Abstract
Arbuscular mycorrhizal fungi (AMFs) and biochar are two common alternatives to chemical fertilizers applied to soil to improve crop growth. However, their interactive effects on maize (Zea mays L.) growth, nutrient absorption, and physiological properties remain poorly understood. In this study, maize plants were grown in pots treated with biochar and AMFs Diversispora eburnea, alone or in combination. The results showed that the individual application of AMFs or biochar increased maize growth and mineral contents in shoots and roots (including P, K, Ca, Na, Mg, Fe, Mn, and Zn). The chlorophyll a, chlorophyll b, and total chlorophyll contents in AMF-treated leaves were significantly higher than those in the control treatment group. However, AMFs had no synergistic effects with biochar on maize growth, nutrient absorption, nor photosynthetic pigments. The application of biochar to the soil significantly reduced mycorrhizal colonization by 40.58% in the root tissues, accompanied by a significant decline in mycorrhizal dependency from 80.57% to −28.67%. We conclude that the application of biochar and AMFs can affect maize growth, nutrient uptake, and physiological properties. Our study can provide vital information for further resource use optimization in agroecosystems.
Keywords: AMF, biochar addition, interactive effect, nutrient uptake, physiological properties
1. Introduction
Given the limited cultivated land area (38% of the global land surface), escalating global population pressure, and extreme weather, the sufficiency of the global food supply to feed the human population has been challenged [1]. To address this challenge, excessive chemical fertilizers have been applied to fields to increase crop yields [2]. According to data from China Statistical Bureau, China is the largest consumer of agricultural chemicals in the world, with more than 30% of global fertilizers being applied to only 9% of the world’s cropland. However, the excessive use of chemical fertilizers has resulted in considerable adverse environmental impacts, such as land and water eutrophication, greenhouse gas emissions, and biodiversity loss [3,4]. Therefore, it is critical to develop alternatives to chemical fertilizers to improve crop productivity without threatening soil health and agricultural ecosystems.
In recent years, soil microorganisms have been presented as safe and effective biofertilizers [5]. Arbuscular mycorrhizal fungi (AMFs) are essential components of the rhizosphere microflora in natural environments, accounting for 20–30% of total soil microbial biomass and forming obligatory mutualism with 80% of vascular plants [6]. In addition to increasing the absorptive surface area of their host plant root systems, AMFs can promote nutrient uptake by transferring nutrients (particularly P) from the soil to the crop via their external mycelium [7]. AMFs can also promote soil photosynthetic activity, improve the water–plant relationship, and cause biochemical and physiological changes in plants using a variety of compounds and molecules, such as auxins, cytokinins, gibberellins, and bioactive organic compounds [8,9].
Biochar is a carbon-rich substance that is produced via the pyrolysis of different organic materials in an oxygen-limited environment. It has a unique porous structure with great biochemical stability, as well as a high adsorption capacity for sorbing and releasing mineral nutrients owing to its large specific surface area [10]. When added to soil, biochar can improve soil organic carbon (SOC) content, cation exchange capacity (CEC), soil porosity, and the bioavailability of minerals such as K, Ca, Mg, and P, resulting in improved root development and nutrient uptake [11,12,13]. Biochar may also affect soil nutrients by altering microbial and fungal metabolism as well as diversity, both of which can influence the availability and quality of soil nutrients [14]. Moreover, the majority of C in biochar persists in the soil for hundreds to thousands of years, making biochar attractive as a soil amendment option to replace mineral fertilizers [15].
Previous studies have shown that soil amendment using biochar can promote the abundance and infection rate of AMFs [16,17] by modifying soil properties and altering microbial activity [16,17,18]. Biochar coupled with AMFs has been demonstrated to boost plant growth, decrease disease severity, and increase productivity [19]. Under soil water deficit conditions, biochar application can increase soil water-holding capacity and promote the growth of AMFs [20]. Using a combination of biochar and AMFs can result in an increase in total root length and root development in strawberry crops [21]. Despite that, knowledge on the combined effect of AMFs and biochar on plant growth, nutrient absorption, and physiological properties is still deficient [22]. Therefore, testing the combination of biochar and AMFs could provide vital information for further resource use optimization in agroecosystems.
Maize (Zea mays L.) is one of the most important cereal crops in agricultural ecosystems, both in terms of cultivated area and amount produced worldwide. The demand for maize is increasing and will likely continue to increase due to the industrial use of maize by-products, biofuels, and food supply for the growing human population [23]. In the present study, maize seedlings grown in soil were inoculated with AMFs or biochar to evaluate whether the use of biochar and AMFs, alone or in combination, improved nutrient uptake, physiological properties, and maize growth. Based on these objectives, we hypothesized that (1) the individual application of AMFs or biochar promotes maize growth, nutrient uptake, and physiological properties compared with the control and (2) the combined application of AMF and biochar promotes maize performance more than either treatment alone.
2. Materials and Methods
2.1. Materials
Topsoil (0–20 cm) was collected from an experimental field at Henan University, Henan Province, China (34°49′3″ N, 114°18′38″ E). To remove plant residue and stones, the soil was homogenized and sieved through a 2 mm mesh and was then autoclaved at 120 °C for 2 h. The soil (pH 8.32) contained 13.6 g kg−1 of organic carbon, 1.1 g kg−1 of total N, 0.50 mg kg−1 of total P, 2.75 mg kg−1 of NH4+-N, 45.39 mg kg−1 of NO3−-N, and 3.7 mg kg−1 of available P.
The AMF species, Diversispora eburnea BGC HK02C, used in the present study was provided by Beijing Academy of Agriculture and Forestry, Beijing, China. The AMF species was identified based on morphological characteristics and 18S rDNA sequence analysis, and the sequences were deposited in GenBank (accession number KT152858).
The inocula were propagated on maize (Zea mays L.) in autoclaved soil/vermiculite (1:1, v/v) substrate for six months. The inoculum was composed of spores (50 spores per gram of soil), hyphae, root pieces, and soil.
The biochar used in this study was prepared using maize straw. Cleaned and air-dried maize straw was cut into small segments (~10 cm in length), fed into a biochar reactor with a tight-fitting lid, and pyrolyzed at 550 °C for 4 h in a muffle furnace. The biochar was then dried, crushed, and passed through a 2 mm mesh. The biochar had a total C content of 597.7 g kg−1, total N content of 13.4 g kg−1, total P content of 2.47 g kg−1, and CEC of 17.0 cmol kg−1.
2.2. Experimental Setup
The experiment was set up using a completely randomized factorial design. Four amendment treatments were included in this experiment: (1) CK (control, without application of AMFs or biochar); (2) A (individual application of AMFs); (3) B (individual application of biochar); and (4) AB (combined application of AMFs and biochar). Each treatment had 5 replicates, yielding 20 experimental pots. Biochar was applied to the soil at a rate of 3% (w/w) in the biochar treatments. Each pot was inoculated with 50 g of the inoculum for mycorrhizal treatments or 50 g of the autoclaved inoculum for non-mycorrhizal treatments. A total of 10 mL of microbial filtrate (10 μm pore size) was applied to provide similar microflora. Maize seeds (variety: Zhengdan 958) were surface-sterilized with 0.5% NaClO solution (v/v) for 5 min, rinsed in distilled water, and then germinated on wet filter paper in a sterile culture dish at 25 °C. Four pre-germinated seeds were sown in each pot (30 cm in height and 13.5 cm in diameter) containing 2 kg of soil. Maize seedlings were thinned to two uniform seedlings per pot after emergence.
