Summary
Background
Co-encapsulated antiretrovirals (ARVs) with ingestible sensor (IS) has the capacity to monitor adherence in real-time using a sensor patch, a mobile device, and supporting software. We evaluated the acceptability, effectiveness, and sustainability of the IS system with real-time text reminders.
Methods
Participants were recruited from HIV clinics in Los Angeles and were randomised 1:1 to IS or usual care (UC) group. Adherence to ARVs (primary outcome) was measured by IS system (IS group only), plasma ARV concentration, and self-report. IS-measured adherence was clustered by group-based trajectory model and was validated by ARV concentration summarized by integrated pharmacokinetic adherence measure (IPAM) score. HIV RNA viral load (VL) was compared between IS and UC group.
Findings
A total of 112 (IS = 54, UC = 58) participants who completed baseline with at least one follow-up data collection were included in analyses. Overall satisfaction rate for the IS system was >90%. The IPAM score was higher (0.018, 95% CI: −0.098–0.134, p = 0.75) and VL decayed faster (−0.020, 95% CI: −0.042–0.002, p = 0.08) in the IS group compared with the UC group. The ingestible sensor system was well tolerated by study participants.
Interpretation
The IS system was well accepted by participants and its use was associated with improved adherence and lower HIV RNA VL. The findings provide a potentially effective strategy for improving adherence.
Funding
This work was supported by grant R01-MH110056 from the National Institute of Mental Health (NIMH)/National Institutes of Health (NIH). Y. Wang was in part supported by the NIMH/NIH award T32MH080634. E. Daar was in part supported by the National Center for Advancing Translational Sciences through UCLACTSI Grant UL1TR001881. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Keywords: Ingestible sensor, Adherence, HIV, Pharmacokinetic, Viral load
Research in context.
Evidence before this study
Higher levels of adherence to antiretrovirals (ARVs) are associated with better plasma HIV RNA control. Most traditional adherence measures only provide inferred estimates of past adherence, and many interventions failed to improve suboptimal adherence. Real-time monitoring of adherence, accompanied with text message reminders may support the development of an effective intervention program. Proteus Digital Health Inc. produced the first FDA-approved digital medicine system with wireless technology to monitor real-time medication ingestion. PubMed searches for clinical trials with patients infected with HIV-1 using the title or abstract search terms “HIV” and “ingestible sensors” were conducted. An unrestricted PubMed search was performed with only three studies identified through June 2021 in the U.S. that applied ingestible sensors on adherence improvements. To the best of our knowledge, this is the only published study that has used an ingestion sensor system with real-time feedback on ARV adherence in HIV-infected individuals.
Added value of this study
The innovative intervention in this study was to integrate co-encapsulated ARVs and ingestible sensors, and the automated personalized text messages. The intervention program offers accurate measurement and real-time monitoring of adherence, together with real-time feedback to improve adherence. The primary outcome is adherence to antiretroviral therapy (ART), and the secondary outcomes included plasma HIV RNA and participant satisfaction/acceptability of the system. This study is among the earliest that utilized an FDA-approved ingestible sensor system for ARV therapy in HIV-infected adults.
Implications of all the available evidence
The improvements in adherence and suppression of plasma HIV RNA during the 16-week intervention period suggest that the ingestible sensor system is effective in this setting. The pattern of adherence was also sustained during the 12-week post-intervention period. The real-time monitoring with text message intervention appeared to enhance adherence during and after ingestion sensor was utilized. The findings in this study support the potential to implement an ingestion sensor intervention to help individuals enhance ARV adherence and viral suppression. Such an intervention could be used to navigate individuals through period of poor adherence and bridge to new therapeutic strategies such as long-acting injection regiments.
Introduction
A recent study indicated many people with HIV (PWHIV) failed to achieve durable viral suppression.1 PWHIV who received antiretroviral therapy (ART) and maintained an undetectable HIV RNA viral load (VL) cannot sexually transmit the virus to others (Undetectable = Untransmittable or U=U).2 It is critically important to identify interventions to enhance adherence in order to achieve viral suppression and optimize outcomes in HIV-infected individuals.3 Extensive research has been done in the past two decades for measuring adherence to ART. The most accurate method is directly observed therapy (DOT),4 which requires an individual observing and documenting when a patient takes the medication, either in-person, by video or artificial intelligence mediated monitoring. Less accurate measures include patient self-report, pill counts, electronic pill-bottle caps, and prescription refill monitoring.5 Biological markers of adherence have the potential to be a highly accurate measure of adherence. For example, measuring drug level concentrations,6 dried blood spot measurements,7 and drug levels in hair,8 but generally not in real-time. The importance of exploring new strategies for monitoring adherence with potential for real-time monitoring to optimize adherence with ARV agents has recently been reviewed by Spinelli and colleagues.9
The Digital pill has emerged as a potential tool for monitoring real-time medication adherence.10 Proteus Digital Health (Redwood City, CA, USA) produced the first FDA-approved digital medicine system to provide the pattern for medication ingestion to both patients and providers (Fig. S1).11, 12, 13 The system includes sensor pill, an adhesive wearable sensor patch, a mobile app, and a provider web portal, with post-dose text messaging. The sensor contains tiny amounts of silicon, copper, and magnesium that pass through the body naturally, like fiber in food.11 When it reaches the stomach, the sensor is activated by stomach fluid and releases an electric signal to the adhesive patch on the patient's torso which records the date and time of the signal as well as the sensor ID (1–2 min after the pills was taken). The patch sends a signal via Bluetooth technology to the mobile app, which stores and uploads data to the secured cloud-based system to display on the web portal when mobile device is within signal range (approximately 10 feet). The system also allows for programing personalized text message reminder that can be sent to patients after a missed dose occurred.
In our earlier work, we analysed the pharmacokinetics of co-encapsulated sensor pills with eight commonly used fixed-dose combination ARVs confirming the safety and bioequivalence/bioavailability for each of the eight ARV formulations in our study: emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF); FTC/tenofovir alafenamide (TAF); efavirenz (EFV)/FTC/TDF; abacavir (ABC)/lamivudine (3 TC); dolutegravir (DTG)/ABC/3TC; rilpivirine (RPV)/TAF/FTC; elvitegravir (EVG)/cobicistat (COBI)/FTC/TAF; and bictegravir (BIC)/FTC/TAF.14 We evaluated the perception of this cutting-edge technology from both study participants and providers to understand the barrier and limitations.15 We further conducted a pilot study with 15 participants to assess the functionality, acceptability and satisfaction with the patch, co-encapsulated ARVs and automatic text message reminders.16 In this paper, we report the findings from our main randomised clinical trial that evaluated the accuracy, effectiveness, sustainability, as well as participant satisfaction with different aspects of the IS system.
