Abstract
One option for new nitrogen sources is industrial liquid side streams containing ammonium nitrogen (NH4-N). Unfortunately, NH4-N often exists in low concentrations in large water volumes. In order to achieve a highly concentrated NH4-Nsolution, scalant removal is needed. In this study, scalant removal by precipitation was investigated. At alkali pH, sodium carbonate (Na2CO3) was used as a precipitation chemical while at acidic pH, the chemical used was oxalic acid (C2H2O4). At alkali pH, high Na2CO3 dose was needed to achieve low content of calcium, which, with sulphate, formed the main scalant in the studied mine water. NH4-N at alkali pH was in the form of gaseous ammonia but it stayed well in the solution during pre-treatment for nanofiltration (NF) and reverse osmosis (RO). However, it was not rejected sufficiently, even via LG SW seawater RO membrane. At acidic pH with CaC2O4 precipitation, NF90 was able to be used for NH4-N concentration up to the volume reduction factor of 25. Then, NH4-N concentration increased from 0.17 g/L to 3 g/L. NF270 produced the best fluxes for acid pre-treated mine water, but NH4-N rejection was not adequate. NF90 membrane with mine water pre-treated using acid was successfully verified on a larger scale using the NF90-2540 spiral wound element.
Keywords: ammonium, nitrogen, membrane, recovery, scaling, precipitation, oxalic acid, sodium carbonate, mine water
1. Introduction
The ability to recover and reuse nutrients from wastewater could reduce dependency on energy-intensive processes used to synthesise mineral fertiliser products, such as nitrogen (N). N, a major component of fertilisers, is manufactured in the present day using a Haber–Bosch process, which accounts for 1–2% of worldwide energy demand, represents 3–5% of global annual natural gas consumption, and generates 4–8 tons of equivalent to carbon dioxide (CO2eq) per ton of N fertiliser per year [1,2,3]. On the other hand, through biological wastewater treatment, it is finally released back into the atmosphere as nitrogen gas (N2). The biochemical conversion of ammonium nitrogen (NH4-N) into N2 at the expense of energy, excludes the potential to recover and reuse N. To this end, more focus has been placed onto the recovery of N from liquid side streams by mature technologies, such as stripping, as well as novel technologies, such as reverse osmosis (RO) and electrodialysis (ED) [1].
Given the limited availability of water in many countries, water reuse in industry is increasing. Apart from that, industry can have a strong environmental impact on surrounding water faces. In the mining industry, most metals are obtained from ore bodies containing sulfidic minerals that are oxidised to sulphate (SO4) during the metal extraction process. Therefore, apart from metals, SO4 is a common impurity in mining waters and wastewaters of hydrometallurgical processing [4], referred to as mine waters in this study. Mine waters can also contain N as an impurity from incomplete detonation of N-rich explosives and from N-containing chemicals used in enrichment processes [5,6]. Environmental limits of impurities when discharging the mine water to surrounding water faces vary from site to site, but there is a trend towards tighter limits in the future. Meanwhile, there is significant potential for raw materials lurking in liquid side streams.
Recently, membrane filtration to purify liquid side streams in terms of different driving forces, such as those that are pressure-driven, osmosis-driven, thermally driven, or electrically driven, has received much attention. Membrane filtration, i.e., nanofiltration (NF) and RO, can be employed to produce high-quality water for reuse or discharge, and it has the advantage of low operating costs [7]. The target today is to gain as much purified water as possible, i.e., to obtain high water recovery (WR), and at the same time, membrane filtration generates impurities at high concentrations to be deposited or utilised. When aiming for high WR, the performance is mostly limited by membrane fouling and scaling. There is a great risk of calcium sulphate (CaSO4), i.e., gypsum scaling, when mine water is purified by pressure-driven NF or RO. Mine water contains originally a lot of SO4, and calcium (Ca) is added as a treatment chemical, thus scaling can occur on the membrane surface when sparingly soluble CaSO4 is concentrated beyond its solubility limit. This leads to significant flux reduction and salt rejection impairment and limits the WR of the desalination process to as low as 50–60% [8,9,10].
The use of antiscalants is a widely adopted technique to prevent scaling of calcium carbonate (CaCO3) and CaSO4. Additionally, pre-treatment of the feed by pH adjustment, ion exchange and chemical precipitation may help to reduce scaling [9]. Chemical precipitation is a process that uses specific chemicals to precipitate targeted sparingly soluble salts prior to clarification and pre-filtration to increase the WR of subsequent NF or RO. An alkaline solution, such as sodium carbonate (Na2CO3), soda ash (NaHCO3), or caustic soda (NaOH) with carbon dioxide (CO2), are the most studied chemicals for RO feed water to precipitate and reduce its hardness [7,11]. CaCO3 precipitation occurs when dissolved Ca and carbonate (CO32−) are mixed at concentrations that exceed the solubility of CaCO3 [12]. The formation rate is controlled by many factors, such as super saturation, temperature and pH. An increase of solution pH significantly enhances CO2 gas absorption to solution, and influences ionisation [13]. At high pH, the CO32− form is dominant [14], which leads to the acceleration of CaCO3 nucleation and crystal growth. The increase in pH favours the formation of the most stable polymorph, calcite, instead of the more soluble, meta-stable vaterite [13,14,15].