The experiment was conducted from 14 May to 14 July 2021. Maize seedlings were watered to 65% of their water-holding capacity daily. The greenhouse temperature was in the range of 15–28 °C during the experimental period. No additional nutrients were used in the experiments. The positions of all trial pots were randomized every two weeks. Maize height, basal diameter, and leaf number were measured every two weeks.
2.3. Harvest and Analysis
During harvest, maize shoots were cut at the soil surface, and roots were carefully separated from the soil. The roots were divided into two subsamples, and the fresh mass was determined for both. The shoots and one root subsample were oven-dried at 80 °C to a constant weight and then weighed. The other root subsample was used for AMF colonization observation, and its dry mass was calculated by multiplying the fresh mass by the dry-to-fresh mass ratio of the oven-dried root subsamples. Samples (0.5 g) of finely ground tissues (roots and shoots) were wet-digested using a mixture of concentrated HNO3 and HClO4 (4:1 v/v, guaranteed reagent), and the digests were adjusted to a final volume of 50 mL using deionized water. The P, K, Ca, Na, Mg, Fe, Mn, and Zn contents in shoots and roots were determined using inductively coupled plasma-atomic emission spectrometry (ICP-AES, Thermo Fisher Scientific, Waltham, England). AMF colonization was quantified under a microscope (×200) using the line intersect method after the roots had been cleared in 10% KOH solution (w/v) and stained with acid fuchsin [24]. Soil pH was measured using a pH meter in a 1:2.5 (w/v) suspension of soil and deionized water. Soil available N (NH4+-N and NO3−-N) was determined using SmartChem 200 automatic chemical analyzer (AMS Group Westco, Rome, Italy) and measured using dual wavelength spectrophotometry and the indophenol blue method. Available P (A-P) was extracted using 0.5 M NaHCO3 at a pH of 8.5 and quantified using SmartChem 200 automatic chemical analyzer. Soil dissolved organic carbon (DOC) was determined using multi N/C 2100/2100S TOC Analyzer (Element, Jena, Germany). Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) were determined using the chloroform fumigation method and quantified using multi N/C 2100/2100S TOC Analyzer (Element, Jena, Germany). The soil alkaline phosphatase activity, and chlorophyll and carotenoid contents in the leaves were determined using BC0285, BC0995, and BC4335 assay kits, respectively (Solarbio, Beijing, China). The enzyme activity unit of alkaline phosphatase was defined as 1 nanomole of phenol released per gram of soil per day at 37 °C (nmol·g−1·d−1).
2.4. Data Analysis
SPSS software (version 25.0) was used for the data analysis. A three-way ANOVA was applied to examine the effects of AMFs, biochar, sampling time, and their interactions on plant height, basal diameter, and leaf number of maize. A two-way ANOVA was applied to examine the effects of AMFs, biochar application, and their interaction on maize shoot, root, and total biomass; root–shoot ratio; photosynthetic pigments; nutrient uptake; soil chemical properties; and alkaline phosphatase activity. A one-way ANOVA followed by a Duncan’s test was used to evaluate significant differences among different treatments at p < 0.05. The mycorrhizal dependency (MD) presents the degree to which a plant relies upon the mycorrhizal condition to produce its maximum growth at a given level of soil fertility. It is calculated using the following formula:
where TBAMF and TBNM represent the total biomass of AMF-inoculated maize and non-inoculated maize, respectively.
3. Results
3.1. Mycorrhizal Colonization Rate
AMF colonization was not observed in the roots of non-inoculated maize at harvest. Inoculated maize had a mycorrhizal colonization rate ranging from 31.52% to 52.77%. The application of biochar decreased the mycorrhizal colonization rate significantly, by 40.58%, relative to that of the AMF-only treatment (Table 1). The mycorrhizal colonization rate was significantly negatively correlated with soil available P and positively correlated with chlorophyll a content, according to the Pearson correlation analysis (Figure S1).
Table 1.
Mycorrhizal colonization rates of maize following different treatments.
| Treatment | Mycorrhizal Colonization Rate |
|---|---|
| A | 52.77 ± 4.74 a |
| AB | 31.52 ± 2.47 b |
Data represent the means of five replicates ±SEs. Values followed by different letters were significantly different according to Duncan’s test at p < 0.05. A, arbuscular mycorrhizal fungi; AB, arbuscular mycorrhizal fungi and biochar.
3.2. Soil Chemical Characteristics and Phosphatase Activity
The two-way ANOVA showed that soil NO3−-N, available P, DOC, and MBC were significantly influenced by AMFs (Table 2). Soil NH4+-N, NO3−-N, available P, DOC, MBC, and MBN were significantly influenced by biochar (Table 2). Soil NH4+-N, NO3−-N, available P, MBC, and alkaline phosphatase activity were significantly influenced by the interaction between AMFs and biochar (Table 2).
Table 2.
Probabilities of significance calculated using a two-way ANOVA for the main treatment effects and treatment interactions of the measured variables.
| Variable Measured | A | B | A × B | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| pH | 0.14 | 0.71 | 0.76 | 0.40 | 0.69 | 0.42 |
| NH4+-N | 2.58 | 0.13 | 12.40 | ** | 18.11 | *** |
| NO3−-N | 159.78 | *** | 200.84 | *** | 135.80 | *** |
| A-P | 6.24 | * | 108.27 | *** | 5.47 | * |
| DOC | 8.48 | * | 5.08 | * | 0.54 | 0.47 |
| MBC | 19.66 | *** | 22.15 | *** | 35.43 | *** |
| MBN | 0.22 | 0.65 | 11.31 | ** | 1.55 | 0.23 |
| Alkaline phosphatase | 0.13 | 0.72 | 1.07 | 0.32 | 5.00 | * |
| Shoot biomass | 32.80 | * | 205.00 | *** | 84.86 | *** |
| Root biomass | 3.33 | 0.09 | 194.54 | *** | 52.22 | *** |
| Total biomass | 23.66 | *** | 216.35 | *** | 81.37 | *** |
| Root–shoot ratio | 3.72 | 0.07 | 58.41 | *** | 6.25 | * |
| Chlorophyll a | 0.97 | 0.34 | 0.46 | 0.51 | 8.70 | ** |
| Chlorophyll b | 0.45 | 0.51 | 2.64 | 0.12 | 4.83 | * |
| Total chlorophyll | 0.58 | 0.46 | 3.08 | 0.10 | 8.51 | ** |
| Carotenoid | 0.88 | 0.36 | 9.47 | ** | 5.80 | * |
| Shoot P content | 11.26 | ** | 82.08 | *** | 22.29 | *** |
| Shoot K content | 23.14 | *** | 343.79 | *** | 37.92 | *** |
| Shoot Ca content | 13.18 | * | 35.70 | *** | 34.25 | *** |
| Shoot Na content | 10.14 | ** | 12.77 | ** | 5.51 | * |
| Shoot Mg content | 49.56 | *** | 25.01 | *** | 96.83 | *** |
| Shoot Fe content | 9.07 | ** | 25.71 | *** | 8.69 | ** |
| Shoot Mn content | 16.84 | *** | 193.23 | *** | 16.84 | *** |
| Shoot Zn content | 22.26 | *** | 39.69 | *** | 54.48 | *** |
| Root P content | 2.12 | 0.17 | 85.91 | *** | 14.89 | ** |
| Root K content | 48.00 | *** | 170.26 | *** | 63.18 | *** |
| Root Ca content | 5.32 | * | 89.56 | *** | 32.50 | *** |
| Root Na content | 3.46 | 0.08 | 137.64 | *** | 56.45 | *** |
| Root Mg content | 2.59 | 0.13 | 187.01 | *** | 48.65 | *** |
| Root Fe content | 2.34 | 0.15 | 96.60 | *** | 42.60 | *** |
| Root Mn content | 1.28 | 0.28 | 242.22 | *** | 69.45 | *** |
| Root Zn content | 0.05 | 0.83 | 45.92 | *** | 19.15 | *** |
A, arbuscular mycorrhizal fungi; B, biochar; A × B, AMFs and biochar interaction; A-P, available phosphorus; DOC, dissolved organic carbon; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen. * p < 0.05; ** p < 0.01; *** p < 0.001.