Methods
Recruitment and randomisation
Study participants were primarily recruited from two urban, safety net HIV clinics at Harbor-UCLA Medical Center and Long Beach Comprehensive Care in Los Angeles from May 2018 to February 2020, along with those referred from other community clinics. The last follow-up study visit happened in October 2020. The inclusion criteria included HIV-infected adults (≥18 years) who were in care and had difficulty adhering to their recommended regimens, defined as self-reported adherence <90%, gaps in treatment, missed appointments, and/or VL elevated in the past six months. In this study, randomisation happened before baseline since extra time was needed for the ARV-sensor co-encapsulation process. Participants were randomised 1:1 to IS or UC groups. We used a stratified urn randomisation procedure by two factors: (i) single/multiple tablet regimen, and (ii) detectable/undetectable VL at baseline with a 2X2 factorial stratification. The randomisation process used centralized randomisation module in REDCap.17 The preparation time varied depending on the regimens and availability of participants. Once randomised to IS group, the participant was scheduled for a baseline visit, typically occurring within 2–4 weeks. Details about co-encapsulation and onboarding process are described in Appendix I.
Ethics
This study was approved by central and site-specific institute review boards or ethics committees. The study protocol was reviewed and approved by the UCLA Institutional Review Boards and the Lundquist Institute at Harbor-UCLA Medical Center. All participants provided written informed consent. The study protocol is included in the website of our lab.18 This study was registered at ClinicalTrials.gov (Identifier: NCT02797262).
Intervention
The IS group used the IS system for 16 weeks during the intervention period and then was followed post-intervention for an additional 12 weeks. We enhanced the existing IS system with an added pre-dose text reminder on top of its original post-dose message and a personalization of the contents of both pre- and post-dose automatic text reminders (e.g., “it is time to eat your pie”) if medication ingestion was not detected. This system enabled real-time adherence intervention whenever a dose signal was not detected within 1 h of scheduled dosing time. When a participant missed a dose, a post-dose reminder was sent after 1 h from the scheduled dosing time. In addition, an automatic pre-dose reminder was sent at all future scheduled dosing times until the participant sent a stop request for these messages.
Outcomes
Primary outcome – adherence measures
We utilized three measures for adherence: daily measured IS adherence, self-reported adherence and ARV concentration. Self-reported adherence was measured monthly for both groups using the question, “in the past seven days, how many doses did you miss?”. In IS group, adherence of selected ARV was additionally measured by IS system during intervention period of study (i.e., first 16 weeks).
The plasma concentration–time data of tenofovir (TFV) from participants who had tenofovir alafenamide (TAF) in their regimen were used to quantify intra-patient pharmacokinetic (PK) variability as a measure of adherence, as previously described.19 This was because the ARV-concentrated adherence required a common drug be used and TAF-based regimens were the most frequently used ARV in our sample (78%). Three samples of blood at baseline (before, then 2 and 6 h following an observed dose) and then at week 4, 8, 12, 16, 20, 24, and 28 were drawn and then shipped to University of Nebraska Medical Center for PK analyses. Plasma concentrations of TFV were measured by liquid chromatography/tandem mass spectrometry. A population PK model was developed using a nonlinear mixed-effects approach (Phoenix NLME software, version 8.0; Certara L.P., St. Louis, MO, USA). The integrated PK adherence score (IPAM) was calculated.19 Briefly, the discrepancy between measured and predicted TFV concentrations at week 4, 8, 12, 16, 20, 24 and 28 were calculated. The acceptable range for each observed concentration was defined as ± 40% of the predicted concentration. The discrepancy was expressed as the ratio of the observed to predicted concentration. The number of ratios within this range was determined for each participant and the IPAM score was defined as the fraction of available ratios within the acceptable deviation range. IPAM scores could therefore range from 0 to 1; a high score indicates concentrations were closer to predicted values, representing higher levels of adherence, while a low score indicates concentrations below predicted value and lower adherence.
Secondary outcomes
Plasma HIV RNA viral load (VL)
A blood draw was scheduled every four weeks during the intervention period (baseline to week 16) and at the end of post-intervention period (week 28). The lower limits of quantitation for VL were designated as 50 copies/mL, so measures marked as “<20 copies/mL” or “<30 copies/mL”, depending upon which commercial laboratory the test was performed, were all treated as 50 copies/mL in the analysis. If VL was missing, we used the last value carried forward (LVCF) method to impute. We aimed to estimate the association between the change of VL in log10 scale and the average of three-month adherence during intervention period and post-intervention period (week 16–28). We then fitted longitudinal mixed-effects models with data from week 0 to week 28, using 1 month as the lag time between adherence and VL.
Acceptability and satisfaction
All participants in IS group filled out questionnaires developed for this study regarding satisfaction with (i) patch wearing, (ii) IS system, (iii) automated text message reminders, and (iv) tolerability of co-encapsulated ARV and sensor pill at weeks 4, 8, 12 and 16. Each question had a Likert scale response with five categories (strongly disagree, disagree, neutral, agree, or strongly agree) with higher scores reflecting more positive attributes of the IS. See Appendix II for details.
Sample size calculation
Our sample size determination (n = 120) was based on practical considerations of potentially available number of patients for recruitment and the level of resource available for the study. Sample size and power analysis were conducted to evaluate the potential effect size that will be detected with the pre-determined sample size based on the primary outcome of adherence to ARVs. Using repeated measures analysis with standard deviation of adherence 21%,20 intra-subject correlation of 0.1, a type I error of 0.05, a type II error of 0.2 (power 80%), and average number of available data points of six, 60 subjects in IS group (intervention) and 60 subjects in UC group (control) can detect an effect size as small as 0.230 between the IS and UC arms, which is small yet statistically and potentially clinically meaningful. With the assumption of about 8% loss of follow-up, 130 patients were needed at randomization.
Statistical analysis
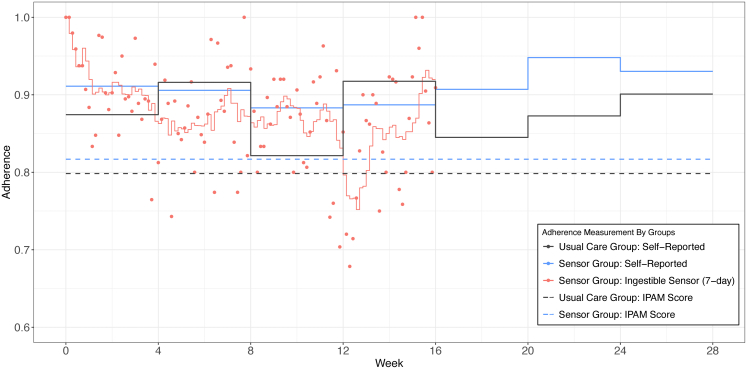
Study participant characteristics between IS group and UC group at baseline were compared with two sample t-tests or Chi-square tests (Fisher test if n < 5). All adherence measures and VL data were summarized for both groups. Bonferroni post-hoc test for multiple comparisons was used and the effective significance level was set accordingly (p < 0.0024). We performed sensitivity analysis for those lost to follow-up and had VL > 50 copies/mL at baseline. All outcomes were summarized in tables by mean (SD), median (IQR) or N (%). We plotted the daily mean IS adherence, self-reported adherence, and ARV concentration adherence. Daily IS-measured was displayed by a scatter plot together with a seven-day moving average during the intervention period. Moving average was calculated by seven-day average adherence with six days’ prior plus the day measured. The seven-day moving average matched with the self-reported adherence administered monthly using 7-day recall interval. The IS adherence and self-reported adherence assessments were compared on the graph. The IPAM score for those on TAF-containing regimen (86 out total of the 112 patients in analytical sample) were also included in the graph.