Oxalic acid (C2H2O4) has long been noticed to precipitate Ca from such solutions as calcium oxalate (CaC2O4) [16]. CaH2O4 is a sparingly soluble salt, which has led to harm for industrial processes. Thus, degradation of C2H2O4 has been studied to avoid precipitation problems [17]. It has not, however, been used as a precipitation chemical for scaling prevention in membrane filtration. With it being more expensive than widely used inorganic precipitation chemicals, it has not been desirable for scaling control unless other benefits would have been obtained, such as the low pH requirement for the subsequent separation process. Low pH can be meaningful for having NH4-N in the solution instead of ammonia (NH3) gas. The NH4/NH3 dissociation equilibrium point is at pKa = 9.24 [18]. Above, the NH4/NH3 dissociation equilibrium point NH4-N is in NH3 form, which is a unionised and small volatile molecule, molecular weight (MW) of 17 Da, which readily diffuses through RO membranes. The retention of uncomplex NH3 can vary between 10% and 40% [19]. On the other hand, the high solubility of NH3 compared to that of other dissolved gases in water, like CO2 or oxygen (O2), and the very low value of the Henry constant may make it difficult to remove free NH3 from solutions [20].
NH3 volatilisation from the aqueous to the gas phase is commonly calculated using Henry’s law. One underlying assumption in these calculations is that Henrys’ law is valid for dilute aqueous systems, which, for NH3, are concentrations up to 1000 mg/L [21]. The driving force for NH3 volatilisation from solution is normally considered to be the difference in NH3 partial pressure between that which is in equilibrium with the liquid phase and that which is in the ambient atmosphere. The equilibrium vapour pressure of NH3 is controlled by the concentration, which, in the absence of other ionic species, is affected by the NH4-N concentration and pH [22]. NH3 volatilisation increases linearly with the concentration, and curvilinearly with temperature and solution pH. At a given pH, the fraction of un-ionised NH3 in solution increases with temperature [21].
To produce a usable chemical or fertiliser of NH4-N from liquid side stream, NH4-N concentration needs to be high. Typically, commercial NH3 stripping systems can achieve ammonium sulphate ((NH4)2SO4) concentration between 25 and 40 m%, equivalent to 6–10% of N, which is marketed as a liquid fertiliser [20]. Hollow fibre liquid–liquid membrane contactor (LLMC) with gas-permeable membrane is an attractive technology for the recovery of volatile compounds, such as NH3, with a low level of impurities [23]. LLMCs have been postulated as an eco-friendly technology for NH3 recovery. The optimum conditions for reject water from wastewater treatment plants has been found to be pH 10 while it is obtaining a 4% (NH4)2SO4 solution [24]. N concentrations of 1.5–3% are required for liquid inorganic macronutrient fertilisers.
In this study, a scalant-controlled concept for maximum NH4-N concentration of mine water from a gold mine was designed and experimentally studied. N existed in large volumes and in low concentrations, thus high WR and volume reduction factor (VRF) was required to obtain maximum NH4-N recovery. Mine water was characterised and based on scalant removal being carried out by precipitation at different pH levels. Clarification and microfiltration (MF) were used to remove precipitates before membrane filtration. The effect of pre-treatment on concentration of N, especially NH4-N, by means of NF and RO, was worked out.
2. Materials and Methods
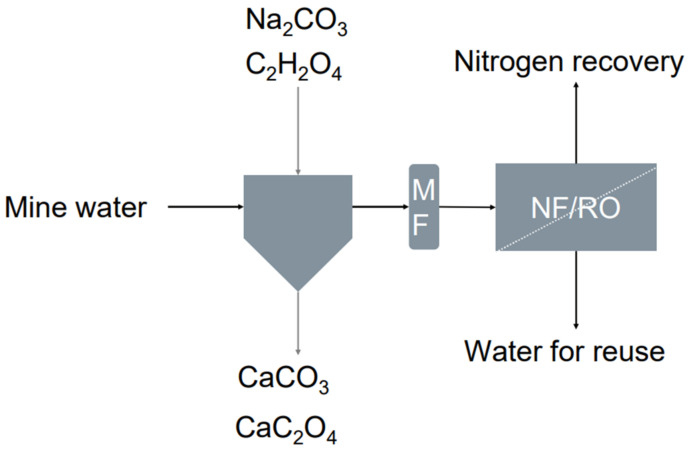
The separation of scalants for the recovery of NH4-N and nitrate nitrogen (NO3-N) was studied at laboratory scale, using mine water sample from a Finnish gold mine according to the scheme in Figure 1. The effect of pH was studied on N content during scalant removal by precipitation aided by clarification and MF, and membrane concentration using either NF or RO.
Figure 1.
Studied process concept for nitrogen (N) concentration at a gold mine. Calcium (Ca) removal prior to nanofiltration (NF) or reverse osmosis (RO) was carried out using sodium carbonate (Na2CO3) and oxalic acid (C2H2O4). Ca was precipitated as calcium carbonate (CaCO3) and calcium oxalate (CaC2O4) and precipitates were removed by clarification and microfiltration (MF).
2.1. Characterisation
Received water samples as well as samples from precipitation and membrane filtration were characterised in terms of water quality and nutrient content. A Hach DR3900 laboratory spectrophotometer (Hach, Loveland, CO, USA) was used for the main analysis [25]. Chemical oxygen demand (COD) was analysed using the cuvette method LCK114, total nitrogen (N total) using the method LCK138, NH4-N using methods LCK302 and LCK303, NO3-N using the method LCK339, potassium (K) using the method LCK228, chloride (Cl) using the method LCK311, Ca and magnesium (Mg) using the method LCK327, as well as SO4 using the method LCK153 [25].
