Abstract
Purpose:
Metastatic hormone receptor-positive (HR-positive), HER2-negative breast cancer is an important cause of cancer mortality. Endocrine treatment with or without additional targeted therapies has been the mainstay of treatment. This trial was designed to evaluate the combination of fulvestrant plus everolimus versus fulvestrant, everolimus and anastrozole compared to fulvestrant alone in the first-line treatment of advanced HR-positive, HER2-negative breast cancer.
Experimental Design:
This randomized placebo-controlled trial included postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had received no prior systemic therapy for metastatic disease. Participants were randomized to one of three treatment arms and the primary outcome was progression-free survival (PFS), comparing combinations of fulvestrant and everolimus with or without anastrozole to fulvestrant alone. Circulating tumor cells (CTC), as measured with two different methods, and circulating tumor DNA (ctDNA) were evaluated serially prior to treatment and the beginning of the second cycle of therapy.
Results:
Due in part to changes in clinical practice, the study was closed after accruing only 37 participants. There was no evidence that everolimus-containing combination treatment improved PFS or overall survival relative to fulvestrant alone. When modeled continuously, an association was observed of baseline CTC and ctDNA with poorer survival.
Conclusion:
Although power of the study was limited, the findings were unable to support the routine use of everolimus combination endocrine therapy in the first-line treatment of advanced hormone-sensitive breast cancer. Prognostic impact of baseline ctDNA and copy number variations in CTC was demonstrated.
Introduction
The mainstay of treatment for endocrine-sensitive metastatic breast cancer has been sequential use of endocrine therapies including selective estrogen receptor modulators, aromatase inhibitors and a selective estrogen receptor down regulator. Recent studies have demonstrated favorable results with endocrine agents used in combination [1] or with the addition of targeted therapies including the mammalian target of rapamycin (mTOR) inhibitor everolimus [2, 3] or inhibitors of cyclin dependent kinase 4 and 6 (CDK4/6) [4, 5, 6, 7]. At initiation of the current study, CDK4/6 inhibitors were not yet FDA-approved therapeutic options. The primary objective of this study was to test the progression-free survival (PFS) benefit of combining fulvestrant with everolimus versus combining fulvestrant with everolimus and anastrozole, each compared with fulvestrant alone in the treatment of postmenopausal women with hormone receptor-positive (HR-positive) metastatic breast cancer. Further objectives included additional comparisons of PFS, overall survival (OS), response rates, toxicities, adherence and feasibility. Translational studies were also planned to investigate molecular determinants of response to treatment and prognosis in components of liquid biopsies: specifically circulating tumor cells (CTC) and circulating tumor DNA (ctDNA).
Design and Methods
Clinical Eligibility and Trial Conduct.
Eligible patients were postmenopausal women with histologically confirmed HR-positive and HER2-negative metastatic breast cancer for which no prior systemic treatment had been received in the metastatic or recurrent setting. Prior chemotherapy and endocrine therapy in the adjuvant or neoadjuvant setting were permitted as long as any aromatase inhibitor therapy was completed more than 12 months prior to randomization. Those with prior treatment with fulvestrant or mTOR inhibitors were ineligible. Participants were required to have adequate cardiac, hepatic, renal and bone marrow function. Those with elevated cholesterol or triglycerides and those with bleeding diathesis or on long-term anti-coagulant therapy were excluded.
Participants were randomized with equal allocation to three arms: fulvestrant plus placebo for both everolimus and anastrozole (Arm 1), fulvestrant plus everolimus with placebo for anastrozole (Arm 2), or fulvestrant plus everolimus and anastrozole (Arm 3). Fulvestrant dosing was 500 mg IM every 4 weeks with an additional 500 mg loading dose day 15 of cycle 1, everolimus was dosed at 10 mg PO daily and anastrozole dose was 1 mg PO daily. Treatment was continued until disease progression, unacceptable toxicity, treatment delay > 4 weeks, if a need for anti-retroviral therapy arose, withdrawal of consent, or study closure. The study was conducted in accordance with recognized ethical guidelines with written informed consent obtained from all participants and approval of local institutional review boards.
Translational Studies.
The identification and enumeration of circulating tumor cells (CTC) has proven to be a clinically useful method of assessing progression in metastatic breast cancer [8, 9]. As an integrated translational study, blood was collected separately into CellSave tubes which were sent to the University of Michigan for CellSearch® analyses and into Streck tubes which were sent to the USC Michelson CSI-Cancer for High Definition Single Cell Analysis (HD-SCA) and cell free DNA (cfDNA) analyses at treatment cycle1 day 1 (baseline), cycle 2 day 1and at progression.