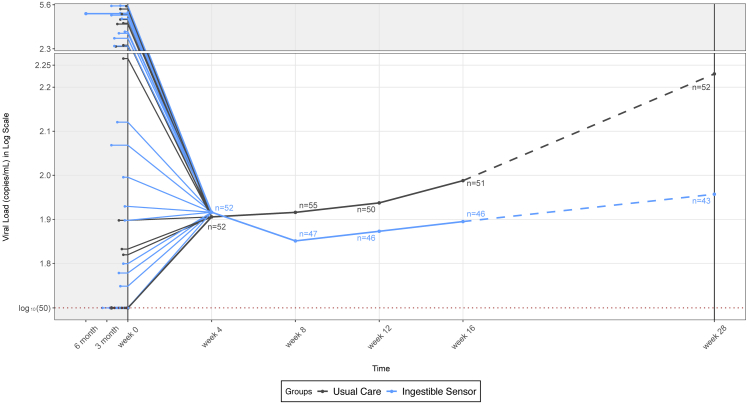
With cluster analysis for the longitudinal IS adherence data series, we applied group-based trajectory model to identify patterns of IS adherence from baseline to week 16, independent from IPAM and self-reported adherence.21 Based on a combination of Bayesian Information Criterion (BIC) and clinical judgement,22 a three-group trajectory model was fitted to best describe the heterogeneity of subgroups with distinct medication taking patterns. We summarized the biological outcomes, such as the plasma ARV concentration adherence assessments and VL, to validate the identified groups by the observed IS adherence and VL. We plotted the longitudinal log-transformed VL to biologically assess how the IS intervention impacted VL. To visualize the entire timeline, we rescaled the time window before week 0 and also used a different y-axis scale for log10(VL) > 2.3 to focus on the change from week 4 to week 28. We modelled the two study periods separately and together: Model 1 analysed the association between VL and adherence during the intervention period (week 4–16); Model 2 analysed the post-intervention period (week 16–28); Model 3 applied longitudinal model for the combined two periods from week 4–28. Due to nonlinearity, a quadratic term of time was added into the model. In Model 3, we controlled for baseline VL and analysed the one-month lag time between adherence level and VL change.
All analyses were conducted using R 4.1.3 and SAS 9.4 software. Statistical significance was determined by p-value<0.05.
Role of the funding source
The funders (National Institute of Health (NIH)/National Institute of Mental Health (NIMH)) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and final responsibility for the decision to submit for publication. Proteus Inc. was contracted as part of this project to provide the Digital Health System and technical assistance, but provided no funding for this project and was not involved in the analysis of the data.
Results
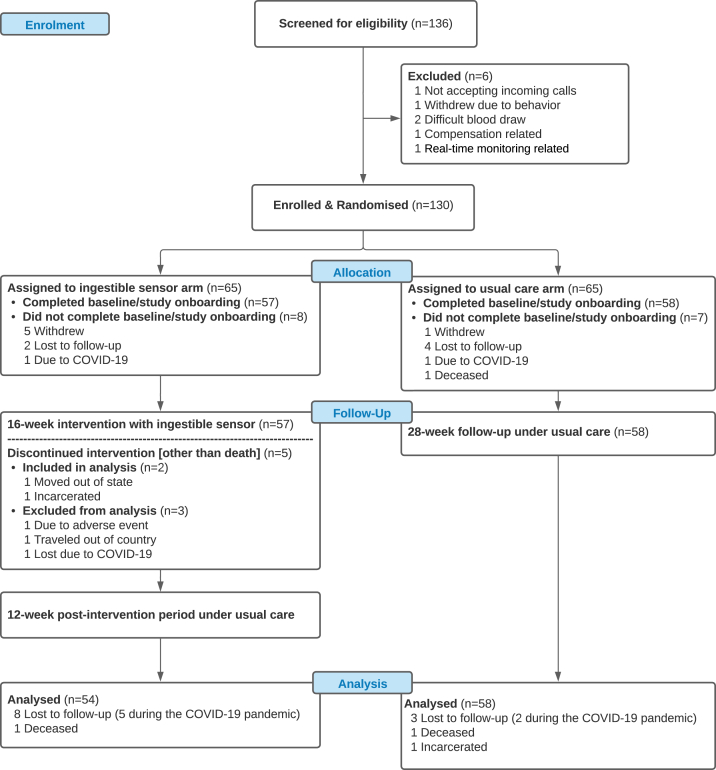
We randomised 130 participants who met the inclusion criteria into IS group (N = 65) or UC group (N = 65). There were 57 participants in the IS group who completed baseline visits (Fig. 1). One participant withdrew due to adverse event (skin irritation after wearing the self-adhesive patch). The irritation resolved after a month without wearing the patch. In UC group, there were 58 participants who completed their baseline visits. Two participants died from HIV progression during the study, none of which were thought to be study-related. A total of seven participants did not complete the study after baseline visits because of the COVID-19 pandemic (five in the IS group and two in the UC group). A total of 112 participants with at least one follow-up data collection after baseline were included in the final analysis with 54 in IS group and 58 in UC group. For IS group, all participants experienced missed doses and the text message intervention was trigged for everyone at some time point during the study. The baseline characteristics are summarized in Table 1 and there was no statistically significant difference between the two study arms.
Fig. 1.
Recruitment and Randomisation of Patients. 136 HIV-infected individuals at least 18 years old with suboptimal ARV adherence at the time of screening were recruited in the main trial. We randomised 130 participants who met the inclusion criteria into IS group (N = 65) or UC group (N = 65). There were 57 participants in the IS group who completed baseline visits. One participant withdrew due to adverse. In UC group, there were 58 participants who completed their baseline visits. Two participants died from HIV progression during the study, none of which were thought to be study-related. A total of seven participants did not complete the study after baseline visits because of the COVID-19 pandemic (five in the IS group and two in the UC group). A total of 112 participants with at least one follow-up data collection after baseline were included in the final analysis with 54 in IS group and 58 in UC group.
Table 1.
