal marker and second primary antibody of interesting targets such as rabbit anti-calcitonin gene-related peptide (anti-CGRP) antibody (1:500, Abcam), or rabbit anti-NGF antibody (1:200, Santa Cruz Biotechnology), or rabbit anti-transient receptor potential vanilloid type-1 (TRPV1) antibody (1:100, Millipore) overnight, followed by incubation with appropriate secondary antibodies coupled to goat anti-mouse Alexa 488 fluorescent antibody conjugate or goat anti-rabbit Alexa 546 fluorescent antibody conjugate (1:300, Life Technologies) for 1 h. Spinal cord sections were incubated overnight with primary mouse anti-glial fibrillary acidic protein (anti-GFAP) antibody (1:500, Millipore) or rabbit anti-ionized calcium–binding adapter molecule 1 (anti-IBA-1) antibody (1:500, Wako), then incubated with goat anti-mouse or goat anti-rabbit Alexa 488 fluorescent antibody conjugate (1:300, Life Technologies) for 1 hour. All samples were counterstained with DAPI for visualization of nuclei. Immunoreactivity was examined under a fluorescence microscope. For each DRG section, the number of immunoreactive neurons and total neurons were counted in the whole field at 200x magnification using a counting grid, and the percentage corresponding to the number of immunoreactive neurons and total neurons was calculated by dividing the total neurons marked by NeuN. For each section of spinal cord, the region defining the spinal cord dorsal horn was outlined and the number of GFAP-immunoreactive (ir) astrocytes and IBA-1-ir microglia per 0.25 mm2 area within the dorsal horn was calculated.
2.6. Nucleus pulposus (NP) cells isolation, culture, treatment, and transfection
Fresh tails from young adult bovine animals (15–18 months old) were purchased from a local slaughterhouse. The NP cells were isolated from coccygeal discs as previously described (Gu et al., 2015; Kim et al., 2012). The isolated bovine NP cells were seeded in 6-well plates at 4 × 105 cells/cm2 and cultured as monolayers in sterile complete medium containing DMEM/F-12 (1:1) with 10% fetal bovine serum and antibiotics. Cells were treated with gradient concentrations of VEGF-A or transfected with siRNA targeting VEGFR-1 or VEGFR-2 using the Lipofectamin 2000 reagent (Thermo Fisher Scientific) according to the protocol and negative control was applied. After successful transfection, the cells in the corresponding wells were treated with 100ng/ml VEGF-A for 24 hours. After the treatment, total RNA was isolated from these wells for further analysis.
2.7. RNA Isolation and Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
The total RNA was extracted using the TRIzol method (Invitrogen Corp.) according to the manufacturer’s instruction and synthesized cDNA with 1μg total RNA. Real-time PCR was performed in triplicate using a Bio-Rad CFX Connect system (Bio-Rad Laboratories, California, USA) and the SYBR Green method (Bio-Rad Laboratories). A threshold cycle (Ct) value was obtained and relative mRNA expression was determined using the ΔΔCT method. Expression of 18s mRNA was used as the internal control. The standard deviation of gene expression values was derived from 3 different sets of bovine tails in 3 separate experiments. The primer sequences are summarized in Table 1.
Table 1.
Primer Sequences for qRT-PCR analysis in bovine disc cell culture.
| Target | Forward Primer | Reverse primer | Primer Sequence |
|---|---|---|---|
| TNF-α | CACGTTGTAGCCGACATCAACTCT | GTTGTCTTCCAGCTTCACACCGTT | NM_173966.2 |
| MMP-13 | GCTTCCTGATGATGATGTCCAAGGGA | AGGGTCACATTTGTCTGGCGTT | NM_174389 |
| NGF | GACCCACACCTTCGTCAAG | GTGTGTGCTCAGCAGGAA | NM_001099362 |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | NR_003286 |
MMp-13: Matrix metallopeptidase 13; TNF-α: Tumor necrosis factor alpha; NGF: Nerve growth factor; CGRP: Calcitonin Gene-related Peptide; 18s: 18S ribosome.
2.8. Western blot analysis
Western blot analysis and ubiquitination assay were performed as described previously (Li et al., 2008). The whole-cell protein was extracted and the concentrations were determined by the bicinchoninic acid protein assay (BCA; Pierce, Rockford, IL). Immunoreactivity of TNF-α (1:2000, Abcam), MMP-13 (1:2000, Abcam), NGF (1:2000, Abcam) and β-actin (1:5000, Abcam) was detected using the ECL system (Amersham Biosciences, Piscataway, NJ) and the Signal Visual Enhancer system (Pierce, Rockford, IL) according to the manufacturer’s instruction.
2.9. Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS version 18; SPSS Inc., Chicago, IL, USA). All data are presented as mean ± SD. Pain data of these four groups were compared and analyzed using the two-factor repeated measures ANOVA. Thermal thresholds from the right and left hind paws were averaged for analysis because of no difference was found. The differences between DDD and sham groups, and between vegfr-1TK−/− and WT animals for histology and immunofluorescent staining were evaluated using two-way ANOVA and a post-hoc Tukey-B test. The results of histology and immunofluorescent staining were independently examined by two observers who were blinded to the grouping. Cohen’s k value was calculated by using an internet-based program. The average values were taken for analysis. The results of qRT-PCR and western-blot were analyzed using one-way ANOVA. P < 0.05 was considered statistically significant for all the analysis.