CTC enumeration and characterization were performed using the CellSearch® CXC Kit and CellSearch® system (Menarini Silicon Biosystems, Inc., Huntingdon Valley, PA) at baseline and then follow-up time points only if elevated at baseline [10] as previously described [8, 10, 11]. CTC levels were enumerated as the average of the CTC levels in the four different aliquots of 7.5 ml whole blood (WB), each of which was used to determine each of the 4 respective CTC-biomarker expressions to calculate the CTC-Endocrine Therapy Index (CTC-ETI) for that blood draw. The CTC-ETI was calculated as described [10]. As per prior studies[8, 9, 11], ≥5 CTC/7.5 ml WB were considered elevated, and 0–4 CTC/7.5ml WB were designated as low.
The HD-SCA method of CTC enumeration and characterization [12] was to be performed for all samples at all three time points (baseline, cycle 2 day 1 and progression). An average volume of 0.55 ml of blood was analyzed per assay and all cells including leukocytes were identified using immunofluorescent stains and enumerated. Cells with high levels of cytokeratin staining were counted as CTCs and scored as a continuous variable ranging from 2.2 to 145.8 CTC/ml blood. Prior to CTC capture, plasma was prepared by centrifugation and archived at −80°C. Cell free DNA was extracted using the QIAamp Kit (QIAGEN) and cfDNA concentration was measured using Qubit (Thermo Scientific) as previously published [12]. Low pass DNA sequencing and copy number profiling were performed as previously described on both cfDNA and isolated single cells[12]. CtDNA tumor fractions were estimated using the ichorCNA statistic [13] and scored as a continuous variable. Multiplex proteomic analysis of individual CTC was performed using the Hyperion Imaging Mass Cytometer (Fluidigm) as published [14].
Statistical Analysis.
The primary outcome was PFS defined as time to progression or death due to any cause. The primary aim was to compare the two combination arms to Arm 1. Secondary outcomes included overall survival (OS) defined as time from registration to death from any cause, as well as CTCAE toxicity. Survival times were compared using log-rank tests for comparisons of treatment and Cox regression analysis for hazard ratio estimation and testing of treatments and biomarkers. Response rates were compared by chi-squared testing.
Predictive testing of the role of liquid biopsy results on treatment and subsequent clinical outcomes were planned, using Cox regression, with a hypothesis that participants with high CTC might benefit from combination therapy while those with low CTC would not.
Results
Clinical Outcomes According to Treatment Assignment.
The original planned sample size of SWOG1222 (NCT02137837) was 825, assuming PFS medians of 15, 21.5, and 25 months for the three arms, respectively. Accrual of 37 participants occurred between May 2014 and February 2015 (Supplement). FDA approval of CDK4/6 inhibitors in the first-line treatment of hormone receptor-positive metastatic breast cancer in February 2015 made the trial not viable. In October 2015, the study sponsor permanently closed the study, offering participants the option to continue their current active drug therapy after unblinding. All study follow-up concluded December 2019.
Patient characteristics are shown in Table 1. One participant received no protocol treatment and is not evaluable for clinical benefit or adverse events. Among 36 evaluable patients, no grade 3 or higher toxicity was observed in the fulvestrant arm; one patient receiving fulvestrant plus everolimus experienced grade 4 toxicity (hypophosphatemia) and an additional 10 participants receiving fulvestrant and everolimus with or without anastrozole experienced grade 3 toxicities. PFS appeared similar for all arms (Figure 1A; log-rank p=0.88) with an overall median of 11.2 months. At the end of study follow-up at 5 years, three patients (one in each arm) were still receiving protocol assigned treatment and had not progressed. There was also no evidence of a difference in OS (Figure 1B; log-rank p=0.81) with an overall median of 42 months. Median follow-up time for those still alive was 56 months. Among those with measurable disease there were 2 responses in 9 patients on Arm 1 (22.2%), 6 in 10 patients on Arm 2 (60.0%), and 4 in 9 patients on Arm 3 (44.4%). Though suggestive of better response on combination therapy, these differences were not statistically different (p=0.25).
Table 1.