Soil NH4+-N, NO3−-N, and MBC were significantly decreased by both individual and dual applications of AMFs and biochar compared with the control treatment (Table 3). Furthermore, MBN was significantly decreased by 33.3% in the biochar treatment group compared with the control treatment group (Table 3). In contrast, the dual application of AMFs and biochar significantly raised soil DOC by 49.9%, while biochar alone and the combination of AMFs and biochar increased available P by factors of 11.1 and 8.1, respectively (Table 3). Soil alkaline phosphatase activity was significantly increased by AMF and biochar application by factors of 4.93 and 5.02, respectively (Table 3).
Table 3.
Effects of arbuscular mycorrhizal fungi and biochar on soil properties and alkaline phosphatase activity.
| Treatment | pH | NH4+-N (mg kg−1) |
NO3−-N (mg kg−1) |
A-P (mg kg−1) |
DOC (mg kg−1) |
MBC (mg kg−1) |
MBN (mg kg−1) |
Alkaline Phosphatase (nmol·g−1·d−1) |
|---|---|---|---|---|---|---|---|---|
| CK | 8.39 ± 0.06 a | 5.51 ± 0.49 a | 23.93 ± 1.3 a | 7.12 ± 0.69 c | 71.41 ± 6.84 b | 299.01 ± 18.18 a | 18.6 ± 1.76 a | 341.23 ± 59.56 b |
| A | 8.49 ± 0.04 a | 3.59 ± 0.35 b | 4.48 ± 0.75 b | 6.14 ± 0.24 c | 86.44 ± 9.04 ab | 147.43 ± 12.9 c | 17.56 ± 1.09 a | 2022.99 ± 253.72 a |
| B | 8.37 ± 0.18 a | 2.97 ± 0.04 b | 3.25 ± 0.37 b | 85.88 ± 12.21 a | 81.9 ± 3.29 b | 143.45 ± 11.88 c | 12.4 ± 1.42 b | 2052.78 ± 792.99 a |
| AB | 8.32 ± 0.05 a | 3.83 ± 0.26 b | 2.46 ± 0.41 b | 55.99 ± 1.74 b | 107.06 ± 7.16 a | 165.61 ± 14.63 c | 14.7 ± 0.96 ab | 1162.71 ± 310.19 ab |
Data are means of five replicates ±SEs. Values within a column followed by different letters were significantly different according to Duncan’s test at p < 0.05. CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar; A-P, available phosphorus; DOC, dissolved organic carbon; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen.
3.3. Maize Growth and Mycorrhizal Dependency
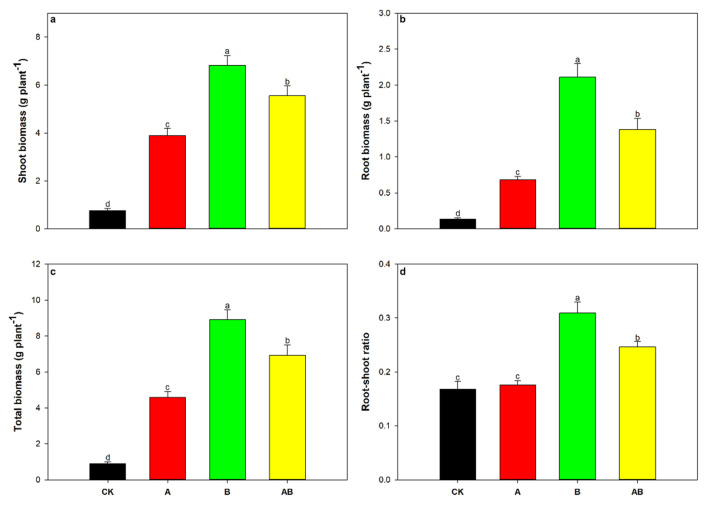
The two-way ANOVA showed that maize shoot biomass, root biomass, total biomass, and root–shoot ratio were significantly influenced by biochar, as well as by the interaction between biochar and AMFs (Table 2, Figure 1a–d). The shoot and total biomass were also significantly influenced by AMFs alone (Table 2, Figure 1a,c). Compared with the control treatment, individual or dual application of AMFs and biochar significantly increased the shoot biomass of maize by factors of 4.2, 8.0, and 6.3, respectively (Figure 1a). Shoot biomass was the greatest when biochar was applied, followed by the combination of AMFs and biochar, AMFs alone, and the control treatment (Figure 1a). A similar trend was also observed in the root and total biomass of maize (Figure 1b,c). Compared with the control treatment, the root–shoot ratio was significantly increased by the application of biochar alone and the dual application by 82.4% and 47.1%, respectively (Figure 1d). Moreover, the mycorrhizal dependency of maize was 80.57% when biochar was absent, but it decreased to −28.67% after biochar addition (Table 4).
Figure 1.
Effects of arbuscular mycorrhizal fungi and biochar on maize (a) shoot biomass, (b) root biomass, (c) total biomass, and (d) root–shoot ratio. Data are means of five replicates ±SEs. Different letters above the columns indicate significant differences according to Duncan’s test at p < 0.05. CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar.
Table 4.
Mycorrhizal dependency of inoculated maize following different biochar treatments.
| Treatment | Mycorrhizal Dependency |
|---|---|
| Without biochar | 80.57 ± 1.63a |
| With biochar | −28.67 ± 3.93b |
Data represent the means of five replicates ±SEs. Values followed by different letters were significantly different according to Duncan’s test at p < 0.05.
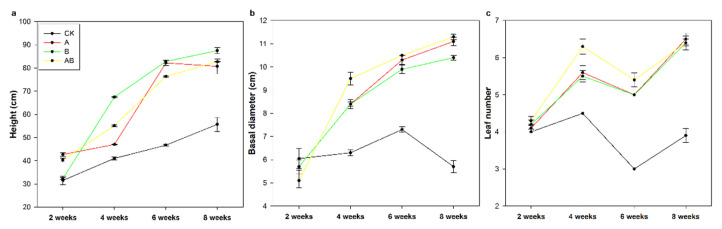
The three-way ANOVA showed that the plant height, basal diameter, and leaf number of maize were significantly influenced by AMFs, biochar, sampling time, and their interactions (Table 5). Maize height was significantly improved by individual and combined applications of AMFs and biochar for the entire growth period, but biochar had no significant effects on maize height during the first two weeks (Figure 2a). Maize basal diameter and leaf number were significantly increased by individual and dual applications of AMFs and biochar after the fourth week (Figure 2b,c). In addition, there were no significant differences in maize height nor leaf number among the different application treatments at harvest (Figure 2a,c). However, the basal diameter of maize was significantly lower in the biochar treatment group than in the AMF application group or the combined treatment group at harvest (Figure 2b).