Conductivity was measured using a VWR Conductivity meter CO 3000 H (VWR, Germany). pH was measured using a VWR pH1000 pH meter (VWR, Darmstadt, Germany). Inductively coupled plasma-atomic emission spectroscopy (ICP-OES) was used with SFS-EN ISO 11885 to analyse aluminium (Al), Ca, copper (Cu), Mg, manganese (Mn), iron (Fe), nickel (Ni), chrome (Cr), phosphorous (P), barium (Ba), sulphur (S), sodium (Na), K, silicon (Si), and zinc (Zn). Osmotic pressure was measured using a Wescor Vapro® Model 5600 Vapor Pressure Osmometer (Wescor Inc, South Logan, UT, USA).
2.2. Precipitation
Based on the mine water characterisation CaSO4, gypsum was the main scalant in the water. Ca removal prior to NF or RO was carried out using Na2CO3 and C2H2O4, both in powder form. The pH of water was increased to 10.1 with 4 g/L Na2CO3, and lowered to 2.2 with 0.77 g/L C2H2O4 in order to achieve sufficient Ca reduction.
Precipitates were separated from the liquid using clarification and subsequent MF using Whatman ME25 filter paper. MF permeate for feed was used as such for NF and RO or pH was decreased by 2 M sulphuric acid (H2SO4) before NF and RO. The solids removal method after precipitation and prior to spiral wound membrane module was carried out by clarification overnight.
2.3. Membrane Filtration
Studied NF membranes were NF270 (DuPont, Wilmington, DE, USA), and NF90 (DuPont), with molecular weight cut-offs (MWCO) of 400 Da and 200 Da, respectively. Studied RO membrane was LG SW (LG Chem, Seoul, Republic of Korea) which is suitable for high salinity seawater RO (SWRO) applications.
The performance of different membranes was evaluated by conducting lab scale filtration tests using a dead-end filtration cell HP4750X (Sterlitech, Auburn, WA, USA), with magnetic agitation for increased shear on a membrane’s surface. Membranes were characterised using manufacturers’ specifications, testing salt rejections using 2 g/L MgSO4 for NF90 and NF270 with 4.8 bar pressure and 15% recovery, and 32 g/L NaCl for LG SW with 55 bar pressure and 8% recovery.
The used feed water volume was 100 mL, the temperature was 23 ± 1 °C (the room temperature), the operating pressure was 20–40 bar for NF and 40–80 bar SWRO, and the effective membrane area was 14.6 cm2. Argon gas was used for the pressurisation of the cell.
Spiral wound membrane module NF90-2540 (DuPont) with membrane area of 2.6 m2 in a 40-bar steel pressure vessel (Sommer & Strassburger GmbH & Co. KG, Bretten, Germany) was used to verify NH4-N concentration on a larger scale. The membrane was characterised as lab scale NF90 membrane.
3. Results
3.1. Liquids Characterisation
The mine water originally had clearly higher NH4-N content than NO3-N content, 170 mg/L and 13 mg/L, respectively (Table 1). NH4-N content was, however, too low for recovery using, e.g., MC technology, which requires at least NH3 500 mg/L. Thus, the solution required concentration for subsequent recovery. In order to achieve the highest N content possible, it was essential to keep NH4-N content as high as possible during pre-treatment and subsequent NF and SWRO. Mine water had high Ca concentration, 390 mg/L, which formed, along with SO4, the main scalant, CaSO4, which was the possible hindrance for high WR and VRF. The concentrations of potential foulants Al and Fe were low enough for NF and SWRO. Mn concentration was higher than that typically guided by membrane manufacturers, 0.12 mg/L and less than 0.05 mg/L, respectively. Other metal concentrations were low in the studied mine water.
Table 1.
Characteristics of mine water. Uncertainty of the measurements was 15–30%.
| Parameter | Unit | Process Water |
|---|---|---|
| pH | 7.1 | |
| Conductivity | mS/cm | 6.1 |
| Osmotic pressure | bar | 1.6 |
| Total-N | mg/L | 174 |
| NH4-N | mg/L | 170 |
| NO3-N | mg/L | 13 |
| Al | mg/L | <0.03 |
| Ca | mg/L | 390 |
| Cu | mg/L | <0.003 |
| Mg | mg/L | 55 |
| Mn | mg/L | 0.12 |
| Fe | mg/L | <0.05 |
| Ni | mg/L | 0.051 |
| Cr | mg/L | <0.003 |
| P | mg/L | <0.01 |
| Ba | mg/L | 0.007 |
| S | mg/L | 920 |
| SO4 | mg/L | 2600 |
| Na | mg/L | 680 |
| K | mg/L | 36 |
| Si | mg/L | 8.2 |
| Zn | mg/L | <0.005 |
3.2. Precipitation
Increasing the dosages of Na2CO3 or C2H2O4 produced lower Ca content in the solutions, as expected. A sufficiently low concentration of Ca was obtained with Na2CO3 when the dosage was as high as 4.0 g/L and the pH was 10.1 (Table 2). C2H2O4 produced somewhat higher final Ca content but the dosage needed for decreasing Ca was lower, 0.77 g/L, than with Na2CO3. C2H2O4 precipitation decreased the solution pH to 2.2. When Ca was reduced at a high pH of 10.1, there was a risk of losing NH3 as a gas from the liquid. Some decrease of NH4-N, maximum 18%, was observed during pre-treatment, but this was within the measurement accuracy.