Patient characteristics by study arm
| Fulvestrant | Fulvestrant + Everolimus | Fulvestrant + Everolimus + Anastrozole | ||||
|---|---|---|---|---|---|---|
| (n=13) | (n=12) | (n=12) | ||||
| AGE | ||||||
| Median | 63.4 | 62.6 | 60.5 | |||
| Minimum | 54 | 45 | 48 | |||
| Maximum | 74 | 87 | 69 | |||
| HISPANIC | ||||||
| Yes | 1 | 8% | 2 | 17% | 0 | 0% |
| No | 12 | 92% | 10 | 83% | 12 | 100% |
| RACE | ||||||
| White | 9 | 69% | 10 | 83% | 9 | 75% |
| Black | 2 | 15% | 0 | 0% | 3 | 25% |
| Asian | 0 | 0% | 1 | 8% | 0 | 0% |
| Multi-Racial | 1 | 8% | 0 | 0% | 0 | 0% |
| Unknown | 1 | 8% | 1 | 8% | 0 | 0% |
| DISEASE | ||||||
| Measurable | 9 | 69% | 10 | 83% | 9 | 75% |
| Evaluable non-measurable disease | 4 | 31% | 2 | 17% | 3 | 25% |
| PRIOR HORMONE | ||||||
| Prior adjuvant hormonal therapy completed more than 5 years ago | 3 | 23% | 1 | 8% | 2 | 17% |
| Prior adjuvant hormonal therapy completed 1–5 years ago | 4 | 31% | 5 | 42% | 6 | 50% |
| De novo presentation of metastatic disease or no prior adjuvant hormonal therapy | 6 | 46% | 6 | 50% | 4 | 33% |
Figure 1.
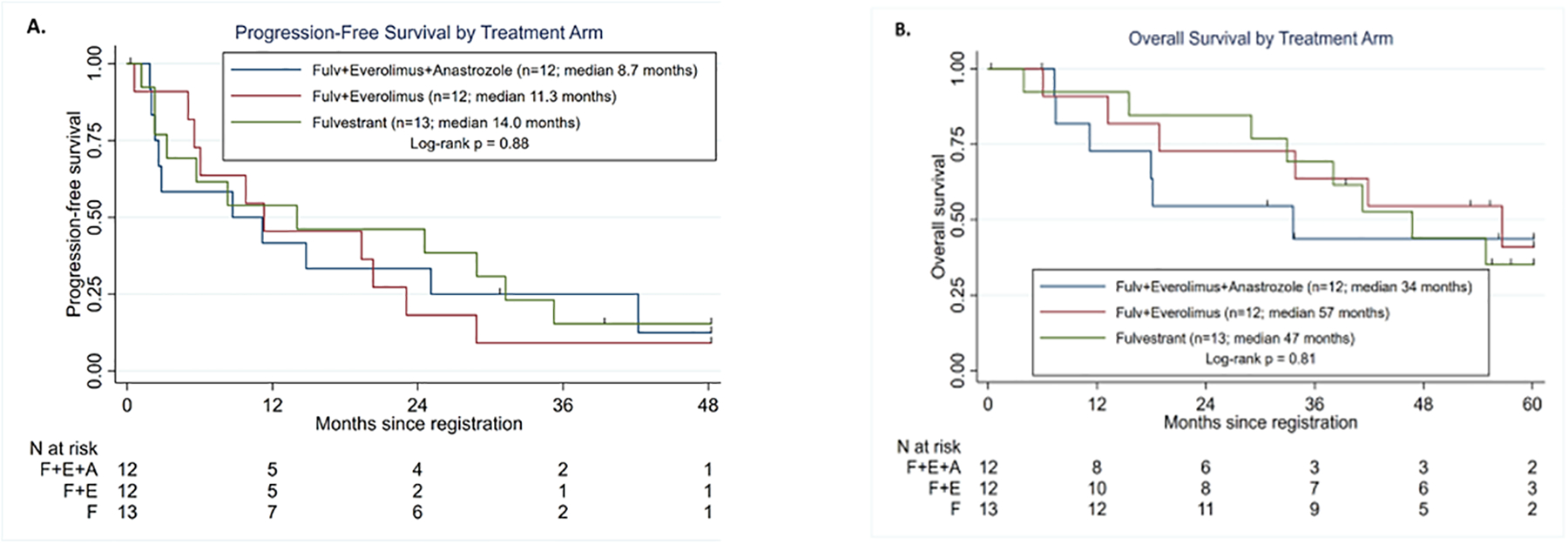
Progression-free and Overall survival by randomized treatment groups. Data cutoff was December 31, 2019. Log-rank test compared all three treatment groups. A) Progression-free survival. B) Overall Survival
Liquid Biopsy Analyses.