Baseline characteristics of participants who had baseline data and at least one follow-up visit.
| Ingestible sensor (N = 54) |
Usual care (N = 58) |
Total (N = 112) |
|
|---|---|---|---|
| Age - mean years (SD) | 46.7 (11.1) | 45.7 (12.4) | 46.2 (11.8) |
| Gender - N (%) | |||
| Female | 5 (9.3%) | 5 (8.6%) | 10 (8.9%) |
| Male | 44 (81.5%) | 43 (74.1%) | 87 (77.7%) |
| Transgender: Male to Female | 5 (9.3%) | 10 (17.2%) | 15 (13.4%) |
| Race and ethnicity - N (%) | |||
| White | 6 (11.1%) | 8 (13.8%) | 14 (12.5%) |
| Black | 24 (44.4%) | 30 (51.7%) | 54 (48.2%) |
| Latino | 19 (35.2%) | 16 (27.6%) | 35 (31.2%) |
| Asian | 1 (1.9%) | 1 (1.7%) | 2 (1.8%) |
| Other | 4 (7.4%) | 3 (5.2%) | 7 (6.3%) |
| Education - N (%) | |||
| 8th grade or less/Some high school but did not graduate | 8 (14.8%) | 10 (17.2%) | 18 (16.1%) |
| High school graduate/Some college but no degree | 38 (70.4%) | 39 (67.2%) | 77 (68.8%) |
| Completed college/More than four-year college degree | 8 (14.8%) | 9 (15.5%) | 17 (15.2%) |
| Employment - N (%) | |||
| Part-time/full-time | 9 (16.7%) | 19 (32.8%) | 28 (25.0%) |
| None/full-time student/retired/disabled | 45 (83.3%) | 39 (67.2%) | 84 (75.0%) |
| HIV + Years - median (IQR) | 14.5 (12.5) | 11.0 (14.0) | 13.5 (13.0) |
| Years under ARV Treatment - median (IQR) | 12.5 (11.5) | 10.0 (9.8) | 10.0 (11.5) |
| History of AIDS Diagnosis - N (%) | |||
| Yes | 12 (22.2%) | 12 (20.7%) | 24 (21.4%) |
| No | 42 (77.8%) | 46 (79.3%) | 88 (78.6%) |
| Detectable VL at Baseline - N (%) | |||
| Less than 50 copies/mL | 35 (64.8%) | 46 (79.3%) | 81 (72.3%) |
| Greater than 50 copies/mL | 19 (35.2%) | 12 (20.7%) | 31 (27.7%) |
| VL (week 0) in Log Scale - median (IQR)a | 1.7 (0.3) | 1.7 (0.0) | 1.7 (0.1) |
| CD4 Cell Count at Baseline (cells/mm3) - mean (SD) | 522.4 (284.4) | 530.5 (283.6) | 526.6 (282.7) |
| Self-reported Adherence (week 0) - mean (SD) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| Current ARV Treatmentb- N (%) | |||
| Single tablet regimens | 39 (72.2%) | 35 (60.3%) | 74 (66.1%) |
| BIC/FTC/TAF | 16 (29.6%) | 17 (29.3%) | 33 (29.5%) |
| 3TC/ABC/DTG | 6 (11.1%) | 8 (13.8%) | 14 (12.5%) |
| EVG/COBI/FTC/TAF | 8 (14.8%) | 6 (10.3%) | 14 (12.5%) |
| FTC/RPV/TAF | 7 (13.0%) | 3 (5.2%) | 10 (8.9%) |
| TDF/FTC/EFV | 2 (3.7%) | 1 (1.7%) | 3 (2.7%) |
| Multiple tablet regimens | 15 (27.8%) | 23 (39.7%) | 38 (33.9%) |
| FTC/TAF + DRV/COBI | 9 (16.7%) | 5 (8.6%) | 14 (12.5%) |
| FTC/TAF + DTG | 1 (1.9%) | 6 (10.3%) | 7 (6.3%) |
| FTC/TAF + DRV + RTV | 1 (1.9%) | 2 (3.4%) | 3 (2.7%) |
| FTC/TDF + DRV/COBI | 0 (0.0%) | 3 (5.2%) | 3 (2.7%) |
| FTC/TAF + ATV/COBI | 1 (1.9%) | 2 (3.4%) | 3 (2.7%) |
| FTC/TDF + DRV + RTV | 0 (0.0%) | 2 (3.4%) | 2 (1.8%) |
| FTC/RPV/TAF + DTG | 1 (1.9%) | 0 (0.0%) | 1 (0.9%) |
| FTC/TDF + DTG | 0 (0.0%) | 1 (1.7%) | 1 (0.9%) |
| 3TC/ABC + DRV + RTV | 1 (1.9%) | 0 (0.0%) | 1 (0.9%) |
| 3TC/ABC + EFV | 1 (1.9%) | 0 (0.0%) | 1 (0.9%) |
| FTC/RPV/TAF + DTG + DRV/COBI | 0 (0.0%) | 1 (1.7%) | 1 (0.9%) |
| FTC/TAF + DTG + DRV/COBI | 0 (0.0%) | 1 (1.7%) | 1 (0.9%) |
VL, viral load; SD, standard deviation; IQR, interquartile range.
For patients with undetectable HIV RNA plasma, viral load were all treated as 50 copies/mL.
ARV, antiretroviral; ABC, abacavir; ATV, atazanavir; BIC, bictegravir; COBI, cobicistat; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; FTC, emtricitabine; RPV, rilpivirine; RTV, ritonavir; 3 TC, lamivudine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
All outcomes of IS and UC groups including adherence measures and VL measures were shown in Table 2. For IS group, the summarized monthly ingestible sensor adherence was from 83.2% to 92.3% during first 16 weeks. The mean IPAM score (TAF-based ARV-concentration adherence) were higher for IS group at 0.817 versus 0.798 for UC group, but not statistically significantly different (0.018, 95% CI: −0.098–0.134, p = 0.75; Student's t-test). Among 54 participants in the IS group, 44 had TAF-based regimens with IPAM score (ARV-concentration adherence) available. In the UC group, 42 out of 58 patients had TAF-based regimens with IPAM score available. We also compared the missing and/or lost to follow-up rate between IS and UC groups, but they are not statistically significantly different. The self-reported adherence was compared at each time point. At week 24, self-reported adherence of IS group is statistically significantly higher than UC group (0.075, 95% CI: 0.004–0.147, p = 0.038; Student's t-test); though it is no longer significant following Bonferroni correction for multiple comparisons. The secondary outcome VL was reported in both continuous, using log transformation, and categorical (VL suppression) in Table 2. At the end of intervention (week 16) and at the end of study (week 28), IS group had lower log VL than UC group, but not statistically significantly different (week 16, geometric mean ratio of VL: 0.807, 95% CI: 0.385–1.690, p = 0.57; week 28, geometric mean ratio of VL: 0.528, 95% CI: 0.209–1.334, p = 0.18). Similar results were found for VL suppression at week 16 (odds ratio: 1.212, 95% CI: 0.387–3.797, p = 0.97) and at week 28 (odds ratio: 2.427, 95% CI: 0.899–6.562, p = 0.12). In addition, we performed the sensitivity analysis to compare VL suppression between IS (N = 19) and UC groups (N = 12) that did not have VL suppression at baseline (Table S2).
Table 2.