Table 2.
Precipitation of mine water sample. Concentrations were measured from the 0.45 µm filtrate.
| Chemical | Dose g/L |
pH | NH4-N mg/L |
Ca mg/L |
Mg mg/L |
|---|---|---|---|---|---|
| - | - | 8.1 | 170 | 390 | 77 |
| Na2CO3 | 0.8 | 8.3 | 140 | 170 | 59 |
| Na2CO3 | 1.7 | 9.5 | 150 | 140 | 23 |
| Na2CO3 | 4.0 | 10.1 | 150 | 7 | 34 |
| C2H2O4 | 0.13 | 6.0 | 140 | 310 | 77 |
| C2H2O4 | 0.77 | 2.2 | 170 | 81 | 56 |
Feed waters to be concentrated using NF and SWRO were those with the lowest Ca content in the solution (Table 3). Mg precipitated with Ca, especially when the pH was high enough for the formation of magnesium hydroxide. Additionally, K was a slightly lower for the precipitated samples compared to the feed with no precipitation. NH4-N as well as NO3-N and SO4 contents after pre-treatment were similar in each water sample. COD increased slightly due to usage of organic acid. Osmotic pressure was double its previous level after Na2CO3 treatment compared to the feed with no precipitation, thus some of the chemical remained in the solution. Osmotic pressure affects the VRF when filtering at the maximum pressure level of the membranes.
Table 3.
Feed water samples for nanofiltration (NF) and seawater reverse osmosis (SWRO).
| Chemical | Dose g/L |
pH | π Bar |
NH4-N mg/L |
NO3-N mg/L |
Ca mg/L |
Mg mg/L |
SO4 mg/L |
K mg/L |
COD mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| - | - | 8.1 | 1.6 | 170 | 13 | 390 | 77 | 2600 | 69 | 160 |
| Na2CO3 | 4.0 | 10.1 | 3.5 | 150 | 12 | 7 | 34 | 2300 | 45 | na |
| C2H2O4 | 0.77 | 2.2 | 2.0 | 170 | 12 | 81 | 56 | 2200 | 41 | 240 |
3.3. Membrane Filtration
Salt rejections in membrane characterisation were 91.5–94.0% for NF270 and NF90 membranes and 92.9–93.0% for LG SW membrane based on conductivity measurements of feed and permeate.
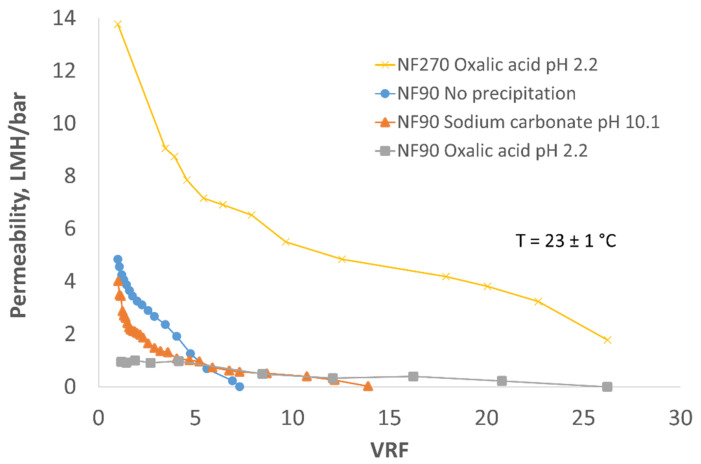
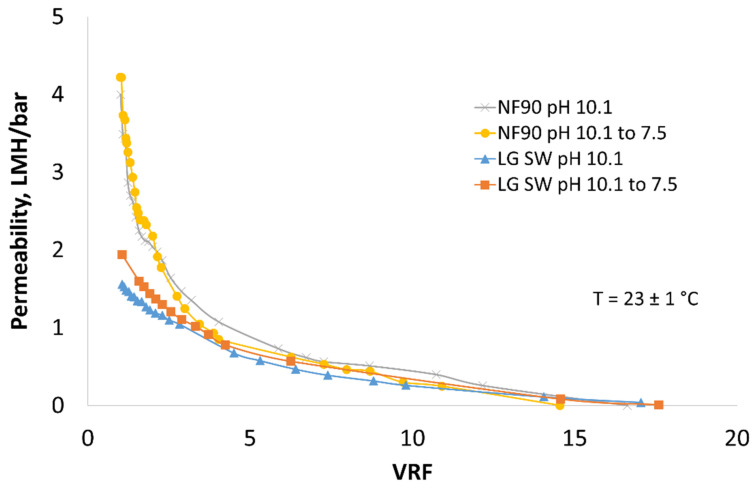
During lab scale membrane filtration, the permeability of mine water filtration was measured as a function of VRF. Pre-treatment for the removal of the main scalant, i.e., CaSO4, did not improve the permeability of the filtrations, but both precipitations significantly assisted in an increase in VRF (Figure 2). C2H2O4-precipitated mine water produced higher VRF than Na2CO3 precipitated. On the other hand, the pH decrease in the feed water from 10.1 to 7.5 did not affect the permeability or VRF of precipitated mine water (Figure 3). The highest permeability was obtained using NF270 membrane. NF90 and LG SW produced the same permeability at pH 10.1, but the pressure needed for filtration when using LG SW was double that of NF90.
Figure 2.