Due to a regulatory issue which delayed the ability of the University of Michigan laboratory to accept specimens, only 13 patients had CTC evaluation by CellSearch® at baseline. Two (15.4%) patients had elevated CTC prior to treatment (Figure 2) and were tested again after one cycle. CTC levels declined dramatically for both patients. For one, assigned to fulvestrant and everolimus, CTC declined from a baseline level of 18 to first follow-up level of 4 CTC/7.5 ml WB. The second patient, assigned to fulvestrant only, had an average of 46 CTC at baseline which declined at first follow-up to 8 CTC/7.5ml WB. PFS did not differ between these two patients with elevated CTC at baseline compared to those without elevated CTC at baseline (log-rank p=0.47). Since only two patients evaluated by the CellSearch® assay had elevated CTC levels, CTC-ETI analysis was determined, but association with outcomes was not performed.
Figure 2.
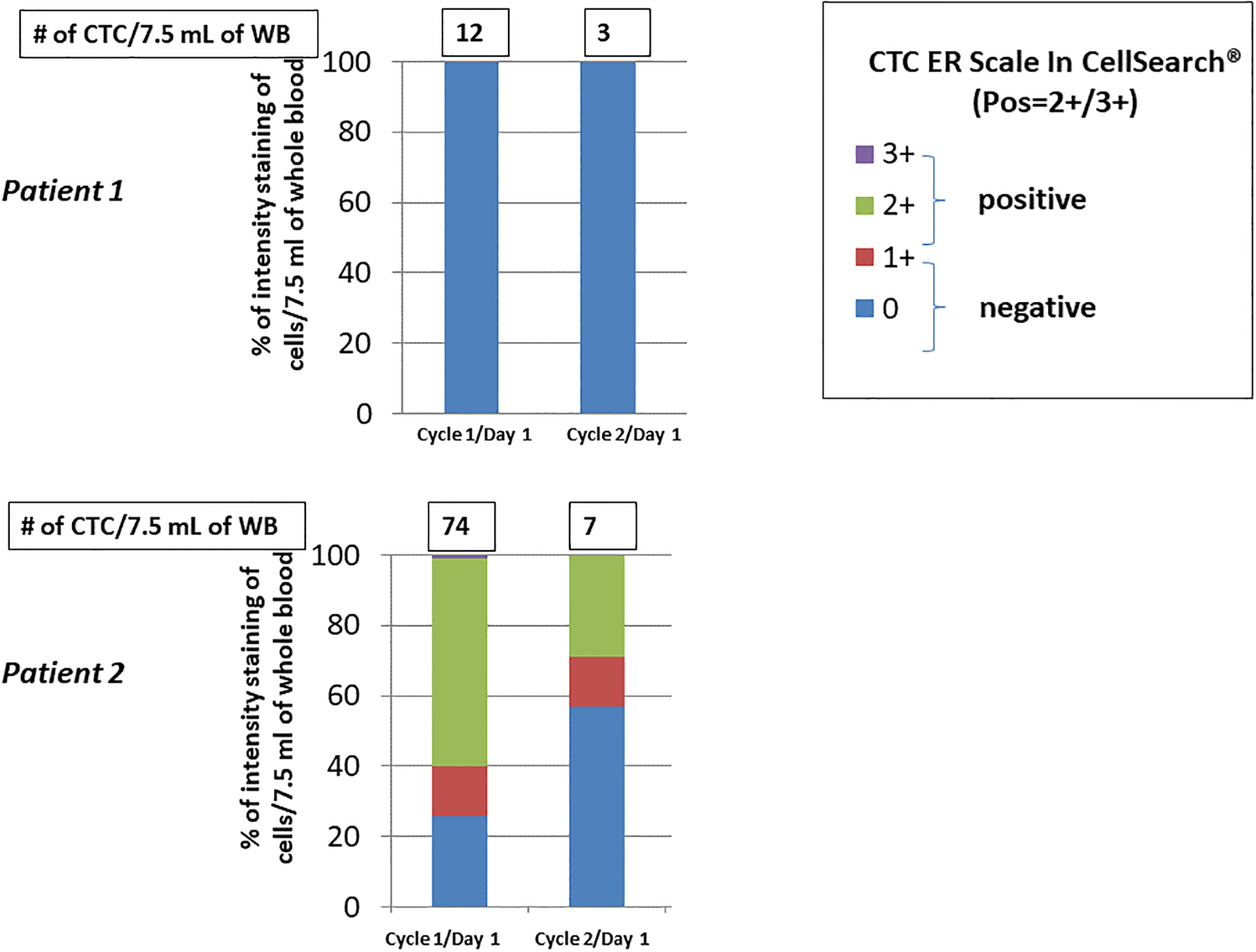
Circulating tumor cell (CTC) expression of estrogen receptor (ER) in two patients with elevated CTC levels. CTC enumeration and ER expression determined using CellSearch®. See text for details.