Table 5.
Probabilities of significance calculated using a three-way ANOVA for the main treatment effects and treatment interactions of maize height, basal diameter, and leaf number.
| Variable Measured | A | B | T | A × B | A × T | B × T | A × B × T | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Maize height | 135.17 | *** | 330.47 | *** | 761.03 | *** | 305.11 | *** | 32.73 | *** | 42.21 | *** | 38.15 | *** |
| Basal diameter | 202.22 | *** | 135.62 | *** | 300.61 | *** | 90.99 | *** | 49.11 | *** | 33.32 | *** | 19.12 | *** |
| Leaf number | 180.04 | *** | 170.04 | *** | 133.18 | *** | 72.32 | *** | 16.99 | *** | 12.70 | *** | 20.70 | *** |
A, arbuscular mycorrhizal fungi; B, biochar; T, sampling time; A × B, AMFs and biochar interaction; A × T, AMFs and sampling time interaction; B × T, biochar and sampling time interaction; A × B × T, AMF, biochar and sampling time interaction. *** p < 0.001.
Figure 2.
Effects of arbuscular mycorrhizal fungi and biochar on (a) maize height, (b) basal diameter, and (c) leaf number. Data are means of five replicates ±SEs. CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar.
3.4. Photosynthetic Pigments
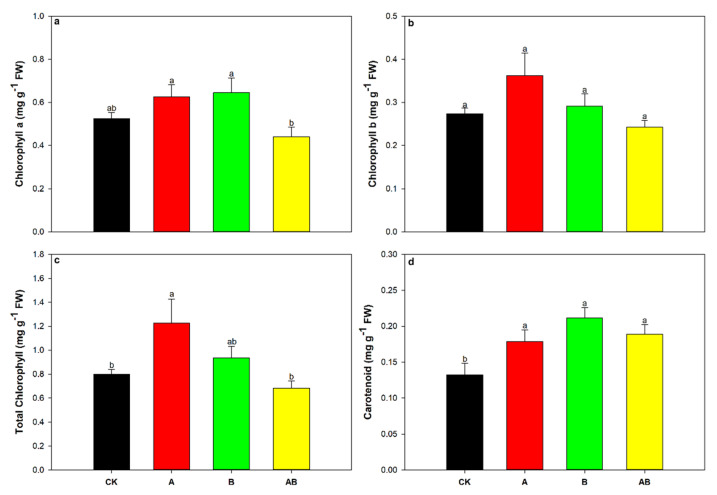
Chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content in maize leaves were significantly influenced by the interaction between AMFs and biochar (Table 2). The carotenoid content was also significantly influenced by biochar alone (Table 2). The chlorophyll a, chlorophyll b, and total chlorophyll contents were significantly enhanced by the individual application of AMFs compared with the control (Figure 3a–c). However, the individual application of biochar had no significant effects on the chlorophyll a, chlorophyll b, nor total chlorophyll content (Figure 3a–c). There was a slight increase in the carotenoid content in the individual and dual application treatments, but the effect was not statistically significant (Figure 3d).
Figure 3.
Effects of AMFs and biochar on (a) chlorophyll a, (b) chlorophyll b, (c) total chlorophyll, and (d) carotenoid contents. Data are means of five replicates ±SEs. Different letters above the columns indicate significant differences according to Duncan’s test at p < 0.05. FW, fresh weight; CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar.
3.5. Mazie Nutrient Uptake
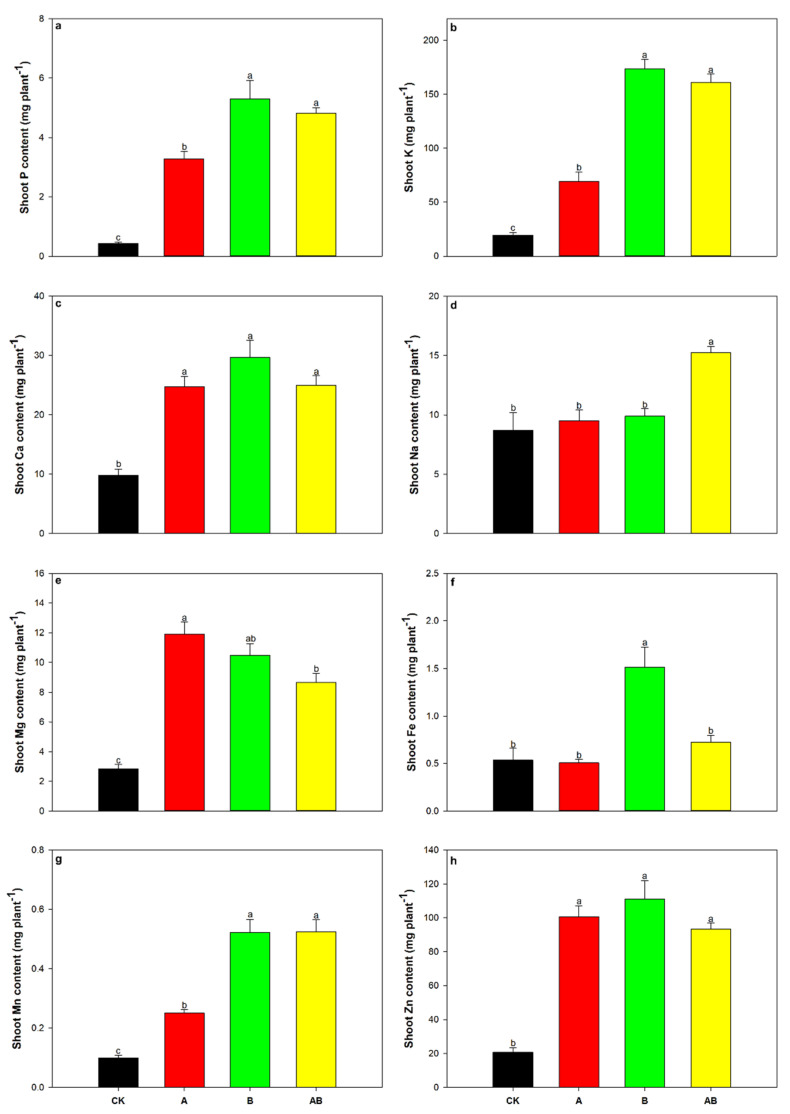
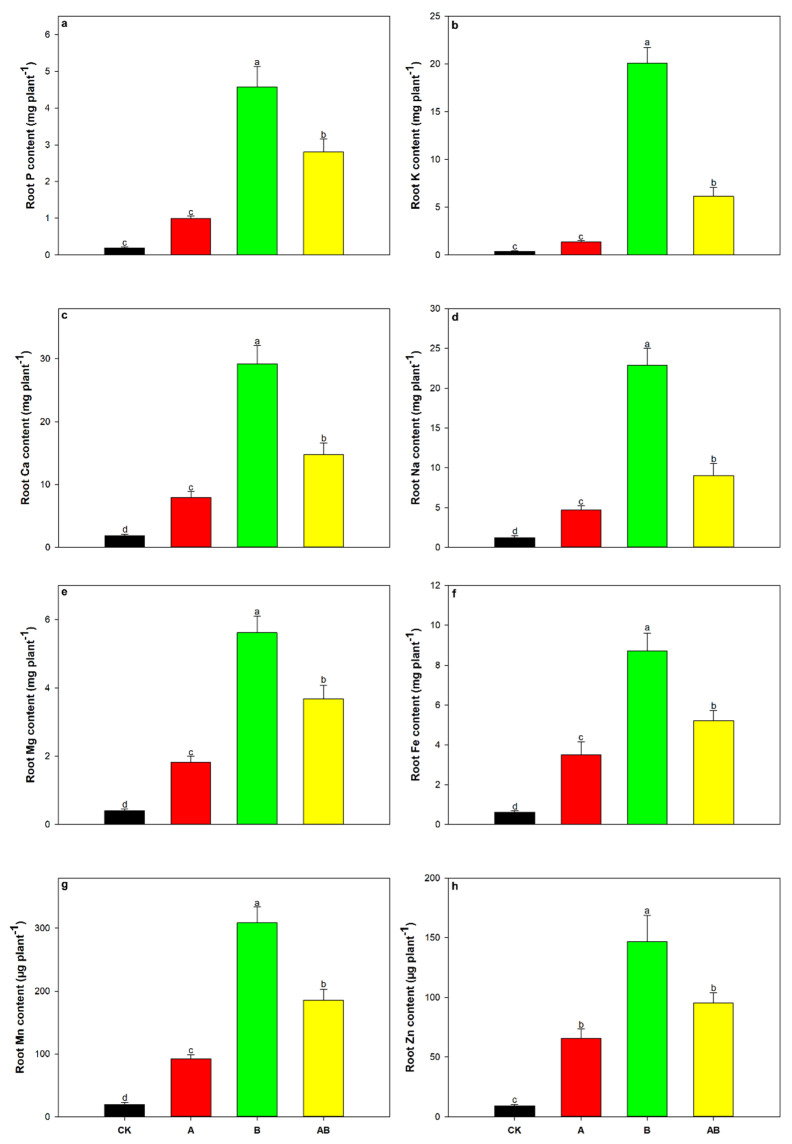
Maize shoot nutrients, including P, K, Ca, Na, Mg, Fe, Mn, and Zn, were significantly influenced by biochar and AMFs, as well as the interaction between biochar and AMFs (Table 2, Figure 4a–h). Compared with the control treatment, shoot P, K, Ca, Mg, Mn, and Zn contents were significantly increased by both the individual and dual applications of AMFs and biochar (Figure 4a–c,e,g,h). The dual application of AMFs and biochar significantly increased shoot Na content by 54.2% (Figure 4d), and the shoot Fe content was significantly increased by a factor of 1.8 by biochar addition compared with the control (Figure 4f). Compared with the control treatment, all shoot nutrient contents were substantially increased by the individual or dual application of AMFs and biochar, although root P and K contents were not significantly affected by AMFs (Figure 5a–h). For all root nutrients, the highest value was found in the biochar treatment group, followed by the dual AMF and biochar treatment, AMF-only treatment, and the control treatment groups (Figure 5a–h).
Figure 4.
Effects of AMFs and biochar on shoot (a) P, (b) K, (c) Ca, (d) Na, (e) Mg, (f) Fe, (g) Mn, and (h) Zn contents. Data are means of five replicates ±SEs. Different letters above the columns indicate significant differences according to Duncan’s test at p < 0.05. CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar.
Figure 5.
Effects of AMFs and biochar on root (a) P, (b) K, (c) Ca, (d) Na, (e) Mg, (f) Fe, (g) Mn, and (h) Zn contents. Data are means of five replicates ±SEs. Different letters above the columns indicate significant differences according to Duncan’s test at p < 0.05. CK, control; A, arbuscular mycorrhizal fungi; B, biochar; AB, arbuscular mycorrhizal fungi and biochar.