Concentration of pre-treated mine water using NF90 with no precipitation, precipitated using Na2CO3 and precipitated C2H2O4 compared to NF270 precipitated with C2H2O4.
Figure 3.
Concentration of pre-treated mine water using NF90, and LG SW precipitated using Na2CO3 without and with pH adjustment.
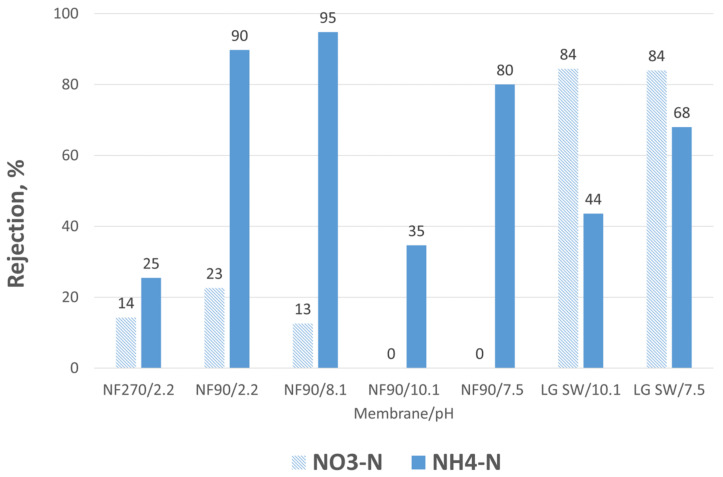
Although NF270 produced the highest permeability, NH4-N rejection at pH 2.2 with NF270 was not as good as with NF90 (Figure 4) due to differences in the MWCOs of the NF270 and NF90 membranes, 400 and 200 Da, respectively. This led to lower NH4-N concentration of NF270 concentrate than NF90 concentrate, 1.5 g/L and 3.0 g/L, respectively, although the VRF was the same, 25 (Table 4). The best rejection was achieved with NF90 with no precipitation. Then, the membrane scaling occurred and VRF was only 5, leading to NH4-N concentration of 0.8 g/L. At a high pH, 10.1, NH3 was not rejected sufficiently even by LG SW membrane. Above the NH4/NH3 dissociation equilibrium point pKa 9.24, NH4-N is in NH3 form, which as a small molecule readily diffused through SWRO membranes. pH adjustment from 10.1 to 7.5 before concentration increased the rejection near the level of C2H2O4 precipitation or no precipitation at all. NO3-N was not rejected well with NF membranes, but rejection with SWRO membrane was 84%. Ca concentration in some concentrates was lower than expected due to precipitation of the sample before analysis.
Figure 4.
Nitrate nitrogen (NO3-N) and ammonium nitrogen (NH4-N) rejections using different pre-treatment and membranes.
Table 4.
Concentrations of feed, permeate and concentrates of the mine water when using the studied membranes.
| Membrane/pH | VRF | Sample | NH4-N mg/L |
NO3-N mg/L |
Ca mg/L |
Mg mg/L |
SO4 mg/L |
K mg/L |
|---|---|---|---|---|---|---|---|---|
| NF270/2.2 | Feed | 170 | 12 | 81 | 56 | 2200 | 41 | |
| 25 | Concentrate | 710 | 16 | 1000 | 1300 | 13,900 | 260 | |
| Permeate | 130 | 10 | 6 | 14 | 550 | 24 | ||
| NF90/2.2 | Feed | 170 | 12 | 81 | 56 | 2200 | 41 | |
| 25 | Concentrate | 2950 | 15 | 620 | 1300 | 22,100 | 860 | |
| Permeate | 9 | 11 | 1 | 4 | 120 | 2 | ||
| NF90/8.1 | Feed | 170 | 13 | 370 | 77 | 2600 | 69 | |
| No precipitation | 7 | Concentrate | 770 | 27 | 1400 | 550 | 9500 | 200 |
| Permeate | 18 | 9.8 | 0 | 3 | 33 | 0 | ||
| NF90/10.1 | Feed | 150 | 12 | 7 | 34 | 2300 | 45 | |
| 17 | Concentrate | 370 | 40 | 100 | 630 | 24,000 | 780 | |
| Permeate | 80 | 12 | 0 | 5 | 30 | 1 | ||
| NF90/7.5 | Feed | 130 | 12 | 18 | 36 | 4200 | 38 | |
| 14 | Concentrate | 1490 | 10 | 600 | 180 | 4400 | 510 | |
| Permeate | 25 | 12 | 0 | 0 | 15 | 6 | ||
| LG SW/10.1 | Feed | 120 | 12 | 7 | 34 | 2300 | 45 | |
| 17 | Concentrate | 440 | 250 | 110 | 800 | 36,300 | 910 | |
| Peremate | 70 | 2 | 0 | 2 | 45 | 3 | ||
| LG SW/7.5 | Feed | 130 | 12 | 18 | 36 | 4200 | 38 | |
| 17 | Concentrate | 2010 | 150 | 290 | 830 | 70,600 | 740 | |
| Permeate | 41 | 2 | 0 | 0 | 90 | 1 |
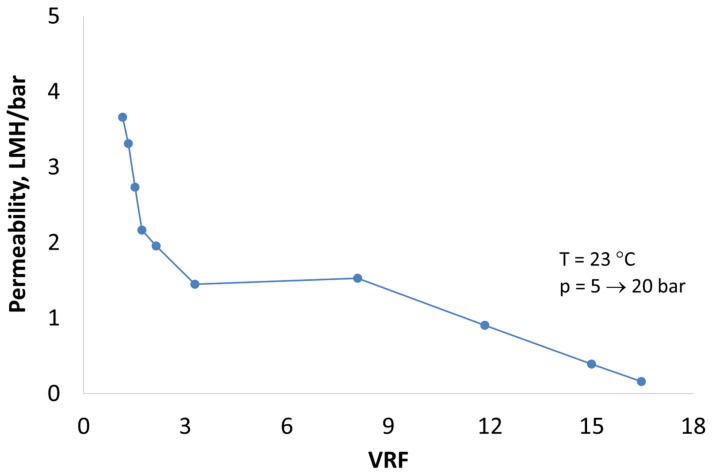
Based on the obtained NH4-N rejection, VRF and energy consumption, NF90 and C2H2O4-precipitated mine water was selected for larger-scale verification studies using a NF 2540 spiral wound element. Verification filtration unfolded similarly to lab scale filtration using NF90 (Figure 5), but the achieved VRF at lower 20 bar pressure used was not as high as in lab filtration, 17 instead of 25. The NH4-N content in the concentrate was 1.9 g/L (Table 5). Obtained permeate quality was good regarding the compounds studied (Table 5 and Table 6), but the pH was low and would need to be increased when reused or discharged.