Using the HD-SCA assay, 25 of 34 cases (74%) had measurable non-leukocyte cell counts and 7 of the 34 (21%) had CTCs with high cytokeratin expression. The presence of CTCs was not associated with poorer PFS (HR=1.40; 95% CI 0.59–3.32; p=0.45). However, if the count of high cytokeratin CTC at baseline is modeled as a continuous variable in the Cox regression, there is a significant decrease in PFS with each unit of CTC by HD-SCA (HR=1.02; 95% CI 1.00–1.04; p=0.043). For draw 2 after one cycle of treatment, 3 of 32 (9.4%) patients measured by the HD-SCA assay were positive for high cytokeratin CTC, including two of the patients elevated at baseline. For draw 3 at the time of progression 5 of 18 (27.8%) patients had positive CTC. For the 13 patients evaluated for CTC by both methods, there was perfect concordance of the two assays: the same 2 patients had elevated CTC by both assays and the remaining 11 did not.
In all detectable cases, copy number profiles of ctDNA represented an aggregate of all clones detected on a single cell level (Figure 3C). Baseline ctDNA (n=25), measured as tumor DNA fraction, was also modeled continuously, and was statistically associated with poorer PFS (HR=1.08;95% CI 1.02–1.15; p=0.005), while total cf DNA as purified from the plasma was not (HR=1.04; 95% CI 0.99–1.09; p=0.11).
Figure 3.
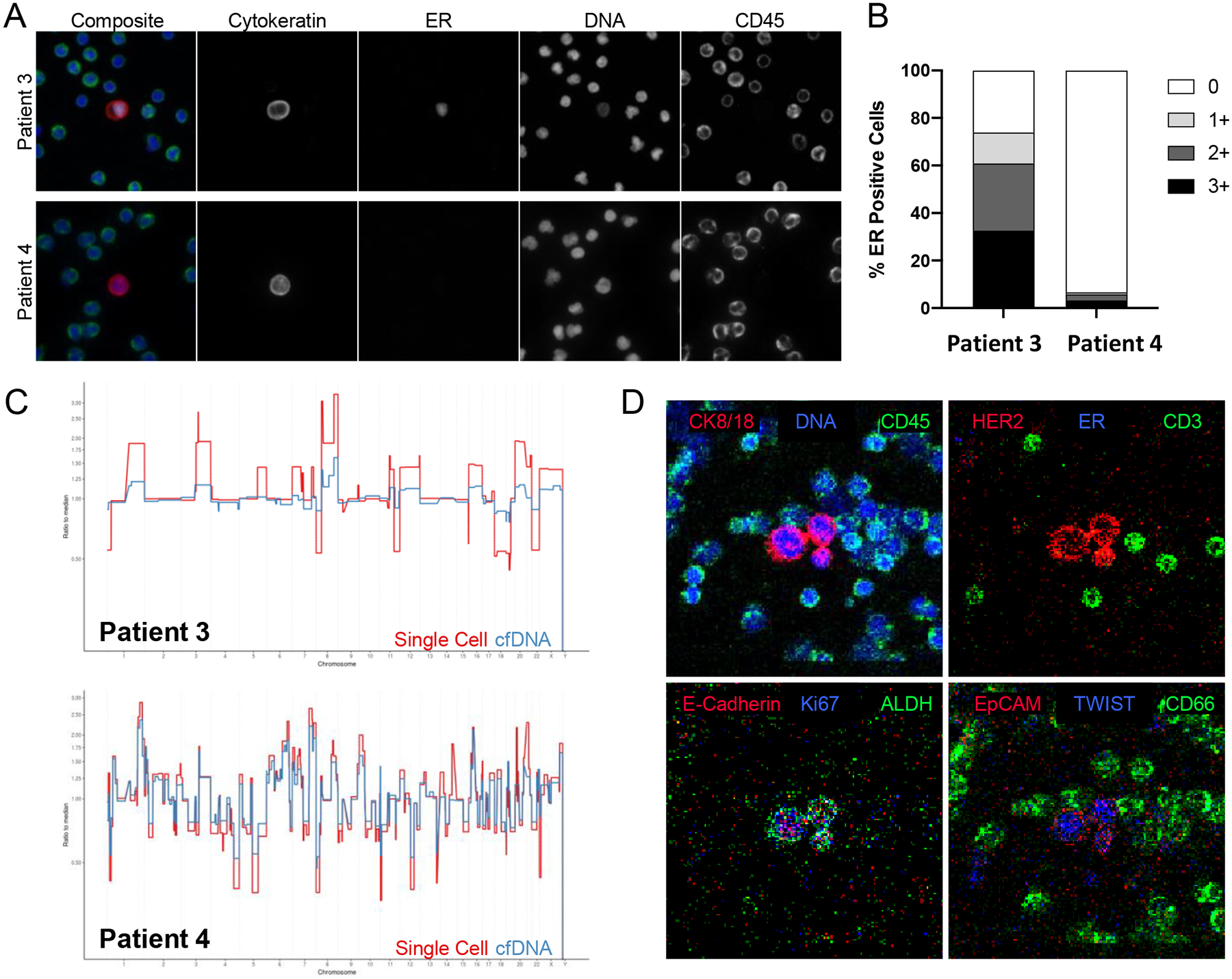
Circulating tumor cell genomic and phenotypic analysis in two patients with elevated CTC levels. CTC enumeration and genomic and phenotypic characterization determined using HD-SCA. A) Galleys of CTC of blood collected from patient AAA, showing cytokeratin, estrogen receptor (ER), DNA, and CD45 expression. B) Estrogen receptor expression in CTC in patients AAA and BBB. C) Genomic analysis of CTC in patients AAA and BBB. D) Galleys of CTC of blood collected from patient BBB showing expression of HER2, e-cadherin, EpCam, and TWIST in 3 epithelial cells. See text for details.
Discussion
Due to the evolving landscape of first-line therapy for metastatic hormone-sensitive breast cancer, the current study was unable to complete accrual or determine the impact of the addition of everolimus to fulvestrant alone or to combination endocrine therapy in this setting. The observed median PFS of 11.2 months in this study (S1222) compares favorably with the 5.6 month PFS with fulvestrant 500 mg in the CONFIRM study which enrolled patients who progressed within 12 months of adjuvant therapy or while on first line endocrine therapy[15], and unfavorably with the median time to progression of 23.4 months observed with first-line fulvestrant alone in the FIRST study[16], highlighting difficulties in cross-study comparisons. The addition