Summarised adherence measures and HIV RNA viral load (VL) for participants who had baseline data and at least one follow-up visit.
| Ingestible sensor group | Usual care group | Treatment difference (95% CI) | P-value | |
|---|---|---|---|---|
| Number of participants - N | ||||
| Baselinea | 54 | 58 | .. | .. |
| Week 4 | 53 | 56 | .. | .. |
| Week 8 | 51 | 56 | .. | .. |
| Week 12 | 46 | 52 | .. | .. |
| Week 16 | 46 | 52 | .. | .. |
| Week 20 | 43 | 51 | .. | .. |
| Week 24 | 44 | 50 | .. | .. |
| Week 28 | 43 | 53 | .. | .. |
| IS measured adherence - mean (SD) | ||||
| Week 4 | 0.871 (0.336) | .. | .. | .. |
| Week 8 | 0.871 (0.336) | .. | .. | .. |
| Week 12 | 0.832 (0.375) | .. | .. | .. |
| Week 16 | 0.923 (0.267) | .. | .. | .. |
| IPAM Scoreb(ARV concentration) - mean (SD) | ||||
| 0.817 (0.276) N = 44 (81.5%) |
0.798 (0.267) N = 42 (72.4%) |
0.018 (−0.098, 0.134) | 0.753 | |
| Self-reported adherence - mean (SD) | ||||
| Baselinea | 0.907 (0.145) | 0.874 (0.132) | 0.033 (−0.019, 0.087) | 0.211 |
| Week 4 | 0.911 (0.157) | 0.874 (0.152) | 0.037 (−0.024, 0.097) | 0.229 |
| Week 8 | 0.906 (0.170) | 0.916 (0.135) | −0.010 (−0.071, 0.050) | 0.738 |
| Week 12 | 0.883 (0.170) | 0.821 (0.249) | 0.062 (−0.026, 0.149) | 0.166 |
| Week 16 | 0.887 (0.175) | 0.917 (0.163) | −0.030 (−0.102, 0.041) | 0.401 |
| Week 20 | 0.907 (0.209) | 0.845 (0.232) | 0.062 (−0.030, 0.154) | 0.186 |
| Week 24 | 0.948 (0.088) | 0.873 (0.224) | 0.075 (0.004, 0.147) | 0.038∗ |
| Week 28 | 0.930 (0.118) | 0.901 (0.198) | 0.029 (−0.037, 0.096) | 0.384 |
| Log VL - mean (SD) | ||||
| Baselinea | 2.273 (1.158) | 2.061 (0.954) | 1.629d (0.650, 4.083) | 0.295 |
| Week 4 | 1.916 (0.793) | 1.906 (0.516) | 1.023 (0.562, 1.866) | 0.936 |
| Week 8 | 1.851 (0.680) | 1.916 (0.711) | 0.861 (0.459, 1.618) | 0.640 |
| Week 12 | 1.873 (0.653) | 1.937 (0.671) | 0.863 (0.465, 1.600) | 0.634 |
| Week 16 | 1.895 (0.691) | 1.988 (0.895) | 0.807 (0.385, 1.690) | 0.567 |
| Week 28 | 1.957 (0.816) | 2.230 (1.117) | 0.528 (0.209, 1.334) | 0.174 |
| VL Suppressionc- N (%) | ||||
| Baselinea | 35 (64.8%) | 46 (79.3%) | 0.481e (0.206, 1.120) | 0.133 |
| Week 4 | 46 (86.8%) | 44 (78.6%) | 1.792 (0.646, 4.968) | 0.379 |
| Week 8 | 41 (80.4%) | 46 (82.1%) | 0.891 (0.337, 2.357) | 1 |
| Week 12 | 41 (89.1%) | 42 (80.8%) | 1.952 (0.614, 6.206) | 0.386 |
| Week 16 | 40 (87.0%) | 44 (84.6%) | 1.212 (0.387, 3.797) | 0.967 |
| Week 28 | 36 (83.7%) | 36 (67.9%) | 2.427 (0.899, 6.562) | 0.123 |
∗ The differences between IS and UC groups were compared by Student's t test. P < 0.05 but above the Bonferroni corrected significance threshold.
Only included participants who completed baseline.
IPAM score is the integrated PK adherence score.
VL suppression is defined as ≤50 per copies/mL.
Geometric mean ratio of VL between IS and UC (all such values).
Odds ratio between IS and UC (all such values).
Adherence measures are displayed in Fig. S2. The difference between IS and self-report adherence measures was larger from week 12 to week 16. The variation of self-reported adherence among the IS group was much smaller than that observed in the UC group. In addition, during the post-intervention period (week 16 to week 28), the self-reported adherence was consistently higher in the IS group.
The three model-generated trajectory groups purely based on daily IS medication ingestion events were plotted in Fig. S3. The best profile was Group 3 (N = 40), with consistent optimal adherence (about 90%) during the intervention period. The mean IPAM score is 0.84, with VL decline of 0.35 log10 copies/mL. This group could benefit the most from the intervention using IS system to monitor and maintain their adherence. There were 94% of participants with VL suppressed in this group at the end of the study. The model classified a small number of patients in Group 2 and Group 1. Group 2 had suboptimal adherence (about 70%–90%), with 100% (five of five) VL suppressed at the end of the intervention period. However, only 50% (two of four) had viral suppression at week 28. Group 1 had the lowest adherence among three groups. Among the individuals of Group 1, adherence improved at the beginning, then dropped quickly during the intervention period. The Group 1's IPAM score was the lowest, consistent with and confirming its lowest IS adherence. The viral suppression rate was 60% (three of five) at both week 16 and 28.
Biological assessment of how the IS intervention impacted VL was also demonstrated by plotting the longitudinal log-transformed VL (Fig. S4). It suggested that the improved adherence during the intervention period from baseline to week 16 was associated with reduction in VL. The decay rate was faster at the beginning and then became slower. Overall, the VL in the IS group (blue line) was lower than the UC group (black line), but not statistically significant. From baseline to week 16, the VL decreased by 16.6% for the IS group and 3.6% for the UC group. During the intervention period, VL also trended to decline faster in IS than UC group (coefficient: −0.020, 95% CI: −0.042–0.002, p = 0.08; linear mixed-effects model). During the post-intervention period from week 16 to week 28 (dotted line), VL measured at week 16 and 28 increased with slopes of 0.005 for the IS group and 0.022 for the UC group, a difference that was not statistically significant.
In the models (Table 3), higher ARV adherence level was statistically associated with lower VL in both intervention period (coefficient: −1.512, 95% CI: −2.740 to −0.284, p = 0.016 for Model 1; linear regression) and post-intervention period (coefficient: −2.073, 95% CI: −3.587 to −0.560, p = 0.008 for Model 2; linear regression). However, the relationship between adherence and VL was not statistically significantly different between IS group and UC group in both the intervention and post-intervention periods (i.e., interaction terms were not statistically significant in either Model 1 or Model 2). In Model 3, the positive coefficient of the quadratic term (Week2) reflected in part the upward trend of the VL curve which is consistent with Fig. S4. Overall, the IS group had a lower VL compared with the UC group (coefficient: −0.726, 95% CI: −1.365 to −0.081, p = 0.028; linear mixed-effects model).