3. Results
3.1. Intervertebral disc degeneration develops in both vegfr-1TK−/− and WT mice after disc injury.
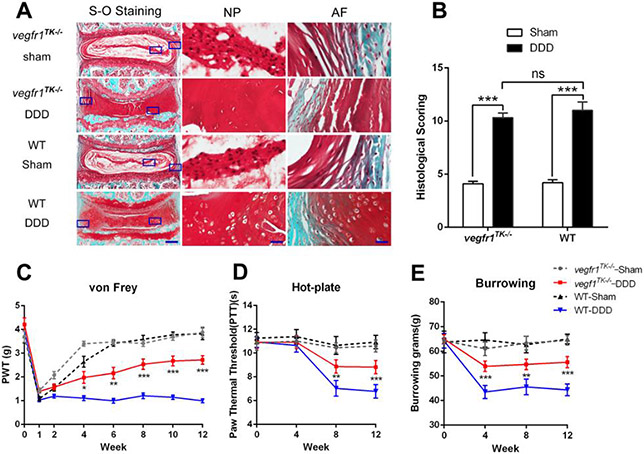
To understand the role of VEGF signaling and its cognate receptors, progressive disc degeneration was surgically induced, using an established mouse model of DDD in both WT and vegfr-1TK−/− mice. Twelve weeks after surgery, the mice were sacrificed and the lumbar spine was harvested for biochemical and morphological analyses. Safranin-O/fast green staining showed normal morphology in both sham groups of vegfr-1TK−/− and WT mice with prominent notochordal cells located in the center of the disc, intact AF with normal patterns of fibrocartilage lamellas, well-defined border between the AF and NP, and preserved proteoglycan content. In both DDD groups (vegfr-1TK−/− and WT), the injured IVDs developed severe degeneration represented by changes of NP cell phenotype into chondrocyte-like cells, reduced NP cell number and proteoglycan content, disorganized AF lamellas and interrupted borders between the AF and NP (Fig. 1A & B) in which the progression of pathological changes are consistent in this DDD murine model as described previously (Shi & Das et al., 2018). Our data suggest that a deficiency in VEGFR-1 does not attenuate the pathological progression of the IVD.
Figure 1.
Deficiency of tyrosine kinase in VEGFR-1 (vegfr1TK−/−) reduces the severity of discogenic low back pain without reversing the intervertebral disc (IVD) degeneration induced by anterior extraperitoneal disc stabbing in 12-week-old vegfr-1TK−/− mice and age-matched wild-type (WT) mice. Each disc shown is representative of a group of mice (n=5). (A) Histological assessment, by Safranin-O/fast green staining, of the proteoglycan induced degradation of the IVD. Each picture shows representative images of the IVDs from the indicated group (bars = 200 μm for the integral IVD and bars = 40μm for magnified NP and AF areas). (B). Quantitative assessment of disc degradation using a modified Masuda’s grading score, comparing sham groups with stabbed groups. Significant differences were found in both DDD groups compared to their respective sham groups (n=5 per group). *P<0.05, **P< 0.01, ***P< 0.001, compared to the sham-operated group. (C) Mechanical hyperalgesia was investigated using the von Frey filament test. The paw withdrawal threshold (PWT) of the DDD group of wt mice (n=10) markedly decreased until 12 weeks post-surgery, while the PWT values of the DDD group of vegfr-1TK−/− mice (n=10) gradually recovered after surgery compared with the DDD group of WT mice. Sham groups (n=10 for each) showed similar values in both WT and vegfr-1TK−/− mice. (D) Thermal hyperalgesia was assessed using the hot-plate test. The paw thermal threshold (PTT) in the DDD group of WT mice(n=10) was significantly decreased at 8 and 12 weeks after surgery while the PTT in the DDD group of vegfr-1TK−/− mice (n=10) gradually recovered during the 8 weeks after surgery. (E) The burrowing test. The DDD group of WT mice (n=10) did less burrowing than sham mice (n=10) at 4, 8, and 12 weeks, while the burrowing of the DDD group of vegfr-1TK−/− mice (n=10) gradually recovered during the 4 weeks after surgery. *P<0.05, **P< 0.01, ***P< 0.001, comparing the DDD group of vegfr-1TK−/− mice to the DDD group of WT mice.
3.2. vegfr-1TK−/− mice display insensitivity to pain compared to WT mice.
We assessed longitudinal pain-related behavioral changes in the vegfr-1TK−/− and WT mice with (DDD group) and without disc injury (sham group), using von Frey filaments (Fig.1C) and hot plate (Fig.1D) for mechanical allodynia and thermal sensitivity, respectively. Borrowing tests were also conducted (Fig.1E) for changes in spontaneous movements as described previously (Shi & Das et al., 2018). Baseline pain tests (before disc injury) showed no differences between vegfr-1TK−/− and WT mice. Despite the development of similar pathological progression in both vegfr-1TK−/− and WT mice after disc injury (Fig. 1A&B), our behavioral pain results clearly demonstrated significantly reduced pain in vegfr-1TK−/− mice compared to those from gender- and age-matched WT mice (Fig. 1C-E, p<0.05 ~ p<0.001). There was no difference in pain response between the right and left hind paw in either DDD group of vegfr-1TK−/− and WT mice.
Taken together, the histological and behavioral studies suggest that VEGFR-1 is involved in discogenic back pain transmission, and thus, blockade of VEGFR-1 tyrosine kinase signaling can reduce pain sensitivity, independently of progression of IVD degeneration in our DDD mouse model.