of everolimus to endocrine therapy in the current study, S1222, was associated with increased toxicity. In spite of an impressive impact on PFS in previous studies, an OS benefit with everolimus in the treatment of metastatic breast cancer has yet to be established. The recent demonstration of improved OS with CDK4/6 inhibitors [17, 18] firmly establishes their inclusion early on in the treatment of metastatic HR-positive breast cancer. Everolimus remains an option in subsequent lines of therapy as suggested by the PreE0102 study, in which the PFS was 10.3 months versus 5.1 months with the addition of everolimus to fulvestrant following progression aromatase inhibitor therapy [19]. Furthermore, everolimus toxicity in the form of stomatitis may be reduced with the use of oral dexamethasone mouthwash [20], which was not mandated in S1222.
The planned translational liquid biopsy studies were likewise severely limited by the low accrual and by a regulatory issue that prevented analysis of the entire population of participants. Nonetheless, enumeration of CTC by two different methods (CellSearch® and HD-SCA) were completely concordant (2 elevated, 11 not). No statistically significant difference in PFS was observed between the two patients with elevated CTC by both methods compared to the 11 with non-elevated CTC at baseline. However, both of these patients experienced a “CTC response,” which has been associated with a better prognosis when compared to patients whose CTC remain elevated at the end of the first cycle of therapy[9, 11]. When modeled as a continuous variable, elevated levels of CTC by HD-SCA were associated with a worse prognosis, consistent with several prior studies that have demonstrated that the presence of CTC enumerated with CellSearch® prior to start of therapy is associated with a worse outcome in metastatic breast cancer[8, 11].
Findings of CTC-ETI analyses of four participants with elevated CTC at baseline are of interest, suggesting that further genomic and proteomic analysis might be valuable in treatment selection and outcomes (Figures 2 anda 3). For patient 1, who was assigned to fulvestrant and everolimus, all of the CTC detected by CellSearch® and by HD-SCA at baseline were negative for ER expression, which we hypothesized would predict for lack of benefit from endocrine therapy (Figure 2A). However, she experienced a “CTC response”, raising the speculation that blocking the mTOR pathway may be successful even if the cancer has reverted to a hormone receptor negative phenotype. By contrast, in patient 2, approximately 60% of the CTC were ER-positive at baseline. She was assigned to fulvestrant alone and also had a CTC response (Figure 2B).