4. Discussion
4.1. Effects of AMFs
Plant nutrients play a critical role in driving a myriad of fundamental ecological processes, such as photosynthesis, plant growth and competition, pathogen infection, decomposition, and coupled biogeochemical cycling [25,26]. The extraradical mycelium of AMFs can expand the root surface, facilitating the acquisition of nutrients from soil [27,28]. There was a decrease in soil nutrient concentrations (e.g., NH4+-N, NO3−-N, and A-P) in the AMF treatment group, which may have been due to the high nutrient uptake promoted by AMFs in both roots and shoots, as shown in this study (particularly P) and earlier ones [29,30,31]. Furthermore, AMFs influence soil phosphatase enzymatic activity and soil physicochemical properties by improving phosphorus availability to the host plant [16]. Alkaline phosphatase activity was significantly higher in mycorrhizal roots than in non-mycorrhizal maize roots. Similarly, Liu et al. [32] detected mycorrhiza-specific phosphatase (MPSase) only in the root extract colonized by mycorrhizal fungi compared with the control treatment.
Photosynthetic pigments, such as chlorophyll and carotenoid pigments, are essential for capturing light energy during photosynthesis [33]. The mycorrhiza treatment resulted in a significant increase in chlorophyll a, chlorophyll b, and total chlorophyll contents, reflecting considerable enhancements in the photosynthetic capacity and efficiency of maize [34]. Our results agree with those obtained by Malik et al. [35], who found that photosynthetic pigments increased in the presence of AMFs. The release of hormonal signals that promote chloroplast formation may potentially play a role, as well as the nutritional intake of elements including phosphorus and magnesium [36].
4.2. Effects of Biochar
Biochar application resulted in increased maize nutrient uptake and growth, which was consistent with findings of previous studies [37,38]. This may be because biochar can act as a nutrient source (e.g., N, P, and K) to directly improve plant growth [39]. In this study, we found that applying biochar to soil significantly enhanced the amount of accessible phosphorus in the soil. Furthermore, biochar can improve the physical and chemical properties of soil, such as bulk density, porosity, and water storage capacity, thus increasing the retention of water and nutrients and decreasing nutrient leaching [40]. In addition, biochar usage greatly increased soil alkaline phosphatase activity, which may have been a reaction to the recycling of organic phosphorus in soil. Biochar application can boost photosynthesis and chlorophyll content in a variety of plants [41,42]. However, neither the chlorophyll nor carotenoid content was influenced by biochar application in our experiment. Similarly, Rehman et al. [43] failed to find any relationship between the use of biochar and chlorophyll content in wheat and rice. The effect of biochar on photosynthetic pigments may depend on biochar type, application rate, soil conditions, and plant species [44].
4.3. Combined Effect of AMFs and Biochar
Biochar-induced increases in the mycorrhizal colonization rate have been observed in previous studies [45,46], as a result of changes in soil properties, alterations in soil microbial activities, or provision of refugia for AMFs [18,47]. Consequently, the combined application of biochar and AMFs may have a synergistic effect on plant growth [48]. However, biochar application significantly diminished the mycorrhizal colonization of maize in this study. Moreover, although the dual application of AMFs and biochar increased maize performance compared with the control, the benefits of dual application never exceeded the unique effect of biochar application alone. Our results are inconsistent with a previous study by Jabborova et al. [49], who found that the combined application of biochar and AMFs had a greater impact on Spinacia oleracea growth, root morphological traits, physiological properties, and soil enzymatic activities than individual application. The results did not support our hypothesis that the dual treatment has synergistic effects on plant performance.