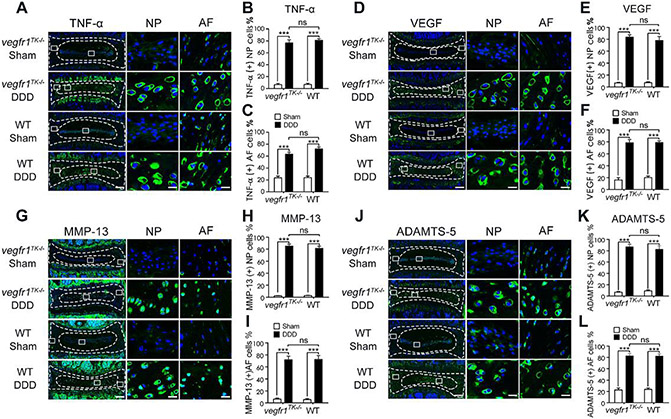
3.3. Similar levels of inflammation and catabolism were identified in the discs for both vegfr-1TK−/− and WT mice.
Inflammatory and angiogenic molecules, such as TNF-α and VEGF (Nagao et al., 2017) are well-known catabolic meditator by stimulating matrix degrading enzymes such as MMPs and ADAMTS which, in turn, lead to acceleration of IVD degeneration. To evaluate the pathological changes during the progression of the IVD degeneration, we examined the expression levels of TNF-α, VEGF, MMP-13 and ADAMTS-5 by comparing vegfr-1TK−/− and WT mice. The immunofluorescence staining showed that the immunoreactivity of these molecules was rather rare in the intact NP cells (sham groups of both vegfr-1TK−/− and WT mice) with only a few TNF-α, VEGF, MMP-13 or ADAMTS-5 immunopositive cells that were mainly confined to the outer layer of the AF. On the other hand, in DDD groups of both vegfr-1TK−/− and WT mice, the expression levels of TNF-α, VEGF, MMP-13 and ADAMTS-5 were markedly increased in the IVD represented by abundant immunopositive cells, not only in the AF but also in the inner NP cells (Fig. 2A-L, sham vs. DDD, p<0.001 for both vegfr-1TK−/− and WT mice). There is no significant difference between vegfr-1TK−/− and WT mice, showing similar expression levels of inflammatory and catabolic factors in both groups. These results suggest that VEGFR-1 tyrosine kinase deficiency has little to no influence on the expression of proinflammatory cytokines or extracellular matrix degrading enzymes such as TNF-α, VEGF, MMP-13 and ADAMTS-5 in the IVD cells.
Figure 2.
Inflammatory cytokine and catabolic enzyme expression in IVDs after disc stabbing surgery. Representative immunofluorescence staining for TNF-α (A), VEGF (D), MMP-13 (G) and Adamts-5 (J) in the discs of DDD and sham mice at 12 weeks post-surgery (bars=200μm for the integral discs, bars=20μm for magnified NP and AF areas). Quantitative analyses showed that TNF-α (B, C), VEGF (E, F), MMP-13 (H, J) and ADAMTS-5 (K, L) expression in the NP and AF in either DDD group mice was markedly increased compared with the respective sham group (n=5 per group), while there were no significant difference between both DDD groups of vegfr-1TK−/− and WT mice(***P< 0.001; ns, not significant).
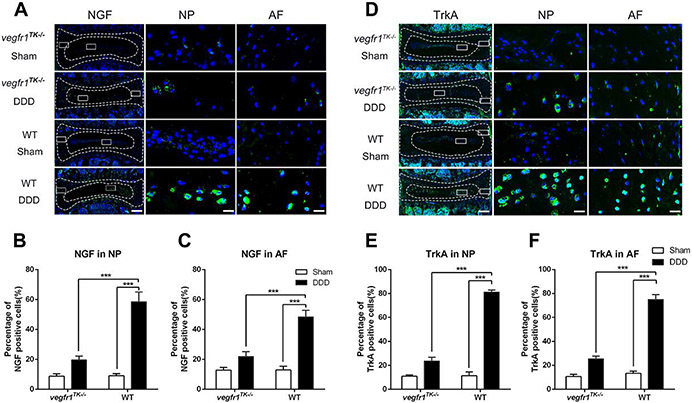
3.4. NGF and TrkA expression was reduced in the discs of the vegfr-1TK−/− mice correlating to the reduced pain behavior.
Studies showed that the level of NGF is abnormally high in many chronically painful conditions in both humans and experimental animals (Hefti et al., 2006; Pezet et al., 2006). These results suggest that NGF acts as a peripheral algogenic mediator (McMahon et al., 2004), including in the degenerative IVDs (Freemont et al., 2002). Increased NGF elicits sensory nerve ingrowth into the injured sites to transmit nociceptive sensation (Aoki et al., 2004; Kc et al., 2016) via its cognate receptor Tropomyosin receptor kinase A (TrkA). Compared to WT, vegfr-1TK−/− mice demonstrated significantly reduced pain, independently of progression of IVD degeneration in our DDD mouse model. Thus, we next examined the expression level of NGF and its cognate receptor TrkA in discs as representative pain mediators at the level of peripheral tissues and sensory neuronal systems. Our immunofluorescence staining results demonstrated that, in sham group, only a few NGF and TrkA immunopositive cells are present in both vegfr-1TK−/− and WT mice. In the DDD groups, the induction of NGF and TrkA immunopositive cells was strikingly increased in WT mice (both in the NP and AF areas). In contrast, this induction of NGF and TrkA was hardly seen in the vegfr-1TK−/− mice at 12 weeks after disc injury (Fig. 3). These results suggest that VEGFR-1 tyrosine kinase deficiency suppresses the expression levels of NGF and TrkA, and that the reduced pain observed in vegfr-1TK−/− mice is, at least in part, due to suppression of the NGF/TrkA signaling.
Figure 3.
NGF and TrkA expression was reduced in the discs of vegfr-1TK−/− mice compared to WT mice of the DDD groups. Representative immunofluorescence staining for NGF (A) and TrkA (D) in the IVD of DDD and sham mice at 12 weeks post-surgery (bars=200μm for the integral discs, bars=20μm for magnified NP and AF areas). Quantitative analyses showed that NGF (B, C) and TrkA (E, F) expression in IVDs of the DDD group of vegfr-1TK−/− mice was significantly lower than that in the IVDs of the DDD group of wt mice at 12 weeks after surgery. (n=5 per group) (***P< 0.001).