Figure 5.
Permeability of C2H2O4-precipitated mine water when using NF90-2540 spiral wound element.
Table 5.
Quality of C2H2O4-precipitated feed, permeate and concentrate in verification filtration using NF90-2540 element.
| pH | π mS/cm |
NH4-N mg/L |
NO3-N mg/L |
Ca mg/L |
K mg/L |
Mg mg/L |
Cl mg/L |
SO4 mg/L |
COD mg/L |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Original | 7.1 | 6.1 | 160 | 12.3 | 440 | 58 | 82 | 19 | 1900 | 160 |
| Feed | 2.1 | 8.4 | 160 | 13.8 | 110 | 73 | 110 | 21 | 2990 | 240 |
| Concentrate | 1.7 | 56 | 1910 | 15.7 | 540 | 660 | 980 | 20 | 36,300 | 110 |
| Permeate | 2.4 | 1.8 | 4.8 | 1.9 | 1 | 4 | 2 | 5.4 | 150 | 160 |
Table 6.
Metal concentration of C2H2O4-precipitated feed and concentrate from verification filtration using spiral wound NF90-2540 element.
| Al mg/L |
Ba mg/L |
Cr mg/L |
Cu mg/L |
Fe mg/L |
Mn mg/L |
Na mg/L |
Ni mg/L |
P mg/L |
Si mg/L |
S mg/L |
Zn mg/L |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | <0.03 | 0.007 | <0.003 | <0.003 | <0.05 | 0.12 | 680 | 0.051 | <0.01 | 8.2 | 920 | <0.005 |
| Concentrate | 0.67 | 0.13 | 0.28 | 5.9 | 24 | 5.3 | 8800 | 1.2 | <5 | 76 | 13,000 | 4.5 |
Based on the mass and NH4-N content, NF90-2540 concentrate and permeate contained 96.3% and 3.7% of NH4-N, respectively. Similarly, in flat sheet tests, the division of NH4-N to concentrate and permeate was 93.3% and 6.7%, respectively.
4. Discussion
Mine water can be considered as a possible N-source for chemicals and fertilisers. N often exists in low concentrations in large water volumes. This is valid for mine waters from a gold mine, although feed water attempts to acquire as concentrated a stream as possible. In order to achieve concentrated NH4-N solution from mine waters and at the same time produce large volumes of purified water by membrane filtration, scaling removal is usually required in order to reach high WR and VRF. In mine waters, Ca often forms the main scalant with SO4, as it was with the studied feed water. Thus, removal of Ca is required, which can be carried out by precipitation with C2H2O4 as well as Na2CO3 chemicals. CaH2O4 and CaCO3 are both sparingly soluble Ca salts.
To achieve low Ca content in the feed solution, a high dose of Na2CO3 may be required, which itself would produce a high-pH feed solution. NH4-N is in a gaseous form in water when pH is 10 or above. In this research the dosage was as high as 4.0 g/L and pH was 10.1. On the other hand, NH3 is a soluble gas and seemed to stay quite well in the solution. It stayed in the solution during precipitation, clarification, and subsequent MF, all of which were carried out as a pre-treatment method to NF and SWRO in this study. However, this research showed that the unionised and small NH3 molecule is not able to be concentrated even by SWRO membrane unless pH is decreased. When low pH is used for scalants removal with C2H2O4, or pH is decreased before concentration to nearly neutral, NF90 is able to concentrate NH4-N. NF270, which had larger MWCO than NF90, could clearly produce higher permeability than NF90, but NH4-N rejection, even at low pH, was not adequate. NF90 and LG SW produced the same permeability at pH 10.1, but the pressure needed for filtration when using SWRO was double that which was needed for NF.
Pre-treatment did not improve the permeability of the NF or SWRO, but both precipitations significantly assisted VRF increase. C2H2O4-precipitated mine water produced higher VRF than Na2CO3 precipitated. Due to high VRF, 25, and high rejection, 90%, NH4-N content in the NF90 concentrate was high at low pH. It was as its best in this research at 3.0 g/L, which would be high enough for the subsequent NH3 recovery, e.g., via stripping or MC technology. NO3-N was not rejected well with either of the NF membranes but rejection with the SWRO membrane was 84%. However, NO3-N concentration in the mine water was originally low. The quality of purified water from NH4-N concentration when using NF90 membrane with C2H2O4 pre-treatment was otherwise good, but the pH of purified water needs to be increased for water reuse or discharge.