Previous studies have shown that the release of biochar P into soil can inhibit AM colonization and external hyphal growth [50], consequently diminishing the host’s nutrient uptake and benefits from AMFs. In this experiment, there was a profound increase in soil available P following biochar application. Furthermore, mycorrhizal root colonization was significantly negatively correlated with soil available phosphorus (Figure S1). The mycorrhizal dependency was 80.57% in non-biochar amended soil, but it decreased to −28.67% after biochar was added to the soil. The negative value of mycorrhizal dependency indicated a parasitic association between mycorrhizal fungi and maize plants when biochar was applied to soil [51,52]. In the present study, we show for the first time that the colonization and benefits of AMFs in maize can be reduced by the presence of biochar. Similarly, LeCroy et al. [53] reported that, under high N conditions, the combined biochar and AMF treatment decreased plant biomass compared with the treatment without biochar. In addition, previous studies showed that the combined application of biochar and AMF showed the significant effect of improving maize growth, especially under nutrient-limited, saline, or water stress [54,55,56]. The combined effect of biochar and AMF on plant growth may vary for different biochar types (i.e., biochar feedstock and production temperature) and application rates [57,58]. For example, biochar produced from leaves improved soil P availability more than that produced from woodchip biochar [59], and biochar produced at a higher temperature increased AMF root colonization and spore germination more than that produced at a lower temperature, due to polycyclic aromatic hydrocarbons (PAHs) produced by biochar at low temperatures [46]. Besides that, wide variability in morphological, physiological, molecular, and biochemical responses to biochar and AMFs have been observed among different maize varieties [60,61,62]. Since only one maize variety (Zhengdan 958) was used in our present experiment, inconsistent results may be obtained with other varieties [54]. The use of AMFs that are compatible with biochar and crop varieties combinations may provide satisfactory results [63]. For example, biochar combined with Claroideoglomus etunicatum showed a more profuse root system in strawberries than biochar combined with C. pellucida or mycorrhizal communities [64]. Our results suggest that applying biochar to soil may reduce AMF development and, consequently, mycorrhizal benefits [65,66,67].
5. Conclusions
Individual application of AMFs and biochar had positive effects on maize shoot, root, and total biomass; plant height; basal diameter; leaf number; the majority of mineral content in shoots and roots (P, K, Ca, Na, Mg, Fe, Zn, and Mn); and alkaline phosphatase activity. In addition, the chlorophyll a, chlorophyll b, and total chlorophyll contents in maize leaves were significantly increased by AMF inoculation. However, the combination of biochar and AMFs had no synergistic effects on maize performance in the present study. Furthermore, we show for the first time that biochar addition to soil significantly reduces mycorrhizal colonization in maize roots, thus reducing mycorrhizal benefits. Our study indicates a parasitic association between mycorrhizal fungi and maize plants when biochar is applied to soil. We conclude that biochar and AMF application can affect maize growth, nutrient uptake, physiological properties, and soil enzymatic activity. Our study can provide vital information for further resource use optimization in agroecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8121275/s1, Figure S1: Pearson correlation analysis (r) between maize growth parameters and soil properties at harvest, Figure S2: Effects of AMFs and biochar on shoot (a) P, (b) K, (c) Ca, (d) Na, (e) Mg, (f) Fe, (g) Mn, and (h) Zn concentrations, Figure S3: Effects of AMFs and biochar on root (a) P, (b) K, (c) Ca, (d) Na, (e) Mg, (f) Fe, (g) Mn, and (h) Zn concentrations.
Author Contributions
N.-N.S. and S.F. conceived and designed the experiments; Q.J., Y.L., T.Z., J.C., Y.R., K.D. and S.X. performed the pot experiments and collected the samples; J.S. analyzed the data and wrote the manuscript; N.-N.S. and S.F. polished the English to improve the quality of this manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Natural Science Foundation of China (No. 31800438), Key Science and Technology Research and Development & Promotion Project of Henan (No. 202102110064), and Interdisciplinary Research for First-class Discipline Construction Project of Henan University (No. 2019YLXKJC04).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lividini K., Masters W.A. Tracing global flows of bioactive compounds from farm to fork in nutrient balance sheets can help guide intervention towards healthier food supplies. Nat. Food. 2022;3:703–715. doi: 10.1038/s43016-022-00585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin H.J., Zhao W.Q., Li T., Cheng X.Y., Liu Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018;81:2695–2702. doi: 10.1016/j.rser.2017.06.076. [DOI] [Google Scholar]
- 3.Bai Y.C., Chang Y.Y., Hussain M., Lu B., Zhang J.P., Song X.B., Lei X.S., Pei D. Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms. 2020;8:694. doi: 10.3390/microorganisms8050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostadi A., Javanmard A., Machiani M.A., Morshedloo M.R., Nouraein M., Rasouli F., Maggi F. Effect of different fertilizer sources and harvesting time on the growth characteristics, nutrient uptakes, essential oil productivity and composition of Mentha x piperita L. Ind. Crops Prod. 2020;148:112290. doi: 10.1016/j.indcrop.2020.112290. [DOI] [Google Scholar]
- 5.Wang Y.F., Chen P., Wang F.H., Han W.X., Qiao M., Dong W.X., Hu C.S., Zhu D., Chu H.Y., Zhu Y.G. The ecological clusters of soil organisms drive the ecosystem multifunctionality under long-term fertilization. Environ. Int. 2022;161:107133. doi: 10.1016/j.envint.2022.107133. [DOI] [PubMed] [Google Scholar]
- 6.Leake J.R., Johnson D., Donnelly D.P., Muckle G.E., Boddy L., Read D.J. Networks of power and influence: The role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004;82:1016–1045. doi: 10.1139/b04-060. [DOI] [Google Scholar]
- 7.Smith S.E., Read D.J. Mycorrhizal Symbiosis. 3rd ed. Academic Press; Cambridge, UK: 2008. pp. 1–787. [Google Scholar]
- 8.Foo E., Ross J.J., Jones W.T., Reid J.B. Plant hormones in arbuscular mycorrhizal symbioses: An emerging role for gibberellins. Ann. Bot. 2013;111:769–779. doi: 10.1093/aob/mct041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard M.L., Belval L., Martin I.R., Roth L., Laloue H., Deglene-Benbrahim L., Valat L., Bertsch C., Chong J.L. Arbuscular mycorrhizal symbiosis triggers major changes in primary metabolism together with modification of defense responses and signaling in both roots and leaves of Vitis vinifera. Front. Plant Sci. 2021;12:721614. doi: 10.3389/fpls.2021.721614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohi S.P., Krull E., Lopez-Capel E., Bol R. A review of biochar and its use and function in soil. In: Sparks D.L., editor. Advances in Agronomy. Volume 105. Elsevier; Amsterdam, The Netherlands: 2010. pp. 47–82. [Google Scholar]