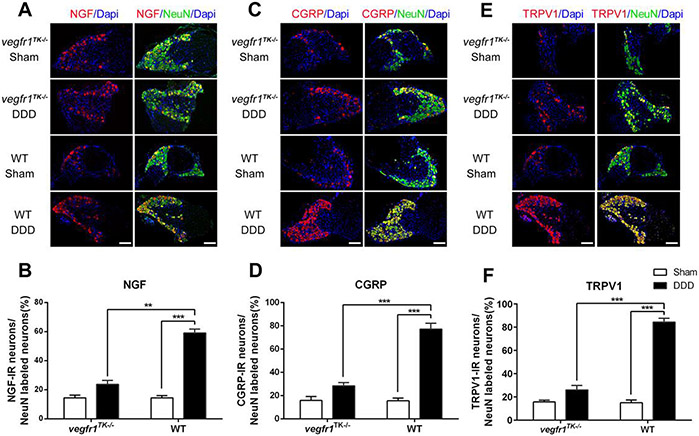
3.5. Plasticity of DRG sensory nervous system and spinal glial activity were better preserved in vegfr-1TK−/− mice.
Nociceptive pain signals from the IVD can be transmitted by DRG sensory nerves to the spinal cord, altering pain perception. To understand the role of VEGFR-1 in neuronal plasticity in discogenic back pain, the L3-5 lumbar DRGs and the spinal cord from the lumbar segments were harvested for biochemical analyses from vegfr-1TK−/− and WT mice, 12-weeks after surgical induction of DDD. In DRG neurons, selective pain-related molecules, including NGF, CGRP and TRPV1 were examined by double-labeling immunofluorescence staining with co-localization of NeuN, a biomarker for neurons (Mullen et al., 1992; Shi & Das et al., 2018). Expression levels of NGF (Fig. 4A & B), CGRP (Fig. 4C & D) and TRPV1 (Fig. 4E & F) in DRG sensory neurons were significantly elevated in WT mice with DDD compared to the corresponding sham group (p<0.001) and those from gender- and age-matched vegfr-1TK−/− mice with DDD (p<0.01~0.001). On the other hand, the induction of NGF, CGRP and TRPV1 expression in DRG sensory neurons was minimal in vegfr-1TK−/− mice with DDD compared with the corresponding sham group.
Figure 4.
VEGFR-1 tyrosine kinase deficiency reduced expression of pain-related targets in the dorsal root ganglion (DRG) after disc stabbing surgery. (A, C & E) Double immunofluorescence staining of NGF (A), CGRP (C) or TRPV1 (E) with NeuN (green) labeled neurons in DRGs of each group of mice. Co-localization of the two stains appears yellow. 4, 6-diamidino-2-phenylindole (DAPI) stains nuclei blue. Bars=20 μm. (B, D &F) Quantitative analyses show that expression of NGF, CGRP and TRPV1 in DRGs from the DDD group of vegfr-1TK−/− mice was significantly lower than that from the DDD group of WT mice at 12 weeks after surgery. (n=5 per group) (Values are mean ± SD. **P< 0.01, ***P< 0.001).
Activation of peripheral nociceptors can result in neuronal plasticity and changes in glial activity in the spinal cord. Especially, the critical roles of glial cells in the pathogenesis of pain have been well documented (Coull et al., 2005; Ji et al., 2013; Milligan et al., 2009). While microglia are important to onset of early phase in neuropathy, injury-induced astroglial activation is more persistent than microglial activation, suggesting an important role for astrocytes in the maintenance of chronic pain (Gao et al., 2010; Scholz et al., 2007; Zhang et al., 2006).
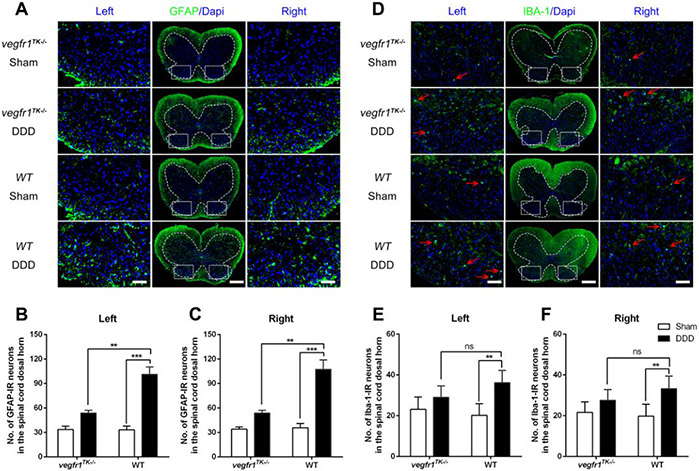
To address the development of neural plasticity in the spinal cord of mice, we examined the expression levels of GFAP, a marker for astrocyte activation, and IBA-1, a marker for microglial activation in the dorsal horn of the lumbar spinal cord. Under chronic LBP conditions (at 12-weeks post-surgery), the expression of GFAP was significantly elevated in WT mice with DDD (p<0.001) as compared with the corresponding sham group and those from gender- and age-matched vegfr-1TK−/− mice with DDD (p<0.01). No significant change in the expression level of GFAP was found in vegfr-1TK−/− mice between the DDD and sham groups (Fig. 5A~C).
Figure 5.
Astrocyte and microglia reactivity in the lumbar part of the spinal cord following disc stabbing surgery. (A & D) Representative immunofluorescence images showing results of staining for glial fibrillary acidic protein (GFAP, an astrocyte marker) (A) and ionized calcium–binding adapter molecule 1 (IBA-1, a microglia marker) (B) in lumbar spinal cord of each group of mice(bars=100μm for the integral spinal cord and bars=20μm for the magnified dorsal horn areas). (B & C) Quantitative analyses show that at 12 weeks post-surgery, although the number of GFAP-ir neurons in both DDD group were increased compared to the respective sham group, the number in the DDD group of vegfr-1TK−/− mice were significantly lower than that in the DDD group of wt mice. (E & F) The number of IBA-1-ir neurons(red arrows) in the DDD group of wt mice markedly increased compared to the respective sham group, while there was no difference between the DDD group and the sham group of vegfr-1TK−/− mice and no difference between both DDD groups of vegfr-1TK−/− and WT mice. (n=5 per group). (**P< 0.01, ***P< 0.001; ns, no significance)
Similarly, the expression of IBA-1, which represents microglial activation, was also increased in WT mice under chronic back pain conditions compared to sham. The DDD group of vegfr-1TK−/−mice, however, showed that the expression level of IBA-1 was almost equivalent to those seen in the sham group at the chronic back pain stage (12 weeks after surgery) (Fig. 5D-F). These results suggest that the plasticity of DRG sensory neurons and glial activity in the spinal cord in vegfr-1TK−/−mice is well preserved compared with the WT mice in our DDD model.