Table 3.
Multivariate analysis of plasma HIV RNA during and post-intervention period.
| Models | Variables | Coefficient | 95% CI | P-value | |
|---|---|---|---|---|---|
| Model 1. Intervention period (week 4–16) |
Intercept | 2.013 | 0.810 | 3.216 | 0.001 |
| log plasma HIV RNA at week 4 | 0.672 | 0.385 | 0.959 | <0.001 | |
| Ingestible sensor (ref: UC) | 0.851 | −0.912 | 2.614 | 0.341 | |
| Average adherence at weeks 4, 8, 12,16 | −1.512 | −2.740 | −0.284 | 0.016 | |
| IS∗Adherence | −0.882 | −2.847 | 1.082 | 0.375 | |
| Model 2. Post-intervention period (week 16–28) |
Intercept | 2.964 | 1.418 | 4.510 | <0.001 |
| Log plasma HIV RNA at week 16 | 0.532 | 0.256 | 0.807 | <0.001 | |
| Ingestible sensor (ref: UC) | −0.734 | −3.180 | 1.713 | 0.553 | |
| Average adherence at weeks 16, 20, 24, 28 | −2.073 | −3.587 | −0.560 | 0.008 | |
| IS∗Adherence | 0.670 | −2.013 | 3.352 | 0.621 | |
| Model 3. Longitudinal mixed-effects (week 4–28) |
Intercept | 1.722 | 1.231 | 2.219 | <0.001 |
| Week | −0.003 | −0.030 | 0.023 | 0.799 | |
| Week2 | 0.0003 | −0.001 | 0.001 | 0.454 | |
| Ingestible sensor (ref: UC) | −0.726 | −1.365 | −0.081 | 0.028 | |
| Weekly adherence | −0.423 | −0.900 | 0.048 | 0.082 | |
| IS∗Adherence | 0.723 | 0.024 | 1.413 | 0.042 | |
| Baseline log plasma HIV RNA | 0.274 | 0.188 | 0.360 | <0.001 | |
IS, ingestible sensor; UC, usual care.
Over the intervention period, more than 90% of participants were very satisfied or satisfied overall with the intervention and IS system. The lowest satisfaction (66%) was with the patch of the IS system (only 50% for Group 2 were very satisfied or satisfied, Table 4). We did not see a statistically significant change in the satisfaction score (overall and by domains) over time (Supplement Table S1). We compared the satisfaction score across three trajectory groups and found that those with lowest IS-measured adherence (red line) had the highest percentage of satisfaction about the system, both overall and across the four domains (Table 4).
Table 4.
Percentage of satisfaction of the ingestible sensor system by trajectory groups (post-hoc results).
| Trajectory Group | N | Percentage of satisfaction across week 4–16 by domain (95% Wilson CI) |
||||
|---|---|---|---|---|---|---|
| Overall | System | Text | Patch | Capsule | ||
| 1 | 6 | 100.0% (55.2%, 100.0%) |
100.0% (55.2%, 100.0%) |
100.0% (55.2%, 100.0%) |
80.0% (29.6%, 90.4%) |
83.3% (41.6%, 98.4%) |
| 2 | 7 | 71.4% (35.2%, 92.1%) |
85.7% (46.4%, 99.0%) |
57.1% (25.1%, 84.0%) |
50.0% (16.0%, 74.9%) |
71.4% (35.2%, 92.1%) |
| 3 | 40 | 92.5% (79.3%, 98.0%) |
92.5% (79.3%, 98.0%) |
73.7% (54.4%, 81.9%) |
66.7% (49.4%, 77.9%) |
92.5% (79.3%, 98.0%) |
| Total | 53 | 90.6% (79.2%, 96.2%) |
92.5% (81.5%, 97.4%) |
74.5% (58.3%, 82.1%) |
66.0% (48.8%, 74.0%) |
88.7% (76.9%, 95.0%) |
See Appendix II for survey questions.
Discussion
This study is the initial formal investigation that we are aware of to use an IS system to measure, monitor and enhance adherence to ART in real-time among HIV-infected adult patients. The collection of real-time adherence data was augmented by personalized automated text message reminders when a missed dose of medication was detected. Adherence measured by the IS system trended with self-report with the greatest discordance being in those with the worst adherence at baseline, suggesting that those who had worst adherence at baseline might benefit the most from this new intervention system. As a supplemental assessment, when participants were grouped by initial adherence levels in early intervention at week 4 as optimal (>95%), sub-optimal (75–95%), and non-optimal (<75%) within and across IS and UC arms, we can see that those with lowest adherence at week 4 in the IS group had the lowest predicted mean of log VL, indicating this group benefited the most from the intervention program (see Table S3). This is further supported by data showing improved adherence at weeks 8 and 16 in the IS group, with data demonstrating a statistically significant relationship between levels of adherence and viral suppression seen in the IS group. Furthermore, when examining the impact of adherence on VL across the entire 16-week intervention period, we see the trajectory Group 3 with the best adherence had the largest drop in VL at week 16 (shown in the table within Fig. S3). In the post-hoc trajectory group analysis, measured drug concentrations (IPAM score) tracked with IS adherence assessments. In addition, overall, participants found all aspects of the IS system to be satisfactory throughout the intervention period.
Prior to the current study, nearly all text reminders were pre-dose reminder except Wisepill which provided real-time feedback. We have previously developed a pre-dose and post-dose reminder system using Wisepill technology.23 Most previous adherence technology was incapable to verify the ingestion of medication, but at best measures device-opening activity and inferred adherence. There have been some small studies utilizing ingestible sensor technology to enhance adherence to ARVs in context of pre-exposure prophylaxis, similarly showing early promise.24 In this study we adapted the IS system that measures actual ingestion to further include a customized, real-time pre-dose and post-dose reminder which has significantly leveraged IT-based adherence measurement method to a new significant level with objectiveness and accuracy. The impact of the IS system in this study is a combined result of ongoing real-time measures of adherence, participation in a clinical trial and the active post and pre-dose text message interventions.