Similarly, two other patients with with the highest level of CTC elevation as identified by the HD-SCA assay provided provocative findings (Figure 3). In a manner similar to Patient 1, Patient 3 had a high percentage of ER positive CTC as well as a copy number profile suggestive of a luminal subtype (Figure 3 A,B,C). This patient was treated with fulvestrant, anastrozole, and everolimus, and had a remarkable PFS of over two years. By contrast, patient 4’s CTC were were almost entirely ER negative with a basal-like genomic subtype (Figure 3 B,C,D). Further, proteomic analysis demonstrated that many of her CTC expressed HER2 and TWIST (Figure 3D). This patient, treated with fulvestrant and everolimus, progressed within 18 days from the time of entry onto the trial.
Taken together, these data suggest an intriguing hypothesis that CTC-ER phenotype might help select patients who could be treated with endocrine therapy alone or who are better treated with combination endocrine and other pathway (such as mTOR or CDK4/6) inhibition. Of course, these speculations require substantial validation. Further assessment of ctDNA showed promise as a prognostic marker for PFS, similar to previously published reports [21].
While the genomic and proteomic analyses are only exploratory given the small number of CTC positive patients, our findings indicate that while CTC enumeration alone can be of prognostic value, deeper characterization of CTC combined with ctDNA analysis may provide further insight into the mechanisms underlying treatment response. Future studies should lead to improved understanding of molecular determinants of response and progression which may help to select which patients are most likely to benefit from the various therapeutic options.
Supplementary Material
Supplemental Figure: Consort Diagram
Statement of translational relevance.
SWOG S1222 is a Phase III randomized clinical trial of fulvestrant, anastrozole and everolimus in the front-line treatment of advanced hormone receptor-positive breast cancer. The study evaluated the hypothesis that addition of everolimus with or without anastrozole would improve progression-free survival compared with fulvestrant alone. Translational studies of circulating tumor cells (CTC) were also conducted with measurement by two distinct methods. The expectation was that patients with high CTC and/or high circulating tumor DNA (ctDNA) might benefit from additional therapy while those with low CTC would not and that CTC phenotype, specifically relative expression of estrogen receptor, BCL2, HER2, and Ki67, would predict benefit from endocrine therapy. Due to early study termination, only the prognostic value of the CTC and ctDNA measures could be evaluated. In this limited sample, CTC measures had high concordance and analysis of ctDNA using genomic copy number was shown to indicate poor prognosis.
Funding:
SWOG Clinical Trials Partnerships (CTP) manages the non-federally funded components of SWOG Cancer Research Network under The Hope Foundation for Cancer Research. This study received support from AstraZeneca plc and from Novartis Pharmaceuticals. DFH received research funding support from Janssen Diagnostics during the conduct of this trial. PK and JH received research funding fromt the Breast Cancer Research Foundation. LW was supported by the C. Vassiliadis fellowship.
Conflicts of Interest:
H. Moore: Research funding to her institution from AstraZeneca, Roche/Genentech, Daiichi-Sankyo, and Sermonix.
W. Barlow: reports that the SWOG Statistical Center received research funding to his institution from AstraZeneca to support this study.
G. Somlo: none to report.
J. Gralow: Roche/Genentech: Personal fees, Steering committee Member, Data Safety Monitoring Committee Member
Astra Zeneca: Personal fees, Advisory Board, Data Safety monitoring committee
Sandoz/Hexal AG: Personal fees, Consultant
Puma: Personal fees, Advisory Board
Novartis: Personal fees, Data Safety Monitoring Committee Member
SeaGen: Personal fees, consultant
Genomic Health/Exact Sciences: Personal fees, Consultant
Radius: Personal fees, Data safety monitoring committee member
Inbiomotion: Personal fees, consultant
A. Schott: none to report
D. Hayes: Related to this work: University of Michigan has received funding to support research on behalf of DFH from Astra Zeneca, manufacturer of anastrozole and fulvestant and from Menarini/Silicon Biosystems, current manufacturer of CellSearch®. CellSearch® was owned by Janssen Diagnostics during the conduct of this trial, which provided financial and technical support. University of Michigan holds a patent on which DFH is the named investigator and which is licensed to Menarini/Silicon Biosystems with annual royalties through January 2021.