3.6. Exogenous VEGF stimulates inflammatory- and catabolic markers through VEGFR-2, but stimulates pain markers through VEGFR-1 in disc cells.
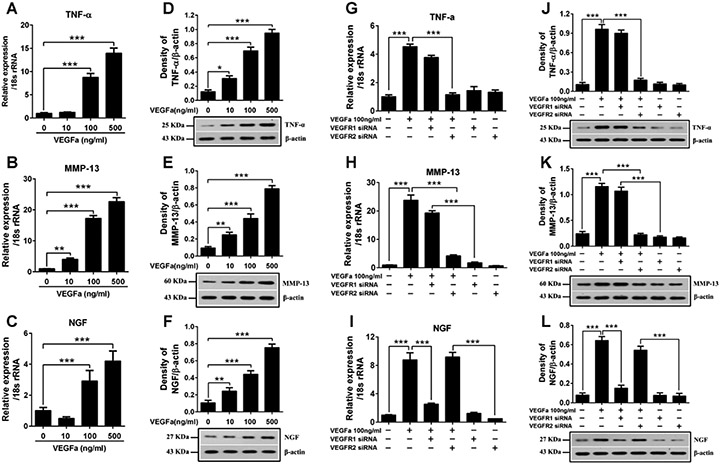
Given the in vivo results above, we next elucidated the detailed molecular mechanism and signaling pathway of VEGF and its cognate receptors using in vitro cell culture system. Bovine NP cells were isolated, cultured as described previously (Ellman et al., 2013; Kim et al., 2010,2013). After exposure to different concentrations (10, 100, 500 ng/ml) of VEGF-A for 24 hours, the cells were subjected to qRT-PCR and western-blot analyses. Stimulation of NP cells with VEGF-A, in a dose-dependent manner, increased the levels of inflammatory cytokines such as TNF-α and cartilage degrading enzymes such as MMP-13, as well as pain-related markers such as NGF (Fig 6 A-F). Similar results were obtained using AF cells in the presence of VEGF-A stimulation (data not shown).
Figure 6.
Analysis of different functions of VEGF mediated by VEGFR-1 and VEGFR-2 on bovine NP cells. (A-F) VEGF-A at different concentration was added to the NP cells and incubated for 24 hours after starving in serum-free medium over night. Real-time PCR and western blot analyses revealed that introduction of VEGF-A to bovine NP cells significantly increased the expression of TNF-α, MMP-13 and NGF in a dose-dependent manner for RNA levels (A-C) and protein levels (D-F). (G-L) Bovine NP cells were transfected with VEGFR-1 siRNA or VEGFR-2 siRNA with Lipofectamine 2000 and then treated with VEGF-A 100ng/ml for 24 hours. Real-time PCR and western blot analyses revealed that VEGFR-1 silencing significantly suppressed the VEGF-A induced over expression of NGF (I, L), while the expression of TNF-α (G, J) and MMP-13 (H, K) was still significantly increased compared to the negative control. And VEGFR-2 silencing markedly reduced the VEGF-A induced over expression of TNF-α (G, J) and MMP-13 (H, K), while the expression of NGF (I, L) still remained at a significantly higher level. All values are mean ± SEM. *P<0.05, **P<0.01, ***P< 0.001.
Since VEGF-A can activate both VEGFR-1 and VEGFR-2, we then delineated the different functional signaling through targeting each receptor by siRNA in the NP cells. NP cells were cultured into monolayers and transfected with siRNA targeting either VEGFR-1 or VEGFR-2 with and without VEGF-A stimulation (100 ng/ml) followed by qRT-PCR and western blotting analyses. The presence of VEGF-A markedly increased the expression of TNF-α, MMP-13 and NGF in a dose-dependent manner.
Silencing vegfr-1 by siRNA significantly suppressed VEGF-A-induced over-expression of NGF, while the expression of TNF-α and MMP-13 was still significantly increased. On the other hand, silencing vegfr-2 markedly reduced VEGF-A-induced over-expression of TNF-α and MMP-13, while the expression of NGF remained significantly high (Fig 6 G-L). These results suggest that VEGF can induce inflammatory and catabolic signaling pathways in disc cells mainly through VEGFR-2, and induce pain pathways through VEGFR-1 activation.
4. Discussion
How DDD develops and progresses to a painful and debilitating disease is unclear, and represents key gaps in our knowledge. A striking feature of IVD degeneration is the dramatic increase in the VEGF levels and in new blood vessel formation in the discs, both of which correlate with the severity of DDD in both human and animal models. In this study, we observed highly elevated VEGF expressions in the discs of DDD groups as compared with the sham groups, which is consistent with the results observed in human degenerative IVDs (David et al., 2010; Koike et al., 2003; Weber et al., 2015) as well as larger animal models of DDD (Salo et al., 2008). These results suggest that VEGF contributes to the pathological progression of disc degeneration.