There has been considerable research demonstrating the relationship between ARV adherence and VL suppression. Previous work has also demonstrated the challenges in accurately assessing adherence to ARVs using standard methods, such as self-report, pill counts, and pharmacy refill records.25 There have also been electronic monitoring devices used that record pill bottle openings.23,26 Although these methods have variable ability to infer actual pill-taking behavior, none assess actual ingestion and are limited in their abilities to provide real-time measurements of adherence. The new technology from Proteus Digital Health Feedback system (Proteus Digital Health Redwood, CA) was approved by the US Food and Drug Administration in 2012 with a specific indication for real-time monitoring of medication ingestion added in 2015, with additional provision for automated short message system text reminders.10 This technology has been used in those treated for a variety of disease states, including hypertension, mental health conditions, tuberculosis and organ transplantation.27, 28, 29 We adapted this system to measure ARV taking behavior. Of note, this first required assuring that co-encapsulation of medications with IS maintained adequate ARV bioavailability of the medications, which we previously reported.14 We further demonstrated in a single-arm pilot study that the system was felt to be acceptable by 15 HIV-infected individuals with perceived poor adherence to ARVs.16 The current study extended upon this experience demonstrating that using a wide range of commonly used ARVs, a larger group of HIV-infected individuals found the system to be tolerable and acceptable. Collectively, this experience was similar to other mostly smaller pilot studies conducted in those with other medical conditions. We only had one person in the current study that stopped using the patch because of intolerance associated with skin irritation, a problem that might already be reduced as new technology is rolled out by companies developing IS monitoring systems.
Since the completion of the study, Proteus Digital Inc. was acquired by Otsuka Pharmaceuticals Inc., with the same system being continued to track mental health medication ingestion.30 In addition, there are currently several other IS systems on the market that have very similar and/or even newer and better functions and can be used to measure, monitor and enhance adherence to ARVs (e.g., the from ID-Cap™ System from etectRx which also has FDA clearance).31 This study was impacted by the COVID-19 pandemic. Among the 112 participants included in the final analysis, 54 (IS: N = 25) completed the study before the pandemic (finished the last visit at week 28 before lockdown); and 58 (IS: N = 28) had at least one study visit after March 19, 2020 when a lockdown order in Los Angeles County was issued. The distribution of number of patients across the IS and UC arms was similar within the before and the after lockdown cohorts. While new enrolment was stopped, all follow-up visits were conducted per protocol. Although challenging, we do not believe there was a significant impact from COVID pandemic on the conduct of this study.
There have been many strategies tested to enhance adherence in people living with HIV, with limited success. The use of short messaging service text messages has been looked at in several studies with some demonstrating improvement when used in resource-limited settings.32 IS system provided real-time monitoring of actual pill taking that was linked to a personalized text message triggered only when a dose was missed, and then provided as a pre-dose reminder prior to the next dose, with the flexibility that the study participants can ask to stop receiving subsequent text messages. This auto-text message reminder system was found to be acceptable to participants in our previous pilot study,16 as well as during the course of this main trial. The entire IS system, including text message reminders, appeared to be associated with increased adherence in the IS group, and associated with lower VL. While it is difficult to determine which part of the IS system impacted the improvements in adherence and viral suppression, the previous experience with text message reminders is certainly compatible with what was observed in this trial.
This study had some limitations, including fact that despite the study demonstrating that the IS system was considered acceptable by most participants and was associated with improved adherence, it included a selected group of individuals willing to participate in the trial. The study was also open-label due to the nature of the IS system. Nevertheless, for both study groups we collected self-reported adherence and checked plasma drug concentrations. Consequently, both groups were aware that they were being closely monitored for their adherence and their HIV RNA VL and would in part be impacted by white coat effects. Additional limitations include that it was designed to be an early study, in a randomised fashion to gather information regarding the acceptability and accuracy of this cutting-edge intervention in HIV-infected individuals. As a result, the sample size was relatively small. In addition, although the study population was diverse and perceived to be poorly adherent by their provider, the goal was not to identify a population that was highly non-adherent with detectable VL. As a result, only approximately 27% had detectable VL at baseline, which limits the sample size for assessing the impact of adherence on changes in VL.
Although the system appears effective, it is important to note that the IS system is an advanced technology requiring substantial commitment by the participants and is associated with considerable cost. Moreover, this IT based intervention may not work well for those with limited literacy as they may not be able to use the system properly, resulting in data that are incorrect or misleading (e.g., if patch was not paired or worn correctly, it may not connect with the system, leading to missing dose in the system). That being said, it is noteworthy that this study enrolled a very diverse population of underserved individuals living with HIV. Regardless, this intervention would optimally be used in those with adherence behavior barriers, at the greatest risk for, or with documented suboptimal adherence, especially if they are persistently viremic on therapy. Further studies can expand the experience described in this study to those with persistently detectable VL. In addition, consideration could be given to use this technology to bridge those during times of high risk for poor adherence, such as during psychological or socially challenging situations, or when dealing with substance misuse. In addition, the recent approval of new long-acting therapies have potential value for those with difficulty adhering with daily pills, but require that individuals not have acquired resistance to any of the drugs in the regimen and be virally suppressed prior to switch.33, 34, 35 Based on the effectiveness of the system, consideration could be given to using the IS intervention to carefully monitor adherence, enhance adherence and reduce HIV RNA VL, making them eligible for new treatment strategies, including long-acting regimens.
Contributors
All authors were involved in the development of the primary manuscript, interpretation of data, and have read and approved the final version. LS, MG, and KC enrolled participants. HL, JS, CVF, MR, and ESD designed the study. YW, YH, DX and VB did the data analyses, which were reviewed and interpreted by HL, YW, JS, CVF, GMS, MR, and ESD. YW and YH have verified the underlying data. The first draft was written by HL, YW, YH, DX, and JS. All co-authors reviewed and revised the manuscript and approved the final version, and are fully responsible for all content and editorial decisions.
Data sharing statement
This project will adhere to the spirit and letter of the NIH guidelines on data sharing as published online at: NIH Data Sharing Policy, http://grants.nih.gov/grants/policy/data_sharing/. De-identified participant Data will be made available to researchers, following publication and approval by University Intellectual Property policy of any formal requests with a defined analysis plan.
Declaration of interests
ESD has grants/contracts from Gilead, Merck, and ViiV, and has received consulting fees from Gilead, Merck, and ViiV. The other authors declare no competing interests.
Acknowledgements
We acknowledge Pedro Chavez, Ramiro Correa and Avon Cuenca for their support in conducting the clinical trial as well as all of the participants involved in this study. We thank former Proteus Digital Health Inc.'s significant contributions and strong support, including hardware, software, and technical support. This work was supported by grant R01-MH110056 from the National Institute of Mental Health (NIMH)/National Institute of Health (NIH). Y. Wang was in part supported by the NIMH/NIH award T32MH080634. E. Daar was in part supported by the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR001881.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104330.
Appendix A. Supplementary data
Fig. S2.
Fig. S4.