Unrelated to this work, DFH reports research financial support provided to his institution during conduct and analysis of this study from Astra Zeneca, Eli Lilly Company, Merrimack Pharmaceuticals, Inc. (Parexel Intl Corp), Menarini/Silicon BioSystems, Veridex and Janssen Diagnostics (Johnson & Johnson), Pfizer, and Puma Biotechnology, Inc. (subcontract Wash Univ St. Louis to Univ Mich). DFH reports personal income related to consulting or advisory board activities from BioVeca, Cellworks, Cepheid, EPIC Sciences, Freenome, L-Nutra, Oncocyte, Predictus BioSciences, Salutogenic Innovations, Turnstone Biologics, and Tempus. DFH reports personally held stock options from InBiomotion.
P. Kuhn and J. Hicks: hold ownership interest in, are consultants to and receive royalties from licensed technology from Epic Sciences. Epic Sciences and the University of Southern California, USC Michelson Center (P. Kuhn and J. Hicks), have entered into a sponsored research agreement to advance next-generation liquid biopsy technology for precision oncology.
L. Welter: none to report.
P. Dy: none to report.
C. Yeon: none to report.
A.Conlin: Personal fees, consultant, SeaGen and AstraZeneca
E. Balcueva: none to report.
D. Lew: reports that the SWOG Statistical Center received research funding to her institution from AstraZeneca to support this study.
D. Tripathy: AstraZeneca: Consultant, Genomic Health: Consultant, Gilead Sciences Inc: Consultant, GlaxoSmithKline: Consultant, Novartis Pharma: Grant/Contract to institution and Consultant, OncoPep: Consultant, Pfizer: Grant/Contract to institution and Consultant.
L. Pusztai: received consulting fees and honoraria from Seagen, Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Genentech, Athenex, Radius, Clovis, Roche, Biotheranostics, and institutional research funding from Seagen, AstraZeneca, Merck, Pfizer and Bristol Myers Squibb, in the past 3 years.
G. Hortobagyi: personal fees as consultant to Novartis Pharmaceuticals
Footnotes
ClinicalTrials.gov identifier: NCT021378737
References:
- 1.Mehta RS, Barlow WE, Albain KS, et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer [published correction appears in N Engl J Med. 2019 Jun 06;380(23):2282]. N Engl J Med. 2019;380(13):1226–1234. doi: 10.1056/NEJMoa1811714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornblum N, Zhao F, Manola J, et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol 2018;36:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn RS, Martin M, Rugo HS et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl J Med 2016;375:1925–36 [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer [published correction appears in Ann Oncol. 2019 Nov;30(11):1842]. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Neven P, Chia S, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 2018;36:2465–2472. [DOI] [PubMed] [Google Scholar]
- 7.Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M, Budd GT, Ellis MJ et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004. Aug 19;351(8):781–91 [DOI] [PubMed] [Google Scholar]
- 9.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500 J Clin Oncol. 2014;32(31):3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paoletti C, Muniz M, Thomas D et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin Cancer Res 2015;21(11):2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paoletti C, Regan MM, Niman SM, Dolce EM, Darga EP, Liu MC, et al. Circulating tumor cell number and endocrine therapy index in ER positive metastatic breast cancer patients. NPJ Breast Cancer 2021;7(1):77 doi 10.1038/s41523-021-00281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welter L, Xu L, McKinley D et al. Treatment response and tumor evolution: lessons from an extended series of multianalyte liquid biopsies in metastatic breast cancer patient. Cold Spring Harb Mol Case Stud. 2020. Dec 17;6(6):a005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adalsteinsson VA, Ha G, Freeman SS, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nature Communications 2017. Nov 6;8(1):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malihi PD, Morikado M, Welter L, et al. Clonal diversity revealed by morphproteomic and copy number profiles of single prostate cancer cells at diagnosis. Converg Sci Phys Oncol 2018. Mar;4(1):015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Leo A, Jerusalem G, Torres R, et al. First-line vs second-line fulvestratnt for hormone receptor-positive advanced breast cancer: A post-hoc analysis of the CONFIRM study. The Breast 2018; 38:144–149. [DOI] [PubMed] [Google Scholar]
- 16.Ellis MJ, Llombart-Cussac A, Feltl D et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II First Study. J Clin Oncol 2015; 33(32):3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im SA, Lu YS, Bardia A, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 18.Sledge GW, Toi M, Neven, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2, a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornblum N, Zhao F, Manola J, et al. Randomized Phase II Trial of Fulbestrant Plus Everolimus or Placebo in Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol 2018; 36:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 2017; 18:654–62. [DOI] [PubMed] [Google Scholar]
- 21.Merker JD, Oxnard GR, Compton C et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol 2018;36(16):1631–41, [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Consort Diagram