VEGF-family ligands signal via multiple VEGFRs, including VEGFR1, VEGFR2 and VEGFR3 (Ferrara et al., 2003; Hamilton et al., 2016; Shibuya et al., 2006). Among VEGF-family ligands, VEGF-A activates both VEGFR-1 and VEGFR-2, while VEGF-B and placental growth factor (PlGF) selectively activate VEGFR-1 (Meyer et al., 1999; Ogawa et al., 1998). VEGFR-2 activation is thought to mediate the main effects of VEGF on endothelial cell proliferation, differentiation, survival, migration and permeability, thus contributing to vasculature development and maintenance in embryonic, fetal, postnatal and adult period (Ferrara et al., 2003; Maharaj et al., 2007; Takahashi et al., 2005), as well as being essential in some pathological process such as tumor angiogenesis, tissue repair and remodeling, etc. Furthermore, accumulating evidence suggests that VEGF can induce and accelerate the development of knee OA through activation of VEGFR-2 (Hamilton et al., 2016; Nagao et al., 2017). In our DDD mouse model, it was noted that both WT and vegfr-1TK−/− mice show similar presentations of disc degeneration. Despite the pathological changes in the disc, pain was sufficiently blocked in vegfr-1TK−/− mice. We further confirmed this phenomenon using in vitro cell culture studies, exogenous stimulation of disc cells with VEGF was able to elevate the expression of pro-inflammatory cytokine TNF-α and cartilage degrading enzyme MMP-13 in a dose-dependent manner. VEGF-induced elevation of TNF-α and MMP-13 production was markedly antagonized by introduction of siRNA targeting VEGFR-2, while introduction of siRNA targeting VEGFR-1 failed to show any significant effect on the VEGF-induced TNF-α and MMP-13 production. Together with our in vivo study, these in vitro studies further suggest that selective activation of VEGFR-1 appears to be the primary pathway involved in pain sensitization, whereas activation of VEGFR-2 plays a key role in disc degeneration processes.
The severity of disc degeneration from radiographic and pathological evidence often does not correlate with perceived levels of pain sensation. Up to 40% of patients with severe radiographic changes in IVD are symptom-free, indicating that DDD pathology can be uncoupled from pain sensation (Chou et al., 2011). Our findings suggesting that selective activation of VEGFR-1 is the primary pathway involving LBP may challenge current research paradigms. It is because, it has long been believed that VEGFR-2 is the primary receptor that mediates the biological effects of VEGF, especially its angiogenic activities, and that VEGFR-1 is less potent and may act as a negative regulator of VEGFR-2. For this reason, the development of drugs has been heavily focused on anti-VEGFR-2 therapy. More recently, it was suggested that VEGFR-1 and VEGFR-2 also play an important role in pain transmission (Im et al., 2010; Ludin et al., 2013). Indeed, our results suggest that VEGFR-1 signaling is selectively involved in disc degeneration-evoked LBP sensation through altering nociceptive pathway by interfering with the expression of pain-related molecules in the peripheral tissue and the DRG sensory neuron along with stimulation of spinal glial activity, which is beyond ‘mere angiogenesis’. Further studies focusing on neuron-glial interactions that potentially lead to enhancement of nociceptive synaptic transmission as spinal mechanisms, may warrant to advance our mechanistic understanding of back pain and may identify VEGFR-1-selective signaling as the major discogenic LBP transmission mechanism, thus, as a drug target for pain.
NGF and VEGF signaling appear to control the expression levels for each other. For example, it is well known that NGF is a potent inducer of VEGF and VEGF strongly arguments the expression of NGF. For instance, the NGF/TrkA axis highly stimulates VEGFR-1 expression in the DRG sensory neurons (Das et al., 2018). VEGF is capable of directly modulating the excitability of primary sensory neurons (Selvaraj et al., 2015); it is likely that stimulation of one of these factors: either NGF or VEGF signaling may be sufficient enough to trigger and develop back pain sensation in the injured discs. Our previous study showed that conjugation of overexpressed NGF in the pathological joint tissues with its cognate receptor TrkA present in the nerve terminals, directly influences on axonal sprouting of the nociceptive nerve in the joint, affecting pain sensation regardless of the degree of joint degeneration (Kc et al., 2016). The immunofluorescence analyses in the current study revealed a deficiency of NGF and TrkA expression in the peripheral disc tissues of vegfr-1TK−/− mice after disc injury (vegfr-1TK−/− DDD) compared to age- and gender-matched WT mice (WT DDD). Immunofluorescence staining results of innervating lumbar DRG also revealed the deficiency of NGF, CGRP and TRPV1 in the peripheral nerves of vegfr-1TK−/− mice, further suggesting less sensory neuronal plasticity in vegfr-1TK−/− compared to WT mice. Similarly, immunofluorescence analyses using the spinal cord demonstrated no/minimal change in the astroglial activity in vegfr-1TK−/− mice compared to age and gender-matched WT mice after disc injury, also suggesting reduced plasticity in the central nervous system. Importantly, the results from these biochemical analyses correlated with the behavioral pain responses in which vegfr-1TK−/− mice clearly demonstrated insensitivity to disc injury-induced LBP.
Taken together, our findings from an established mouse model of DDD and in vitro studies indicate that VEGF modulates LBP sensation by activation of the VEGFR-1 pathways while VEGRF-2 signaling pathway mainly contributes to IVD degeneration. Thus, blockage of VEGF or combined targeting of VEGFR-1 and VEGFR-2 may have therapeutic effects in degenerative diseases of discs.
Acknowledgements
Funding: This work was supported by an NIH/NIAMS R21 grant, USA (1R21AR067935-01) to HJI; a Veterans Affairs (VA) BLD & R Merit Review Award, USA (I01BX002647-01) to HJI; a Natural Science Foundation of Guangdong Province, China (2014A030313348) to SM; a Science and Technology Project of Guangzhou, China (201607010266) to SM; a Scientific Research Foundation of Southern Medical University, Guangzhou, China (PY2014NO64) to SQ; a National Natural Science Foundation of China to CS (81802120) and a Shanghai Sailing Program to CS (18YF1423100); Canadian Institutes of Health Research to FM.