References
- 1.Han W.M., Law M.G., Egger M., et al. Global estimates of viral suppression in children and adolescents and adults on antiretroviral therapy adjusted for missing viral load measurements: a multiregional, retrospective cohort study in 31 countries. Lancet HIV. 2021;8(12):e766–e775. doi: 10.1016/S2352-3018(21)00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisinger R.W., Dieffenbach C.W., Fauci A.S. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321(5):451–452. doi: 10.1001/jama.2018.21167. [DOI] [PubMed] [Google Scholar]
- 3.Schaecher K.L. The importance of treatment adherence in HIV. Am J Manag Care. 2013;19(12 Suppl):s231–s237. [PubMed] [Google Scholar]
- 4.Subbaraman R., de Mondesert L., Musiimenta A., et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health. 2018;3(5) doi: 10.1136/bmjgh-2018-001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonacini M., Kim Y., Pitney C., McKoin L., Tran M., Landis C. Wirelessly observed therapy to optimize adherence and target interventions for oral hepatitis C treatment: observational pilot study. J Med Internet Res. 2020;22(4) doi: 10.2196/15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rode R., Vrijens B., Niemi K., Wikstrom K., Heuser R., Podsadecki T. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. 2005. 2005. Differences in treatment compliance between lopinavir/ritonavir (LPV/r) given once (QD) versus twice (BID) daily do not affect virologic or immunologic outcomes; pp. 2012–2016. Washington, DC. [Google Scholar]
- 7.Castillo-Mancilla J.R., Zheng J.-H., Rower J.E., et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retrovir. 2013;29(2):384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olds P.K., Kiwanuka J.P., Nansera D., et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care. 2015;27(3):327–332. doi: 10.1080/09540121.2014.983452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spinelli M.A., Haberer J.E., Chai P.R., Castillo-Mancilla J., Anderson P.L., Gandhi M. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV AIDS Rep. 2020;17(4):301–314. doi: 10.1007/s11904-020-00502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafezi H., Robertson T.L., Moon G.D., Au-Yeung K.-Y., Zdeblick M.J., Savage G.M. An ingestible sensor for measuring medication adherence. IEEE (Inst Electr Electron Eng) Trans Biomed Eng. 2014;62(1):99–109. doi: 10.1109/TBME.2014.2341272. [DOI] [PubMed] [Google Scholar]
- 11.Frias J., Virdi N., Raja P., Kim Y., Savage G., Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res. 2017;19(7):e246. doi: 10.2196/jmir.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Miguel Beriain I., Morla González M. Digital pills' for mental diseases: an ethical and social analysis of the issues behind the concept. J Law Biosci. 2020;7(1):lsaa040. doi: 10.1093/jlb/lsaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiCarlo L., Moon G., Intondi A., et al. A digital health solution for using and managing medications: wirelessly observed therapy. IEEE Pulse. 2012;3(5):23–26. doi: 10.1109/MPUL.2012.2205777. [DOI] [PubMed] [Google Scholar]
- 14.Liu H., Daar E., Wang Y., et al. Pharmacokinetics of coencapsulated antiretrovirals with ingestible sensors. AIDS Res Hum Retrovir. 2020;36(1):65–74. doi: 10.1089/aid.2019.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamal S., Rosen M.I., Lazar C., et al. Perceptions of people living with HIV and HIV healthcare providers on real-time measuring and monitoring of antiretroviral adherence using ingestible sensors: a qualitative study. AIDS Res Treat. 2020;2020 doi: 10.1155/2020/1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daar E.S., Rosen M.I., Wang Y., et al. Real-time and wireless assessment of adherence to antiretroviral therapy with Co-encapsulated ingestion sensor in HIV-infected patients: a pilot study. Clin Transl Sci. 2020;13(1):189–194. doi: 10.1111/cts.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright A. REDCap: a tool for the electronic capture of research data. J Electron Resour Med Libr. 2016;13(4):197–201. [Google Scholar]
- 18.Daar E., Liu H. 2018. Measuring and Monitoring Adherence to ART with Pill Ingestible Sensor System.https://dentistry.ucla.edu/sites/default/files/2021-10/Liu%27s%20Lab_Investigator%20Protocol%20-%2012-26-18.pdf [Google Scholar]
- 19.Brundage R.C., Yong F.H., Fenton T., Spector S.A., Starr S.E., Fletcher C.V. Intrapatient variability of efavirenz concentrations as a predictor of virologic response to antiretroviral therapy. Antimicrob Agents Chemother. 2004;48(3):979–984. doi: 10.1128/AAC.48.3.979-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams E.C., McGinnis K.A., Rubinsky A.D., et al. Alcohol use and antiretroviral adherence among patients living with HIV: is change in alcohol use associated with change in adherence? AIDS Behav. 2021;25(1):203–214. doi: 10.1007/s10461-020-02950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones B.L., Nagin D.S., Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Socio Methods Res. 2001;29(3):374–393. [Google Scholar]
- 22.Raftery A.E. Bayesian model selection in social research. Socio Methodol. 1995:111–163. [Google Scholar]
- 23.Moore B.A., Rosen M.I., Wang Y., et al. A remotely-delivered CBT and contingency management therapy for substance using people with HIV. AIDS Behav. 2015;19(2):156–162. doi: 10.1007/s10461-014-0990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai P.R., Mohamed Y., Bustamante M.J., et al. DigiPrEP: a pilot trial to evaluate the feasibility, acceptability, and accuracy of a digital pill system to measure PrEP adherence in men who have sex with men who use substances. JAIDS. 2022;89(2):e5–e15. doi: 10.1097/QAI.0000000000002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterberg L., Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 26.Blaschke T.F., Osterberg L., Vrijens B., Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 27.Eisenberger U., Wüthrich R.P., Bock A., et al. Medication adherence assessment: high accuracy of the new Ingestible Sensor System in kidney transplants. Transplantation. 2013;96(3):245. doi: 10.1097/TP.0b013e31829b7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters-Strickland T., Pestreich L., Hatch A., et al. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatric Dis Treat. 2016;12:2587. doi: 10.2147/NDT.S116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiCarlo L.A., Weinstein R.L., Morimoto C.B., et al. Patient-centered home care using digital medicine and telemetric data for hypertension: feasibility and acceptability of objective ambulatory assessment. J Clin Hypertens. 2016;18(9):901–906. doi: 10.1111/jch.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration . 2017. FDA approves pill with sensor that digitally tracks if patients have ingested their medication.https://www.fda.gov/news-events/press-announcements/fda-approves-pill-sensor-digitally-tracks-if-patients-have-ingested-their-medication [Google Scholar]
- 31.etectRx . 2019. etectRx Announces U.S. FDA Clearance of Novel Ingestible Event Marker.https://etectrx.com/etectrx-announces-u-s-fda-clearance-of-novel-ingestible-event-marker/ [Google Scholar]
- 32.Lester R., Karanja S. Mobile phones: exceptional tools for HIV/AIDS, health, and crisis management. Lancet Infect Dis. 2008;8(12):738–739. doi: 10.1016/S1473-3099(08)70265-2. [DOI] [PubMed] [Google Scholar]
- 33.Overton E.T., Richmond G., Rizzardini G., et al. Cabotegravir+ rilpivirine every 2 Months is noninferior to monthly: ATLAS-2M study. Infect Chemother. 2020;52 [Google Scholar]
- 34.Swindells S., Andrade-Villanueva J.-F., Richmond G.J., et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382(12):1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 35.Orkin C., Arasteh K., Górgolas Hernández-Mora M., et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.