- 11.Jeffery S., Verheijen F.G.A., van der Velde M., Bastos A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011;144:175–187. doi: 10.1016/j.agee.2011.08.015. [DOI] [Google Scholar]
- 12.Haider F.U., Coulter J.A., Cai L.Q., Hussain S., Cheema S.A., Wu J., Zhang R.Z. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere. 2022;32:107–130. doi: 10.1016/S1002-0160(20)60094-7. [DOI] [Google Scholar]
- 13.Razzaghi F., Obour P.B., Arthur E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma. 2020;361:114055. doi: 10.1016/j.geoderma.2019.114055. [DOI] [Google Scholar]
- 14.Lehmann J., Rillig M.C., Thies J., Masiello C.A., Hockaday W.C., Crowley D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011;43:1812–1836. doi: 10.1016/j.soilbio.2011.04.022. [DOI] [Google Scholar]
- 15.Schmidt M.W.I., Torn M.S., Abiven S., Dittmar T., Guggenberger G., Janssens I.A., Kleber M., Kogel-Knabner I., Lehmann J., Manning D.A.C., et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 16.Vanek S.J., Lehmann J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil. 2015;395:105–123. doi: 10.1007/s11104-014-2246-y. [DOI] [Google Scholar]
- 17.Mickan B.S., Abbott L.K., Stefanova K., Solaiman Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza. 2016;26:565–574. doi: 10.1007/s00572-016-0693-4. [DOI] [PubMed] [Google Scholar]
- 18.Warnock D.D., Lehmann J., Kuyper T.W., Rillig M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil. 2007;300:9–20. doi: 10.1007/s11104-007-9391-5. [DOI] [Google Scholar]
- 19.Yang W., Zhao Y., Yang Y., Zhang M., Mao X., Guo Y., Li X., Tao B., Qi Y., Ma L., et al. Co-application of biochar and microbial inoculants increases soil phosphorus and potassium fertility and improves soil health and tomato growth. J. Soils Sediments. 2022 doi: 10.1007/s11368-022-03347-0. [DOI] [Google Scholar]
- 20.Jabborova D., Annapurna K., Azimov A., Tyagi S., Pengani K.R., Sharma P., Vikram K.V., Poczai P., Nasif O., Ansari M.J., et al. Co-inoculation of biochar and arbuscular mycorrhizae for growth promotion and nutrient fortification in soybean under drought conditions. Front. Plant Sci. 2022;13:947547. doi: 10.3389/fpls.2022.947547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa R., Calvete E.O., Spengler N., Chiomento J., Paula J.J.A.S.A. Morpho-phenological and agronomic performance of strawberry cultivars with different photoperiodic flowering responses. Sci. Hortic. 2021;43:e45189. [Google Scholar]
- 22.Jabborova D., Annapurna K., Choudhary R., Bhowmik S.N., Desouky S.E., Selim S., Azab I.H.E., Hamada M.M.A., Nahhas N.E., Elkelish A. Interactive impact of biochar and arbuscular mycorrhizal on root morphology, physiological properties of fenugreek (Trigonella foenum-graecum L.) and soil enzymatic activities. Agronomy. 2021;11:2341. doi: 10.3390/agronomy11112341. [DOI] [Google Scholar]
- 23.Cairns J.E., Prasanna B.M. Developing and deploying climate-resilient maize varieties in the developing world. Curr. Opin. Plant Biol. 2018;45:226–230. doi: 10.1016/j.pbi.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGonigle T.P., Miller M.H., Evans D.G., Fairchild G.L., Swan J.A. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 25.Lambers H., Raven J.A., Shaver G.R., Smith S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008;23:95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Bahram M., Netherway T., Hildebrand F., Pritsch K., Drenkhan R., Loit K., Anslan S., Bork P., Tedersoo L. Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol. 2020;227:1189–1199. doi: 10.1111/nph.16598. [DOI] [PubMed] [Google Scholar]
- 27.Hodge A., Campbell C.D., Fitter A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature. 2001;413:297–299. doi: 10.1038/35095041. [DOI] [PubMed] [Google Scholar]
- 28.Etesami H., Jeong B.R., Glick B.R. Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021;12:699618. doi: 10.3389/fpls.2021.699618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodge A., Storer K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil. 2015;386:1–19. doi: 10.1007/s11104-014-2162-1. [DOI] [Google Scholar]
- 30.Hodge A., Fitter A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA. 2010;107:13754–13759. doi: 10.1073/pnas.1005874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavagnaro T.R., Barrios-Masias F.H., Jackson L.E. Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil. 2012;353:181–194. doi: 10.1007/s11104-011-1021-6. [DOI] [Google Scholar]
- 32.Liu Y., Zhang G., Luo X., Hou E., Zheng M., Zhang L., He X., Shen W., Wen D. Mycorrhizal fungi and phosphatase involvement in rhizosphere phosphorus transformations improves plant nutrition during subtropical forest succession. Soil Biol. Biochem. 2021;153:108099. doi: 10.1016/j.soilbio.2020.108099. [DOI] [Google Scholar]
- 33.Dashwood R.H. Chlorophylls as anticarcinogens (review) Int. J. Oncol. 1997;10:721–727. doi: 10.3892/ijo.10.4.721. [DOI] [PubMed] [Google Scholar]
- 34.Ghani M.I., Ali A., Atif M.J., Ali M., Amin B., Cheng Z. Arbuscular mycorrhizal fungi and dry raw garlic stalk amendment alleviate continuous monocropping growth and photosynthetic declines in eggplant by bolstering its antioxidant system and accumulation of osmolytes and secondary metabolites. Front. Plant Sci. 2022;13:849521. doi: 10.3389/fpls.2022.849521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik J.A., AlQarawi A.A., Dar B.A., Hashem A., Alshahrani T.S., AlZain M.N., Habib M.M., Javed M.M., Abd-Allah E.F. Arbuscular mycorrhizal fungi isolated from highly saline “sabkha habitat” soil alleviated the NaCl-induced stress and improved Lasiurus scindicus Henr. growth. Agriculture. 2022;12:337. doi: 10.3390/agriculture12030337. [DOI] [Google Scholar]
- 36.Cortleven A., Schmulling T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015;66:4999–5013. doi: 10.1093/jxb/erv132. [DOI] [PubMed] [Google Scholar]
- 37.Liang J.F., An J., Gao J.Q., Zhang X.Y., Song M.H., Yu F.H. Interactive effects of biochar and AMF on plant growth and greenhouse gas emissions from wetland microcosms. Geoderma. 2019;346:11–17. doi: 10.1016/j.geoderma.2019.03.033. [DOI] [Google Scholar]
- 38.Efthymiou A., Jensen B., Jakobsen I. The roles of mycorrhiza and Penicillium inoculants in phosphorus uptake by biochar-amended wheat. Soil Biol. Biochem. 2018;127:168–177. doi: 10.1016/j.soilbio.2018.09.027. [DOI] [Google Scholar]
- 39.Hossain M.Z., Bahar M.M., Sarkar B., Donne S.W., Ok Y.S., Palansooriya K.N., Kirkham M.B., Chowdhury S., Bolan N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar. 2020;2:379–420. doi: 10.1007/s42773-020-00065-z. [DOI] [Google Scholar]
- 40.Laird D., Fleming P., Wang B., Horton R., Karlen D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma. 2010;158:436–442. doi: 10.1016/j.geoderma.2010.05.012. [DOI] [Google Scholar]