Mass balance of NH4-N from spiral wound membrane test resulted in 96% and 4% division of NH4-N to concentrate and permeate, respectively. Permeate amount was 96% of the feed with low concentration of NH4-N, i.e., 4.8 mg/L. In the future, industry can utilise the results for water reuse and minimising surface water uptake. The gold mine produces 70 m3/h of the studied mine water, from which it is possible to draw 66 m3/h of pure water with low NH4-N concentration. The developed concept is also usable by producing concentrated NH4-N stream in low volume.
This study is part of circular economy development. When producing NH4-N concentrate from mine water, all the streams from the developed process need to be designed. Clarifier underflow from solids removal could go to a filter press, from which the filtrates from filtration and pressing stages could be returned back to the clarifier, and the filter cake is considered a suitable material for, e.g., mine backfilling or cement additive. Residual water from NH4-N recovery consists mainly of Na and SO4 and could be processed into usable chemicals, e.g., acid and base by electrodialysis (ED). These uses can be a part of future studies.
5. Conclusions
Mine water from a metal enrichment process featuring NH4-N was studied as a possible option for N recovery and water reuse. N existed in a low concentration in a large water volume and in order to achieve concentrated NH4-N solution, scalant removal by precipitation was needed. Precipitation was carried out at alkali pH 10.1 using Na2CO3 and at acidic pH 2.2 using C2H2O4 as a precipitation chemical, which both functioned well in removal.
Low concentration of Ca was obtained with Na2CO3 at high pH when N was in gaseous NH3 form. Instead, C2H2O4 precipitation produced a solution where N was in ionic NH4-N form. Soluble NH3 remained well in the solutions during pre-treatment with either of the chemicals but pH had a significant influence on the N rejection. Good NH4-N rejection was achieved at low pH using NF90, but at the high pH, gaseous NH3 was not rejected sufficiently even by means of an LG SW membrane. Pre-treatment did not improve the permeability of the NF or SWRO but both precipitations significantly assisted VRF increase. Good rejection, 90%, at acidic pH and high VRF, 25, when using NF90 membrane produced NH4-N concentration 3 g/L, which is a suitable concentration for subsequent N recovery. Concentration was successfully verified at pilot scale using NF90-2540 element.
Author Contributions
Studies at laboratory scale, H.K., J.H., E.J. and A.G.; writing—original draft preparation, H.K., J.H. and E.J.; writing—review and editing, H.K. and A.G.; project administration, H.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was done in TYPKI project funded by Business Finland grant number [43649/31/2020], VTT Technical Research Centre of Finland Ltd. and the companies involved in the TYPKI project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deng Z., Van Linden N., Guillen E., Spanjers H., Van Lier J.B. Recovery and applications of ammoniacal nitrogen from nitrogen-loaded residual streams: A review. J. Environ. Manag. 2021;295:113096. doi: 10.1016/j.jenvman.2021.113096. [DOI] [PubMed] [Google Scholar]
- 2.Van der Hoek J.P., Duijff R., Reinstra O. Nitrogen Recovery from Wastewater: Possibilities, Competition with Other Resources, and Adaptation Pathways. Sustainability. 2018;10:4605. doi: 10.3390/su10124605. [DOI] [Google Scholar]
- 3.Iddya A., Hou D., Khor C.M., Ren Z., Tester J., Posmanik R., Gross A., Jassby D. Efficient ammonia recovery from wastewater using electrically conducting gas stripping membranes. Environ. Sci. 2020;7:1759. doi: 10.1039/C9EN01303B. [DOI] [Google Scholar]
- 4.Kinnunen P., Kyllönen H., Kaartinen T., Mäkinen J., Heikkinen J., Miettinen V. Sulphate removal from mine water with chemical, biological and membrane technologies. Water Sci. Technol. 2017;1:194–205. doi: 10.2166/wst.2018.102. [DOI] [PubMed] [Google Scholar]
- 5.Jermakka J., Wendling L., Sohlberg E., Heinonen H., Merta E., Laine-Ylijoki J., Kaartinen T., Mroueh U.-M. Nitrogen compounds at mines and quarries, Sources, behaviour and removal from mine and quarry waters—Literature study. VTT Technol. 2015;226:1–144. [Google Scholar]
- 6.Häyrynen K., Pongrácza E., Väisänen V., Pap N., Mänttäri M., Langwaldt J., Keiski R.L. Concentration of ammonium and nitrate from mine water by reverse osmosis and nanofiltration. Desalination. 2009;240:280–289. doi: 10.1016/j.desal.2008.02.027. [DOI] [Google Scholar]
- 7.Ogunbiyi O., Saththasivam J., Al-Masri D., Manawi Y. Sustainable brine management from the perspectives of water, energy and mineral recovery: A comprehensive review. Desalination. 2021;513:115055. doi: 10.1016/j.desal.2021.115055. [DOI] [Google Scholar]