Footnotes
Conflict of interest statement
All authors have no conflict of interest to declare.
References:
- Aoki Y, Ohtori S, Ino H, Douya H, Ozawa T, Saito T, Moriya H, & Takahashi K (2004). Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine (Phila Pa 1976), 29, 2621–2626 [DOI] [PubMed] [Google Scholar]
- Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Cross AK, & Le Maitre CL (2014). Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther, 16, 416. doi: 10.1186/s13075-014-0416-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D, Samartzis D, Bellabarba C, Patel A, Luk KD, Kisser JM, & Skelly AC (2011). Degenerative magnetic resonance imaging changes in patients with chronic low back pain: a systematic review. Spine (Phila Pa 1976), 36, S43–S53. doi: 10.1097/BRS.0b013e31822ef700 [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, & De Koninck Y (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature, 438, 1017–1021. doi: 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- Dagenais S, Caro J, & Haldeman S (2008). A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J, 8, 8–20. doi: 10.1016/j.spinee.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Das V, Kc R, Li X, O-Sullivan I, van Wijnen AJ, Kroin JS, Pytowski B, Applegate DT, Votta-Velis G, Ripper RL, Park TJ, & Im HJ (2018). Blockade of Vascular Endothelial Growth Factor Receptor-1 (Flt-1), Reveals a Novel Analgesic For Osteoarthritis-Induced Joint Pain. Gene Rep, 11, 94–100. doi: 10.1016/j.genrep.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Ciurea AV, Iencean SM, & Mohan A (2010). Angiogenesis in the degeneration of the lumbar intervertebral disc. J Med Life, 3, 154–161 [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Duda DG, Chae SS, Fukumura D, & Jain RK (2009). VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS One, 4, e6525. doi: 10.1371/journal.pone.0006525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman MB, Kim J, An HS, Chen D, Kc R, Li X, Xiao G, Yan D, Suh J, van Wjnen AJ, Wang JH, Kim SG, & Im HJ (2013). Lactoferricin enhances BMP7-stimulated anabolic pathways in intervertebral disc cells. Gene, 524, 282–291. doi: 10.1016/j.gene.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, & LeCouter J (2003). The biology of VEGF and its receptors. Nat Med, 9, 669–676. doi: 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O'Brien JP, & Hoyland JA (2002). Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol, 197, 286–292. doi: 10.1002/path.1108 [DOI] [PubMed] [Google Scholar]
- Gao YJ, & Ji RR (2010). Targeting astrocyte signaling for chronic pain. Neurotherapeutics, 7, 482–493. doi: 10.1016/j.nurt.2010.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SX, Li X, Hamilton JL, Chee A, Kc R, Chen D, An HS, Kim JS, Oh CD, Ma YZ, van Wijnen AJ, & Im HJ (2015). MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene, 555, 80–87. doi: 10.1016/j.gene.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, & Im HJ (2016). Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J Bone Miner Res, 31, 911–924. doi: 10.1002/jbmr.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro H, Kato T, Komori H, Osada M, & Shinomiya K (2002). Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J Orthop Res, 20, 409–415. doi: 10.1016/S0736-0266(01)00150-4 [DOI] [PubMed] [Google Scholar]
- Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, & Davies AM (2006). Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci, 27, 85–91. doi: 10.1016/j.tips.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, & Shibuya M (1998). Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A, 95, 9349–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevre J, & Kroin JS (2010). Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum, 62, 2995–3005. doi: 10.1002/art.27608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Berta T, & Nedergaard M (2013). Glia and pain: is chronic pain a gliopathy? Pain, 154 Suppl 1, S10–S28. doi: 10.1016/j.pain.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamouzian S, Eskandary H, Faramarzee M, Saba M, Safizade H, Ghadipasha M, Malekpoor AR, & Ohadi A (2010). Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine (Phila Pa 1976), 35, 881–886. doi: 10.1097/BRS.0b013e3181b9c986 [DOI] [PubMed] [Google Scholar]
- Kc R, Li X, Kroin JS, Liu Z, Chen D, Xiao G, Levine B, Li J, Hamilton JL, van Wijnen AJ, Piel M, Shelly DA, Brass D, Kolb E, & Im HJ (2016). PKCdelta null mutations in a mouse model of osteoarthritis alter osteoarthritic pain independently of joint pathology by augmenting NGF/TrkA-induced axonal outgrowth. Ann Rheum Dis, 75, 2133–2141. doi: 10.1136/annrheumdis-2015-208444 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim JS, Ellman MB, An HS, van Wijnen AJ, Borgia JA, & Im HJ (2010). Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum, 62, 3706–3715. doi: 10.1002/art.27733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Ellman MB, An HS, Yan D, van Wijnen AJ, Murphy G, Hoskin DW, & Im HJ (2012). Lactoferricin mediates anabolic and anti-catabolic effects in the intervertebral disc. J Cell Physiol, 227, 1512–1520. doi: 10.1002/jcp.22867 [DOI] [PubMed] [Google Scholar]
- Kim JS, Ellman MB, Yan D, An HS, Kc R, Li X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, Buechter DD, Van Wijnen AJ, & Im HJ (2013). Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J Cell Physiol, 228, 1884–1896. doi: 10.1002/jcp.24350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike Y, Uzuki M, Kokubun S, & Sawai T (2003). Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine (Phila Pa 1976), 28, 1928–1933. doi: 10.1097/01.BRS.0000083324.65405.AE [DOI] [PubMed] [Google Scholar]