- 41.Shakeel H., Jahan S., Rafiq K., Iqbal S., Rasul F. Efficacy of biochar-supplemented soil for modification of physio-biochemical attributes of Canola (Brassica napus L.) genotypes under different moisture regimes. J. Soil Sci. Plant Nutr. 2022;22:3667–3684. doi: 10.1007/s42729-022-00918-5. [DOI] [Google Scholar]
- 42.Guo L., Yu H., Kharbach M., Wang J. The response of nutrient uptake, photosynthesis and yield of tomato to biochar addition under reduced nitrogen application. Agronomy. 2021;11:1598. doi: 10.3390/agronomy11081598. [DOI] [Google Scholar]
- 43.Rehman M.Z.u., Rizwan M., Khalid H., Ali S., Naeem A., Yousaf B., Liu G., Sabir M., Farooq M. Farmyard manure alone and combined with immobilizing amendments reduced cadmium accumulation in wheat and rice grains grown in field irrigated with raw effluents. Chemosphere. 2018;199:468–476. doi: 10.1016/j.chemosphere.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 44.He Y.H., Yao Y.X., Ji Y.H., Deng J., Zhou G.Y., Liu R.Q., Shao J.J., Zhou L.Y., Li N., Zhou X.H., et al. Biochar amendment boosts photosynthesis and biomass in C3 but not C4 plants: A global synthesis. Glob. Chang. Biol. Bioenergy. 2020;12:605–617. doi: 10.1111/gcbb.12720. [DOI] [Google Scholar]
- 45.Rafique M., Ortas I., Rizwan M., Chaudhary H.J., Gurmani A.R., Munis M.F.H. Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere. 2020;238:124710. doi: 10.1016/j.chemosphere.2019.124710. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q., Ravnskov S., Andersen M.N. Nutrient uptake and growth of potato: Arbuscular mycorrhiza symbiosis interacts with quality and quantity of amended biochars. J. Soil Sci. Plant Nutr. 2020;183:220–232. doi: 10.1002/jpln.201900205. [DOI] [Google Scholar]
- 47.Yang X., Ran Z., Li R., Fang L., Zhou J., Guo L. Effects of biochar on the growth, ginsenoside content, and soil microbial community composition of Panax quinquefolium L. J. Soil Sci. Plant Nutr. 2022;22:2670–2686. doi: 10.1007/s42729-022-00835-7. [DOI] [Google Scholar]
- 48.Abou El Seoud I.I.A. Effect of biochar rates on A-mycorrhizal fungi performance and maize plant growth, Phosphorus uptake, and soil P availability under calcareous soil conditions. Commun. Soil Sci. Plant Anal. 2021;52:815–831. [Google Scholar]
- 49.Jabborova D., Annapurna K., Paul S., Kumar S., Saad H.A., Desouky S., Ibrahim M.F.M., Elkelish A. Beneficial features of biochar and arbuscular mycorrhiza for improving spinach plant growth, root morphological traits, physiological properties, and soil enzymatic activities. J. Fungi. 2021;7:571. doi: 10.3390/jof7070571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott L.K., Robson A.D. The effect of root density, inoculum placement and infectivity of inoculum on the development of vesicular arbuscular mycorrhizas. New Phytol. 1984;97:285–299. doi: 10.1111/j.1469-8137.1984.tb04133.x. [DOI] [Google Scholar]
- 51.Cavagnaro R.A., Oyarzabal M., Oesterheld M., Grimoldi A.A. Species-specific trade-offs between regrowth and mycorrhizas in the face of defoliation and phosphorus addition. Fungal Ecol. 2021;51:101058. doi: 10.1016/j.funeco.2021.101058. [DOI] [Google Scholar]
- 52.Johnson N.C., Graham J.H., Smith F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997;135:575–586. doi: 10.1046/j.1469-8137.1997.00729.x. [DOI] [Google Scholar]
- 53.LeCroy C., Masiello C.A., Rudgers J.A., Hockaday W.C., Silberg J.J. Nitrogen, biochar, and mycorrhizae: Alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 2013;58:248–254. doi: 10.1016/j.soilbio.2012.11.023. [DOI] [Google Scholar]
- 54.Javeed H.M.R., Naeem R., Ali M., Qamar R., Sarwar M.A., Nawaz F., Atiqueur R., Shehzad M., Farooq A., Haseebur R., et al. Coupling biochar with microbial inoculants improves maize growth and nutrients acquisition under phosphorous-limited soil. Acta Physiol. Plant. 2022;44:110. doi: 10.1007/s11738-022-03440-4. [DOI] [Google Scholar]
- 55.Ndiate N.I., Saeed Q., Haider F.U., Liqun C., Nkoh J.N., Mustafa A. Co-application of biochar and arbuscular mycorrhizal fungi improves salinity tolerance, growth and lipid metabolism of maize (Zea mays L.) in an alkaline soil. Plants. 2021;10:2490. doi: 10.3390/plants10112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M.Y., Cai L.Q. Biochar and arbuscular mycorrhizal fungi play different roles in enabling maize to uptake phosphorus. Sustainability. 2021;13:3244. doi: 10.3390/su13063244. [DOI] [Google Scholar]
- 57.Han Y., Douds D.D., Jr., Boateng A.A. Effect of biochar soil-amendments on Allium porrum growth and arbuscular mycorrhizal fungus colonization. J. Plant Nutr. 2016;39:1654–1662. doi: 10.1080/01904167.2015.1089903. [DOI] [Google Scholar]
- 58.Koide R.T. Biochar-arbuscular mycorrhiza interaction in temperate soils. In: Johnson N.C., Gehring C., Jansa J., editors. Mycorrhizal Mediation of Soil. Elsevier; Berlin, Germany: 2017. pp. 461–477. [Google Scholar]
- 59.Zhou C., Heal K., Tigabu M., Xia L., Hu H., Yin D., Ma X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. Forest. Ecol. Manag. 2020;455:117635. doi: 10.1016/j.foreco.2019.117635. [DOI] [Google Scholar]
- 60.Bashir M.A., Wang X.K., Naveed M., Mustafa A., Ashraf S., Samreen T., Nadeem S.M., Jamil M. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int. J. Environ. Res. Public Health. 2021;18:4461. doi: 10.3390/ijerph18094461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright D.P., Scholes J.D., Read D.J., Rolfe S.A. European and African maize cultivars differ in their physiological and molecular responses to mycorrhizal infection. New Phytol. 2005;167:881–896. doi: 10.1111/j.1469-8137.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- 62.Merlos M.A., Zitka O., Vojtech A., Azcon-Aguilar C., Ferrol N. The arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci. 2016;253:68–76. doi: 10.1016/j.plantsci.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Amery F., Debode J., Ommeslag S., Visser R., De Tender C., Vandecasteele B. Biochar for circular horticulture: Feedstock related effects in soilless cultivation. Agronomy. 2021;11:629. doi: 10.3390/agronomy11040629. [DOI] [Google Scholar]
- 64.dos Santos Trentin T., Dornelles A.G., dos Santos Trentin N., Huzar-Novakowiski J., Calvete E.O., Trevizan Chiomento J.L. Addition of arbuscular mycorrhizal fungi and biochar in the cultivation substrate benefits macronutrient contents in strawberry plants. J. Soil Sci. Plant Nutr. 2022;22:2980–2991. doi: 10.1007/s42729-022-00861-5. [DOI] [Google Scholar]
- 65.Warnock D.D., Mummey D.L., McBride B., Major J., Lehmann J., Rillig M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010;46:450–456. doi: 10.1016/j.apsoil.2010.09.002. [DOI] [Google Scholar]
- 66.Liu M., Zhao Z., Chen L., Wang L., Ji L., Xiao Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicol. Environ. Saf. 2020;196:110537. doi: 10.1016/j.ecoenv.2020.110537. [DOI] [PubMed] [Google Scholar]
- 67.Liu C., Liu F., Ravnskov S., Rubæk G.H., Sun Z., Andersen M.N. Impact of wood biochar and its interactions with mycorrhizal fungi, phosphorus fertilization and irrigation strategies on potato growth. J. Agron. Crop Sci. 2017;203:131–145. doi: 10.1111/jac.12185. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.