- 8.Zhao Y., Zhang Y., Liu J., Gao J., Ji Z., Xiaofu G.X., Liu J., Yuan J. Trash to treasure: Seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination. 2017;407:85–92. doi: 10.1016/j.desal.2016.12.018. [DOI] [Google Scholar]
- 9.Shenvi S., Isloor A., Ismail A. A review on RO membrane technology: Developments and challenges. Desalination. 2015;368:10–26. doi: 10.1016/j.desal.2014.12.042. [DOI] [Google Scholar]
- 10.Kyllönen H., Grönroos A., Järvelä E., Heikkinen J., Tang C. Experimental Aspects of Scaling Control in Membrane Filtration of Mine Water. Mine Water Environ. 2016;36:193–198. doi: 10.1007/s10230-016-0415-3. [DOI] [Google Scholar]
- 11.Zhao Y., Cao H., Xie Y., Yuan J., Ji Z., Yan Z. Mechanism studies of a CO2 participant softening pretreatment process for seawater desalination. Desalination. 2016;393:166–173. doi: 10.1016/j.desal.2015.12.014. [DOI] [Google Scholar]
- 12.Ubbink B.J. Master’s Thesis. Delft University of Technology; Delft, The Netherlands: 2013. Controlled Precipitation of Calcium Carbonate in Spatial Dimension with Multiple Calcium Chloride and Sodium Carbonate Pulses. [Google Scholar]
- 13.Aghajanian S., Nieminen H., Koiranen T. Precipitation of calcium carbonate in highly alkaline solution through carbonated water Precipitation of calcium carbonate in highly alkaline solution through carbonated water; Proceedings of the 2nd International Process Intensification Conference; Leuven, Belgium. 27 May 2019. [Google Scholar]
- 14.Ryu M.Y., You K.S., Ahn J.W., Kim H. Effect of the pH and Basic Additives on the Precipitation of Calcium Carbonate during Carbonation Reaction. Resour. Process. 2007;54:14–18. doi: 10.4144/rpsj.54.14. [DOI] [Google Scholar]
- 15.Kontrec J., Tomaši N., Mlinari N.M., Kralj D., Džakula B.N. Effect of pH and Type of Stirring on the Spontaneous Precipitation of CaCO3 at Identical Initial Supersaturation, Ionic Strength and (Ca2+)/(CO32−) Ratio. Crystals. 2021;11:1075. doi: 10.3390/cryst11091075. [DOI] [Google Scholar]
- 16.Verma A., Kore R., Corbin D.R., Shiflett M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019;58:15381–15393. doi: 10.1021/acs.iecr.9b02598. [DOI] [Google Scholar]
- 17.Nilvebrand N.-O., Reimann A., De Sousa F., Cassland P., Larsson S., Hong F., Jönsson L.J. Enzymatic degradation of oxalic acid for prevention of scaling. In: Viikari L., Lantto R., editors. Biotechnology in the Pulp and Paper Industry 8th ICBPPI. Volume 21. Elsevier Science B.V.; Amsterdam, The Netherlands: 2002. pp. 231–238. [Google Scholar]
- 18.Damtie M., Volpin F., Yao M., Tijing L.D., Hailemariam R.H., Bao T., Park K.-D., Shon H.K., Choi J.-S. Ammonia recovery from human urine as liquid fertilizers in hollow fiber membrane contactor: Effects of permeate chemistry. Environ. Eng. Res. 2021;26:190523. doi: 10.4491/eer.2019.523. [DOI] [Google Scholar]
- 19.Masse L., Masse D.I., Pellerin Y. Review Paper: SE—Structures and Environment The use of membranes for the treatment of manure: A critical literature review. Biosyst. Eng. 2007;98:371–380. doi: 10.1016/j.biosystemseng.2007.09.003. [DOI] [Google Scholar]
- 20.Vecino X., Reiga M., Bhushana B., Giberta O., Valderramaa C., Cortinaa J.L. Liquid fertilizer production by ammonia recovery from treated ammonia rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019;360:890–899. doi: 10.1016/j.cej.2018.12.004. [DOI] [Google Scholar]
- 21.Arogo J., Westerman P.W., Heber A.J., Robarge W.P., Classen J.J. Ammonia Emissions from Animal Feeding Operations. White Paper Prepared for: National Center for Manure and Animal Waste Management North Carolina State University 2014, Raleigh, N.C. [(accessed on 1 August 2022)]. Available online: https://www.researchgate.net/publication/238176842_Ammonia_Emissions_from_Animal_Feeding_Operations.
- 22.Freney J.R., Simpson J.R., Denmead O.T. Volatilization of Ammonia, Gaseous Loss of Nitrogen from Plant-Soil Systems. Volume 9. Springer Science + Business Media; Dordrecht, The Netherlands: 1983. pp. 1–32. (Part of the Developments in Plant and Soil Sciences Book Series). [DOI] [Google Scholar]
- 23.Kyllönen H., Heikkinen J., Järvelä E., Sorsamäki L., Siipola V., Grönroos A. Wastewater Purification with Nutrient and Carbon Recovery in a Mobile Resource Container. Membranes. 2021;11:975. doi: 10.3390/membranes11120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noriega-Hevia G., Serralta J., Seco A., Ferrer J. Economic analysis of the scale-up and implantation of a hollow fibre membrane contactor plant for nitrogen recovery in a full-scale wastewater treatment plant. Sep. Purif. Technol. 2021;275:119128. doi: 10.1016/j.seppur.2021.119128. [DOI] [Google Scholar]
- 25.LCK Cuvette Test System|HACH. [(accessed on 16 November 2022)]. Available online: https://uk.hach.com/lck.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.