- Li X, An HS, Ellman M, Phillips F, Thonar EJ, Park DK, Udayakumar RK, & Im HJ (2008). Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther, 10, R48. doi: 10.1186/ar2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li G, Den X, Xu C, Liu S, Gao Y, Liu H, Zhang J, Li X, & Liang S (2010). VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X(2)(/)(3) receptor of primary sensory neurons. Brain Res Bull, 83, 284–291. doi: 10.1016/j.brainresbull.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Ludin A, Sela JJ, Schroeder A, Samuni Y, Nitzan DW, & Amir G (2013). Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthritis Cartilage, 21, 491–497. doi: 10.1016/j.joca.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Maharaj AS, & D'Amore PA (2007). Roles for VEGF in the adult. Microvasc Res, 74, 100–113. doi: 10.1016/j.mvr.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, & An HS (2005). A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976), 30, 5–14 [DOI] [PubMed] [Google Scholar]
- McMahon SB, & Jones NG (2004). Plasticity of pain signaling: role of neurotrophic factors exemplified by acid-induced pain. J Neurobiol, 61, 72–87. doi: 10.1002/neu.20093 [DOI] [PubMed] [Google Scholar]
- Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, & Dehio C (1999). A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J, 18, 363–374. doi: 10.1093/emboj/18.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, & Watkins LR (2009). Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci, 10, 23–36. doi: 10.1038/nrn2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, & Smith AM (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development, 116, 201–211 [DOI] [PubMed] [Google Scholar]
- Nagai T, Sato M, Kobayashi M, Yokoyama M, Tani Y, & Mochida J (2014). Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res Ther, 16, 427. doi: 10.1186/s13075-014-0427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Hamilton JL, Kc R, Berendsen AD, Duan X, Cheong CW, Li X, Im HJ, & Olsen BR (2017). Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci Rep, 7, 13027. doi: 10.1038/s41598-017-13417-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, & Shibuya M (1998). A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem, 273, 31273–31282 [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, & Claesson-Welsh L (2006). VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol, 7, 359–371. doi: 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- Pai RR, D'Sa B, Raghuveer CV, & Kamath A (1999). Neovascularization of nucleus pulposus. A diagnostic feature of intervertebral disc prolapse. Spine (Phila Pa 1976), 24, 739–741 [DOI] [PubMed] [Google Scholar]
- Pezet S, & McMahon SB (2006). Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci, 29, 507–538. doi: 10.1146/annurev.neuro.29.051605.112929 [DOI] [PubMed] [Google Scholar]
- Ratsep T, Minajeva A, & Asser T (2013). Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur Spine J, 22, 2474–2480. doi: 10.1007/s00586-013-2842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo J, Kaigle HA, Indahl A, Mackiewicz Z, Sukura A, Holm S, Jamsen E, & Konttinen YT (2008). Expression of vascular endothelial growth factor receptors coincide with blood vessel in-growth and reactive bone remodelling in experimental intervertebral disc degeneration. Clin Exp Rheumatol, 26, 1018–1026 [PubMed] [Google Scholar]
- Sato J, Inage K, Miyagi M, Sakuma Y, Yamauchi K, Koda M, Furuya T, Nakamura J, Suzuki M, Kubota G, Oikawa Y, Sainoh T, Fujimoto K, Shiga Y, Abe K, Kanamoto H, Inoue M, Kinoshita H, Norimoto M, Umimura T, Takahashi K, Ohtori S, & Orita S (2017). Inhibiting Vascular Endothelial Growth Factor in Injured Intervertebral Discs Attenuates Pain-Related Neuropeptide Expression in Dorsal Root Ganglia in Rats. Asian Spine J, 11, 556–561. doi: 10.4184/asj.2017.11.4.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawano A, Takahashi T, Yamaguchi S, Aonuma M, & Shibuya M (1996). Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ, 7, 213–221 [PubMed] [Google Scholar]
- Sawano A, Takahashi T, Yamaguchi S, & Shibuya M (1997). The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCgamma. Biochem Biophys Res Commun, 238, 487–491. doi: 10.1006/bbrc.1997.7327 [DOI] [PubMed] [Google Scholar]
- Scholz J, & Woolf CJ (2007). The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci, 10, 1361–1368. doi: 10.1038/nn1992 [DOI] [PubMed] [Google Scholar]
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, & Bogduk N (1994). The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Phila Pa 1976), 19, 801–806 [DOI] [PubMed] [Google Scholar]
- Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, & Kuner R (2015). A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell, 27, 780–796. doi: 10.1016/j.ccell.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Das V, Li X, Kc R, Qiu S, O-Sullivan I, Ripper RL, Kroin JS, Mwale F, Wallace AA, Zhu B, Zhao L, van Wijnen AJ, Ji M, Lu J, Votta-Velis G, Yuan W, & Im HJ (2018). Development of an in vivo mouse model of discogenic low back pain. J Cell Physiol, 233, 6589–6602. doi: 10.1002/jcp.26280 [DOI] [PubMed] [Google Scholar]
- Shi C, Qiu S, Riester SM, Das V, Zhu B, Wallace AA, van Wijnen AJ, Mwale F, Iatridis JC, Sakai D, Votta-Velis G, Yuan W, & Im HJ (2018). Animal models for studying the etiology and treatment of low back pain. J Orthop Res, 36, 1305–1312. doi: 10.1002/jor.23741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, & Claesson-Welsh L (2006). Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res, 312, 549–560. doi: 10.1016/j.yexcr.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Takahashi H, & Shibuya M (2005). The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond), 109, 227–241. doi: 10.1042/CS20040370 [DOI] [PubMed] [Google Scholar]
- Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, & Karaharju EO (2006). Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J, 15, 588–596. doi: 10.1007/s00586-005-0930-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Satoh S, Alipui DO, Virojanapa J, Levine M, Sison C, Quraishi S, Bloom O, & Chahine NO (2015). Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res, 63, 170–180. doi: 10.1007/s12026-015-8709-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, & De Koninck Y (2006). Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem, 97, 772–783. doi: 10.1111/j.1471-4159.2006.03746.x [DOI] [PubMed] [Google Scholar]
- Zhang YG, Guo TM, Guo X, & Wu SX (2009). Clinical diagnosis for discogenic low back pain. Int J Biol Sci, 5, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]